Abstract
Backgroung: This study investigated the efficacy and safety of TNF antagonists in sarcoid uveitis in unselected cases. Design: This is a multicentre study on patients with sarcoidosis who received TNF antagonists in pneumology and internal medicine departments in France. We present here the subgroup of patients with biopsy-proven sarcoid uveitis included in the nationwide registry STAT (Sarcoidosis treated with TNF AnTagonists). Results: Among the 132 patients included in this multicenter study, 18 patients with refractory uveitis were treated as a first-line TNF antagonist with infliximab (n=14), adalimumab (n=3) and certolizumab (n=1). Before anti-TNF initiation, the median duration of sarcoidosis was 42 months and 83% of the patients have been treated with at least one immunosuppressive drug. Six patients switched for a second-line TNF antagonist. After a mean time under treatment of 29 months, the treatment resulted in a significant decrease of the ophthalmic extrapulmonary Physician Organ Severity Tool (ePOST) (mean score: 4.2 vs. 2.6) scores and a steroid sparing effect (29.4±20.7 vs. 6.2±5.2 mg/d). Overall, the ophthalmic response, either complete or partial, was 67%. Nine patients (50%) presented adverse events, including severe infectious complications in 5 patients, which required anti-TNF treatment interruption in 6 cases (33%). Among the 7 responder patients who discontinued anti-TNF therapy, 71% relapsed. Finally, 12 patients (67%) could continue TNF antagonist treatment. Conclusions: TNF antagonists were efficient in 67% of biopsy-proven refractory sarcoid uveitis. Severe adverse events, mainly infectious complications, were frequent. The high frequency of relapses after anti-TNF-α discontinuation requires a close patient follow-up thereafter. (Sarcoidosis Vasc Diffuse Lung Dis 2017; 34: 74-80)
Keywords: efficacy, safety, sarcoidosis, TNF antagonists, uveitis
Introduction
Uveitis is a frequent (20 to 50%) feature of sarcoidosis (1). Typical sarcoid uveitis presents with mutton-fat keratic precipitates, iris nodules, anterior and posterior synechiae. Posterior involvement includes vitritis, vasculitis, and choroidal lesions. Cystoid macular oedema is the most sight-threatening consequence. Corticosteroids are the mainstay treatment for sarcoidosis (1). Systemic corticosteroids are indicated when uveitis does not respond to topical corticosteroids or in case of bilateral posterior involvement. In up to 15% of corticosteroid resistance or cases requiring unacceptable dosages to maintain remission, immunosuppressive agents are used, mostly methotrexate (1, 2).
In case of failure, agents able to block the tumor necrosis factor alpha (TNF-α) may prove efficient (3, 4). Most literature reports include single or a few cases. To date, only three case studies, reporting conflicting results, have suggested that targeting TNF may be efficient in refractory chronic sarcoid uveitis (2, 5, 6).
The aim of the STAT study (Sarcoidosis treated with TNF AnTagonists) was to evaluate the effect of TNF antagonists in a large case series of sarcoidosis patients. We present here the subgroup of patients with sarcoid uveitis included in this nationwide registry.
Patients and methods
Descriptive study
STAT is a French national drug registry of patients with histologically-proven sarcoidosis treated with TNF antagonists. We e-mailed pneumology and internal medicine departments by using the networks of Groupe Sarcoïdose Francophone (GSF) and Société Nationale Française de Médecine Interne (SNFMI), to gather data on sarcoidosis patients who had received at least one TNF antagonist infusion. The registrations started July 2014 and ended July 2015.
To be included, a patient had to have clinical features consistent with sarcoidosis and a biopsy analysis revealing non-caseating granuloma (7). The records of patients with other granuloma-forming diseases (such as tuberculosis or fungal infections) were excluded.
According to the current French Legislation (Loi Huriet-Sérusclat 88-1138), an observational study that does not change the management of the patients does not need to be declared to a research ethics board. The authors observed a strict accordance to the Helsinki Declaration guidelines.
Data collection
The participating physicianscollected: i) personal data: sex, age, date of diagnosis, comorbidities, ii) disease phenotype: pulmonary and extrapulmonary data, iii) treatment data: previous treatments, types of TNF antagonists, dates of initiation and discontinuation, concomitant treatments, and iv) adverse events (AE) data: date, type, and severity.
