Abstract
Background: Previous studies have demonstrated a relationship between biomass of fungi exposure in the home and the risk of sarcoidosis. β-glucan was present in the bronchial alveolar lavage fluid (BALF) of patients with sarcoidosis. The Kveim-Siltzbach test reagent (KSTR) induces a sarcoidosis specific, granulomatous, cutaneous response and was used to establish the diagnosis. To date, the granuloma-inducing component of KSTR is still unknown. The present study was undertaken to investigate the presence of β-glucan in the lymph nodes of patients with sarcoidosis and to determine the relationship between the amounts of this agent with disease severity and to investigate the presence of β-glucan in KSTR. Materials and methods: Lymph node aspirations were collected by transbronchial needle aspiration (TBNA) in region R4 or 7 from patients with newly diagnosed sarcoidosis. The samples were treated to isolate β-glucan and analyzed using a Limulus-based assay. Cultures of Propionibacterium ac. and Mycobacterium gordonae as well as samples of Kveim-Siltzbach test reagent were analyzed to determine β-glucan content. Results: A significant relationship was observed between the amount of the β-glucan in the lymph nodes and the extent of granuloma formation in the lung parenchyma, and the size of the lymph nodes in the mediastinum (r=0.787, p=0.0001 and r=0.664, p<0.001 respectively, Spearman’s test). The samples of Kveim-Siltzbach test reagent contained high levels of β-glucan. Cultures of Propionibacterium ac. and Mycobacterium gordonae contained β-glucan, the levels of which were lower in the Mycobacterium cultures. Comments: The results support the hypothesis that β-glucan, and thus fungal exposure, are involved in the pathogenesis of sarcoidosis. (Sarcoidosis Vasc Diffuse Lung Dis 2017; 34: 130-135)
Keywords: sarcoidosis, β-glucan, lymph nodes, granuloma, Kveim-Siltzbach test reagent
Introduction
Sarcoidosis is a multisystem inflammatory disorders associated with noncaseating granuloma inflammation at the sites of disease (1). Although a general consensus regarding the identity of the causative agent has not been reached, several studies have suggested that fungi play an important role in the pathogenesis of sarcoidosis. Anti-fungal antibodies have been found in the bronchial alveolar lavage fluid (BALF) of patients with sarcoidosis (2). Additionally, sarcoidosis has been associated with the presence of Propionibacterium acne and non-tuberculosis Mycobacterium, and it has been suggested that non-infectious mechanisms may explain the causative role (3, 4).
Previous studies have reported a relationship between the severity of sarcoidosis, evaluated using an X-ray score, and the presence of β-glucan in BALF (5). Certain pro-inflammatory cytokine levels are usually increased in patients with sarcoidosis; however, one study reported that the amount of IL-10 in the serum was decreased, with high levels of β-glucan in the BALF (6).
Fungi contain β-glucan in their cell walls, similar to some bacteria. There are water-soluble and insoluble forms of β-glucan as well as different types of glucoside linkages, which determine the cell reactivity induced by exposure (7). This polyglucose agent has several effects on the innate immune system and adaptive immune responses (8-10). Particulate β-glucans induce stronger immune responses than soluble β-glucans by clustering of Dectin-1 receptors (10, 11). The effects are mediated by the Dectin-1 receptor on macrophages that particulate β-glucan is capable of stimulating innate and adaptive immune responses via a dectin-1 pathway while soluble β-glucan initiates an inflammatory reaction (10).
Based on the above information, it can be hypothesized that inhaled β-glucan is taken up by macrophages in the airways and transported to regional lymph nodes where it would initially stimulate the production of IL-10 and iNKT cells, which are important constituents of the anti-microbial defense of the body (12). At higher levels of β-glucan, there would be a suppression of IL-10 production and the formation of iNKT cells, resulting in an increased risk of granuloma formation (13).
