Abstract
Background: Pectoralis muscle area (PMA) is an easily derived computed tomography-based assessment that can provide insight into clinical features of other skeletal muscles. Respiratory and locomotor muscle dysfunction has been increasingly recognized in patients with interstitial lung disease (ILD). Its contribution to exercise performance has been controversial. Objective: We aimed to investigate if PMA is related with respiratory and locomotor skeletal muscle strength in ILD patients, and if skeletal muscle function is compromised and independently related with exercise capacity and dyspnea. Methods: Cross-sectional study where subjects performed incremental cycling cardiopulmonary exercise testing with maximal inspiratory (MIP) and expiratory (MEP) pressure measurements, and quadriceps maximal voluntary contraction (MVC) before and after exercise. Results: Thirty ILD patients (forced vital capacity [FVC] and lung diffusing capacity [DLCO] of 60±15% and 38±10% of predicted, respectively) and 15 healthy control subjects were studied. Patients presented significantly lower MIP and qMVC compared to controls. PMA was significantly associated with qMVC only (r=0.506; p<0.01). Only expiratory muscles showed a significant strength decline after exercise, both in patients and controls. In multivariate regression analysis, only FVC remained as independent predictor of peak aerobic capacity and MEP post exercise remained as independent predictor of peak exercise dyspnea even adjusting for FVC. Conclusion: ILD patients exhibited reduced inspiratory and quadriceps strength, but PMA was associated with the later only. Muscle strength was not associated with exercise capacity while expiratory muscle fatigue might underlie exertional dyspnea. (Sarcoidosis Vasc Diffuse Lung Dis 2017; 34: 200-208)
Keywords: interstitial lung diseases, diffuse parenchymal lung diseases, skeletal muscle, respiratory muscles, exercise, dyspnea
Abbreviation list:
- Bronchodilator (BD)
- Body mass index (BMI)
- Breathing frequency (f)
- Carbon dioxide output (CO2)
- Chronic obstructive pulmonary disease (COPD)
- Computed tomography (CT)
- Coefficient of variation (CV)
- Delta, variable change from rest (Δ)
- Forced expiratory volume in one second (FEV1)
- Forced vital capacity (FVC)
- Functional residual capacity (FRC)
- Heart rate (HR)
- Hounsfield unit (HU)
- Inspiratory capacity (IC)
- Interstitial lung disease (ILD)
- Lung diffusion capacity for carbon monoxide (DLCO)
- Maximal cardiopulmonary exercise testing (CPET)
- Maximal expiratory pressure (MEP)
- Maximal inspiratory pressure (MIP)
- Minute ventilation (E)
- Modified medical research council (mMRC)
- Maximum voluntary ventilation (MVV)
- Oxyhemoglobin saturation by pulse oximetry (SpO2)
- Oxygen uptake (O2)
- Pectoralis muscle area (PMA)
- Peak of oxygen uptake (pO2)
- Quadriceps maximal voluntary contraction (qMVC)
- Residual volume (RV)
- Total lung capacity (TLC)
- Tidal volume (VT)
Introduction
Pectoralis muscle area (PMA) is a computed tomography (CT)-based assessment easily obtained from evaluation of patients with interstitial lung disease (ILD). PMA may provide insight into clinical features of other skeletal muscles. Accordingly, PMA recently shown association with more severe disease (1), diminished exercise capacity, respiratory muscle force, and mortality in chronic obstructive pulmonary disease (COPD) (2).
Exercise intolerance and dyspnea are cardinal symptoms of ILD. Reduced exercise capacity is multifactorial, but restrictive pulmonary mechanics, severe gas-exchange derangements and intolerable exertional symptoms are often considered the primary mechanisms (3). Although respiratory (4-7) and locomotor muscle (7-12) dysfunction has been increasingly recognized, its contribution to exercise limitation in ILD has been controversial. Impaired exercise capacity was reported to be related to respiratory muscle dysfunction (5, 11) or quadriceps weakness (7, 8), while other studies did not identify such relationships (10, 13).Additionally, expiratory muscle fatigue was observed and correlated with peak exercise capacity in ILD patients, which was not observed with inspiratory muscle strength (14). Finally, whether quadriceps fatigue occurs following exercise or contributes to exercise capacity in ILD is unknown.
Therefore, we reasoned that PMA could be associated with strength of other skeletal muscles in patients with ILD. We further considered that skeletal muscle force would be reduced in these patients and related to exercise capacity and dyspnea. Therefore, we also aimed to investigate if skeletal muscle function (strength and fatigue) is compromised and independently related to those clinical outcomes.
