Abstract
Background: Some data suggest that anti-inflammatory macrolides may be effective to treat organizing pneumonia (OP) and prevent relapses, but no formal comparison with prednisone alone is available. To explore this issue, we retrospectively compared the efficacy of a 12-week combined regimen of clarithromycin and prednisone with a 24-week prednisone alone regimen in OP. Methods: A standard 12-week regimen of combined clarithromycin and prednisone was designed for the treatment of cryptogenic or radiation-induced OP, aiming at reducing the cumulated prednisone dose and the relapse rate. Its use was left to the discretion of the treating physicians, members of the Groupe d’Etudes et de Recherche sur les Maladies Orphelines Pulmonaires. Data were compared to a historical control group treated with a standard 24-week prednisone alone regimen. Results: 16 patients were treated with combined therapy and 21 with prednisone alone. Complete radiological remission was achieved in 63% of the combined therapy group and 81% of the prednisone alone group (p=0.38). Symptomatic relapses occurred in 81% of the combined therapy group, and 52% of the prednisone alone group (p=0.14). No side effect of clarithromycin was reported. Conclusions: In patients with cryptogenic or radiation-induced OP, a 12-week regimen of clarithromycin and prednisone showed no benefit on remission rate and relapse rate as compared to a 24-week prednisone only regimen. (Sarcoidosis Vasc Diffuse Lung Dis 2018; 35: 230-238)
Keywords: cryptogenic organizing pneumonia, lung diseases, interstitial, glucocorticoids, clarithromycin, treatment outcome, recurrence
Introduction
Organizing pneumonia (OP) is a distinct clinicopathological syndrome characterized by the subacute onset of cough, fever, dyspnea, patchy alveolar infiltrates at chest imaging, and buds of granulation tissue filling distal airspaces with overall preservation of the lung architecture (1-9). OP may be associated with various clinical conditions (secondary OP, SOP) or occur without detectable cause (cryptogenic OP, COP).
Corticosteroids are a very effective treatment of OP, but side effects occur in 25% of patients and relapses are common when steroids are tapered or stopped (3, 4, 10). We previously reported a standardized corticosteroid treatment regimen for COP (10), which proved highly effective and allowed a 50% reduction of the cumulative dose of prednisone as compared to other regimens. However, little attempts have been made to investigate the efficacy of other treatment modalities in OP.
In addition to antimicrobial effects, some macrolide antibiotics have anti-inflammatory properties, and several studies have demonstrated the beneficial effects of azithromycin in various conditions including cystic fibrosis (CF) (11), non-CF bronchiectasis (12), diffuse pan-bronchiolitis (13, 14), bronchiolitis obliterans syndrome after lung transplantation (15, 16) and chronic obstructive pulmonary disease (17-19). Besides inflammatory airway diseases, case reports and small retrospective series have suggested that erythromycin and clarithromycin could be of interest for the treatment of OP as an adjunct or an alternative to prednisone, and could reduce the relapse rate (20-24). Three studies also reported that macrolides decrease pro-inflammatory cytokine production alveolar macrophages and neutrophils in OP (24-26). However, macrolide treatment in OP has hitherto not been standardized and has not been formally compared to prednisone therapy.
The objective of the present study was to determine whether a standardized combination of clarithromycin and prednisone could allow to further reduce both the cumulative dose of prednisone and the relapse rate in OP, as compared to the previously published regimen of prednisone alone.
