Abstract
Introduction: Interstitial lung diseases (ILDs) are a heterogeneous group of lung disorders characterized by dyspnea on exertion, exercise-induced hypoxemia and reduced exercise tolerance. There are some evidences that pulmonary rehabilitation (PR) successfully treats these manifestations. Objective: To identify if pulmonary rehabilitation can achieve a clinically significant improvement of functional exercise capacity measured by 6-minute walk test (6-MWT) and arterial blood gases analysis (ABG) in patients with ILDs. Methods: A retrospective secondary analysis of the patients’ records from in-patients of a pulmonary rehabilitation center between 2012 and 2015. Profiles of 38 patients who had completed 4-6 PR weeks were included. 6-MWT distance, dyspnea and fatigue Borg score and ABG at baseline and the end of the pulmonary rehabilitation were compared. Results: There was a statistically and clinically significant improvement in 6-MWT distance after PR with a mean difference for change in distance walked of 68.5±54.2 m. The pre post PR variation of dyspnea and fatigue Borg score significantly improved (-2.3±3.7, and -1.7±2.9, always p≤0.001). Among the ABG parameters, only the resting PaO2 showed a significant improvement after PR (Delta PaO2= 4.6±8.5 mmHg, p=0.005). Conclusions: A 4-6 week of PR improves functional exercise capacity and hypoxemia in patients with ILDs. (Sarcoidosis Vasc Diffuse Lung Dis 2018; 35: 245-251)
Keywords: interstitial pulmonary diseases, rehabilitation, exercise, 6-minute walk test
Introduction
The interstitial lung diseases (ILDs) are an incapacitating group of chronic respiratory diseases characterized by symptoms such as dyspnoea, cough, fatigue, that tends to worsen with disease progression (1). In particular, dyspnoea on exertion, limited exercise capacity and dry cough are the clinical distinguishing marks of ILDs (2, 3). Consequently, they progressively reduce their activity levels and this, in turn, worsens exercise capacity, increases symptoms (4)and, finally, impair health-related quality of life (4). There are few effective and/or well-tolerated pharmaco-therapies available today for many ILD subtypes, sometimes, being oxygen therapy the only treatment applied (2, 5). Therefore, other therapeutic options such as pulmonary rehabilitation (PR) which may relieve symptoms, without changing disease course, have been recently considered (6, 7).
However, although there is plenty of data showing a significant and clinically meaningful improvement in functional exercise capacity and psychological condition after PR in subjects with chronic obstructive pulmonary disease (COPD), only limited information is available to date regarding its role in subjects with ILD (2). In fact, only low-grade recommendations on exercise training for subjects with ILD are provided in official guidelines for PR (8-10).
In contrast to these recommendations mounting evidence indicate that PR can result in clinically relevant improvements also in subjects with ILD (1, 5, 11-13), although these improvements may vary greatly, depending on a number of factors in primis the clinico-radiological subtype (14). Furthermore, the effect of PR on arterial blood gases analysis (ABG) have been barely studied in ILD subjects and the few data available in the literature have been conflicting.
The aim of this study was to assess the short-term effects of a 4-6 week PR program on functional exercise capacity measured by six-minute walk test (6-MWT) and ABG in a group of patients with ILD.
Patients and methods
The current study was a retrospective secondary analysis of a database of ILD participants in an in-patients hospital-based pulmonary rehabilitation program in Pulmonary Rehabilitation Unit of IRCCS Salvatore Maugeri Foundation (n=45). The database included profiles of pulmonary rehabilitation participants from 2012-2015.
Inclusion criteria. The inclusion criteria for the current study were as follows: documented diagnosis of ILD as defined by the ATS/ERS/JRS/ALAT statement (9); clinically stable patients for the previous four weeks; absence of exacerbation during PR; absence of co-morbidities interfering with exercise training (such as severe neurologic deficits, inability to walk unassisted because of orthopedic deficit, unstable cardiac disease); absence of participation in PR program in the past 12 months.
Pulmonary function test. Flow rates were determined using automated equipment (V Max 22 System SensorMedics, Milan, Italy) according to ATS/ERS recommendations (15). Static lung volumes were determined by the helium dilution method (16).