Method
Design
Cross-sectional study approved by Institutional Research Ethics Committee performed from April 2014 to October 2015. All patients signed written informed consent. Maximal inspiratory pressure (MIP), maximal expiratory pressure (MEP), and quadriceps maximal voluntary contraction (qMVC) were performed before and 3-15 minutes after maximal cardiopulmonary exercise testing (CPET).
Participants
Patients were recruited from a specialized ILD clinic. Clinically stable patients with a diagnosis of fibrotic ILD and free from other disorders that could interfere with exercise performance were invited to participate. Exclusion criteria include home oxygen therapy or any clinical features suggesting myopathy (abnormal muscle enzymes, muscle pain or fatigue). Healthy controls were recruited from the community by invitation. The specific diagnosis of ILD was established by consensus among two pulmonologists and one experienced thorax radiologist based on clinical features, thorax CT and, in some cases, lung biopsy (15, 16).
Study procedures
Lung function
Spirometry, plethysmographic lung volumes and single breathe lung diffusing capacity for carbon monoxide (DLCO) were measured with an automated system(Jaeger®, Wüerzburg, Germany) and obtained from medical records of the routine clinical assistance of the patients.
Pectoralis muscle area (PMA)
Quantitative CT assessment of PMA was performed on the first axial image above the aortic arch. The left and right pectoralis major and minor muscles were manually identified using a predefined attenuation range of -50 and 90 HU (although modifications were allowed when excluded muscle regions were identified) and measures of area were performed for each subject. PMA was presented as the aggregate area (in cm2) of the right and left pectoralis major and minor muscles (1).
Respiratory muscle dtrength
MIP and MEP were measured in sitting position with a pressure transducer (MVD-300®, Globalmed, Porto Alegre, Brazil) at residual volume and total lung capacity, respectively. At least five measurements were performed and the highest values (peak values) were recorded with a difference lower than 10% between the two highest values. Measurements were presented in absolute (cmH2O) and percentage of predicted value (17).
Quadriceps strength
qMVC was measured during bilateral isometric contractions with a load cell (BTS-200kg®, Weightech, São Paulo, Brazil) attached to a fixed knee extension chair (NT-840®, Buik, Rio de Janeiro, Brazil). Measurements were presented in absolute (Kg) and percentage of predicted values, adjusted for (Nm)(18) and adapted to unilateral contraction (19). The knee joint was aligned with the axis of rotation. After familiarization with the procedure and a practice trial, the subjects were instructed to increase force in 1s and held the maximal force during approximately 5 s while the investigators provided strong verbal encouragement (20, 21). Three to four trials were performed with at least 3 min of rest between trials. The peak force was recorded as the measure of MVC (Kg) with a difference lower than 10% between the two highest values.
Cardiopulmonary exercise testing
Incremental CPET (5-10 w/min) were performed on cycle ergometer (Ergoline-900®, Jaeger, Wüerzburg, Germany) using a breath-by-breath metabolic system (Oxycon Pro®, Jaeger). Dyspnea was obtained every 2 min during exercise (22). To adjust for different exercise capacities and peak ventilation, dyspnea at peak exercise was divided by peak ventilation (23). Peak aerobic capacity was presented as absolute and percent predicted values (24).
Pedaling rates were maintained ~60 revolutions per minute. Oxyhemoglobin saturation (SpO2) was measured by pulse oximetry (Takaoka Oxicap®, São Paulo, Brazil). Maximum voluntary ventilation (MVV) was estimated as 37.5 x forced expiratory volume in the first second (FEV1) (25).
Statistical analysis
Baseline characteristic were compared with Student t test or Mann-Whitney test. Values before and after exercise were compared using generalized estimating equation. Pearson or Spearman correlation coefficients were used for correlation analyzes. The reproducibility of muscle strength measurements was evaluated with coefficient of variation (CV; standard deviation/mean).
Skeletal muscle variables to predict peak aerobic capacity (pO2) (% predicted) and exercise dyspnea (peak Borg dyspnea/peak ventilation) were analyzed in a multivariate linear regression model adjusted for forced vital capacity (FVC) and DLCO (both % predicted).
Twenty-three patients would be necessary to detect a moderate association (r=0.50) between primary outcomes with a statistical power of 80%. In addition, the inclusion of 27 patients and 14 controls were estimated to detect a difference in maximal expiratory pressure of 20 cmH2O (SD~20) between groups as previously described (11, 14). With an error type I and II of 5 and 20%, respectively.
The probability of a type I error was set at 5%. Data were analyzed using SPSS®V18.0; Chicago, IL).
