Abstract
Idiopathic dendriform diffuse pulmonary ossification (DPO) is a rare disorder. High resolution CT (HRCT) with appropriate osteoporosis window setting reveals the diagnosis. We report the features of eight patients, of whom two brothers, with HRCT findings compatible with predominant DPO in a bibasal subpleural distribution (usual interstitial pneumonia (UIP)-like distribution) and review the literature for DPO in this UIP-like distribution. DPO in a UIP-like distribution seems to be a disorder of the very old (age 75-87 (mean 83.6) male (8 out of 8), with familial occurrence, with associated cardiovascular disease and frequent use of anticoagulants as common findings, and with a slowly progressive nature and the absence of radiological honeycombing despite long lasting disease contrasting with idiopathic pulmonary fibrosis (IPF). Idiopathic pulmonary fibrosis (IPF) should be differentiated from predominant DPO in a UIP-like distibution. (Sarcoidosis Vasc Diffuse Lung Dis 2017; 34: 251-256)
Keywords: pulmonary ossification, idiopathic pulmonary fibrosis, phenotype, interstitial lung disease, genetic
Introduction
Pulmonary ossification is a rare indolent chronic process characterized by progressive, metaplastic ossification leading to small bone fragments in lung tissue. Ossification can be localized in lung tissue injured by any pathological process. Diffuse pulmonary ossification refers to disseminated formation of spicules of bone affecting diffuse areas of the lung (1,2). Nodular pulmonary ossifications are radiological distinctive round and were seen historically in the lower lobes of patients with chronic passive congestion especially in mitral stenosis. In dendriform diffuse pulmonary ossification (DPO), dendriform refers to the dendritic, branching appearance of the heterotopic mature bone (3). The striking HRCT appearance of irregular branching lines with bone density distinguishes DPO from other forms of pulmonary ossification. HRCT in an osteoporosis window setting (window width 818, level 273) detected DPO as tiny calcifications in bibasilar subpleural lungs with a 100% specificity when compared to histopathologic findings (4).
Idiopathic pulmonary fibrosis is an inexorably progressive pulmonary disease diagnosed in the presence of usual interstitial pneumonia (UIP) in the absence of known cause. HRCT Criteria for a “definite” UIP pattern include a predominant subpleural and basilar distribution of reticular pattern without inconsistent features (including profuse micronodules) and associated with honeycombing. A “possible” UIP pattern is identical with the omission of honeycombing. In the presence of a radiographical possible UIP pattern, histopathology and multidisciplinary approach is needed to confirm the diagnosis of UIP (5). However in a large number of patients, several reasons do not allow to proceed to biopsy.
We report on eight patients with HRCT findings compatible with diffuse DPO in a UIP-like distribution. A literature review is provided, but current information is sparse.
Methods
Our cases were acquired over a 4-year period at ZNA Antwerp in a tertiary referral non-university center that discusses approximately 150 new cases of diffuse interstitial lung disease (ILD) per year in a multidisciplinary setting. Inclusion criteria comprised cases showing features of predominant DPO in a UIP-like distribution pattern as concluded by the multidisciplinary discussion. Informed consent was obtained.
Eight subjects were identified from the multidisciplinary ILD database. Clinical data including demographic and clinical findings, laboratory and lung functional data, exposure history and comorbidities were obtained from medical records. Furthermore all available chest imaging was evaluated. Histopathology was not available, except for one patient, in whom diagnosis was confirmed postmortem at autopsy. The HRCT appearances were reviewed for associated findings of fibrosis, honeycombing, ground glass opacification, pleural plaques and emphysema.
Results
Demographic findings, clinical features, treatment, comorbidities and exposure history are summarized in Table 1. There were eight males and no female. Age ranged from 75 to 87 years (mean 83 years). Only two patients had never smoked, 6 were ex-smokers and none was a current smoker. There was no significant other exposure history nor there were clues for associated rheumatic disorder. Family history for ILD was absent, except for the very important finding that two of the patients were brothers. There was mild productive cough in two patients, all but one had some dyspnea on exertion. Bibasilar crackles were heard in all, but were minimal in the majority. Only one patient had mild clubbing. Signs of congestive heart failure were absent in all. A medical history of hypertension was present in six, diabetes mellitus in three and coronary atheromatosis in seven. Five patients suffered from gastroesophageal reflux. All took antiplatelet medication or warfarin. No patient had severe renal impairment. Calcium metabolism was always normal. There was no obstructive lung function impairment. FVC was preserved, while DLCO was diminished in a variable degree.
Table 1.
