Abstract
Introduction: Necrotizing sarcoid granulomatosis (NSG) is a very rare disease of unknown etiology characterized by sarcoid-like granulomas, vasculitis and necrosis in pulmonary and extrapulmonary localizations. Case report: We describe a case of a 34-year-old Caucasian male with fever, pleural pain, and nodular pulmonary opacities on chest radiograph. Histological examination of the lung tissue confirmed NSG. Diagnostically, infectious causes, vasculitis, and malignancy were excluded. A tendency to partial regression was observed, without the need for corticosteroid treatment. Conclusion: NSG is a rare disease which must be distinguished from other systemic diseases including vasculitides. The key to diagnosis, emphasized in our paper, is the histopathological finding. The course of NSG is similar to sarcoidosis. Corticosteroids are considered the treatment of choice, but the disease exhibits a tendency towards spontaneous regression. (Sarcoidosis Vasc Diffuse Lung Dis 2018; 35: 395-398)
Keywords: differential diagnosis, histopathological diagnosis, necrotising sarcoid granulomatosis
Introduction
Necrotizing sarcoid granulomatosis (NSG) is a rare systemic disease characterized by sarcoid-like granulomas, central necrosis and vasculitis (1-3). It was first described by American pathologist, Averill Abraham Liebow in 1973 (1).
Case report
A 34-year-old Caucasian male, an ex-smoker, presented with fever and breathing-related pleural pain. The patient had a long-term antihypertensive medication. His family history was negative. Physical examination revealed pleural friction rub on the right side of the chest; the rest of the physical examination was unremarkable. The posteroanterior chest radiograph showed nodular opacities mostly in the right lung. Multiple subpleural nodular opacities, the largest 13mm in diameter, were seen on the chest CT (Figures 1, 2, 3). Pulmonary function testing revealed no ventilation or diffusion defect. Arterial blood gas analysis was also normal. Laboratory tests at admission showed (reference values between brackets): C-reactive protein (CRP) 45mg/L (0-5), serum calcium 2.57 mmol/l (2.25-2.55), IgE 1.281 g/l (reference value <90 g/l), angiotensin-converting enzyme (ACE) 8 U/L (20-70) – the low ACE probably being influenced by the ACE inhibitor medication for high blood pressure. Total IgG, IgA, IgM concentrations were normal. The IgG4 subtype concentration was increased to 2.46 g/L (0.08-1.40 g/L). Serum protein electrophoresis complete with paraprotein and clonal IgE was normal. Complete blood count including white blood cell differential was normal, without eosinophilia. The liver function tests were normal.
Fig. 1.
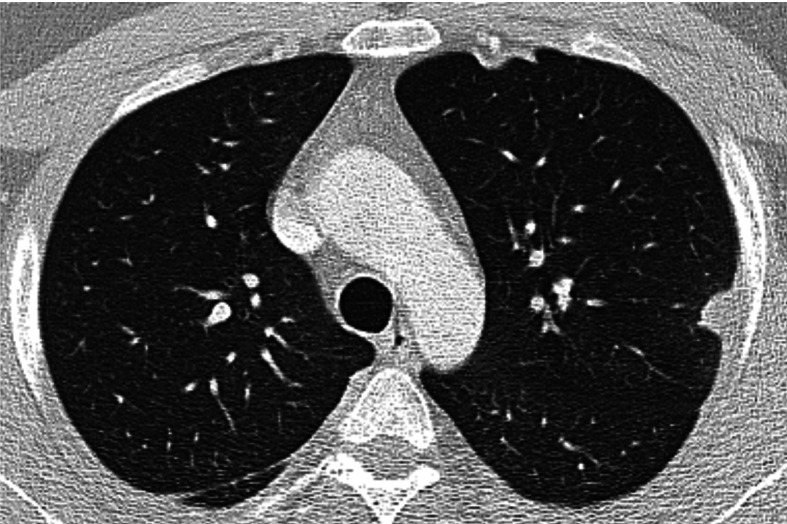
Chest HRCT, transverse plane, initial examination. At the aortic arch level, a subpleural nodule in the apical S1+2 segments of the left upper lobe with pleural thickening in the adjacent area. Subpleural lesions are quite typical for necrotizing sarcoid granulomatosis (NSG) without predilection for upper or lower lobe involvement. In the right lung dorsally, a slight thickening of the pleura and of the major interlobar fissure
Fig. 2.
