Abstract
Background: Although some articles have described renal sarcoidosis, the incidence among biopsy cases remains unclear. Here, we defined the incidence of renal sarcoidosis among renal biopsy cases and analyzed the clinical course. Methods: We performed an epidemiological study examining renal biopsy cases treated at 5 centers between January 2000 and September 2015 and identified 16 cases (7 men, 9 women; mean (±SD) age, 59.4±18.6 years) out of a total of 14191 renal biopsy cases. Renal involvement of sarcoidosis was defined as granulomatous tubulointerstitial nephritis, tubulointerstitial nephritis without granulomatous lesions, and renal calcinosis. Fifteen of the cases were treated with steroid therapy. One case initially received steroid pulse therapy. The outcome was evaluated based on the estimated glomerular filtration rate (eGFR), CKD stage, and the change in eGFR (ΔeGFR) after treatment. A favorable response was defined as ΔeGFR ≥25%. Results: The incidence of renal sarcoidosis was 0.11%. The mean eGFR was 28.2±16.1 mL/min/1.73 m2. At the last observation, the mean eGFR was 43.7±19.7 mL/min/1.73 m2. Although a favorable response to steroid therapy was found in the majority of cases (10/15, 67%), 12 of the 15 cases (80%) had residual renal dysfunction at the last observation and 8 cases (53%) had moderate to severe renal dysfunction. Conclusion: Renal sarcoidosis is extremely rare among renal biopsy cases. Among cases with an unfavorable response to steroid therapy, pathogenetic mechanisms other than sarcoidosis and severe nephron damage were observed. (Sarcoidosis Vasc Diffuse Lung Dis 2018; 35: 252-260)
Keywords: renal biopsy, renal sarcoidosis, tubulointerstitial nephritis, epidemiological study
Introduction
Sarcoidosis is a systemic inflammatory disease characterized by granulomatous lesions of unknown pathogenesis. The most commonly affected organs are the lungs and peripheral lymph nodes, followed by the eyes, liver, and muscles, etc. The incidence of renal sarcoidosis is lower than that of other organs. Post-mortem studies have reported incidences of renal sarcoidosis of 3%-19% among autopsy cases (1, 2). A diagnosis of the renal involvement of sarcoidosis is usually confirmed by a renal biopsy. In some case series of renal sarcoidosis, the initial number of renal biopsies was not described, and the incidence of renal sarcoidosis among renal biopsies remains unclear. Here, we reviewed renal biopsy cases and selected individuals with renal sarcoidosis based on defined criteria to investigate the epidemiology of this disease. Most of the cases had moderate to severe renal dysfunction even after treatment. We examined the correlations between treatment outcome, clinical characteristics, and pathology, especially among refractory cases, and compared the results with those of other reports.
Reserch design and methods
Diagnosis of renal sarcoidosis
Renal biopsies performed between January 2000 and September 2015 were reviewed. The cases diagnosed as renal sarcoidosis were selected from among 5 medical departments and affiliated hospitals (Tohoku University, JCHO Sendai Hospital, Nihon Medical University, Yamagata University, and Kitamurayama Hospital). We first examined whether these cases actually fulfilled the criteria for sarcoidosis based on the 2014 WASOG Statement (3).
Renal involvement of sarcoidosis was defined according to the presence of the following criteria:
Granulomatous tubulointerstitial nephritis
Tubulointerstitial nephritis without granulomatous lesions
Renal calcinosis
When a renal sarcoidosis candidate did not exhibit any granulomatous lesions in a renal biopsy, we also examined whether granulomatous lesions were present in other organs; cases without granulomatous lesions in any organ were excluded. Other diagnoses resulting in exclusion from the study were as follows: infectious diseases, connective tissue diseases, malignancy, and drug allergies. These diagnoses were made based on a clinical chart review, and 16 cases were finally enrolled in the study.