Results
Thirty patients with ILD and 15 control subjects took part in the study. ILD sub-diagnosis included 6 patients with idiopathic pulmonary fibrosis, 10 with nonspecific interstitial pneumonia (NSIP) (without a recognizable potential causes, e.g. connective tissue disease [CTD] or drug exposure), 7 with fibrotic hypersensitivity pneumonitis (HP), 6 with associated CTD (4 with rheumatoid arthritis, one with systemic sclerosis and another with undifferentiated CTD) and one with lymphocytic interstitial pneumonia (LIP). Four patients with associated CTD, 4 with NSIP, and 3 with HP were receiving oral prednisone (10-20 mg/day) plus a second immunosuppressive drug (azathioprine; 50-100 mg/day) at the time of study procedures. There was no significant difference in muscle strength comparing patients with and without current use of immunosuppressive therapy.
The majority of patients (24/30; 74%) had restrictive ventilatory pattern as evidenced by reduced TLC (<80% of predicted). On average, patients presented a mild reduction in total lung capacity (TLC) and exercise capacity, with a severe reduction in DLCO. Baseline characteristics of subjects are given in tables 1 and 2. The CV for MIP, MEP and qMVC were 7.6%±0.6%, 7.3%±0.9% and 8.2%±0.8%, respectively.
Table 1.
Baseline characteristics of patients and controls
| Variables | Patients (N=30) | Controls (N=15) |
| Male sex, n° (%) | 14 (46.7%) | 7 (46.7%) |
| Age, years | 61.7±8.7 | 60.2±7.9 |
| Weight, Kg | 72.3±13.5 | 67.2±11.4 |
| Height, cm | 160±8 | 161±6 |
| BMI, Kg/m2 | 28.1±4.2 | 25.5±3.2* |
| mMRC Scale | 2.5 (1-4) | 0 (0-0)* |
| Resting Lung Function | ||
| FEV1, L (%pred) | 1.62±0.37 (62±15) | 2.85±0.63 (102±13)* |
| FEV 1 post BD, L (%pred) | 1.61±0.39 (62±14) | - |
| FVC, L (%pred) | 2.04±0.59 (60±15) | 3.67±0.73 (104±12)* |
| FVC post BD, L (%pred) | 1.92±0.48 (59±15) | - |
| FEV1/FVC, % | 81±1 | 77±1 |
| FEV1/FVC post BD, % | 85±0.7 | - |
| TLC, L (%pred) | 3.70±0.58 (71±15) | - |
| FRC, L (%pred) | 2.31±0.49 (81±22) | - |
| RV, L (%pred) | 1.77±0.46 (92±30) | - |
| IC, L (%pred) | 1.32±0.35 (52±15) | 2.44±0.57 (88±14)* |
| DLco, mmol/min/kPa (%pred) | 2.93±0.8 (38±10) | - |
| MIP, cmH2O (%pred) | 81±25 (87±24) | 109±36 (106±33)* |
| MEP, cmH2O (%pred) | 94±32 (98±23) | 107±40 (111±30) |
| SpO2, % | 96±2 | 98±1 |
| Muscle strength and size | ||
| qMVC kg | 21.8 (7.8-73.0) | 42.5 (17.7-90.0)* |
| qMVC % pred | 38.8 (22-97) | 63.7 (31-145)* |
| PMA cm2 | 33.4±11.5 | - |
Data are presented as mean±SD or median (range), unless otherwise stated.
Definition of abbreviations: BMI: body mass index; mMRC: modified medical research council; FEV1: forced expiratory volume in one second; %pred: % of predicted; BD: bronchodilator; FVC: forced vital capacity; TLC: total lung capacity; FRC: functional residual capacity; RV: residual volume; IC: Inspiratory capacity; DLCO: lung diffusing capacity for carbon monoxide; MIP: maximal inspiratory pressure; MEP: maximal expiratory pressure; SpO2: oxyhemoglobin saturation by pulse oximetry; qMVC: Quadriceps isometric maximal voluntary contraction; PMA: Pectoralis muscle area* P<0.05.
Table 2.