Predominant DPO in a peripheral bibasilar distribution. Demographics and clinical findings
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | |
| Demographics | ||||||||
| Male/Female | Male | Male | Male | Male | Male | Male | Male | Male |
| Age in years | 87 | 86 | 75 | 85 | 79 | 86 | 85 | 87 |
| Risk factors | ||||||||
| Smoking/pack-years | Ex; 43 | Ex; 10 | Ex; 32 | never | Ex; 20 | Ex; 10 | Ex; 25 | never |
| Occupation | baker | clerk | clerk | Rx sheets | Carp. | police | clerk | clerk |
| Familial | no | Father lungca | no | no | Brother DPO | Brother DPO | no | Brother lungca |
| Medication | W, M, S | W, PPI | ASA, S | ASA, S, M | ASA | W, PPI, S | ASA, M | ASA, S |
| Symptoms/clinical | ||||||||
| Cough | no | no | mild | no | no | mild | no | mild |
| Dyspnea (MRC) | 2 | 2 | no | 2 | 2 | 2 | 2-3 | 2 |
| Finger clubbing | no | no | no | mild | no | no | no | no |
| Bibasilar crackles | mild | minimal | minimal | minimal | mild | mild | mild | mild |
| Comorbidities | ||||||||
| GERD | yes | yes | yes | no | no | yes | yes | no |
| AHT | yes | yes | yes | yes | no | no | no | no |
| Diabetes | yes | no | no | yes | no | no | yes | no |
| Coronary atheromatosis | yes | no | yes | yes | yes | yes | yes | no |
| Overt heart failure | no | no | no | no | no | no | no | no |
Rx sheets means exposed to production of roentgen rolls; Carp.=carpenter; Lungca=lungcancer; W=Warfarin; M=Metformin; PPI=proton pomp inhibitor; S=Statin; AHT=arterial hypertension
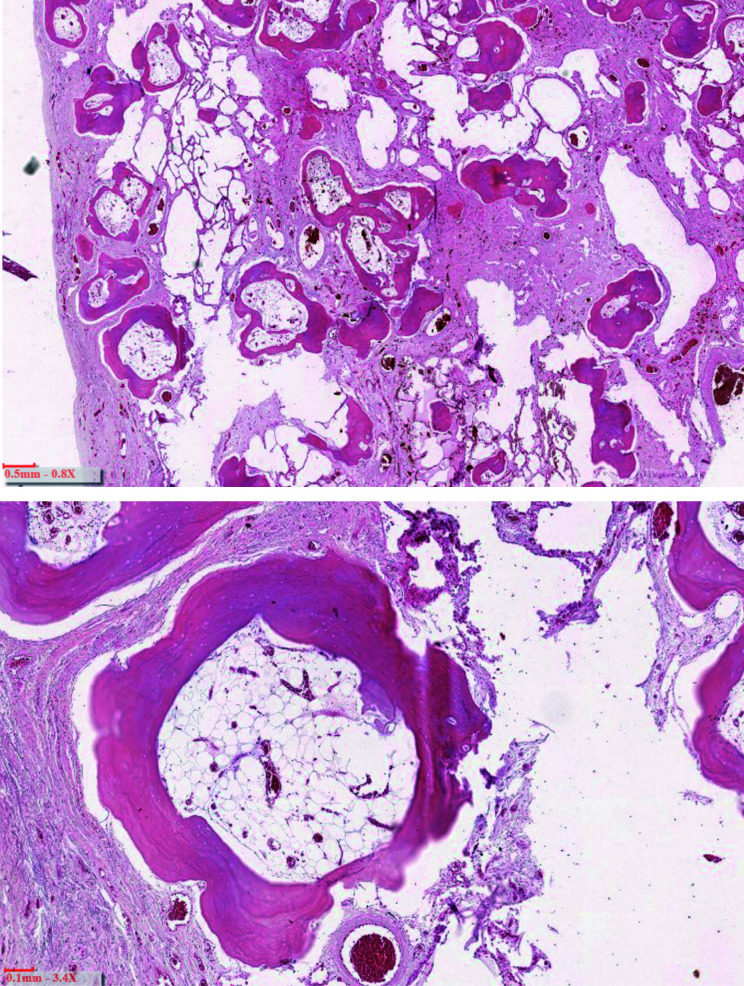
The HRCT findings are summarized in Table 2 and some images are shown in figure 1. Conform to the inclusion criteria, there was a predominant dendritic, branching appearance of mature bone in a bibasal subpleural distribution in all. However, signs of fibrosis such as traction bronchiectasis and honeycombing were remarkably absent. Ground glass opacities were minimally present in one. There were no pleural plaques. Two patients had minimal paraseptal emphysema. In retrospect, changes on previous images were visible three to thirteen years before referral for multidisciplinary diagnosis. In one patient bone scinitigraphy was performed revealing subpleural captation (figure 2). Bronchoalveolar lavage was available for one patient revealing 88% non-pigmented macrophages. The histopathology, available for one patient at autopsy, confirmed extensive DPO (figure 3).
