Abstract
Background: Tumor necrosis factor (TNF) antagonists have been reported as an efficient third-line therapy for sarcoidosis but there is no data regarding patients who do not respond to this treatment. Objective: To report the characteristics, the outcome and the response to therapy of patients with sarcoidosis resistant to TNF antagonists. Methods: Patients from the French STAT (Sarcoidosis Treatment with Anti-TNF) registry who were classified as non-responders and who were followed-up for >1 year were included. The response to further therapies was classified as complete response, or partial response, and the others were classified as non-responders. Results: Among the 132 patients from the registry, 14 were considered as non-responders to anti-TNF. Nine patients (66% of women; mean age 48 years) were analyzed. The mean number of organs involved was 4.2. Seven patients were previously treated with more than 2 immunosuppressive treatments. The mean duration of the anti-TNF treatment was 9 months (range, 3-24). After a mean follow-up duration of 58 months (median, 35; range, 19-128) a complete response was observed in 2/9 cases, a partial response in 5/9 cases, and 2/9 cases were considered as non-responders. In all but one patient, the immunosuppressant that allowed the clinical response had previously been used. Furthermore, the dosage was not necessarily increased to gain efficacy. Non-responders were treated by corticosteroids only because of their comorbidities or noncompliance. Conclusion: In patients who do not respond to TNF antagonists, previously used immunosuppressants may be useful. Excluding a differential diagnosis, assessing compliance and testing for anti-drug antibodies should be systematic. (Sarcoidosis Vasc Diffuse Lung Dis 2018; 35: 371-375)
Keywords: sarcoidosis, anti-TNF, infliximab, immunosuppressant, treatment, outcome
Introduction
Sarcoidosis is a systemic granulomatous disease that mainly affects the lungs and the lymphatics but any organ or system can be involved (1). The disease may go into remission spontaneously or upon treatment but it has a chronic course in about 25% of the patients. Corticosteroids (CS) are the mainstay of treatment but their long-term use is hindered by a cumulative toxicity (1). The efficacy of hydroxychloroquine, methotrexate (MTX), azathioprine (AZA), mycophenolate mofetil (MMF), leflunomide (LEF) and cyclophosphamide (CYC) as adjunctive or alternative therapies has been reported (2).
Because some patients are non responsive to these therapies or may develop adverse events, tumor necrosis factor (TNF) antagonists have been proposed as a third-line option (3). Several retrospective studies have reported the efficacy of TNF antagonists for the treatment of sarcoidosis (4-10). In a previous retrospective multicentric study, the STAT (Sarcoidosis Treated with Anti-TNF) study, reporting on 132 sarcoidosis patients treated with TNF antagonists, we found that TNF antagonists were efficient in about two-thirds of the patients and allowed a substantial reduction in prednisone dosages (from 23 to 11 mg/day) (11).
Although some patients do not respond to TNF antagonists (12), there is no data in the literature regarding the characteristics of these patients, the outcome and the subsequent treatment options.
Thus, our aim was to describe patients included in our registry who did not respond to TNF antagonists.
Methods
Patients from the French STAT registry were previously described (11). Patients who were classified as non-responders to TNF antagonists (clinical or radiological progression) and who were followed-up for more than one year were included.
Patients were not included if the outcome was not reported. Special attention was held to exclude differential diagnoses such as tuberculosis, other mycobacterial infections, Whipple’s disease, Crohn’s disease and lymphoma.
The response to therapies was classified as complete response (CR), or partial response (PR). Complete response was defined as a resolution at any time of clinical signs along with a CS dosage <10 mg/day. Partial response was defined as an improvement in clinical and paraclinical parameters and a reduction of >50% of initial corticosteroid dosages. Other patients were classified as non-responders (NR, stable or progressive disease).
Results
Initial characteristics of patients with a sarcoidosis resistant to TNF antagonists
Among the 132 patients included in the STAT registry, 14 patients had been classified as non-responders to TNF antagonists. Five patients were not included because: (i) three were lost to follow-up; (ii) one patient died; and (iii) one had an alternative final diagnosis.
Nine patients (6 women) were finally included in the study; six were Caucasian, one was North African, one was African, and one was Asian.