Measurements during incremental cardiopulmonary exercise testing
| Variables | Patients (N=30) | Controls (N=15) |
| Peak exercise | ||
| O2, mL/min (% pred) | 1088±277 (75±13) | 1595±550 (110±19)* |
| O2, mL/Kg/ min | 14.6±2.6 | 23.3±4.5* |
| CO2, mL/min | 1138±334 | 1985±707* |
| E L/min | 46.4±14.7 | 64.6±25.7* |
| VT, L | 1.02±0.24 | 1.82±0.47* |
| f breaths/ min | 46±11 | 34±7* |
| E/MW | 0.76±0.15 | 0.59±0.13* |
| ΔE/ΔCO2 | 38.5±9.8 | 27.8±3.1* |
| IC, L (% pred) | 1.40±0.27 (55±13) | 2.61±0.62 (95±13)* |
| ΔIC, L | 0.11 (-0.6-0.4) | 0.19 (-0.08-0.5) |
| HR, beats/min (% pred) | 123±20 (76±11) | 144±12 (103±28)* |
| O2 pulse, mL/beats | 8.8±2.1 | 11.1±3.8 |
| ΔO2/Δwork, mL/min/watts | 9.7±3.5 | 9.4±1.7 |
| Peak workload, watts (%pred) | 49±18 (51±18) | 109±35 (106±18)* |
| SpO2, % | 88±7 | 98±1* |
| ΔSpO2, % | -8.2±5.3 | 0.2±1.2* |
| Borg dyspnoea score | 5.9 (1-10) | 3.9 (1-10)* |
| Dyspnoea/E, Borg/L/min | 0.14 (0.01-0.30) | 0.05 (0.01-0.20)* |
| Borg leg effort score | 6.3 (1-10) | 5.9 (1-10) |
| Leg effort/CO2, Borg/L/min | 5.5 (1.9-12.7) | 2.8 (0.3-7.3)* |
| Anaerobic threshold | ||
| O2, mL/min (% predicted) | 771±173 (53±6) | 1083±417 (74±19)* |
Data presented as means ± SÇ or median (range).
Definition of abbreviations: O2: oxygen uptake; %pred: % of predicted CO2: carbon dioxide output; E: minute ventilation; VT: tidal volume; y=breathing frequency; MMV: maximum voluntary ventilation; IC: inspiratory capacity; ΔI& IC change from rest; HR: heart rate; SpO2: oxyhemoglobin saturation by pulse oximetry. *P<0.05
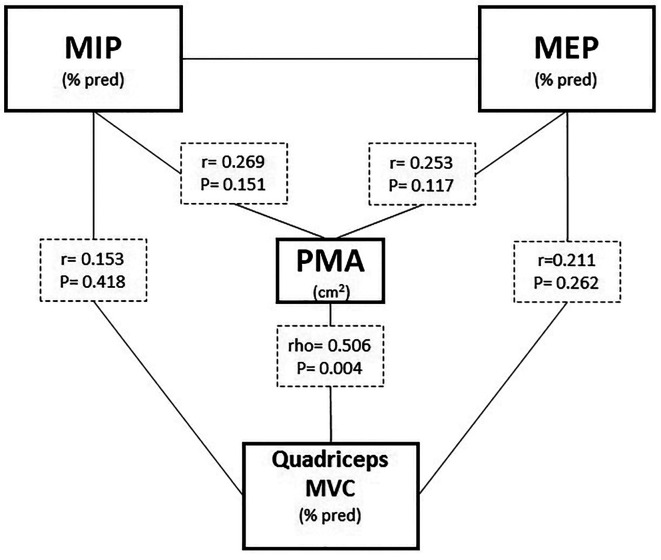
In patients, PMA was significantly associated with qMVC only (Figure 1). Peak of O2 significantly correlated just with MIP (pre and post exercise) but not with exercise changes (Δ; post-pre exercise) of any skeletal muscle strength assessed. In multivariate analysis, however, only FVC remained as independent predictor of pO2 (Table 3). Exercise dyspnea was related with MIP and MEP post exercise. In multivariate regression analysis, MEP post exercise remained as independent predictor of peak exercise dyspnea even adjusting for FVC (Table 4).
Fig. 1.
Relationship between pectoralis muscle area (PMA) and maximal respiratory and locomotor muscle force. Definition of abbreviations: see Table 1.
Table 3.
Linear regression analyses to predict peak aerobic capacity in patients with ILD
| Variables | Unstandardized coefficient | r value | p value |
| Univariate model | |||
| FVC, %pred | 0.440 | 0.51 | 0.005 |
| DLco, %pred | 0.557 | 0.45 | 0.039 |
| MIP pre exercise, %pred | 0.212 | 0.40 | 0.030 |
| MEP pre exercise, %pred | 0.139 | 0.25 | 0.174 |
| qMVC pre exercise, %pred | - 16.228 | 0.23 | 0.205 |
| PMA, cm2 | -0.393 | 0.35 | 0.059 |
| Multivariable model* | |||
| FVC, %pred | 0.398 | ------ | 0.024 |
| DLco, %pred | ------ | ------ | 0.133 |
Definition of abbreviations: see Table 1.
* Constant (intercept): 36.3; Coefficient of regression (R): 0.64
Table 4.
