Abstract
Background: Hypercalcemia, a common feature in sarcoidosis, is due to the excessive production of active Vitamin D metabolite, 1,25(OH)2D. Levels of 25(OH) Vitamin D however may not be appropriate. Objectives: To assess Vitamin D status and its clinical associations in sarcoidosis patients compared to a general respiratory diseases out-patient clinic population, serving as controls. Methods: 64 sarcoidosis cases and 53 control cases with other than sarcoidosis respiratory diseases, matched for age and sex were included in the study. Serum 25(OH)D, 1,25(OH)2D, calcium, angiotensin converting enzyme (ACE) were measured. 25(OH) Vitamin D was described as deficient when <20 ng/ml and insufficient when <30 ng/ml. Clinical parameters were recorded for sarcoidosis cases. Results: Overall 41/64 sarcoidosis cases (64%) had low 25(OH) D, 7/64 (11%) had high 1,25(OH)2D and 2/64 had hypercalcaemia (3%). Sarcoidosis subjects likely exhibited deficient (39%) or normal 25(OH)D levels (36%) in comparison to controls (p=0.018). 25(OH) Vitamin D deficiency in sarcoidosis was associated with race and radiological stage I disease, with regression analysis identifying African-American race as the only significant risk factor (p=0.03). An inverse correlation between ACE and 25(OH)D levels was found (p=0.052). 1,25(OH)2D was significantly elevated in sarcoidosis compared to controls. Among sarcoidosis patients, those with insufficient 25(OH)D levels exhibited higher calcium levels in serum. Conclusions: 25(OH) Vitamin D deficiency is prevalent in sarcoidosis, particularly in African-Americans and likely those with active disease. However, concomitant 1,25(OH)2D elevation and associated hypercalcaemia make Vitamin D supplementation dangerous in sarcoidosis. (Sarcoidosis Vasc Diffuse Lung Dis 2018; 35: 154-159)
Keywords: sarcoidosis, vitamin D, hypercalcaemia
Introduction
Calcium metabolism abnormalities are a well studied phenomenon in sarcoidosis. Prevalence of hypercalcaemia varies from 4% reported by ACCESS (A Case Control Etiologic Study of Sarcoidosis) investigators to 11% reported in older studies (1, 2). It is known that an active metabolite of Vitamin D, 1,25(OH)2D, is mainly responsible for hypercalcaemia in sarcoidosis (3). Cholecalciferol and ergocalciferol collected through diet and sun exposure are firstly hydroxylated in the liver to 25(OH)D. A second hydroxylation occurs normally in the kidneys by 1a-hydroxylase to 1,25(OH)2D. In sarcoidosis, abnormal extra-renal intracrine 1a-hydroxylase (CYP27B1) production by granulomas’ macrophages leads to excessive, non parathormone related dysregulated production of 1,25(OH)2D, which through increased intestinal absorption and bone resorption of calcium causes hypercalcaemia (4, 5). Hypercalcaemia requires systemic treatment, and sarcoidosis patients are often advised to decrease Vitamin D uptake.
On the other hand, Vitamin D is recently shown to possess serious immune-regulating properties. The active metabolite 1,25(OH)2D through Vitamin D Receptor (VDR) interact with innate immunity cells (monocyte/macrophage, dendritic cells) in order to enhance adaptive immunity and potent antibacterial enzymes (cathelicidin) against microbes like Mycobacterium Tuberculosis or induce immune tolerance. The ideal level of serum Vitamin D to promote innate immunity is not known; it seems, however, that sarcoidosis Th-1 cytokine milieu (IFN-γ, TNF-α) may not enhance Vitamin D regulated innate immunity (6, 7).
Recently, several studies have reported on Vitamin D status in sarcoidosis. Baughman and colleagues reported 80% prevalence of 25(OH)D deficiency in their sarcoidosis cohort (8). Burke et al report low (<28 ng/ml) 25(OH)D in 97% of 59 sarcoidosis patients (9). We conducted this study in order to investigate whether decreased 25(OH)D is in sarcoidosis as prevalent as in patients suffering from a variety of acute and chronic respiratory diseases. We then tried to elucidate which risk factors best predicted 25(OH)D deficiency among our sarcoidosis patients. Due to the deranged calcium metabolism described above, we then examined 1,25(OH)2D and calcium levels.