Table 2.
Predominant DPO in a peripheral bibasilar distribution. Imaging and lung function findings.
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | |
| Interstitial changes | ||||||||
| Subpleural, bibasilar distribution | yes | yes | yes | yes | yes | yes | yes | yes |
| Reticular opacities | yes | yes | yes | yes | yes | yes | yes | yes |
| DPO | yes | yes | yes | yes | yes | yes | yes | yes |
| Honeycombing | Min. | mild | Min. | no | no | no | mild | Min. |
| Traction bronchiectasies | no | mild | no | no | no | mild | no | Min. |
| Ground glass opacities | no | no | no | no | no | mild | no | no |
| Associated findings | ||||||||
| Emphysema | no | parasep | parasep | no | no | no | no | no |
| Pleural plaques | no | no | no | no | no | no | no | no |
| Interval since initial changes in years | ||||||||
| Chest X-ray or CT | 5 | 5 | 3 | 7 (CT) | 3 | 13 | 8 | NA |
| Pulmonary function | ||||||||
| FVC (%) | NA | 116 | 92 | 82 | 69 | NA | 80 | 79 |
| FEV1 (%) | 62 | 114 | 92 | 89 | 76 | 98 | 81 | 94 |
| FEV1/FVC | 69 | NA | 76 | 80 | 121 | 102 | 73 | 120 |
| DLCO | 36 | 84 | 72 | 59 | 62 | 55 | 52 | 47 |
Min.= minimal; parasep=paraseptal emphysema
Fig. 1.
(A) Axial HRCT findings case 5. Left images: Diffuse interstitial lung disease preferentially located in a peripheral bibasilar distribution with a coral-like, branching, dendritic pattern. Despite extensive changes, there is a remarkable absence of radiological signs of fibrosis (no honeycombing nor traction bronchiectasis). Right images: CT findings with osteoporosis window settings (window width 818; Level 273) at the same levels confirm the bone density of the coral-like densities. (B) Axial HR CT findings in case 6 , whom is the brother of case 5, showing similar findings. X-ray changes had been observed 13 years before. (C) Axial HR CT findings in case 7
Fig. 2.

Moderate subpleural tracer capitation in a 99m technetium-methylene diphosphate (99mTc-MDP) bone scintigraphy (case 5)
Fig. 3.
Histological appearance of autopsy findings in case 5 (H&E stain) includes numerous branching osseous structures within a fibrotic expanded interstitium. Some of the bone nodules contain fat marrow
Discussion
We report eight elderly patients with HRCT findings pathognomonic for predominant DPO in a UIP-like distribution. In one patient, DPO was subsequently confirmed by autopsy. The patients lacked the presence of radiological important signs of fibrosis such as honeycombing and traction bronchiectasis despite long-lasting disease. Two patients were brothers, suggesting a genetic predisposition. Progression in our patients was slow. One patient had been followed elsewhere for 12 years without significant progression.
Only a handful of cases with DPO in a UIP-like distribution have been reported in the literature, with the majority being mild DPO. Of 84 cases of so-called Hamman-Rich syndrome collected from the literature, pulmonary ossification was found in 8% (6). In one of the more recent large adult autopsy studies, 5 out of 11 cases with DPO were diagnosed with “IPF” (7). In a study assessing radiologic-pathologic correlations, the frequency of biopsy-proven mild DPO was 5 of 75 patients with UIP, whereas none of 44 patients with NSIP in that series had DPO (4).
Reports on DPO in a UIP-like distribution as a predominant finding is even more uncommon. In the largest series of patients with DPO, Felson et al. presented 8 cases with predominatly bibasilar branching linear shadows of mainly calcific composition on chest X-ray. Subsequent histological examination at autopsy proved the DPO nature of these lesions. Interestingly, they pointed out that in these patients there was no evidence of fibrosis (8).
