Abstract
Background: Sarcoidosis is an inflammatory disease with pulmonary and extrapulmonary manifestations. In such pathologic conditions, increased oxidative stress and rearrangement of high-density lipoprotein (HDL) and low-density lipoprotein (LDL) may occur. Objective: This study evaluated association of oxidative stress and lipoprotein subclasses in severe forms of pulmonary and pulmonary plus extrapulmonary sarcoidosis. Methods: Lipid parameters, LDL and HDL subclass distributions, high-sensitivity C-reactive protein (hsCRP), serum amyloid A (SAA), paraoxonase 1 (PON1), malondialdehyde (MDA), total-oxidant status (TOS), sulfhydryl (SH) groups, pro-oxidant anti-oxidant balance (PAB) were determined in 77 patients (53 isolated pulmonary and 24 pulmonary plus extrapulmonary) and 139 controls. Results: Both pulmonary and extrapulmonary sarcoidosis patients had significantly higher levels of triglycerides and TOS (P<0.05) and more LDL II, LDL III, LDL IVA particles (P<0.01), but lower HDL size, SH groups (P<0.001), PON1 activity and less LDL I subclasses (P<0.05) than controls. In isolated pulmonary disease, HDL-cholesterol (P<0.01) was significantly lower whereas proportions of HDL 3a and PAB were significantly higher (P<0.05) when compared with the control group. PON1 was significantly higher in pulmonary than in combined pulmonary-extrapulmonary disease (P<0.05). In pulmonary sarcoidosis, TOS and PON1 correlated significantly with small-sized HDL particles (P<0.05). Conclusions: Both patient groups were characterized by adverse lipoprotein profile and elevated oxidative stress. In isolated pulmonary group significant associations of oxidative stress and HDL particles distribution was demonstrated. Pulmonary sarcoidosis was associated with higher PON1 activity and rearrangement of LDL particles did not depend on disease localization. (Sarcoidosis Vasc Diffuse Lung Dis 2018; 35: 198-205)
Keywords: lipoproteins, oxidative stress, paraoxonase-1, sarcoidosis manifestations
Introduction
Sarcoidosis is an inflammatory disease that most often affects the lungs, but there are also extrapulmonary manifestations involving the eye, skin, nervous system and lymph nodes (1). The patients show evidence of increased oxidative stress and exhausted and/or deficient anti-oxidative defence enzymes (2, 3). Recent data suggest that sarcoidosis is also associated with dyslipidemia (2, 4) and adverse changes in low-density (LDL) and high-density lipoprotein (HDL) particle size and subclasses distributions (5).
Pulmonary disease may be accompanied by extrapulmonary involvement and this is characterized as a more severe form of disease because of a higher extent of inflammation (6). These manifestations of disease usually require higher doses of medications to be used. Corticosteroids are the first line treatment in sarcoidosis having anti-inflammatory effects (7). Suppression of inflammation may lead to changes in oxidative stress (2, 3) and lipid status (2, 8), but there is still no data describing the extent of these changes in pulmonary and pulmonary plus extrapulmonary manifestations of the disease, taking into account different dosage of corticosteroids.
Overproduction of free radicals under pathophysiological conditions plays an important role in the development of atherosclerosis and its complications, particularly cardiovascular disease (CVD). In conditions of increased oxidative stress, LDL is more prone to oxidative modifications, thus creating oxidatively-modified particles which accumulate in blood vessel walls (9). Besides its role in the process of reverse cholesterol transport (RCT), HDL also has anti-inflammatory and antioxidative properties. Antioxidative properties originate from major proteins of HDL, such as apoAI, AII and paraoxonase 1 (PON1). However, these properties of HDL could be compromised in various pathological conditions (9). There are reports indicating a decrease in HDL-cholesterol (HDL-c) concentration in sarcoidosis patients (2, 4), however no previous studies have investigated whether there is a specific interplay of oxidative stress, inflammation and atherogenic dyslipidemia according to presentation of the disease.
Chronic inflammatory disorders have been associated with higher cardiovascular disease (CVD) risk (10). In sarcoidosis patients increased risk for CVD is attributable to inflammation, oxidative stress and dyslipidemia (2), although there is a lack of data regarding different manifestations of disease. Sarcoidosis may vary from milder forms, characterized by spontaneous remissions, to severe and progressive forms that require treatment (6, 7). Since the majority of sarcoidosis patients are characterized by milder forms of disease (7), less data is available on patients with severe forms of sarcoidosis. To our knowledge, oxidative stress and dyslipidemia have not yet been studied in severe and progressive sarcoidosis, with respect to its manifestations.
