Abstract
Background: Chronic hypersensitivity pneumonitis (CHP) is characterized by varying degrees of inflammation and fibrosis of the lungs caused by a variety of inhaled antigens. Despite extensive efforts to minimize exposure to the antigens, patients with CHP sometimes experience rapid deterioration of their pulmonary functions, resulting in death within a few years. Objectives: This study aimed to define clearly the clinical and molecular features of patients with rapidly progressive CHP. Methods: Annual decline in pulmonary functions and its association with clinical variables was evaluated in 43 patients with CHP. The RNA from frozen lung specimens of nine patients with rapidly progressive CHP and normal control subjects was profiled using Illumina HumanWG-6 v3 Expression BeadChips, and an Ingenuity Pathway Analysis was performed to identify the altered functional and canonical signaling pathways. Results: Patients with more than 10% annual decline in forced vital capacity and those with more than 15% annual decline in diffusion capacity for carbon monoxide showed significantly poor overall survival rates (p=0.002 and p=0.001, respectively). According to the gene expression analysis, 160 genes, including cystatin SN (CST1), ephrin-A2 (EFNA2), and wingless-type MMTV integration site family, member 7B (WNT7B) were upregulated, and pathways related to inflammatory responses and autoimmune diseases were differentially expressed. Conclusion: Greater annual decline in pulmonary function can predict poorer prognosis of patients with CHP. Genes and pathways related to inflammatory responses and autoimmune diseases have potential roles in the pathogenesis of rapidly progressive CHP, suggesting their potential as diagnostic biomarkers and/or therapeutic targets. (Sarcoidosis Vasc Diffuse Lung Dis 2017; 34: 48-57)
Keywords: autoimmune diseases, biomarkers, gene expression, hypersensitivity pneumonitis, interstitial lung disease
Introduction
Chronic hypersensitivity pneumonitis (CHP) is characterized by varying degrees of inflammation and progressive fibrosis of the lungs caused by persistent exposure to a variety of inhaled antigens, including fungi, animal or bacterial proteins, and low-molecular-weight chemical compounds (1-3). Identification of the causative antigen and efforts to avoid or minimize exposure to it are key actions in the management of CHP. Pharmacological therapeutics, including corticosteroids, have limited efficacy in patients with CHP (3), and patients sometimes experience rapid deterioration of pulmonary function, resulting in death within a few years (4-8). Moreover, patients with usual interstitial pneumonia (UIP)-like or fibrotic non-specific interstitial pneumonia (f-NSIP)-like pattern on surgically resected lung tissue have shown a clinical outcome similar to that of patients with idiopathic pulmonary fibrosis (IPF) (4, 5, 7). Since surgical biopsy is a highly invasive procedure, it is not usually tolerable in all patients. Thus, a less invasive clinical biomarker for predicting the outcome of patients with CHP as well as a novel effective therapeutic agent is urgently required.
Serial changes in forced vital capacity (FVC) and diffusion capacity for carbon monoxide (DLco) have been reported to predict the outcome of patients with IPF in several previous studies (9-14). Because IPF and CHP have significant clinical, radiological, and pathological overlaps (15, 16), we hypothesized that annual declines in FVC and DLco can predict the functional deterioration as well as prognosis of patients with CHP.
Genome-wide microarray analysis enabled us to obtain comprehensive gene expression profiles related to detailed phenotypic and biological information for several diseases (17). This approach is also useful to identify unknown molecules in the pathways involved in various types of pulmonary fibrosis (18-27). However, few such analyses have been performed for CHP (19).
This study aimed to define the clinical and molecular features of patients with CHP and to identify potential therapeutic target molecules, especially in cases of rapidly progressive CHP with poor prognosis. First, we investigated the association between the annual decline in pulmonary function and the prognosis of patients with CHP. Second, we identified the genes and pathways that are differentially expressed in patients with rapidly progressive CHP as compared to the controls.
Material and Methods
Study subjects
Between November 2001 and June 2012, 43 patients with newly diagnosed CHP at Hiroshima University Hospital (Hiroshima, Japan) were enrolled in this study. Diagnoses of CHP were made according to the criteria proposed by Yoshizawa et al (1, 28, 29). In brief, the diagnostic criteria requires that three or more of the following conditions (including either i) or ii), either iii) or iv), and either v) or vi)) should be met: i) reproduction of symptoms by environmental provocation or inhalation of the antigen, ii) antibodies and/or lymphocyte proliferation targeting the specific antigen, iii) evidence of pulmonary fibrosis with or without granulomas on histopathological analysis, iv) honeycombing on computed tomography (CT), v) progressive deterioration of a restrictive impairment of pulmonary function over 1 year, and vi) persistence of respiratory symptoms related to the disease for more than 6 months. This study was approved by the Ethics Committee of Hiroshima University Hospital (approval numbers 326 and M33) and conducted in accordance with the ethical standards established in the Helsinki Declaration of 1975. All patients provided written informed consent to use their samples for this study.
