Abstract
Primary pulmonary extra-nodal MALT-lymphomas are very uncommon. Clinical-radiological pattern is variable and usually non-specific and a correct diagnosis usually requires the histopathological examination. Herein we report a case of a 59-year-old man presented with dyspnea at the slightest effort and dry cough. At imaging multiple pulmonary consolidations with interlobular septal thickenings and ground-glass opacities were disclosed, defining a crazy paving pattern. The surgical approach was necessary to reach the diagnosis of primary pulmonary low-grade marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALT-lymphoma). Immunotherapy (Rituximab) and chemotherapy (Bendamustine) were started leading to a progressive improvement of the disease. (Sarcoidosis Vasc Diffuse Lung Dis 2017; 34: 260-263)
Keywords: MALT-lymphoma, lung lymphoma, crazy-paving pattern, lung disease, immunotherapy
Introduction
Pulmonary mucosa-associated lymphoid tissue-derived (MALT) lymphoma is a rare disease, but is the most frequent subset of primary pulmonary lymphoma (represents 90% of PPL).
Usually this entity is characterized by a non-specific radiological pattern, consisting of hilar and/or mediastinal lymphadenopathy or single/multiple, bilateral pulmonary solid nodules.
Herein we report a very challenging case of a primary MALT-lymphoma presenting with a very uncommon radiological pattern (“crazy-paving”) characterized by bilateral multiple pulmonary consolidations and mimicking an (sub)acute lung infectious disease.
Case report
A 59-year-old, former smoker, man, presented at the Emergency Department with a sub-acute onset of increasing dyspnea and persistent dry cough. Previous medical history consisted of an occupational exposure to fine dusts and other toxic inhalants (ex pottery maker), and acute myocardial infarction previously treated with PTCA. No symptoms of collagen vascular/autoimmune disease were present.
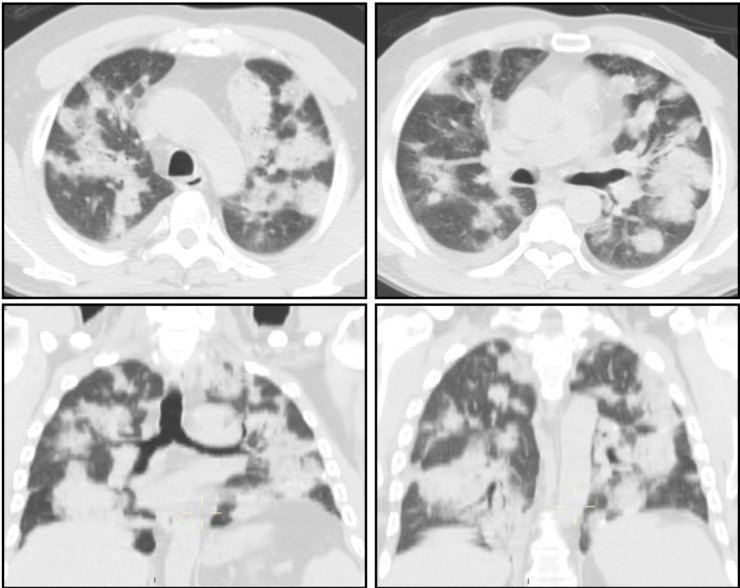
At physical examination, digital clubbing was observed and inspiratory basal crackles were detected. Chest X-rays documented multiple bilateral pulmonary consolidations and a High-Resolution Computed Tomography (HRCT) confirmed the presence of bilateral multiple areas of consolidations with frequent air bronchograms, interlobular septal thickenings and ground-glass opacities (Fig. 1). Imaging was consistent with a crazy paving pattern.
Fig. 1.
High-resolution Computed Tomography (HRCT) shows multiple areas of consolidations, interlobular septal thickenings and ground-glass opacities, defining a peculiar “crazy-paving” pattern.
Sputum examination was negative for pathogenic microbes, while pulmonary function tests revealed a moderate restrictive disease (VEMS 66,5%, FVC 61,1%, TLC 56,8%).