Renal pathology
The renal biopsies of each department were examined by a pathologist, and all the samples were reviewed by one specialized pathologist (J.K.). The biopsy specimens were fixed in formalin-alcohol, embedded in paraffin, and sectioned. Thin-slice sections were stained with hematoxylin-eosin, periodic acid-Schiff, Masson trichrome, and periodic acid-methenamine-silver. Immunostaining for IgG, IgA, IgM, C3, and C4 was performed using thin sections from frozen specimens or formalin-alcohol embedded specimens. The grades of tubulointerstitial fibrosis were defined according to the Banff classification (4), as follows: 0%-5% was defined as F0, 6%-25% was defined as F1 (mild), 26%-50% was defined as F2 (moderate), and >50% was defined as F3 (severe).
Clinical data at time of renal biopsy
Proteinuria was defined as a daily urinary protein level ≥300 mg/day or a urinary protein-urinary creatinine ratio ≥0.3 g/g·Cr, hematuria was defined as a red blood cell count of ≥5 per high-power field, and leukocyturia was defined as a white blood cell count of ≥5 per high-power field. The serum creatinine level was measured using an enzymatic method in each department, and the eGFR was calculated based on the serum creatinine level and patient age according to the “Revised equations for estimated GFR from serum creatinine in Japan” (5). The eGFR values were then graded according to the KDIGO 2012 Guidelines (6). We analyzed the relations between the eGFR at each time point and the grade of interstitial fibrosis. Hypercalcemia was defined as a corrected total plasma calcium level ≥10.5 mg/dL. The levels of serum angiotensin-converting enzyme and the blood cell counts were recorded. Anemia was defined as a hemoglobin level <13 g/dL for men and <11 g/dL for women.
Follow-up and Response to Therapy
We evaluated the outcomes and the responses to therapy based on the eGFR. The response to therapy was evaluated by the ΔeGFR at the final observation compared with the eGFR at the start of therapy. A favorable response was defined as ΔeGFR ≥25%. The CKD stage was also used to predict the patient outcome.
Statistics
We used SPSS Ver. 19 (IBM Corporation) and Statview Ver. 5 (SAS Institute) for the statistical analysis. Clinical data were described as the mean±standard deviation. The correlations among the variables were analyzed using the Spearman rank correlation coefficient. The differences in measurements between the groups were analyzed using the Mann-Whitney test. The distribution of elements among the groups was analyzed using the chi-square test.
Results
Case profiles at the time of renal biopsy
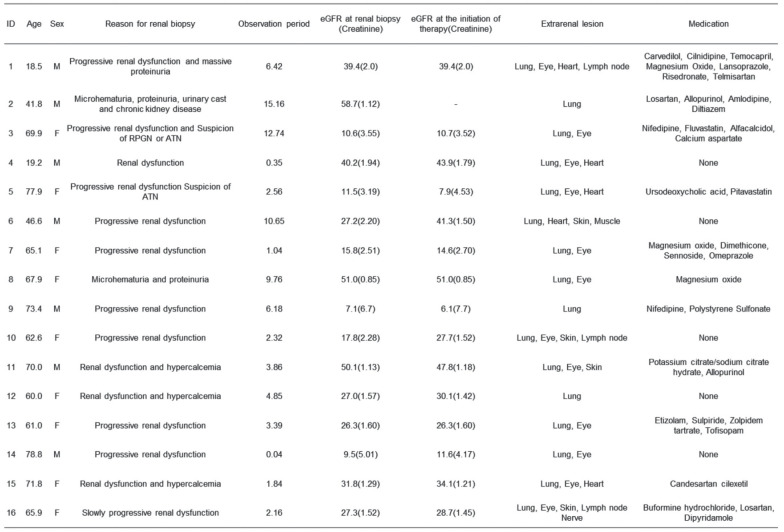
Table 1 shows the case profiles, as follows: reason for biopsy, eGFR at the time of biopsy and the initiation of therapy, extrarenal lesions, and medication. The average patient age was 59.4±18.6 years (18.5-78.8 years). The mean observation period was 5.2±4.6 years. At the time of the renal biopsy, the mean eGFR was 28.2±16.1 mL/min/1.73 m² (range, 7.1-58.7 mL/min/1.73 m²). At the initiation of therapy, the mean eGFR was 28.1±15.0 mL/min/1.73 m² (range, 6.1-51.0 mL/min/1.73 m²).
Table 1.
