Abstract
Sarcoidosis is a systemic inflammatory disease characterized by granulomatous inflammation. The soluble interleukin-2 receptor (sIL-2R) is used as a biomarker for disease severity in sarcoidosis. Moreover, rather than just a biomarker, evidence indicates that sIL-2R could be of biological significance in this disease. The aim of this review is to investigate both its qualities as a biomarker and a potential biological role in sarcoidosis. As a biomarker, the serum level of sIL-2R can be used to distinguish patients from healthy controls, active from inactive disease and to assess treatment success. Additionally, sIL-2R correlates with other biomarkers, including angiotensin-converting enzyme, and with lung function tests and nuclear imaging studies. In sarcoidosis T helper cells and alveolar macrophages are the most likely sources of sIL-2R. While most of the evidence indicates that sIL-2R is generated through proteolytic cleavage of membrane-bound IL-2Rα, no endogenous enzyme has been found to be clearly responsible for sIL-2R formation. It is unclear if sIL-2R has immunostimulatory, immunomodulatory or no functional effects, since conflicting results have been reported. Several potential mechanisms of sIL-2R’s biological functions include IL-2 sequestration, prolonging IL-2 half-life, preventing activation of resting T cells or increasing affinity of IL-2Rβ for IL-2. The most likely function of sIL-2R is to modify IL-2 signaling. Increased levels of sIL-2R could either promote disease processes, represent an ineffective attempt to resolve the inflammation or have no effect at all. Further research is required to determine its exact role in the disease and thus its usefulness as a therapeutic target. (Sarcoidosis Vasc Diffuse Lung Dis 2017; 34: 122-129)
Keywords: sarcoidosis, soluble interleukin-2 receptor, biomarker, immunopathology
Introduction
Sarcoidosis is defined as “a multisystem disease of unknown cause characterized by the formation in all or several affected tissues of epithelioid-cell tubercles without caseation” (1). The lungs are the organ most often affected and symptoms of pulmonary sarcoidosis include cough, dyspnea, wheezing and chest pain (2). Diagnostic criteria for sarcoidosis include radiological features, such as bilateral hilar lymphadenopathy and pulmonary infiltration, presence of non-caseating granulomatous inflammation and exclusion of similar diseases (3,4). A widely used biomarker for disease activity in sarcoidosis is the soluble IL-2 receptor (sIL-2R). The full human membrane-associated IL-2 receptor consists of three subunits (α, β and γ) and is expressed on activated T cells, while an intermediate affinity form containing only the β and γ chains is found on resting T cells (5). Under certain conditions the α-chain is released from the membrane as sIL-2R. The serum level of this molecule is used to assess disease severity in a number of inflammatory conditions, including sarcoidosis. While the value of sIL-2R as a biomarker is reasonably well established, little is known about its biological role in sarcoidosis. After a brief discussion of sIL-2R as a biomarker, this paper will review the molecular and cellular origin of sIL-2R, its functional effects and potential mechanisms of action, and finally, its potential role in sarcoidosis.
sIL-2R as a Biomarker for sarcoidosis severity
The serum level of sIL-2R is assessed using a quantitative ELISA and has been found to correlate with various disease aspects. Several investigations have shown that the serum level of sIL-2R is significantly higher in sarcoidosis patients than in healthy controls (6-9). Additionally, sIL-2R levels are higher in patients with active disease compared to inactive disease (7, 8, 10-13). In contrast, the level of sIL-2R does not clearly differentiate between patients with different radiological stages (12) and, additionally, data reported on sIL-2R in relation to disease manifestation conflict (14,15). Furthermore, serum sIL-2R levels are significantly lower in treated than in untreated patients (10, 11, 16) and significantly decrease after treatment (6, 17, 18). Interestingly, while some reports indicate a correlation between the serum levels of sIL-2R and ACE, another biomarker for sarcoidosis, (10, 12, 19), others could not reproduce these findings (6, 14, 20). Associations between sIL-2R and other sarcoidosis biomarkers, such as soluble CD27 (21), C-reactive protein (12), α1-acid glycoprotein (12), chitotriosidase (9) and soluble CD163 (22), have been reported, but these findings still need to be reproduced. The serum sIL-2R level has also been associated with certain lung function parameters including vital capacity and carbon monoxide diffusion (8), and, improvements in lung function as a result of methotrexate therapy were found to correlate with decreases in sIL-2R levels (18). Finally, a relation has been reported between the serum sIL-2R level and outcome of 67Gallium scans (6, 23) and, while sIL-2R levels were significantly higher in patients with positive 18F-FDG PET scan results (24, 25), they did not directly correlate with standardized uptake values (17, 19). Thus, while above data indicate that sIL-2R can be a valuable biomarker for diagnosis and therapy of sarcoidosis, the exact role of sIL-2R in the immunopathology of sarcoidosis has not been elucidated.