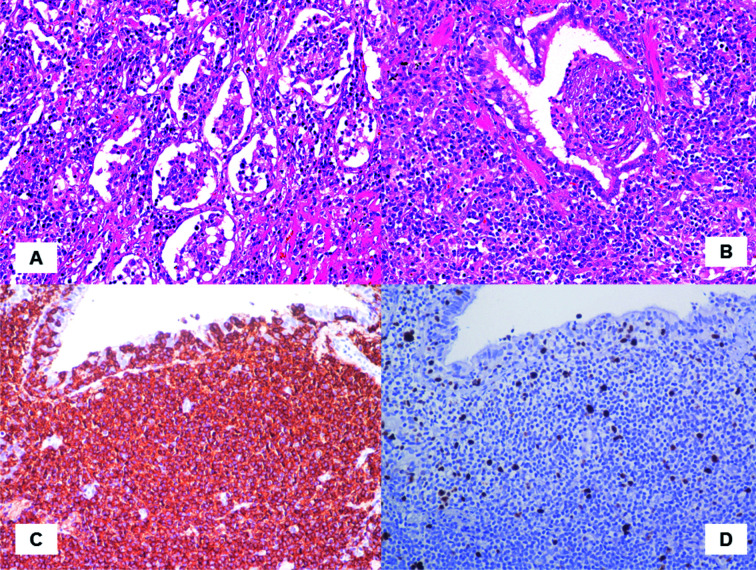
The post-operative course was unremarkable and the patient was discharged in 4th post-op day. At histology, the lung parenchyma showed nodular lesions characterized by an infiltration monomorphic small lymphocites and plasma-cells obliterating the alveolar spaces (Fig. 2A) associated with lymphoepithelial lesions (Fig. 2B). At immunohistochemistry, neoplastic lymphocytes show a B-cell clonal phenotype (CD20 immunohistochemistry, Hematoxylin counterstain, 20X (Fig. 2C)); moreover, stain for MIB1 shows a low proliferative index of the neoplastic infiltrate (MIB1 immunohistochemistry, Hematoxylin counterstain, 20X (Fig. 2D)) accounting for 3-5%. The final diagnosis of mucosa-associated lymphoid tissue-derived (MALT) lymphoma was finally made. The patient underwent chemotherapy with Rituximab and Bendamustine, with gradual and progressive improvement of the overall clinical scenario.
Fig. 2.
At histology the pulmonary lesion are characterized by a dense and diffuse infiltrate of monomorphic lymphocytes and plasma-cells. The lymphoid population is composed of small lymphocytes with a moderate rim of clear cytoplasm obliterating the alveolar spaces (A). The mucosa of bronchial wall reveal lympho-epithelial lesions strongly indicative of extra-nodal MALT lymphoma (B). At immunohistochemistry, neoplastic cells are diffusely positive for CD20, demonstrating the B-cell phenotype and the clonal nature (CD20 immunohistochemistry, Hematoxylin counterstain, 20X (C). Mitotic activity evaluated with MIB-1/Ki-67 is low (3-5%) (MIB1 immunohistochemistry, Hematoxylin counterstain, 20X (D)
Discussion
MALT-lymphoma is a monoclonal lymphoid proliferation classified as low-grade marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue. A part from the nodal and splenic forms, MALT lymphoma prevalently affects the stomach and a causative relationship is recognized between the chronic antigenic stimulation elicited by Helicobacter pylori infections and the disease. MALT-lymphoma may also involve the pulmonary parenchyma, especially in certain autoimmune disorders leading to chronic antigenic stimulation, particularly Sjogren Syndrome. Despite its rarity MALT-lymphoma is the most frequent primary pulmonary lymphomas (1). Half of patients are often asymptomatic. When present, symptoms are unspecific, including most frequently cough, chest pain fatigue, weight loss, fever and night sweats (2). In the current case, despite a severe radiological scenario, clinical symptoms were scarce and more indicative of an inflammatory process.
