Abstract
Rationale: Sarcoidosis is an inflammatory disorder of unclear etiology with historical significance in the U.S. Department of Defense (DoD). Objectives: This study sought to characterize the sarcoidosis population within the DoD Military Health System (MHS). Methods: Adult patients with sarcoidosis were identified in the DoD MHS database from 01-JAN-2004 through 31-DEC-2013. Patients required ≥3 encounters with a sarcoidosis diagnosis and continuous MHS eligibility. Index was defined as date of first sarcoidosis encounter. Comorbidities were assessed within the pre-index and follow-up periods. Additionally, a subset of sarcoidosis patients was identified as having conditions that can be associated with cardiac sarcoidosis. Measurements and Main Results: The final sarcoidosis cohort was 9,908 patients, 57% female, and had a mean (SD) age of 53.1 (13.6) years. The region with the largest population was the east coast (45.6%). The top 5 pre-index comorbidities were hypertension (51.7%), fatigue (27.0%), anemia (21.4%), diabetes, type II (19.6%), and coronary heart disease (16.5%). Prevalence of the following conditions increased ≥2-fold from pre-index to follow-up: leukocytopenia, pulmonary hypertension, chronic kidney disease, thrombocytopenia, hypercalcemia, venous thromboembolism, congestive heart failure, seizure disorder, stroke/TIA, hypercalciuria, and arthritis. Of the sarcoidosis cohort, 21.8% (n=2,164) were identified as having cardiac conditions that can be associated with cardiac sarcoidosis. The top conditions in this cohort were cardiac arrhythmia (75.6%), congestive heart failure (20.4%), and cardiomyopathy (13.6%). Conclusions: The MHS has a large population of sarcoidosis patients, of which 22% had cardiac conditions that can be associated with granulomatous inflammation of the heart. Prevalence of numerous comorbid conditions increased after sarcoidosis diagnosis. (Sarcoidosis Vasc Diffuse Lung Dis 2018; 35: 261-267)
Keywords: sarcoidosis, cardiomyopathies, military
Introduction
Sarcoidosis is a systemic granulomatous disease of unknown etiology that typically affects young and middle-aged adults. The diagnosis is normally established with clinicoradiological findings and histopathologic specimens that demonstrate evidence of non-caseating epithelioid cell granulomas. Other causes of collections of non-caseating granulomas, such as infections or localized sarcoidal reactions must be ruled out (1). Any organ system can be affected, although the lung is affected in over 90% of people, followed by the skin, lymph nodes, eye, and liver, respectively (2). There is often a delay in diagnosis from symptom onset, and only about 50% of people will be diagnosed within three months of symptoms onset (3). The natural history of sarcoidosis is poorly understood, which makes the decision to treat difficult. Some patients will have spontaneous resolution, while others will develop chronic and progressive disease. First line therapy in treatment of sarcoidosis is systemic steroids. When steroid therapy fails other agents such as methotrexate, azathioprine, hydroxychloroquine, leflunomide, or infliximab can be used with variable success (4). Studies have suggested environmental exposures, such as insecticides and microbial bioaerosols (2), genetics, and occupations in the military and agriculture (3) may be associated with sarcoidosis.
Sarcoidosis has received historical attention in the United States (U.S.) Department of Defense (DoD). In 1974, a 21-year-old enlisted Navy service member was diagnosed with sarcoidosis based on chest radiographs with bilateral hilar adenopathy and a lymph node biopsy demonstrating non-caseating granulomas. He also had dyspnea, cough, and chest and joint pain, which he attributed to his work of grinding anti-skid materials from aircraft carrier decks. He ultimately received a medical discharge for sarcoidosis in 1975. However, in 1987, physicians at the U.S. Department of Veterans Affairs changed his diagnosis to pneumoconiosis after mineral dust deposits were found on lung biopsy. The CDC’s National Institute for Occupational Safety and Health launched an investigation at the request of the patient to see if his case and others like his were related to occupational exposure while in the service of the U.S. Navy (5). A case-control study by Jajosky demonstrated increased odds of having sarcoidosis in those individuals assigned to industrial or “dirty” ships, the majority of which were aircraft carriers (6). Another study by Gorham and others of 674 sarcoidosis hospitalizations again demonstrated increased odds for diagnosis of sarcoidosis in those U.S. Navy personnel assigned to an aircraft carrier. This led to the conclusion that some people may have been mislabeled as sarcoidosis or that sarcoidosis had a previously unrecognized occupational exposure (7).
