Abstract
Background: Although sarcoidosis is commonly considered a restrictive disorder, more recent studies demonstrated opposite results. Objectives: To determine the prevalent functional pattern in patients with intrathoracic sarcoidosis and to assess the role of granulomatous inflammation in determining ventilatory disturbances. Methods: We included 144 consecutive newly diagnosed patients with sarcoidosis, who were evaluated by chest radiography, chest high resolution computer tomography, pulmonary function tests and dyspnea score. Additionally, endobronchial and transbronchial biopsies were performed to a subset of 78 patients. Results: We obtained a wide range of ventilatory abnormalities that characterize airways impairment: FEV1/FVC<70% - in 14 (9.7%) cases, low MMEF25-75 - in 69 (47.9%) patients, increased RV/TLC - in 65 (45.1%) subjects, while the subjects with restrictive defects was observed in a minority of cases - 7 (4.9%). Decreased DLCO was found in 100 (69.4%) individuals, in the majority of cases with mild changes. Patients in whom endobronchial biopsy showed granuloma had worse ventilatory results versus those in whom they have not been detected, with significant differences in FEV1 and MMEF25-75. We found significant correlations between radiological stage and pulmonary function tests. Dyspnea score (mMRC scale) in our cohort reflected lung volumes and DLCO modifications. Conclusion: The dominant functional abnormality in patients with intrathoracic sarcoidosis is obstruction, which affects the entire length of the bronchial tree causing a wide range of airways impairment and altered gas diffusion. These functional disturbances are prevalent from early stages of the disease and have a tendency to coexist with restriction as the disease advances. Granulomatous inflammation seems to have an important role in determining obstructive defect, even from “infra-radiological” stages. (Sarcoidosis Vasc Diffuse Lung Dis 2017; 34: 58-67)
Keywords: sarcoidosis, spirometry, obstruction
Introduction
Sarcoidosis is a systemic chronic granulomatous disease of unknown cause that affects mainly the lungs (1). Currently, several tools are used for a comprehensive clinical evaluation of a patient with sarcoidosis: physical, functional, imaging and endoscopic exploration, all being part of the routine clinical assessment.
Although widely used, the informativity of chest radiography (CXR) is clearly exceeded by the high resolution computer tomography (HRCT), which, besides the fact that facilitates the diagnostic process, it can also be helpful in distinguishing active inflammation from irreversible fibrosis (2). Criado et al. stated that active, reversible granulomatous inflammation is suggested by the presence of nodules, “ground-glass” opacities, alveolar opacities, interlobular septal thickening and intralobular linear opacities, while honeycombing, bullae, broad and coarse septal bands, architectural distortion, volume loss, and traction bronchiectasis are considered irreversible fibrotic lesions (2). However, guidelines (1) recommend CXR for routine use, thus the radiographic classification of sarcoidosis is still used after more than half a century, since it was proposed by Scadding (3).
In symptomatic sarcoidosis patients, dyspnea and cough is a common finding (4). While pulmonary function tests (PFTs) are an important instrumental tool in determining the severity of the disease (5), evaluating breathlessness might become an inexpensive and an easier method to assess the severity, since it seems to correlate with spirometric indices (6).
As an interstitial lung disease, sarcoidosis is typically considered to show a restrictive pattern on PFTs (7, 8). Several studies found restriction as the dominant ventilatory abnormality (9, 10), while other researches point out that obstruction in these patients is found in a significant proportion (11-13). Since it has been stated that obstruction in sarcoidosis is correlated with increased morbidity and higher risk of mortality (OR=1.9) (14), emphasizing the prevalent pulmonary functional pattern is crucial for the management of these patients.
Gas diffusion alterations is usually related to restrictive disease, althought several studies suggest that sarcoidosis induced pulmonary hypertension play an important role in altering DLCO (15, 16).
The aim of the study was to establish the dominant functional pattern in patients with intrathoracic sarcoidosis, and to assess the role of granulomatous inflammation in determining the ventilatory disorder.
Methods
This was a prospective cohort study, which included 144 consecutive subjects newly diagnosed with sarcoidosis, collected during 2010-2013 period, from the Phthisiopneumology Institute “Chiril Draganiuc”, Chisinau, Republic of Moldova. Subjects in the study were recruited either by direct addressing, or following an abnormal prophylactic CXR. Research protocol was approved by the Ethical Committee of the “Nicolae Testemitanu” State Medical and Pharmaceutical University from Chisinau, Republic of Moldova.
We included in the study patients with intrathoracic sarcoidosis, diagnosed according to the ATS/ERS/WASOG criteria (1). Exclusion criteria from the study: stage 0 subjects; patient’s desire to leave the study; eligible subjects with documented current lung infection or neoplasia; previous chronic lung disease; significant comorbidities (eg. preexisting cardiac disease).
Each patient underwent a physical examination and answered a questionnaire for shortness of breath. The investigation protocol included CBC count, serum chemistry panel, urine analysis, a CXR, a chest HRCT, PFTs, and echocardiography with Doppler. These investigations were performed to all the subjects included in the study at the time of diagnosing the disease within a period of 2 weeks.