Uveitis was classified according to the Standardization of Uveitis Nomenclature (SUN) criteria. Chronic uveitis was defined as lasting more than 3 months (8).
Organ assessment was performed using the extrapulmonary Physician Organ Severity Tool (ePOST) (9), which examined sarcoidosis extrapulmonary involvement by scoring 17 organs on a scale from 0 (not affected) to 6 (very severely affected). To determine whether the organs with the most important clinical impacts responded similarly to the treatment, separate ePOST scores were analysed.
The SUN for clinical data, in conjunction with the results on the International Criteria for the Diagnosis of Ocular Sarcoidosis (IWOS), was used to score the location and activity of uveitis (8,10). The main outcome was a complete response when all the scored inflammatory signs showed complete response, and partial response when at least one of the scored inflammatory signs improved in the absence of deterioration of the others. In addition, “unchanged disease” was considered in case all inflammatory signs remained unchanged and “progression” in case at least one inflammatory sign increased. ePOST and ocular inflammation were assessed at inclusion, at months 3, 6, 12 and at end-point, i.e., anti-TNF discontinuation or end of follow-up if biologic therapy is continued.
The secondary outcomes included corticosteroids sparing (evaluated by comparing prednisone dosage at baseline and at end-point) and treatment safety (assessed by the occurrence of AE). An AE was considered as serious when it led to treatment interruption, hospitalization or death.
Statistical analyses
Categorical variables were expressed as numbers (percentages), continuous variables as means (standard deviations) or medians (range or interquartile range, IQR) and were compared using non-parametric tests (Wilcoxon test for continuous variables and Fisher test for categorical variables). Logistic regression model was used for adjusted analyses. Results were expressed as odds ratios (OR) with their 95% confidence intervals. A p-values < 0.05 was considered for statistical significance. Analyses were performed with R statistical software, version 3.0.2 (http://www.R-project.org).
Results
Patient characteristics
Among the 132 patients listed in STAT registry, 25 had active uveitis. Seven patients with possible neurosarcoidosis according to Zajicek’s classification (11), without histological proof, were excluded. The baseline characteristics of the 18 patients with biopsy-proven sarcoid uveitis are shown in Table 1.
Table 1.
Main characteristics of the 18 patients with sarcoid uveitis
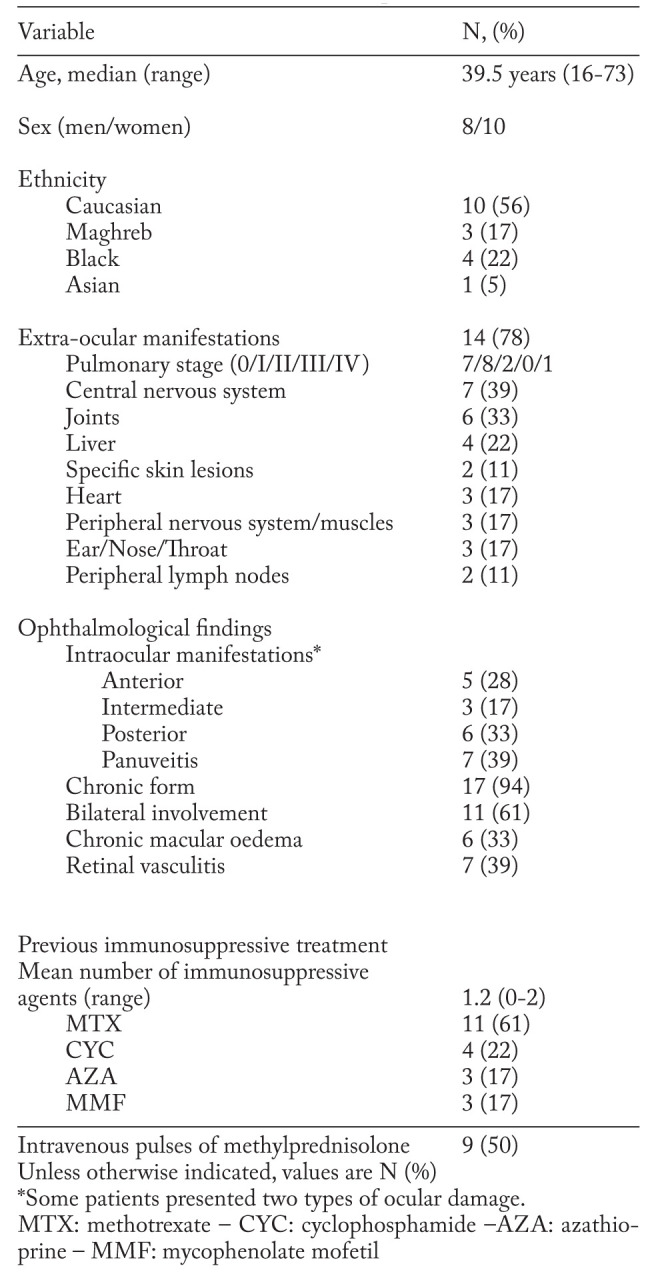
The patient group had a majority of women (56%) and persons of Caucasian origin (56%). The mean age was 42+17 years, the median duration of sarcoidosis, before TNF antagonist initiation, was 42 months (range:11-216, IQR:22-84).