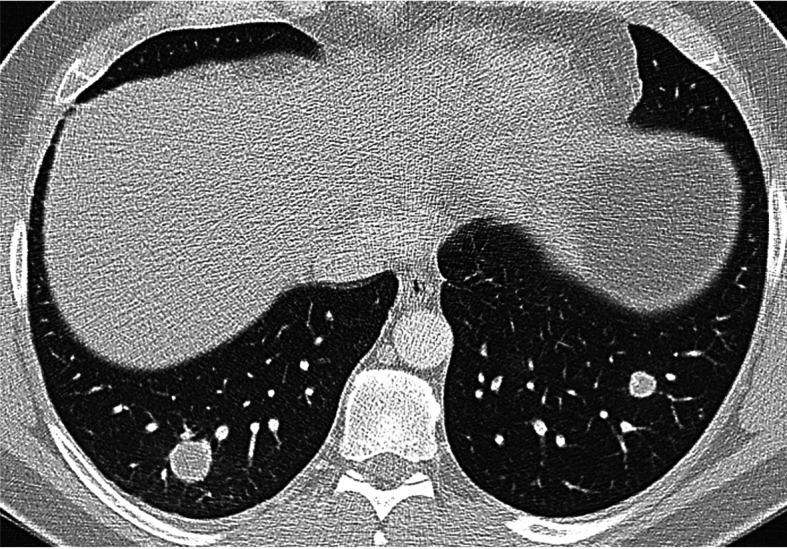
Chest HRCT, initial examination, transversal plane at the level of lower lobes shows spherical nodules with smooth edges, with no apparent spiculations extending into the surrounding pulmonary parenchyma. A larger, 18 mm nodule in the S10 segment of the right lower lobe; a smaller one, 11 mm, in the S9 segment of the left lower lobe. Among frequent findings in NSG are nodular lesions with smooth or slightly irregular borders that may exhibit cavitations
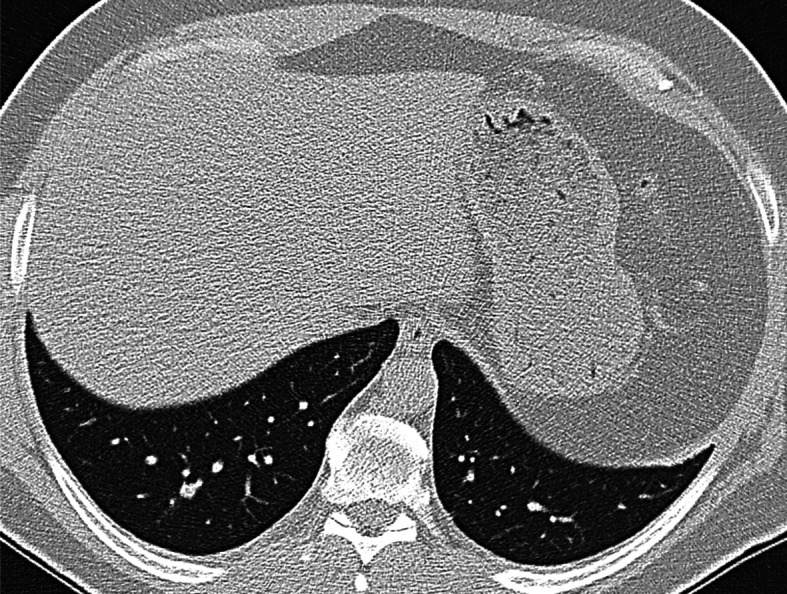
Fig. 3.