Cellular origin of sIL-2R
While various studies have reported that T cells produce sIL-2R (26-33), they only produce notable amounts of sIL-2R after activation (26, 31, 32), with the exception of regulatory T cells which do not require (in vitro) activation (31, 32). Other cells of the immune system, including dendritic cells (34), macrophages and monocytes (29, 35) and B cells (26) can produce sIL-2R as well. While T cells are considered the main source of sIL-2R in sarcoidosis (13, 18, 21), there is some evidence indicating that macrophages also contribute to sIL-2R production in sarcoidosis (7, 8).
Generation of sIL-2R
The soluble form of the IL-2 receptor is most likely produced through enzymatic cleavage of membrane IL-2Rα. Experimental evidence argues against other mechanisms, such as cell death (36), separate genes for membrane IL-2R and sIL-2R (37) or differential mRNA splicing (37). Furthermore, it has been reported that membrane IL-2Rα levels decrease as sIL-2R levels increase, which is consistent with enzymatic cleavage (38, 39). In contrast, continuous synthesis and degradation of membrane IL-2Rα has been reported and, consequently, not all membrane IL-2Rα is released as sIL-2R (28). Additionally, using an enzymology approach, it was estimated that sIL-2R terminates between residues 187 and 192 of the membrane IL-2Rα molecule (40). Several cellular structures potentially involved in sIL-2R production, including lysosomal enzymes, proteases and serum components, were studied, but none of these processes were found to be essential for sIL-2R production (40). Furthermore, based on analysis of enzyme kinetics, it has been suggested that IL-2Rα release as sIL-2R is a first-order, nonsaturable process, which could be consistent with both enzymatic proteolysis and autocatalysis (41). Several enzymes responsible for cleavage of membrane IL-2R have been proposed: Der p 1 produced by Dermatophagoides pteronyssinus (42), neutrophil elastase (43) and metalloproteinases 2 and 9 (30, 44). Regardless of all data above, it has to be concluded that despite over 30 years of research, the exact molecular mechanism leading to generation of sIL-2R remains to be determined.
Functional effects of sIL-2R
Theoretically, sIL-2R could have three functional effects on the immune response. It could inhibit the immune response, stimulate the immune response or have no effect. The majority of studies (30, 32-34, 45-47) reported inhibitory effects, such as reduced T cell proliferation, decreased cytotoxicity and increased apoptosis. On the other hand, some studies observed immunostimulatory effects, including increased peripheral blood monocyte proliferation (48), STAT5 phosphorylation (39) and Th17 cell numbers (49). Furthermore, two studies (31, 50) observed no effect of sIL-2R on the immune response. However, Lindqvist and colleagues (32) pointed out a methodological limitation of these studies, namely that their sIL-2R was derived from an in vivo source, and consequently, might be bound to IL-2. Certain variables, such as the number of cells in culture (46) and the medium constituents and stimulatory signals (30) may modulate sIL-2R’s effect. Since none of the cells in these studies were obtained from sarcoidosis patients, these data should be considered broadly applicable, not specific to sarcoidosis.
Mechanisms of action
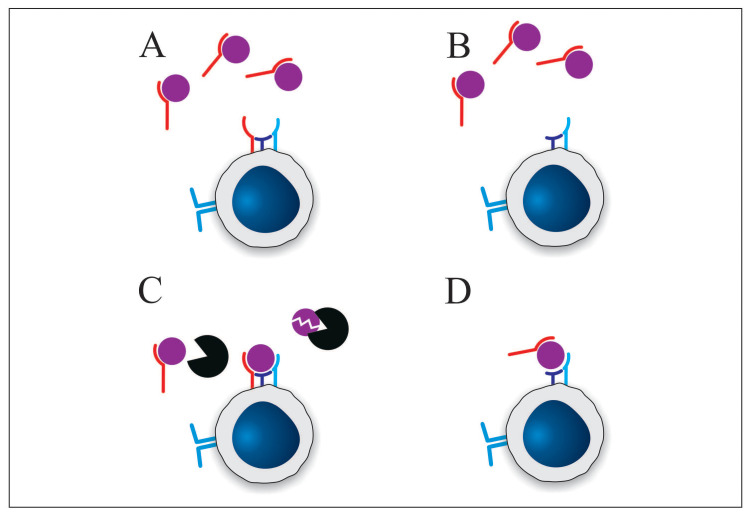
Since the functional effects of sIL-2R remain subject to controversy, the underlying mechanism of those functional effects likewise is unknown. However, a wide range of theories has been proposed including reduced IL-2 receptor density (51), local confinement of the inflammation (51), interaction with membrane IL-2 receptor (46), prevention of IL-2-mediated activation-induced cell death (48), interference with IL-2 feedback (49, 52) and inhibition of membrane IL-2Rα expression (47). While there is evidence in favor of and against any of these mechanisms, the following discussion is limited to the three most plausible mechanisms, namely IL-2 sequestration, prolongation of IL-2 half-life and induction of a structural change in IL-2 (Figure 1).
Fig. 1.