Extraocular manifestations of sarcoidosis were observed in most cases (78%). The most frequent involvements concerned the lungs (61%) and the central nervous system (39%).
In most cases, uveitis was bilateral (61%) and had a chronic course (94%). Panuveitis was the most frequent pattern of ocular involvement (39%).
83% of patients had been given at least one immunosuppressive drug before TNF antagonists, over a median duration of 17 months (IQR:7.6-34). The median doses and treatment times were: 20 mg/week during 18.8 months for methotrexate (MTX) (n=11), 1000 mg/month or infusion during 8.5 months for cyclophosphamide (CYC) (n=4), 2.5 g/day over an unknown duration for mycophenolate mofetil (MMF) (n=3), 150 mg/day during 2.4 months for azathioprine (AZA) (n=3).
The clinical indications for biologic therapy are shown in Table 2.
Table 2.
Clinical indications and treatment by biologic therapy of the 18 patients with biopsy-proven sarcoid uveitis
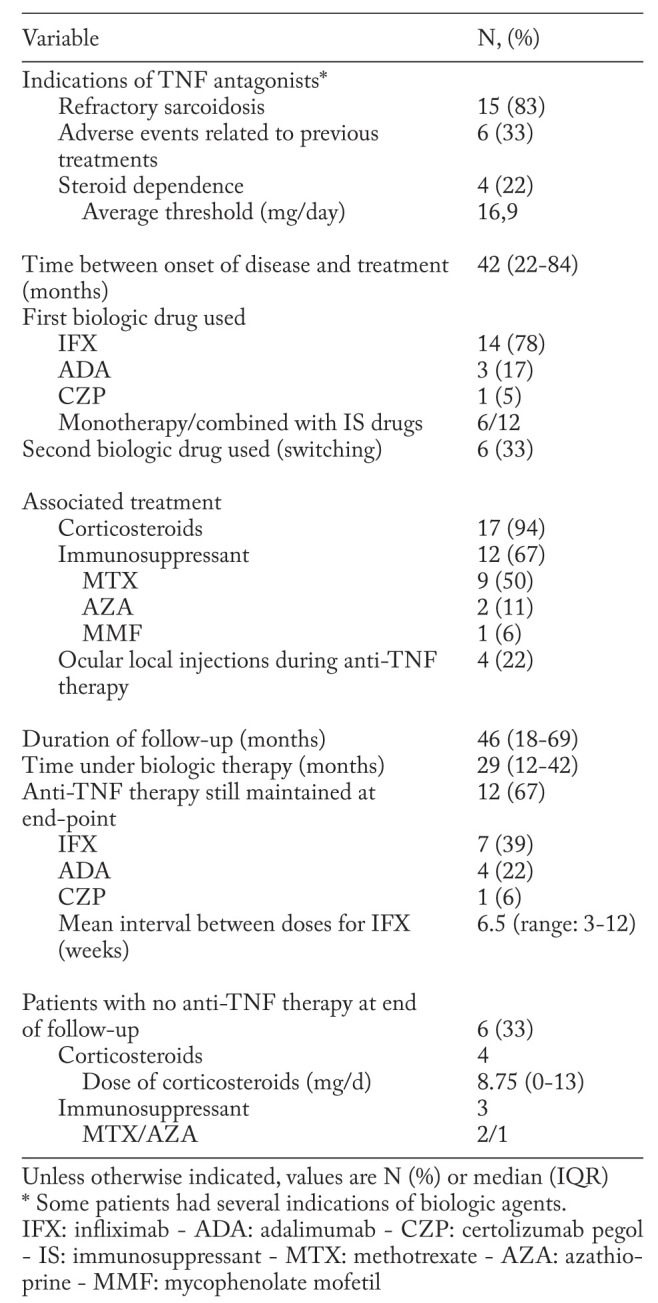
The first-line anti-TNF therapy was infliximab (IFX) (n=14), adalimumab (ADA) (n=3), or certolizumab pegol (CZP) (n=1). IFX intravenous infusions were given at 3 to 5 mg/kg at weeks 0, 2 and 6, then every 4–8 weeks. ADA dosing regimen was 40 mg subcutaneous injection every other week. CZP regimen was subcutaneous injection of 400 mg at weeks 0, 2 and 4, then 200 mg every other week. Six patients (33%) switched for a second-line TNF antagonist (5 from IFX to ADA, 1 from ADA to IFX).
In association with anti-TNF therapy, seventeen patients (94%) received corticosteroids (median dose 20mg/day (range:10-70)) and twelve patients (67%) received an immunosuppressant (IS) (MTX (n=9), AZA (n=2) and MMF (n=1)).