Pulmonary function tests and bronchoalveolar lavage (BAL)
Spirometry and DLco measurements were performed by specialized technicians in accordance with the recommendations of the American Thoracic Society, as previously described (30-33). The rate of annual decline in FVC or DLco was calculated by dividing baseline FVC or DLco by the slope of regression line, although six patients missed follow-up measurements for DLco. BAL was performed under local anesthesia, by injecting 50 mL of saline thrice at the more severely affected area in the right middle lobe or lingula, as observed by high-resolution CT performed just before BAL (34).
RNA isolation and gene expression profiling
Gene expression profiles of frozen specimens derived from the central part of the lung by surgical biopsy in nine patients with CHP who showed more than 10% annual decline in FVC was analyzed by GP Biosciences Ltd. (Kanagawa, Japan). Control lung specimens consisted of total RNA from three lungs (Caucasians aged 32-61 years, without any concomitant lung disease, cause of death: sudden death) purchased from BD Biosciences Clontech (Lot Number 7080277; Palo Alto, CA, USA). RNA quality was checked using RNA6000 Nano Assay on Agilent Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA, USA). The Illumina BeadArrays Human WG-6 v3 (Illumina Inc., San Diego, CA, USA) with about 48,000 transcripts was used for RNA profiling, according to the manufacturer’s instructions. The Illumina TotalPrep RNA Amplification Kit (Ambion, Inc., Austin, TX, USA) was used to obtain biotin-labeled cRNA from 500 ng of total RNA. As a control probe, normal human lung poly (A) RNA (BD Biosciences Clontech) was amplified using the same amplification conditions. cRNA was synthesized overnight (18 h), labeled, and hybridized to the chip at 58°C overnight. Hybridized arrays were stained with streptavidin-Cy3 (PA43001; AmershamTM, Buckinghamshire, UK) and scanned with an Illumina BeadArray Reader (Illumina Inc.). The scanned images were imported into BeadStudio v3 software (Illumina Inc.) for extraction, quality control, and quintile normalization. Satisfactory quality of the arrays and samples was observed in all cases.
Microarray data analysis
Cluster analysis was performed using Gene Cluster 3.0 and Java TreeView software developed by Eisen et al (35, 36). The analysis included 515 genes for which valid data were obtained in 80% of the experiments, and whose expression ratios varied by standard deviations of >2.5. Gene lists were further categorized into functional and pathway analysis, using the Ingenuity Pathway Analysis (IPA; Ingenuity Systems, Redwood City, CA, USA).
Statistical analysis
Data were analyzed with SPSS for Windows, version 18.0 (SPSS Inc., Chicago, IL, USA). Data for individual variables from two groups were tested by the Mann-Whitney U test, with the level of significance set at P<0.05. Survival time was defined as the period from the date of initial consultation to the date of death due to any cause. Survival curves were analyzed by the Kaplan-Meier method, and the log-rank tests stratified for annual decline in FVC or DLco and baseline BALF lymphocyte count were performed. The division thresholds were set at annual declines of 10% and 15% for FVC and DLco, respectively, which were previously reported to be the appropriate prognostic thresholds for predicting the outcome of patients with IPF (9-13). In addition, the division threshold for BALF lymphocyte count was set at a median value of 4.0×104/mL. The receiver operating curves (ROC) were drawn for these three factors to confirm their ability to predict the five-year survival. The selection of covariates included in multivariate Cox proportional hazards models was based on previous studies that demonstrated the importance of gender, age, and pulmonary function as the prognostic factors for chronic interstitial lung diseases (ILDs) including CHP (37, 38).
Results
Baseline characteristics
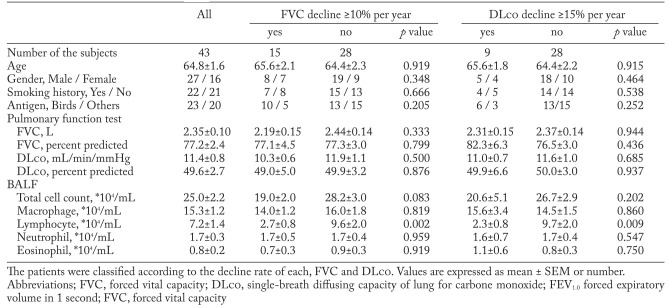
The baseline characteristics of 43 patients with CHP are presented in Table 1. There was no significant difference in age, gender, smoking status and baseline pulmonary functions between the patients with greater annual decline in pulmonary functions and those with less annual decline. On the other hand, BALF lymphocyte count was significantly higher in patients who showed less than 10% annual decline in FVC and less than 15% annual decline in DLco, as compared to those who showed greater rates of decline.