A Case-Control Study of Sarcoidosis (ACCESS) study enrolled 736 incident cases from 10 participating centers to assess the demographics of sarcoidosis patients. The study population was heterogeneous in terms of race (53% white, 44% black), sex (64% female, 36% male), and age (46% <40 years old, 54% ≥40 years old) (2). Women were more likely to have eye and neurologic involvement, have erythema nodosum, and be ≥40 years old. Men were more likely to be hypercalcemic. Black patients were more likely to have skin involvement other than erythema nodosum, eye, liver, bone marrow, and extrathoracic lymph node involvement. The study found that lower income, the absence of private or Medicare health insurance, and other barriers to care were associated with sarcoidosis severity at presentation, as were race, sex, and age (2).
Since the natural history of sarcoidosis is poorly understood, decisions regarding treatment can be difficult. This study sought to determine the disease burden of sarcoidosis within the U.S. DoD Military Health System (MHS).
Methods
This study was conducted using the U.S. DoD MHS data contained within the MHS Data Repository (MDR), which provides a longitudinal, electronic health record for all eligible beneficiaries and includes integrated administrative, medical, clinical, and pharmacy data. This database covers nearly 10 million active MHS beneficiaries, comprised of active or retired service members and their dependents, receiving care throughout the country. The MHS consists of care delivered within any military facility, including 65 hospitals and over 400 clinics, as well as within the vast network of civilian facilities that bill TRICARE, the insurance arm of the DoD, for delivery of care.
Research data were derived from an approved Naval Medical Center Portsmouth institutional review board (IRB) protocol with waiver of consent. All data were de-identified prior to analysis, and the research was conducted in compliance with federal and state laws, including the Health Insurance Portability and Accountability Act of 1996 (HIPAA) and Safe Harbor guidelines.
This study queried all MHS eligible adult beneficiaries for the presence of sarcoidosis over a 10-year period covering January 01, 2004 through December 31, 2013. Sarcoidosis patients were required to have ≥3 medical encounters occurring on separate dates with a sarcoidosis diagnosis (ICD-9-CM code 135) present in any available diagnosis field with the earliest encounter considered index, continuous MHS eligibility covering a minimum of 12 months prior to index through 6 months post index, ≥18 years of age at index, and no presence of diagnosis codes over the entire study period for any of the following: mycobacteria, tuberculosis, histoplasmosis, blastomycosis, pneumocysotis, hypersensitivity pneumonitis, berylliosis, common variable immunodeficiency, eosinophilic granuloma, granulomatosis polyangitis, polylymphomatoid granulomatosis, immune reconstitution inflammatory syndrome.
Among those meeting all sarcoidosis cohort requirements, a subset of patients with cardiac involvement additionally required ≥1 encounter with a diagnosis and/or procedure code for any of the following: atrioventricular block, bundle branch block, cardiac arrhythmia, cardiomyopathy, congestive heart failure, heart block, implantable cardiac device/pacemaker, pulmonary hypertension, and no presence of diagnosis codes over the entire study period for coronary artery disease.
This retrospective observational study was descriptive in nature. Patient characteristics were tallied among all sarcoidosis patients, where age and region were defined as of index and assessment of baseline comorbid conditions of interest were respective of the 12 months prior to index. These same conditions were also assessed during follow-up, i.e., post index, to determine to what extent the prevalence of each condition changed among our cohort after initial sarcoidosis diagnosis. Among the sarcoidosis cohort with cardiac involvement, the frequency of each cardiac criterion used in identification of the cohort was tallied.