No significant difference was observed with respect to the demographic, ophthalmic and main features of sarcoidosis in patients treated with IFX and ADA (data not shown).
Efficacy of anti-TNFα therapy
The mean ophthalmic ePOST values at months 6, 12, and end-point are shown in Figure 1. After a median follow-up of 46 months (IQR:18-69) the mean ophthalmic ePOST at end-point was significantly lower than at baseline (4.2 (range:2-6) vs. 2.6 (0-6), p=0.0007). Anti-TNF agents allowed ophthalmic improvement in 67% of the patients: 5 patients (28%) showed complete response and 7 (39%) partial response. In six patients (33%), treatment led to no change in the course of the disease. No significant difference was found in efficacy between anti-TNF alone and associated with an immunosuppressive agent (data not shown).
Fig. 1.
Mean ePOST scores at 6 months, 12 months and end-point
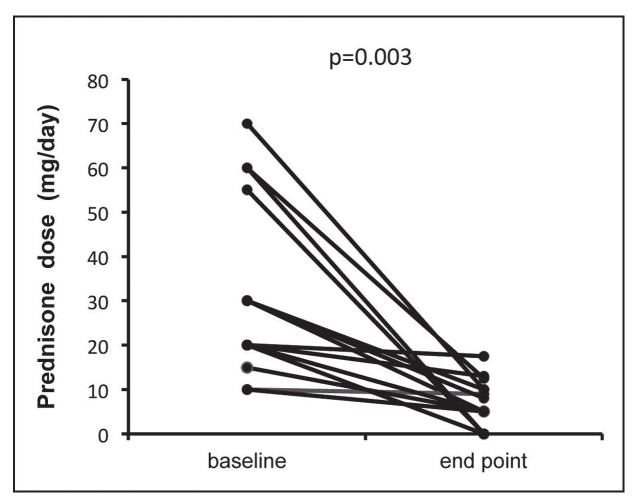
TNF antagonists had a significant corticosteroid sparing effect (Fig. 2). The mean daily prednisone dose decreased from 29.4±20.7 mg/day at the study start to 6.2±5.2 mg/day at the end of follow-up (p=0.003).
Fig. 2.
Corticosteroid sparing effect in sarcoidosis patients treated with TNF-antagonists
Six patients received a second-line anti-TNFα because of lack of efficacy (n=1), the occurrence of an AE (n=3), or the physicians decision after a favorable response (n=2), with a response rate of 5 patients out of 6. The difference in efficacy between first- and second-line TNF antagonists could not be analyzed because of the small sample sizes.