Therefore, the aim of our study was to determine the extent of inflammation and oxidative stress, as well as LDL and HDL subclass distribution in patients with severe forms of pulmonary and pulmonary plus extrapulmonary sarcoidosis. In addition, we evaluated the relationship among lipoprotein subclass distribution and oxidative stress status according to the presentation of the disease.
Methods
Study participants
In this study, we included 77 severe sarcoidosis patients (23 males and 54 females) from the Clinic for Pulmonary Diseases and Tuberculosis, Clinical Centre of Serbia, Belgrade. The diagnosis was established by clinical, radiological and histological findings (7). Patients underwent biopsy analysis that confirmed noncaseating epithelioid granulomatous inflammation in the appropriate organ/tissue. Other non-infectious and infectious causes of granulomas had been excluded. There were 53 patients with only pulmonary sarcoidosis and 24 patients with additional extrapulmonary presentations (with eye - 6, cardiac - 6, neurological - 9, and skin - 3 involvement). Of these patients, 77.9% were receiving prednisolone and 22.1% a combination of prednisolone and methotrexate. A low dose of prednisolone (median: 10 mg; interquartile range: 5–10 mg) was used in the treatment during 24.5±16.4 months (median: 24 months; interquartile range: 12–39 months) and some patients were additionally treated weekly with methotrexate (median: 5 mg; interquartile range: 5-10 mg) during 9.4±4.2 months. No patient received any lipid-lowering medications. Our 77 patients were selected after applying exclusion criteria and these criteria were the presence of cardiovascular disease, pulmonary (any other pulmonary disease except sarcoidosis), neurological, renal, hepatic, endocrine or malignant disease. We also recruited 139 gender-matched healthy controls (51 males and 88 females). They attended regular medical check-ups at health centres in Belgrade and inclusion criteria were the absence of any pulmonary, gastrointestinal, hepatic, renal, cardiovascular, malignant or endocrine disease.
The study was planned according to the ethical guidelines stated in the Helsinki declaration. Written informed consent was obtained from all subjects prior to study entry. The research was approved by the institutional committee (Ethics Board) of Clinical Centre of Serbia (number 617/7).
Sample collection
After a 12-hour fasting period, venous blood samples were collected into serum and EDTA plasma sample tubes and then centrifuged (1500xg, 10 min at 40C) to obtain serum and plasma. Samples were aliquoted and stored at -800C. Aliquots were thawed immediately before analyses.
Biochemical analysis
Serum glucose, total protein, urea and creatinine were assayed by routine laboratory methods. Concentrations of lipid status parameters (total cholesterol (TC), LDL-c, HDL-c and triglycerides (TG)) were measured by standard laboratory procedures (ILAB 300+analyzer, Instrumentation Laboratory, Milan, Italy). The latex-enhanced immunoturbidimetry method (Quantex hsCRP kit, BIOKIT, Barcelona, Spain) was used to measure the concentration of high-sensitive C-reactive protein (hsCRP) on an ILAB 600 analyzer. The concentration of serum amyloid A (SAA) was determined by a commercially-available two-site enzyme linked immunosorbent assay (ELISA) kit (Immunology Consultants Laboratory, Portland, OR, USA).
Paraoxonase 1 (PON1) was assessed using paraoxone as a substrate. The conversion of paraoxone to p-nitrophenol by the hydrolytic activity of PON1 was measured at 405 nm using an ILAB 300+ analyzer (Instrumentation Laboratory, Milan, Italy) according to Richter and Furlong (11). Paraoxon was purchased from Santa Cruz Biotechnology (Dallas, Texas, USA). Malondialdehyde (MDA) concentration was determined in the assay described by Girotti and colleagues (12) based on spectrophotometric measurement of red-coloured MDA-thiobarbituric acid adduct at 535 nm. The Ellman method, used originally for measuring tissue sulfhydryl (SH) groups (13), has already been applied for measurement of serum or plasma SH groups in our laboratory (14, 15) and by others (16). Total oxidative status (TOS) was determined by Erel’s method (17). The assay is based on oxidation of ferrous ion-o-dianizidine complex to ferric ion in the presence of various oxidant species in serum. The pro-oxidant-anti-oxidant balance (PAB) was measured according to a previously-published method (18).
LDL and HDL subclass determination
The method of Rainwater et al. (19) was used for separating plasma LDL and HDL particles. A detailed description of the procedure has been published (20). The migration distance for each absorbance peak was determined and the particle diameter corresponding to each peak was calculated from the calibration curve. The estimated diameter of the major peak in the LDL and HDL regions of each scan was referred to as the dominant particle diameter. To estimate the relative content of each LDL and HDL subclass, areas under the peaks of densitometric scans stained with Sudan black were determined (21). The criteria used to define the regions which comprised small, dense LDL (sdLDL) and small-sized HDL (ssHDL) were areas under the peak in the LDL region below 25.5 nm and in the HDL region below 8.8 nm (20).