Results
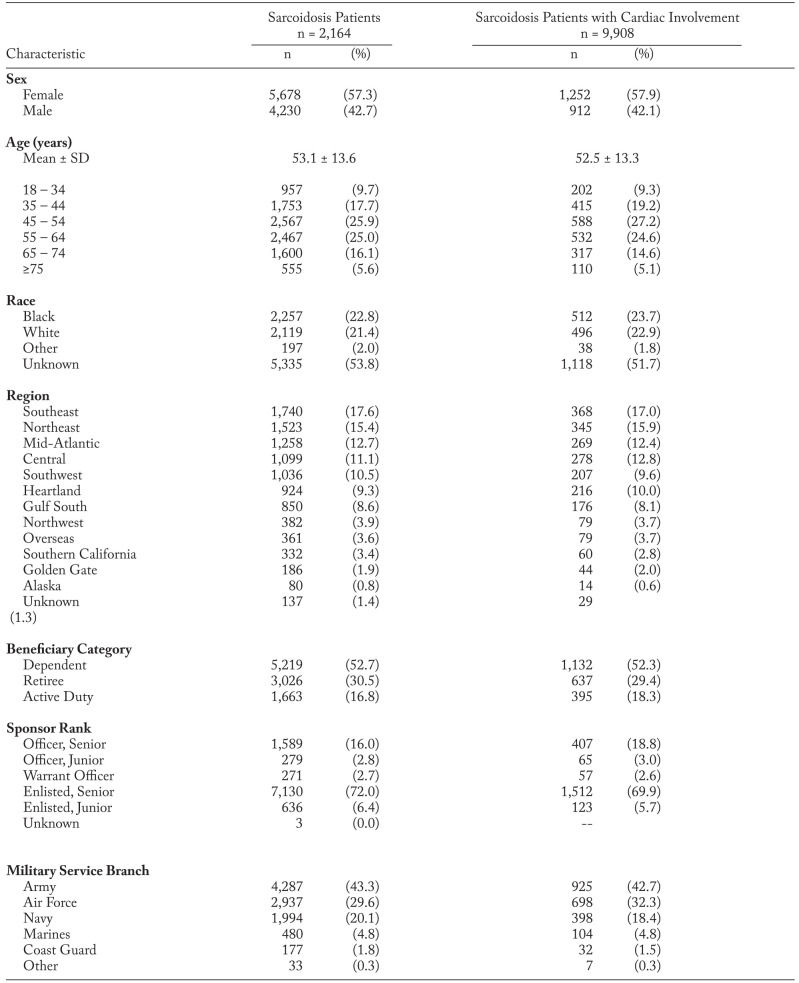
On initial query there were close to thirty thousand adult individuals with ≥1 diagnosis of sarcoidosis. Upon applying all requirements for case identification, the final study cohort consisted of 9,908 sarcoidosis patients, which equates to a prevalence of 77.5 per 100,000 persons across the 10-year period. The cohort was predominantly female (57.3%) and had a mean (SD) age of 53.1 (13.6) years. Race was unknown for the majority of patients (53.8%) along with 22.8% black, 21.4% white, and 2.0% other. The largest population of sarcoidosis patients at index was found on the east coast (45.6%), which encompasses the northeast, mid-Atlantic, and southeast MHS regions.
Over half (52.7%) of the sarcoidosis cohort were dependents of active or retired service members with the remaining patients split between retired (30.5%) and active (16.8%). In terms of military significance, 43.3% of patients were members or sponsored by members of the Army, followed by the Air Force (29.7%) and the Navy (20.1%), and the majority of sarcoidosis patients were enlisted or sponsored by enlisted personnel (78.4%) as compared to officers. These same trends were also seen among the subset of sarcoidosis patients with cardiac involvement. Patient characteristics for both the sarcoidosis cohort and the cardiac subset of patients are presented in Table 1.
Table 1.