Methods
Study sesign
This was a prospective cross sectional cohort study undertaken from July 2009 through July 2011 in the clinics at University of Southern California-University Hospital (USC-UH) and Los Angeles County+USC (LAC+USC) hospital in Los Angeles, California. The study was approved by the ethics committee of these hospitals and was in accordance with the declaration of Helsinki regarding research in human subjects. All patients signed an informed consent form.
In USC-UH, consecutive sarcoidosis subjects were recruited to participate in the study as they presented at the sarcoidosis outpatient clinic in the above time period. Inclusion criterion was a diagnosis of sarcoidosis based on American Thoracic Society/European Respitory Society/World Association of Sarcoidosis and Other Granulomatous Disorders published criteria (10). These cases were matched for age and gender with subjects from LAC+USC general pulmonary medicine out-patient clinic (controls). Controls were selected from a different hospital because the larger clinic there allowed faster recruitment. Control subjects were included in the study consecutively if they had a diagnosis other than sarcoidosis. All study subjects were excluded from the study if they had comorbidities associated with Vitamin D deficiency, namely small bowel disease, gastrectomy, pancreatic insufficiency, nephrotic syndrome, renal failure, rickets, cirrhosis, hypoparathyroidism, or anticonvulsant therapy. Final exclusion criterion was exogenous calcium and Vitamin D supplementation.
Methods
A blood sample was collected from all study subjects. Laboratory investigations included 25(OH)D, 1,25(OH)2D, calcium and albumin. Reference values for hydroxy-vitamin D was 30-55 ng/ml and for 1,25(OH)2D 24-65 pg/ml. 25(OH)D status was described as deficient for values <20 ng/ml (D), insufficient for values <30 ng/ml (I) and normal for values ≥30 ng/ml (N). In sarcoidosis subjects we recorded Pulmonary Function Tests (PFT’s), serum Angiotensin Converting Enzyme (ACE), chronicity of sarcoidosis, status of current treatment and radiological stage of disease (Scadding).
Statistics
Continuous variables were analyzed with two-tailed t test. Categorical variables between sarcoidosis subjects and controls were evaluated by chi-square test. Pearson’s correlation coefficient (r) was calculated among measured variables and regression analysis was performed for predictors of hydroxy-vitamin D deficiency. Statistical significance was defined as a p value <0.05. IBM SPSS software (version 22.0) and GraphPad Prism 5.0 were used.
Results
Vitamin D
Participants’ entry flowchart is shown in Figure 1. Study subjects demographics and Vitamin D levels are shown in Table 1. African-Americans were more prevalent in the sarcoidosis group. Importantly, sarcoidosis patients exhibited higher values of 1,25(OH)2D (p<0.05). 25(OH)D and calcium levels were not significantly different between sarcoidosis and controls. Only 2 sarcoidosis patients had hypercalcaemia (3%). The overall prevalence of decreased hydroxy-Vitamin D in sarcoidosis was (41/64) 64%, comparable to controls’ overall prevalence (43/53) 81%. However, sarcoidosis cases were more likely to have deficient (39%) or normal (36%) 25(OH)D levels, while controls likely had insufficient 25(OH)D (p=0.018) (Figure 2).
Fig. 1.

Flow chart of participants in the study. Abbreviations: C: Cirrhosis, NS: Nephrotic syndrome, H: Hypoparathyroidism, G: Gastrectomy, RF: Renal failure, VDS: Vitamin D supplementation, ILD: Interstitial Lung Diseases, TBC: Tuberculosis, LRTI: Lower Respiratory Tract Infections, COPD: Chronic Obstructive Pulmonary Disease, BA: Bronchial Asthma, BE: Bronchiectasis, OSA: Obstructive Sleep Apnoea, LC: Lung Cancer, AIDS: Acquired Immunodeficiency Syndrome
Table 1.
Subjects’ demographics and Vitamin D status (measured values are presented as mean ± standard deviation)

Fig. 2.