Proposed mechanisms of action of soluble IL-2 receptor. A) Sequestration of IL-2 from the heterotrimeric IL-2 receptor by sIL-2R. As discussed above the affinity of sIL-2R for IL-2 in comparison to the affinity of the heterotrimeric IL-2 receptor argues against this mechanism. B) Sequestration of IL-2 from the intermediate affinity IL-2 receptor by sIL-2R. C) sIL-2R competes with plasma enzymes for IL-2 resulting in prolonged IL-2 half-life. D) sIL-2R binding to IL-2 increases the affinity of IL-2 for the intermediate affinity receptor. (s)IL-2Rα: red; IL-2Rβ: purple; IL-2Rγ: blue; IL-2: purple sphere; serum protease: black sphere.
Probably the most commonly proposed mechanism of action of sIL-2R is sequestration of IL-2. This was already suggested in the original publication on the discovery of sIL-2R: “the release of soluble IL-2R by activated lymphocytes might serve an immunoregulatory role by competing with cellular IL-2R for the growth factor IL-2 and thus down-regulating the immune response” (36). Support for this theory is found in sIL-2R’s ability to bind IL-2 (38, 53) with an affinity between 10 (40) and 30 nM (54). The major limitation is that this reported affinity of sIL-2R for IL-2 is approximately 1000 times lower than the affinity of the full membrane IL-2 receptor for IL-2 (10-11M, 5). As such, it is unlikely that sIL-2R can effectively compete for binding of IL-2 with the full membrane receptor. However, only activated T cells express the full heterotrimeric IL-2 receptor, while resting T cells express only the IL-2Rβγ. The affinity of this dimeric receptor is only 10 times higher than that of sIL-2R (10-9 M, 5). Consequently, while sIL-2R may not be able to affect activated T cells, it might negatively impact activation of resting T cells by limiting IL-2 availability. One fundamental question related to this mechanism is whether or not resting T cells expressing only the intermediate affinity receptor can respond to IL-2. Since IL-2Rα is not associated with intracellular signaling pathways, it could be argued that cells should still be able to respond to IL-2 in its absence. Additionally, since T-cell activation results in IL-2 expression and, in turn, intracellular signaling mediated by the IL-2 receptor results in expression of IL-2Rα, it is possible that the intermediate affinity receptor mediates this process, which leads to expression of the heterotrimeric IL-2 receptor. However, if resting T cells do not respond to IL-2 in the same way as active T cells, it is unlikely that competition by sIL-2R for IL-2 would have an effect.
sIL-2R theoretically also has immunostimulatory potential. Binding of sIL-2R to IL-2 does not have to prevent IL-2 from interacting with the membrane IL-2 receptor, but rather may protect it from degradation by serum proteases. As such, sIL-2R would behave as a carrier protein for IL-2. This theory was originally proposed by Caruso and colleagues (51) and experimental evidence was provided by Kobayashi and associates (55), who conducted both in vivo and in vitro experiments that indicate that sIL-2R prolongs IL-2’s half-life.
Finally, Maier and colleagues (48) suggested that binding of sIL-2R to IL-2 might promote IL-2 signaling, by analogy with the IL-6 and IL-15 systems (56, 57). Indeed, it was found that membrane IL-2Rαinduces a conformational change in the IL-2 molecule which increases its affinity for IL-2Rβ (58). Consequently, it is possible that sIL-2R induces the same conformational change and, as such, the affinity of IL-2Rβ would be higher for the sIL-2R-IL-2 complex than for IL-2 alone.
IL-2 and sIL-2R in sarcoidosis
While the immunopathology of sarcoidosis is very complex and many aspects are still unclear, it is in essence a Th1-mediated granulomatous inflammatory disorder (1). There is a considerable amount of evidence indicating that IL-2 plays a vital role in sarcoidosis. First of all, through upregulation of the IL-12 receptor β2 subunit IL-2 promotes the development of Th1 cells (59, 60). Secondly, abnormalities in the IL-2 system have been observed in sarcoidosis, including increased IL-2 release by T cells (61) and increased expression of membrane IL-2Rα (62-64). Thirdly, therapeutic administration of IL-2 results in development of sarcoidosis-like disease in HIV patients (65, 66) and a case study reported worsening of symptoms in a patient with sarcoidosis after IL-2 immunotherapy (67). Finally, a recent study reported dysregulation of IL-2 release in sarcoidosis and, interestingly, that disease resolution was accompanied by restoration of IL-2 release (68). As such, these studies highlight the importance of IL-2 in the immunopathology of sarcoidosis.