The present study was undertaken to determine weather β-glucan is present in the lymph nodes of patients with sarcoidosis and weather the levels are related to the severity of the disease. Additionally, weather the levels of β-glucan are present in samples of Kveim-Siltzbach test reagent, which is a preparation of the lymph nodes or the spleen which is used as a diagnostic test for sarcoidosis following its subcutaneous injection, granulomas develop in patients with sarcoidosis (14, 15). The β-glucan levels were also analyzed in cultures of Propionibacterium acne and Mycobacterium gordonae.
Materials and methods
2.1. Subjects
The subjects were recruited from the Department for Respiratory and Allergic Diseases at the University Medical Centre, Ljubljana, Slovenia, from January 2012 to December 2015. The department is one of the national centers for patients with sarcoidosis.
The American Thoracic Society/European Respiratory Society/World Association for Sarcoidosis and Other Granulomatous Disorders (ATS/ERS/WASOG) criteria were used for the diagnosis (1). The routine procedure involved performing a bronchoscopy with 5 to 10 transbronchial biopsies of the lung parenchyma and TBNA of the mediastinal lymph nodes in regions R4 and 7. BALF was collected, and the CD4+/CD8+ ratio was determined. The presence of non-caseating granulomas in the lung was histologically verified. If a biopsy was not considered representative, the patient underwent a surgical pulmonary or lymph node biopsy. The right upper lobe was washed with 20 ml of saline to culture pathogenic fungi and bacteria, including tuberculosis bacillus (TB). The levels of IgA, IgM, and IgG antibodies against Candida spp. and Aspergillus spp. as well as mannan antigen in the blood were determined. Diagnostic BALF and sputum in subjects with sarcoidosis demonstrated an absence of pathogenic fungi and TB. We have isolated 1 CFU of Aspergilus niger and 1 CFUof Aspergillus fumigatus from two patients and from three patients Prevotella spp. Staphylococcus aureus was isolated once from BALF. There was no isolation of Propionibacterium acne from BALF nor of Non-tubeculosis mycobacterium.
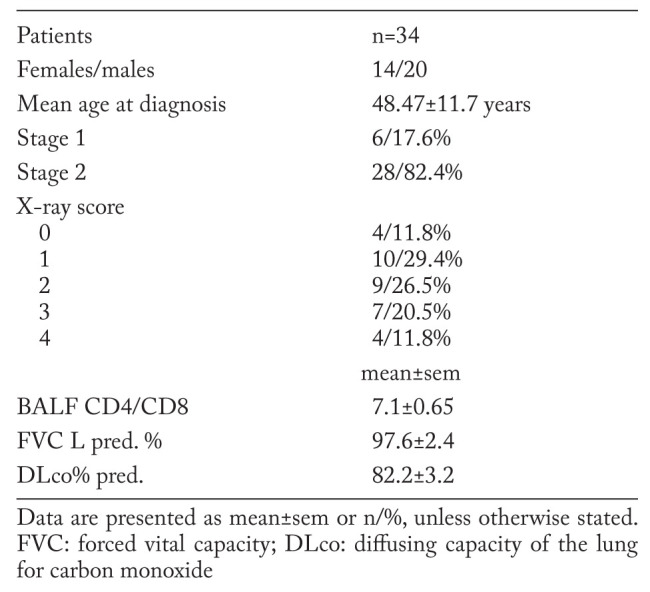
This study comprised newly diagnosed cases of pulmonary sarcoidosis (n=34). All subjects were non-smokers. The study was approved by the Ethical Committee at the University Medical Centre, Ljubljana (198/05/04), and written informed consent was obtained from all subjects. The demographic characteristics are shown in Table 1.
Table 1.
Demographic characteristics of the 34 patients with sarcoidosis
2.2 Pulmonary X-ray scores
The degree of granuloma infiltration in the lung parenchyma was used to evaluate the severity of the disease. Chest radiograms were obtained and a grading score for the presence of granuloma infiltration was used as previously described (2, 5). The chest radiograms were interpreted by two experienced radiologists who were unaware of the status of the patient. They assessed the granulomas using a numerical score (0-4) to describe the size and extension of the infiltrates (X-ray score: 0=normal, 1=approximately 25% involvement of the lung field, 2=up to 50% involvement of the lung field, 3=up to 75% involvement of the lung field, and 4=100% involvement of the lung field). Repeated evaluations by different radiologists showed only minor deviations in the score classification.