Linear regression analyses to predict peak exercise dyspnea (adjusted to peak exercise ventilation) in patients with ILD
| Variables | Unstandardized coefficient | r value | p value |
| Univariate model | |||
| FVC, %pred | -0.002 | 0.49 | 0.005 |
| DLco, %pred | 0.001 | 0.04 | 0.836 |
| MIP post exercise, %pred | -0.001 | 0.46 | 0.009 |
| MEP post exercise, %pred | -0.001 | 0.37 | 0.045 |
| qMVC post exercise, %pred | 0.039 | 0.11 | 0.545 |
| PMA, cm2 | 0.000 | 0.05 | 0.773 |
| Multivariable model* | |||
| FVC, %pred | -0.003 | 0.001 | |
| MEP post exercise, %pred | -0.001 | 0.042 | |
For abbreviations definitions see Table 1.
* Constant (intercept): 0.390; Coefficient of regression (R): 0.72
Patients presented lower values of MIP and qMVC compared to controls. Only expiratory muscles strength showed a significant decline after exercise, without difference between groups (Figure 2). ΔMEP was not related with pO2 or exercise dyspnea in both groups.
Fig. 2.
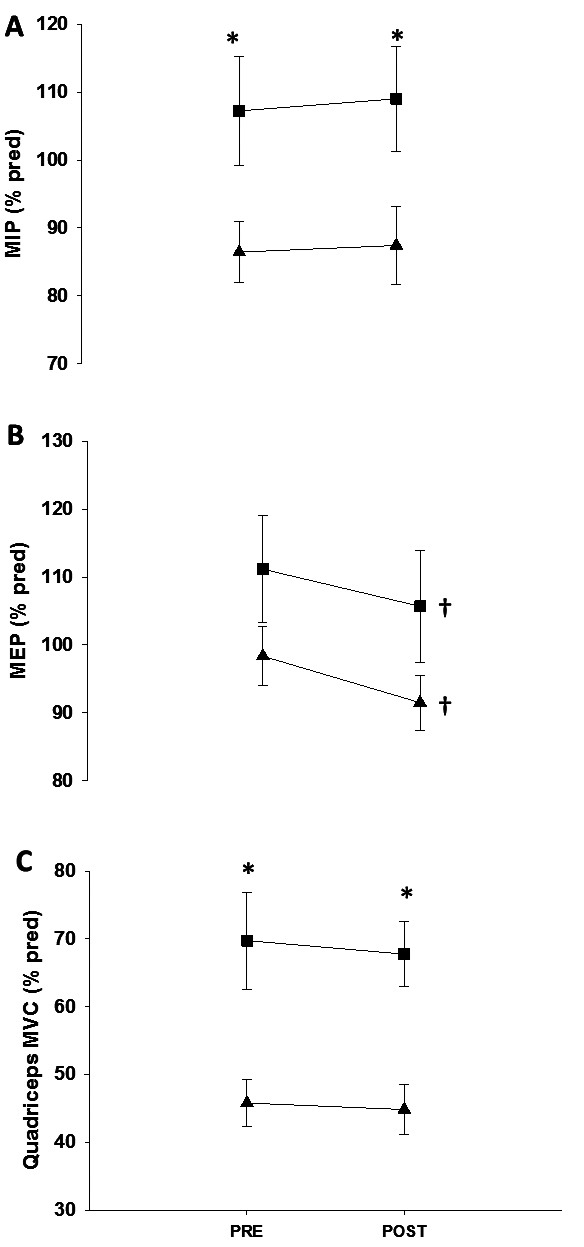
Skeletal muscle force before and after maximal exercise testing in patients (▲) and healthy controls (■).
Definition of abbreviations: see Table 1.
* P<0.05 between groups in a given moment.
† P<0.05 intra-group.
Discussion
In ILD patients, PMA was related to qMVC without significant relationship with MIP or MEP. Although qMVC and MIP were lower in ILD compared to controls, only MIP was correlated with pO2. Nevertheless, when adjusted for other variables related with disease pathophysiology (FVC and DLCO), just FVC remained as predictor of pO2. Only expiratory muscle fatigue was observed after exercise, but it was not associated with pO2. Nevertheless, MEP post exercise was an independent predictor of exercise dyspnea even after adjusting for potential confounders.
The significant association between PMA and qMVC supports the potential usefulness of PMA to evaluate skeletal muscle function from other parts of the body. In contrast to previous reports (7, 9), qMVC was not related with exercise capacity, maybe due to the more severe disease of the patients in the present study (mean FVC: ~75 vs 60% predicted). Additionally, the assessment of trunk muscles based on PMA was related to strength of peripheral but not respiratory muscles. Hence, PMA seems not provide a comprehensive assessment of whole body muscle function in our patients.