Fiberbronchoscopy was performed in a subgroup of 78 randomly selected subjects with intrathoracic sarcoidosis during which transbronchial biopsy (TBB) and endobronchial biopsy (EBB) were carried out. Randomization of selection was stipulated in the research protocol which assigned for fiberbronchoscopy investigation each second patient diagnosed with sarcoidosis in order of appearance. Thus, from an initial number of 156 subjects, we had 78 subjects assigned for bronchoscopy. For reasons stated in the exclusion criteria 12 patients left the study, and 144 remained.
According to the endoscopic biopsy protocol, 4-5 samples were taken. All subjects were histologically proven sarcoidosis patients. Depending on the case, biopsy confirmation was obtained from other affected organs: skin, extrathoracic lymph nodes, liver, etc.
Posteroanterior CXR was carried out on a Siemens digital radiography system, evaluated and staged according to Scadding classification (3). Chest HRCT was part of the diagnosis protocol, and also used as a screening method for compressive hilar adenopathy.
Dyspnea was evaluated according to the modified Medical Research Council scale (mMRC) (17), which gives scores from 0 (no dyspnea) to 4 (almost complete incapacity) to the degree of shortness of breath during daily activity: 0 – no dyspnea at all; 1- breathlessness when hurrying on the level or walking up a slight hill; 2 - dyspnea while walking at a rapid pace on the level ground; 3 - the person stops for breath after walking about 100 m or after a few minutes on the level; 4 - too breathless to leave the house or to dress.
PFTs were performed using bodyplethismograph Jaeger® Master Screen Body (Germany). We have assessed the following ventilatory parameters: forced vital capacity (FVC), forced expiratory volume in first second (FEV1), maximum midexpiratory flow (MMEF25-75); as well as lung volumes: the residual volume (RV), and total lung capacity (TLC). Carbon monoxide diffusion capacity of the lungs (DLCO) was measured using the single breath maneuver.
In order to assess the prevalent functional pattern, we have used several definitions: obstruction, as FEV1/FVC <70%, defined by GOLD (18). We have stratified the severity of central airways obstruction according to GOLD severity classification (18).
Data obtained from PFTs were presented both as parameters alone and as functional patterns. The functional patterns were defined according to ERS/ATS task force on Interpretative strategies for lung function tests (19). A reduced small airways flow was expressed as low MMEF25-75, in cases of normal and reduced FEV1/FVC. Obstructive abnormalities, such as air-trapping/ hyperinflation, were expressed as increased RV/TLC ratio with TLC values within the normal range or increased (19). For assessing the incidence of the restrictive pattern we have applied the following definitions: reduced FVC and increased FEV1/FVC; and reduced TLC and a normal FEV1/FVC ratio (19). A mixed ventilatory defect was defined as decreased FEV1/FVC and low TLC (19).
Echocardiography with Doppler was performed to screen for pulmonary hypertension, measuring the systolic pressure in the pulmonary artery.
Statistics
Continuous variables were expressed as means and standard deviations, while not normally distributed variables were expressed as medians and interquartile ranges (IQR=25-75 quartiles).
For comparative analysis of lung volumes according to radiological stage we have used Kruskal-Wallis test and ANOVA, as appropriate. Man Whithey U test and Tukey post-hoc test were used for pairwise comparison, as appropriate.
The presence of associations between ventilatory parameters and dyspnea score were studied using Spearman’s rank correlation.
Comparison between positive and negative biopsy groups according to functional parameters was made using Student t test.
A value of p<0.05 was considered as statistically significant. Data were analyzed using the Statistical Package for the Social Sciences version 17.