Table 1.
Baseline characteristics of the patients
Annual decline in pulmonary function and prognosis of patients with CHP
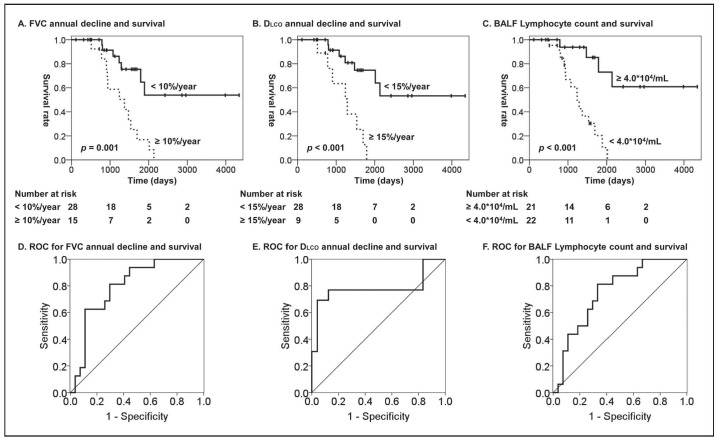
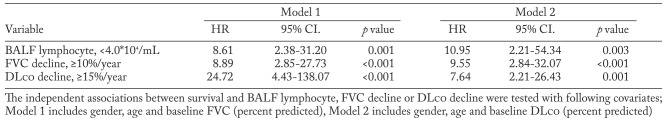
The mean annual decline in FVC was 156.2±62.9 mL/year, which accounted for 7.7%± .1% of baseline FVC, and the mean annual decline in DLco was 0.67±0.40 mL/min/mmHg/year, which accounted for 5.6%±3.8% of baseline DLco. As shown in Figure 1, the log-rank analyses revealed that more than 10% annual decline in FVC, more than 15% annual decline in DLco, and less than 4.0×104/mL of BALF lymphocyte count were significant predictors of poor overall survival of patients with CHP (P<0.001, P=0.001, and P<0.001, respectively). ROC analysis confirmed the significant abilities of annual decline in FVC or DLco and BALF lymphocyte count to discriminate the patients who died within five year from those who survive. Further, multivariate Cox proportional hazard analyses revealed that annual decline in both FVC and DLco, as well as the baseline BALF lymphocyte count, are significantly associated with the survival of CHP patients independently from other clinical factors including gender, age, and baseline pulmonary function (Table 2).
Fig. 1.
Overall survival of the forty-three patients with CHP was assessed in relation to (A) annual decline in FVC, (B) annual decline in DLco and (C) BAL lymphocyte count. Differences between the two groups were evaluated using a log-rank test. Number at risk is the number of patients whose follow-up continues at each time point. ROC analysis for (D) annual decline in FVC (area under the curve (AUC) = 0.796, p = 0.001), (E) annual decline in DLco (AUC = 0.782, p = 0.005) and (F) BAL lymphocyte count (AUC = 0.752, p = 0.006) to discriminate the patients who die within five year from the patients who survive
Table 2.
Multivariate Cox proportional hazard analyses in two models
Cluster analysis of gene expression profiles
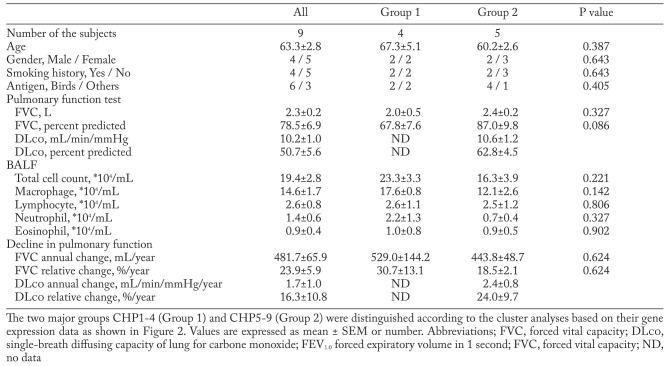
The clinical characteristics of the nine patients with CHP who were enrolled in the gene expression analysis are shown in Table 3. An unsupervised two-dimensional hierarchical clustering algorithm was used to analyze the similarities among the samples and genes (Figure 2). The two major groups CHP1-4 (Group 1) and CHP5-9 (Group 2) were distinguished based on their expression data. However, there were no significant differences in the clinical characteristics between the groups (Table 3).