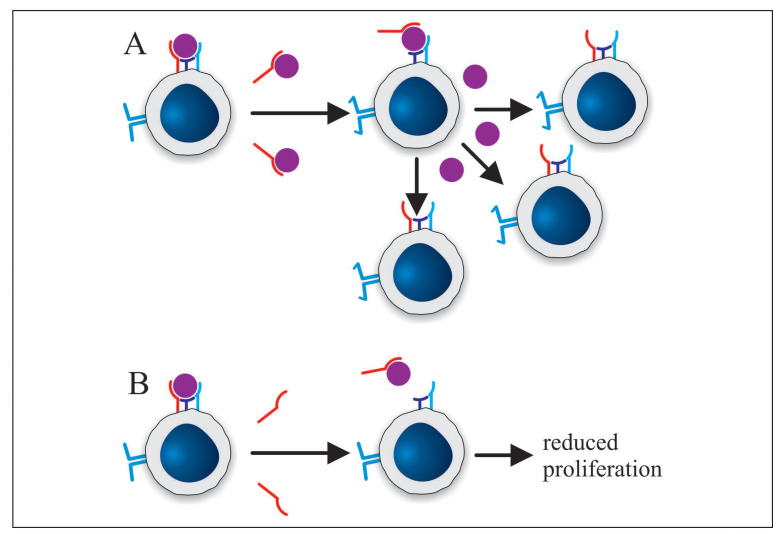
Although the exact functional effect of sIL-2R is not yet fully understood, it is most likely that in sarcoidosis sIL-2R interacts with IL-2. Three possible roles for sIL-2R in the immunopathology of sarcoidosis can be envisioned. First of all, sIL-2R could contribute directly to the immunopathology of sarcoidosis. By prolonging the half-life of IL-2 or by increasing its affinity for IL-2Rβ sIL-2R could stimulate IL-2 signaling, which would promote the inflammatory process and might also result in proliferation of T cells specific for other antigens (Figure 2A). Alternatively, sIL-2R might inhibit T cell proliferation through IL-2 sequestration which would impair clearance of disease-promoting antigens (Figure 2B). On the other hand, the presence of sIL-2R might reflect an ongoing process to limit inflammation, which apparently in sarcoidosis is ineffective. By preventing activation of resting T cells through IL-2 sequestration, sIL-2R would reduce inflammation. However, since the inflammation in sarcoidosis does not resolve despite high sIL-2R levels, it is apparently ineffective. Finally, it is possible that sIL-2R does not exert a major biological effect and is only useful as a biomarker.
Fig. 2.
Potential mechanisms explaining how sIL-2R might contribute to the immunopathology of sarcoidosis. A) Binding of sIL-2R to IL-2 increases the affinity of IL-2 for the intermediate affinity receptor. Consequently, the sIL-2R-IL-2 complex can activate resting (potentially autoreactive) T cells, which then proliferate. B) Binding of sIL-2R to IL-2 sequesters IL-2 from resting cells only expressing the intermediate affinity receptor. As a result, T cell proliferation is reduced and antigen clearance is affected. (s)IL-2Rα: red; IL-2Rβ: purple; IL-2Rγ: blue; IL-2: purple sphere
Future perspectives
While the existing literature provides substantial information on sIL-2R and sarcoidosis, there are several unresolved issues. First of all, efforts should be made to identify the enzyme responsible for the cleavage of membrane IL-2Rα, as this might lead to the development of drugs that could inhibit this enzyme and, consequently, selectively block sIL-2R production. Furthermore, the inconsistencies reported on the immunological effect of sIL-2R impede our understanding of its role in sarcoidosis and a study investigating factors that moderate sIL-2R’s effect is highly desirable. Additionally, while there is some evidence for the various mechanisms of action of sIL-2R, more substantial experimental proof is required. Finally, studies in patients could determine if administration of sIL-2R has a functional role in the immunopathology of sarcoidosis, but due to ethical constraints those studies would not be feasible.
Conclusion
Based on its correlation with various disease aspects, including disease activity, response to treatment, lung function, radiological examinations and other biomarkers, sIL-2R is a valuable biomarker for sarcoidosis. Additionally, based on the immunological effect of sIL-2R, it is possible that sIL-2R has a biological role in sarcoidosis. However, several aspects of sIL-2R’s function remain unclear and future research will have to address these. Depending on its role, sIL-2R might become a therapeutic target. If sIL-2R is found to be play a protective role, future therapies might include administration of exogenous sIL-2R. Alternatively, if sIL-2R actively promotes disease development, inhibitors of the enzyme responsible for sIL-2R production could be therapeutically useful. In conclusion, aside from its potential as a biomarker, sIL-2R might play a biological role in sarcoidosis and become a target for future therapies.