Results
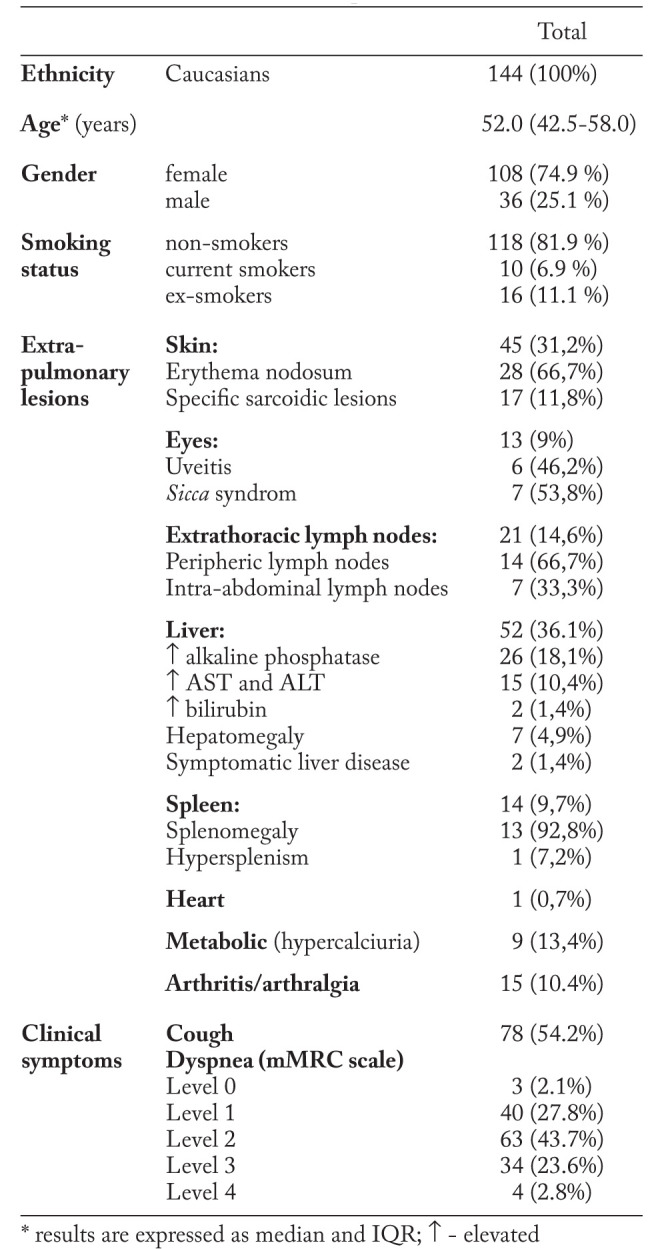
The study group consisted of Caucasian adults, with a median age of 52 years (IQR 42.5-58.0), mainly females (74.9%), mostly non-smokers (81.9%).
Clinical symptoms of lung involvement were: cough - present in 78 (54.2%) cases, and dyspnea with a wide range of severity degree (table 1). Extrapulmonary manifestations of sarcoidosis were dominated by skin lesions, observed in 45 (31.2%) individuals, and liver abnormalities - in 52 (36.1%) subjects. Enlarged extrathoracic lymph nodes - found is 21 (14.6%) cases, hypercalciuria - in 9 (13.4%) patients, joint and spleen involvement - in 15 (10.4%) and 14 (9.7%) cases respectively, were less common.
Table 1.
Features of the study group
Radiographic examination (table 2) revealed 56 (38.9%) patients with bilateral hilar adenopathy (stage 1); 62 (43%) subjects showed both nodal and parenchymal involvement (stage 2); 22 (15.3%) subjects demonstrated only parenchymal involvement (stage 3), and 4 (2.8%) cases with pulmonary fibrosis (stage 4).
Table 2.
Radiological staging and HRCT findings in the group
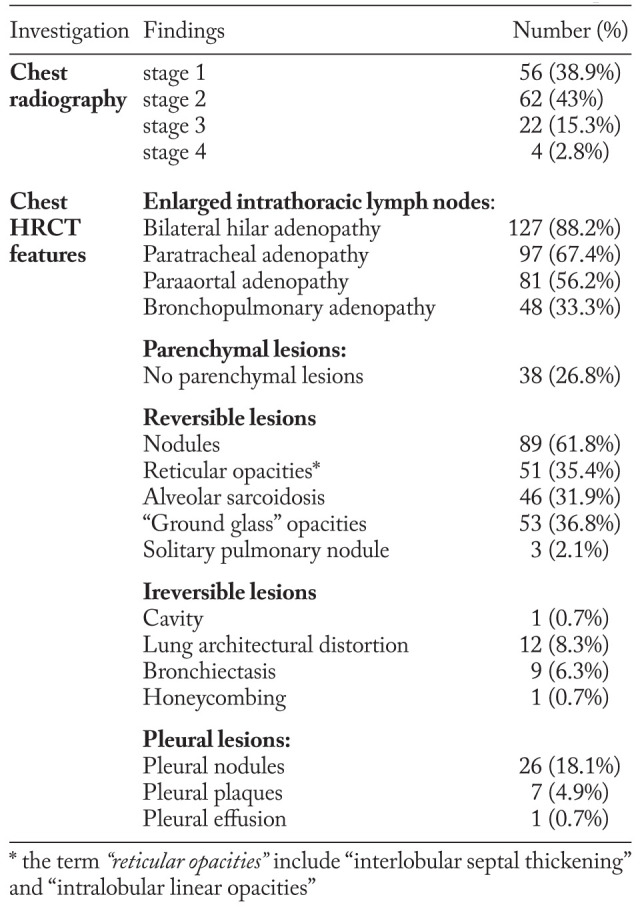
While CXR analysis revealed 56 subjects eligible for stage 1 intrathoracic sarcoidosis, according to Scadding radiological classification (3), chest HRCT scans identified 18 patients among them with isolated pulmonary nodules.
Chest HRCT scans revealed that bilateral hilar adenopathy was the most frequent lesion, found in 127 (88.2%) cases (table 2). Compressive lymphadenopathy was observed in only one patient.
