Abstract
Background: Idiopathic interstitial pneumonia (IIP) is associated with increased risk of acute exacerbation after lung surgery, which has a poor prognosis and can be fatal. Although some studies have investigated acute exacerbation of IIP after lung surgery, the incidence and risks of acute exacerbation of IIP after nonpulmonary surgery have not been reported. The aim of present study to evaluate the characteristics and risk factors for acute exacerbation of IIP after nonpulmonary surgery. Methods: We retrospectively reviewed the clinical characteristics of 2908 consecutive patients (1620 men, 1288 women; mean age, 61.7) who underwent nonpulmonary surgery under general anesthesia between April 2008 to April 2013. Using preoperative chest computed tomography images, we identified IIP cases and compared preoperative characteristics, laboratory findings, and anesthesia conditions among patients who did and did not develop AE. Results: We extracted 103 IIP patients who underwent nonpulmonary surgery; postoperative acute exacerbation of IIP developed in 8 (7.8%). Univariate analysis identified several risk factors, namely, emergency surgery, preoperative prednisolone use, high serum C-reactive protein, lactate dehydrogenase, white blood cell count, low serum albumin and propofol use during anesthesia. Conclusion: The results suggest that the incidences of postoperative acute exacerbation of IIP are similar after nonpulmonary and pulmonary surgery. In addition, propofol use during anesthesia is a possible risk factor for acute exacerbation of IIP after nonpulmonary surgery. (Sarcoidosis Vasc Diffuse Lung Dis 2017; 34: 156-164)
Keywords: acute exacerbation, idiopathic interstitial pneumonia, risk factors, propofol
Introduction
Acute exacerbation of idiopathic interstitial pneumonia (IIP) is a serious complication and is associated with high mortality (1-6). Acute exacerbation of IIP after surgery for lung cancer found that the existence of IIP is associated with increased risk of acute exacerbation, a sometimes fatal postoperative complication with a poor prognosis. Some reports indicate that acute exacerbation of IIP is often triggered by thoracotomy (7, 8). The incidence of acute exacerbation of IIP after lung surgery is 5.8% to 9.3% (9-12). Putative predictive factors for acute exacerbation of IIP after lung resection include 1) type of surgical procedure, 2) male sex, 3) history of exacerbation, 4) preoperative steroid use, 5) elevation of serum KL-6 level, 6) usual interstitial pneumonia (UIP) appearance on computed tomography (CT) scanning, and 7) diminished percentage predicted vital capacity. Although acute exacerbation of IIP is often fatal, the incidence and risk factors for acute exacerbation of IIP after surgery other than lung surgery have not been reported. The present study investigated the characteristics and risk factors for acute exacerbation of IIP after surgery other than lung surgery.
Methods
Study design
This single-center retrospective cohort study reviewed clinical and laboratory data from patient medical records. We analyzed data from 2908 consecutive patients (1620 men, 1288 women; mean age, 61.7 yrs) who underwent nonpulmonary surgery under general anesthesia at Toho University Omori Medical Center during the period from April 2008 to April 2014. Case histories, anesthesiology records, surgical records, and CT images were analyzed. Anesthesiology and surgical records were reviewed to determine surgical method, anesthesia time, oxygen concentration, propofol use, and amounts of blood transfused, bleeding, fluid infused, and urine excreted during mechanical ventilation. Preoperative chest CT images were used to identify IIP cases. Acute exacerbation of IIP after nonpulmonary surgery was defined using the criteria proposed by Sato et al (12), 1) onset within 30 days after surgery (exacerbations on day 31 or later were defined as chronic exacerbations), 2) worsening dyspnea, 3) an increase in the interstitial shadow on chest CT scan, 4) a decrease of greater than 10 mm Hg in arterial oxygen tension under similar conditions, 5) absence of pulmonary infection, and 6) exclusion of alternative causes, such as cardiac failure, pulmonary embolism, or other identifiable causes of lung injury. Using these criteria, we identified patients with postoperative acute exacerbation of IIP and compared preoperative patient characteristics, laboratory findings, and anesthesia conditions between those who developed acute exacerbation and those who did not. Univariate and multivariate Cox analyses were used to examine the characteristics and risk factors for acute exacerbation of IIP after nonpulmonary surgery.
