Abstract
Background and objective: Generally, a disease-specific health-related quality of life (HRQOL) measurement is more useful than generic measures in assessing perceived physical and mental health characteristic of a particular disease. The idiopathic pulmonary fibrosis (IPF)-specific version of St. George’s Respiratory Questionnaire (SGRQ-I) has been recently developed for patients with IPF. We proposed to evaluate associations between the SGRQ-I and other clinical indices, as well as its prognostic value in patients with IPF. Methods: Fifty-two patients with IPF were recruited in this prospective cohort study. HRQOL was assessed using the SGRQ-I and the Medical Outcomes Study 36-item Short Form, dyspnea using the modified Medical Research Council (mMRC) dyspnea scale, and psychological status using the Hospital Anxiety and Depression Scale (HADS). We then evaluated the relationship between the SGRQ-I and other clinical measures, as well as one-year clinical deterioration defined as a hospital admission due to respiratory exacerbation or all-cause death. Results: Stepwise multiple-regression analyses revealed that the mMRC dyspnea scale, the HADS anxiety or depression, and minimum oxygen saturation during a six-minute walk test significantly contributed to the Total and three components of the SGRQ-I. In multivariate Cox proportional-hazards analyses, the Total score of SGRQ-I predicted clinical deterioration independent of forced vital capacity, the six-minute walk distance, or partial pressure of arterial oxygen on room air. Conclusions: The SGRQ-I is a multidisciplinary instrument representing physical, functional and psychological impairments in patients with IPF. The SGRQ-I is a significant predictor of short-term disease progression independent of physiological measurements. (Sarcoidosis Vasc Diffuse Lung Dis 2017; 34: 226-235)
Keywords: idiopathic pulmonary fibrosis, health-related quality of life, IPF-specific version of St. George’s Respiratory Questionnaire, clinical deterioration
Abbreviations:
- ABG, arterial blood gas
- COPD, chronic obstructive pulmonary disease
- CPFE, combined pulmonary fibrosis and emphysema
- DLCO, diffusing capacity of the lung for carbon monoxide
- %DLCO, percentage of the predicted DLCO
- FVC, forced vital capacity
- %FVC, percentage of the predicted FVC
- HADS, Hospital Anxiety and Depression Scale
- HRQOL, health-related quality of life
- IPF, idiopathic pulmonary fibrosis
- mMRC, modified Medical Research Council
- 6MWD, six-minute walk distance
- 6MWT, six-minute walk test
- PaO2, partial pressure of arterial oxygen
- PFT, pulmonary function test
- PRO, patient-reported outcome
- PSQI, Pittsburgh Sleep Quality Index
- ROC, receiver operating characteristic
- Rs, Spearman’s rank correlation coefficient
- SF-36, Medical Outcomes Study 36-item Short Form
- SGRQ, St. George’s Respiratory Questionnaire
- SGRQ-I, IPF-specific version of SGRQ
- SpO2, oxygen saturation measured by pulse oximetry.
Introduction
Idiopathic pulmonary fibrosis (IPF) is a progressive fibrotic pulmonary disease with a poor prognosis (1). IPF has diverse features and various clinical courses. Patients with IPF demonstrate refractory progressive fibrosis, with a median survival of approximately three years, and sometimes develop acute exacerbations (1, 2), whereas some patients with IPF remain stable over the long term (3). In clinical trials, pulmonary function measurements such as forced vital capacity (FVC) often serve as primary endpoints. Recently, however, multidisciplinary and comprehensive assessments including not only pulmonary function measurements but also patient-reported outcomes (PROs) such as health-related quality of life (HRQOL) and dyspnea have been recognized as important in IPF (4), given that chronic progressive symptoms that may not be well controlled by novel IPF drugs adversely affect the HRQOL (5, 6).
The St. George’s Respiratory Questionnaire (SGRQ) is one of the most common instruments for evaluating HRQOL in patients with IPF (7, 8). Although the SGRQ was originally developed for patients with chronic airflow limitation such as chronic obstructive pulmonary disease (COPD) or asthma (9), it has been validated for use in other pulmonary diseases, including IPF (7, 10, 11). Recently, an IPF-specific version of SGRQ (SGRQ-I), a simpler version of the SGRQ, has been developed for patients with IPF, with certain SGRQ items removed because of their lesser relevance to patients with IPF (12). However, the characteristics of the SGRQ-I have not been well established.
Although the SGRQ has been shown to predict mortality in patients with COPD (13, 14), no such relationship has been demonstrated in patients with IPF (15). We previously reported that the relationship between HRQOL and mortality in COPD was partly dependent on the questionnaire used (14, 16). We hypothesized that the SGRQ-I, which is more specific to IPF than the SGRQ, would be associated with disease progression in patients with IPF. In this prospective single-center cohort study of IPF, we sought to identify clinical indices associated with the SGRQ-I cross-sectionally and to determine the association between the SGRQ-I and the short-term progression of IPF longitudinally.
Methods
Patients
We prospectively enrolled 52 consecutive patients with IPF who attended the Department of Respiratory Medicine at Kyoto University Hospital from April 2013 to October 2014. The diagnosis of IPF was made according to the criteria for IPF (1). Patients who were <20 years old and had active malignant disease or cognitive impairment were excluded. This study was approved by the Ethics Committee of Kyoto University (approval number; E1765), and all patients provided written informed consent. This study was registered on UMIN.org (UMIN000011142).