Abbreviations
- ACE
angiotensin-converting enzyme
- IL-2
interleukin-2
- IL-2Rα
interleukin-2 receptor alpha subunit
- IL-2Rβ
interleukin-2 receptor beta subunit
- IL-2Rγ
interleukin-2 receptor gamma subunit
- sIL-2R
soluble interleukin-2 receptor alpha subunit
References
- 1.Mitchell D, Wells A, Spiro S, Moller D. Sarcoidosis. London: Hodder Arnold; 2012. [Google Scholar]
- 2.Judson MA. The clinical features of sarcoidosis: A comprehensive review. Clinic Rev Allerg Immunol. 2015;49:63–78. doi: 10.1007/s12016-014-8450-y. [DOI] [PubMed] [Google Scholar]
- 3.American Thoracic Society. Statement on sarcoidosis. Am J Respir Crit Care Med. 1999;160:736–55. doi: 10.1164/ajrccm.160.2.ats4-99. [DOI] [PubMed] [Google Scholar]
- 4.Valeyre D, Prasse A, Nunes H, Uzunhan Y, Brillet P, Müller-Quernheim J. Sarcoidosis. Lancet. 2014;383:1155–67. doi: 10.1016/S0140-6736(13)60680-7. [DOI] [PubMed] [Google Scholar]
- 5.Lin J, Leonard WJ. Interleukin-2. The Cytokine Handbook. In: Thomson AW, Lotze MT, editors. 4th. London: Academic Press; 2003. pp. 167–99. [Google Scholar]
- 6.Lawrence EC, Brousseau KP, Berger MB, Kurman CC, Marcon L, Nelson DL. Elevated concentrations of soluble interleukin-2 receptors in serum samples and bronchoalveolar lavage fluids in active sarcoidosis. Am Rev Respir Dis. 1988;137:759–64. doi: 10.1164/ajrccm/137.4.759. [DOI] [PubMed] [Google Scholar]
- 7.Ina Y, Takada K, Sato T, Yamamoto M, Noda M, Morishita M. Soluble interleukin 2 receptors in patients with sarcoidosis. Chest. 1992;102:1128–33. doi: 10.1378/chest.102.4.1128. [DOI] [PubMed] [Google Scholar]
- 8.Pforte A, Brunner A, Gais P, et al. Concomitant modulation of serum-soluble interleukin-2 receptor and alveolar macrophage interleuking-2 receptor in sarcoidosis. Am Rev Respir Dis. 1993;147:717–22. doi: 10.1164/ajrccm/147.3.717. [DOI] [PubMed] [Google Scholar]
- 9.Bargagli E, Bianchi N, Margollicci M, et al. Chitotriosidase and soluble IL-2 receptor: Comparison of two markers of sarcoidosis severity. Scand J Clin Lab Invest. 2008;68:479–83. doi: 10.1080/00365510701854975. [DOI] [PubMed] [Google Scholar]
- 10.Keicho N, Kitamura K, Takaku F, Yotsumoto H. Serum concentration of soluble interleukin-2 receptor as a sensitive parameter of disease activity in sarcoidosis. Chest. 1990;98:1123–9. doi: 10.1378/chest.98.5.1125. [DOI] [PubMed] [Google Scholar]
- 11.Müller-Quernheim J, Pfeifer S, Strausz J, Ferlinz R. Correlation of clinical and immunologic parameters of the inflammatory activity of pulmonary sarcoidosis. Am Rev Respir Dis. 1991;144:1322–9. doi: 10.1164/ajrccm/144.6.1322. [DOI] [PubMed] [Google Scholar]
- 12.Hrycaj P, Wurm K, Mennet P, Müller W. Microheterogeneity of acute-phase glycoproteins in patients with pulmonary sarcoidosis. Eur Respir J. 1996;9:313–8. doi: 10.1183/09031936.96.09020313. [DOI] [PubMed] [Google Scholar]
- 13.Miyoshi S, Hamada H, Kadowaki T, et al. Comparative evaluation of serum markers in pulmonary sarcoidosis. Chest. 2010;137:1391–7. doi: 10.1378/chest.09-1975. [DOI] [PubMed] [Google Scholar]
- 14.Grutters JC, Fellrath J, Mulder L, Janssen R, Van den Bosch, JM, Van Velzen-Blad H. Serum soluble interleukin-2 receptor measurement in patients with sarcoidosis. Chest. 2003;124:186–95. doi: 10.1378/chest.124.1.186. [DOI] [PubMed] [Google Scholar]
- 15.Zurkova M, Kolek V, Tomankova T, Kriegova E. Extrapulmonary involvement in patients with sarcoidosis and comparison of routine laboratory and clinical data to pulmonary involvement. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2014;158:613–20. doi: 10.5507/bp.2014.026. [DOI] [PubMed] [Google Scholar]
- 16.Rothkranz-Kos S, Van Dieijen-Visser MP, Mulder PG, Drent M. Potential usefulness of inflammatory markers to monitor respiratory function impairment in sarcoidosis. Clin Chem. 2003;49:1510–7. doi: 10.1373/49.9.1510. [DOI] [PubMed] [Google Scholar]
- 17.Keijsers RG, Verzijlbergen JF, Van Diepen DM, Van den Bosch JM, Grutters JC. 18F-FDG PET in sarcoidosis: an observational study in 12 patients treated with infliximab. Sarcoidosis Vasc Diffuse Lung Dis. 2008;25:143–50. [PubMed] [Google Scholar]