Diagnostic criteria for IIP
A diagnosis of IIP was made using a combination of clinical and radiological findings, as proposed by the American Thoracic Society (ATS)/European Respiratory Society (ERS)/Japanese Respiratory Society (JRS)/Asociación Latinoamericana de Tórax (ALAT) guidelines. Chest CT images were classified based on the ATS/ERS/JRS/ALAT guidelines(13). Cases were classified into 2 groups according to radiological findings on chest CT, as follows: cases with the UIP pattern or possible UIP pattern were classified as the UIP group, and those with findings inconsistent with the UIP pattern were classified as the non-UIP group.
Chest CT scanning
Chest CT scanning was performed before (within 1 month) and after surgery, using the SOMATOM Definition AS, Flash, and Edge devices (Siemens Co., Ltd., Munich, Germany). After development of acute exacerbation of IIP, the entire lung was routinely scanned, in 5.0-mm-thick sections. Additional thin-section CT was used to obtain images with a thickness of 1.0–2.0 mm. A limitation of the present CT image analysis is that thin-section CT images were reconstructed with a fixed window setting. The CT scans were independently reviewed by one thoracic radiologist and two pulmonologists, all of whom were blinded to the identity and clinical, physiological, and pathological characteristics of the patients.
Pulmonary function tests
Lung volumes and forced expiratory volume in 1 second (FEV1) were measured by standard methods using the Chestac-8800 and Chestac-8900 devices (Chest Co., Ltd., Tokyo, Japan) and are expressed as a percentage of the predicted value. These measurements were conducted before (within 1 month of) surgery but could not be repeated at or after acute exacerbation of IIP, due to the poor general condition of the patients. Arterial blood gas analysis was done using the ABL510 and ABL800 FLEX devices (Radiometer Co., Ltd., Copenhagen, Denmark) before (within 1 month), during, and after surgery, in all patients.