Six minute walk test. 6-MWT was performed according to ATS recommendations (17). The test was performed along the same air-conditioned, 15-meter long hospital corridor. The walk was symptom limited, so patients were allowed to stop, if necessary; but they had to continue again once they were rested. The distance covered during the test (6-MWD) was expressed in meters. During the test, the oxygen saturation was continuously monitored by pulse oxymeter via a finger electrode, and minimum value recorded for oxyhaemoglobin saturation was considered. Patients were asked to rate their symptoms both at the beginning and end of the test using the modified Borg CR10 scale (18).
Arterial blood gases analysis. ABG was performed at resting by means of an automated analyzer (Gas analyzer ABL 330; Radiometer, Copenhagen, Denmark), while the subject was breathing room air for at least 30 minutes in the sitting position.
Pulmonary rehabilitation program. The pulmonary rehabilitation program in this study took place in a hospital between 2012 and 2015. PR program included educational intervention (once per week) and endurance training sessions (five times per week) for 4-6 weeks. Endurance training sessions were performed under the supervision of an experienced PR physiotherapist. The educational session was delivered as a talk by experienced physiotherapist and topics included breathing control, airway clearance, relaxation, nutrition, devices, and oxygen use. The exercise session consisted in: 1) general exercise, including mobilization and prevention of chest rigidity using respiratory education techniques (abdominal-diaphragmatic ventilation, thoracic mobilization, control of dyspnea); 2) endurance training session performed on treadmill or cycle ergometer. Exercise intensity was personalized on the base of individual tolerance, physiological parameters or physiotherapist judgment. Baseline exercise intensity on treadmill was set at 70% of the average speed of the 6-MWT and subsequently enhanced by increasing duration, speed, and possibly, slope. Differently, bicycle exercise intensity was guided by the patients’ self-assessment using the Borg scale (score between 4 to 6 for dyspnea and fatigue). The length of exercise session was of 30 minutes or less when the subject deemed it necessary. Supplemental oxygen was provided during exercise session if necessary to achieve SaO2 above 90%.
Data collection procedures. Institutional review board approval was obtained for this study from the Maugeri foundation. The records were cleaned, reviewed and analyzed. The variation (post – pre PR treatment) of six parameters (the 6-MWT distance covered, the effort induced dyspnea Borg scores, the effort induced fatigue Borg scores as well as the resting PaO2, PaCO2 and arterial blood pH) were evaluated to assess the effect of the PR program. These differences (delta) between post- and pre- treatment values were considered as the outcomes of the rehabilitation program.
Statistical Analysis
Student t test for paired data was used to assess the significance of the outcomes after PR. Multiple linear regression analysis was used to assess, on each of the significant outcome variations, the possible effect of the confounders/effect-modifiers, namely age, sex, FVC, FEV1, TLC, CRF and DLCO. Each of the models included the pre-PR values. All analyses were performed with 0.05 type I error using STATA 14 software (Stata Corp. LP College Station, Texas, USA).
Results
Sixty-one patients were selected. Twenty-one patients were excluded due to non-compliance to treatment or missing data. Two patients were excluded for exacerbation before completion of PR. Thirty-eight patients were included. Thirty patients were diagnosed as IPF, four had combined pulmonary fibrosis and emphysema, two had non-specific interstitial pneumonia, and pulmonary fibrosis was associated to arthritis rheumatoid in two patients.
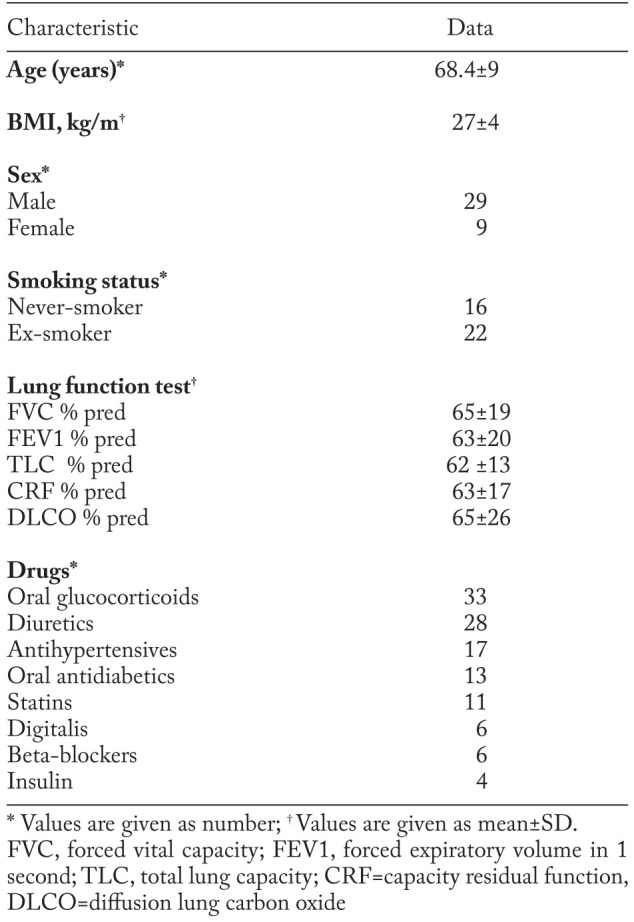
Clinical and functional characteristics of patients are reported in table 1. Oxygen therapy was required in all subjects during exercise, and in 25 of them at rest. Five of the studied patients were not taking drugs.