Methods
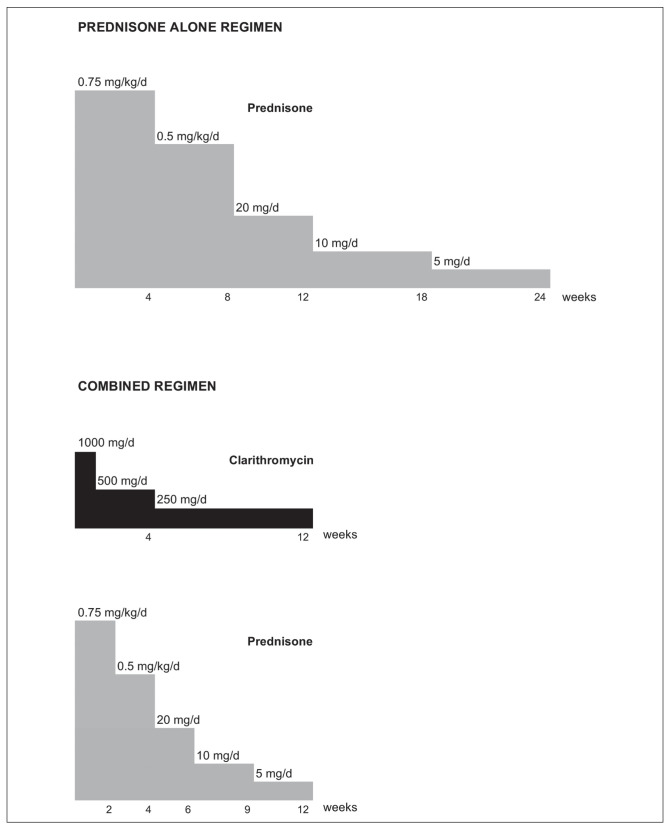
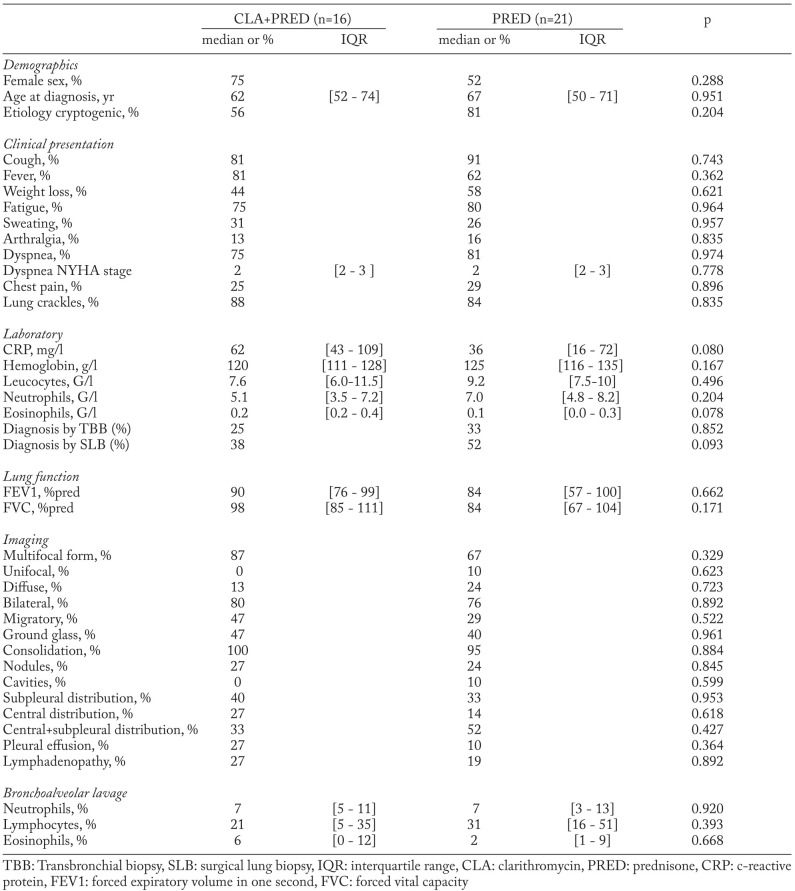
In 2008, a standard treatment protocol was defined by the Groupe d’Etudes et de Recherche sur les Maladies “Orphelines” Pulmonaires (GERM”O”P), a French group dedicated to the study of rare pulmonary diseases. This combined treatment protocol was based on the previously published prednisone alone protocol (10) with 2 changes: 1) addition of clarithromycin to prednisone, and 2) reduction of the treatment duration from 24 to 12 weeks. This duration was based on previous reports showing that macrolides may provide response in this time frame (20, 22, 24, 27). Prednisone was added to provide a rapid relief of symptoms, because clarithromycin alone appeared to have a slow onset of action in previous reports, and it was considered unethical to treat patients without prednisone given its rapid effect and high efficacy in OP. The combined treatment regimen is shown in figure 1. Relapses occurring under or after combined therapy were treated with prednisone alone according to a previously published 24-weeks protocol for relapses (20 mg/d prednisone for 12 weeks, then 10 mg/d for 6 weeks, then 5 mg/d for 6 weeks) (10). The combined treatment protocol was proposed to GERM”O”P members as a treatment option which could be offered to patients with OP, at the discretion and under the responsibility of the treating physician, in the absence of contra-indication to clarithromycin. Protocol modifications or switches to prednisone alone were allowed when deemed useful.