No other demographic, clinical, or treatment variable was associated with disease improvement.
Safety
Nine patients (50%) presented 31 AE, which required anti-TNF interruption in 6 patients (33%). Infections were the most frequent AE (25 out of 31), in 7 patients (39%), requiring hospitalization or treatment interruption in 5 patients (28%). These infections included 11 pulmonary infections, 4 recurrent urinary infections or prostatitis, 3 herpes zoster virus infections, and 7 bacterial sepsis cases. No difference was seen relative to age, geographic origin, dose and longer duration of steroids, severity score, choice of TNF antagonists between infected and non-infected patients.
Other remarkable AE during anti-TNF therapy were severe allergic reactions in two patients, the production of TNF α inhibitors antibodies in one patient, a serum sickness-like reaction in another, and one paradoxical cutaneous granulomatous reaction; all led to anti-TNF discontinuation.
No significant difference was observed with respect to the toxicity between IFX and ADA used in first-line therapy.
Follow-up
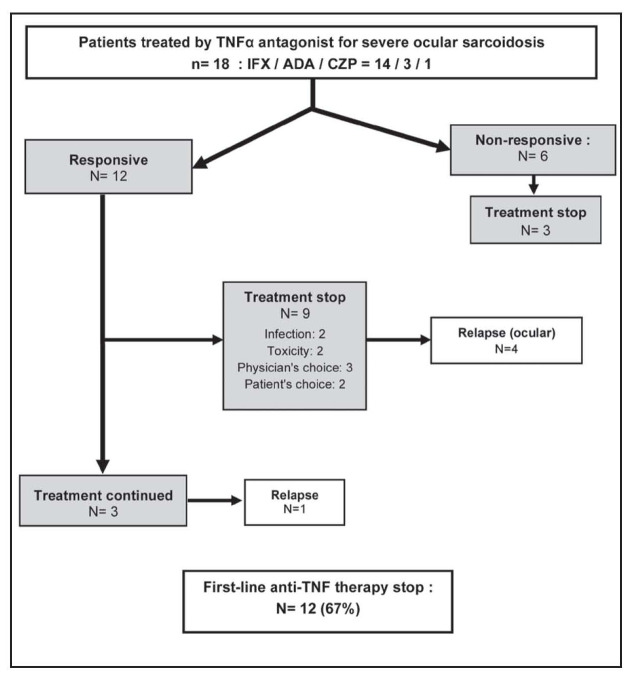
First-line TNF antagonist was interrupted in 12 cases (67%) (Fig. 3). In two patients, the interval between doses was shortened because of lack of efficacy. In contrast, intervals were extended in four patients who presented clinical improvement; however, three of them relapsed and were successfully treated thereafter by reducing the infusion intervals. Ocular relapses were also observed in 5 out of 7 responders (71%) who stopped first- or second-line TNF antagonist, after a median follow-up of 21 months (range:14-34). These five patients were successfully treated by retreatment with IFX (3 cases), the use of Rituximab (one case), or of topical corticosteroids (one case).
Fig. 3.
Outcome of first-line TNF-antagonists therapy
At the end of the study, after a median follow-up of 46 months (IQR:18-69), 12 patients (67%) were still receiving anti-TNF therapy, but 6 patients stopped this treatment, because of toxicity (n=3), lack of efficacy (n=1) or after a favorable response (n=2).
Discussion
In this multicentre study, we analyzed an unselected series of patients with resistant sarcoid uveitis, treated with TNF antagonists, followed-up over a median duration of 45 months. The main conclusions are: i) TNF antagonists were efficient in most cases and allowed substantial reductions of prednisone doses; ii) Relapses were frequent after TNF antagonists withdrawal; and iii) Anti-TNF treatment was associated with a high rate of AE, especially severe infections, which led to drug discontinuation in most cases.
TNF antagonists have been assessed in refractory pulmonary and extrapulmonary sarcoidosis, IFX being the most extensively studied (9, 12-14). Data on uveitis are scarce. Four main studies have been recently published (2, 5, 6 13).