Data collection
At baseline, all patients underwent blood tests including an arterial blood gas (ABG), pulmonary function tests (PFTs), a six-minute walk test (6MWT), and evaluations of HRQOL, dyspnea, psychological status, and sleep quality. Patients who developed acute events such as acute exacerbations or pulmonary infections within four weeks of these baseline measurements were excluded. We evaluated the time from baseline to admission due to respiratory exacerbation or all-cause death within one year, which we arbitrarily defined as clinical deterioration.
Blood tests and physiological measurements
All patients underwent an ABG on room air. The PFT and 6MWT were performed according to published guidelines (17, 18). The diffusing capacity of the lung for carbon monoxide (DLCO) was mandatory wherever possible. When performing the 6MWT, the patients were permitted to use supplemental oxygen at a concentration equal to that available during exertion. The GAP index was calculated by age, gender, and the percentages of the predicted FVC (%FVC) and DLCO (%DLCO) and had a range of 0 to 8 (19). We also evaluated the extent of emphysema according to previous reports (20, 21). Combined pulmonary fibrosis and emphysema (CPFE) was diagnosed on the basis of having 10% or more emphysema area by visual assessment in patients with IPF. The presence of CPFE was independently assessed by two observers (K.I. and T.K.) who were blinded to clinical information, and interobserver disagreements were resolved by consensus.
Patient-reported outcomes
HRQOL was assessed using the Japanese versions of the Medical Outcomes Study 36-item Short Form (SF-36) (22), a generic questionnaire, and the SGRQ-I (12), a disease-specific questionnaire. SF-36 consists of eight subscales: Physical functioning, Role physical, Bodily pain, General health, Vitality, Social functioning, Role emotional, and Mental health. Each subscale is scored with a range from 0-100. Higher scores indicate better HRQOL. The SGRQ-I consists of three components: Symptoms, Activity, and Impacts. Each component and the Total are scored with a range from 0-100. Higher scores indicate worse HRQOL. Dyspnea was evaluated by the Japanese version of the modified Medical Research Council (mMRC) dyspnea scale graded from 0-4 (23, 24), and psychological status was assessed by the Hospital Anxiety and Depression Scale (HADS) (24, 25). Patients’ sleep quality was assessed by the Japanese version of the Pittsburgh Sleep Quality Index (PSQI) (24, 26).
Statistical analysis
Data are expressed as the median and interquartile range, unless otherwise stated. Statistical analyses were performed using JMP 10.0 (SAS Institute Inc., Cary, NC, USA). Correlations between variables were evaluated using Spearman’s rank correlation coefficient tests. Stepwise multiple-regression analyses were performed to identify the clinical variables that significantly contributed to the SGRQ-I scores. Clinical variables whose p-value was less than 0.10 in correlation to HRQOL in univariate analyses were included in stepwise multiple-regression analyses. Univariate and multivariate Cox regression analyses were performed to identify factors predicting clinical deterioration within one year, which was defined as a hospital admission due to respiratory exacerbation or all-cause death. A receiver operating characteristic (ROC) analysis of SGRQ-I (Total score) was performed to determine the threshold for predicting clinical deterioration. All analyses were considered statistically significant when p < 0.05.
Results
Study population
Fifty-two consecutive patients with IPF were enrolled in this study (Table 1). Seven patients (13.5%) were diagnosed by histology, confirming usual interstitial pneumonia pattern. The median age of the patients was 73.0 years, and 44 patients (84.6%) were male. Forty-seven patients (90.4%) had a smoking history. The medians of %FVC and %DLCO were 86.5% and 39.7%, respectively. Seventeen (32.7%) patients were diagnosed with CPFE.
Table 1.
Patient Characteristics (n = 52)
| Median (interquartile range) or number (%) | |
| Age, y | 73.0, (66.3-76.8) |
| Gender, male | 44 (84.6%) |
| Smoking History | 47 (90.4%) |
| BAL, performed | 22 (42.3%) |
| SLB confirmation | 7 (13.5%) |
| CPFE | 17 (32.7%) |
| %FVC, % | 86.5, (71.9-99.9) |
| %Dlco, % | 39.7, (33.0-50.0) |
| 6MWD, m | 491, (416-529) |
| Minimum SpO2 during 6MWT, % | 86.0, (80.5-89.3) |
| PaO2 on room air, Torr | 83.1, (75.7-91.5) |
| KL-6, U/mL | 784, (625-1190) |
| SP-D, ng/mL | 232, (176-232) |
| mMRC (0 to 4) | 1, (0-2) |
| HADS (0 to 21) | |
| Anxiety | 3, (1-5) |
| Depression | 4, (2-7) |
| PSQI (0 to 21) | 4, (2-8) |
Abbreviations: IPF, idiopathic pulmonary fibrosis; BAL, bron- choalveolar lavage; SLB, surgical lung biopsy; CPFE, combined pulmonary fibrosis and emphysema; %FVC, percentage of the predicted forced vital capacity; %DLCO, percentage of the predicted diffusing capacity of the lung for carbon monoxide; 6MWD, six- minute walk distance; SpO2, oxygen saturation measured by pulse oximetry; 6MWT, six-minute walk test; PaO2 partial pressure of arterial oxygen; KL-6, Krebs von den lungen-6; SP-D, surfactant protein-D; mMRC, modified Medical Research Council; HADS, Hospital Anxiety and Depression Scale; PSQI, Pittsburgh Sleep Quality Index.