Fig. 1.
Prednisone alone and combined clarithromycin and prednisone protocols
In 2012, a detailed questionnaire was sent to physicians having reported cases of OP treated with the clarithromycin + prednisone combination, to analyze its efficacy retrospectively. Questionnaires were filled by reviewing medical records. Retrieved items included demographic data, co-morbidities, cigarette smoking, medication, symptoms, laboratory values, pulmonary function testing, results of biopsies, bronchoalveolar lavage, chest X-rays and CT-scan results, and follow-up information. All previously published causes of OP such as infections, drugs, or collagen-vascular diseases where explicitly looked for.
Cases were included if they met all the following criteria:
Clinical and imaging features characteristic of OP, with no follow-up event which could challenge the initial diagnosis.
Diagnosis of COP, or OP due to radiation therapy for breast cancer. This particular cause of OP was accepted as its clinical presentation is highly similar to COP, and because its cause can be confidently identified.
Histopathological proof of OP pattern at surgical or transbronchial lung biopsy in COP. This criterion was not required for OP due to radiation therapy for breast cancer, as the clinical and imaging features in this particular context allow a confident diagnosis in the absence of lung biopsy.
Treatment with a combination of clarithromycin and prednisone according to the 2008 protocol prescribed for the first episode of OP, on an intention-to-treat basis.
Cases were excluded if they met any of the following criteria:
OP other than cryptogenic or secondary to radiation therapy for breast cancer
Lack of histological proof of OP pattern in COP.
Treatment of clarithromycin and prednisone prescribed for a relapse.
A historical control group consisted of consecutive patients treated with the previously published prednisone alone protocol for COP or OP due to radiotherapy for breast cancer in GERM”O”P participating centers before 2008 and/or after 2012.
The main endpoints were the radiological response rates and the relapse rate. Treatment efficacy was categorized by the treating physician as follows based on radiological assessment: 1) complete remission, 2) more than 50% improvement, 3) less than 50% improvement, 4) no improvement.
Numerical data were expressed as medians and interquartile range. Comparisons between continuous variables were made by the Mann-Whitney test. Proportions were compared by the chi-square test.
Results
Case selection and study population
Twenty-nine questionnaires were available for analysis. Thirteen cases were excluded for the following reasons: OP associated with systemic sclerosis (n=1), combined treatment given for a relapse (n=7), and COP without histological proof (n=5). The remaining 16 cases (9 COP and 7 OP due to radiation therapy for breast cancer) were included. All patients had a compatible clinicoradiological picture and no event during follow-up which could challenge the diagnosis of OP. Histopathological specimens were available in all cases of COP, either by surgical lung biopsy (6 cases), or transbronchial biopsy (3 cases).
The control group consisted of 21 patients treated for a first episode of OP with the standard prednisone alone protocol. There were 4 cases of OP secondary to radiation therapy and 17 COP. Seven cases of COP were proven by surgical lung biopsy, and 10 by transbronchial biopsy.
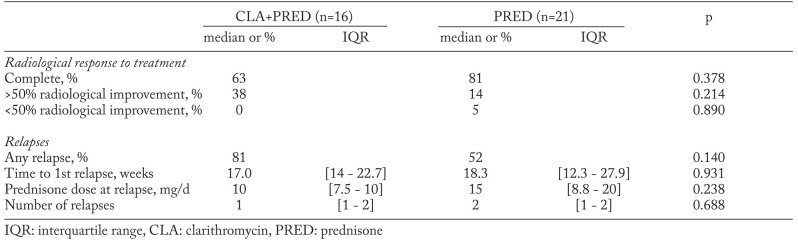
Patients’ baseline characteristics are shown in Table 1. There were no statistically significant differences between groups for demographic variables, clinical features, laboratory data, lung function tests, chest imaging and BAL. The median follow-up duration was similar in both groups (1.7 vs 1.3 years, p=0.510).
Table 1.