Statistical analysis
All values are expressed as mean ± SD, and differences between groups were analyzed using the χ2 test and Mann-Whitney nonparametric U test for two independent samples. All p values are two-sided and were considered statistically significant when less than 0.05. All statistical analyses were performed using SPSS version 11.0 (SPSS Inc, Chicago, IL, USA).
Ethics
This study was approved by the Institutional Review Board of Toho University Omori Medical Center (project approval number 25-65). All medical records were reviewed with the approval of the Institutional Review Board.
Results
Patients studied
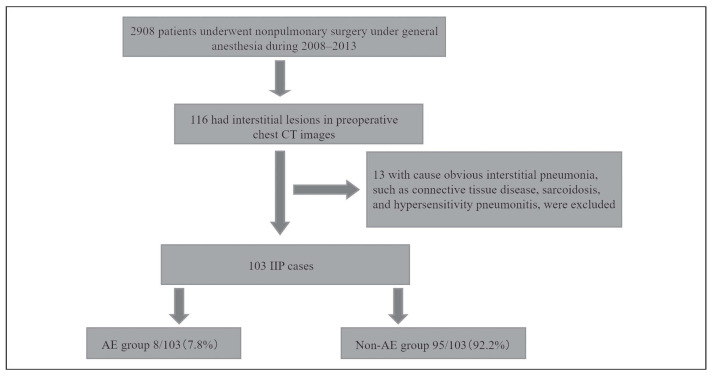
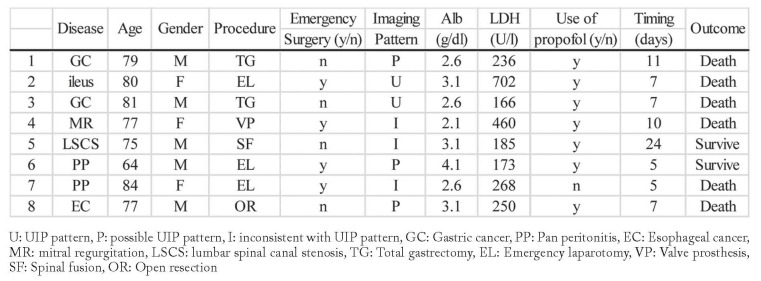
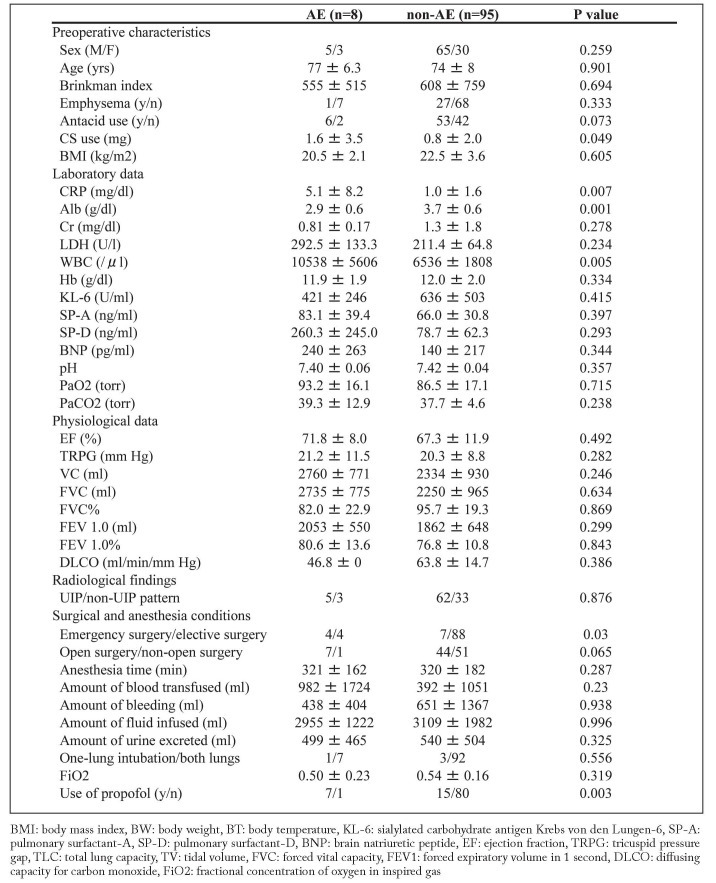
We identified 116 patients with preoperative interstitial lesions in chest CT scans. We excluded 13 patients with interstitial pneumoniae other than IIP, namely, 11 with connective tissue disease, 1 with sarcoidosis, and 1 with chronic hypersensitivity pneumonitis (Figure 1). Acute exacerbation of IIP developed in 8 of the 103 patients (7.8%) who underwent nonpulmonary surgery (Table 1). We compared preoperative patient characteristics, laboratory findings, and anesthesia conditions between those who did and did not develop acute exacerbation (Table 2).
Fig. 1.
Study design. We analyzed data from 2908 consecutive patients who underwent nonpulmonary surgery under general anesthesia. We identified 137 patients with preoperative interstitial lesions in chest CT scans. We excluded 34 patients with interstitial pneumoniae other than IIP. We compared preoperative patient characteristics, laboratory findings, and anesthesia conditions between AE group and Non-AE group
Table 1.
Patient’ characteristics who developed acute exacerbation of IIP
Table 2.