Table 1.
Baseline subject characteristics
There were no serious adverse events during PR.
Outcome evaluation
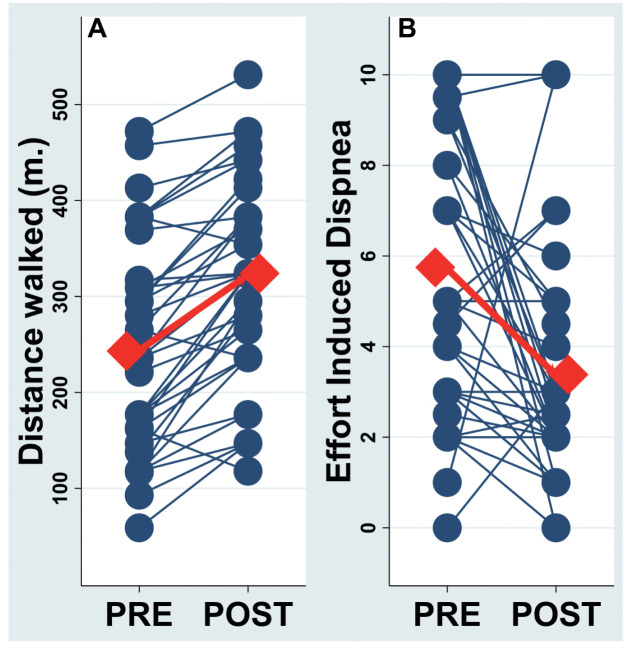
The pre- PR 6-MWT distance (6-MWD) was 240±110 m (range 59-557 m) and significantly increased to 309±106 m (range 118-531 m) (p<0.0001) after PR. The mean difference for change in distance walked was 68±54 m (figure 1 panel A).
Fig. 1.
Panel A: Individual and mean difference for change in distance walked at 6 minute-walking test before (pre) and after (post) pulmonary rehabilitation. Panel B: Individual and mean changes of the effort induced dyspnea before (pre) and after (post) pulmonary rehabilitation
Thirty-five patients showed an increase in 6-MWD after PR (11 increase 1-50 m; 13 increase 51-100 m; 9 increase 101-150 m and 2 increase >151 m). An improvement greater than 25m was observed in thirty-one subjects. Three subjects showed a decreasing 6-MWD after PR (all showed a decrease <50 m).
The pre- and post- PR resting dyspnea Borg score (0.8±1.3 and 0.45±0.9, respectively), as expected, significantly (p≤0.001) increased after effort (6.5±3.4 and 3.8±2.9, respectively). Of note, albeit resting values were not significantly different, the pre post PR variation of the effort induced dyspnea (EID), i.e. the rest-effort variation modulated by the PR, was (pre PR EID=5.7±3.1, post PR EID=3.4±2.6, Delta EID=-2.3±3.7, p≤0.001. Fig 1 panel B).
Similarly the pre- and post- PR resting fatigue score were 1.3±1.7 and 0.6±1.4, and both significantly increased at the end of 6-MWT to 3.9±3.8 and 2.2±2.6 respectively (always p≤0.001). The pre post PR variation of the effort induced fatigue score significantly improved (-1.7±2.9, p≤0.001).