Baseline HRQOL scores
Baseline HRQOL scores are shown in Table 2. Regarding SF-36, median scores of six among eight subscales were over 70, and 36-49% of patients had the maximal scores (best health) in four subscales. Regarding SGRQ-I, the median of the Total score was 28.4, slightly skewed toward a better score, and 17.3% scored worst on the Activity component, whereas 23.1% scored best on the Impacts component.
Table 2.
Baseline HRQOL scores
| Median (interquartile range) | Patients with minimal score, n (%) | Patients with maximal score, n (%) | |
| SF-36 | |||
| Physical functioning | 80.0 (45.0-88.8) | 0 (0.0) | 1 (1.9) |
| Role physical | 75.0 (43.8-100) | 0 (0.0) | 19 (36.5) |
| Bodily pain | 84.0 (64.0-100) | 0 (0.0) | 25 (48.1) |
| General health | 50.0 (31.3-57.0) | 0 (0.0) | 0 (0.0) |
| Vitality | 56.3 (43.8-75.0) | 1 (1.9) | 1 (1.9) |
| Social functioning | 75.0 (50.0-100) | 0 (0.0) | 20 (38.5) |
| Role emotional | 79.2 (50.0-100) | 1 (1.9) | 19 (36.5) |
| Mental health | 70.0 (50.0-83.8) | 0 (0.0) | 3 (5.8) |
| SGRQ-I | |||
| Symptoms | 43.7 (18.6-66.8) | 4 (7.7) | 3 (5.8) |
| Activity | 48.1 (21.9-78.2) | 4 (7.7) | 9 (17.3) |
| Impacts | 18.5 (3.2-40.4) | 12 (23.1) | 0 (0.0) |
| Total | 28.4 (14.3-57.9) | 0 (0.0) | 0 (0.0) |
Higher scores indicate better status on the SF-36, and lower scores indicate better status on the SGRQ-I.
Abbreviations: HRQOL, health-related quality of life; SF-36, Medical Outcomes Study 36-item Short Form; SGRQ-I, idiopathic pulmonary fibrosis-specific version of the St. George’s Respiratory Questionnaire
Correlations between SGRQ-I and other clinical measurements
The three components and the Total of the SGRQ-I had significant weak-to-moderate correlations with physiological parameters including %FVC, %DLCO, minimum oxygen saturation measured by pulse oximetry (SpO2) during the 6MWT, partial pressure of arterial oxygen (PaO2) on room air (absolute Spearman’s rank correlation coefficient (Rs)=0.38-0.59, p<0.01) except for the non-significant correlation between 6MWD and the Symptoms of the SGRQ-I (Table 3). The three components and the Total of the SGRQ-I had moderate to strong correlations with the mMRC dyspnea (Rs=0.58-0.82, p<0.001) and weak to moderate correlations with HADS anxiety and depression (Rs=0.39-0.66, p<0.01), except for a non-significant correlation between HADS anxiety and the Activity of the SGRQ-I. Relationships between the SGRQ-I and SF-36 are also shown in Table 3. The Total of the SGRQ-I was moderately to strongly related to subscales of the SF-36 (absolute Rs=0.53-0.85, p<0.05), except for an insignificant relationship with the SF-36 Bodily pain. A similar trend was found for the three components of SGRQ-I.
Table 3.
Correlations between the SGRQ-I and other indicators, including physical measurements and patient-reported outcomes
| SGRQ-I Symptoms | SGRQ-I Activity | SGRQ-I Impacts | SGRQ-I Total | ||
| %FVC | -0.44‡ | -0.52‡ | -0.44† | -0.50‡ | |
| %Dlco | -0.38† | -0.56‡ | -0.56‡ | -0.57‡ | |
| 6MWD | -0.22 | -0.59‡ | -0.53‡ | -0.51‡ | |
| Minimum | SpO2 during 6MWT | -0.42† | -0.55‡ | -0.54‡ | -0.57‡ |
| PaO2 on room air | -0.44† | -0.48‡ | -0.57‡ | -0.54‡ | |
| mMRC | 0.58‡ | 0.82‡ | 0.66‡ | 0.77‡ | |
| HADS Anxiety | 0.44† | 0.27 | 0.44† | 0.39† | |
| HADS Depression | 0.50‡ | 0.54‡ | 0.66‡ | 0.64‡ | |
| PSQI | 0.17 | 0.15 | 0.25 | 0.22 | |
| SF-36 | |||||
| Physical functioning | -0.57‡ | -0.90‡ | -0.80‡ | -0.85‡ | |
| Role physical | -0.49‡ | -0.67‡ | -0.64‡ | -0.65‡ | |
| Bodily pain | -0.24 | -0.21 | -0.21 | -0.24 | |
| General health | -0.55‡ | -0.70‡ | -0.71‡ | -0.72‡ | |
| Vitality | -0.48‡ | -0.63‡ | -0.71‡ | -0.67‡ | |
| Social functioning | -0.47‡ | -0.53‡ | -0.64‡ | -0.59‡ | |
| Role emotional | -0.57‡ | -0.58‡ | -0.67‡ | -0.64‡ | |
| Mental health | -0.46‡ | -0.47‡ | -0.55‡ | -0.53‡ | |
Data are presented as Spearman’s rank correlation coefficient.