Resting pulmonary function tests neither exclude nor quantify the extent of exercise limitation in ILD (26, 27). It raises the question if other alternative mechanisms could contribute to exercise intolerance. Patients with chronic lung disease are prone to peripheral muscle dysfunction resulting from deconditioning, systemic inflammation, oxidative stress, blood gas disturbances, and sarcopenia (28). Inspiratory muscle strength was related to pO2 while quadriceps force did not. However, when ventilatory and lung diffusing parameters were included in multivariate model, only FVC remained as independent predictor of pO2. The type and severity of ILD patients may explain the discrepancy between the present study and previous reports.
Exertional dyspnea in various lung diseases generally occurs due to an imbalance between ventilatory demand and capacity(3). In this context, lower space to increase ventilation (i.e. ↓ FVC) and lower maximal inspiratory force to produce the required level of ventilation are potential mechanisms of exercise dyspnea. Notwithstanding, MIP was not independently associated with exercise performance and dyspnea. Short-term incremental exercise to exhaustion did not alter stimulated transdiaphragmatic pressure, probably because of too short exercise duration for the cumulative work history of the diaphragm to reach fatiguing levels (29, 30). The role of expiratory muscles in respiratory sensations was described as less important than inspiratory muscles in COPD (31), but was less examined in ILD. The higher proportion of more easily fatigable fast-twitch fibers (32), in combination with the fact that the expiratory muscles are engaged to a larger extent than inspiratory muscles during dynamic exercise, suggest that the expiratory muscles may be particularly prone to fatigue and contribute to exercise intolerance and dyspnea (33, 34). In the present study, similar MEP decline after exercise was observed in both groups. While ΔMEP was not related with exercise capacity (in contrast with previous report) (14) or dyspnea, post exercise MEP remained as independent predictor of dyspnea. It could be inferred that, during exercise, abdominal muscles are vigorously contracting to achieve high expiratory flow rate and to facilitate subsequent inspiration, contributing to expiratory muscle fatigue. Greater recruitment of expiratory muscle would result in lower values of MEP post exercise which was related to exercise dyspnea.
To minimize selection bias and at same time recruit our planned sample size, a prospective approach was undertaken in which all-consecutive patients with chronic fibrotic interstitial lung disease followed at our ambulatory clinic were recruited. As a result, the present study includes patients with a variety of histological diagnoses. However, our sample is still relatively small, and do not allow analysis of subgroups according to the ILD etiology. Nevertheless, due to the relatively infrequent nature of such diseases, other recent studies (35, 36) included ILD with similar etiological heterogeneity to explore mechanisms of exercise limitation and dyspnea. Significant correlations in transversal analysis do not necessarily imply causal relationships and future studies evaluating the effect of interventions to improve muscle function (e.g. muscle strength or endurance training) on clinical outcomes are recommended. On the other hand, one strength of our study, not previously performed, was include in the main regression analyses expected confounders regarding lung mechanics (FVC) and gas exchange (DLCO).
Finally, the validity of effort-dependent muscle force evaluations has been questioned (37), mainly because it may be influenced by sub-maximal effort and patient motivation. However, the reproducibility of muscle strength measurements in the present study was good (all CV <10%) and the likelihood of sub-maximal efforts associated with such a level of reproducibility is low. In addition, our aim was to evaluate muscle strength and fatigue using measurements easily accessible in clinical practice (i.e. maximum voluntary strength).
Conclusion
ILD patients exhibited reduced inspiratory and quadriceps strength, but PMA was associated with the later only. Muscle weakness was not associated to exercise capacity while expiratory muscle fatigue might underlie exertional dyspnea.
Acknowledgements
The study was supported by a grant received from Incentive Fund of Research of Hospital de Clínicas de Porto Alegre (FIPE/HCPA). PKM received a CAPES Fellowship, Graduation Program in Pulmonology, Universidade Federal do Rio Grande do Sul (UFRGS) (2014–2015).
Footnotes
Our data were preliminarily presented during the 7th International Wasog Conference on Diffuse Parenchymal Lung Diseases and 10th Brazilian Thoracic Society course on ILD, held on 04-06 june, 2015 at the city of Sao Paulo, Brazil. Sao Paulo - Brazil, June 6th, 2015: Respiratory and peripheral muscle force after maximal exercise in patients with fibrotic interstitial lung diseases vs healthy authored by Pietro Merola; Gabriela Fischer; Igor Gorsky Benedetto; Ricardo Gass; Rui Gustavo Paulus Nene Dorneles; Marcelo Basso Gazzana; Danilo Cortozi Berton.