- 18.Vorselaars AD, Van Moorsel CH, Zanen P, et al. ACE and sIL-2R correlate with lung function improvement in sarcoidosis during methotrexate therapy. Respir Med. 2015;109:279–85. doi: 10.1016/j.rmed.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 19.Keijsers RG, Verzijlbergen FJ, Oyen WJ, et al. 18 F-FDG PET, genotype-corrected ACE and sIL-2R in newly diagnosed sarcoidosis. Eur J Nucl Med Mol Imaging. 2009;3:1131–7. doi: 10.1007/s00259-009-1097-x. [DOI] [PubMed] [Google Scholar]
- 20.Ziegenhagen MW, Benner UK, Zissel G, Zabel P, Schlaak M, Müller-Quernheim J. Sarcoidosis: TNF-α release from alveolar macrophages and serum level of sIL-2R are prognostic markers. Am J Respir Crit Care Med. 1997;156:1586–92. doi: 10.1164/ajrccm.156.5.97-02050. [DOI] [PubMed] [Google Scholar]
- 21.Hol BE, Hintzen RQ, Van Lier RA, Alberts C, Out TA, Jansen HM. Soluble and cellular markers of T cell activation in patients with pulmonary sarcoidosis. Am Rev Respir Dis. 1993;148:643–9. doi: 10.1164/ajrccm/148.3.643. [DOI] [PubMed] [Google Scholar]
- 22.Tanimura H, Mizuno K, Okamoto H. Serum levels of soluble CD163 as a useful marker of macrophage/monocyte activity in sarcoidosis patients. Sarcoidosis Vasc Diffuse Lung Dis. 2015;32:99–105. [PubMed] [Google Scholar]
- 23.Tamotsu K, Watanabe S, Yano F, et al. Clinical significance of the serum IL-2R level and Ga-67 scan findings in making a differential diagnosis between sarcoidosis and non-Hodgkin’s lymphoma. Ann Nucl Med. 2007;21:499–503. doi: 10.1007/s12149-007-0060-9. [DOI] [PubMed] [Google Scholar]
- 24.Mostard RL, Vöö S, Van Kroonenburgh MJ, et al. Inflammatory activity assessment by F18 FDG-PET/CT in persistent symptomatic sarcoidosis. Respir Med. 2011;105:1917–24. doi: 10.1016/j.rmed.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 25.Mostard RL, Van Kuijk SM, Verschakelen JA, et al. A predictive tool for an effective use of 18F-FDG PET in assessing activity in sarcoidosis. BMC Pulm Med. 2012;57(12) doi: 10.1186/1471-2466-12-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nelson DL, Rubin LA, Kurman CC, Fritz ME. Boutin, B. An analysis of the cellular requirements for the production of soluble interleukin-2 receptors in vitro. J Clin Immunol. 1986;6:114–20. doi: 10.1007/BF00918743. [DOI] [PubMed] [Google Scholar]
- 27.Wagner DK, Wong HL, Gately MK, Nelson DL. Cellular source of soluble interleukin 2 receptors in serum of mice after recombinant interleukin 2 administration. Cytokine. 1990;2:337–43. doi: 10.1016/1043-4666(90)90063-y. [DOI] [PubMed] [Google Scholar]
- 28.Sayar D, Ketzinel M, Gerez L, Silberberg C, Reshef A, Kaempfer R. Expression of the human IL-2 receptor on lymphocytes involves rapid turnover of its p55 α-subunit (Tac) J Immunol. 1990;145:2946–9. [PubMed] [Google Scholar]
- 29.Kniep EM, Strelow I, Lohmann-Matthes ML. The monocyte interleukin-2 receptor light chain: Production of cell associated and soluble interleukin-2 receptor by monocytes. Immunology. 1992;75:299–304. [PMC free article] [PubMed] [Google Scholar]
- 30.Brusko TM, Wasserfall CH, Hulme MA, Cabrera R, Schatz D, Atkinson MA. Influence of membrane CD25 stability on T lymphocyte activity: implications for immunoregulation. PLOS ONE. 2009;4(11) doi: 10.1371/journal.pone.0007980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pedersen AE, Lauritsen JP. CD25 shedding by human natural occurring CD4+CD25+ regulatory T cells does not inhibit the action of IL-2. Scand J Immunol. 2009;70:40–3. doi: 10.1111/j.1365-3083.2009.02268.x. [DOI] [PubMed] [Google Scholar]
- 32.Lindqvist CA, Christiansson LH, Simonsson B, Enblad G, Olsson-Strömberg U, Loskog AS. T regulatory cells control T-cell proliferation partly by the release of soluble CD25 in patients with B-cell malignancies. Immunology. 2010;131:371–6. doi: 10.1111/j.1365-2567.2010.03308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cabrera R, Ararat M, Cao M, et al. Hepatocellular carcinoma immunopathogenesis: Clinical evidence for global T cell defects and an immumodulatory role for soluble CD25 (sCD25) Dig Dis Sci. 2010;55:484–95. doi: 10.1007/s10620-009-0955-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Von Bergwelt-Baildon MS, Popov A, Saric T, et al. CD25 and indoleamine 2,3-dioxygenase are up-regulated by prostaglandin E2 and expressed by tumor-associated dendritic cells in vivo: Additional mechanisms of T-cell inhibition. Blood. 2006;108:228–37. doi: 10.1182/blood-2005-08-3507. [DOI] [PubMed] [Google Scholar]