The size of the mediastinal lymph nodes on CT scan was measured and evaluated using a scoring system. No visible lymph nodes = 0, 1= 1<2 cm, 2=2-3 cm, 3>3 cm.
The size of the mediastinal lymph nodes on X-ray images was evaluated using a scoring system (zero=no visible lymph nodes, 1= 1<2 cm, 2=2-3 cm, 3>3 cm).
2.3 Lymph node aspiration
The procedures were performed as part of routine clinical care. Patients were deeply sedated for all procedures. Flexible fiberoptic bronchoscopy was performed through the orotracheal tube using an Olympus BF-TE2 bronchofiberscope (Olympus, Japan) with a 2.8 mm working channel. TBNA was performed using a 21-gauge, 13-mm-long cytology needle (Olympus, Japan). The TBNA procedure included positioning the bronchoscope with respect to the target site and visualization of the needle sheath, followed by needle exit and puncture of the tracheobronchial wall. TBNA was performed in the mediastinal region of lymph nodes R4 and 7. Each aspiration consisted of approximately 1-2 mm3 of lymph node tissue. After the successful puncture of the tracheobronchial wall, suction was manually applied to the TBNA needle using a 20 cc syringe with needle agitation within the lymph node. Three samples were obtained from each lymph node station. Direct smears were obtained from the first sample for Papanicolaou staining, and air-dried smears were obtained for May-Grunewald-Giemsa staining. Next, the samples were assessed by an experienced cytopathologist. The adequacy of sampling was defined by the presence of lymphocytes in the smear. The second sample was used for the analysis of β-glucan and placed into 1 ml of sterile saline solution. The third sample was added to 4 ml of saline solution to culture pathogenic fungi and bacteria.
2.4 Cultures of Propionibacterium and Mycobacterium
Cultures were prepared from Mycobacterium gordonae, and three different CFU suspensions were prepared. For Propionibacterium, three different dilutions were prepared; however, CFU determinations were not made. One millilitre of each preparation was placed in 1 ml of saline and analysed to determine β-glucan content.
2.5. Analysis of ß-glucan
The saline solutions of lymph node aspiration by TBNA, Kveim-Siltzbach test reagent and bacteria samples, Propionebacterium ac. and non-tuberculosis Mycobacterium gordonae, were added to 4 ml of a solution containing 0.15 mol/l KOH and 0.3 mol/l KCl and incubated at 37°C for 10 minutes. It was necessary to first solubilize β-glucan for a commercially available method based on the reactivity of Limulus extract was used for the analysis of β-glucan (Endosafe PTS, Charles River, Charleston, USA). The lymph node preparations were diluted in a protein-blocking buffer and further diluted in endotoxin-free water. Then, 25 μl of the preparations were added to each of the four wells of a plate prepared with a Limulus reagent specific to β-glucan and read in an automatic analyzer. The results are expressed as ng/ml of lymph node suspension. The lower limit for detection was 1 ng/ml. Samples yielding a readout value of <100 ng/ml were assigned the uniform value of 90 to save on reagents and recorded as not containing β-glucan. Analyses were performed without knowledge of the results of other analyses.
2.6 Kveim-Siltzbach test reagent
Samples of Kveim-Siltzbach test reagent were suspended in 0.15 mol/L KOH and 0.3 mol/L KCL and incubated at 37°C for 10 minutes to solubilize β-glucan and analyzed as described above. The Kveim-Siltzbach test reagent preparation was a gift from Adam Morgenthau, MD; Director, Sarcoidosis Program; Assistant Professor of Medicine; Icahn School of Medicine at Mount Sinai Hospital, New York, USA.
2.7. Statistical analysis
The data were analyzed using SPSS (version 21, Inc., Armonk, NY, USA). Group data were reported as mean and standard error of the mean and the relationship between the X-ray scores and the β-glucan content in the lymph nodes was analyzed using Spearman’s test. A P-value of ≤0.05 was considered statistically significant.