In each of three review articles on interstitial lung disease and/or lung calcification, one of the enclosed figures represent DPO in a UIP-like distribution (9-11). Recently four case reports have been published of DPO in a UIP-like distribution (2,12-14). In one patient diagnosis was made based on the typical HRCT findings, in the absence of honeycombing (12). One patient was diagnosed with transbronchial biopsy showing DPO and some fibrosis (2). In two patients VATS lung biopsy revealed DPO with temporally heterogeneous fibrosis. Presence of fibroblast foci was not mentioned (13,14). In an unpublished abstract presented at the ERS, one patient suffered from unilateral DPO in a peripheral subpleural basal distribution (15).
The pathophysiology of heterotopic ossification in dendriform DPO is unknown, with calcium metabolism being normal. The most extreme cases of heterotopic ossification are observed in fibrodysplasia ossificans progressiva (FOP), characterized by formation of cartilage and bone in inflamed muscle tissue. Patients with FOP carry an autosomal dominant heterozygous germ-line mutation in the transforming growth factor-beta/bone morphogenetic protein (TGFbeta/BMP) type I receptor ALK2 gene. This mutation causes the receptor to be constitutively active (16). In response to this activating mutation in ALK2, endothelial cells from capillary blood vessels in muscle tissue undergo endothelial mesenchymal transition before differentiating into chondrocytes and osteoblasts in response to inflammatory signals. Chondrogenesis is followed by endochrondral ossification (16). Lung capillary endothelial cells also give rise to significant numbers of fibroblasts through an endothelial-mesenchymal transition in bleomycin-induced lung fibrosis model (17). Although dendriform DPO is not a feature of FOP, there might be a potential role for this pathway in DPO. A genetic cause for DPO seems plausible, as there were two brothers in our series and there is one more report of familial clustering of DPO (18).
Peros-Golubicic et al. suggested a central role of diabetes mellitus in DPO, as several patients suffered from diabetes in their literature review (2). In our series, some patients suffered from diabetes, but more patients had cardiovascular disorders and were treated with warfarin or antiplatelet therapy. There might be a role of minor bleeding at the onset of ossification. In support of this hypothesis, Ohtsuki found iron deposits in vascular elastic fibers in lung biopsy samples of patients with dendriform ossification (19).
The majority of patients with IPF have inexorably progression with median survival estimated to be approximately 3 years. However the clinical course of individual patients is often unpredictable. Some patients remain stable for several months to years, while others have acute deteriorations or rapidly progressive respiratory insufficiency. Accurately defining phenotypes might be important to predict prognosis, to select patients for inclusion into clinical trials, and to provide a basis for studying the pathobiology of disorders. Distinct morphological phenotypes of IPF have been proposed including combined pulmonary fibrosis and emphysema (20). In accordance with the clinical significance of defining phenotypes of IPF, it is of main importance to differentiate IPF from resembling disorders, such as DPO in a UIP-like distribution.
In conclusion, we present eight patients, of whom two brothers, with DPO in a UIP-like distribution. HRCT with appropriate osteoporosis window setting revealed the diagnosis. Although DPO is an uncommonly reported interstitial lung disease, it seems highly probable that the routine use of HRCT in the evaluation of fibrotic lung diseases, the use of cryobiopsy and the awareness of the disorder, will increase the diagnosis of DPO.
The aetiology remains obscure, but it seems likely that external triggers (such as minor bleeding), and genetic predisposition may be involved, with DPO just being one of the limited possible healing processes. In line with the well-studied pathogenesis of heterotopic ossification in FOP, the TGF-β/ BMP pathway could be involved in DPO. Studying a larger cohort of patients and further genetic analysis might resolve several of the raised questions.
The slowly progressive nature of the disorder contrasting with IPF, the absence of radiological honeycombing despite long-lasting disease, the preferentially occurrence in the very old male, and the highly likelihood of a genetic predisposition highlight the importance of distinguishing IPF from DPO in a UIP-like distribution.
