Abstract
Systemic therapy is administered to 50% of patients and the need for long-term use of therapy is quite variable (1,2). Prednisone is often administered for many months with risk for multiple side effects, immunomodulators as steroid sparing agents have a delayed onset of action and have risks for infection and malignancies, and infliximab increases the risk for infection (1). In light of these issues, alternative options for therapy are desirable. We made a comparison between sarcoidosis and Crohn’s disease in that in each disease there is unregulated lymphocyte activity, a common unique pathological finding of non-caseating granulomas, and a similar approach to medical therapy (3,4). Low dose naltrexone (LDN) has been utilized for many conditions (5). Efficacy has been documented in Crohn’s disease with randomized controlled studies showing mucosal healing and histologic improvement (6-7). LDN is compounded in 1/10th to 1/20th the dose used for the FDA-approved indications of narcotic and alcohol dependence (8). Neuropeptides (e.g., enkephalins and endorphins) are present in the gastrointestinal tract and endocrine cells and modulate immune responses (9). Up-regulation of met-enkephelin and opioid receptors can be induced by a rebound effect by short-acting LDN (10). Higher levels of endogenous opioids and receptors inhibit cell proliferation which suppress T and B lymphocyte responses (11,12) and decrease production of pro-inflammatory interleukins-6 and -12 (13). In light of the Crohn’s disease LDN literature and similar experiences with other inflammatory conditions in our clinic (14,15), LDN was administered to a sarcoidosis patient with severe fatigue, sarcoid rash, and marked radiographic evidence of gastrointestinal involvement. (Sarcoidosis Vasc Diffuse Lung Dis 2017; 34: 184-187)
Keywords: sarcoidosis, low-dose naltrexone, LDN
Case Report
The patient is an African American who first developed sarcoidosis at age 31, with supraglottic and cervical lymphadenopathy, and relapsing parotitis. At age 64, a tracheostomy was required owing to tracheal obstruction due to progressive supraglottic web formation and paratracheal lymphadenopathy. After numerous reconstructive surgeries, dilations, and laser therapy in addition to prednisone and methotrexate, the tracheostomy was able to be removed 6 years later. Methotrexate led to peripheral neuropathy and was discontinued. She also had a painful facial rash which was responsive to daily minocycline. Fatigue has been a major chronic problem. Chest radiography showed calcified granulomas. Pulmonary function testing showed reactive airway disease.
At age 70, she has an ultrasound to screen for abdominal aortic aneurysm. This revealed periaortic lymphadenopathy and a CT showed low density hepatic and splenic lesions as well. Endoscopic ultrasound with needle aspiration revealed non-caseating granulomas in the lymph nodes. No treatment was given for these asymptomatic lesions and follow up 3 years later showed stability of the lymph nodes but worsening of the splenic and liver lesions (Fig. 1A, 1B; Fig. 2A, 2B). We first evaluated her one month later on January, 31, 2015. Her chief complaints were severe fatigue and moderate dyspnea on exertion. Physical examination was remarkable for thickening of the facial skin. Medications included albuterol, famotidine, daily minocycline, diclofenac 1% topical gel (for peripheral neuropathy), and amoxicillin for recent parotitis. The ACE level, calcium, and liver chemistries were normal. Naltrexone 1 mg/day was started in early February 2015. Two months later, she reported less fatigue and dyspnea and was able to stop using inhalers and minocycline without relapse of the rash. The naltrexone dose was increased over 12 days to 4.5 mg in an attempt to prevent recurrent parotitis. Two months later, she reported further improvement in dyspnea and more energy although she had another bout of parotitis. Five months after starting LDN the CT showed reduction in the size of the splenic and the liver lesions with stability of periportal lymph nodes (Fig. 1C, 2C). Twelve months after starting LDN, she has maintained improvement in energy and prevention of the skin rash. The CT showed complete improvement in the splenic and liver lesions (Fig. 1D; 2D). Pulmonary function tests were unchanged after 5 months of the LDN.