Clinical characteristics in patients who develop AE (AE) and did not develop AE (non-AE)
Preoperative patient’ characteristics
Prednisolone dosage was significantly higher in the acute exacerbation (AE) group than in the non-AE group (1.6 ± 3.5 mg vs. 0.8 ± 2.0 mg, respectively). Although the proportion of antacid users was higher in the AE group than in the non-AE group (75% vs. 56%), the difference was not significant. There were no significant differences between the groups in the other preoperative characteristics, including sex, age, and smoking index.
Preoperative Laboratory findings
In the AE and non-AE groups, serum C-reactive protein (CRP) was 5.1 ± 8.2 mg/dl versus 1.0 ± 1.6 mg/dl (P = 0.007), white blood cell count (WBC) count was 10,538 ± 5606/μl versus 6536 ± 1808/μl (P = 0.005), and albumin (Alb) was 2.9 ± 0.6 g/dl versus 3.7 ± 0.6 g/dl (P = 0.001), respectively. Serum levels of markers of pneumocyte damage (KL-6, SP-A [surfactant-A], and SP-D [surfactant-D]) did not significantly differ between the groups.
Preoperative Physiological findings
There was no significant difference between the AE and non-AE groups in baseline pulmonary function tests, including forced vital capacity (FVC), %predicted FVC, %predicted diffusing capacity (DLCO), and FEV1%. Estimated pulmonary artery pressure did not significantly differ between the groups.
Preoperative Radiological findings
In the AE group, 5 patients had a UIP pattern and 3 had a non-UIP pattern on high-resolution CT, as compared with 62 and 33 patients, respectively, in the non-AE group. The difference was not significant.
Surgical and anesthesia conditions
Emergency surgery was required for 4 of 8 patients (50%) in the AE group and for 7 of 85 patients (8%) in the non-AE group. The rate of emergency surgery was significantly higher in the AE group (P = 0.03). Most patients were anesthetized with oxygen, sevoflurane, and propofol. The proportion of patients receiving propofol was higher in the AE group (88%) than in the non-AE group (16%) (P = 0.003). There were no significant differences between groups in fluid balance or FiO2.
Risk factor analysis of acute exacerbation of IIP
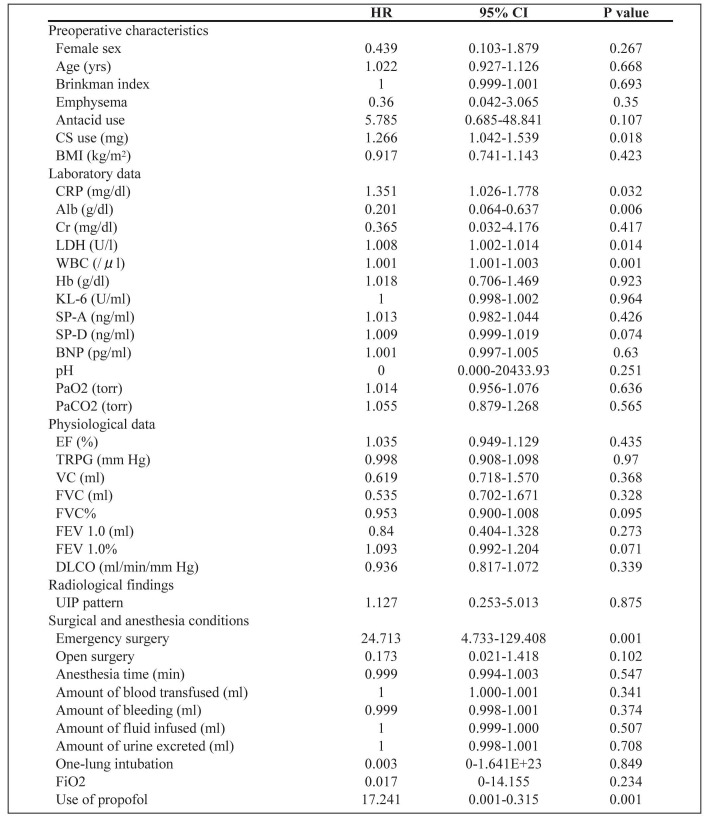
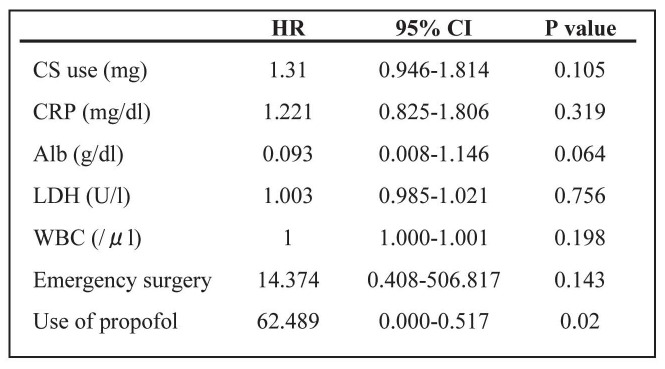
We used univariate and multivariate Cox analyses to examine the characteristics of and risk factors for acute exacerbation of IIP after nonpulmonary surgery. The univariate Cox proportional hazards regression model showed that the predictive factors were emergency surgery (HR, 24.713; P = 0.001), preoperative corticosteroid (CS) use (HR, 1.266; P = 0.018), increased serum CRP (HR, 1.351; P = 0.032), lactate dehydrogenase (LDH) (HR, 1.008; P = 0.014) and WBC count (HR, 1.001; P = 0.001), low serum Alb (HR, 0.201; P = 0.006), and use of propofol for general anesthesia (HR, 17.241; P = 0.001) (Table 3). In multivariate analysis only use of propofol was indentified as a possible risk factor (HR, 62.489; P = 0.020) (Table 4).