* p<0.05, † p<0.01, ‡ p<0.001.
Abbreviations: SGRQ-I, idiopathic pulmonary fibrosis-specific version of the St. George’s Respiratory Questionnaire; %FVC, percentage of the predicted forced vital capacity; %Dlco, percentage of the predicted diffusing capacity of the lung for carbon monoxide; 6MWD, six- minute walk distance; SpO2, oxygen saturation measured by pulse oximetry; 6MWT, six-minute walk test; PaO2 partial pressure of arterial oxygen; mMRC, modified Medical Research Council; HADS, Hospital Anxiety and Depression Scale; PSQI, Pittsburgh Sleep Quality Index; SF-36, Medical Outcomes Study 36-item Short Form
Factors contributing to SGRQ-I
Stepwise multiple-regression analyses were used to identify those variables that contribute most to the SGRQ-I scores (Table 4). The mMRC dyspnea scale, HADS anxiety or depression, and minimum SpO2 during the 6MWT significantly explained the Total and the three components of the SGRQ-I. PaO2 on room air also weakly but significantly explained the Impact of the SGRQ-I. These factors accounted for 60-83% of the variance. Neither pulmonary function tests (%FVC and %DLCO) nor the 6MWD were significant predictors.
Table 4.
Exploration of factors contributing to the SGRQ-I in patients with IPF using stepwise multivariate analyses
| Variables | Symptoms | Activity | Impacts | Total |
| %FVC | ||||
| %Dlco | ||||
| 6MWD | ||||
| Minimum SpO2 during 6MWT | 0.24 | 0.15 | 0.21 | 0.21 |
| PaO2 on room air | 0.11 | |||
| mMRC | 0.15 | 0.50 | 0.23 | 0.34 |
| HADS Anxiety | 0.21 | |||
| HADS Depression | 0.14 | 0.28 | 0.23 | |
| PSQI | ||||
| Cumulative R2 | 0.60 | 0.79 | 0.83 | 0.78 |
Data are presented as the coefficients of determination (R2).
R2 is shown when p < 0.05.
Abbreviations: SGRQ-I, idiopathic pulmonary fibrosis-specific version of the St. George’s Respiratory Questionnaire; IPF, idiopathic pulmonary fibrosis; %FVC, percentage of the predicted forced vital capacity; %DLCO, percentage of the predicted diffusing capacity of the lung for carbon monoxide; 6MWD, six-minute walk distance; SpO2, oxygen saturation measured by pulse oximetry; 6MWT, six-minute walk test; PaO2 partial pressure of arterial oxygen; mMRC, modified Medical Research Council; HADS, Hospital Anxiety and Depression Scale; PSQI, Pittsburgh Sleep Quality Index.
Relationship of SGRQ-I to clinical deterioration within one year
Eleven patients developed clinical deterioration within one year. Ten patients were hospitalized due to respiratory exacerbation, and one died from septic shock due to a urinary tract infection during the one-year observational period. Among the ten hospitalized patients, three patients died from acute exacerbation, one died from lung cancer, and one died from pulmonary infection, while five patients survived during the observational period. Univariate analyses revealed that PFT measurements, 6MWD, minimum SpO2 during the 6MWT, PaO2 on room air, and the GAP index were significant predictors of clinical deterioration (Table 5). Regarding HRQOL, the Total and the three components of the SGRQ-I significantly predicted clinical deterioration (p<0.001). Five of eight subscales of the SF-36 were also predictive of clinical deterioration (p<0.05). The mMRC was related to clinical deterioration (p=0.002), but the HADS anxiety and depression and the PSQI were not.
Table 5.
Univariate Cox proportional hazards analyses to predict clinical deterioration within one year
| Variables | Univariate analysis | ||
| HR | 95% CI | p-value | |
| Age | 1.01 | 0.93-1.10 | 0.861 |
| Male | 0.40 | 0.12-1.82 | 0.209 |
| Smoking History | 0.19 | 0.05-0.83 | 0.031 |
| CPFE | 0.73 | 0.16-2.54 | 0.641 |
| %FVC | 0.93 | 0.89-0.96 | <0.001 |
| %Dlco | 0.89 | 0.83-0.96 | 0.001 |
| 6MWD | 0.996 | 0.992-1.000 | 0.035 |
| Minimum SpO2 during 6MWT | 0.88 | 0.82-0.93 | <0.001 |
| PaO2 on room air | 0.92 | 0.87-0.96 | <0.001 |
| GAP index | 4.68 | 1.79-13.2 | 0.002 |
| SGRQ-I | |||
| Symptoms | 1.04 | 1.02-1.07 | <0.001 |
| Activity | 1.05 | 1.02-1.09 | <0.001 |
| Impacts | 1.04 | 1.02-1.06 | <0.001 |
| Total | 1.05 | 1.03-1.08 | <0.001 |
| SF-36 | |||
| Physical functioning | 0.97 | 0.95-0.99 | 0.002 |
| Role physical | 0.98 | 0.96-1.00 | 0.028 |
| Bodily pain | 1.01 | 0.98-1.04 | 0.586 |
| General health | 0.96 | 0.93-0.99 | 0.018 |
| Vitality | 0.97 | 0.95-0.99 | 0.015 |
| Social functioning | 0.98 | 0.96-1.00 | 0.040 |
| Role emotional | 0.98 | 0.96-1.00 | 0.058 |
| Mental health | 0.99 | 0.96-1.01 | 0.333 |
| mMRC | 2.28 | 1.37-3.95 | 0.002 |
| HADS Anxiety | 0.94 | 0.73-1.15 | 0.588 |
| HADS Depression | 1.03 | 0.88-1.18 | 0.670 |
| PSQI | 1.02 | 0.87-1.16 | 0.837 |
Abbreviations: HR, hazard ratio; CI, confidence interval; CPFE, combined pulmonary fibrosis and emphysema; %FVC, percentage of the predicted forced vital capacity; %Dlco, percentage of the predicted diffusing capacity of the lung for carbon monoxide; 6MWD, six-minute walk distance; SpO2, oxygen saturation measured by pulse oximetry; 6MWT, six-minute walk test; PaO2 partial pressure of arterial oxygen; SGRQ-I, idiopathic pulmonary fibrosis -specific version of the St. George’s Respiratory Questionnaire; SF-36, Medical Outcomes Study 36-item Short Form; mMRC, modified Medical Research Council; HADS, Hospital Anxiety and Depression Scale; PSQI, Pittsburgh Sleep Quality Index.