Table 3.
Clinical characteristics of the patients for gene expression analysis
Fig. 2.
Cluster analysis of gene expression profile was performed for nine patients with rapidly progressive CHP. The dendrogram (top) indicates similarities between cases, with shorter branch indicating higher similarity. Within the heatmap (bottom), red represents the upregulated gene expression and green represents the downregulated gene expression
Identification of genes upregulated in rapidly progressive CHP
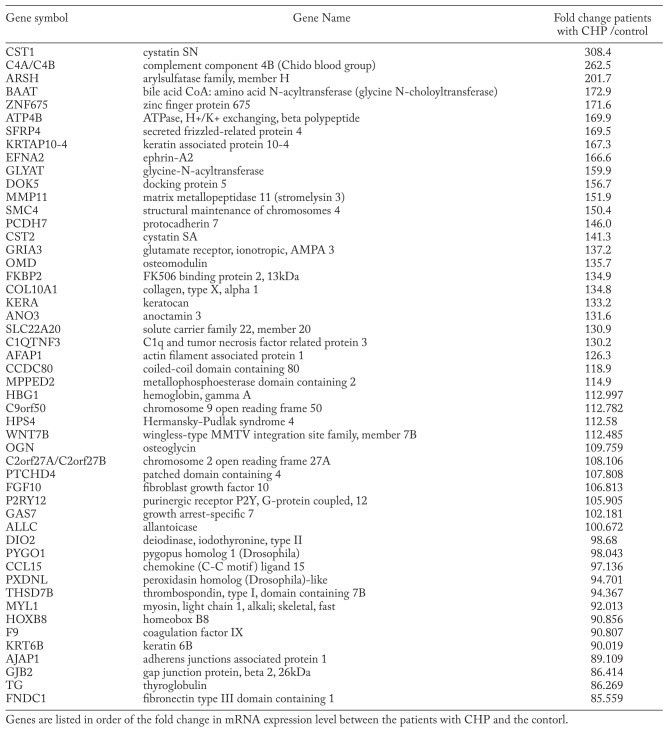
In total, 160 genes were upregulated while 832 were downregulated in the lung tissues of patients with rapidly progressive CHP as compared to control subjects (expression ratio >20.0 and <0.05, respectively), in at least75% (i.e., seven out of nine) of the patients; the top 50 upregulated genes are listed in Table 4. Some of these genes, namely secreted frizzled-related protein 4 (SFRP4), docking protein 5 (DOK5), wingless-type MMTV integration site family, member 7B (WNT7B), are known to be involved in cell differentiation; some, namely ephrin-A2 (EFNA2), protocadherin 7 (PCDH7), osteomodulin (OMD), are known to be involved in cell-cell interaction or cell adhesion; and the others, namely matrix metallopeptidase 11 (MMP11), cystatin SN (CST1), and cystatin SA (CST2), are known proteases or antiproteases. Of these, WNT7B and CST1 have also been previously reported as serum biomarkers for ILDs (39, 40).
Table 4.
Top 50 upregulated genes in the rapid progressive CHPs
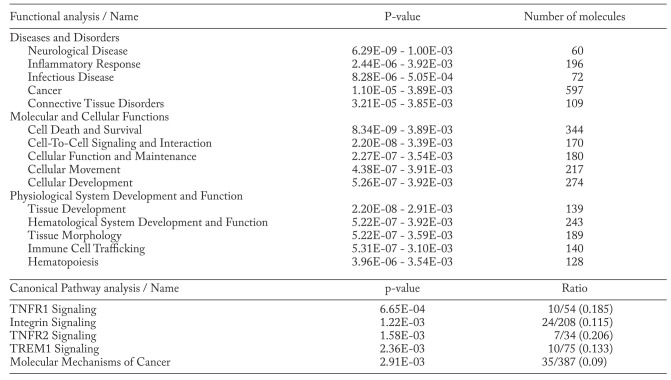
Biological pathway analysis
As shown in Table 5, IPA software estimated the differently expressed gene sets based on three categories: 1) diseases and disorders; 2) molecular and cellular functions; and 3) physiological system development and function. The top entry in each category was neurological disease, cell death and survival, and tissue development, respectively. The top five canonical signalingpathways comprised tumor necrosis factor receptor-1 (TNFR1) signaling, TNFR2 signaling, integrin signaling, triggering receptor expressed on myeloid cells-1 (TREM1) signaling, and those implicated in molecular mechanisms of cancer.