Case profiles
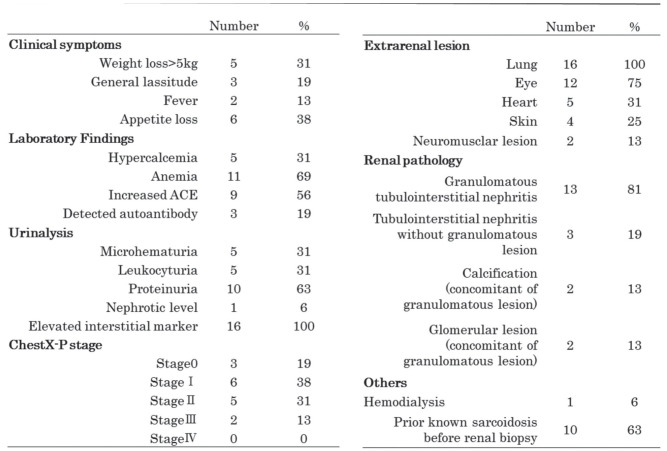
Clinical and laboratory findings and chest X-ray stage at the time of renal biopsy
Table 2 shows the clinical, laboratory, chest X-ray findings and extrarenal lesions at the time of renal biopsy. One case initially received temporary hemodialysis on two occasions because of severe renal dysfunction. After steroid therapy, hemodialysis was no longer required.
Table 2.
Clinical and laboratory findings, extrarenal involvement of sarcoidosis and renal pathology at presentations. Elevated interstitial marker means urinary level of α1-MG or β2·MG was above normal limit
Pathology
Among the main clinical findings leading to a renal biopsy, renal dysfunction was the most common finding (15 cases, 94%), and progressive renal dysfunction was the reason for the renal biopsy in 10 of these cases. In one case, persistent microhematuria and proteinuria were the main symptoms leading to the renal biopsy (Case 2).
The pathological findings were as follows: granulomatous tubulointerstitial nephritis was found in 13 cases (81%), tubulointerstitial nephritis without granuloma was observed in 3 cases (19%), renal calcinosis was observed in 2 cases (13%), and glomerular lesions were observed in 2 cases (13%). Glomerular lesions were observed in two cases (Case 1 and Case 2) with concomitant tubulointerstitial nephritis. Case 1 had focal segmental glomerulonephrosclerosis. After the initiation of treatment with an antihypertensive agent and subsequent steroid therapy, the renal function improved and the proteinuria decreased. Case 2 had hypertensive nephrosclerosis.
The degree of tubulointerstitial fibrosis was as follows: 10 cases (63%) were classified as F3, and the mean eGFR was 22.1±14.7 mL/min/1.73 m2 at the time of biopsy. The eGFR values of the cases classified as F3 were significantly lower than those of the 6 cases classified as F1 or F2 (mean eGFR, 38.4±13.7 mL/min/1.73 m2).
Treatment and Outcome
The mean daily loading dose of prednisolone (PSL) was 27.0 mg/day (0.5 mg/kg). One month after the initiation of treatment, the mean PSL dose was 19.7 mg (0.4 mg/kg). At 1 year after the initiation of treatment, the dose was 7.1 mg (0.2 mg/kg) in 11 cases, while one case (case 15) received 5 mg of PSL every other day. Among the cases with favorable responses, the mean daily loading dose of PSL was 29.5 mg/day (0.6 mg/kg) and the mean daily dose of PSL at 1 month after the initiation of treatment was 23.5 mg/day (0.5 mg/kg). Among the cases with unfavorable responses, the mean daily loading dose was 22.0 mg/day (0.4 mg/kg), and the mean daily dose of PSL at 1 month after the initiation of treatment was 12.0 mg/day (0.2 mg/kg). The period of steroid tapering was as follows: tapering was performed after 1 month in 10 cases, after less than 3 weeks in 4 cases, and after 6 weeks in 1 case. The mean duration of PSL treatment was 3.49 years.