To compare the ability of the SGRQ-I to predict clinical deterioration independent of each physiological measurement previously shown to be related to mortality, multivariate-regression analyses were performed (Table 6). When the %FVC or the GAP index were entered into the analyses with the Total of the SGRQ-I, both were significantly related to clinical deterioration (Models 1 and 7). When the SGRQ-I and the %DLCO were entered as explanatory variables, neither the SGRQ-I nor the %DLCO was significant (Model 2). When the mMRC, 6MWD or PaO2 were used as explanatory variables in addition to the Total of the SGRQ-I, only the SGRQ-I was a significant predictor of clinical deterioration (Models 3, 5, and 6). However, when the minimum SpO2 during the 6MWT was used, only the minimum SpO2 was significant (Model 4).
Table 6.
Multivariate Cox proportional hazards analyses for the SGRQ-I versus other variables to predict clinical deterioration within one year
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | Model 7 | ||
| SGRQ-I Total | 1.04 (1.01-1.07)* |
1.03 (0.99-1.06) |
1.05 (1.02-1.08)* |
1.02 (0.98-1.06) |
1.04 (1.01-1.07)* |
1.05 (1.01-1.09)* |
1.05 (1.02-1.09)* |
|
| %FVC | 0.95 (0.91-0.99)* |
|||||||
| %Dlco | 0.94 (0.85-1.01) |
|||||||
| 6MWD | 1.000 (0.995-1.006) |
|||||||
| Minimum SpO2 during 6MWT | 0.91 (0.83-0.99) |
|||||||
| PaO2 at room air | 0.97 (0.91-1.03) |
|||||||
| mMRC | 1.16 (0.48-2.61) |
|||||||
| GAP index | 3.28 (1.24-9.53)* |
Data are shown as the hazard ratio (95% confidence interval).
* p<0.05.
Abbreviations: SGRQ-I, idiopathic pulmonary fibrosis-specific version of the St. George’s Respiratory Questionnaire; %FVC, percentage of the predicted forced vital capacity; %Dlco, percentage of the predicted diffusing capacity of the lung for carbon monoxide; 6MWD, six- minute walk distance; SpO2, oxygen saturation measured by pulse oximetry; 6MWT, six-minute walk test; PaO2 partial pressure of arterial oxygen; mMRC, modified Medical Research Council.
An ROC analysis was performed to determine the cut-off value of the SGRQ-I Total score for predicting clinical deterioration within one year. The area under the curve was 0.88, and the cut-off value was 51.2. When 50 was set as the threshold, patients with an SGRQ-I Total score ≥50 developed clinical deterioration within one year more frequently than patients with a SGRQ-I Total score <50 (hazard ratio, 25.5 [95% confidence interval, 4.8-468]; p<0.001) (Figure 1).
Fig. 1.
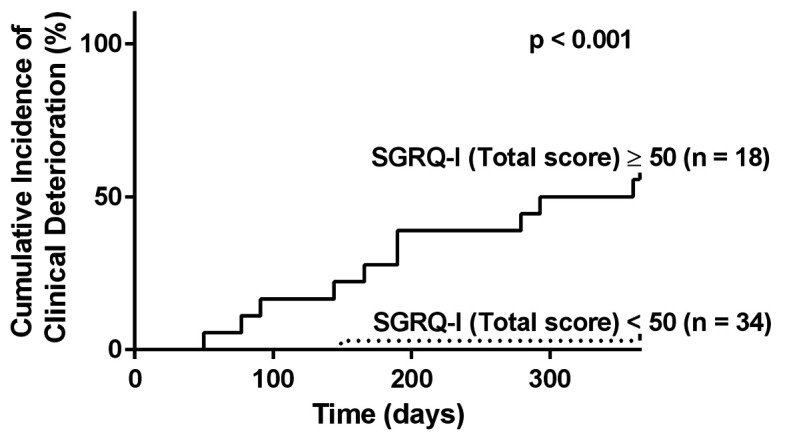
The cumulative incidence of clinical deterioration. One-year probability curves for patients with SGRQ-I Total scores ≥50 and <50. Patients with SGRQ-I Total scores ≥50 developed clinical deterioration within one year more frequently than patients with scores <50 (log rank test, p<0.001).