Table 5.
IPA analysis (Top 5 Functional and canonical pathway analyses)
Discussion
In this study, we evaluated the clinical and molecular features of patients with CHP. Patients with CHP who showed a greater decline in FVC and/or DLco showed significantly poorer prognosis. To our knowledge, this is the first report demonstrating the independent association between changes in FVC or DLco over time and the prognosis of patients with CHP. Further, a gene expression analysis in patients with rapidly progressive CHP identified several genes that could be deeply involved in the molecular pathogenesis of CHP and could be useful diagnostic biomarkers or therapeutic targets.
Multivariate Cox proportional hazard analyses demonstrated that annual declines in FVC and DLco, as well as BALF lymphocyte count were independent prognostic factors in patients with CHP (Table 2). Vourlekis et al. previously reported that a decreased BALF lymphocyte count in patients with CHP is significantly associated with lung fibrosis as well as with poor survival, which is in accordance with our results (5). Since BAL and surgical lung biopsy are invasive and sometimes intolerable procedures, our results suggest that monitoring the changes in FVC or DLco may be sufficient to predict the outcome of patients with CHP. To the best of our knowledge, this is the first time that the predictive ability of serial changes in FVC or DLco in patients with CHP has been demonstrated. Based on these findings, we believe that it is important to further investigate the molecular background of patients with CHP who show a greater annual decline in FVC, in order to clarify the molecular pathogenesis of CHP and to identify potential therapeutic targets.
Among the several highly upregulated genes listed in Table 4, transmembrane/secretory genes such as CST1, SFRP4, EFNA2, DOK5, MMP11, PCDH7, CST2, and WNT7B may be useful biomarkers and potential therapeutic targets. Among them, MMP11 is one of the members of the matrix metalloproteinase family that may play an important role in the pathogenesis of pulmonary fibrosis through extracellular matrix remodeling, basement-membrane breakdown, epithelial cell apoptosis, cell migration, and angiogenesis (41). EFNA2 is a cell surface glycosyphosphatidylinositol (GPI)-bound ligand for ephrin receptors, a family of receptor tyrosine kinases, which are crucial for migration and adhesion during neuronal, vascular, and epithelial development (42). To verify the biological and clinicopathological significance of the candidate gene products, further validation of their expression at protein level in the lung tissues and loss-of-function assays, using siRNA, are warranted. Further, evaluation of their usefulness as a potential diagnostic serum biomarker by enzyme-linked immunoassay (ELISA) systems is also necessary (17).
Interestingly, IPA demonstrated that several pathways related to inflammatory responses and immunological diseases were differentially expressed in patients with rapidly progressive CHP (Table 5). Among the most prominent pathways in rapidly progressive CHP, there are several interesting pathways related to inflammatory and autoimmune diseases, such as TNFR signaling and TREM1 signaling pathways, which is consistent with the results of several previous investigations (43). Further, several agents that inhibit the TNFR signaling pathway, such as adalimumab, infliximab, etanercept, golimumab, and certolizumab, are available (44). Although a recent clinical trial using soluble TNF-alpha receptor agonist has failed to improve survival in patients with IPF (45), such agents that alter inflammatory pathways may be beneficial for patients with rapidly progressive CHP but not for patients with IPF. Further clinical studies are required to determine whether these agents can actually alter the progression of CHP.
In this study, the nine patients with rapidly progressive CHP were divided into two groups according to their transcriptional profiles (Figure 2), although no significant difference was identified in the clinical backgrounds between these groups (Table 3). These results may suggest the possibility of inter-individual heterogeneity in the molecular pathogenesis of rapidly progressive CHP. To further investigate this possibility, patients with stable CHP without progression should also be included in the gene expression analysis. However, in clinical practice, such stable patients tend to be followed up without surgical lung biopsy; therefore, we could not include these patients in the present gene expression analysis.
Although this study showed promising results, it has some limitations. First, this study was conducted in a retrospective manner. Therefore, some information such as changes in DLco was not obtained from all the patients studied during follow-ups. Second, the number of patients included in the study was not sufficient for a valid statistical analysis. Further prospective studies are required to clarify whether the annual decline in lung function can predict the prognosis of patients with CHP in a large multi-institutional setting. Third, only Japanese patients were studied. Considering the ethnic differences in the occurrence of drug-induced interstitial pneumonia and acute exacerbation in patients with IPF (46, 47), the application of these results to non-Japanese patients should be carefully extrapolated.