The most common parenchymal lesion, found in 89 (61.8%) cases, was represented by nodules. Other features, such as: reticular opacities, “alveolar sarcoidosis” and “ground glass” opacities were detected with an incidence that ranges from 31.9% to 36.8%. In a minority of cases we have found lung architectural distortion - 12 (8.3%) patients, traction bronchiectasis - 9 (6.3%) cases, 1 patient with a cavity and a singular case with honeycombing (table 2).
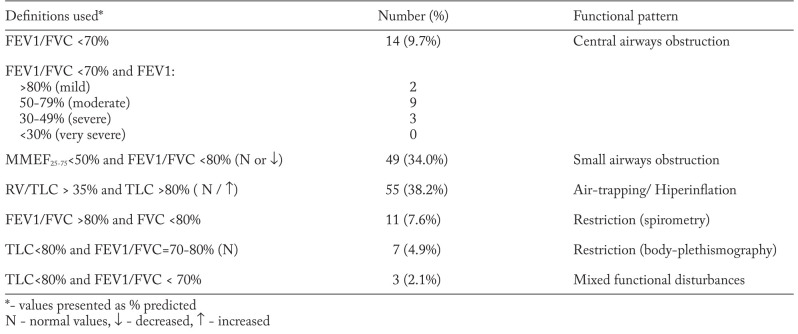
PFTs results identified 14 (9.7%) patients with obstruction, defined as FEV1/FVC <70% of predicteds (table 3). Although, when obstruction was defined as FEV1/FVC<80%, we obtained a higher prevalence of obstructive disorders, found in 56 (38.9%) cases. Impairment of central airways, expressed as decreased FEV1 was observed in 43 (29.9%) subjects. In 69 (47.9%) cases we have determined a reduced flow of small airways, expressed as low MMEF25-75. Plethysmographic assessment of lung volumes revealed an increased RV/TLC ratio in 65 (45.1%) cases, suggesting other obstructive abnormalities such as air-trapping or lung hyperinflation. A decreased TLC was found in 13 (9%) subjects, suggesting a restrictive abnormality. That the most common functional disorder in our group is altered gas diffusion, expressed as decreased DLCO, detected in 100 (69.4%) individuals (table 3). However mild changes (78% of cases) outweighed more severe alterations.
Table 3.
Spirometry, lung volumes and gas diffusion in patients with intrathoracic sarcoidosis
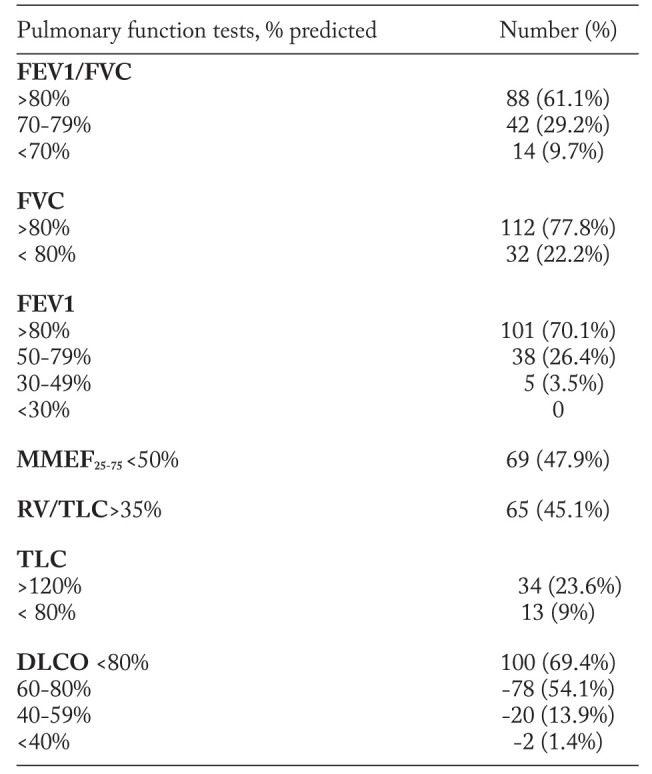
Because guidelines on interpreting the functional disturbances (19) recommend the use of a constellation of modified lung volumes, rather than a spirometric parameter alone, in table 4 we have presented the frequency of functional patterns found in patients with intrathoracic sarcoidosis. When small airways obstruction was defined as decreased MMEF25-75 and a normal or low FEV1/FVC ratio, we obtained 49 (34.0%) cases. Air-trapping or hyperinflation defined as increased RV/TLC and a normal or increased TLC (19) was revealed in 55 (38.2%) subjects.
Table 4.
Respiratoryfunctional patterns in patients with intrathoracic sarcoidosis
The presence of a restrictive pattern (reduced FVC and increased FEV1/FVC) was observed in 11 (7.6%) subjects; using the other definition (reduced TLC and a normal FEV1/FVC) we found only 7 (4.9%) patients.
A mixed functional pattern, defined as decreased FEV1/FVC and low TLC (19) was detected only in 3 subjects.
Among patients with restrictive defect, decreased DLCO was found in 85.7% of cases; while in patients with obstruction (FEV1/FVC<70%) DLCO was reduced in 57% of cases.