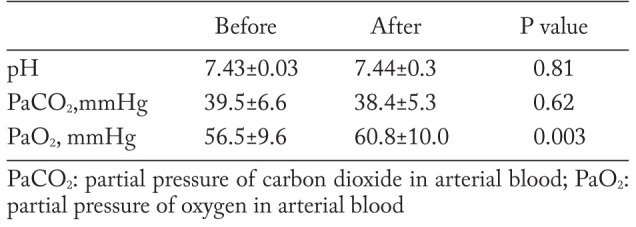
The resting PaO2 showed a significant improvement after PR (Delta PaO2=4.6±8.5 mmHg, p=0.005) (figure 2), while the resting pH and PaCO2 did not (Delta pH=0.008±0.03; Delta PaCO2= -1.2±5.0) (table 2).
Fig. 2.
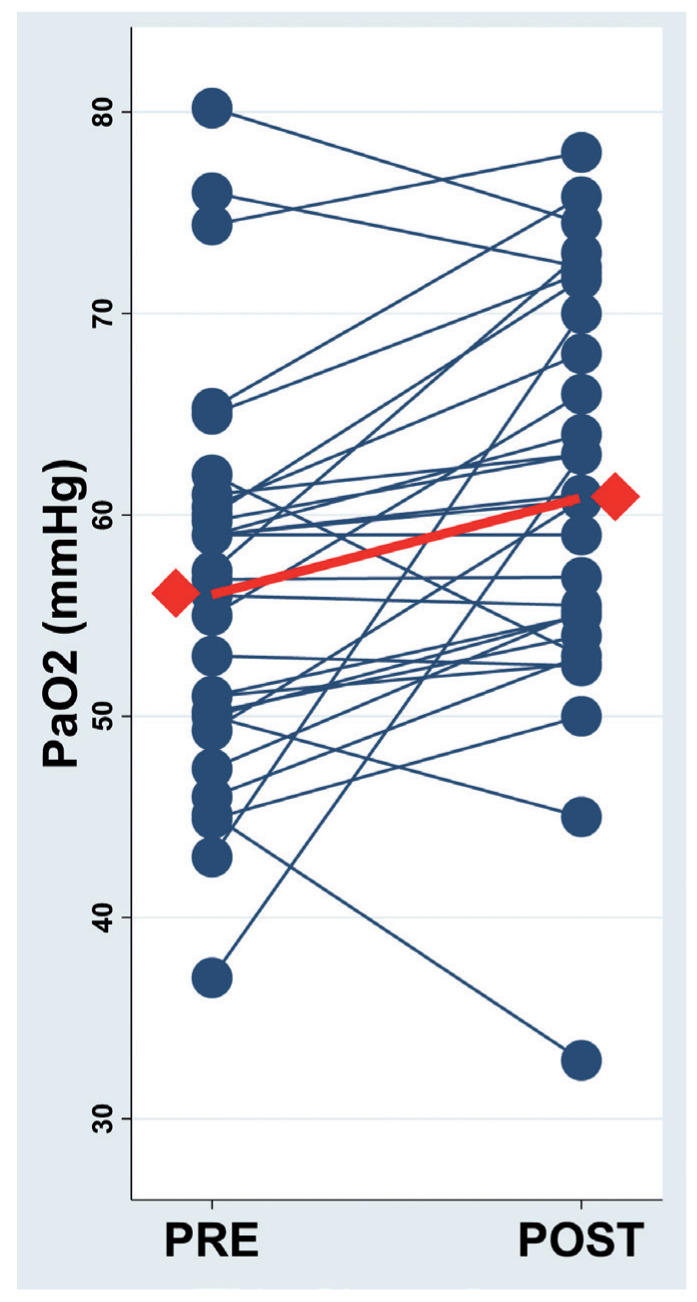
Resting PaO2 before (pre) and after (post) pulmonary rehabilitation.
Table 2.
Results of resting arterial blood gas analysis before and after pulmonary rehabilitation
With multiple regression analysis the pre-post PR variations (delta) of both the 6-MWD and EID were associated only with the pre-treatment respective values (Delta 6-MWD → pre-treatment 6-MWD r2=0.15, coef=-0.19±0.09, p≤0.05; Delta EID → pre-treatment EID r2=0.55, coef=-0.9±0.16, p≤0.001 (figures 3 and 4).
Fig. 3.