Results
The levels of β-glucan in the lymph node samples ranged from 90 to 3020 pg/ml, with a mean of 705 pg/ml and an SEM of 125 pg/ml.
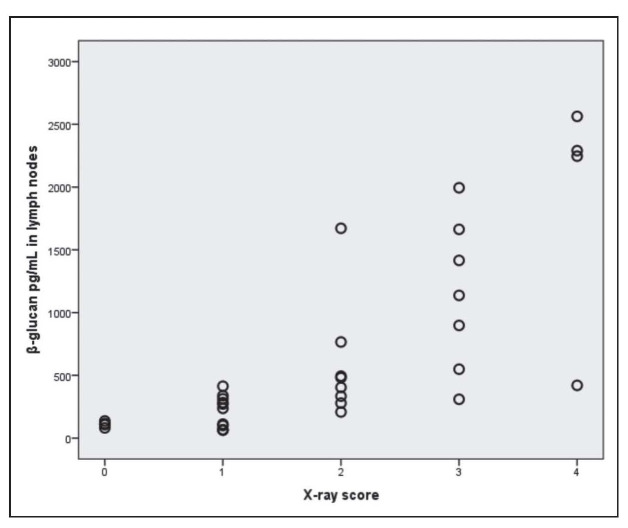
The relationship between β-glucan in the lymph nodes in mediastinum and the X-ray scores is illustrated in Figure 1.
Fig. 1.
Relationship between β-glucan in the lymph nodes in mediastinum and X-ray scores
The figure illustrates a significant relationship between the amount of β-glucan in the lymph nodes in mediastinum and lymph node size andX-ray scores (r=0.801, p<0.001, Spearman’s test).
Points are slightly randomly perturbed in order to see tied points in figure.
Chest radiograms were taken and evaluated with a numerical score (0-4) regarding the size and extent of the infiltrates (x-ray score: 0 normal, 1 approximately 25% of the lung field involved, 2 up to 50%, 3 up to 75%, and 4 virtually the whole lung field involved).
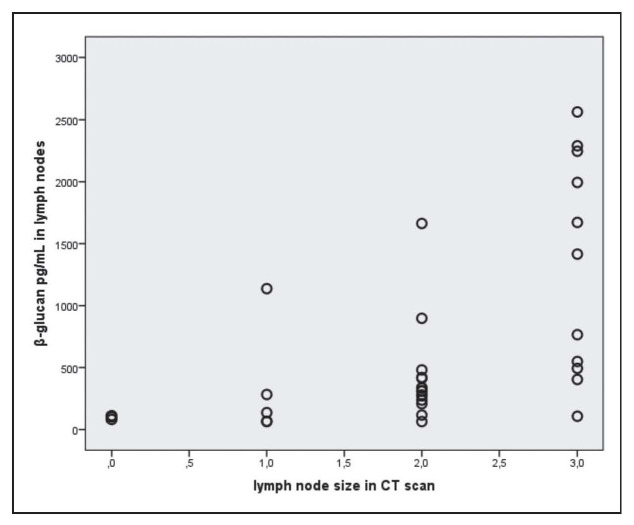
The relationship between β-glucan in the lymph nodes in mediastinum and the lymph node size in CT scan is illustrated in Figure 2.
Fig. 2.
Relationship between β-glucan in the lymph nodes in mediastinum and lymph node size in CT scan
The figure illustrates a significant relationship between the amount of β-glucan in the lymph nodes and lymph node size in CT scan (r=0.664, p=0.0001, Spearman’s test). The size of the mediastinal lymph nodes on CT scan was evaluated using a scoring system (lymph node size: zero=no visible lymph nodes, 1<2 cm, 2=2-3 cm, 3>3 cm). Points are slightly randomly perturbed in order to see tied points in figure.
The samples of Kveim-Siltzbach test reagent were analyzed to determine the water non-soluble β-glucan content. The amounts of β-glucan in the Kveim-Siltzbach test reagent were similar to those found in the lymph nodes and are illustrated in Table 2.
Table 2.