In conclusion, the greater annual decline in FVC and/or DLco is an independent predictor of the poorer prognosis of patients with CHP. Further, genes and pathways related to inflammatory responses and autoimmune diseases have been demonstrated to be differentially expressed in patients with rapidly progressive CHP as compared to the controls. The findings of this study could offer a powerful strategy for rapid identification and further evaluation of target molecules for personalized treatment of patients with rapidly progressive CHP.
Financial support:
This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1.Yoshizawa Y, Ohtani Y, Hayakawa H, Sato A, Suga M, Ando M. Chronic hypersensitivity pneumonitis in Japan: a nationwide epidemiologic survey. J Allergy Clin Immunol. 1999;103:315–20. doi: 10.1016/s0091-6749(99)70507-5. [DOI] [PubMed] [Google Scholar]
- 2.Churg A, Muller NL, Flint J, Wright JL. Chronic hypersensitivity pneumonitis. Am. J Surg Pathol. 2006;30:201–8. doi: 10.1097/01.pas.0000184806.38037.3c. [DOI] [PubMed] [Google Scholar]
- 3.Selman M, Pardo A, King TE., Jr Hypersensitivity pneumonitis: insights in diagnosis and pathobiology. Am J Respir Crit Care Med. 2012;186:314–24. doi: 10.1164/rccm.201203-0513CI. [DOI] [PubMed] [Google Scholar]
- 4.Pérez-Padilla R, Salas J, Chapela R, et al. Mortality in Mexican patients with chronic pigeon breeder’s lung compared with those with usual interstitial pneumonia. Am Rev Respir Dis. 1993;148:49–53. doi: 10.1164/ajrccm/148.1.49. [DOI] [PubMed] [Google Scholar]
- 5.Vourlekis JS, Schwarz MI, Cherniack RM, et al. The effect of pulmonary fibrosis on survival in patients with hypersensitivity pneumonitis. Am J Med. 2004;116:662–8. doi: 10.1016/j.amjmed.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 6.Miyazaki Y, Tateishi T, Akashi T, Ohtani Y, Inase N, Yoshizawa Y. Clinical predictors and histologic appearance of acute exacerbations in chronic hypersensitivity pneumonitis. Chest. 2008;134:1265–70. doi: 10.1378/chest.08-0866. [DOI] [PubMed] [Google Scholar]
- 7.Churg A, Sin DD, Everett D, Brown K, Cool C. Pathologic patterns and survival in chronic hypersensitivity pneumonitis. Am J Surg Pathol. 2009;33:1765–70. doi: 10.1097/PAS.0b013e3181bb2538. [DOI] [PubMed] [Google Scholar]
- 8.Gaxiola M, Buendía-Roldán I, Mejía M, et al. Morphologic diversity of chronic pigeon breeder’s disease: clinical features and survival. Respir Med. 2011;105:608–14. doi: 10.1016/j.rmed.2010.11.026. [DOI] [PubMed] [Google Scholar]
- 9.Latsi PI, du Bois RM, Nicholson AG, et al. Fibrotic idiopathic interstitial pneumonia: the prognostic value of longitudinal functional trends. Am J Respir Crit Care Med. 2003;168:531–7. doi: 10.1164/rccm.200210-1245OC. [DOI] [PubMed] [Google Scholar]
- 10.Collard HR, King TE, Jr, Bartelson BB, Vourlekis JS, Schwarz MI, Brown KK. Changes in clinical and physiologic variables predict survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2003;168:538–42. doi: 10.1164/rccm.200211-1311OC. [DOI] [PubMed] [Google Scholar]
- 11.Jegal Y, Kim DS, Shim TS, et al. Physiology is a stronger predictor of survival than pathology in fibrotic interstitial pneumonia. Am J Respir Crit Care Med. 2005;171:639–44. doi: 10.1164/rccm.200403-331OC. [DOI] [PubMed] [Google Scholar]
- 12.Flaherty KR, Andrei AC, Murray S, et al. Idiopathic pulmonary fibrosis: prognostic value of changes in physiology and six-minute-walk test. Am J Respir Crit Care Med. 2006;174:803–9. doi: 10.1164/rccm.200604-488OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zappala CJ, Latsi PI, Nicholson AG, et al. Marginal decline in forced vital capacity is associated with a poor outcome in idiopathic pulmonary fibrosis. Eur Respir J. 2010;35:830–6. doi: 10.1183/09031936.00155108. [DOI] [PubMed] [Google Scholar]