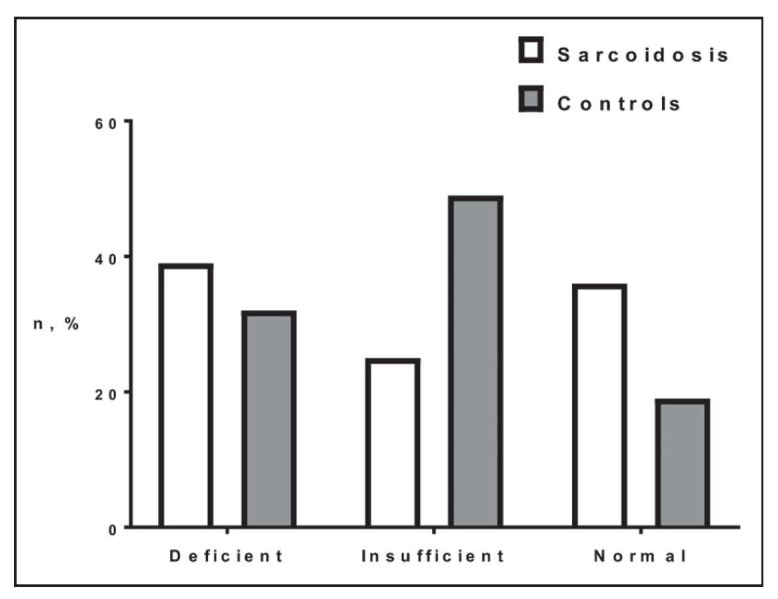
Comparison of sarcoidosis and controls based on 25(OH)D serum levels (Deficient <20 ng/ml; Insufficient <30 ng/ml; Normal ≥30 ng/ml) [p=0.018]
Sarcoidosis
Disease characteristics of sarcoidosis cases according to 25(OH)D status are shown in Table 2. Race and stage of sarcoidosis varied between groups, with African-American race and stage I disease being associated with deficient 25(OH)D (p<0.05). Multiple regression analysis identified race as the only significant risk factor for such deficiency (p=0.03). Correlation analysis revealed a negative association of 25(OH)D value with ACE (r=-0.331) close to statistical significance (p=0.052). Lastly, relatively increased 1,25(OH)2D and significantly higher serum calcium levels (exceeding the normal range) were observed in patients with insufficient 25(OH)D levels, 44% of whom were on treatment.
Table 2.
Characteristics of Sarcoidosis subjects by 25(OH)D status [numerics (% of total), measured variables(±standard deviation)]
Discussion
Our study shows that in sarcoidosis, low 25(OH)-Vitamin D is as prevalent (64%) as in patients with other diseases following an outpatient county respiratory clinic, with deficiency though being more prevalent in sarcoidosis. Risk factors associated with this deficiency in sarcoidosis were African-American race and maybe disease activity as determined by ACE. However, sarcoidosis patients had increased 1,25(OH)2D compared to controls. Further, significant hypercalcaemia was found in sarcoidosis subjects with insufficient 25(OH)D in the serum, highlighting the limited clinical significance of measuring 25(OH)D in sarcoidosis.
The high prevalence of 25(OH) Vitamin D deficiency and insufficiency combined (64%) was expected and is similar to rates reported in all recent research in the field (8, 9). Capolongo et al report abnormally low 25(OH)D in 86,3% and 73.8% of their American and Italian cohorts, respectively (11). Respective prevalence of 61% is reported by Sodhi et al in a community based setting (12). The significant rates of severe deficiency found in our study between sarcoidosis and controls, after matching for sex and age, might be related to the racial heterogeneity with many African-Americans in the sarcoidosis group (Table 1), to nutritional and/or other demographic variances among two groups and not to sarcoidosis per se.
African-American race was found the significant risk factor for 25(OH)D deficiency among our sarcoidosis group. This is a new finding, although Kavvathia et al, similarly, found lower 25(OH)D serum levels in African-Americans than Caucasians (13). African-American race due to dark skin pigmentation, has well established relation to hypovitaminosis D. National Health and Nutrition Examination Survey III (NHANES III) large population study by Zadshir and colleagues reported a prevalence of 34% for Vitamin D deficiency and insufficiency in white men, this being greatly higher in females and minorities (14). This finding is further confirmed in healthy African-Americans compared to healthy Caucasians, as shown in more recent studies implicating genetic polymorphisms in Vitamin D-binding protein (15, 16). This finding prompts special attention to a specific population in danger for 25(OH)D deficiency.