SGRQ-I, idiopathic pulmonary fibrosis-specific version of the St. George’s Respiratory Questionnaire
Discussion
We evaluated the relationships between HRQOL and other clinical measurements cross-sectionally and between HRQOL and future outcomes longitudinally in patients with IPF. One of the characteristics of this study was the use of the SGRQ-I, which is a more disease-specific measure than the original SGRQ. Significant contributing factors to the SGRQ-I included minimum SpO2 during the 6MWT, mMRC dyspnea scale, and HADS, indicating physiological, functional, and psychological impairments, respectively. This finding suggests that the SGRQ-I is an indicator with multidisciplinary properties. Additionally, the SGRQ-I predicted clinical deterioration within one year independent of several important clinical measurements.
One important feature of assessing HRQOL is the ability to predict future outcomes (predictive property) (13-16, 27). We first demonstrated that HRQOL measured with the SGRQ-I predicted clinical deterioration, defined as hospitalization due to respiratory exacerbation or all-cause mortality. The most notable result was that the SGRQ-I predicted clinical deterioration independent of %FVC, the 6MWD, and the GAP index, which are well-known predictors of mortality in IPF (19, 28, 29). This finding indicated that HRQOL is complementary to pulmonary function and the GAP index in predicting outcomes for patients with IPF and that HRQOL should be measured besides physiological measurements such as pulmonary function tests and 6MWT. These findings will be helpful in multi-dimensional assessment in patients with IPF.
The level of dyspnea is related to mortality in several diseases including IPF (30-32) and even in elderly people (33). However, in this study, the SGRQ-I was more significantly related to clinical deterioration than was the mMRC dyspnea scale. This finding may have several causes. First, HRQOL provides a more comprehensive assessment of symptoms other than dyspnea during daily activities. In this study, contributing factors to the SGRQ-I included physiological and psychological factors in addition to the mMRC scale. Second, while the SGRQ-I is more specific to IPF than the respiratory-specific SGRQ, the uni-dimensional five-point mMRC scale may lack the precision and complexity required to detect a correlation between dyspnea and clinical deterioration. A more sophisticated method of assessing dyspnea may be needed. Thus, HRQOL and dyspnea assessments using appropriate measurements are important.
As a respiratory-specific HRQOL measure, the SGRQ is associated with a number of physiological parameters in patients with IPF (7, 8, 34), and changes in the SGRQ scores are partly correlated with changes in physiological parameters and CT indices in IPF (7). Thus, the SGRQ has been evaluated in several clinical trials of IPF (6, 35, 36). The SGRQ-I, modified from SGRQ, was developed as a disease-specific measure to evaluate HRQOL in patients with IPF (12). A previous study revealed that each component of the SGRQ-I corresponding to the SGRQ was significantly associated with %FVC, %DLCO, 6MWD, and all subscales of SF-36, including Bodily pain, except for a non-significant association between the Symptoms of the SGRQ-I and the 6MWD (12). Accordingly, in this study, most components of the SGRQ-I were significantly associated with physiological functions including PFTs, 6MWD, and minimum SpO2 during the 6MWT.
The mMRC contributed to the SGRQ-I score. These results are in accordance with a previous study in which the dyspnea scale, represented by a baseline dyspnea index, was found to be the most significant contributing factor to the SGRQ in patients with IPF (34). Conversely, PFT parameters such as vital capacity and DLCO were not significant (34). The HADS was also a significant factor in the SGRQ-I. Although depression is relatively common in patients with IPF (37), its relationship with HRQOL has not been fully investigated. We found that depression or anxiety was the important determinant of HRQOL in patients with IPF, as with other respiratory diseases (38-40). Sleep quality was also associated with HRQOL and was assessed by the SF-36 in patients with IPF (41). Poor sleep quality is common and results in a poor QOL in patients with IPF (42). In this study, however, sleep quality was not significantly related to the SGRQ-I.
This study has some limitations. First, the cohort size was small and the observational period was short. To investigate the independent influence of the SGRQ-I on clinical deterioration or survival, additional studies with a larger multicenter cohort and longer observational period are necessary. Second, the enrolled patients had relatively mild pulmonary impairment, with a median %FVC of 86.5%, which likely contributed to the low death rate. Therefore, the generalization of the results may be limited. Third, we chose clinical deterioration (arbitrarily defined as hospitalization due to respiratory failure or death) as our measure of disease progression, rather than survival. However, no composite outcome of disease progression specific for patients with IPF has yet been established. Further studies based on other clinical outcomes may be needed.
In patients with IPF, the SGRQ-I is a multidisciplinary instrument representing physiological, functional, and psychological impairment. HRQOL assessed by the SGRQ-I may be an independent predictor of short-term disease progression.
Conflicts of interest:
K. Tanizawa, T. Oga, and K. Chin belong to the Department of Respiratory Care and Sleep Control Medicine, which is funded by endowments from Philips-Respironics, Teijin Pharma, Fukuda Denshi, and Fukuda Lifetec Keigji to Kyoto University, but they have no other conflict of interest to disclose. All other authors have no conflict of interest to disclose.