Chest HRCT, transverse plane, two-month follow-up. A tiny ‘consolidation’ of the pulmonary parenchyma with isolated calcifications and fibrous streaks extending towards the thickened pleura is present at the upper left lobe level in S1+2. The original S1+2 nodule adjacent to the pleura has partially regressed – a sign of a certain development of the disease over time
Treatment with antibiotics (amoxicillin) was started – without any effect on the pulmonary lesions. As the finding persisted and metastatic disease has to be excluded, video-assisted thoracoscopic surgery (VATS) with pulmonary biopsy was indicated. Histopathological examination then confirmed necrotizing sarcoid granulomatosis. Pulmonary parenchyma with peribronchovascular epitheloid granulomas including a few multinucleated giant cells, with a sparse perifocal lymphocytic inflammatory infiltrate. Minute, spotty areas of necrosis are present in the centre of some granulomas. Granulomas are well-circumscribed, well-formed, of the sarcoid type. In some places, granulomas merge into nodules with large central necrosis with chromatin dust. Granulomas are visible both in the lung parenchyma and, abundantly, in the vessel wall (with focal destruction). No cavitation is present. While granulomatous vasculitis is predominant, focally the inflammation is lymphocytic in character. The pleura exhibits identical lesions. Special staining on mycobacteria and fungi are negative (Figure 4). Searching for NSC’s extrapulmonary manifestions, hypodense deposits were discovered in the liver and renal cortex. A tiny (6mm) deposit was also found in a non-enlarged spleen. Further investigations were focussed on the exclusion of infectious causes or systemic diseases including vasculitides. The immunological screening including ANCA (antineutrophil cytoplasmatic antibodies) was negative. For the detection of elevated serum IgG4, immunohistological examination of the lung tissue was performed but no increase in the number of IgG4-positive plasma cells was found, and the histopathological finding did not meet the criteria of IgG4-related disease. Infectious examination including serology of chlamydophila, mycoplasma, and athropozoonoses was negative. The allergologic examination confirmed dust mite allergy. Serum Aspergillus galactomannan antigen testing by ELISA yielded negative results in pulmonary tissue, serum and BALF (bronchoalveolar fluid obtained by bronchoalveolar lavage). Specific IgE, IgG against Aspergillus were not detected. Evaluation of panfugal antigen in BALF and serum was also negative. The macroscopic finding in bronchoscopy was normal. The bronchoalveolar lavage samples collected for cytological examination revealed increased neutrophil granulocyte count (9.2%; reference range ≤3%) and a slightly increased eosinophil granulocyte count (2.2%; reference range≤0.5%); macrophages 83% (reference range ≥80%), lymphocytes 4.9% (reference range ≤15%). The CD4+/CD8+ ratio was not increased.
Fig. 4.

Histopathological findings. Microscopically, confluent non-caseating granulomas with extensive necrosis are seen in the lung parenchyma. The granulomas are of the sarcoid type, palisaded by Langerhans-type giant cells and mononuclear lymphohistiocytes with central necrosis. Another typical finding is the presence of vasculitis, usually granulomatous, and a large zone of necrosis. a) Large zone of necroris (×50); b) granulomatous vasculitis (×100); c) confluent granulomas and vasculitis (× 100).
Three months later, regression of nodular opacities on chest HRCT was observed (Figure 5). The control abdominal ultrasound was normal. Currently, the patient is asymptomatic – without dyspnea, chest pain, or fever. The patient is being monitored, without the need for corticosteroid therapy.