Therefore, regardless of the definitions used, results presentation method (parameters alone or functional pattern), - it seems that obstructive abnormalities prevail over the restrictive ones.
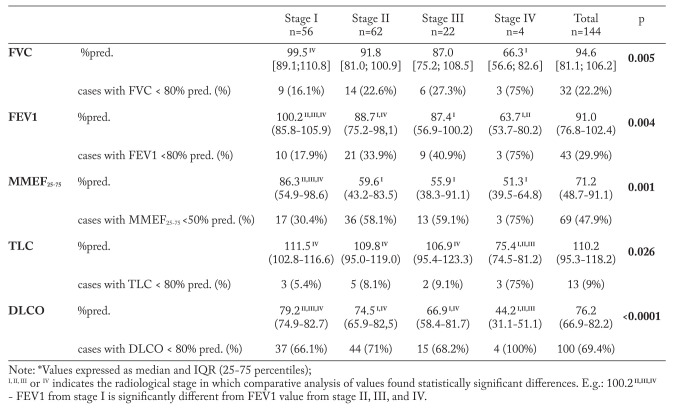
The relationship between PFTs and radiological stage is presented in table 5. We found that, as the disease advances the lung volumes tend to decrease in all analyzed parameters. Pairwise analysis revealed that unlike stage I, in stage IV patients, FVC median is significantly reduced (p=0.005), thus, indicating the prevalence of restrictive defects in these subjects. This finding is confirmed by plethysmographic measurements, which showed a significant reduction of TLC in stage IV subjects, compared to those in other stages (p=0.026). Furthermore, stage IV patients have significantly reduced most of the ventilatory parameters, including FEV1 (p=0.004) and MMEF25-75 (p=0.001), which suggests that in stage IV obstructive disease coexists with restrictive abnormalities.
Table 5.
Relationship between PFTs* and radiological stage
An interesting finding was detection of several functional impairments in stage I patients, which by definition does not involve lung parenchyma. The most prevalent alterations were represented by reduced MMEF25-75 in 17 (30.4%) cases, which suggests the presence of obstructive phenomena in the small airways from early stages of the disease and decreased DLCO - in 37 (66.1%) stage I subjects, indicating parenchymal involvement even in “infra-radiological” stages.
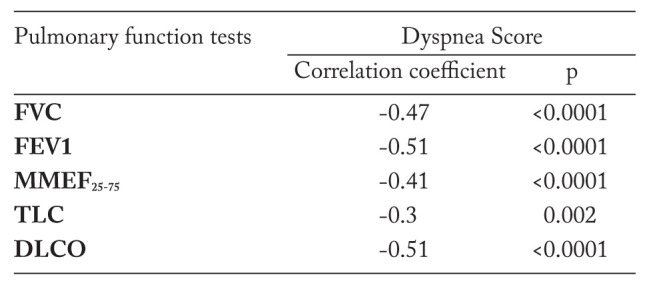
Multiple comparative analysis of PFTs and dyspnea score (table 6) pointed out the tendency downward of lung volumes and DLCO as the breathlessness became more severe. Table 7 confirms the presence of linear correlations between PFTs and dyspnea score, and emphasizes moderate correlations with FEV1 and DLCO, while FVC, MMEF25-75, and TLC correlated weakly.
Table 6.
Relationship between PFTs* and dyspnea score
Table 7.
Correlations between dyspnea score and pulmonary functional parameters
Echocardiography with Doppler screening revealed 7 (4.9%) patients with increased systolic pressure in the pulmonary artery (mean pressure 43.42±2.3 mm Hg). Because of the small number of subjects, we found the influence of sarcoidosis induced pulmonary hypertension on functional parameters insignificant.
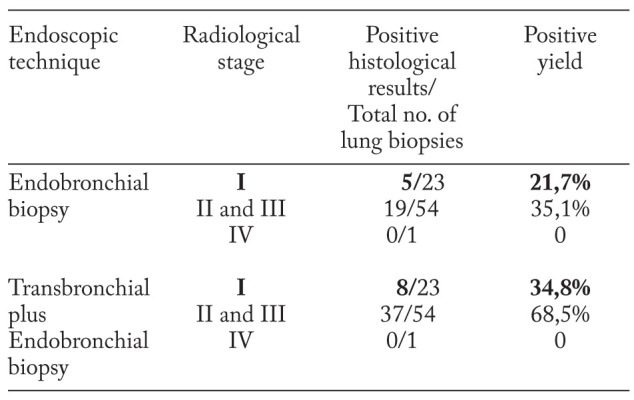
In order to find if determined the functional disturbances, we have performed diagnostic bronchoscopy with transbronchial and endobronchial biopsies in a subgroup of 78 patients. An interesting finding was the presence of sarcoid granuloma in the lung parenchyma and in the bronchial mucosa even in patients with stage I, which could explain the high incidence of obstructive disorders in these patients (table 8).
Table 8.