- 14.Taniguchi H, Kondoh Y, Ebina M, et al. The clinical significance of 5% change in vital capacity in patients with idiopathic pulmonary fibrosis: extended analysis of the pirfenidone trial. Respir Res. 2011;12:93. doi: 10.1186/1465-9921-12-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fink JN, Ortega HG, Reynolds HY, et al. Needs and opportunities for research in hypersensitivity pneumonitis. Am J Respir Crit Care Med. 2005;171:792–8. doi: 10.1164/rccm.200409-1205WS. [DOI] [PubMed] [Google Scholar]
- 16.Hodnett PA, Naidich DP. Fibrosing interstitial lung disease. A practical high-resolution computed tomography-based approach to diagnosis and management and a review of the literature. Am J Respir Crit Care Med. 2013;188:141–9. doi: 10.1164/rccm.201208-1544CI. [DOI] [PubMed] [Google Scholar]
- 17.Daigo Y, Nakamura Y. From cancer genomics to thoracic oncology: discovery of new biomarkers and therapeutic targets for lung and esophageal carcinoma. Gen Horac Cardiovasc Surg. 2008;56:43–53. doi: 10.1007/s11748-007-0211-x. [DOI] [PubMed] [Google Scholar]
- 18.Zuo F, Kaminski N, Eugui E, et al. Gene expression analysis reveals matrilysin as a key regulator of pulmonary fibrosis in mice and humans. Proc Natl Acad Sci USA. 2002;99:6292–7. doi: 10.1073/pnas.092134099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Selman M, Pardo A, Barrera L, et al. Gene expression profiles distinguish idiopathic pulmonary fibrosis from hypersensitivity pneumonitis. Am J Respir Crit Care Med. 2006;173:188–98. doi: 10.1164/rccm.200504-644OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Selman M, Carrillo G, Estrada A, et al. Accelerated variant of idiopathic pulmonary fibrosis: clinical behavior and gene expression pattern. PLoS One. 2007;2:e482. doi: 10.1371/journal.pone.0000482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang IV, Burch LH, Steele MP, et al. Gene expression profiling of familial and sporadic interstitial pneumonia. Am J Respir Crit Care Med. 2007;175:45–54. doi: 10.1164/rccm.200601-062OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Konishi K, Gibson KF, Lindell KO, et al. Gene expression profiles of acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;180:167–75. doi: 10.1164/rccm.200810-1596OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boon K, Bailey NW, Yang J, et al. Molecular phenotypes distinguish patients with relatively stable from progressive idiopathic pulmonary fibrosis (IPF) PLoS One. 2009;4:e5134. doi: 10.1371/journal.pone.0005134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang IV, Coldren CD, Leach SM, et al. Expression of cilium-associated genes defines novel molecular subtypes of idiopathic pulmonary fibrosis. Thorax. 2013;68:1114–21. doi: 10.1136/thoraxjnl-2012-202943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel NM, Kawut SM, Jelic S, Arcasoy SM, Lederer DJ, Borczuk AC. Pulmonary arteriole gene expression signature in idiopathic pulmonary fibrosis. Eur Respir J. 2013;41:1324–30. doi: 10.1183/09031936.00084112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanaoka M, Ito M, Droma Y, et al. Comparison of gene expression profiling between lung fibrotic and emphysematous tissues sampled from patients with combined pulmonary fibrosis and emphysema. Fibrogenesis Tissue Repair. 2012;5:17. doi: 10.1186/1755-1536-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DePianto DJ, Chandriani S, Abbas AR, et al. Heterogeneous gene expression signatures correspond to distinct lung pathologies and biomarkers of disease severity in idiopathic pulmonary fibrosis. Thorax. 2015;70:48–56. doi: 10.1136/thoraxjnl-2013-204596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohtani Y, Kojima K, Sumi Y, et al. Inhalation provocation tests in chronic bird fancier’s lung. Chest. 2000;118:1382–9. doi: 10.1378/chest.118.5.1382. [DOI] [PubMed] [Google Scholar]
- 29.Ohtani Y, Saiki S, Kitaichi M, et al. Chronic bird fancier’s lung: histopathological and clinical correlation. An application of the 2002 ATS/ERS consensus classification of the idiopathic interstitial pneumonias. Thorax. 2005;60:665–71. doi: 10.1136/thx.2004.027326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iwamoto H, Yokoyama A, Kitahara Y, et al. Airflow limitation in smokers is associated with subclinical atherosclerosis. Am J Respir Crit Care Med. 2009;179:35–40. doi: 10.1164/rccm.200804-560OC. [DOI] [PubMed] [Google Scholar]
