Abstract
Up to one fourth of sarcoidosis patients may have cardiac involvement, what is potentially a life-threatening condition and requires aggressive treatment. Corticosteroids are generally effective in cardiac sarcoidosis, however may have significant short and long term adverse effects. We present a case of a 42-year-old male, who was diagnosed with pulmonary and cardiac sarcoidosis. He was treated initially with corticosteroids and satisfactory improvement was achieved in the lungs but not in the heart. Methotrexate was added as a second line therapy, being beneficial for the heart as well as steroid sparing agent. Cardiac improvement was documented during serial CMR imaging. (Sarcoidosis Vasc Diffuse Lung Dis 2018; 35: 178-181)
Keywords: sarcoidosis, cardiac sarcoidosis, corticosteroids, methotrexate
Introduction
Sarcoidosis is a multisystem granulomatous disease of unknown cause. Granulomatous inflammation from sarcoidosis may occur in any organ (1-3). Clinically evident sarcoidosis involving the heart has been noted in at least 2-7% of patients with sarcoidosis, but latent involvement is much higher and exceeds 20% (4-6).
Early diagnosis and treatment of cardiac sarcoidosis (CS) is critical and may be lifesaving (7, 8). Symptoms, ECG abnormalities or cardiac failure are nonspecific. Currently, gadolinium-enhanced cardiovascular magnetic resonance (CMR) imaging is a well-accepted imaging modality, which determine the presence and extent of cardiac involvement (2, 9).
Despite the lack of randomized trials, extensive clinical experience supports aggressive treatment for CS. Systemic corticosteroids are typically first line therapy. Other immunosuppressants may be useful, although standards in this field are still lacking (10). There remains controversy regarding the clinical efficacy, optimal dose and duration of corticosteroid treatment for cardiac sarcoidosis (11, 12). Based on general consensus, alternative agents may be given to patients who do not respond to corticosteroids or who have significant adverse effects from the steroids.
Case report
We present a case of a 42-year-old male, who was admitted to our hospital in June 2014 due to symmetrical hilar and mediastinal lymphadenopathy and features of interstitial lung disease. He did not complain of palpitations or syncope. Physical examination did not reveal any abnormalities. The ECG at the time of admission showed a single supraventricular premature beat, nonspecific T-wave alterations of the inferior wall. Laboratory testing (including BNP, TSH) were normal. The serum ACE level was elevated (136 IU/l). Histopathological examination of lymph nodes and pulmonary parenchymal obtained from transbronchial biopsy supported the diagnosis of sarcoidosis. Pulmonary function tests (PFTs) revealed severe airway obstruction and desaturation (95-85%) during a 6 minute walk test (6MWT). Transthoracic echocardiography (ECHO) showed hypokinesis of hyperechoic interventricular septum (IVS) and inferior wall with left ventricular ejection fraction (LVEF) of 40%. The pulmonary artery systolic pressure was 32 mmHg. A gadolinium enhanced CMR confirmed decreased global contractibility (LVEF-43%), global edema of left ventricle, focal thickening and edema in the right ventricular (RV) free wall, small areas of delayed enhancement (DE) in the IVS and inferolateral wall. Pulmonary and cardiac sarcoidosis was diagnosed. Due to severe lung impairment and active heart inflammation, steroid treatment (prednisone 60 mg/day with gradually decreasing dose to 25mg/day after 6 months, 15mg/day after 12 months) was started with good tolerance. At follow-up after 6, 12 and 18 months, there was a gradual to complete regression of diffuse lung infiltrations, mediastinal and hilar lymphadenopathy (confirmed in CT scan) together with improvement in PFTs (improvement to moderate obstruction and 90m longer distance in 6MWT without desaturation). ECHO showed a LVEF – 56%, without pulmonary hypertension. Despite improvement in the CMR after 18 months of treatment with LVEF - 53% at the dose of prednisone 15mg/day features of active inflammation of the myocardium persisted (intramural DE pattern in the IVS, intramural and subendocardial DE in the infero-lateral wall, globally increased signal intensity (oedema) of the left and right ventricular myocardium). A CT coronary angiogram revealed normal epicardial coronary vessels. We decided to continue steroid treatment in a reduced dose (prednisone 10 mg/day) but add methotrexate with folate as a second agent (target dose 15 mg/week). A CMR 6 months later showed a resolution of the active inflammatory changes in the myocardium (significantly smaller DE areas in the IVS, normalization of the myocardial signal, no features of oedema) and this persisted for the next 6 months (with improvement of EF to 57%, see fig. 1, 2 and 3, 4).
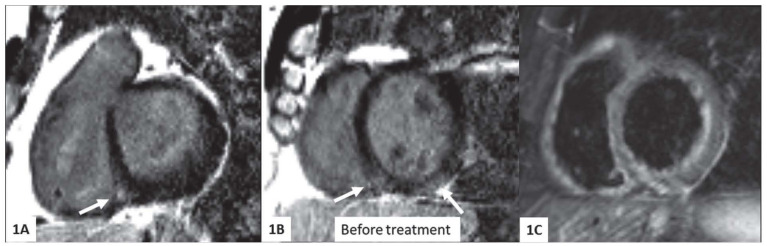
Fig. 1.