Histological results of the EBB and TBB according to radiological stage
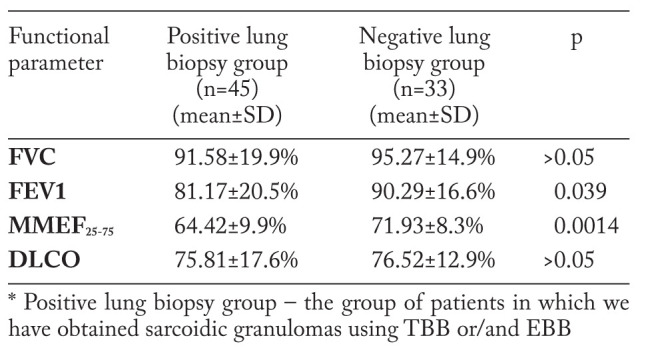
To assess the influence of endobronchial changes on ventilatory indicators, we have divided the group of subjects that have undergone transbronchial/endobronchial biopsies into 2 groups, according to the histological results, following this endoscopic procedure (table 9). Results show that lung volumes and DLCO are lower in the positive lung biopsy group, with statistically significant difference of FEV1 (p=0.039) and MMEF25-75 values (p=0.0014). These data emphasize the role of endobronchial granulomas, as well as of those situated along the bronchovascular bundles, in determining obstructive disorders in patients with intrathoracic sarcoidosis.
Table 9.
Comparative results of pulmonary functional parameters in positive and negative lung biopsy groups
Discussions
Several earlier publications (8-10) suggest that restrictive functional disturbances in patients with sarcoidosis are a common finding. Contrary to this view, in our research we identifical only 11 (7.6%) subjects who showed this type of ventilatory abnormality established by spirometry, while bodyplethismographic measurement revealed the presence of restriction in only 7 (4.9%) of these individuals. Other recent similar works confirm that restriction in sarcoidosis is a rare phenomenon (20, 21).
However, our results show that sarcoidosis is predominantly obstructive, prior studies indicate incidences ranging from 4 to 63% (10-13, 22). An explanation regarding the large variation of the incidente from study to study could be the various definitions and reference values used by different authors. Thus, Sharma et al.reported that obstruction, defined as FEV1/FVC below 75% was found in 63% of sarcoidosis non-smoking negroid patients (13). The ACCES study presents a high incidence of obstruction (53%) when considered as FEV1/FVC <80%, while when defined as FEV1/FVC <70%, they found only 14%. Other researches, as well as our study, showed similar results (22, 23). A more recent trial presented a higher incidence of this parameter (FEV1/FVC<70%) - affecting 27% subjects with sarcoidosis (24) of patients with sarcoidosis, probably due to patients selection criteria (Japanese population).
Involvement of the central airways, defined by ATS/ERS as decreased FEV1 (25), was detected in a proportion of 29.9% in our study, data similar to those reported by several authors (11, 26). Experts explain the large airway lesions due to chronic granulomatous inflammation, endobronchial masses and extrinsic compression of massive lymphadenopathy (27).
A more prominent functional disturbance in our study group is lower airways flow limitation. The prevalence of small airway obstruction is showed by the high incidence of decreased MMEF25-75 in almost half of the investigated subjects (47.9%). Although this parameter is frequently considered to have a limited use, due to its wide variability, the obtained data were confirmed by plethysmographic measurements (RV/TLC) with similar results.
While the substrate of lower airways impairment and bronchiolitis secondary to sarcoidosis cannot be confirmed by endoscopic procedures due to technical limitations, the significant differences of FEV1 and MMEF25-75 values between positive and negative lung biopsy groups, and also the nodules present at HRCT with a high frequency, suggest the importance of granulomatous inflammation in determining an obstructive spirometric pattern. Unlike us, in an earlier study, Stjernberg et al. failed to detect any correlation of endoscopic findings with severity of airflow limitation (28). Emphasizing the role of granulomatous inflammation in determining the obstructive impairment in patients with sarcoidosis is very important, since airways obstruction can be completely or partially reversed with steroid treatment, thus improving symptoms (29).
Based on our results, we can conclude that as the disease advances, the restrictive defect becomes more prevalent, while obstructive disturbances are present from early stages, mainly affecting small airways, and in end-stage disease obstructive impairment coexists with restrictive disorders. Mixed abnormalities were found only in 3 (2.1%) cases, a result that corresponds to data from other similar studies (10, 12, 30).
Gas diffusion abnormalities list among functional parameters that are commonly decreased in patients with sarcoidosis, suggestive of altered lung insterstitium (8). Our study found an incidence of 100 (69.4%) cases of impaired DLCO. Prior studies stated that low carbon monoxide diffusion can be found in approx. 2/3 of sarcoidosis patients with abnormal radiographs (31). The high incidence of decreased DLCO in the majority of patients with restrictive ventilatory defect, but also in subjects with obstructive disturbances, suggests that in our cohort altered DLCO could be due to both granulomatous alveolitis, and small airways involvement that impede the alveolar gas exchange. Pulmonary hypertension induced by sarcoidosis in our study doesn’t seem to have a significant role in determining reductions in DLCO since its incidence in our group is low. In a recent review Shino and the colleagues found incidences of pulmonary hypertension associated to sarcoidosis that ranged 6-74% (32).