The mean eGFR changed from 28.1±15.0 mL/min/1.73 m2 at the initiation of therapy to 43.5±20.4 mL/min/1.73 m2 at the final observation, and both eGFRs were significantly correlated with each other (r = 0.693, P = 0.004). Case 2 did not receive steroid therapy because nephrosclerosis was determined to be the main cause of renal injury. Case 8 received methotrexate because of PSL sparing. Oral PSL treatment was discontinued in 6 cases (Cases 6, 9, 12, 13, 15, and 16) because of the stabilization of renal function in 2 cases (Cases 6 and 9) and the lack of a response to treatment in 4 cases (Cases 12, 13, 15, and 16). The mean period until the discontinuation of treatment was 2.26 years. Recurrence was observed in one case (Case 11). Case 11 had received oral PSL, 30 mg/day, as an initial therapy. The eGFR increased from 47.8 mL/min/1.73 m2 to 64.3 mL/min/1.73 m2 at 1 month after the start of steroid therapy. At 1.5 years after steroid initiation, the eGFR had decreased from 54.1 to 42.3 mL/min/1.73 m2 during the 3-month period during which the oral PSL dose was tapered to 1 mg/day. Then, the oral PSL dose was increased to 15 mg/day, and the eGFR recovered to 56.4 mL/min/1.73 m2. Eight months later, the PSL dose was tapered. Two months after the temporary suspension, the eGFR decreased from 48.3 to 40.6 mL/min/1.73 m2. PSL at 10 mg/day was restarted, and maintenance therapy at 5 mg/day of PSL was continued until the final observation period. Immunosuppressants were not administered in this case.
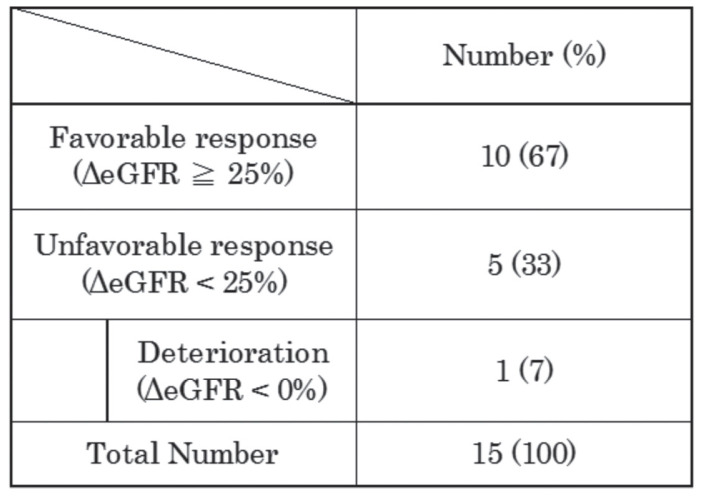
We evaluated the response to steroid therapy based on the ΔeGFR. A favorable response was observed in 60% of the cases that received steroid therapy (Table 3a). The distribution of cases with severe tubulointerstitial fibrosis did not differ according to the degree of the response to steroid therapy when examined using a chi-square test.
Table 3a.
Change in eGFR (ΔeGFR) at the final observation, compared with the eGFR at the start of steroid therapy among the cases that received the steroid therapy
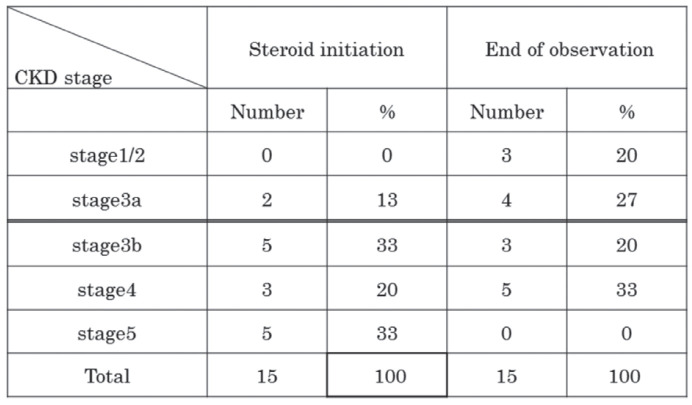
Table 3b shows the number of cases with each CKD stage at the start and end of steroid therapy. At steroid initiation, most of the cases had severe renal dysfunction (Stage 4/5 CKD). The number of cases with severe renal dysfunction decreased over time after steroid therapy.
Table 3b.