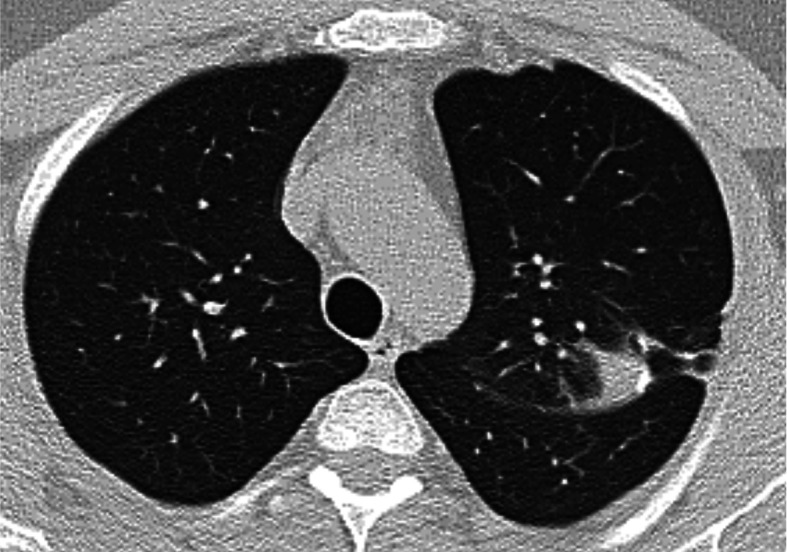
Fig. 5.
Chest HRCT, transverse plane, two-month follow-up. Compared with the initial examination, multiple nodules in the pulmonary parenchyma, predominantly in subpleural localization, have partially or completely disappeared. An 8 mm nodule (previously 18 mm) is present in S10 of the left lower lobe (indicated by the arrow)
Discussion
Necrotizing sarcoid granulomatosis (NSG) is a disease showing features of both vasculitis and granulomatous disease. Whether NSG is a separate entity or a variant of sarcoidosis is not yet clear (4,5,6,7). Etiopathogenesis is not known; infectious causes such as Chlamydophila pneumoniae, Mycoplasma pneumoniae and Aspergillus fumigatus (4,8,9) have been mooted. NSG primarily affects younger patients (median, 42 years of age) (10). Family occurrence has not been reported. Clinical symptoms are nonspecific and include pleural pain, weight loss, cough, dyspnea and fever. Extrapulmonary involvement can mimick sarcoidosis (10). Radiological findings vary; most often, nodular opacities are observed in the lung parenchyma, either solitary or multiple, with or without hilar and mediastinal lymphadenopathy (11,12). No specific laboratory biomarker for NSG diagnosis is available. ACE is usually normal (4). Pulmonary function tests are either normal or demonstrate a variety of abnormal patterns (13). The CD4/CD8 ratio in the bronchoalveolar fluid may be normal – in contrast to sarcoidosis (4).
The histopathological finding is central to, and indispensable for, the diagnosis of NSG. NSG should meet the following criteria: granulomas, necrosis and granulomatous vasculitis with no evidence to support an infectious etiology (7). The differential diagnosis includes granulomatous infection, nodular sarcoidosis and Wegener’s granulomatosis (granulomatosis with polyangiitis). The most difficult – and mostly impossible – to exclude is granulomatous infection that can cause necrotizing granulomatous inflammation and vasculitis resembling NSG. However, histological features typical of pulmonary sarcoidosis – non-necrotizing confluent granulomas surrounded by hyaline fibrosis and necrosis – are not characteristically seen in NSG. Still, some experts believe that NSG is essentially the same disease as sarcoidosis. Necrotizing granulomatous inflammation with vasculitis is also seen in Wegener’s granulomatosis. However, the presence of sarcoid-like granulomas and absence of the classic pattern of necrosis in Wegener’s granulomatosis, i.e.geographical areas of basophilic necrosis, favour NGS (10),
The clinical course of NSG is mostly benign with a tendency to spontaneous remission except for potentially lethal neurological lesions (14). Corticosteroids are the treatment of choice (4).
Conclusion
This work describes a rare systemic disease called necrotizing sarcoid granulomatosis (NSG). Emphasis is placed on histopathological findings and differentiation from other vasculitides or granulomatous diseases of the sarcoidosis type.