In a review of the literature (1998-2011), Maneiro et al. reported an improvement in 85.7% of ocular sarcoidosis treated with IFX (13). Erckens et al. showed successful responses to ADA in 85% of 26 sarcoidosis patients with refractory posterior uveitis over one year (5). Recently, Riancho-Zarrabeitia et al. reported significant improvements and corticosteroid-sparing effects in patients treated with ADA (n=10) or IFX (n=7) for refractory sarcoid uveitis, followed-up for more than 2 years (6). Baughman et al. reported opposite results on 25 patients with ocular sarcoidosis treated with IFX (n=19) or ADA (n=6); all patients responded to therapy initially but only ten experienced a sustained response (2). Overall, TNF antagonists seem to be efficient in severe and refractory sarcoid uveitis. The lower proportion of patients who improved in our study may be explained by a higher rate of long-lasting and highly-treated uveitis: i.e., at onset of biologic therapy, the daily median dose of prednisone was 20 mg versus 10 mg in Riancho-Zarrabeitia’s and 15.6 mg in Erckens’s studies. The duration of the disease and of immunosuppression before anti-TNF initiation were not indicated in these two studies. In contrast to these studies, we only included patients with histologic proof. Recently, the IWOS has established criteria for the diagnosis of intraocular sarcoidosis that includes a combination of suggestive ophthalmological findings and laboratory tests when a biopsy is not carried out or is negative (10). The IWOS criteria have been retrospectively validated in Japan with a control group mainly composed of patients with Behçet’s or Vogt-Koyanagi-Harada’s disease (15). Excepting Japan, no other country has validated these criteria yet while ophthalmological features differ according to demographic factors (1). Moreover, only very rarely does sarcoid uveitis fail to respond to corticosteroids and methotrexate (1). Based on these data, we require histologic proof and exclusion of other granulomatous diseases for inclusion in our study.
In our series, we could not assess the difference in ocular response between IFX and ADA in first-line therapy. In noninfectious uveitis, there are very few data about differential efficacy between IFX and ADA, head-to-head studies are not available in adult patients. Vallet et al. recently reported 160 patients with refractory uveitis treated with TNF antagonists (IFX (n=98) and ADA (n=40)), included in an observational multicenter study (16). The overall response rate at 6 and 12 months was 87% and 93%, respectively and did not differ between the two drugs. For now, IFX remains the reference anti-TNF treatment of refractory sarcoid uveitis, due to the largest available experience and the good outcomes it has provided (2-4, 17).
Several studies have described the beneficial effect of certolizumab (a pegylated humanized monoclonal antibody Fab’ fragment) in non-infectious uveitis refractory to conventional immunosuppressants and to other anti-TNF agents (18). We report here efficacy and good tolerance of CZP in one case of sarcoid uveitis.
Here, 50% of patients had AE, requiring treatment interruption in 33% of them, with no difference between IFX and ADA. In line with previous studies on sarcoidosis (13) or other inflammatory diseases (19), infections were the most frequent AE. Chapelon-Abric et al. recently reported infections in 44% of 16 patients with severe and refractory sarcoidosis, more frequently with long use of corticosteroids and immunosuppressants (20). Similarly, Baughman et al. reported toxicity, including repeated infections, necessitating drug withdrawals in 44% of 11 patients with ocular sarcoidosis (2). However, no infection was reported in Riancho-Zarrabeitia’s and Erckens’s series (5, 6). Though we could not identify the factors associated with infections, these discordant results might be explained in our patient series by a long-term and high-dose immunosuppression, in connection with the severity of the disease. As one of our patients, paradoxical granulomatous reactions related to TNF-α inhibitors have been frequently reported (21).
Contrary to some previous studies, none of our patients developed neoplasia (12, 13).
Responders are at risk of relapse during treatment and/or after TNF antagonist withdrawal. Judson et al. reported that IFX dose escalation was needed in the majority of patients (9). Although not confirmed here, the combination of TNF antagonist and methotrexate might improve the therapeutic response while limiting the progressive resistance induced by immunization (22).
Vorselaars et al. and Panselinas et al. reported 62 and 86% of relapses after IFX discontination in their series, with a median time of 11 and 3 months respectively (23, 24). Here, 71% of the patients experienced relapses, after a median time of 21 months. Longer treatment durations might be needed but expose the patients to high risks of AE.