Chronic kidney disease (CKD) stage at steroid initiation and end of observation
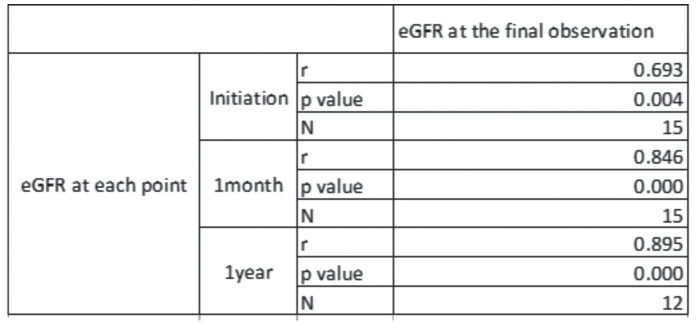
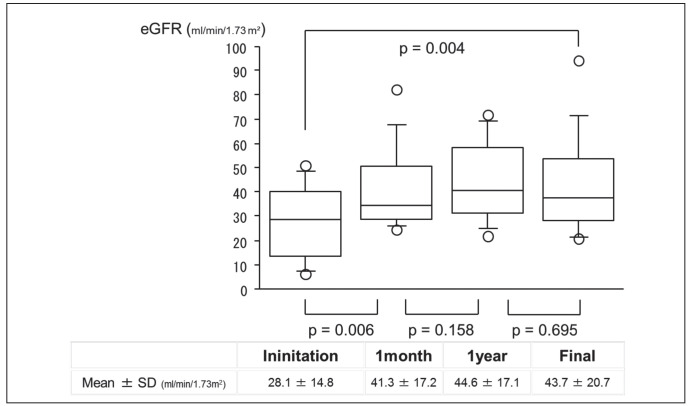
The eGFR values at the final observation were significantly correlated with the eGFRs at steroid initiation and at 1 month and 1 year after initiation (Table 4). Among the 13 cases with observation periods of over 1 year, we analyzed the time courses of eGFR after the steroid initiation. The eGFR values improved significantly after the steroid initiation, and this improvement was maintained until the final observations (Figure 1).
Table 4.
Correlations in eGFR between each time point and at the final observation. Data were analyzed by determining the Spearman Rank-order correlations coefficients.
Fig. 1.
Time course of estimated glomerular filtration rate (eGFR) values after steroid initiation among cases observed for over 1 year. Each value are analyzed using the Mann-Whitney test
The presence of hypercalcemia, hypertension, and diabetes mellitus did not have a significant impact on the steroid response. Furthermore, cases without renal granulomatous lesions did not respond to steroid therapy.
Discussion
Epidemiology
Most reports on renal sarcoidosis in which the diagnosis was based on a renal biopsy are case reports or case series. However, these reports did not describe the total number of renal biopsies performed during the study periods, and the incidence of renal sarcoidosis among renal biopsies has been unclear. Reports on the incidence of granulomatous tubulointerstitial nephritis may provide a clue to this issue (7, 8, 10). In these previous studies, the reported incidences of renal sarcoidosis were 0.11% (11 cases out of 9779 renal biopsy cases in the USA between 1987-2004 ), 0.18% (19 cases out of 10023 renal biopsy cases in the USA between 2000-2011), and 0.64% (20 cases out of 3132 renal biopsy cases in France between 1991-2004), which are all extremely low. The incidence of renal sarcoidosis was similar in these countries even though the annual incidences of sarcoidosis varied (Table 5). However, these studies did not include tubulointerstitial nephritis without granuloma or renal calcinosis lesion with renal involvement. In contrast, the present study also included interstitial nephritis cases without granulomatous lesions and cases with renal calcinosis lesions. Although the renal biopsies included in the present study were aggregated from multiple institutions in eastern Japan, the data likely reflect the actual condition of renal sarcoidosis in Japan because the rate of tubulointerstitial nephritis among the renal biopsies in the present study was 3.3%, which was equal to data from the Japan Renal Biopsy Registry (15). The correct diagnosis of renal involvement requires a renal biopsy. However, a biopsy is not always performed because of variations in the indications for performing a biopsy among institutions (16). Furthermore, the preferred indications for performing renal biopsy differ among countries (17). Therefore, the actual incidence of renal involvement is difficult to determine. In any case, renal sarcoidosis seems to be relatively rare among renal biopsy cases.
Table 5.