CMR images before treatment, short axis plane. 1A, 1B - intramural delayed enhancement (DE) pattern in interventricular septum, intramural and subendocardial DE in the infero-lateral wall (arrows). 1C- globally increased signal intensity (oedema) of the left and right ventricular myocardium, present on T2-weigted STIR image
Fig. 2.
CMR images obtained one year after treatment modification showing significant improvement. 2A, 2B - significantly smaller DE areas in the IVS (arrows), 2C - normalization of the myocardial signal on T2-weigted STIR image
Fig. 3.
Cine images in four chamber plane obtained before treatment onset showing decreased systolic LV function: LVEF assessed from short axis planes was 43%. 3A - end systolic image; 3B - end diastolic image
Fig. 4.
Images assessed one year after treatment modification showing normalization of systolic LV function: LVEF assessed from short axis plains was 57 %. 4A - end systolic image; 4B - end diastolic image
Conclusion
Early treatment with corticosteroids is a well-accepted treatment strategy in cardiac sarcoidosis. However, it can be limited by significant short term and long term side effects.
In our case corticosteroids appeared not effective enough to stop inflammatory process in the heart, despite their efficacy in treating pulmonary disease. Alternative agents can be used as steroid sparing or steroid minimising options. In our case presented methotrexate was an effective addition in the treatment of CS.
References
- 1.Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160(2):736–55. doi: 10.1164/ajrccm.160.2.ats4-99. [DOI] [PubMed] [Google Scholar]
- 2.Judson MA, Costabel U, Drent M, Wells A, Maier L, Koth L, et al. The WASOG Sarcoidosis Organ Assessment Instrument: An update of a previous clinical tool. Sarcoidosis Vasc Diffuse Lung Dis. 2014;31(1):19–27. [PubMed] [Google Scholar]
- 3.Judson MA. The three tiers of screening for sarcoidosis organ involvement. Respir Med. 2016;113:42–49. doi: 10.1016/j.rmed.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 4.Martusewicz-Boros MM, Boros PW, Wiatr E, Zych J, Piotrowska-Kownacka D, Roszkowski-Sliz K. Prevalence of cardiac sarcoidosis in white population: a case-control study: Proposal for a novel risk index based on commonly available tests. Medicine (Baltimore) 2016;95(32):e4518. doi: 10.1097/MD.0000000000004518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silverman KJ, Hutchins GM, Bulkley BH. Cardiac sarcoid: a clinicopathologic study of 84 unselected patients with systemic sarcoidosis. Circulation. 1978;58(6):1204–11. doi: 10.1161/01.cir.58.6.1204. [DOI] [PubMed] [Google Scholar]
- 6.Baughman RP, Teirstein AS, Judson MA, Rossman MD, Yeager H, Jr, Bresnitz EA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164(10 Pt 1):1885–9. doi: 10.1164/ajrccm.164.10.2104046. [DOI] [PubMed] [Google Scholar]
- 7.Mantini N, Williams B, Stewart J, Rubinsztain L, Kacharava A. Cardiac Sarcoid: A Clinician’s Review on How to Approach the Patient With Cardiac Sarcoid. Clinical Cardiology. 2012;35(7):410–415. doi: 10.1002/clc.21982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yazaki Y, Isobe M, Hiroe M, Morimoto S, Hiramitsu S, Nakano T, et al. Prognostic determinants of long-term survival in Japanese patients with cardiac sarcoidosis treated with prednisone. Am J Cardiol. 2001;88(9):1006–10. doi: 10.1016/s0002-9149(01)01978-6. [DOI] [PubMed] [Google Scholar]
- 9.Birnie DH, Sauer WH, Bogun F, Cooper JM, Culver DA, Duvernoy CS, et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014;11(7):1305–1323. doi: 10.1016/j.hrthm.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 10.Nagai S, Yokomatsu T, Tanizawa K, Ikezoe K, Handa T, Ito Y, et al. Treatment with Methotrexate and Low-dose Corticosteroids in Sarcoidosis Patients with Cardiac Lesions. Intern Med. 2014;53(5):427–433. doi: 10.2169/internalmedicine.53.0794. [DOI] [PubMed] [Google Scholar]
- 11.Nagai T, Nagano N, Sugano Y, Asaumi Y, Aiba T, Kanzaki H, et al. Effect of Corticosteroid Therapy on Long-Term Clinical Outcome and Left Ventricular Function in Patients With Cardiac Sarcoidosis. Circ J. 2015;79(7):1593–1600. doi: 10.1253/circj.CJ-14-1275. [DOI] [PubMed] [Google Scholar]
- 12.Chapelon-Abric C, de Zuttere D, Duhaut P, Veyssier P, Wechsler B, Huong DL, et al. Cardiac sarcoidosis: a retrospective study of 41 cases. Medicine (Baltimore) 2004;83(6):315–34. doi: 10.1097/01.md.0000145367.17934.75. [DOI] [PubMed] [Google Scholar]