This study has several limitations. The lack of patient and treatment homogeneity are due to the retrospective design of the study. Secondly, the efficacy and safety of IFX, ADA, and CZP could not be correctly fully assessed because of the small number of patients.
In conclusion, this series of refractory sarcoid uveitis reports the efficacy of TNF antagonists in 67% of the patients. Severe adverse AE, mainly infections, were frequent, requiring anti-TNF interruption in 33% of the patients. Discrepancies regarding efficacy and safety with other reports may be due to a larger proportion of highly treated sarcoidosis and a longer exposition to prior immunosuppressants. Prospective studies are needed to better define the place of TNF antagonists in sarcoid uveitis.
Acknowledgments
We thank Pr Laurent Kodjikian (Department of Ophthalmology, Hôpital Croix-Rousse, Lyon) for his collaboration to this study and Nicolaï Johnson for editing assistance.
Contributions:
AM and PS contributed to the conception of the study. AM collected the data. AM, DMB, and PS analyzed the data. FCA, LP, LB, SA, PB, DB, MA, NN, BB, AP, SV, BB, FSR, DS, DV and PS were all involved in patient follow-up. AM, FCA, DV, and PS contributed to the writing of the article.
References
- 1.Jamilloux Y, Kodjikian L, Broussolle C, et al. Sarcoidosis and uveitis. Autoimmun Rev. 2014;13:840–9. doi: 10.1016/j.autrev.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Baughman RP, Lower EE, Ingledue R, et al. Management of ocular sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis Off J WASOG World Assoc Sarcoidosis Granulomatous Disord. 2012;29:26–33. [PubMed] [Google Scholar]
- 3.Saadoun D, Bodaghi B, Bienvenu B, et al. Biotherapies in inflammatory ocular disorders: Interferons, immunoglobulins, monoclonal antibodies. Autoimmun Rev. 2013;12:774–83. doi: 10.1016/j.autrev.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Sánchez-Cano D, Callejas-Rubio JL, Ruiz-Villaverde R, et al. Off-Label Uses of Anti-TNF Therapy in Three Frequent Disorders: Behçet’s Disease, Sarcoidosis, and Noninfectious Uveitis. Mediators Inflamm. 2013;2013:286857. doi: 10.1155/2013/286857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erckens RJ, Mostard RLM, Wijnen PAHM, et al. Adalimumab successful in sarcoidosis patients with refractory chronic non-infectious uveitis. Graefes Arch Clin Exp Ophthalmol. 2012;250:713–20. doi: 10.1007/s00417-011-1844-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riancho-Zarrabeitia L, Calvo-Río V, Blanco R, et al. Anti-TNF-α therapy in refractory uveitis associated with sarcoidosis: Multicenter study of 17 patients. Semin Arthritis Rheum. 2015;45:361–8. doi: 10.1016/j.semarthrit.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 7.Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160:736–55. doi: 10.1164/ajrccm.160.2.ats4-99. [DOI] [PubMed] [Google Scholar]
- 8.Trusko B, Thorne J, Jabs D, et al. The Standardization of Uveitis Nomenclature (SUN) Project. Development of a clinical evidence base utilizing informatics tools and techniques. Methods Inf Med. 2013;52:259–65. doi: 10.3414/ME12-01-0063. S1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Judson MA, Baughman RP, Costabel U, et al. Efficacy of infliximab in extrapulmonary sarcoidosis: results from a randomised trial. Eur Respir J. 2008;31:1189–96. doi: 10.1183/09031936.00051907. [DOI] [PubMed] [Google Scholar]
- 10.Herbort CP, Rao NA, Mochizuki M. members of Scientific Committee of First International Workshop on Ocular Sarcoidosis. International criteria for the diagnosis of ocular sarcoidosis: results of the first International Workshop On Ocular Sarcoidosis (IWOS) Ocul Immunol Inflamm. 2009;17:160–9. doi: 10.1080/09273940902818861. [DOI] [PubMed] [Google Scholar]