While we found that lung volumes trend downward as the disease advances, experts consider the correlation between radiographic staging and lung function imprecise (33), as in individual cases it is hard to predict the patient’s functional status from a radiograph (34).
Dyspnea, one of the most common respiratory symptoms in patients with sarcoidosis, in our trial to correlates with functional parameters, among them FEV1 and DLCO obtained higher correlation coefficients. Based on these results, the mMRC scale could be used as an easy and inexpensive method of assessment of disease severity.
There is a wide range of functional disorders even in patients with stage I of the disease, which by definition doesn’t involve the lung parenchyma. Our results show that small airways are affected in 17 (30.4%) cases while decreased DLCO was detected in more than half of radiological stage I subjects - 37(66.1%) cases. Other authors have noted that ventilatory defects are present in only 20% of these patients (31, 35). Moreover, chest HRCT scans detected 18 patients out of 56 radiologically assigned as stage I patients, according to Scadding classification, who had isolated nodules. Furthermore, TBB and EBB found granulomas in these patients. Thus, these findings suggest that granulomatous inflammation could determine lung volumes and DLCO alterations.
Conclusions
Our study reveals that the dominant functional abnormality in patients with intrathoracic sarcoidosis is obstruction that affects the entire length of the bronchial tree causing a wide range of airways impairment and altered gas diffusion. These functional disturbances are prevalent from early stages of the disease and have a tendency to coexist with restriction as the disease advances. Granulomatous inflammation seems to have an important role in determining obstructive defect, even from “infra-radiological” stages.
References
- 1.Hunnighake GW, Costabel U, Ando M, Baughman R, Cordier JF, du Bois R, et al. ATS/ERS/ WASOG Statement on sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16(2):149–73. [PubMed] [Google Scholar]
- 2.Criado E, Sánchez M, Ramírez J, et al. Pulmonary Sarcoidosis: Typical and Atypical Manifestations at High-Resolution CT with Pathologic Correlation. RadioGraphics. 2010;30:1567–86. doi: 10.1148/rg.306105512. [DOI] [PubMed] [Google Scholar]
- 3.Scadding JG. Prognosis of intrathoracic sarcoidosis in England. BMJ. 1961;4:1165–72. doi: 10.1136/bmj.2.5261.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.du Bois RM, Richeldi L. Interstitial lung diseases. Eur Resp Mon. 2009;46:126–55. [Google Scholar]
- 5.Lynch JP III, Ma YL, Koss MN, White ES. Pulmonary Sarcoidosis. Semin Respir Crit Care Med. 2007;28(1):53–74. doi: 10.1055/s-2007-970333. [DOI] [PubMed] [Google Scholar]
- 6.Bhanurekha B, Sasisekhar TVD, Saireddy Y, Indrakeela GM. Correlation of MRC Dyspnoea Scale and Forced Expiratory Volume in First Second (FEV1) In Chronic Obstructive Pulmonary Diseases. Journal of Dental and Medical Sciences. 2013;7(2):55–7. [Google Scholar]
- 7.Gibson GJ. Interstitial lung diseases: pathophysiology and respiratory function. Eur Resp Mon. 2000;14:15–28. [Google Scholar]
- 8.Lynch JP III, White ES. Pulmonary sarcoidosis. Eur Resp Mon. 2005;32:105–29. [Google Scholar]
- 9.Levinson RS, Metzger LF, Stanley NN, et al. Airway function in sarcoidosis. The American Journal of Medicine. 1977;62(1):51–9. doi: 10.1016/0002-9343(77)90349-7. [DOI] [PubMed] [Google Scholar]
- 10.Loddenkemper R, Kloppenborg A, Schoenfeld N, Grosser H, Costabel U. Clinical findings in 715 patients with newly detected pulmonary sarcoidosis: results of a cooperative study in former West Germany and Switzerland. WATL Study Group. Wissenschaftliche Arbeitgemeinschaft fur die Therapie von Lungenkrankenheitan. Sarcoidosis Vasc Diffuse Lung Dis. 1998;15:178–82. [PubMed] [Google Scholar]
- 11.Baughman RP, Teirstein AS, Judson MA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164:1885–9. doi: 10.1164/ajrccm.164.10.2104046. [DOI] [PubMed] [Google Scholar]
- 12.Harrison BD, Shaylor JM, Stokes TC, Wilkes AR. Airflow limitation in sarcoidosis - a study of pulmonary function in 107 patients with newly diagnosed disease. Respir Med. 1991;85(1):59–64. doi: 10.1016/s0954-6111(06)80211-8. [DOI] [PubMed] [Google Scholar]
- 13.Sharma OP, Johnson R. Airway obstruction in sarcoidosis. A study of 123 nonsmoking black American patients with sarcoidosis. Chest. 1988;94:343–56. doi: 10.1378/chest.94.2.343. [DOI] [PubMed] [Google Scholar]
- 14.Viskum K, Vestbo J. Vital prognosis in intrathoracic sarcoidosis with special reference to pulmonary function and radiological stage. Eur Respir J. 1993;6:349–53. [PubMed] [Google Scholar]
- 15.Sulica R, Teirstein AS, Kakarla S, Nemani N, Behnegar A, Padilla ML. Distinctive clinical, radiographic, and functional characteristics of patients with sarcoidosis-related pulmonary hypertension. Chest. 2005;128:1483–9. doi: 10.1378/chest.128.3.1483. [DOI] [PubMed] [Google Scholar]
- 16.Baughman RP, Engel PJ, Meyer CA, Barrett AB, Lower EE. Pulmonary hypertension in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2006;23:108–16. [PubMed] [Google Scholar]
- 17.Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93:580–86. doi: 10.1378/chest.93.3.580. [DOI] [PubMed] [Google Scholar]
- 18.Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. (Updated 2010) http://www.goldcopd.org .