Table 3.
Results of univariate Cox analysis of acute exacerbation of IIP
Table 4.
Results of multivariate Cox analysis of potential risk factors for acute exacerbation of IIP
Discussion
Acute exacerbation of IIP is a rapidly progressive and sometimes fatal postoperative complication. Although there are some reports of acute exacerbation of IIP after lung surgery under general anesthesia (12) and a case series that included nonpulmonary surgery (14), there are no published studies of acute exacerbation of IIP after nonpulmonary surgery. We found that preoperative CS use was an independent risk factor for acute postoperative exacerbation of IIP (12). Although steroids are used as a treatment for IIP, high-dose CS was a risk factor in the present study. Although we were unable to determine baseline IIP severity, due to the lack of data for some patients, IIP severity might be a prognostic factor, as it is possible that more-severe cases were treated with CS. In addition, immunosuppression caused by CS may have resulted in infection and thus AE development. Elevated serum CRP and LDH are significant risk factors for acute exacerbation of IIP after lung surgery (1, 3). In the present study, elevated CRP, LDH, and WBC count and low Alb were identified as risk factors for acute exacerbation of IIP after nonpulmonary surgery. These markers reflect
systemic inflammation and the general condition of patients before surgery and are directly related to possible development of postoperative acute exacerbation of IIP. Serum levels of markers of damaged pneumocytes (KL-6, SP-A, and SP-D) were elevated in patients who developed acute exacerbation. Serum KL-6, SP-A, and SP-D are believed to reflect the number of regenerating type II epithelial cells in patients with IIP, as well as IIP disease activity and prognosis (15, 16), and were identified as independent risk factors for acute exacerbation of IIP after lung surgery (8-12). In the present study, these markers were not independent risk factors. However, because the present study investigated nonpulmonary surgery, some patients had missing data for these markers, which might have affected the results. Low %FVC and DLCO are reliable indicators of restrictive ventilator impairment and might thus be risk factors for acute exacerbation of IIP after lung surgery (17, 18). In contrast to the findings of a previous study, low %predicted FVC and DLCO were not independent risk factors for acute exacerbation of IIP after nonpulmonary surgery in the present study.