References
- 1.Liebow AA. The J. Burns Amberson lecture – pulmonary angiitis and granulomatosis. Am Rev Respir Dis. 1973;108(1):1–18. doi: 10.1164/arrd.1973.108.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Churg A. Pulmonary angiitis and granulomatosis revisited. Hum Pathol. 1983;14(10):868–883. doi: 10.1016/s0046-8177(83)80162-2. [DOI] [PubMed] [Google Scholar]
- 3.Churg A, Carrington GB, Gupta R. Necrotizing sarcoid granulomatosis. Chest. 1979;76(4):406–413. doi: 10.1378/chest.76.4.406. [DOI] [PubMed] [Google Scholar]
- 4.Quaden C, Tillie-Leblond I, Delobbe A, et al. Necrotising sarcoid granulomatosis: clinical, functional, endoscopical and radiographical evaluations. Eur Respir J. 2005;26(5):778–85. doi: 10.1183/09031936.05.00024205. [DOI] [PubMed] [Google Scholar]
- 5.Bouman KP, Slabbynck H, Cuykens JJ, Galdermans D, Coolen D, Kock M. Necrotising sarcoid granulomatosis with uveitis: a variant of sarcoidosis. Acta Clin Belg. 1997;52(6):367–370. doi: 10.1080/17843286.1997.11718602. [DOI] [PubMed] [Google Scholar]
- 6.Rolfes DB, Weiss MA, Sanders MA. Necrotizing sarcoid granulomatosis with suppurative features. J Clin Pathol. 1978;38(5):602–607. doi: 10.1093/ajcp/82.5.602. [DOI] [PubMed] [Google Scholar]
- 7.Popper HH, Klemen H, Colby TV, Churg A. Necrotizing sarcoid granulomatosis--is it different from nodular sarcoidosis. Pneumologie. 2003;57(5):268–271. doi: 10.1055/s-2003-39365. [DOI] [PubMed] [Google Scholar]
- 8.Tauber E, Wojnarowski C, Horcher E, Dekan G, Frischer T. Necrotizing sarcoid granulomatosis in a 14-yr-old female. Eur Respir J. 1999;13(3):703–705. doi: 10.1183/09031936.99.13370399. [DOI] [PubMed] [Google Scholar]
- 9.Koss MN, Hochholzer L, Feigin DS, Garancis JC, Ward PA. Necrotizing sarcoid-like granulomatosis: clinical, pathologic, and immunopathologic findings. Hum Pathol. 1980;11(5 Suppl):510–519. [PubMed] [Google Scholar]
- 10.Karpathiou G, Batistatou A, Boglou P, Stefanou D, Froudarakis ME. Necrotizing sarcoid granulomatosis: A distinctive form of pulmonary granulomatous disease. Clin Respir J. 2017 Jul 29 doi: 10.1111/crj.12673. [DOI] [PubMed] [Google Scholar]
- 11.Alliberti S, Falleni M, Tarsia P, et al. A 13-year-old female with shortness of breath and pleuritic chest pain. Eur Respir J. 2006;28(4):876–882. doi: 10.1183/09031936.06.00021006. [DOI] [PubMed] [Google Scholar]
- 12.Niimi H, Hartmann TE, Müller NL. Necrotising sarcoid granulomatosis: computed tomography and pathologic findings. J Comput Assist Tomogr. 1995;19(6):920–923. [PubMed] [Google Scholar]
- 13.Chittock DR, Joseph MG, Paterson NM, McFaden RG. Necrotizing sarcoid granulomatosis with pleural involvement. Clinical and radiographic features. Chest. 1994;106(3):672–676. doi: 10.1378/chest.106.3.672. [DOI] [PubMed] [Google Scholar]
- 14.Beach RC, Corrin B, Scopes JW, Graham E. Necrotizing sarcoid granulomatosis with neurologic lesions in a child. J Pediatr. 1980;97(6):950–953. doi: 10.1016/s0022-3476(80)80431-8. [DOI] [PubMed] [Google Scholar]