Contributions:
All authors played a role in the content of the manuscript and approved its final version. In addition: DCB, PKM, MBG and BH had input into the study design and conception; PKM, RG,RGPND and FFG collected the data; DCB, PKM and SV performed data analysis. DCB, PKM and SV prepared the first draft of the manuscript.
References
- 1.McDonald ML, et al. Quantitative computed tomography measures of pectoralis muscle area and disease severity in chronic obstructive pulmonary disease. A cross-sectional study. Ann Am Thorac Soc. 2014;11(3):326–34. doi: 10.1513/AnnalsATS.201307-229OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mendonca D, Washko GR, Estepar RSJ, Criner GJ, Kim V. Pectoralis muscle area measurements are associated with degree of exercise capacity and mortality in individuals with advanced emphysema. American journal of respiratory and critical care medicine. 2015 [Google Scholar]
- 3.Palange P, Carlsen KH, Casaburi R, Gallagher CG, Gosselink R, O’Donnell DE, Puente-Maestu L, Schols AM, Singh S, Whipp BJ. Recommendations on the use of exercise testing in clinical practice. Eur Respir J. 2007;29(1):185–209. doi: 10.1183/09031936.00046906. [DOI] [PubMed] [Google Scholar]
- 4.Baydur A, et al. Respiratory muscle strength, lung function, and dyspnea in patients with sarcoidosis. Chest. 2001;120(1):102–8. doi: 10.1378/chest.120.1.102. [DOI] [PubMed] [Google Scholar]
- 5.Kabitz HJ, et al. Impact of impaired inspiratory muscle strength on dyspnea and walking capacity in sarcoidosis. Chest. 2006;130(5):1496–502. doi: 10.1378/chest.130.5.1496. [DOI] [PubMed] [Google Scholar]
- 6.Marcellis RG, et al. Exercise capacity, muscle strength and fatigue in sarcoidosis. Eur Respir J. 2011;38(3):628–34. doi: 10.1183/09031936.00117710. [DOI] [PubMed] [Google Scholar]
- 7.Spruit MA, et al. Skeletal muscle weakness in patients with sarcoidosis and its relationship with exercise intolerance and reduced health status. Thorax. 2005;60(1):32–8. doi: 10.1136/thx.2004.022244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishiyama O, et al. Quadriceps weakness is related to exercise capacity in idiopathic pulmonary fibrosis. Chest. 2005;127(6):2028–33. doi: 10.1378/chest.127.6.2028. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe F, et al. Quadriceps weakness contributes to exercise capacity in nonspecific interstitial pneumonia. Respir Med. 2013;107(4):622–8. doi: 10.1016/j.rmed.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 10.Mendoza L, et al. Quadriceps strength and endurance in fibrotic idiopathic interstitial pneumonia. Respirology. 2014;19(1):138–43. doi: 10.1111/resp.12181. [DOI] [PubMed] [Google Scholar]
- 11.Walterspacher S, et al. Respiratory muscle function in interstitial lung disease. Eur Respir J. 2013;42(1):211–9. doi: 10.1183/09031936.00109512. [DOI] [PubMed] [Google Scholar]
- 12.Mendes P, et al. Skeletal muscle atrophy in advanced interstitial lung disease. Respirology. 2015;20(6):953–9. doi: 10.1111/resp.12571. [DOI] [PubMed] [Google Scholar]
- 13.Brancaleone P, et al. Clinical impact of inspiratory muscle impairment in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2004;21(3):219–27. [PubMed] [Google Scholar]
- 14.Elia D, et al. Respiratory muscle fatigue following exercise in patients with interstitial lung disease. Respiration. 2013;85(3):220–7. doi: 10.1159/000338787. [DOI] [PubMed] [Google Scholar]
- 15.Travis WD, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188(6):733–48. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raghu G, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neder JA, et al. Reference values for lung function tests. II. Maximal respiratory pressures and voluntary ventilation. Braz J Med Biol Res. 1999;32(6):719–27. doi: 10.1590/s0100-879x1999000600007. [DOI] [PubMed] [Google Scholar]
- 18.Hogrel JY, Ollivier G, Tanant V, Attarian S, Couillandre A, Dupeyron A, Lacomblez L, Doppler V, Meininger V, Tranchant C, Pouget J, Desnuelle C. Development of a French isometric strength normative database for adults using quantitative muscle testing. Arch Phys Med Rehabil. 2007;88(10):1289–97. doi: 10.1016/j.apmr.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 19.Van Dieen JH, Ogita F, De Haan A. Reduced neural drive in bilateral exertions: a performance-limiting factor. Med Sci Sports Exerc. 2003;35(1):111–8. doi: 10.1097/00005768-200301000-00018. [DOI] [PubMed] [Google Scholar]