Comparison of present study results and those of biopsy based studies examing granulomatous tubulointerstitial nephritis
Clinical course
The majority of cases with renal sarcoidosis responded to steroid therapy. However, the renal outcomes were not ideal, since 12 cases (80%) exhibited residual renal dysfunction and 8 cases (53%) had moderate to severe renal dysfunction (Stage 3b/4). Most of the cases already had severe renal damage at the time of the renal biopsy. Ten cases (67%) had severe fibrosis (F3), and the mean eGFR of these cases was 22.1±14.7 mL/min/1.73 m2 before treatment. Furthermore, 8 cases (50%) were classified as Stage 4/5 CKD. Recovery to an eGFR above 60 mL/min/1.73 m2 only occurred in 3 cases, similar to the results reported by Singer (18). These results suggest that renal sarcoidosis was not detected at an early stage. In patients with renal sarcoidosis, especially tubulointerstitial nephritis, the predominant urinalysis findings are non-nephritic proteinuria or microhematuria. These findings might not be sufficient for physicians to perform a renal biopsy. Consequently, the onset of renal failure might be the predominant indication for renal biopsy, enabling treatment to only begin after the renal injury has already progressed.
The characteristics of the cases with unfavorable responses were as follows. Case 13 showed renal deterioration after treatment. This case had severe tubulointerstitial fibrosis and severe tubulointerstitial infiltration. Cases 15 and 16 had diffuse glomerular sclerosis (global sclerosis/total glomeruli: Case 15 = 85/92, Case 16 = 28/38). In Case 15, the renal pathology results showed severe tubulointerstitial fibrosis and severe inflammatory cell infiltration. In Case 16, the renal pathology results showed that renal ischemia was the main type of pathogenesis. Case 12 responded to steroid therapy at first, and the PSL dose was tapered. The eGFR had decreased slightly at the final observation (ΔGFR, 24%). Case 11 experienced a recurrence and showed an unfavorable response at the final observation, but the patient’s renal function remained within the category of mild dysfunction. Case 11 showed an improvement in renal function at 1 month after steroid initiation. Rajakariar reported that continuous low-dose steroid treatment can preserve renal function (19). The continuance of low-dose steroid treatment might enable a better outcome in this case.
Steroid therapy is effective for suppressing active inflammation, but other drugs that suppress subsequent fibrosis may be needed to further improve the renal prognosis. An anti-fibrotic agent that has been used for the treatment of interstitial pneumonia is one possible candidate for such treatment (20).
A differential diagnosis of granulomatous nephritis should include drug interactions, infectious diseases, connective tissue diseases, and malignancy (21). In the present study, all the registered cases had lung involvement and/or other organ involvement, and these systemic findings could not be explained by drug effects alone. A culture test, the biopsy specimens, and the course after treatment did not provide any evidence of bacterial infection in any of the cases. In 3 cases, autoantibodies (Case 3, anti-GBM antibody; Case 7, anti SS-A and SS-B antibodies; and Case16, anti-nuclear antibody) were detected, but symptoms typical of autoimmune diseases were not found. Case 9 had previously undergone a total gastrectomy for the treatment of gastric cancer, and a whole-body CT did not show the recurrence of gastric cancer.
In the present study, the renal granulomas were located in the tubulointerstitum, and not the glomerulus. Some cases with glomerular nephritis concomitant with sarcoidosis have been reported (22), but granulomatous lesions of the glomerulus have never been reported.
Comparison with other studies
In the present study, over 60% of the cases showed a favorable response, similar to the results reported by Löffler (23). The mean eGFRs before and after treatment in each study were as follows: Rajakariar (19), 26.8±14 and 47.9±6.8 mL/min/1.73 m2; Mahévas (24): 20±19 and 49.13±25 mL/min/1.73 m2; Löffler: 38±21 and 57±26 mL/min/1.73 m2; present study: 28.1±15.0 and 43.3±20.6 mL/min/1.73 m2. In Rajariar’s and Mahévas’s reports, the renal functions of most of the cases were severely impaired at the time of presentation. In Rajariar’s case series, the indication for biopsy was progressive renal impairment. In Mahévas’s and Löffler’s reports, the indications for biopsy were unclear. Therefore, we could not adequately explain what caused these variations in renal function at the time of presentation and at the final observation. A considerable number of cases seemed to have severe renal dysfunction at the time of the renal biopsy, similar to the present study. Among the above-mentioned reports (19, 23, 24), one case required maintenance dialysis.