The imaging of MALT-lymphoma is unspecific and characterize by hilar and/or mediastinal lymphadenopathy (30% of cases) or single, multiple or bilateral pulmonary solid nodules (about 70% of cases). Sometimes the presence of air bronchograms within the nodules could support the radiologic hypothesis of MALT-lymphoma. When the pulmonary consolidations are the main radiological features, the differential diagnosis should be mainly oriented to more common etiologies such as chronic eosinophilic pneumonia, organizing pneumonia or pulmonary infarction; conversely, when thickened septal lines are detectable in association with ground glass opacities, as in our case, a pulmonary infection (such as Pneumocystis pneumonia) or diffuse alveolar disease (alveolar proteinosis) should be considered as the first etiology in the differential diagnosis (3).
Usually, bronchoscopy do not reveal per se specific abnormalities (sometimes bronchial edema, inflammation or external compression may be observed), but bronchiolo-alveolar lavage (BAL) could be very useful in selected instances.
Indeed, the result of BAL may indicate the presence of pathogens pointing towards a specific infection; moreover, the lymphocyte rate (LF-Rate) calculated on bronchiolo-alveolar lavage could be suggestive of pulmonary lymphoma, if LF-Rate results to be more than 20% of total cells and, above all, the B-cells are more than 10% (4). In our case, the BAL results were negative for fungal/bacterial pathogens (such as Pneumocystis jirovecii) and, surprisingly, the lymphocyte rate was very low (about 2%), to support the initial hypothesis of a primary pulmonary lymphoma.
To establish a certain diagnosis of pulmonary MALT lymphoma, the histopatological examination is often required. From a histological point of view, the pulmonary lesions comprised sheets of monoclonal B-cell lymphocytes with variable number of plasma-cells, forming a mass-like lesion and obliterating the alveolar spaces. In addition, invasion of the bronchial epithelium by lymphoid cells, referred to as lymphoepitelial lesion is a key feature of MALT-lymphoma (5).
The immunohistochemical demonstration of a B-clonal origin of the lymphoid population together with a light chain restriction is necessary to differentiate benign/reactive conditions from MALT-lymphoma.
In conclusion, there is no non-invasive test specific enough to make a correct diagnosis of MALT-lymphoma. A proper diagnosis may be established by a histopathological examination; notably, histological diagnosis of lymphoma can be difficult on small biopsy specimens, and a surgical approach may be necessary in some cases.
The present challenging case should alert the physicians in taking into account such rare entity in differential diagnosis of bilateral pulmonary consolidation with a specific radiological pattern (“crazy paving”). In fact, MALT-lymphoma has a good prognosis (up to 80% at 5 year survival) and it could be successfully treated with surgery or radiotherapy in case of localized forms and with chemotherapy and immunotherapy for disseminated ones.
References
- 1.Bi L, Li J, Dan W, Lu Z. Pulmonary MALT lymphoma: A case report and review of the literature. Exp Ther Med. 2015 Jan;9(1):147–150. doi: 10.3892/etm.2014.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nahorecki A, Chabowski M, Straszak E, Teplicki A, Szuba A, Langfort R, Janczak D. Primary pulmonary MALT lymphoma - case report and literature overview. Eur Rev Med Pharmacol Sci. 2016 May;20(10):2065–9. [PubMed] [Google Scholar]
- 3.Carter BW, Wu CC, Khorashadi L, Godoy MC, de Groot PM, Abbott GF, Lichtenberger JP., 3rd Multimodality imaging of cardiothoracic lymphoma. Eur J Radiol. 2014 Aug;83(8):1470–82. doi: 10.1016/j.ejrad.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 4.Cadranel J, Wislez M, AntoIne M. Primary pulmonary lymphoma. Eur Respir J. 2002;20:750–62. doi: 10.1183/09031936.02.00404102. [DOI] [PubMed] [Google Scholar]
- 5.Hare SS, Souza CA, Bain G, Seely JM, Frcpc, Gomes MM, Quigley M. The radiological spectrum of pulmonary lymphoproliferative disease. Br J Radiol. 2012 Jul;85(1015):848–64. doi: 10.1259/bjr/16420165. [DOI] [PMC free article] [PubMed] [Google Scholar]