Clinical trial registration:
UMIN000011142 at www.umin.ac.jp
References
- 1.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Natsuizaka M, Chiba H, Kuronuma K, Otsuka M, Kudo K, Mori M, et al. Epidemiologic survey of Japanese patients with idiopathic pulmonary fibrosis and investigation of ethnic differences. Am J Respir Crit Care Med. 2014;190(7):773–9. doi: 10.1164/rccm.201403-0566OC. [DOI] [PubMed] [Google Scholar]
- 3.Ley B, Collard HR, King TE., Jr Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;183(4):431–40. doi: 10.1164/rccm.201006-0894CI. [DOI] [PubMed] [Google Scholar]
- 4.Swigris JJ, Gould MK, Wilson SR. Health-related quality of life among patients with idiopathic pulmonary fibrosis. Chest. 2005;127(1):284–94. doi: 10.1378/chest.127.1.284. [DOI] [PubMed] [Google Scholar]
- 5.King TE, Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2083–92. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 6.Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2071–82. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 7.Peng S, Li Z, Kang J, Hou X. Cross-sectional and longitudinal construct validity of the Saint George’s Respiratory Questionnaire in patients with IPF. Respirology. 2008;13(6):871–9. doi: 10.1111/j.1440-1843.2008.01359.x. [DOI] [PubMed] [Google Scholar]
- 8.Tzanakis N, Samiou M, Lambiri I, Antoniou K, Siafakas N, Bouros D. Evaluation of health-related quality-of-life and dyspnea scales in patients with idiopathic pulmonary fibrosis. Correlation with pulmonary function tests. Eur J Intern Med. 2005;16(2):105–12. doi: 10.1016/j.ejim.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 9.Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George’s Respiratory Questionnaire. Am Rev Respir Dis. 1992;145(6):1321–7. doi: 10.1164/ajrccm/145.6.1321. [DOI] [PubMed] [Google Scholar]
- 10.Wilson CB, Jones PW, O’Leary CJ, Cole PJ, Wilson R. Validation of the St. George’s Respiratory Questionnaire in bronchiectasis. Am J Respir Crit Care Med. 1997;156(2):536–41. doi: 10.1164/ajrccm.156.2.9607083. [DOI] [PubMed] [Google Scholar]
- 11.Al-Shair K, Atherton GT, Kennedy D, Powell G, Denning DW, Caress A. Validity and reliability of the St. George’s Respiratory Questionnaire in assessing health status in patients with chronic pulmonary aspergillosis. Chest. 2013;144(2):623–31. doi: 10.1378/chest.12-0014. [DOI] [PubMed] [Google Scholar]
- 12.Yorke J, Jones PW, Swigris JJ. Development and validity testing of an IPF-specific version of the St. George’s Respiratory Questionnaire. Thorax. 2010;65(10):921–6. doi: 10.1136/thx.2010.139121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Domingo-Salvany A, Lamarca R, Ferrer M, Garcia-Aymerich J, Alonso J, Felez M, et al. Health-related quality of life and mortality in male patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166(5):680–5. doi: 10.1164/rccm.2112043. [DOI] [PubMed] [Google Scholar]
- 14.Oga T, Nishimura K, Tsukino M, Sato S, Hajiro T. Analysis of the factors related to mortality in chronic obstructive pulmonary disease: role of exercise capacity and health status. Am J Respir Crit Care Med. 2003;167(4):544–9. doi: 10.1164/rccm.200206-583OC. [DOI] [PubMed] [Google Scholar]
- 15.Nishiyama O, Taniguchi H, Kondoh Y, Kimura T, Kataoka K, Nishimura K, et al. Health-related quality of life does not predict mortality in idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis. 2012;29(2):113–8. [PubMed] [Google Scholar]
- 16.Oga T, Nishimura K, Tsukino M, Sato S, Hajiro T, Ikeda A, et al. Health status measured with the CRQ does not predict mortality in COPD. Eur Respir J. 2002;20(5):1147–51. doi: 10.1183/09031936.02.00303702. [DOI] [PubMed] [Google Scholar]
- 17.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–7. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 18.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948–68. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 19.Ley B, Ryerson CJ, Vittinghoff E, Ryu JH, Tomassetti S, Lee JS, et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med. 2012;156(10):684–91. doi: 10.7326/0003-4819-156-10-201205150-00004. [DOI] [PubMed] [Google Scholar]
- 20.Ikezoe K, Handa T, Tanizawa K, Kubo T, Oguma T, Hamada S, et al. Bone mineral density in patients with idiopathic pulmonary fibrosis. Respir Med. 2015;109(9):1181–7. doi: 10.1016/j.rmed.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 21.Ryerson CJ, Hartman T, Elicker BM, Ley B, Lee JS, Abbritti M, et al. Clinical features and outcomes in combined pulmonary fibrosis and emphysema in idiopathic pulmonary fibrosis. Chest. 2013;144(1):234–40. doi: 10.1378/chest.12-2403. [DOI] [PubMed] [Google Scholar]
- 22.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–83. [PubMed] [Google Scholar]