Baseline Characteristics of Sarcoidosis Patients in the MHS, 2004 - 2013
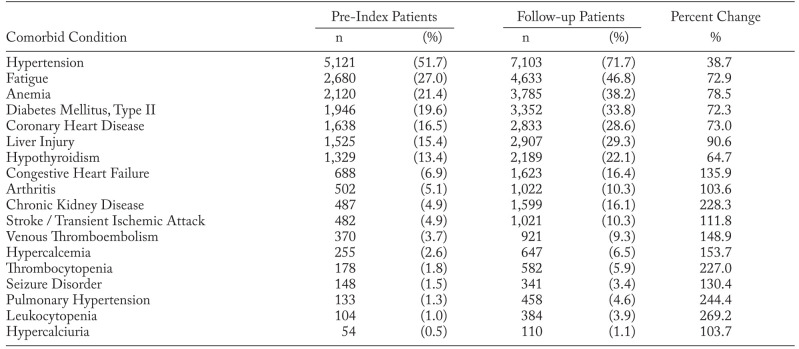
The most prevalent baseline comorbid conditions of interest were: hypertension (51.7%), fatigue (27.0%), anemia (21.4%), diabetes, type II (19.6%), and coronary heart disease (16.5%). Compared to baseline, the prevalence of the following conditions increased ≥2-fold in the follow-up period: leukocytopenia, pulmonary hypertension, chronic kidney disease, thrombocytopenia, hypercalcemia, venous thromboembolism, congestive heart failure, seizure disorder, stroke/TIA, hypercalciuria, and arthritis. The full list of conditions under study is presented in Table 2.
Table 2.
Change in Prevalence of Comorbid Conditions from Prior to Sarcoidosis Diagnosis to Follow-up
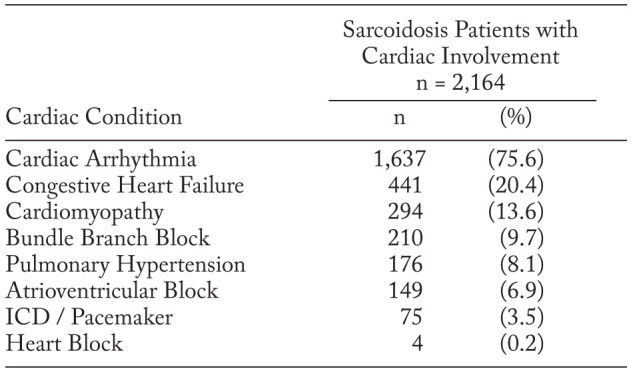
Among the sarcoidosis cohort, 21.8% (n=2,164) had diagnoses that could be consistent with cardiac sarcoidosis. These patients were predominantly female (57.9%) and had a mean (SD) age of 52.5 (13.3) years. Over half (51.7%) of race data were unknown followed by 22.9% white, 23.7% black, and 1.8% other. As outlined in Table 3, cardiac arrhythmia was the top cardiac condition used for identification with 75.6% of patient reporting, followed by congestive heart failure (20.4%) and cardiomyopathy (13.6%).
Table 3.
Prevalence of Cardiac Conditions Among Sarcoidosis Patients with Cardiac Involvement
Discussion
Over a 10-year period, almost ten thousand individuals in the MHS carried a diagnosis of sarcoidosis equating to a prevalence of 77.5 per 100,000 persons. The sex distribution in this study was similar to the ACCESS study with the MHS documenting 57% of the sarcoidosis population as female compared to 64% in ACCESS (2). In a study of sarcoidosis among 1.2M U.S. Navy recruits, the highest incidence was seen in the southeastern U.S. with the lowest in the western region of the country (8). In the current study close to half of the sarcoidosis patients were found on the east coast, with the largest percentage in the southeast region along with a relatively low number of patients in the western regions. Similar geographic patterns also were seen in a large study of U.S. Navy and Marine Corps patients as well as among U.S. Medicare Part B beneficiaries over the age of 65 (9, 10).
Symptomatic cardiac sarcoidosis is estimated to only affect about 5% of sarcoidosis patients in the U.S. However, autopsy studies report that approximately 20% of patients have pathologic evidence of cardiac sarcoidosis (11). This is an important diagnosis to make as the first manifestation of myocardial involvement can be sudden death (12). After excluding coronary artery disease, almost a quarter of the patients in our study had a cardiac condition that could be linked to cardiac sarcoidosis. The case definition used to identify sarcoidosis patients with cardiac involvement relied solely on the presence of diagnoses and/or procedures. As advanced cardiac imaging is needed for a definitive diagnosis, the true prevalence of cardiac sarcoidosis in this population is suspected to be lower.