- 20.Tracy BL, Enoka RM. Steadiness training with light loads in the knee extensors of elderly adults. Med Sci Sports Exerc. 2006;38(4):735–45. doi: 10.1249/01.mss.0000194082.85358.c4. [DOI] [PubMed] [Google Scholar]
- 21.Petrella JK, et al. Age differences in knee extension power, contractile velocity, and fatigability. J Appl Physiol (1985) 2005;98(1):211–20. doi: 10.1152/japplphysiol.00294.2004. [DOI] [PubMed] [Google Scholar]
- 22.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–81. [PubMed] [Google Scholar]
- 23.O’Donnell DE, et al. Reliability of ventilatory parameters during cycle ergometry in multicentre trials in COPD. Eur Respir J. 2009;34(4):866–74. doi: 10.1183/09031936.00168708. [DOI] [PubMed] [Google Scholar]
- 24.Neder JA, et al. Prediction of metabolic and cardiopulmonary responses to maximum cycle ergometry: a randomised study. Eur Respir J. 1999;14(6):1304–13. doi: 10.1183/09031936.99.14613049. [DOI] [PubMed] [Google Scholar]
- 25.Hansen JE, Wasserman K. Pathophysiology of activity limitation in patients with interstitial lung disease. Chest. 1996;109(6):1566–76. doi: 10.1378/chest.109.6.1566. [DOI] [PubMed] [Google Scholar]
- 26.Bye PT, et al. Bicycle endurance performance of patients with interstitial lung disease breathing air and oxygen. Am Rev Respir Dis. 1982;126(6):1005–12. doi: 10.1164/arrd.1982.126.6.1005. [DOI] [PubMed] [Google Scholar]
- 27.Marciniuk DD, Watts RE, Gallagher CG. Dead space loading and exercise limitation in patients with interstitial lung disease. Chest. 1994;105(1):183–9. doi: 10.1378/chest.105.1.183. [DOI] [PubMed] [Google Scholar]
- 28.Maltais F, et al. An official American Thoracic Society/European Respiratory Society statement: update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;189(9):e15–62. doi: 10.1164/rccm.201402-0373ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Romer LM, Haverkamp HC, Pegelow DF, Dempsey JA. Inspiratory muscles do not limit maximal incremental exercise performance in healthy subjects. Respir Physiol Neurobiol. 2007;156:353–61. doi: 10.1016/j.resp.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bachasson D, et al. Quadriceps and respiratory muscle fatigue following high-intensity cycling in COPD patients. PLoS One. 2013;8(12):e83432. doi: 10.1371/journal.pone.0083432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laveneziana P, Wadell K, Neder JA, O’Donnell DE. Does increased expiratory muscle activity contribute to dyspnea during exercise in COPD. Respir Physiol Neurobiol. 2014;199:24–33. doi: 10.1016/j.resp.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 32.Keens TG, et al. Developmental pattern of muscle fiber types in human ventilatory muscles. J Appl Physiol Respir Environ Exerc Physiol. 1978;44(6):909–13. doi: 10.1152/jappl.1978.44.6.909. [DOI] [PubMed] [Google Scholar]
- 33.Verges S, et al. Expiratory muscle fatigue impairs exercise performance. Eur J Appl Physiol. 2007;101(2):225–32. doi: 10.1007/s00421-007-0491-y. [DOI] [PubMed] [Google Scholar]
- 34.Taylor BJ, How SC, Romer LM. Exercise-induced abdominal muscle fatigue in healthy humans. J Appl Physiol (1985) 2006;100(5):1554–62. doi: 10.1152/japplphysiol.01389.2005. [DOI] [PubMed] [Google Scholar]
- 35.Faisal A, et al. Common Mechanisms of Dyspnea in Chronic Interstitial and Obstructive Lung Disorders. Am J Respir Crit Care Med. 2015 doi: 10.1164/rccm.201504-0841OC. [DOI] [PubMed] [Google Scholar]
- 36.Degani-Costa LH, et al. Pulmonary vascular response patterns during exercise in interstitial lung disease. Eur Respir J. 2015 doi: 10.1183/09031936.00191014. [DOI] [PubMed] [Google Scholar]
- 37.NHLBI Workshop summary. Respiratory muscle fatigue. Report of the Respiratory Muscle Fatigue Workshop Group. Am Rev Respir Dis. 1990;142(2):474–80. doi: 10.1164/ajrccm/142.2.474. [DOI] [PubMed] [Google Scholar]