The mean age at TNF antagonist initiation was 48 (range, 29-70) years. The disease was severe and the mean number of organs involved was 4.2 (2-7). 7 patients were previously treated with at least two immunosuppressive therapies (Table 1). Eight patients were treated with only one TNF antagonist [infliximab (IFX), n=8] while one patient received 3 different TNF antagonists [etanercept (ETN), IFX, and adalimumab (ADA)]. The mean duration of treatment was 9 months (range, 3-24). In addition to TNF antagonists, five patients received CS (mean dosage, 34 mg/day) and four patients received an immunosuppressant (IS: MTX, n=2; AZA, n=1; and MMF, n=1).
Table 1.
Treatments before, during and after TNF antagonists
| Before anti-TNF | During anti-TNF | After anti-TNF | Response to treatment |
| 1 MMF, CYC | IFX, AZA | MTX (25 mg/w) | PR |
| 2 MTX, CYC | IFX | CYC, AZA, RTX, CYC | PR |
| 3 MTX (10 mg/w), plaquenil | IFX,MTX (20 mg/w) | MTX (25 mg/w), AZA (150 mg/d) | CR |
| 4 MTX, CYC | IFX | CYC, AZA (200 mg/d) | CR |
| 5 MTX (15 mg/w), plaquenil, thalidomide | IFX, MTX (10 mg/w), | MTX (20 mg/w), LEF (20 mg/d) | PR |
| 6 MTX (20 mg/w), MMF, plaquenil (400 mg/d), thalidomide (150 mg/d) | IFX, MMF | MTX (20 mg/w), AZA (150 mg/d), LEF (20 mg/d) | PR |
| 7 MMF (3 g/d), CYC, plaquenil | IFX | MMF (2 g/d), plaquenil, AZA (75 mg/d) | PR |
| 8 CYC | IFX | CS | NR |
| 9 MTX | ETN→IFX→ADA | MTX (15 mg/w) | NR |
ADA, adalimumab; AZA, azathioprine; CS, corticosteroids; CYC, cyclophosphamide; ETN, etanercept; IFX, infliximab, LEF, leflunomide; MMF, mycophenolate mofetil; MTX, methotrexate; TNF, tumor necrosis factor; RTX, rituximab; CR: complete response; PR: partial response; NR : non-responder
Outcome of patients with a sarcoidosis resistant to TNF antagonists
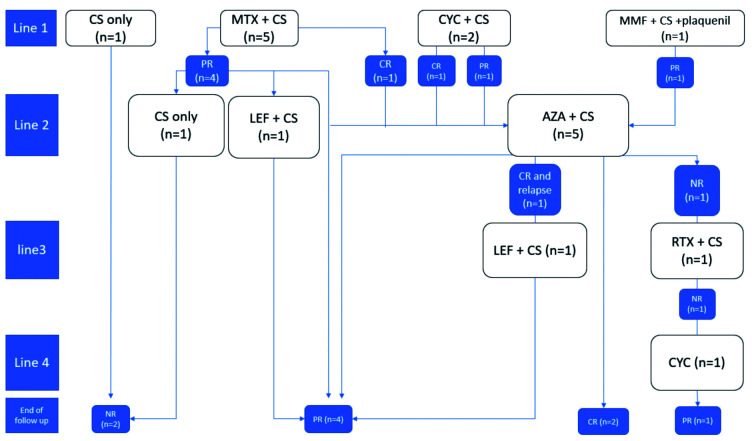
The mean follow-up duration was 58 months (median, 35; range, 19-128) and patients received a median of 2 IS treatments (range, 0-4), including MTX, CYC, MMF, AZA, LEF, and rituximab (Figure 1).
Fig. 1.
Response to therapy in sarcoidosis patients resistant to TNF antagonists.
MTX was the most frequent first-line IS (n=5) while AZA was more frequently used as a second-line therapy (n=5).
All patients were alive at last follow-up and a CR was observed in two cases, a PR in five cases, and the disease was progressive in two cases.
The first patient had a CR with MTX but had to be switched to AZA because of side-effects. The second patient had a CR with CYC, which was maintained under AZA. Both patients had previously been treated by these IS, but the MTX dosage was increased from 20 to 25 mg/week.
In patients with a PR, all had the same IS as before except for one patient.
The disease was progressive in two cases that were treated by CS only because of their comorbidities or noncompliance to IS treatment.
Discussion
In this retrospective study, we describe for the first time the characteristics, outcome and response to therapy of sarcoidosis patients who do not respond to TNF antagonists. Among the 9 patients, 7 had a clinical response (CR or PR) with additional IS therapy. The disease was only progressive in patients treated with CS without adjunctive IS.