Statistical analysis
To describe the data, we used means±standard deviation for normally-distributed data, geometric means and 95% confidence intervals for log-transformed normally-distributed data, median and interquartile range for non-normally distributed data and relative frequencies for categorical variables. The normality of distribution of parameters was assessed using the Kolmogorov Smirnov test. Continuous variables having normal distribution were compared using ANOVA test. As age differed significantly between isolated pulmonary and combined pulmonary and extrapulmonary sarcoidosis patients, ANCOVA test was employed to determine whether age affected the studied parameters in these two groups. The Mann Whitney test was used for comparisons of non-normally-distributed data. Differences between categorical variables were tested with the Chi-square test for contingency tables.
Spearman’s correlation analysis was employed to determine possible correlations between the parameters. Multiple linear regression analysis (enter selection) was used to estimate the independent contribution of age, gender, prednisolone dosage, oxidative stress, lipid status parameters and PON1 activity on HDL subclasses proportion. Model in the multiple regression analysis included parameters whose P values for Spearman’s correlation coefficient were ≤0.1. Multicolinearity between variables was also tested for these models and none of the variables significantly influenced another variable in the models. All statistical analyses were performed using MS Excel, PASW Statistics Version 18.0 and MedCalc (version 11.4 Software, Belgium) software. The 0.05 probability level was considered significant in all statistical tests.
Results
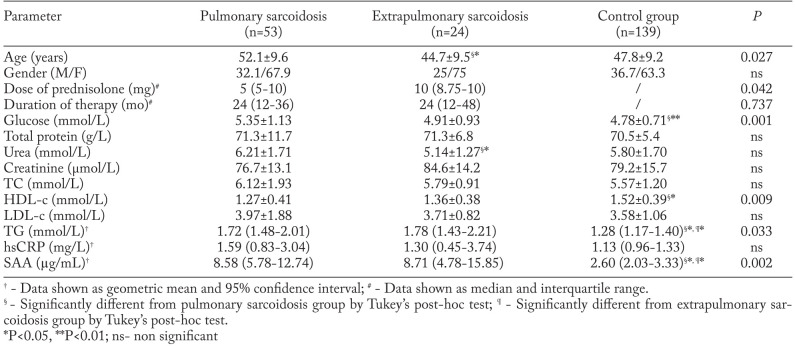
General characteristics, lipid and inflammatory parameters of the study participants are shown in Table 1. All patients and controls were matched according to gender (χ2=1.391, P=0.499). As patients with pulmonary sarcoidosis were older than patients with extrapulmonary disease, group differences were compared after an appropriate adjustment. Age had no significant influence on parameters presented in Table 1. Dosage of prednisolone was significantly higher in the extrapulmonary group whereas treatment duration showed no significant difference between the groups. The pulmonary sarcoidosis group had significantly higher levels of glucose, TG and SAA, but significantly lower HDL-c concentration when compared with controls. The extrapulmonary group of patients had significantly higher TG and SAA concentrations than the control group. Both groups of patients had higher hsCRP concentrations than controls, although not significantly. Distribution of patients with respect to the type of therapy (prednisolone or prednisolone and methotrexate) did not differ significantly between pulmonary and extrapulmonary disease groups (P=0.428, data not shown).
Table 1.