- 31.Ishikawa N, Hattori N, Tanaka S, et al. Levels of Surfactant Proteins A and D and KL-6 Are Elevated in the Induced Sputum of Chronic Obstructive Pulmonary Disease Patients: A Sequential Sputum Analysis. Respiration. 2011;82:10–8. doi: 10.1159/000324539. [DOI] [PubMed] [Google Scholar]
- 32.Ishikawa N, Hattori N, Kohno N, Kobayashi A, Hayamizu T, Johnson M. Airway inflammation in Japanese COPD patients compared with smoking and nonsmoking controls. Int J Chron Obstruct Pulmon Dis. 2015;10:185–92. doi: 10.2147/COPD.S74557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.American Thoracic Society. Standardization of Spirometry, 1994 Update. Am J Respir Crit Care Med. 1995;152:1107–36. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 34.Kohno N, Awaya Y, Oyama T, et al. KL-6, a mucin-like glycoprotein, in bronchoalveolar lavage fluid from patients with interstitial lung disease. Am Rev Respir Dis. 1993;148:637–42. doi: 10.1164/ajrccm/148.3.637. [DOI] [PubMed] [Google Scholar]
- 35.Page RD. TreeView: an application to display phylogenetic trees on personal computers. Comput Appl Biosci. 1996;12:357–8. doi: 10.1093/bioinformatics/12.4.357. [DOI] [PubMed] [Google Scholar]
- 36.Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA. 1998;95:14863–8. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ryerson CJ, Vittinghoff E, Ley B, et al. Predicting survival across chronic interstitial lung disease: the ILD-GAP model. Chest. 2014;145:723–8. doi: 10.1378/chest.13-1474. [DOI] [PubMed] [Google Scholar]
- 38.Ley B, Ryerson CJ, Vittinghoff E, et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med. 2012;156:684–91. doi: 10.7326/0003-4819-156-10-201205150-00004. [DOI] [PubMed] [Google Scholar]
- 39.Fietta A, Bardoni A, Salvini R, et al. Analysis of bronchoalveolar lavage fluid proteome from systemic sclerosis patients with or without functional, clinical and radiological signs of lung fibrosis. Arthritis Res Ther. 2006;8:R160. doi: 10.1186/ar2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meuten T, Hickey A, Franklin K, et al. WNT7B in fibroblastic foci of idiopathic pulmonary fibrosis. Respir Res. 2012;13:62. doi: 10.1186/1465-9921-13-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pardo A, Selman M. Matrix metalloproteases in aberrant fibrotic tissue remodeling. Proc Am Thorac Soc. 2006;3:383–8. doi: 10.1513/pats.200601-012TK. [DOI] [PubMed] [Google Scholar]
- 42.Coulthard MG, Morgan M, Woodruff TM, et al. Eph/Ephrin signaling in injury and inflammation. Am J Pathol. 2012;181:1493–503. doi: 10.1016/j.ajpath.2012.06.043. [DOI] [PubMed] [Google Scholar]
- 43.Chen B, Tong Z, Ye Q, Nakamura S, Costabel U, Guzman J. Expression of tumour necrosis factor receptors by bronchoalveolar cells in hypersensitivity pneumonitis. Eur Respir J. 2005;25:1039–43. doi: 10.1183/09031936.05.00084704. [DOI] [PubMed] [Google Scholar]
- 44.van Schouwenburg PA, Rispens T, Wolbink GJ. Immunogenicity of anti-TNF biologic therapies for rheumatoid arthritis. Nat Rev Rheumatol. 2013;9:164–72. doi: 10.1038/nrrheum.2013.4. [DOI] [PubMed] [Google Scholar]
- 45.Raghu G, Brown KK, Costabel U, et al. Treatment of idiopathic pulmonary fibrosis with etanercept: an exploratory, placebo-controlled trial. Am J Respir Crit Care Med. 2008;178:948–55. doi: 10.1164/rccm.200709-1446OC. [DOI] [PubMed] [Google Scholar]
- 46.Kudoh S, Kato H, Nishiwaki Y, et al. Interstitial lung disease in Japanese patients with lung cancer: a cohort and nested case-control study. Am J Respir Crit Care Med. 2008;177:1348–57. doi: 10.1164/rccm.200710-1501OC. [DOI] [PubMed] [Google Scholar]
- 47.Kondoh Y, Taniguchi H, Katsuta T, et al. Risk factors of acute exacerbation of idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis. 2010;27:103–10. [PubMed] [Google Scholar]