Most importantly, the IS that allowed the clinical response had previously been used, except for one patient. Furthermore, the dosage was not necessarily increased to gain efficacy (same galenic and dosage in 4 cases).
IFX has been the most extensively studied TNF antagonist in sarcoidosis (5, 12-18) and is considered as the main anti-TNF treatment for refractory sarcoidosis. Nevertheless, several open-label studies have also shown that ADA was efficient for skin, ocular or pulmonary sarcoidosis (9, 17). Crommelin et al. have reported the efficacy of ADA in 39% of sarcoidosis patients who developed an intolerance or a resistance to IFX (10). In other inflammatory diseases, it has been shown that patients who have anti-infliximab antibodies have an increased risk of infusion reactions and a 3-fold higher risk of losing clinical response (19-22). Switching from one anti-TNF to another may thus be a reasonable strategy, mostly for ETN because immunization against this fusion protein is much less frequent. Unfortunately, in our multicentric retrospective study, we did not have data describing immunization against IFX because anti-IFX antibodies were not systematically tested. Moreover, we cannot exclude noncompliance to explain the initial resistance to IS in our patients since drug dosages were not performed.
Although the number of patients included may seem small, the data was recorded as part of the STAT registry, which is the largest cohort of patients with sarcoidosis treated with TNF antagonists. Further larger studies are warranted to confirm our findings.
Conclusion
Our study reports for the first time the outcome of sarcoidosis patients refractory to TNF antagonists. The prognosis was good for the majority of the patients who were treated with IS. Most importantly, the dosage and galenic were not systematically modified indicating that, when sarcoidosis remains refractory to TNF antagonists, treating with previously used IS may still be useful. Excluding a differential diagnosis, assessing compliance, and testing for anti-drug antibodies should be systematic before classifying patients as non-responders. Finally, switching to another TNF antagonist could be useful.
Acknowledgments
The authors thank Dr. Audrey de Parisot for editing assistance.
References
- 1.Valeyre D, Prasse A, Nunes H, Uzunhan Y, Brillet P-Y, Müller-Quernheim J. Sarcoidosis. Lancet. 2014;383(9923):1155–67. doi: 10.1016/S0140-6736(13)60680-7. [DOI] [PubMed] [Google Scholar]
- 2.Baughman RP, Nunes H, Sweiss NJ, Lower EE. Established and experimental medical therapy of pulmonary sarcoidosis. Eur Respir J. 2013;41(6):1424–38. doi: 10.1183/09031936.00060612. [DOI] [PubMed] [Google Scholar]
- 3.Vorselaars ADM, Verwoerd A, van Moorsel CHM, Keijsers RGM, Rijkers GT, Grutters JC. Prediction of relapse after discontinuation of infliximab therapy in severe sarcoidosis. Eur Respir J. 2014;43(2):602–9. doi: 10.1183/09031936.00055213. [DOI] [PubMed] [Google Scholar]
- 4.Baughman RP, Lower EE, Drent M. Inhibitors of tumor necrosis factor (TNF) in sarcoidosis: who, what, and how to use them. Sarcoidosis Vasc Diffuse Lung Dis. 2008;25(2):76–89. [PubMed] [Google Scholar]
- 5.Baughman RP, Drent M, Kavuru M, et al. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am J Respir Crit Care Med. 2006;174(7):795–802. doi: 10.1164/rccm.200603-402OC. [DOI] [PubMed] [Google Scholar]
- 6.Hostettler KE, Studler U, Tamm M, Brutsche MH. Long-term treatment with infliximab in patients with sarcoidosis. Respir Int Rev Thorac Dis. 2012;83(3):218–24. doi: 10.1159/000328738. [DOI] [PubMed] [Google Scholar]
- 7.Pariser RJ, Paul J, Hirano S, Torosky C, Smith M. A double-blind, randomized, placebo-controlled trial of adalimumab in the treatment of cutaneous sarcoidosis. J Am Acad Dermatol. 2013;68(5):765–73. doi: 10.1016/j.jaad.2012.10.056. [DOI] [PubMed] [Google Scholar]