Conclusion
Although previously unclear, the incidence of renal sarcoidosis among the renal biopsy cases in the present series was extremely low. Among cases with an unfavorable response to steroid therapy, the existence of another form of pathogenesis other than sarcoidosis and the presence of tubulointerstitial nephritis without granulomatous lesions were observed. The initial number of renal biopsies was thought to be large enough to define the incidence of renal sarcoidosis based on renal biopsy cases, and the present study is also the first epidemiological study to focus on renal biopsy cases of renal sarcoidosis in Asia.
Acknowledgements
The authors appreciate Yoshio Taguma, Isao Kubota, Satoko Honda, Rica Domon, Sachiko Fukase, and Naoki Akiyu, who contributed to the data collection.
Contributions:
Hiroshi Sato, Tsuneo Konta and Yoshinori Tsuchiya were responsible for the work in the field of nephrology. Kensuke Joe, Akira Shimizu and Shinobu Kunugi were responsible for the work in the field of renal pathology. Arata Azuma and R.P. Baughman reviewed and revised the manuscript as supervisors.
Ethics approval:
This study was approved by the ethics committees of each of the involved departments.
Availability of data and materials:
All data generated or analyzed during this study are included in this published article.
All the clinical data of patients was presented anonymously. No additional data is available.
References
- 1.Longcope WT, Freiman DG. A study of sarcoidosis; based on a combined investigation of 160 cases including 30 autopsies from The Johns Hopkins Hospital and Massachusetts General Hospital. Medicine (Baltimore) 1952 Feb;31(1):1–132. [PubMed] [Google Scholar]
- 2.Ricker W, Clark M. Sarcoidosis; a clinicopathologic review of 300 cases, including 22 autopsies. Am J Clin Pathol. 1949 Aug;19(8):725–49. doi: 10.1093/ajcp/19.8.725. [DOI] [PubMed] [Google Scholar]
- 3.Judson MA, Costabel U, Drent M, et al. The WASOG Sarcoidosis Organ Assessment Instrument: An update of a previous clinical tool. Sarcoidosis Vasc Diffuse Lung Dis. 2014 Apr 18;31(1):19–27. [PubMed] [Google Scholar]
- 4.Racusen LC, Solez K, Colvin RB, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999 Feb;55(2):13–23. doi: 10.1046/j.1523-1755.1999.00299.x. [DOI] [PubMed] [Google Scholar]
- 5.Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009 Jun;53(6):982–92. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 6.Adeera Levin, Paul E. Stevens. Summary of KDIGO 2012 CKD Guideline: behind the scenes, need for guidance, and a framework for moving forward. Kidney Int. 2014;85:49–61. doi: 10.1038/ki.2013.444. [DOI] [PubMed] [Google Scholar]
- 7.Bijol V, Mendez GP, Nosé V, Rennke HG. Granulomatous interstitial nephritis: a clinicopathologic study of 46 cases from a single institution. Int J Surg Pathol. 2006 Jan;14(1):57–63. doi: 10.1177/106689690601400110. [DOI] [PubMed] [Google Scholar]
- 8.Bagnasco SM, Gottipati S, Kraus E, et al. Sarcoidosis in native and transplanted kidneys: incidence, pathologic findings, and clinical course. PLoS One. 2014 Oct 20;9(10) doi: 10.1371/journal.pone.0110778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henke CE, Henke G, Elveback LR, Beard CM, Ballard DJ, Kurland LT. The epidemiology of sarcoidosis in Rochester, Minnesota: a population-based study of incidence and survival. Am J Epidemiol. 1986 May;123(5):840–5. doi: 10.1093/oxfordjournals.aje.a114313. [DOI] [PubMed] [Google Scholar]