- 19.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;25:948–68. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 20.Boros PW, Enright PL, Quanjer PH, Borsboom GJ, Wesolowski SP, Hyatt RE. Impaired lung compliance and DLCO but no restrictive ventilatory defect in sarcoidosis. Eur Resp J. 2010;36(6):1315–22. doi: 10.1183/09031936.00166809. [DOI] [PubMed] [Google Scholar]
- 21.Rasheed A, Vasudevan V, Arjomand F. Patterns of Pulmonary Function Test (PFT) Abnormalities in Sarcoidosis. Chest. 2012;142(4_MeetingAbstracts):446A. [Google Scholar]
- 22.Handa T, Nagai S, Fushimi Y, et al. Clinical and radiographic indices associated with airflow limitation in patients with sarcoidosis. Chest. 2006;130:1851–6. doi: 10.1378/chest.130.6.1851. [DOI] [PubMed] [Google Scholar]
- 23.Cieslicki J, Zych D, Zielinski J. Airways obstruction in patients with sarcoidosis. Sarcoidosis. 1991;8(1):42–4. [PubMed] [Google Scholar]
- 24.Hirano R, Yoshida Y, Matsumoto T, Tashiro N, Harada T, et al. Obstructive ventilatory impairment in sarcoidosis. Jpn J Sarcoidosis other Granulomatous Disorders. 2013;33:79–82. [Google Scholar]
- 25.Pellegrino R, Viegi G, Brusasco V, et al. Series ‘‘ATS/ERS task force: standardisation of lung function testing’’. Eur Respir J. 2005;26:948–68. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 26.Subramanian I, Flaherty K, Martinez F. Pulmonary function testing in sarcoidosis. In: Sarcoidosis. 2006;210:415–33. [Google Scholar]
- 27.Costabel U. Sarcoidosis: clinical update. Eur Respir J. 2001;18(Suppl 32):56s–68s. [PubMed] [Google Scholar]
- 28.Stjernberg N, Thunell M. Pulmonary function in patients with endobronchial sarcoidosis. Acta Med Scand. 1984;215:121–26. doi: 10.1111/j.0954-6820.1984.tb04981.x. [DOI] [PubMed] [Google Scholar]
- 29.Lavergne F, Clerici C, Sadoun D, et al. Airway obstruction in bronchial stenosis. Outcome with treatment. Chest. 1999;116:1194–9. doi: 10.1378/chest.116.5.1194. [DOI] [PubMed] [Google Scholar]
- 30.Coates R, Neville E. The development of airways obstruction in sarcoidosis among smokers and non-smokers. Sarcoidosis. 1993;10(2):115–7. [PubMed] [Google Scholar]
- 31.Alhamad EH, Lynch JP, Martinez FJ. Pulmonary function tests in interstitial lung disease: what role do they have. Clin Chest Med. (3rd) 2001;22:715–50. doi: 10.1016/s0272-5231(05)70062-9. [DOI] [PubMed] [Google Scholar]
- 32.Shino MY, Lynch JP III, Fishbein MC, et al. Sarcoidosis-associated pulmonary hypertension and lung transplantation for sarcoidosis. Semin Respir Crit Care Med. 2014;35(3):362–71. doi: 10.1055/s-0034-1376863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nunes H, Uzunhan Y, Gille T, Lamberto C, Valeyre D, Brillet P. Imaging of sarcoidosis of the airways and lung parenchyma and correlation with lung function. Eur Respir J. 2012;40:750–65. doi: 10.1183/09031936.00025212. [DOI] [PubMed] [Google Scholar]
- 34.Baydur A, Alsalek M, Louie SG, Sharma OP. Respiratory Muscle Strength, Lung Function, and Dyspnea in Patients With Sarcoidosis. Chest. 2001;120:102–8. doi: 10.1378/chest.120.1.102. [DOI] [PubMed] [Google Scholar]
- 35.Lynch JP III, Kazerooni EA, Gay SE. Pulmonary sarcoidosis. Clin Chest Med. 1997;18:755–85. doi: 10.1016/s0272-5231(05)70417-2. [DOI] [PubMed] [Google Scholar]