- 23.National Collaborating Centre for Chronic Conditions. Chronic obstructive pulmonary disease. National clinical guideline on management of chronic obstructive pulmonary disease in adults in primary and secondary care. Thorax. 2004;59(1):1–232. [PMC free article] [PubMed] [Google Scholar]
- 24.Aihara K, Oga T, Yoshimura C, Hitomi T, Chihara Y, Harada Y, et al. Measurement of dyspnea in patients with obstructive sleep apnea. Sleep Breath. 2013;17(2):753–61. doi: 10.1007/s11325-012-0759-2. [DOI] [PubMed] [Google Scholar]
- 25.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 26.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 27.Oga T, Taniguchi H, Kita H, Tsuboi T, Tomii K, Ando M, et al. Analysis of the relationship between health status and mortality in hypercapnic patients with noninvasive ventilation. Clin Respir J. 2015. Dec 28 doi: 10.1111/crj.12415. [DOI] [PubMed] [Google Scholar]
- 28.Latsi PI, du Bois RM, Nicholson AG, Colby TV, Bisirtzoglou D, Nikolakopoulou A, et al. Fibrotic idiopathic interstitial pneumonia: the prognostic value of longitudinal functional trends. Am J Respir Crit Care Med. 2003;168(5):531–7. doi: 10.1164/rccm.200210-1245OC. [DOI] [PubMed] [Google Scholar]
- 29.Caminati A, Bianchi A, Cassandro R, Mirenda MR, Harari S. Walking distance on 6-MWT is a prognostic factor in idiopathic pulmonary fibrosis. Respir Med. 2009;103(1):117–23. doi: 10.1016/j.rmed.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 30.Nishiyama O, Taniguchi H, Kondoh Y, Kimura T, Kato K, Kataoka K, et al. A simple assessment of dyspnoea as a prognostic indicator in idiopathic pulmonary fibrosis. Eur Respir J. 2010;36(5):1067–72. doi: 10.1183/09031936.00152609. [DOI] [PubMed] [Google Scholar]
- 31.Nishimura K, Izumi T, Tsukino M, Oga T. Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest. 2002;121(5):1434–40. doi: 10.1378/chest.121.5.1434. [DOI] [PubMed] [Google Scholar]
- 32.Ekman I, Cleland JG, Swedberg K, Charlesworth A, Metra M, Poole-Wilson PA. Symptoms in patients with heart failure are prognostic predictors: insights from COMET. J Card Fail. 2005;11(4):288–92. doi: 10.1016/j.cardfail.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 33.Ahmed T, Steward JA, O’Mahony MS. Dyspnoea and mortality in older people in the community: a 10-year follow-up. Age Ageing. 2012;41(4):545–9. doi: 10.1093/ageing/afs049. [DOI] [PubMed] [Google Scholar]
- 34.Nishiyama O, Taniguchi H, Kondoh Y, Kimura T, Ogawa T, Watanabe F, et al. Health-related quality of life in patients with idiopathic pulmonary fibrosis. What is the main contributing factor. Respir Med. 2005;99(4):408–14. doi: 10.1016/j.rmed.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 35.Zisman DA, Schwarz M, Anstrom KJ, Collard HR, Flaherty KR, Hunninghake GW. A controlled trial of sildenafil in advanced idiopathic pulmonary fibrosis. N Engl J Med. 2010;363(7):620–8. doi: 10.1056/NEJMoa1002110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.King TE, Jr, Behr J, Brown KK, du Bois RM, Lancaster L, de Andrade JA, et al. BUILD-1: a randomized placebo-controlled trial of bosentan in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;177(1):75–81. doi: 10.1164/rccm.200705-732OC. [DOI] [PubMed] [Google Scholar]
- 37.De Vries J, Kessels BL, Drent M. Quality of life of idiopathic pulmonary fibrosis patients. Eur Respir J. 2001;17(5):954–61. doi: 10.1183/09031936.01.17509540. [DOI] [PubMed] [Google Scholar]
- 38.Hajiro T, Nishimura K, Tsukino M, Ikeda A, Koyama H, Izumi T. Comparison of discriminative properties among disease-specific questionnaires for measuring health-related quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157(3):785–90. doi: 10.1164/ajrccm.157.3.9703055. [DOI] [PubMed] [Google Scholar]
- 39.Nishimura K, Oga T, Ikeda A, Hajiro T, Tsukino M, Koyama H. Comparison of health-related quality of life measurements using a single value in patients with asthma and chronic obstructive pulmonary disease. J Asthma. 2008;45(7):615–20. doi: 10.1080/02770900802127014. [DOI] [PubMed] [Google Scholar]
- 40.Tanizawa K, Handa T, Nagai S, Oga T, Kubo T, Ito Y, et al. Validation of the Japanese version of the Sarcoidosis Health Questionnaire: a cross-sectional study. Health Qual Life Outcomes. 2011;9(1):34. doi: 10.1186/1477-7525-9-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krishnan V, McCormack MC, Mathai SC, Agarwal S, Richardson B, Horton MR, et al. Sleep quality and health-related quality of life in idiopathic pulmonary fibrosis. Chest. 2008;134(4):693–8. doi: 10.1378/chest.08-0173. [DOI] [PubMed] [Google Scholar]
- 42.Milioli G, Bosi M, Poletti V, Tomassetti S, Grassi A, Riccardi S, et al. Sleep and respiratory sleep disorders in idiopathic pulmonary fibrosis. Sleep Med Rev. 2016;26:57–63. doi: 10.1016/j.smrv.2015.03.005. [DOI] [PubMed] [Google Scholar]