An UIP pattern on chest CT was found to be a risk factor for acute exacerbation of IIP after lung surgery (12, 19). However, in the present study there was no association between imaging pattern and risk of postoperative acute exacerbation of IIP, perhaps because of the small number of AE cases. Patients with interstitial lesions, regardless of imaging pattern, should undergo careful preoperative assessment. These results thought to be caused that patients’ general condition and the state of surgical disease are have an effect more than the state of the IIP other than lung surgery as described above. Univariate Cox analysis showed that emergency surgery is a risk factor for acute exacerbation of IIP after nonpulmonary surgery. This is the first study to identify emergency surgery as a risk factor for postoperative acute exacerbation of IIP. Patients requiring emergency surgery often have severe disease, which more often require emergency surgery. Worse general health and the possibility of cytokine storm are risk factors for acute exacerbation of IIP after nonpulmonary surgery. High surgical stress could lead to postoperative acute exacerbation of IIP. The possibility of acute exacerbation of IIP should be considered in patients with interstitial lung disease on preoperative chest CT, especially those undergoing emergency surgery. High oxygen concentration was reported to cause lung damage and to lead to acute exacerbation of IIP after lung surgery(20). The toxic effects of oxygen on the lung have been previously reported, and the results suggest that intraoperative FiO2 should be minimized (21). However, some studies found only a weak correlation between FiO2 and lung damage(22). In the present study, we found no correlation between FiO2 and risk of postoperative acute exacerbation, which suggests that, although a high oxygen concentration can cause lung damage, a short exposure time – as in the present surgery – may not be a concern. A previous study compared the incidence of lung injury in patients receiving inhaled anesthetics (sevoflurane, desflurane) or intravenous anesthetics (propofol) for anesthesia maintenance. The effects of propofol on the lung are controversial. Propofol is thought to protect the lung by inhibiting free radicals. However, propofol may trigger opening of mitochondrial permeability transition pores (mPTP) through negative feedback with glycogen synthase kinase 3 (GSK-3), and could have a role in apoptosis (23-25). Our analysis showed that propofol was a risk factor for acute exacerbation of IIP. Desflurane and sevoflurane were reported to exert cardioprotective effects by suppressing mPTP opening, which decreased morbidity and mortality (26). A previous report found that, in patients undergoing one-lung ventilation, inhaled anesthetics were likely to have an attenuated inflammatory reaction at the organ level in the lung, due to immunoregulatory activity (27). We believe the present results are due to the cytoprotective effect of inhaled anesthetics rather than to any deleterious effect of propofol. It may be that inhaled anesthetics protect against ischemia-reperfusion injury, which thus suppresses development of acute exacerbation of IIP.
Limitations
This study has several limitations that should be considered when interpreting the results. First, this study was retrospective and undertaken at a single center, and it was difficult to determine the cause of acute exacerbation of IIP after nonpulmonary surgery. Second, although intraoperative FiO2 had adopted that longest period administered during surgery, it is difficult to say it accurate because anesthetic machine with semiclosed circle was used.
Conclusions
This is the first retrospective study of acute exacerbation of IIP after nonpulmonary surgery. The results suggest that the incidences of postoperative acute exacerbation of IIP are similar after nonpulmonary and pulmonary surgery. In addition, propofol use during anesthesia is a possible risk factor for acute exacerbation of IIP after nonpulmonary surgery. A future multicenter study should attempt to identify additional preoperative risk factors for acute exacerbation of IIP after nonpulmonary surgery.
Acknowledgments
This study is partially supported by the Practical Research Project for Rare Intractable Diseases from Japan Agency for Medical Research and development, AMED, and partially supported by a grant from the Ministry of Health, Labour and Welfare of Japan awarded to the Study Group on Diffuse Pulmonary Disorders, Scientific Research/Research on intractable diseases.