General characteristics, lipid and inflammatory parameters in pulmonary, extrapulmonary sarcoidosis and control groups
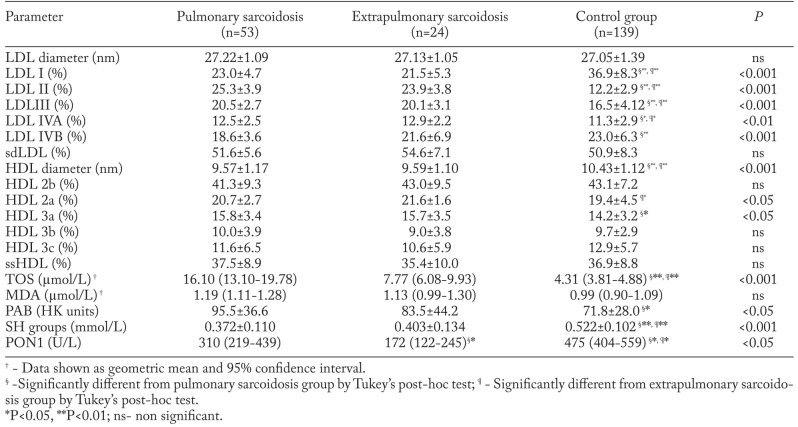
Lipoprotein subclass distributions, oxidative stress status parameters and PON1 activity are given in Table 2. Both pulmonary and extrapulmonary sarcoidosis groups had more LDL II, LDL III and LDL IVA, but less LDL I particles than the control group. HDL particle size was significantly lower in the patient groups. Also, pulmonary sarcoidosis patients had significantly lower proportions of LDL IVB, but significantly higher proportions of HDL 3a subclasses when compared with controls. The extrapulmonary sarcoidosis group was characterized by a higher proportion of HDL 2a subclasses than the control group. Both patient groups had significantly higher concentrations of TOS and lower levels of SH groups and PON1 activity than control subjects. Additionally, in the pulmonary group of patients, significantly higher levels of PAB were found in comparison with control subjects. The pulmonary sarcoidosis group had significantly higher PON1 activity than extrapulmonary subjects.
Table 2.
Lipoprotein subclass distributions, oxidative stress status parameters and PON1 in pulmonary, extrapulmonary sarcoidosis and control groups
We performed Spearman’s correlation analysis to test associations of LDL and HDL particle sizes and subclass distributions with lipid, inflammatory, oxidative stress parameters and PON1, both in pulmonary and extrapulmonary sarcoidosis. In pulmonary sarcoidosis, LDL diameter was negatively correlated with concentration of SAA (r=-0.513; P=0.035). Also, LDL IVA subclass proportion was significantly positively associated with TC (r=0.308; P=0.047), while LDL IVB were positively associated with levels of MDA (r=0.296; P=0.031).
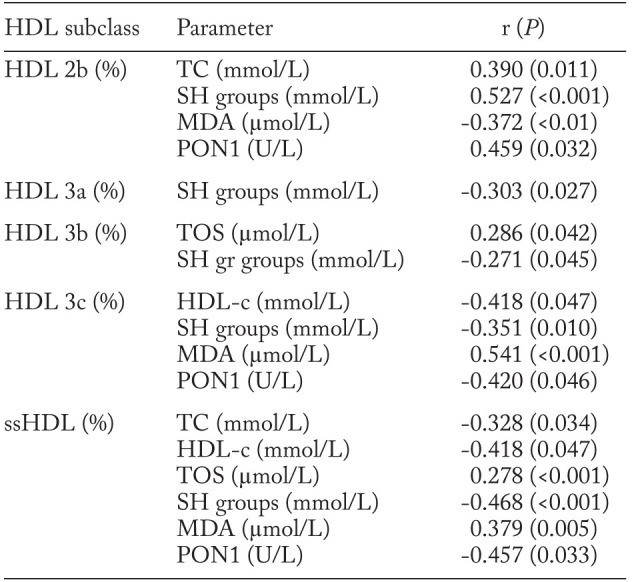
Significant associations of HDL subclass proportions with lipid, oxidative stress and PON1 status parameters in the pulmonary sarcoidosis group are presented in Table 3. The relative proportion of HDL 2b particles was significantly positively correlated with TC and SH group concentrations and PON1 activity, while negatively correlated with MDA level. The proportion of HDL 3b particles was positively correlated with TOS and negatively correlated with the concentration of SH groups. Both relative proportion of HDL 3c subclasses and ssHDL particles were positively correlated with MDA level and negatively correlated with HDL-c and SH group concentrations as well as PON1 activity. In addition, the level of TOS was positively and TC negatively associated with the proportion of ssHDL particles.
Table 3.
Correlations of HDL subclasses with lipid, oxidative stress status parameters and PON1 activity in the pulmonary sarcoidosis group
In extrapulmonary sarcoidosis, MDA levels negatively correlated with LDL I (r=-0.433; P=0.039), while positively correlated with proportions of LDL IVA (r=0.536; P=0.008) and sdLDL particles (r=0.415; P=0.049). A significant positive correlation was also found between LDL IVA proportion and hsCRP level (r=0.807; P=0.028). Regarding HDL particle size and distributions in extrapulmonary sarcoidosis, we found that dominant HDL diameter was negatively associated with PAB (r=-0.433; P=0.034) and relative proportion of the HDL 3c subclass was positively associated with TG level (r=0.622; P=0.004).