Patients characteristics
Response to treatment and relapse rate
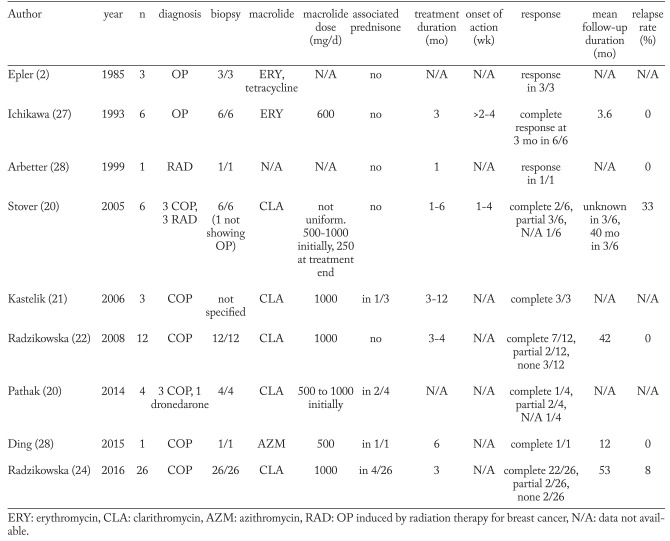
Treatment response data were available for all patients. The response rates and the relapse rates are shown in Table 2. Fifteen patients (94%) received exactly the planned doses of clarithromycin and prednisone, and in one case the combination therapy was altered at the discretion of the treating physician. Complete remission was observed in 63% of the combined treatment group and in 81% of the prednisone alone group (p=0.38). All the remaining patients had a >50% improvement, except one in the prednisone alone group who was considered to have <50% improvement.
Table 2.
Response to treatment
One or more relapses occurred in 13 (81%) patients of the combined treatment group and in 11 (52 %) of the prednisone alone group (p=0.14). The diagnosis of relapse was clinical and radiological in all but one patient who had a surgical lung biopsy. The time to the first relapse was similar in both groups (median 17 vs 18.3 weeks, p=0.931). Among the patients who relapsed, the median number of relapses was 1 in the combination group and 2 in the prednisone group (range 1 to 5, p=0.688). In the combined treatment group, 3/13 (23%) patients were still on prednisone (5 to 10mg/d) and clarithromycin (250 to 500mg/d) at the time of first relapse. In 10/13 (77%), the first relapse occurred after the end of therapy. Of the 11/21 patients who relapsed in the prednisone alone group, 9/11 (82%) were still on prednisone at the time of relapse (doses ranging from 2.5 to 20mg/d, median 10mg/d). All relapses were treated with the same protocol of prednisone only, starting with 20mg/d of prednisone, except patients who relapsed on higher doses of prednisone.
Outcome
No death or respiratory failure occurred. Thirty-three percent of the patients in the prednisone group, and 20% of patients in the combination group were still on prednisone when last seen. 73% in the combination group and 67% in the prednisone group had normal chest X-ray at last visit (p=0.951). No relevant side effects were reported.
Discussion
This retrospective study is the first to compare the standard treatment regimen to an alternative regimen for OP. Contrary to our hypothesis, we found that a 12-week combined treatment regimen of clarithromycin and prednisone resulted in a lower response rate and a higher relapse rate as compared to a previously published 24-week prednisone alone regimen. These findings suggest that clarithromycin cannot replace prednisone in the initial treatment of OP, and challenge previous reports suggesting a beneficial effect of anti-inflammatory macrolides in this condition.
Ishikawa et al. first reported the use of erythromycin in organizing pneumonia (27) soon after a publication showing its efficacy in chronic panbronchiolitis. In the following years, case reports and small series suggested that macrolide antibiotics such as erythromycin and clarithromycin could be of interest for the treatment of OP as an adjunct or an alternative to prednisone, and could reduce the relapse rate (20-24). Two in vitro studies also showed that macrolides decrease pro-inflammatory cytokine production by alveolar macrophages and neutrophils in OP (25, 26). The findings of the clinical studies are summarized in table 3.
Table 3.