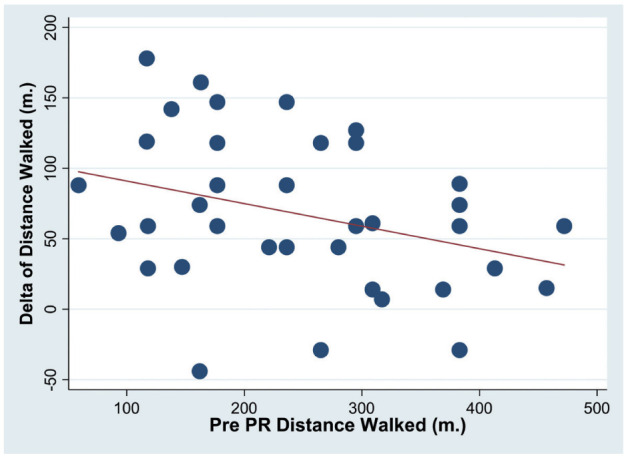
Association of pre-post (Delta) rehabilitation variations of the 6-MWD with pre PR 6-MWD
Fig. 4.
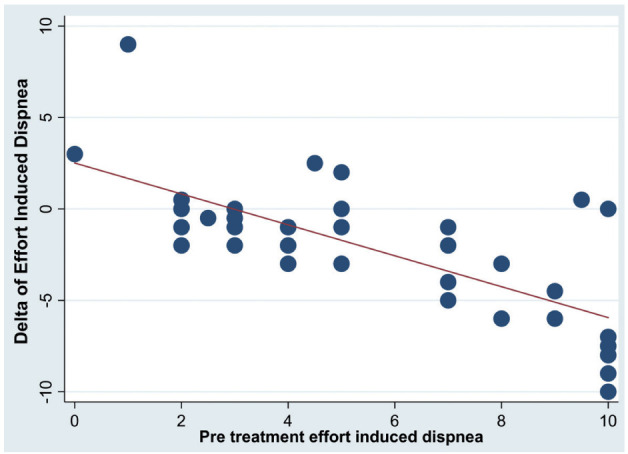
Association of pre-post (Delta) rehabilitation variations of the effort induced dyspnea (EID) with pre PR value of EID
The pre-post PR variations of the resting PaO2 were associated with the TLC and FVC pre-PR values (Delta PaO2 r2=0.34, TLC coef=47.5±16.8, p=0.01; FVC coef=-40.4±14.1, p=0.01).
Discussion
The present study shows that substantial improvement in functional exercise capacity and PaO2 value can be obtained in subjects with ILD following a PR program of 4-6 weeks. This is in line with most part of data reported in previous studies on this topic, although there are only weak recommendations in international guidelines for considering exercise training in ILDs (8-10).
A Cochrane review (19) analysed the effects of PR in ILDs. Most studies included participants with several types of ILD. These studies had differing lengths of PR programme, from 5 to 12 weeks. Meaningful improvements in exercise capacity were seen following PR, with a weighted mean difference for change in 6-MWD of 44 m. In particular, Nishiyama et al. observed an increase of 46 m in a group of subjects with IPF after a PR program (12). Huppmann et al. in a cohort of 402 consecutive subjects with interstitial lung disease reported an increase of 46 m in 6-MWD (2) and Holland et al. in a group of 57 subjects with ILD reported that 6-MWD increased of a mean of 35 m compared with controls after 8 weeks of exercise training (5).
Recently Dowman et al.(20) in a randomised, controlled trial evaluated the effectiveness of 8 week PR in 142 stable patients with exertional dyspnoea and a variety of ILD aetiologies. The Authors found that following the PR programme, exercise capacity and health status had improved compared with usual care, though whole group improvement in 6-MWD was below the minimum clinically important difference for ILDs, suggesting that benefits of PR in terms of exercise capacity in this group are relatively modest.
In our study 6-MWD improved of 68 m at the end of PR. This value is well above the results reported by the majority of previous studies but are in line with those by Swigris et al. reporting a mean increase of 61 m in a small group of IPF subjects (21). Salhi et al. assessed the effectiveness of a PR in 31 patients with restrictive lung disease 11 of whom had ILD. Overall, all subjects improved their functional exercise capacity, but those with ILD experienced an impressive improvement in 6-MWD increasing by 79 m at 12 weeks and 102 m after the full 24 weeks, compared with their baseline values (3). The effects of PR in ILD subjects are highly variable depending on a number of factors such as subtype, extent of exercise desaturation, presence of pulmonary hypertension, and baseline exercise performance (19). The differences between our results and those reported in the studies mentioned above might result either from differences in the characteristics of the subjects studied or by differences in the structure of PR program provided or from both.