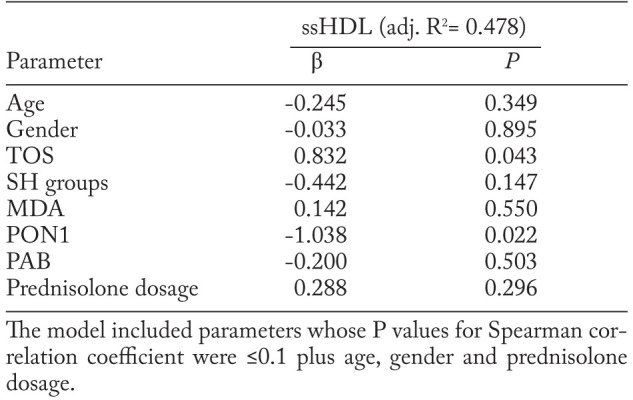
The association between ssHDL particles and oxidative stress parameters in pulmonary sarcoidosis was further evaluated in multiple linear regression analysis. When age, gender, oxidative stress parameters and prednisolone dosage were included in the model, TOS and PON1 activity remained independently associated with the variations in proportion of ssHDL particles. Even 47.8% of variance in ssHDL proportions in isolated pulmonary sarcoidosis patients was associated with increased level and reduced PON1 activity. The results are shown in Table 4.
Table 4.
Predictors of ssHDL particles proportion in pulmonary sarcoidosis patients according to multiple regression analysis
Discussion
In the presented study, we found that both severe forms of isolated pulmonary and combined pulmonary and extrapulmonary sarcoidosis patients were characterized by inflammation, enhanced oxidative stress, exhausted anti-oxidative mechanisms and adverse lipoprotein subclass profiles. The interplay of these pathological conditions was more evident in the pulmonary group.
Sarcoidosis is an inflammatory disease (1, 6) and it could be expected that inflammatory reactions are more severe and pronounced in pulmonary sarcoidosis with extrapulmonary manifestations than in the isolated pulmonary form (6). However, hsCRP and SAA did not differ significantly between these two groups (Table 1). This may be due to the medications used in both groups of patients. Nevertheless, when isolated pulmonary or combined pulmonary and extrapulmonary patient groups were compared with controls, SAA was significantly higher in both groups of patients (Table 1). These results fit into conclusions of Rothkrantz-Kos et al. (1), who reported that, although immunosuppressive drugs such as corticosteroids may decrease the extent of inflammation through decrease of hsCRP levels, SAA may remain increased because of a lower sensitivity to these drugs.
Recently-published data have shown that sarcoidosis patients have increased proportions of HDL 3a and 2a particles (4, 5) and increased oxidative stress (2, 3). A shift in LDL and HDL subclass distribution toward smaller particles and increased oxidative stress (higher TOS) with diminished antioxidative protection (decreased SH groups and PON1 activity) in patients compared with controls was also observed in the current study (Table 2). Disturbances in LDL and HDL subclass distributions may arise as a consequence of elevated inflammation, oxidative stress and dyslipidemia (22, 23). However, it is still unknown whether the changes in lipoprotein profiles and oxidative stress might vary according to different manifestations of sarcoidosis. Our current data have shown that isolated pulmonary sarcoidosis patients had increased proportions of smaller HDL 3a particles and higher PAB levels, when compared with controls (Table 2). Moreover, in the same group, relative proportions of each HDL 3 subclasses, as well as of ssHDL particles, were positively related to the markers of oxidative stress and inversely related with parameters of antioxidative protection (Table 3). These observations regarding a potential link between small HDL particles and intensity of oxidative stress in pulmonary sarcoidosis were further confirmed by the results of multiple linear regression analysis, indicating that TOS concentration and PON1 activity were variables independently associated with the proportion of ssHDL particles, even when prednisolone dosage was included in the model (Table 4). The possible explanation for interactions that were present between oxidative stress and smaller HDL particles is elevated peroxides that could cause impaired lecithin-cholesterol acyl-transferase (LCAT) activity, thereby provoking defects in HDL maturation (24, 25). Additionally, different oxidants may directly cause loss of the structural and functional properties of HDL (26). This further may contribute to increase the catabolism of HDL particles (4) which is evident through decreased HDL-c concentrations in the pulmonary group of our patients (Table 1). Also, the drug dosage implemented in the pulmonary group did not affect the association between small HDL subclasses and oxidative stress (Table 4). Giving that several studies (27-29) demonstrated no significant differences in serum lipid status parameters between untreated patients with rheumatoid arthritis and those on low-dose long-term corticosteroid treatment, it is possible that observed changes in HDL particles in our sarcoidosis patients could arise as a consequence of increased oxidative stress and inflammation and not only as a result of applied corticosteroids.