The amount of β-glucan in the Kveim-Siltzbach test reagent (pg/ml preparation)
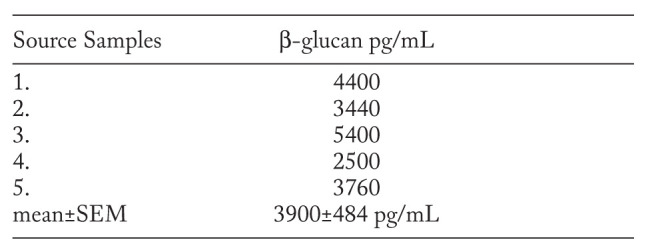
The results showed that all samples contained β-glucan. The amount of β-glucan in KSTR ranged from 2500 to 5400 pg/mL, mean 3900 pg/mL. The values for the Kveim-Siltzbach test reagent were similar to those found in the lymph nodes.
The amount of β-glucan in cultures of non-tuberculosis Mycobacterium gordonae and Propionibacterium acne in different CFU 106, in CFU 107 and in CFU 108 were 90 pg/ml, 194 pg/ml, 432 pg/ml.
The amount of β-glucan in cultures of Propionibacterium in different concentration I, II and III. There were 3020 pg/ml, 1150 pg/ml and 334 pg/ml. The results indicate that β-glucan was present in both bacterial preparations. The values were considerably higher in the Propionibacterium ac. preparation.
Discussion
The main finding in this study was the identification of a relationship between the amount of β-glucan in lymph nodes and the severity of sarcoidosis, expressed as the degree of granuloma infiltration in the lung parenchyma and mediastinal lymph node size.
A methodological shortcoming of the study was the variation in lymph node sampling. Due to technical reasons, the amount of lymph node mass that was extracted may have varied, although a standardized protocol was applied. The potential variations in cell mass could be one reason for the variation of β-glucan levels in lymph nodes with the same X-ray score (Figure 1).
Other studies of the immune response after exposure to β-glucan support the relationship between exposure to β-glucan and granuloma formation. Chronic inhalation in guinea pigs was shown to suppress antibody responses induced by aerosol exposure to the antigen ovalbumin in combination with endotoxin (16). In a murine model, Dectin-1, which is expressed on all monocytes and macrophages, was demonstrated to be the major receptor for β-glucan (17). This receptor triggers IL-12 production, which induces iNKT cells to secrete interferon-γ. Data from one study demonstrated that this response was blocked by higher levels of β-glucan exposure, resulting in increased disease severity (6). In another study, the absence of iNKT cells and IL-10 increased the risk of granuloma formation (18).
The lymph nodes were prepared to solubilize insoluble fraction of β-glucan, as described in Methods. This is important as the immunomodulatory effects of β-glucan are mediated by the water insoluble fraction. An analysis of the water-soluble fraction alone will thus yield a severe underestimation of the total amount present in the homes of patients with sarcoidosis.
The validity of the β-glucan model for the pathogenesis of sarcoidosis is further supported by the Kveim-Siltzbach test reagent data. A preparation that has been shown to induce granulomas in patients with sarcoidosis. KSTR was shown to contain β-glucan, which has been associated with an increased risk of granuloma formation. An interesting result was obtained in a study in which BALF was intracutaneously injected, after which granulomas developed (19). According to the data presented in this study, this result was most likely caused by the β-glucan in the BALF. Additionally, microbial agents other than fungi have been associated with sarcoidosis. Propionibacterium has been found in the lungs of patients with sarcoidosis (20, 21). The presence of a sarcoid reaction in these patients can be explained by the presence of the β-glucan in Propionibacterium, as reported in this study.
The results of this study confirm our previous findings of the influence of the fungal biomass in domestic environment as well as the role of β-glucan as a potential ethiopatogenetic factor in sarcoidosis. We have found the patients with chronic sarcoidosis to have higher concentration of β-glucan in their homes (22) and have established “in vitro” that only the water insoluble β-glucan exerts a significant influence on some of certain inflammatory cytokines (23). It is known that Propionebacterium ac. is present in sarcoidosis patients in higher quantities than in other subjects. De-vitalised Propionebacterium ac. injected in PMNC in medium “in vitro” caused an increase of certain inflammatory cytokines (24, 25) that is similar to what we have achieved with the water insoluble β-glucan “in vitro” in PMNC from sarcoidosis patients (23).