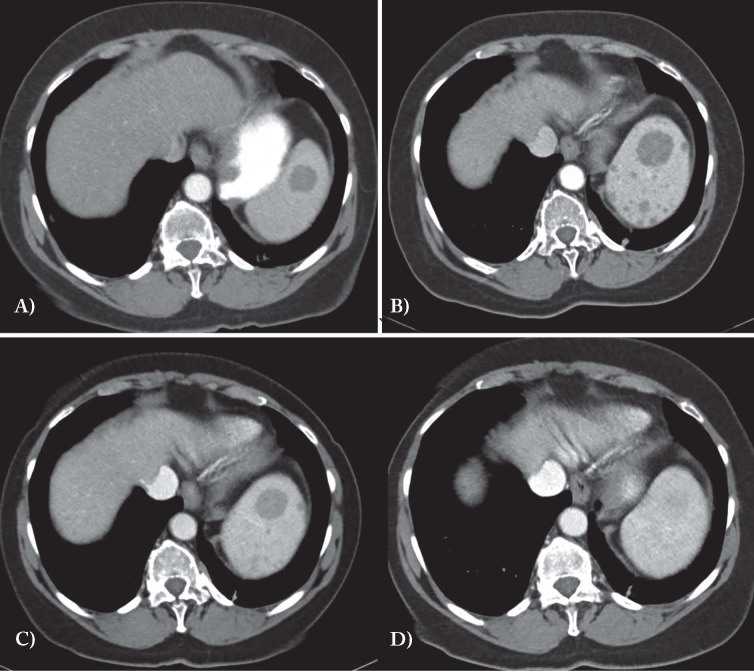
Fig. 1.
CT images in A and B show progression of splenic involvement over 3 years with an increase in the large central lesion and development of multiple smaller low density lesions. Treatment with low dose naltrexone results in significant radiographic improvement: at 5 months (C) the central lesion is smaller and the small lesions are much less apparent and at 10 months (D) the spleen is virtually normal. The images show the same area of the spleen based on level of the vertebral body and the apparent different in liver size is an artifact based on respiratory phase
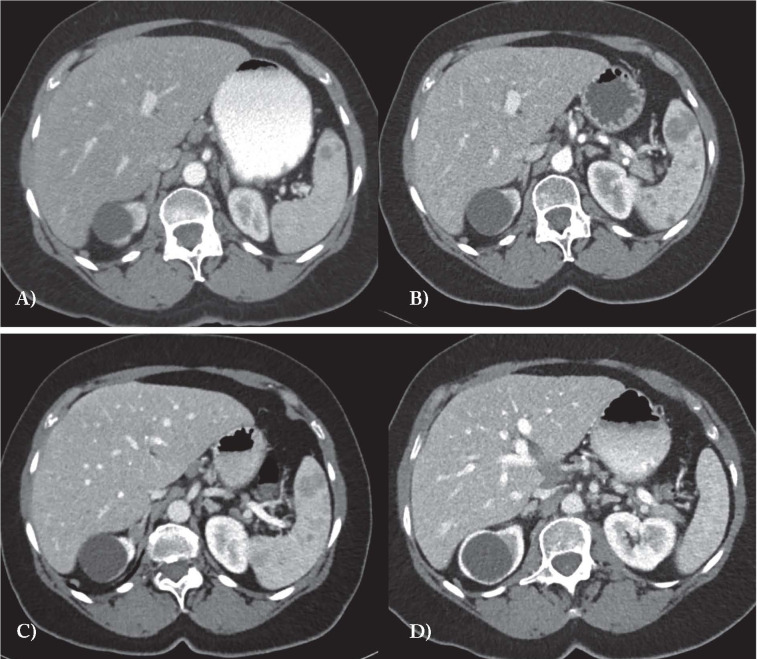
Fig. 2.
CT images in A and B show progression of lower splenic involvement over 3 years with an increase in the lesion and development of multiple smaller low density lesions. The liver images in A and B show general congestion and compression of blood vessels and bile ducts. Treatment with low dose naltrexone results in significant radiographic improvement: at 5 months (C) all lesions are much less apparent and at 10 months (D) the spleen appears normal. The liver progressively has returned to normal enhancement of the parenchyma, vasculature and blood vessels
Discussion
Granulomatous inflammation of the spleen and the liver is common in sarcoidosis (16). In this report, a patient with systemic sarcoidosis was treated with LDN which increases endorphin levels and helps regulate inflammation including lymphocyte activity (11,12). This mechanism of action could play a role in the treatment of sarcoidosis (3). The radiographic improvement helped substantiate the therapeutic efficacy in the case study. Clinical improvement was seen in her fatigue and ability to stop taking antibiotic therapy for the sarcoid rash. It is unclear if the perceived improvement in dyspnea was directly responsible from LDN. The pulmonary function test did not improve but this is not surprising since cardiopulmonary exercise testing may be the most discriminant test in sarcoidosis (17).
After seeing success in this case, we administered LDN to a 64 year old African American female with pulmonary sarcoidosis. She had required steroids and oxygen on and off over the past few years. Two months after initiating therapy with LDN she reported less dyspnea and was able to reduce the need for oxygen. As was seen in the case report, the most dramatic improvement was noted in her fatigue.