We also demonstrated that the pulmonary sarcoidosis group had significantly higher PON1 activity than the group with combined pulmonary and extrapulmonary disease (Table 2). PON1 is an enzyme located on HDL particles reported to have an antioxidative role by neutralizing lipid hydroperoxides, thereby protecting LDL from oxidative modifications (30). As a consequence of elevated oxidative stress and inflammation, down-regulation of PON1 in patients, when compared with controls, has been observed (31, 32). On the other hand, previous reports have suggested that anti-inflammatory/antioxidative genes may be up-regulated as a result of chronic augmented oxidative stress (33). Hence, higher PON1 activity in the isolated pulmonary group when compared with the combined pulmonary and extrapulmonary disease group could be a consequence of up-regulated PON1 gene expression. In addition, an inverse association between PON1 and small HDL particles in the isolated pulmonary disease (Tables 3 and 4) may further indicate a compensatory increase in enzyme activity to combat defective HDL maturation. Taken together, it seems that various mechanisms are responsible for the complex structural and functional rearrangement of HDL particles and consequent altered activity of HDL-associated proteins in the pulmonary manifestation of sarcoidosis.
Regarding LDL, we found that the proportion of smaller LDL particles correlated positively with MDA in both pulmonary and combined pulmonary and extrapulmonary sarcoidosis. These correlations were completely in line with the findings of Kondo et al. (34) that subjects with sdLDL particles and high TG concentrations had an elevated concentration of MDA-modified LDL particles (Tables 1 and 2). In addition, these findings suggest that the previously-mentioned mechanism of modifying LDL particles did not depend on disease localization.
To the best of our knowledge, this is the first study investigating the differences and associations between inflammatory and oxidative stress status parameters and lipoprotein size heterogeneity in severe forms of isolated pulmonary and combined pulmonary and extrapulmonary sarcoidosis. One limitation of this study is that all included patients had more severe forms of the disease and were treated with prednisolone. Since we could not recruit patients free of the therapy, our findings cannot be extrapolated to untreated patients. Hence, the observations from this study are only applicable to more severe cases of sarcoidosis. Also, a relatively small number of subjects included in our study may limit generalization of our results. Finally, our conclusions are based upon a cross-sectional design and the possible impact of associations we observed on the disease progression should be further addressed in future work.
Previous reports regarding immune-mediated inflammatory diseases have indicated an increased CVD risk (35, 36) and extensive evidence has shown that the elevated presence of smaller LDL and HDL subclasses and elevated oxidative stress are deeply involved in initiation and enhancement of atherosclerotic plaques (15, 37). The findings obtained in our study may arise as a consequence of disease process, corticosteroid treatment or both factors. Nevertheless, dyslipidemia and oxidative stress in different manifestations of severe sarcoidosis may indicate higher CVD risk, regardless of the cause.
In conclusion, our data have shown that both isolated pulmonary and pulmonary plus extrapulmonary severe sarcoidosis patients have adverse profile of lipoprotein subclasses, elevated oxidative stress and diminished anti-oxidative protection. In pulmonary sarcoidosis group significant associations of oxidative stress and HDL subclasses distribution were found. LDL particle size distribution did not depend on disease localization, suggesting the involvement of other complex mechanisms, not only redox pathways, in their rearrangement.
Acknowledgements
This work was financially supported by the Ministry of Education, Science and Technological Development, Republic of Serbia (Project number 175035).
Funding:
This work was financially supported by the Ministry of Education, Science and Technological Development, Republic of Serbia (Project number 175035).