References
- 1.Simon-Blancal V, Freynet O, Nunes H, et al. Acute exacerbation of idiopathic pulmonary fibrosis: outcome and prognostic factors. Respiration. 2012;83(1):28–35. doi: 10.1159/000329891. [DOI] [PubMed] [Google Scholar]
- 2.Kondoh Y, Taniguchi H, Katsuta T, et al. Risk factors of acute exacerbation of idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis. 2010;27(2):103–10. [PubMed] [Google Scholar]
- 3.Song JW, Hong SB, Lim CM, Koh Y, Kim DS. Acute exacerbation of idiopathic pulmonary fibrosis:incidence, risk factors and outcome. Eur Respir J. 2011;37(2):356–63. doi: 10.1183/09031936.00159709. [DOI] [PubMed] [Google Scholar]
- 4.Usui Y, Kaga A, Sakai F, et al. A cohort study of mortality predictors in patients with acute exacerbation of chronic fibrosing interstitial pneumonia. BMJ open. 2013;31(3) doi: 10.1136/bmjopen-2013-002971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park IN, Kim DS, Shim TS, et al. Acute exacerbation of interstitial pneumonia other than idiopathic pulmonary fibrosis. Chest. 2007;132(1):214–20. doi: 10.1378/chest.07-0323. [DOI] [PubMed] [Google Scholar]
- 6.Silva CI, Müller NL, Fujimoto K, et al. Acute exacerbation of chronic interstitial pneumonia: high-resolution computed tomography and pathologic findings. J Thorac Imaging. 2007;22(3):221–9. doi: 10.1097/01.rti.0000213588.52343.13. [DOI] [PubMed] [Google Scholar]
- 7.Kumar P, Goldstraw P, Yamada K, et al. Pulmonary fibrosis and lung cancer: risk and benefit analysis of pulmonary resction. J Thorac Cardiovasc Surg. 2003;125(6):1321–7. doi: 10.1016/s0022-5223(03)00028-x. [DOI] [PubMed] [Google Scholar]
- 8.Chida M, Kobayashi S, Karube Y, et al. Incidence of acute exacerbation of interstitial pneumonia in operated lung cancer. Ann Thorac Cardiovasc Surg. 2012;18(4):314–7. doi: 10.5761/atcs.oa.11.01839. [DOI] [PubMed] [Google Scholar]
- 9.Chiyo M, Sekine Y, Iwata T, et al. Impact of interstitial lung disease on surgical morbidity for lung cancer: an analyses of short-term and long-term outcomes. J Thorac Cardiovasc Surg. 2003;126(4):1141–6. doi: 10.1016/s0022-5223(03)00791-8. [DOI] [PubMed] [Google Scholar]
- 10.Koizumi K, Hirata T, Hirai K, et al. Surgical treatment of lung cancer combined with interstitial pneumonia: the effect of surgical approach on postoperative acute exacerbation. Ann Thorac Cardiovasc Surg. 2004;10(6):340–6. [PubMed] [Google Scholar]
- 11.Watanabe A, Kawaharada N, Higami T. Postoperative acute exacerbation of IPF after lung resection for primary lung cancer. Pulm Med. 2011 doi: 10.1155/2011/960316. doi: 10.1155/2011/960316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sato T, Teramukai S, Kondo H, et al. Impact and predictors of acute exacerbation of interstitial lung diseases after pulmonary resection for lung cancer. J Thorac Cardiovasc Surg. 2014;147(5):1604–11. doi: 10.1016/j.jtcvs.2013.09.050. [DOI] [PubMed] [Google Scholar]
- 13.Raghu G, Collard HR, Egan JJ, et al. Idiopathic Pulmonary Fibrosis: Evidence-based Guidelines for Diagnosis and Management. Am J Respir Crit Care Med. 2011;183(6):788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghatol A, Ruhl AP, Danoff SK. Exacerabation in Idiopathic Pulmonary Fibrosis Triggered by Pulmonary and Nonpulmonary Surgery: A Case Series and Comprehensive Review of the Literature. Lung. 2012;190(4):373–80. doi: 10.1007/s00408-012-9389-5. [DOI] [PubMed] [Google Scholar]