Limitations
This study was a retrospective analysis that utilized data points originally collected for electronic health record and accounting/claims purposes, rather than research. We relied on the presence of ICD-9 codes to make the diagnosis. As sarcoidosis is a diagnosis of exclusion there could be misdiagnosed patients in our cohort, though, the likelihood of misclassification is expected to be diminished as patients were required to carry a diagnosis of sarcoidosis over at least three visits to a healthcare professional. Given that sarcoidosis is a rare entity in the pediatric population, we limited our cohort to those patients 18 years of age and older, which likely does not greatly impact the number in our cohort (13). The case definition used to identify the sarcoidosis cohort with cardiac involvement was based upon conditions known to be associated with granulomatous infiltration of the myocardium (14). Accurate diagnosis of cardiac sarcoidosis requires histologic evidence of noncaseating granulomatous inflammation of the myocardium or findings on advanced cardiac imaging consistent with cardiac sarcoidosis. Myocardial biopsies and cardiac imaging results were not available in this study. Race is not a mandated demographic field for DoD beneficiaries, which leads to a predominance of incomplete race data in the database and this study.
Conclusion
Sarcoidosis is an inflammatory multisystem disease with unclear etiology and is characterized by the presence of noncaseating granulomas in the affected organs. Our retrospective analysis of U.S. DoD beneficiaries with sarcoidosis showed patient characteristics similar to those found in past studies of sarcoidosis patients in the U.S. It was noted that almost 22% of sarcoidosis patients in this study had cardiac conditions that can be associated with cardiac sarcoidosis with the most common condition being arrhythmia, followed by congestive heart failure, and cardiomyopathy. This does not definitively give a diagnosis of cardiac sarcoidosis but suggests that clinically significant cardiac sarcoidosis may be higher in this cohort than reported in other studies. There was a predominance of sarcoidosis patients on the east coast which includes the northeast, mid-Atlantic, and southeast MHS regions. Just over half of the population were dependents of active or retired service members, and in terms of military branch, most were sponsors or sponsored by the Army, followed by the Air Force and Navy, respectively.
Acknowledgements
The authors would like to thank the Navy and Marine Corps Public Health Center for its support during the conduct of this study.
At a Glance Commentary:
This study identifies a significant population of sarcoidosis patients within the Military Health System. The prevalence of multiple comorbid conditions increased after a patient was diagnosed with sarcoidosis. Patients with cardiac conditions that can be related to granulomatous inflammation of the myocardium was 20% of the sarcoidosis cohort, which matches previous autopsy studies, but is much higher than that reported in clinical studies.
Disclaimers:
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Air Force, Department of the Army, Department of the Navy, Department of Defense, nor the U. S. Government. The opinions and research contained herein are the private ones of the authors and are not to be considered as official or reflecting the views of Health ResearchTx LLC. All authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Copyright Statement:
LCDR Thuy Lin is a military service member. This work was prepared as part of her official duties. Title 17 U.S.C. 105 provides that “Copyright protection under this title is not available for any work of the United States Government.” Title 17 U.S.C. 101 defines a United States Government work as a work prepared by a military service member or employee of the United States Government as part of that person’s official duties.
Statement of Ethics:
Research data derived from an approved Naval Medical Center, Portsmouth, Virginia Institutional Review Board (Institutional Animal Care and Use Committee) protocol number NMCP.2014.0009.
Funding Statement:
This study was funded by Health ResearchTx, LLC.