Previous studies reported that PR is more beneficial in ILD subjects more severely impaired (13). In the present study, baseline functional exercise capacity of study subjects was more severely impaired as compared to the majority of the above referenced studies. Furthermore, in those studies, PR was performed as an outpatient program and the frequency of endurance training session was 2 per week. In our study PR program was performed as in-patients and the frequency of endurance training sessions was 5 per week. In the present study PR was structured in 20-30 sessions of endurance training over a period of 4-6 weeks, while in most of the previous studies, a smaller number of sessions was distributed over a longer period. Similarly to previous studies (3, 5) (6, 21), the gains in exercise capacity in the present study were paralleled with an improvement in both dyspnea and fatigue rating scores. In another study (2), although PR had positive effects on functional exercise capacity dyspnea rating scores were unchanged.
The short-term gain in functional exercise capacity we observed can result from overcoming the deconditioned physical status generally presented by subjects with ILD. In fact, exercise limitation in ILDs is multifactorial, with contributions including impairment of gas exchange and pulmonary circulation, ventilatory and muscle dysfunction. The latter is an emerging area that might be particularly amenable to amelioration with PR (1). In consideration that exercise capacity has been recognized as a relevant prognostic factors in subjects with IPF (22), its improvement can have great clinical relevance.
Only few reports have studied the effects of PR on blood gases in ILDs and their results have been conflicting. Holland et al. reported a statistically significant improvement in both PaO2 and PaCO2 (6), while no significant improvement at the end of PR in ABG was reported by others (12). We found a statistically significant improvement in PaO2 value but no change in PaCO2 value was observed.
As in other respiratory diseases, we found that baseline clinical variables were poorly predictive of the response to exercise training, and this work provides no basis for using pulmonary function markers of severity to exclude individuals with ILD from exercise training. Interestingly, in our study we found that the pre-post treatment variations of both the 6-MWD and EID were inversely associated only with the pre-treatment respective values. These results confirm previous studies, which reported that PR is more beneficial in IPF patients more severely impaired (13) and those most limited at baseline tended to have the greatest short-term benefit from PR, a pattern that is also recognised in COPD and has been noted previously in ILD (23).
Some limitations of the present study must be underlined. The primary limitation of this study is the retrospective non-randomized design without a control group. Second the sample size of the study population was limited, but because of the rarity of the disease. However, the magnitude of the observed differences between baseline and PR allowed a statistically significant detection. Third, the 6-MWT was performed by several examiners, although all were experienced physiotherapists and the tests were conducted in accordance with current standards. Finally, in this study the long-term effects of PR have not been investigated. Despite these limitations, this study adds data to the mounting literature in favor of PR in patients with ILD.
In conclusion, our results demonstrate that PR improve functional exercise capacity in subjects with ILD. Prospective controlled studies are needed to address unanswered questions, such as how to optimize training to increase benefit, how to maintain the benefit obtained and prevent its decline, and most importantly, what strategy to adopt to promote long-term adherence to exercise training.
Acknowledgements
We thank Anna Ciullo, Maria Rosaria Guarente for technical help.