References
- 1.Rothkrantz-Kos S, Van Dieijen-Visser MP, Mulder PGH, Drent M. Potential usefullness of inflammatory markers to monitor respiratory functional impairment in sarcoidosis. Clin Chem. 2003;49:1510–7. doi: 10.1373/49.9.1510. [DOI] [PubMed] [Google Scholar]
- 2.Ivanisevic J, Kotur-Stevuljevic J, Stefanovic A, et al. Dyslipidemia and oxidative stress in sarcoidosis patients. Clin Biochem. 2012;45:677–82. doi: 10.1016/j.clinbiochem.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 3.Koutsokera A, Papaioannou AI, Malli F, et al. Systemic oxidative stress in patients with pulmonary sarcoidosis. Pulm Pharm Therap. 2009;22:603–7. doi: 10.1016/j.pupt.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Salazar A, Pinto X, Mana J. Serum amyloid A and high-density lipoprotein cholesterol: serum markers of inflammation in sarcoidosis and other systemic disorders. Eur J Clin Invest. 2001;31:1070–7. doi: 10.1046/j.1365-2362.2001.00913.x. [DOI] [PubMed] [Google Scholar]
- 5.Vekic J, Zeljkovic A, Jelic-Ivanovic Z, et al. Distribution of low-density lipoprotein and high-density lipoprotein subclasses in patients with sarcoidosis. Arch Pathol Lab Med. 2013;137:1780–7. doi: 10.5858/arpa.2012-0299-OA. [DOI] [PubMed] [Google Scholar]
- 6.Gvozdenović B, Mihailović-Vučinić V, Ilić-Dudvarski A, Žugic V, Judson M.A. Differences in symptom severity and health status impairment between patients with pulmonary and pulmonary plus extrapulmonary sarcoidosis. Resp Med. 2008;102:1636–42. doi: 10.1016/j.rmed.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 7.The joint statement of the American Thoracic Society (ATS), the European Respiratory Society (ETS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) Statement on sarcoidosis. Am J Resp Crit Care Med. 1999;160:736–55. doi: 10.1164/ajrccm.160.2.ats4-99. [DOI] [PubMed] [Google Scholar]
- 8.Salazar A, Mañá J, Pintó X, Argimón J.M, Hurtado I, Pujol R. Corticosteroid therapy increases HDL-cholesterol concentrations in patients with active sarcoidosis and hypoalphalipoproteinemia. Clin Chim Acta. 2002;320:59–64. doi: 10.1016/s0009-8981(02)00046-3. [DOI] [PubMed] [Google Scholar]
- 9.Barter PJ, Nicholls SJ, Rye K-A, Anantharamaiah GM, Navab M, Fogelman AM. Antiinflammatory properties of HDL. Circ Res. 2004;95:764–72. doi: 10.1161/01.RES.0000146094.59640.13. [DOI] [PubMed] [Google Scholar]
- 10.Roifman I, Beck PL, Anderson TJ, Eisenberg MJ, Genest J. Chronic inflammatory diseases and cardiovascular risk: a systematic review. Can J Cardiol. 2011;27(2):174–182. doi: 10.1016/j.cjca.2010.12.040. [DOI] [PubMed] [Google Scholar]
- 11.Richter R, Furlong CE. Determination of paraoxonase (PON1) status requires more than genotyping. Pharmacogenetics. 1999;9:745–53. [PubMed] [Google Scholar]
- 12.Girotti MJ, Khan N, Mc Lellan BA. Early measurement of systemic lipid peroxidation products in plasma of major blunt trauma patients. J Trauma. 1991;31:32–5. doi: 10.1097/00005373-199101000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Ellman G. Tissue sulfhydryl groups. Arc Biochem Biophys. 1952;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 14.Stefanovic A, Kotur Stevuljevic J, Spasic S, Vekic J, Bujisic N. Association of oxidative stress and paraoxonase status with PROCAM risk score. Clin Biochem. 2009;42:617–23. doi: 10.1016/j.clinbiochem.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Kotur Stevuljevic J, Spasic S, Jelic Ivanovic Z, et al. PON1 status is influenced by oxidative stress and inflammation in coronary heart disease patients. Clin Biochem. 2008;41:1067–73. doi: 10.1016/j.clinbiochem.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 16.Esen R, Aslan M, Kucukoglu MH, et al. Serum paraoxonase activity, total thiols levels, and oxidative status in patients with acute brucellosis. Wien Klin Wochenschr. 2015;127:427–33. doi: 10.1007/s00508-015-0720-z. [DOI] [PubMed] [Google Scholar]
- 17.Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38:1103–11. doi: 10.1016/j.clinbiochem.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 18.Hamidi Alamdari D, Ghayour-Mobarhan M, Tavallaie S, et al. Prooxidant–antioxidant balance as a new risk factor in patients with angiographically defined coronary artery disease. Clin Biochem. 2008;41:375–80. doi: 10.1016/j.clinbiochem.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Rainwater DL, Moore PH, Gamboa IO. Improved method for making nondenaturing composite gradient gels for the electrophoretic separation of lipoproteins. J Lipid Res. 2004;45:773–5. doi: 10.1194/jlr.D300035-JLR200. [DOI] [PubMed] [Google Scholar]