- 15.Kohno N, Kyoizumi S, Awaya Y, Fukuhara H, Yamakido M, Akiyama M. New serum indicator of interstitial pnuemonitis activity. Sialyted carbohydrate antigen KL-6. Chest. 1989;96(1):68–73. doi: 10.1378/chest.96.1.68. [DOI] [PubMed] [Google Scholar]
- 16.Yokoyama A, Kohno N, Hamada H, et al. Circulating KL-6 predicts the outcome of rapidly progressive idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1998;158(5 pt 1):1680–4. doi: 10.1164/ajrccm.158.5.9803115. [DOI] [PubMed] [Google Scholar]
- 17.Utz JP, Ryu JH, Douglas WW, et al. High short-term mortality following lung biopsy for usual interstitial pneumonia. Eur Respir J. 2001;17(2):175–9. doi: 10.1183/09031936.01.17201750. [DOI] [PubMed] [Google Scholar]
- 18.Ley B, Collard HR. King TE Jr. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;1834:431–40. doi: 10.1164/rccm.201006-0894CI. [DOI] [PubMed] [Google Scholar]
- 19.Sakamoto S, Homma S, Mun M, Fujii T, Kurosaki A, Yoshimura K. Acute exacerbation of idiopathic interstitial pneumonia following lung surgery in 3 of 68 consecutive patients: a retrospective study. Internal Medicine. 2011;50(2):77–85. doi: 10.2169/internalmedicine.50.3390. [DOI] [PubMed] [Google Scholar]
- 20.Okamoto T, Gotoh M, Masuya D, et al. Clinical analysis of interstitial pneumonia after surgery for lung cancer. Jpn J Thorac Cardiovasc surg. 2004;52(7):323–9. doi: 10.1007/s11748-004-0063-6. [DOI] [PubMed] [Google Scholar]
- 21.Clark JM, Lambertsen CJ. Pulmonary oxygen toxicity: a review. Pharmacol Rev. 1971;23(2):37–133. [PubMed] [Google Scholar]
- 22.Edmark L, Kostova-Aherdan K, Enlund M, Hedenstierna G. Optimal oxygen concentration during induction of general anesthesia. Anesthesiology. 2003;98(1):28–33. doi: 10.1097/00000542-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Grimm S, Brdiczka D. The permeability transition pore in cell death. Apotosis. 2007;12(5):841–55. doi: 10.1007/s10495-007-0747-3. [DOI] [PubMed] [Google Scholar]
- 24.Yeh CH, Cho W, So EC, et al. Propofol inhibits lipopolysaccharide-induced lung epithelial cell injury by reducing hypoxia-inducible factor-1α expression. British journal of Anesthesia. 2011;106(4):590–9. doi: 10.1093/bja/aer005. [DOI] [PubMed] [Google Scholar]
- 25.Hsing CH, Chen YH, Chen CL, et al. Anesthetic Propofol Causes Glycogen Synthase Kinase-3β-regulated Lysosomal/Mitochondrial Apoptosis in Macrophages. Anesthesiology. 2012;116(4):868–81. doi: 10.1097/ALN.0b013e31824af68a. [DOI] [PubMed] [Google Scholar]
- 26.Landoni G, Biondi-Zoccai GG, Zangrillo A, et al. Desflurane and sevoflurane in cardiac surgery: a meta-analysis of randomized clinical trials. J CariothoracVasc Anesth. 2007;21(4):502–11. doi: 10.1053/j.jvca.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 27.De Conno E, Steurer MP, Wittlinger M, et al. Anesthetic-induced Improvement of the Inflammatory Response to One-lung Ventilation. Anesthesiology. 2009;110(6):1316–26. doi: 10.1097/ALN.0b013e3181a10731. [DOI] [PubMed] [Google Scholar]