This is the first time that a specific agent, β-glucan, is shown as a possible ethiologic factor in sarcoidosis, and is also associated with the level of disease activity.
Conclusion
In summary, we demonstrate that β-glucan is present in the lymph nodes of patients with sarcoidosis and that there is a relationship between the amounts of this agent and the disease severity. The Kveim-Siltzbach test reagent, shown to induce granulomas in patients with sarcoidosis, is shown to contain β-glucan. The data from the present and previous studies collectively suggest that a high level of exposure to β-glucan perturbs normal immune defense mechanisms and increases the risk of granuloma formation. As a result, β-glucan should be recognized as a potential causative agent for sarcoidosis. Other as yet undefined microbial-derived agents that are recognized by microbial pattern recognition receptors expressed on cells of the innate immune system may have similar effects.
Acknowledgements
The Kveim-Siltzbach test reagent preparation was a gift from Adam Morgenthau, MD; Director, Sarcoidosis Program; Assistant Professor of Medicine; Icahn School of Medicine at Mount Sinai Hospital, New York, USA.
References
- 1.Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160(2):736–55. doi: 10.1164/ajrccm.160.2.ats4-99. [DOI] [PubMed] [Google Scholar]
- 2.Suchankova M, Paulovicova E, Paulovicova L, et al. Increased anti-fungal antibodies in bronchoalveolar lavage fluid and serum in pulmonary sarcoidosis. Scand J Immunol. 2015;81:259–64. doi: 10.1111/sji.12273. [DOI] [PubMed] [Google Scholar]
- 3.Zhou Y, Hu Y, Li H. Role of Proprionibacterium acnes in sarcoidosis – a meta-analysis. Sarc Vasc Dioff Lung Dis. 2013;30(4):262–7. [PubMed] [Google Scholar]
- 4.Mortaz E, Adcock IM, Barnes PJ. Sarcoidosis: Role of non-tuberculosis mycobacteria and Mycobacterium tuberculosis. Int J Mycobacteriol. 2014;3(4):225–9. doi: 10.1016/j.ijmyco.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 5.Terčelj M, Stopinšek S, Ihan A, Salobir B, Simčič S, Rylander R. Fungal exposure and low level of IL-10 in patients with sarcoidosis. Pulm Med 2014. 2014;164565 doi: 10.1155/2014/164565. doi: 10.1155/2014/164565. Epub 2014 Aug 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Terčelj M, Salobir B, Zupancic M, Wraber B, Rylander R. Inflammatory markers and pulmonary granuloma infiltration in sarcoidosis. Respirology. 2014;19:225–30. doi: 10.1111/resp.12199. [DOI] [PubMed] [Google Scholar]
- 7.Rylander R. Human exposures to β-glucan in the environment. Biology and Chemistry of Beta Glucan. In: Vetvicka V, Novak M, editors. Vol. 2. New York: Nova Scientific Publishers; 2013. pp. 131–53. [Google Scholar]
- 8.Goodridge HS, Wolf AJ, Underhill DM. β-Glucan recognition by the innate immune system. Immunol Rev. 2009;230:38–50. doi: 10.1111/j.1600-065X.2009.00793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noss I, Doekes G, Thorne PS, et al. Comparison of the potency of a variety of β-glucans to induce cytokine production in human whole blood. Innate Immun. 2013;19(1):10–9. doi: 10.1177/1753425912447129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qi C, Cai Y, Gunn L, et al. Differential pathways regulating innate and adaptive antitumor immune responses by particulate and soluble yeast-derived β-glucans. Blood. 2011;117(25):6825–36. doi: 10.1182/blood-2011-02-339812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sahasrabudhe NM, Dokter-Fokkens J, de Vos P. Particulate β-glucans synergistically activate TLR4 and Dectin-1 in human dendritic cells. Mol Nutr Food Res. 2016 Jun 30 doi: 10.1002/mnfr.201600356. doi: 10.1002/mnfr.201600356. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Cohen NR, Tatituri RVV, Rivera A, et al. Innate recognition of cell wall β-glucans drives invariant natural killer T cell responses against fungi. Cell Host and Microbe. 2011;10(5):437–50. doi: 10.1016/j.chom.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crawshaw A, Kendrick YR, McMichael AJ. Ho L-P. Abnormalities in iNKT cells are associated with impaired ability of monocytes to produce IL-10 and supress T-cell proliferation in sarcoidosis. Eur J Immunol. 2014;44(7):2165–74. doi: 10.1002/eji.201344284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nelson CT. The Kveim reaction in sarcoidosis. J Chron Dis. 1957;6:158–77. doi: 10.1016/0021-9681(57)90050-4. [DOI] [PubMed] [Google Scholar]
- 15.Kataria YP, Holter JF. Sarcoidosis: a model of granulomatous inflammation of unknown etiology associated with a hyperactive immune system. Methods. 1996;9:268–94. doi: 10.1006/meth.1996.0033. [DOI] [PubMed] [Google Scholar]
- 16.Rylander R, Holt PG. (1→3)-b-D-glucan and endotoxin modulate immune response to inhaled allergen. Mediat Infl. 1998;7:105–10. doi: 10.1080/09629359891252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Willment JA, Marshall ASJ, Reid DM, et al. The human β-glucan receptor is widely expressed and functionally equivalent to murine Dectin-1 on primary cells. Eur J Immunol. 2005;35:1539–47. doi: 10.1002/eji.200425725. [DOI] [PubMed] [Google Scholar]
- 18.Synder-Cappione JE, Nixon DF, Chi JC, et al. Evidence of Invariant natural killer T (iNKT) cell exhaustion in sarcoidosis. Eur J Immunol. 2013;43:2194–205. doi: 10.1002/eji.201243185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holter JF, Park HK, Sioerdsma KW, Kataria YP. Nonviable, autologous bronchoalveolar lavage cell preparations induce intradermal epitheloid cell granulomas in sarcoidosis patients. Am Rev Resp Dis. 1992;145:864–71. doi: 10.1164/ajrccm/145.4_Pt_1.864. [DOI] [PubMed] [Google Scholar]
- 20.Eishi Y. Etiologic aspect of sarcoidosis as an allergic endogenous infection caused byProprionibacterium acnes. Biomed Res Int 2013. 2013;935289 doi: 10.1155/2013/935289. doi: 10.1155/2013/935289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deutsch SM, Parayre S, Bouchoux A, et al. Contribution of surface β-glucan polysaccharide to physicochemical and immunomodulatory properties of Proprionibacterium freudenerichii. Appl Env Microbiol. 2012;78:1765–75. doi: 10.1128/AEM.07027-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Terčelj M, Salobir B, Harlander M, Rylander R. Fungal exposure in homes of patients with sarcoidosis - an environmental exposure study. Environ Health. 2011 Jan 20;10(1):8. doi: 10.1186/1476-069X-10-8. doi: 10.1186/1476-069X-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Terčelj M, Stopinšek S, Ihan A, et al. In vitro and in vivo reactivity to fungal cell wall agents in sarcoidosis. Clin Exp Immunol. 2011;166(1):87–93. doi: 10.1111/j.1365-2249.2011.04456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schupp JC, Tchaptchet S, Lützen N. e al. Immune response to Propionibacterium acnes in patients with sarcoidosis-in vivo and in vitro. BMC Pulm Med. 2015;15:75. doi: 10.1186/s12890-015-0070-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaede KI, Kataria YP, Mamat U, Müller-Quernheim J. Analysis of differentially regulated mRNAs in monocytic cells induced by in vitro stimulation with Kveim-Siltzbach test reagent. Exp Lung Res. 2004;30(3):181–92. doi: 10.1080/01902140490276267. [DOI] [PubMed] [Google Scholar]