- 11.Zajicek JP, Scolding NJ, Foster O, et al. Central nervous system sarcoidosis - diagnosis and management. QJM. 1999;92:103–17. doi: 10.1093/qjmed/92.2.103. [DOI] [PubMed] [Google Scholar]
- 12.Baughman RP, Drent M, Kavuru M, et al. Infliximab Therapy in Patients with Chronic Sarcoidosis and Pulmonary Involvement. Am J Respir Crit Care Med. 2006;174:795–802. doi: 10.1164/rccm.200603-402OC. [DOI] [PubMed] [Google Scholar]
- 13.Maneiro JR, Salgado E, Gomez-Reino JJ. BIOBADASER Study Group. Efficacy and safety of TNF antagonists in sarcoidosis: data from the Spanish registry of biologics BIOBADASER and a systematic review. Semin Arthritis Rheum. 2012;42:89–103. doi: 10.1016/j.semarthrit.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Pariser RJ, Paul J, Hirano S, et al. A double-blind, randomized, placebo-controlled trial of adalimumab in the treatment of cutaneous sarcoidosis. J Am Acad Dermatol. 2013;68:765–73. doi: 10.1016/j.jaad.2012.10.056. [DOI] [PubMed] [Google Scholar]
- 15.Takase H, Shimizu K, Yamada Y, et al. Validation of international criteria for the diagnosis of ocular sarcoidosis proposed by the first international workshop on ocular sarcoidosis. Jpn J Ophthalmol. 2010;54:529–36. doi: 10.1007/s10384-010-0873-2. [DOI] [PubMed] [Google Scholar]
- 16.Vallet H, Seve P, Biard L, et al. Infliximab versus adalimumab in the treatment of refractory inflammatory uveitis: multicenter study from the French uveitis network. Arthritis Rheum. 2016 Mar 25 doi: 10.1002/art.39667. doi: 10.1002/art.39667. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 17.Valeyre D, Prasse A, Nunes H, et al. Sarcoidosis. Lancet Lond Engl. 2014;383:1155–67. doi: 10.1016/S0140-6736(13)60680-7. [DOI] [PubMed] [Google Scholar]
- 18.Llorenç V, Mesquida M, Sainz de la Maza M, et al. Certolizumab Pegol, a New Anti-TNF-α in the Armamentarium against Ocular Inflammation. Ocul Immunol Inflamm. 2014;0:1–6. doi: 10.3109/09273948.2014.967779. [DOI] [PubMed] [Google Scholar]
- 19.Ramiro S, Gaujoux-Viala C, Nam JL, et al. Safety of synthetic and biological DMARDs: a systematic literature review informing the 2013 update of the EULAR recommendations for management of rheumatoid arthritis. Ann Rheum Dis. 2014;73:529–35. doi: 10.1136/annrheumdis-2013-204575. [DOI] [PubMed] [Google Scholar]
- 20.Chapelon-Abric C, Saadoun D, Biard L, et al. Long-term outcome of infliximab in severe chronic and refractory systemic sarcoidosis: a report of 16 cases. Clin Exp Rheumatol. 2015;33:509–15. [PubMed] [Google Scholar]
- 21.Vigne C, Tebib J-G, Pacheco Y, et al. Sarcoidosis: an underestimated and potentially severe side effect of anti-TNF-alpha therapy. Jt Bone Spine Rev Rhum. 2013;80:104–7. doi: 10.1016/j.jbspin.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Baughman RP, Lower EE, Drent M. Inhibitors of tumor necrosis factor (TNF) in sarcoidosis: who, what, and how to use them. Sarcoidosis Vasc Diffuse Lung Dis Off J WASOG World Assoc Sarcoidosis Granulomatous Disord. 2008;25:76–89. [PubMed] [Google Scholar]
- 23.Vorselaars ADM, Verwoerd A, van Moorsel CHM, et al. Prediction of relapse after discontinuation of infliximab therapy in severe sarcoidosis. Eur Respir J. 2014;43:602–9. doi: 10.1183/09031936.00055213. [DOI] [PubMed] [Google Scholar]
- 24.Panselinas E, Rodgers JK, Judson MA. Clinical outcomes in sarcoidosis after cessation of infliximab treatment. Respirol Carlton Vic. 2009;14:522–8. doi: 10.1111/j.1440-1843.2009.01518.x. [DOI] [PubMed] [Google Scholar]