- 20.Vekic J, Topic A, Zeljkovic A, Jelic-Ivanovic Z, Spasojevic-Kalimanovska V. LDL and HDL subclasses and their relationship with Framingham risk score in middle-aged Serbian population. Clin Biochem. 2007;40:310–6. doi: 10.1016/j.clinbiochem.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 21.Zeljkovic A, Vekic J, Spasojevic-Kalimanovska V, et al. LDL and HDL subclasses in acute ischemic stroke: prediction of risk and short-term mortality. Atherosclerosis. 2010;210:548–54. doi: 10.1016/j.atherosclerosis.2009.11.040. [DOI] [PubMed] [Google Scholar]
- 22.Berneis KK, Krauss RM. Metabolic origins and clinical significance of LDL heterogeneity. J Lipid Res. 2002;43:1363–79. doi: 10.1194/jlr.r200004-jlr200. [DOI] [PubMed] [Google Scholar]
- 23.Tsompanidi EM, Brinkmeier MS, Fotiadou EH, Giakoumi SM, Kypreos KE. HDL biogenesis and functions: role of HDL quality and quantity in atherosclerosis. Atherosclerosis. 2010;208:3–9. doi: 10.1016/j.atherosclerosis.2009.05.034. [DOI] [PubMed] [Google Scholar]
- 24.Vekić J, Kotur Stevuljević J, Jelić Ivanović Z, et al. Association of oxidative stress and PON1 with LDL and HDL particle size in middle-aged subjects. Eur J Clin Invest. 2007;37:715–23. doi: 10.1111/j.1365-2362.2007.01849.x. [DOI] [PubMed] [Google Scholar]
- 25.Kontush A, Chapman J. Functionally defective high-density lipoprotein: a new therapeutic target at the crossroads of dyslipidemia, inflammation and atherosclerosis. Pharmacol Rev. 2006;58:342–74. doi: 10.1124/pr.58.3.1. [DOI] [PubMed] [Google Scholar]
- 26.Feretti G, Bacchetti T, Negre-Salvayre A, Salvayre R, Dousset N, Curatola G. Structural modifications of HDL and functional consequences. Atherosclerosis. 2006;184:1–7. doi: 10.1016/j.atherosclerosis.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Hafstrom I, Rohani M, Deneberg S, et al. Effects of low-dose prednisolone on endothelial function, atherosclerosis, and traditional risk factors for atherosclerosis in patients with rheumatoid arthritis–a randomized study. J Rheumatol. 2007;34:1810–6. [PubMed] [Google Scholar]
- 28.Asanuma Y, Kawai S, Aoshima H, et al. Serum lipoprotein(a) and apolipoprotein(a) phenotypes in patients with rheumatoid arthritis. Arthritis Rheum. 1999;42:443–7. doi: 10.1002/1529-0131(199904)42:3<443::AID-ANR8>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 29.Dahlqvist SR, Engstrand S, Berglin E, et al. Conversion towards an atherogenic lipid profile in rheumatoid arthritis patients during long-term infliximab therapy. Scand J Rheumatol. 2006;35:107–11. doi: 10.1080/03009740500474578. [DOI] [PubMed] [Google Scholar]
- 30.Goswani B, Tayal D, Gupta N, Malika V. Paraoxonase: a multifaceted biomolecule. Clin Chim Acta. 2009;410:1–12. doi: 10.1016/j.cca.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 31.Gergely T, Molnar V, Semsei AF, et al. Gene expression profiling of experimental asthma reveals a possible role of paraoxonase-1 in the disease. Intern Immun. 2009;21:967–75. doi: 10.1093/intimm/dxp063. [DOI] [PubMed] [Google Scholar]
- 32.Selek A, Cosar N, Kocyigit N, et al. PON1 activity and total oxidant status in patients with active pulmonary tuberculosis. Clin Biochem. 2008;41:140–4. doi: 10.1016/j.clinbiochem.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 33.Rahman I. Regulation of glutathione in inflammation and chronic lung diseases. Mutat Res-Fund Mol M. 2005;79:58–80. doi: 10.1016/j.mrfmmm.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 34.Kondo A, Muranaka Y, Ohta I, et al. Relationship between triglycerides concentrations and LDL size evaluated by malondialdehyde-modified LDL. Clin Chem. 2001;47:893–900. [PubMed] [Google Scholar]
- 35.Roifman I, Beck PL, Anderson TJ, Eisenberg MJ, Genest J. Chronic inflammatory diseases and cardiovascular risk: a systematic review. Can J Cardiol. 2011;27:174–82. doi: 10.1016/j.cjca.2010.12.040. [DOI] [PubMed] [Google Scholar]
- 36.Kizer JR, Zisman DA, Blumenthal NP, et al. Association between pulmonary fibrosis and coronary artery disease. Arch Intern Med. 2004;164:551–6. doi: 10.1001/archinte.164.5.551. [DOI] [PubMed] [Google Scholar]
- 37.Rizzo M, Berneis K, Zeljkovic A, Vekic J. Should we routinely measure low-density and high-density lipoprotein subclasses. Clin Lab. 2009;55:421–9. [PubMed] [Google Scholar]