- 10.Ungprasert P, Carmona EM, Utz JP, Ryu JH, Crowson CS, Matteson EL. Epidemiology of Sarcoidosis 1946-2013: A Population-Based Study. Mayo Clin Proc. 2016 Feb;91(2):183–8. doi: 10.1016/j.mayocp.2015.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Javaud N, Belenfant X, Stirnemann J, et al. Renal granulomatoses: a retrospective study of 40 cases and review of the literature. Medicine (Baltimore) 2007 May;86(3):170–80. doi: 10.1097/MD.0b013e3180699f55. [DOI] [PubMed] [Google Scholar]
- 12.Turiaf J, Brun J, Meyer A. Epidemiologic investigation on sarcoidosis in France. Acta Med Scand Suppl. 1964;425:129. doi: 10.1111/j.0954-6820.1964.tb05722.x. [DOI] [PubMed] [Google Scholar]
- 13.Morimoto T, Azuma A, Abe S, et al. Epidemiology of sarcoidosis in Japan. Eur Respir J. 2008 Feb;31(2):372–9. doi: 10.1183/09031936.00075307. [DOI] [PubMed] [Google Scholar]
- 14.Yamaguchi M, Hosoda Y, Sasaki R, Aoki K. Epidemiological study on sarcoidosis in Japan. Recent trends in incidence and prevalence rates and changes in epidemiological features. Sarcoidosis. 1989 Sep;6(2):138–46. [PubMed] [Google Scholar]
- 15.Sugiyama H, Yokoyama H, Sato H, et al. Japan Renal Biopsy Registry and Japan Kidney Disease Registry: Committee Report for 2009 and 2010. Clin Exp Nephrol. 2013 Apr;17(2):155–73. doi: 10.1007/s10157-012-0746-8. [DOI] [PubMed] [Google Scholar]
- 16.McQuarrie EP, Mackinnon B, Young B, et al. Centre variation in incidence, indication and diagnosis of adult native renal biopsy in Scotland. Nephrol Dial Transplant. 2009 May;24(5):1524–8. doi: 10.1093/ndt/gfn677. [DOI] [PubMed] [Google Scholar]
- 17.Fiorentino M, Bolignano D, Tesar V, et al. Renal Biopsy in 2015-From Epidemiology to Evidence-Based Indications. Am J Nephrol. 2016;43(1):1–19. doi: 10.1159/000444026. [DOI] [PubMed] [Google Scholar]
- 18.Singer DR, Evans DJ. Renal impairment in sarcoidosis: granulomatous nephritis as an isolated sarcoidosis (two case reports and review of the literature) Clin nephrol. 1986;26(5):250–6. [PubMed] [Google Scholar]
- 19.Rajakariar R, Sharples EJ, Raftery MJ, Sheaff M, Yaqoob MM. Sarcoid tubulo-interstitial nephritis: long-term outcome and response to corticosteroid therapy. Kidney Int. 2006 Jul;70(1):165–9. doi: 10.1038/sj.ki.5001512. [DOI] [PubMed] [Google Scholar]
- 20.Fukagawa M, Noda M, Shimizu T, Kurokawa K. Chronic progressive interstitial fibrosis in renal disease--are there novel pharmacological approaches. Nephrol Dial Transplant. 1999 Dec;14(12):2793–5. doi: 10.1093/ndt/14.12.2793. [DOI] [PubMed] [Google Scholar]
- 21.Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999 Aug;160(2):736–55. doi: 10.1164/ajrccm.160.2.ats4-99. [DOI] [PubMed] [Google Scholar]
- 22.Stehlé T, Joly D, Vanhille P, et al. Clinicopathological study of glomerular diseases associated with sarcoidosis: a multicenter study. Orphanet J Rare Dis. 2013 Apr 30;8:65. doi: 10.1186/1750-1172-8-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Löffler C, Löffler U, Tuleweit A, Waldherr R, Uppenkamp M, Bergner R. Renal sarcoidosis: epidemiological and follow-up data in a cohort of 27 patients. Sarcoidosis Vasc Diffuse Lung Dis. 2015 Jan 5;31(4):306–15. [PubMed] [Google Scholar]
- 24.Mahévas M, Lescure FX, Boffa JJ, et al. Renal sarcoidosis: clinical, laboratory, and histologic presentation and outcome in 47 patients. Medicine (Baltimore) 2009 Mar;88(2):98–106. doi: 10.1097/MD.0b013e31819de50f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
All the clinical data of patients was presented anonymously. No additional data is available.