Footnotes
Three cases of this case series of eight patients have been the subject of a personal communication (poster session) at the ERS in Munchen in Sept ‘14
References
- 1.Chan ED, Morales DV, Welsh CH, McDermott MT, Schwarz MI. Calcium deposition with or without bone formation in the lung. Am J Respir Crit Care Med. 2002;165(12):1654–69. doi: 10.1164/rccm.2108054. [DOI] [PubMed] [Google Scholar]
- 2.Peros-Golubicić T, Tekavec-Trkanjec J. Diffuse pulmonary ossification: an unusual interstitial lung disease. Curr Opin Pulm Med. 2008;14:488–92. doi: 10.1097/MCP.0b013e3283043e1b. [DOI] [PubMed] [Google Scholar]
- 3.Müller K-M, Friemann J, Stichnoth E. Dendriforn Pulmonary Ossification. Pathol Res Pract. 1980;168(1-3):163–72. doi: 10.1016/s0344-0338(80)80215-9. [DOI] [PubMed] [Google Scholar]
- 4.Kim TS, Han J, Chung MP, Chung MJ, Choi YSl. Disseminated dendriform pulmonary ossification associated with usual interstitial pneumonia: Incidence and thin-section CT-pathologic correlation. Eur Radiol. 2005;15(8):1581–5. doi: 10.1007/s00330-005-2671-7. [DOI] [PubMed] [Google Scholar]
- 5.Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188(6):733–48. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mendeloff J. Disseminated nodular pulmonary ossification in the Hamman-Rich lung. Am Rev Respir Dis. 1971;103(2):269–74. doi: 10.1164/arrd.1971.103.2.269. [DOI] [PubMed] [Google Scholar]
- 7.Tseung J, Duflou J. Diffuse pulmonary ossification: an uncommon incidental autopsy finding. Pathology. 2006;38(1):45–8. doi: 10.1080/00313020500464912. [DOI] [PubMed] [Google Scholar]
- 8.Felson B, Schwarz J, Lukin RR, Hawkins HH. Idiopathic pulmonary ossification. Radiology. 1984;153(2):303–10. doi: 10.1148/radiology.153.2.6435169. [DOI] [PubMed] [Google Scholar]
- 9.Marchioril E, Souza AS, Franquet T, Muller NL. Diffuse high-attenuation pulmonary abnormalities: A pattern-oriented diagnostic approach on high-resolution CT. AJR Am J Roentgenol. 2005;184(1):273–82. doi: 10.2214/ajr.184.1.01840273. [DOI] [PubMed] [Google Scholar]
- 10.Brown K, Mund DF, Aberle DR, Batra P, Young DA. Intrathoracic calcifications: radiographic features and differential diagnosis. Radiographics. 1994;14(6):1247–61. doi: 10.1148/radiographics.14.6.7855339. [DOI] [PubMed] [Google Scholar]
- 11.Souza CA, Müller NL, Flint J, Wright JL, Churg A. Idiopathic pulmonary fibrosis: Spectrum of high-resolution CT findings. AJR Am J Roentgenol. 2005;185(6):1531–9. doi: 10.2214/AJR.04.1599. [DOI] [PubMed] [Google Scholar]
- 12.Burkett A, Coffey N, Voduc N. Diffuse pulmonary ossification as a rare cause of interstitial lung disease. Can Respir J. 2014;21(1):23–4. doi: 10.1155/2014/640419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandez Crisosto CA, Quercia Arias O, Bustamante N, Moreno H, Echevarria U. Diffuse pulmonary ossification associated with idiopathic pulmonary fibrosis. Arch Bronconeumol. 2004;40(12):595–8. doi: 10.1016/s1579-2129(06)60380-7. [DOI] [PubMed] [Google Scholar]
- 14.Kim DU, Guinee D, Mohammed TLH. Case of the season: usual interstitial pneumonia with dendriform pulmonary ossification. Semin Roentgenol. 2015;50(1):4–7. doi: 10.1053/j.ro.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Gruden J, Panse P, Trahan A. Dendriform pulmonary ossification in the absence of interstitial fibrosis: CT findings and clinical correlates [abstract] Eur Respir J Suppl. 2011;38(Suppl 55):578. [Google Scholar]
- 16.Medici D, Olsen BR. The role of endothelial-mesenchymal transition in heterotopic ossification. J Bone Miner Res. 2012;27(8):1619–22. doi: 10.1002/jbmr.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hashimoto N, Phan SH, Imaizumi K, et al. Endothelial-mesenchymal transition in bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2010;43(2):161–72. doi: 10.1165/rcmb.2009-0031OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Azuma A, Miyamoto H, Enomoto T, Usuki J, Kudoh S. Familial clustering of dendriform pulmonary ossification. Sarcoidosis Vasc Diffuse Lung Dis. 2003;20(2):152–4. [PubMed] [Google Scholar]
- 19.Ohtsuki Y, Yamanaka A, Ohyama H, et al. Histochemical demonstration of aluminum and iron deposition in pulmonary bony tissues in three cases of diffuse pulmonary ossification. Histol Histopathol. 2008;23(2):137–41. doi: 10.14670/HH-23.137. [DOI] [PubMed] [Google Scholar]
- 20.Cottin V, Nunes H, Brillet , et al. Combined pulmonary fibrosis and emphysema: A distinct underrecognised entity. Eur Respir J. 2005;26(4):586–93. doi: 10.1183/09031936.05.00021005. [DOI] [PubMed] [Google Scholar]