Another finding of this study is the inverse correlation between 25(OH)D levels and sarcoidosis activity as determined by serum ACE, with borderline statistical significance. Two other studies have made similar observations; Kamphuis et al using scintigraphy to evaluate sarcoidosis activity and Seidenberg et al which used clinical parameters and ACE (17, 18). 25(OH)D deficiency observed in Stage I predominantly disease, has been reported in other studies as well, and might relate to newly diagnosed acute disease.
Contrary to controls, our sarcoidosis cases exhibited high levels of the active Vitamin D metabolite, 1,25(OH)2D. In our opinion, this is a very important finding, as reported elsewhere (8, 9). Further, the higher levels of serum calcium and of 1,25(OH)2D were observed in the sarcoidosis subgroup of insufficient 25(OH)D levels (20-30 ng/ml). This observation means that counting only the inactive component of Vitamin D in order to guide possible supplementation is inadequate in sarcoidosis, where extrarenal paracrine active metabolite production takes place, and that Vitamin D supplementation may be unsafe and cause hypercalcaemia, even when 25(OH)D levels are below normal. Desired 25(OH)D levels may be lower in sarcoidosis than in the general population.
In line with this observation, in their study of bone fragility in sarcoidosis, Seidenberg et al found that levels of 25(OH) Vitamin D between 20-30 ng/ml were associated with increased risk of bone fractures, while lesser amounts were associated with higher bone mineral density (18). In their randomized controlled trial of Vitamin D supplementation in sarcoidosis patients with Vitamin D deficiency, Bolland et al found no benefit on skeletal health surrogate markers and a mild increase of dangerous hypercalcaemia (19). 2-fold increased risk of hypercalcaemia has been observed in sarcoidosis patients on Vitamin D supplementation therapy (12). This important issue has been highlighted by other authors as well (20, 21).
Unfortunately, the effect of treatment on sarcoidosis-associated hypercalcaemia was not addressed in our study, as did Baughman and coworkers (8). However, our patients with insufficient 25(OH)D and hypercalcaemia, had the highest levels of 1,25(OH)2D and the highest percentage of patients being on treatment (44%), albeit both not statistically significant. This observation points out the therapeutic implications of calcium metabolism abnormalities in sarcoidosis.
The advantage of our study is that it managed to show, prospectively and in relation to a control population, that albeit low 25(OH)D is as prevalent in sarcoidosis, especially in African-Americans, yet hypercalcaemia occurs and 1,25(OH)2D is elevated in sarcoidosis. The limitations of our study, on the other hand, are mainly the relatively small amount of patients and, as we acknowledged, the heterogeneity of the control group in relation to sarcoidosis group, in racial and demographic terms, since they were recruited in different hospitals, which may have affected our results.
Conclusions
Sarcoidosis patients, especially those of African-American decent, maybe with active disease, exhibit low 25(OH)D similar to control population. Unlike controls, however, they demonstrate high 1,25(OH)2D; associated hypercalcaemia is shown to occur in the event of insufficient 25(OH)D. Thus, determination of Vitamin D status solely by 25(OH)D is inadequate and Vitamin D supplementation should largely be withheld in sarcoidosis.