- 35.Scheibenbogen C, Keilholz U, Richter M, Andreesen R, Hunstein W. The interleukin-2 receptor in human monocytes and macrophages: regulation of expression and release of the α and β chains (p55 and p75) Res Immunol. 1992;143:33–7. doi: 10.1016/0923-2494(92)80077-x. [DOI] [PubMed] [Google Scholar]
- 36.Rubin LA, Kurman CC, Fritz ME, et al. Soluble interleukin 2 receptors are released from activated human lymphoid cells in vitro. J Immunol. 1985;135(5):3172–7. [PubMed] [Google Scholar]
- 37.Rubin LA, Galli F, Greene WC, Nelson DL, Jay G. The molecular basis for the generation of the human soluble interleukin 2 receptor. Cytokine. 1990;2:330–6. doi: 10.1016/1043-4666(90)90062-x. [DOI] [PubMed] [Google Scholar]
- 38.Diamantstein T, Osawa H, Mouzaki A, Josimovic-Alasevic O. Regulation of interleukin-2 receptor expression and receptor release. Mol Immunol. 1986;23:1165–72. doi: 10.1016/0161-5890(86)90147-1. [DOI] [PubMed] [Google Scholar]
- 39.Yang ZZ, Grote DM, Ziesmer SC, et al. Soluble IL-2Rα facilitates IL-2-mediated immune responses and predicts reduced survival in follicular B-cell non-Hodgkin lymphoma. Blood. 2011;118:2809–20. doi: 10.1182/blood-2011-03-340885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robb RJ, Kutny RM. Structure-function relationships for the IL 2-receptor system. IV. Analysis of the sequence and ligand-binding properties of soluble Tac protein. J Immunol. 1987;139:855–62. [PubMed] [Google Scholar]
- 41.Junghans RP, Waldmann TA. Metabolism of Tac (IL2Rα): Physiology of cell surface shedding and renal catabolism, and suppression of catabolism by antibody binding. J Exp Med. 1996;183:1587–1602. doi: 10.1084/jem.183.4.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schulz O, Sewell HF, Shakib F. Proteolytic cleavage of CD25, the α subunit of the human T cell interleukin 2 receptor, by Der p 1, a major mite allergen with cysteïne protease activity. J Exp Med. 1998;187:271–5. doi: 10.1084/jem.187.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bank U, Reinhold D, Schneemilch C, Kunz D, Synowitz H, Ansorge S. Selective proteolytic cleavage of IL-2 receptor and IL-6 receptor ligand binding chains by neutrophil-derived serine proteases at foci of inflammation. J Interferon Cytokine Res. 1999;19:1277–87. doi: 10.1089/107999099312957. [DOI] [PubMed] [Google Scholar]
- 44.Sheu B, Hsu S, Ho H, Lien H, Huang S, Lin R. A novel role of metalloproteinase in cancer-mediated immunosuppression. Cancer Res. 2001;61:237–42. [PubMed] [Google Scholar]
- 45.Kondo N, Kondo S, Shimizu A, Honjo T, Hamuro J. A soluble ‘anchorminus’ interleukin 2 receptor suppresses in vitro interleukin 2-mediated immune responses. Immunol Lett. 1988;19:299–308. doi: 10.1016/0165-2478(88)90159-9. [DOI] [PubMed] [Google Scholar]
- 46.Zorn U, Dallmann I, Große J, Kirchner H, Poliwoda H, Atzpodien J. Soluble interleukin 2 receptors abrogate IL-2 induced activation of peripheral mononuclear cells. Cytokine. 1994;6:358–64. doi: 10.1016/1043-4666(94)90058-2. [DOI] [PubMed] [Google Scholar]
- 47.Cabrera R, Ararat M, Eksioglu EA, et al. Influence of serum and soluble CD25 (sCD25) on regulatory and effect T-cell function in hepatocellular carcinoma. Scand J Immunol. 2010;72:293–301. doi: 10.1111/j.1365-3083.2010.02427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maier LM, Anderson DE, Severson CA, et al. Soluble IL-2Rα levels in multiple sclerosis subjects and the effects of soluble IL-2RA on immune responses. J Immunol. 2009;182:1541–7. doi: 10.4049/jimmunol.182.3.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Russell SE, Moore AC, Fallon PG, Walsh PT. Soluble IL-2Rα (sCD25) exacerbates autoimmunity and enhances the development of Th17 responses in mice. PLOS ONE. 2012;7(10) doi: 10.1371/journal.pone.0047748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pizzolo G, Vincenzi C, Vinante F, et al. Highly concentrated urine-purified Tac peptide fails to inhibit IL-2-dependent cell proliferation in vitro. Cell Immunol. 1992;141:253–9. doi: 10.1016/0008-8749(92)90144-e. [DOI] [PubMed] [Google Scholar]
- 51.Caruso C, Di Lorenzo G, Modica MA, et al. Soluble interleukin-2 receptor release defect in vitro in elderly subjects. Mech Aging Dev. 1991;59:27–35. doi: 10.1016/0047-6374(91)90071-7. [DOI] [PubMed] [Google Scholar]