Fatigue is a common and significant problem in sarcoidosis and can be secondary to pulmonary hypertension (1), chronic inflammation, infections, and drug side effects. LDN may have improved fatigue by reducing the inflammatory burden as was suggested by the case report where progressive organ involvement was returned to normal with a 10 month course of LDN. Further studies are required to substantiate the efficacy of LDN in a variety of sarcoidosis involvement.
References
- 1.Baughman RP, Grutters JC. New treatment strategies for pulmonary sarcoidosis: antimetabolites, biological drugs, and other treatment approaches. Lancet Respir Med. 2015;3:813–22. doi: 10.1016/S2213-2600(15)00199-X. [DOI] [PubMed] [Google Scholar]
- 2.Baughman RP, Judson MA, Teirstein A, et al. Presenting characteristics as predictors of duration of treatment in sarcoidosis. QJM. 2006;99:307–15. doi: 10.1093/qjmed/hcl038. [DOI] [PubMed] [Google Scholar]
- 3.Iida K, Kadota J, Kawakami K, Matsubara Y, et al. Analysis of T cell subsets and beta chemokines in patients with pulmonary sarcoidosis. Thorax. 1997;52:431–7. doi: 10.1136/thx.52.5.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith JP, Weinstock LB. Inflammatory Bowel Disease. The LDN Book. In: Elsegood L, editor. Chelsea Green Publishing; 2016. [Google Scholar]
- 5.Elsegood L, editor. The LDN Book. Chelsea Green Publishing; 2016. [Google Scholar]
- 6.Smith JP, Bingaman SI, Ruggiero F, et al. Therapy with the opioid antagonist naltrexone promotes mucosal healing in active Crohn’s disease: a randomized placebo-controlled trial. Dig Dis Sci. 2011;56:2088–97. doi: 10.1007/s10620-011-1653-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith JP, Field D, Bingaman SI, et al. Safety and tolerability of low-dose naltrexone therapy in children with moderate to severe Crohn’s disease: a pilot study. J Clin Gastroenterol. 2013;47:339–45. doi: 10.1097/MCG.0b013e3182702f2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sudakin D. Naltrexone: Not Just for Opioids Anymore. J Med Toxicol. 2016;12:71–5. doi: 10.1007/s13181-015-0512-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gross K, Pothoulakis C. The role of neuropeptides in inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:918–32. doi: 10.1002/ibd.20129. [DOI] [PubMed] [Google Scholar]
- 10.Zagon IS, McLaughlin PJ. Targeting opioid signaling in Crohn’s disease: new therapeutic pathways. Expert Rev Gastroenterol Hepatol. 2011;5:555–8. doi: 10.1586/egh.11.62. [DOI] [PubMed] [Google Scholar]
- 11.Zagon IS, Donahue RN, Bonneau RH, McLaughlin PJ. T lymphocyte proliferation is suppressed by the opioid growth factor ([Met(5)]-enkephalin)-opioid growth factor receptor axis: implication for the treatment of autoimmune diseases. Immunobiology. 2011;216:579–90. doi: 10.1016/j.imbio.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 12.Zagon IS, Donahue RN, Bonneau RH, McLaughlin PJ. B lymphocyte proliferation is suppressed by the opioid growth factor-opioid growth factor receptor axis: Implication for the treatment of autoimmune diseases. Immunobiology. 2011;216:173–83. doi: 10.1016/j.imbio.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Matters GL, Harms JF, McGovern C, Fitzpatrick L, Parikh A, Nilo N, Smith JP. The opioid antagonist naltrexone improves murine inflammatory bowel disease. J Immunotoxicol. 2008;5:179–87. doi: 10.1080/15476910802131469. [DOI] [PubMed] [Google Scholar]
- 14.Weinstock LB. Naltrexone Therapy for Crohn’s Disease and Ulcerative Colitis. J Clin Gastroenterol. 2014;48:742. doi: 10.1097/MCG.0000000000000093. [DOI] [PubMed] [Google Scholar]
- 15.Weinstock LB, Myers TL, Walters AS, et al. Identification and treatment of new inflammatory triggers for complex regional pain syndrome: small intestinal bacterial overgrowth and obstructive sleep apnea. A&A Case Reports. 2016. Feb 10 doi: 10.1213/XAA.0000000000000292. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Giovinale M, Fonnesu C, Soriano A, et al. Atypical sarcoidosis: case reports and review of the literature. Eur Rev Med Pharmacol Sci. 2009;13:37–44. [PubMed] [Google Scholar]
- 17.Kallianos A, Zarogoulidis P, Ampatzoglou F, et al. Reduction of exercise capacity in sarcoidosis in relation to disease severity. Patient Prefer Adherence. 2015;9:1179–88. doi: 10.2147/PPA.S86465. [DOI] [PMC free article] [PubMed] [Google Scholar]