References
- 1.Nakazawa A, Cox NS, Holland AE. Current best practice in rehabilitation in interstitial lung disease. Ther Adv Respir Dis. 2017;11:115–28. doi: 10.1177/1753465816676048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huppmann P, Sczepanski B, Boensch M, et al. Effects of inpatient pulmonary rehabilitation in patients with interstitial lung disease. Eur Respir J. 2013;42:444–53. doi: 10.1183/09031936.00081512. [DOI] [PubMed] [Google Scholar]
- 3.Salhi B, Troosters T, Behaegel M, et al. Effects of pulmonary rehabilitation in patients with restrictive lung diseases. Chest. 2010;137:273–9. doi: 10.1378/chest.09-0241. [DOI] [PubMed] [Google Scholar]
- 4.Collard HR, King TE, Jr, Bartelson BB, et al. Changes in clinical and physiologic variables predict survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2003;168:538–42. doi: 10.1164/rccm.200211-1311OC. [DOI] [PubMed] [Google Scholar]
- 5.Holland AE, Hill CJ, Conron M, et al. Short term improvement in exercise capacity and symptoms following exercise training in interstitial lung disease. Thorax. 2008;63:549–54. doi: 10.1136/thx.2007.088070. [DOI] [PubMed] [Google Scholar]
- 6.Holland AE, Hill CJ, Glaspole I, et al. Predictors of benefit following pulmonary rehabilitation for interstitial lung disease. Respir Med. 2012;106:429–35. doi: 10.1016/j.rmed.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 7.Swigris JJ, Brown KK, Make BJ, et al. Pulmonary rehabilitation in idiopathic pulmonary fibrosis: a call for continued investigation. Respir Med. 2008;102:1675–80. doi: 10.1016/j.rmed.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradley B, Branley HM, Egan JJ, et al. Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and New Zealand and the Irish Thoracic Society. Thorax. 2008;63(Suppl 5):v1–58. doi: 10.1136/thx.2008.101691. [DOI] [PubMed] [Google Scholar]
- 9.Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spruit MA, Singh SJ, Garvey C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188:e13–64. doi: 10.1164/rccm.201309-1634ST. [DOI] [PubMed] [Google Scholar]
- 11.Johnson-Warrington V, Williams J, Bankart J, et al. Pulmonary rehabilitation and interstitial lung disease: aiding the referral decision. J Cardiopulm Rehabil Prev. 2013;33:189–95. doi: 10.1097/HCR.0b013e31828db112. [DOI] [PubMed] [Google Scholar]
- 12.Nishiyama O, Kondoh Y, Kimura T, et al. Effects of pulmonary rehabilitation in patients with idiopathic pulmonary fibrosis. Respirology. 2008;13:394–9. doi: 10.1111/j.1440-1843.2007.01205.x. [DOI] [PubMed] [Google Scholar]
- 13.Ryerson CJ, Cayou C, Topp F, et al. Pulmonary rehabilitation improves long-term outcomes in interstitial lung disease: a prospective cohort study. Respir Med. 2014;108:203–10. doi: 10.1016/j.rmed.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 14.Pisano V, Fuschillo S, Balzano G. Histopathology and exercise: a winning combination in pulmonary fibrosis: a case report. Respir Care. 2014;59:e31–4. doi: 10.4187/respcare.02520. [DOI] [PubMed] [Google Scholar]
- 15.Brusasco V, Crapo R, Viegi G. Coming together: the ATS/ERS consensus on clinical pulmonary function testing. Eur Respir J. 2005;26:1–2. doi: 10.1183/09031936.05.00034205. [DOI] [PubMed] [Google Scholar]
- 16.Wanger J, Clausen JL, Coates A, et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26:511–22. doi: 10.1183/09031936.05.00035005. [DOI] [PubMed] [Google Scholar]
- 17.Holland AE, Spruit MA, Troosters T, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014;44:1428–46. doi: 10.1183/09031936.00150314. [DOI] [PubMed] [Google Scholar]
- 18.Mahler DA, Horowitz MB. Perception of breathlessness during exercise in patients with respiratory disease. Med Sci Sports Exerc. 1994;26:1078–81. [PubMed] [Google Scholar]
- 19.Dowman L, Hill CJ, Holland AE. Pulmonary rehabilitation for interstitial lung disease. Cochrane Database Syst Rev: CD006322. 2014 doi: 10.1002/14651858.CD006322.pub3. [DOI] [PubMed] [Google Scholar]
- 20.Dowman LM, McDonald CF, Hill CJ, et al. The evidence of benefits of exercise training in interstitial lung disease: a randomised controlled trial. Thorax. 2017;72:610–9. doi: 10.1136/thoraxjnl-2016-208638. [DOI] [PubMed] [Google Scholar]
- 21.Swigris JJ, Fairclough DL, Morrison M, et al. Benefits of pulmonary rehabilitation in idiopathic pulmonary fibrosis. Respir Care. 2011;56:783–9. doi: 10.4187/respcare.00939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Triantafillidou C, Manali E, Lyberopoulos P, et al. The Role of Cardiopulmonary Exercise Test in IPF Prognosis. Pulm Med. 2013:514817. doi: 10.1155/2013/514817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferreira A, Garvey C, Connors GL, et al. Pulmonary rehabilitation in interstitial lung disease: benefits and predictors of response. Chest. 2009;135:442–7. doi: 10.1378/chest.08-1458. [DOI] [PubMed] [Google Scholar]