- 52.Christiakov DA, Christiakova EI, Voronova NV, Turakulov RI, Savost’anov KV. A variant of the Il2ra/Cd25 gene predisposing to Graves’ disease is associated with increased levels of soluble interleukin-2 receptor. Scand J Immunol. 2011;74:496–501. doi: 10.1111/j.1365-3083.2011.02608.x. [DOI] [PubMed] [Google Scholar]
- 53.Rubin LA, Jay G, Nelson DL. The released interleukin 2 receptor binds interleukin 2 efficiently. J Immunol. 1986;137:3841–4. [PubMed] [Google Scholar]
- 54.Jacques Y, Le Mauff B, Boeffard F, Godard A, Soulillou J. A soluble interleukin 2 receptor produced by a normal alloreactive human T cell clone binds interleukin 2 with low affinity. J Immunol. 1987;139:2308–16. [PubMed] [Google Scholar]
- 55.Kobayashi H, Tagaya Y, Han E, et al. Use of an antibody against the soluble interleukin 2 receptor α subunit can modulate the stability and biodistribution of interleukin-2. Cytokine. 1999;11:1065–75. doi: 10.1006/cyto.1999.0509. [DOI] [PubMed] [Google Scholar]
- 56.Jones SA, Horiuchi S, Topley N, Yamamoto N, Fuller GM. The soluble interleukin 6 receptor: Mechanisms of production and implications in disease. FASEB. 2001;15:43–58. doi: 10.1096/fj.99-1003rev. [DOI] [PubMed] [Google Scholar]
- 57.Rubinstein MP, Kovar M, Purton JF, et al. Converting IL-15 to a superagonist by binding to soluble IL-15Rα. Proc Natl Acad Sci USA. 2006;103:9166–71. doi: 10.1073/pnas.0600240103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang X, Rickert M, Garcia KC. Structure of the quaternary complex of interleukin-2 with its α, β, and γc receptors. Science. 2005;310:1159–63. doi: 10.1126/science.1117893. [DOI] [PubMed] [Google Scholar]
- 59.Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol. 2012;12:180–90. doi: 10.1038/nri3156. [DOI] [PubMed] [Google Scholar]
- 60.Liao W, Lin J, Wang L, Li P, Leonard WJ. Cytokine receptor modulation by interleukin-2 broadly regulates T helper cell lineage differentiation. Nat Immunol. 2011;12:551–9. doi: 10.1038/ni.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hunninghake GW, Bedell GN, Zavala DC, Monick M, Brady M. Role of interleukin-2 release by lung T cells in active pulmonary sarcoidosis. Am Rev Respir Dis. 1983;128:634–8. doi: 10.1164/arrd.1983.128.4.634. [DOI] [PubMed] [Google Scholar]
- 62.Semenzato G, Agostini C, Trentin L, et al. Evidence of cells bearing interleukin-2 receptor at sites of disease activity in sarcoid patients. Clin Exp Immunol. 1984;57:331–7. [PMC free article] [PubMed] [Google Scholar]
- 63.Hancock WW, Muller WA, Cotran RS. Interleukin 2 receptors are expressed by alveolar macrophages during pulmonary sarcoidosis and are inducible by lymphokine treatment of normal human lung macrophages, blood monocytes, and monocyte cell lines. J Immunol. 1987;138:185–91. [PubMed] [Google Scholar]
- 64.Konishi K, Moller DR, Saltini C, Kirby M, Crystal RG. Spontaneous expression of the interleukin 2 receptor gene and presence of functional interleukin 2 receptors on T lymphocytes in the blood of individuals with active pulmonary sarcoidosis. J Clin Invest. 1988;82:775–81. doi: 10.1172/JCI113678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Naccache J, Antoine M, Wislez M, et al. Sarcoid-like pulmonary disorder in human immunodeficiency virus-infected patients receiving antiretroviral therapy. Am J Respir Crit Care Med. 1999;159:2009–13. doi: 10.1164/ajrccm.159.6.9807152. [DOI] [PubMed] [Google Scholar]
- 66.Blanche P, Gombert B, Rollot F, Salmon D, Sicard D. Sarcoidosis in a patient with acquired immunodeficiency syndrome treated with interleukin-2. Clin Infect Dis. 2000;31:1493–4. doi: 10.1086/317475. [DOI] [PubMed] [Google Scholar]
- 67.Logan TF, Bensadoun ES. Increased disease activity in a patient with sarcoidosis after high dose interleukin 2 treatment for metastatic renal cancer. Thorax. 2005;60:610–1. doi: 10.1136/thx.2004.024018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Oswald-Richter KA, Richmon BW, Braun NA, et al. Reversal of global CD4+ subset dysfunction is associated with spontaneous clinical resolution of pulmonary sarcoidosis. J Immunol. 2013;190:5446–53. doi: 10.4049/jimmunol.1202891. [DOI] [PMC free article] [PubMed] [Google Scholar]