References
- 1.Statement on Sarcoidosis. The joint statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS), and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) was adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160:736–755. doi: 10.1164/ajrccm.160.2.ats4-99. [DOI] [PubMed] [Google Scholar]
- 2.Baughman RP, Teirstein AS, Judson MA, Rossman MD, Yeager H, Jr, Bresnitz EA, DePalo L, Hunninghake G, Iannuzzi MC, Johns CJ, McLennan G, Moller DR, Newman LS, Rabin DL, Rose C, Rybicki B, Weinberger SE, Terrin ML, Knatterud GL, Cherniak R. Case Control Etiologic Study of Sarcoidosis (ACCESS). Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164:1885–1889. doi: 10.1164/ajrccm.164.10.2104046. [DOI] [PubMed] [Google Scholar]
- 3.Judson MA, Thompson BW, Rabin DL, Steimel J, Knattereud GL, Lackland DT, Rose C, Rand CS, Baughman RP, Teirstein AS. ACCESS Research Group. The diagnostic pathway to sarcoidosis. Chest. 2003;123:406–412. doi: 10.1378/chest.123.2.406. [DOI] [PubMed] [Google Scholar]
- 4.Baughman RP, Nunes H. Therapy for sarcoidosis: evidence-based recommendations. Expert Rev Clin Immunol. 2012;8(1):95–103. doi: 10.1586/eci.11.84. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC) MMWR Morb Mortal Wkly Rep. 1997;46(23):539–543. [PubMed] [Google Scholar]
- 6.Jajosky P. Sarcoidosis diagnoses among U.S. military personnel: trends and ship assignment associations. Am J Prev Med. 1998;14(3):176–183. doi: 10.1016/s0749-3797(97)00063-9. [DOI] [PubMed] [Google Scholar]
- 7.Gorham ED, Garland CF, Garland FC, Kaiser K, Travis WD, Centeno JA. Trends and occupational associations in incidence of hospitalized pulmonary sarcoidosis and other lung diseases in Navy personnel: a 27-year historical prospective study, 1975-2001. Chest. 2004;126(5):1431–1438. doi: 10.1378/chest.126.5.1431. [DOI] [PubMed] [Google Scholar]
- 8.Sartwell P, Edwards L. Epidemiology of sarcoidosis in the U.S. Navy. American Journal of Epidemiology. 1974;99(4):250–257. doi: 10.1093/oxfordjournals.aje.a121609. [DOI] [PubMed] [Google Scholar]
- 9.Gundelfinger BF, Briten SA. Sarcoidosis in the United States Navy 1,2. Am Rev Respir Dis. 1961;84:5P2. doi: 10.1164/arrd.1961.84.1.109a. [DOI] [PubMed] [Google Scholar]
- 10.Wills A, Adjemian J, Manganiello VC, Fontana JR, Prevots DR. Prevalence of sarcoidosis among U.S. Medicare Part B beneficiaries aged ≥65 years, 2000-2007. Am J Respir Crit Care Med. 2015:A3751. [Google Scholar]
- 11.Lynch JP, Hwang J, Bradfield J, Fishbein M, Shivkumar K, Tung R. Cardiac involvement in sarcoidosis: evolving concepts in diagnosis and treatment. Semin Respir Crit Care Med. (3rd) 2014;35:372–390. doi: 10.1055/s-0034-1376889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tavora F, Cresswell N, Li I, Ripple M, Solomon C, Burke A. Comparison of necropsy findings in patients with sarcoidosis dying suddenly from cardiac sarcoidosis versus dying suddenly from other causes. Am J Card. 2009;104(4):571–577. doi: 10.1016/j.amjcard.2009.03.068. [DOI] [PubMed] [Google Scholar]
- 13.Nathan N, Marcelo P, Houdouin V, Epaud R, de Blic J, Valeyre D, Houzel A, Busson PF, Corvol H, Deschildre A, Clement A. RespiRare and the French Sarcoidosis groups. Lung sarcoidosis in children: update on disease expression and management. Thorax. 2015;70(6):537. doi: 10.1136/thoraxjnl-2015-206825. [DOI] [PubMed] [Google Scholar]
- 14.Greulich S, Deluigi CC, Gloekler S, Wahl A, Zürn C, Kramer U, Nothnagel D, Bültel H, Schumm J, Grün S, Ong P, Wagner A, Schneider S, Nassenstein K, Gawaz M, Sechtem U, Bruder O, Mahrholdt H. CMR imaging predicts death and other adverse events in suspected cardiac sarcoidosis. JACC Cardiovasc Imagin. 2013;6(4):501–511. doi: 10.1016/j.jcmg.2012.10.021. [DOI] [PubMed] [Google Scholar]