- 8.Erckens RJ, Mostard RLM, Wijnen PA, HM, Schouten JS, Drent M. Adalimumab successful in sarcoidosis patients with refractory chronic non-infectious uveitis. Graefes Arch Clin Exp Ophthalmol. 2012;250(5):713–20. doi: 10.1007/s00417-011-1844-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sweiss NJ, Noth I, Mirsaeidi M, et al. Efficacy Results of a 52-week Trial of Adalimumab in the Treatment of Refractory Sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2014;31(1):46–54. [PMC free article] [PubMed] [Google Scholar]
- 10.Crommelin HA, van der Burg LM, Vorselaars ADM, et al. Efficacy of adalimumab in sarcoidosis patients who developed intolerance to infliximab. Respir Med. 2016;115:72–7. doi: 10.1016/j.rmed.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 11.Jamilloux Y, Cohen-Aubart F, Chapelon-Abric C, et al. Efficacy and safety of tumor necrosis factor antagonists in refractory sarcoidosis: A multicenter study of 132 patients. Semin Arthritis Rheum. 2017;47(2):288–294. doi: 10.1016/j.semarthrit.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Maneiro JR, Salgado E, Gomez-Reino JJ, Carmona L. BIOBADASER Study Group. Efficacy and safety of TNF antagonists in sarcoidosis: data from the Spanish registry of biologics BIOBADASER and a systematic review. Semin Arthritis Rheum. 2012;42(1):89–103. doi: 10.1016/j.semarthrit.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Judson MA, Baughman RP, Costabel U, et al. Efficacy of infliximab in extrapulmonary sarcoidosis: results from a randomised trial. Eur Respir J. 2008;31(6):1189–96. doi: 10.1183/09031936.00051907. [DOI] [PubMed] [Google Scholar]
- 14.Rossman MD, Newman LS, Baughman RP, et al. A double-blinded, randomized, placebo-controlled trial of infliximab in subjects with active pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2006;23(3):201–8. [PubMed] [Google Scholar]
- 15.Zajicek JP, Scolding NJ, Foster O, et al. Central nervous system sarcoidosis--diagnosis and management. QJM. 1999;92(2):103–17. doi: 10.1093/qjmed/92.2.103. [DOI] [PubMed] [Google Scholar]
- 16.Wijnen PA, Cremers JP, Nelemans PJ, et al. Association of the TNF-α G-308A polymorphism with TNF-inhibitor response in sarcoidosis. Eur Respir J. 2014;43(6):1730–9. doi: 10.1183/09031936.00169413. [DOI] [PubMed] [Google Scholar]
- 17.Riancho-Zarrabeitia L, Calvo-Río V, Blanco R, et al. Anti-TNF-α therapy in refractory uveitis associated with sarcoidosis: Multicenter study of 17 patients. Semin Arthritis Rheum. 2015;45(3):361–8. doi: 10.1016/j.semarthrit.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 18.Chapelon-Abric C, Saadoun D, Biard L, et al. Long-term outcome of infliximab in severe chronic and refractory systemic sarcoidosis: a report of 16 cases. Clin Exp Rheumatol. 2015;33(4):509–15. [PubMed] [Google Scholar]
- 19.Colombel J-F, Feagan BG, Sandborn WJ, Van Assche G, Robinson AM. Therapeutic drug monitoring of biologics for inflammatory bowel disease. Inflamm Bowel Dis. 2012;18(2):349–58. doi: 10.1002/ibd.21831. [DOI] [PubMed] [Google Scholar]
- 20.Steenholdt C, Svenson M, Bendtzen K, Thomsen OØ, Brynskov J, Ainsworth MA. Severe infusion reactions to infliximab: aetiology, immunogenicity and risk factors in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2011;34(1):51–8. doi: 10.1111/j.1365-2036.2011.04682.x. [DOI] [PubMed] [Google Scholar]
- 21.Ordás I, Feagan BG, Sandborn WJ. Therapeutic drug monitoring of tumor necrosis factor antagonists in inflammatory bowel disease. Clin Gastroenterol Hepatol. 2012;10(10):1079–87. doi: 10.1016/j.cgh.2012.06.032. [DOI] [PubMed] [Google Scholar]
- 22.Nanda KS, Cheifetz AS, Moss AC. Impact of antibodies to infliximab on clinical outcomes and serum infliximab levels in patients with inflammatory bowel disease (IBD): a meta-analysis. Am J Gastroenterol. 2013 Jan;108(1):40–47. doi: 10.1038/ajg.2012.363. [DOI] [PMC free article] [PubMed] [Google Scholar]