References
- 1.Siltzbach L, James D, Neville E, et al. Course and prognosis of sarcoidosis around the world. Am J Med. 1974;57:847–52. doi: 10.1016/0002-9343(74)90160-0. [DOI] [PubMed] [Google Scholar]
- 2.Baughman R, Teirstein A, Judson M, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164:1885–9. doi: 10.1164/ajrccm.164.10.2104046. [DOI] [PubMed] [Google Scholar]
- 3.Sharma OP. Vitamin D, calcium, and sarcoidosis. Chest. 1996;109:535–9. doi: 10.1378/chest.109.2.535. [DOI] [PubMed] [Google Scholar]
- 4.Adams JS, Sharma OP, Gacad MA, Singer FR. Metabolism of 25-hydroxyvitamin D3 by cultured pulmonary alveolar macrophages in sarcoidosis. J Clin Invest. 1983;72:1856–60. doi: 10.1172/JCI111147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bell NH, Stern PH, Pantzer E, Sinha TK, DeLuca HF. Evidence that increased circulating 1 alpha, 25-dihydroxyvitamin D is the probable cause for abnormal calcium metabolism in sarcoidosis. J Clin Invest. 1979;64:218–25. doi: 10.1172/JCI109442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barna BP, Culver DA, Kanchwala A, et al. Alveolar macrophage cathelicidin deficiency in severe sarcoidosis. J Innate Immun. 2012;4:569–78. doi: 10.1159/000339149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lagishetty V, Liu NQ, Hewison M. Vitamin D metabolism and innate immunity. Mol Cell Endocrinol. 2011;347:97–105. doi: 10.1016/j.mce.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baughman RP, Janovcik J, Ray M, Sweiss N, Lower EE. Calcium and vitamin D metabolism in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2013;30:113–20. [PubMed] [Google Scholar]
- 9.Burke RR, Rybicki BA, Rao DS. Calcium and vitamin D in sarcoidosis: how to assess and manage. Semin Respir Crit Care Med. 2010;31:474–84. doi: 10.1055/s-0030-1262215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunninghake GW, Costabel U, Ando M, et al. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16:149–73. [PubMed] [Google Scholar]
- 11.Capolongo G, Xu LH, Accardo M, et al. Vitamin-D status and mineral metabolism in two ethnic populations with sarcoidosis. J Investig Med. 2016;64:1025–34. doi: 10.1136/jim-2016-000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sodhi A, Aldrich T. Vitamin D supplementation: not so simple in sarcoidosis. Am J Med Sci. 2016;352:252–7. doi: 10.1016/j.amjms.2016.05.027. [DOI] [PubMed] [Google Scholar]
- 13.Kavathia D, Buckley JD, Rao D, Rybicki B, Burke R. Elevated 1, 25-dihydroxyvitamin D levels are associated with protracted treatment in sarcoidosis. Respir Med. 2010;104:564–70. doi: 10.1016/j.rmed.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zadshir A, Tareen N, Pan D, Norris K, Martins D. The prevalence of hypovitaminosis D among US adults: data from the NHANES III. Ethn Dis. 2005;15:97–101. [PubMed] [Google Scholar]
- 15.Nesby-O’Dell S, Scanlon KS, Cogswell ME, et al. Hypovitaminosis D prevalence and determinants among african american and white women of reproductive age: third national health and nutrition examination survey, 1988-1994. Am J Clin Nutr. 2002;76:187–92. doi: 10.1093/ajcn/76.1.187. [DOI] [PubMed] [Google Scholar]
- 16.Powe CE, Evans MK, Wenger J, et al. Vitamin D-binding protein and vitamin D status of black americans and white americans. N Engl J Med. 2013;369:1991–2000. doi: 10.1056/NEJMoa1306357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamphuis LS, Bonte-Mineur F, van Laar JA, van Hagen PM, van Daele PL. Calcium and vitamin D in sarcoidosis: is supplementation safe. J Bone Miner Res. 2014;29:2498–503. doi: 10.1002/jbmr.2262. [DOI] [PubMed] [Google Scholar]
- 18.Saidenberg-Kermanach N, Semerano L, Nunes H, et al. Bone fragility in sarcoidosis and relationships with calcium metabolism disorders: a cross sectional study on 142 patients. Arthritis Res Ther. 2014;16:R78. doi: 10.1186/ar4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bolland MJ, Wilsher ML, Grey A, et al. Randomised controlled trial of vitamin D supplementation in sarcoidosis. BMJ Open. 2013;3:e003562. doi: 10.1136/bmjopen-2013-003562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sweiss NJ, Lower EE, Korsten P, Niewold TB, Favus MJ, Baughman RP. Bone health issues in sarcoidosis. Curr Rheumatol Rep. 2011;13:265–72. doi: 10.1007/s11926-011-0170-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burke RR, Rybicki BA, Rao DS. Calcium and vitamin D in sarcoidosis: how to assess and manage. Semin Respir Crit Care Med. 2010;31:474–84. doi: 10.1055/s-0030-1262215. [DOI] [PMC free article] [PubMed] [Google Scholar]