Previous reports of macrolide use in OP
Most previous reports suggested that the initial response to macrolide therapy in OP is less predictable than the response to prednisone. In a series of 12 patients treated with clarithromycin for 3 months, only 7 (58%) had complete response and 3 (25%) had to be switched to prednisone because they showed no improvement at all (22). Stover et al reported complete response in only 2/6 patients (33%) and improvement in 3/6 (50%), while the response was not well specified in one case (20). Pathak et al. reported complete response in only one of 4 cases (25%) (23). In contrast, Ishikawa and Kastelik reported a complete response in respectively 6/6 and 3/3 cases (21, 27), and in one recent study, a complete response was reported in 22/26 cases treated with clarithromycin 1000 mg/day for 3 months (24). The complete response rate observed in the present study with a combined clarithromycin and prednisone regimen (58%) is similar or higher than in most previous reports with clarithromycin alone, but appears lower than with the prednisone alone regimen (81%). One could hypothesize that the lower cumulative dose of prednisone and the shorter treatment duration in the combined regimen, which were both reduced 50% as compared to the prednisone alone regimen, were the cause of a lesser disease control and the lower rate of complete treatment response.
The relapse rate observed in our study with combined therapy (81%) was the highest among all series of macrolide therapy in OP, and also one of the highest reported in OP overall. However, the relapse rate could have been underestimated in some previous studies because of short follow-up. For instance, in the study by Ichikawa et al., the mean follow-up was only 3.6±1.8 months (27). In contrast to our findings, Radzikowska et al. reported no relapse in 11 patients treated with clarithromycin alone for 3 to 4 months and followed for a mean duration of 42 months (22). More recently, the same authors reported only 2 relapses in 26 patients with COP treated with clarithromycin alone 1000 mg/day for 3 months and followed for a mean duration of 53 months (24). The cause of this striking difference in relapse rate is unclear. One could hypothesize that the patients reported in these 2 series (22, 24) had a less severe disease and were less prone to relapses. Another hypothesis is that prednisone used in our protocol in addition to clarithromycin could have favored the occurrence of relapses after treatment tapering as compared to clarithromycin alone. However, the relapse rate in the combined regimen (81%) was higher than in the prednisone alone regimen (52%), whereas the opposite would have been expected if prednisone therapy was favoring the occurrence of relapses. Alternatively, the different relapse rate could be explained by different doses of clarithromycin, which was tapered in our protocol but maintained at 1000 mg/day for 3 months in the study by Radzikowska (24).
Our study represents real-life experience with a standardized clarithromycin-prednisone protocol in several centers. We only included well-documented cases of OP, all with compatible clinico-radiological features, and all cases of COP with histological proof of diagnosis. 94% of patients received the combination treatment as planned. All cases treated with the combination treatment by GERM”O”P members were reported, thus reducing the bias resulting from overreporting of successful cases.
Our study also had weaknesses. The treatment allocation was not randomized. As the choice of treatment was left to the physician, a selection bias could have occurred. The treatment efficacy was evaluated by the treating physician. A review of all radiological files by an independent radiologist would have been preferable but could not be done for practical reasons. Follow-up pulmonary function tests were not available for analysis. Due to small sample size, no formal comparison between COP and radiation-induced OP was made. Despite these limitations, we believe that the very high relapse rate observed with the combined regimen could not be explained by chance or bias alone, and that we can confidently conclude that this combined regimen does not prevent relapses effectively.
In summary, the present study does not suggest a role for a combination of clarithromycin and prednisone in the treatment of OP, neither to reduce the cumulative dose of prednisone, nor to reduce the relapse rate. However, as suggested by other authors (24), it is possible that clarithromycin alone at higher doses is of interest in the situation. A randomized controlled trial comparing clarithromycin alone with prednisone alone would be needed to address this issue.
Acknowledgements
The following physicians contributed to this study by reporting one case: Dr M.-L. Braud (Bourg-en-Bresse), Dr G. Rolachon (Bourgoin Jallieu). The authors thank Nathalie Bacco for figure 1 and administrative work.
Ethics approval and consent to participate:
This retrospective study was approved by the Review Board of the French Learned Society for Respiratory Medicine (Société de Pneumologie de Langue Française) under the reference CEPRO 2012 019.
Authors’ contributions
Study conception and design: all authors; data acquisition: all authors; data analysis and interpretation: NP, VC, JFC, RL; manuscript drafting: NP, VC, JFC, RL; critical manuscript revision: all authors; final manuscript approval: all authors.
References
- 1.Davison AG, Heard BE, McAllister WA, Turner-Warwick ME. Cryptogenic organizing pneumonitis. Q J Med. 1983;52(207):382–94. [PubMed] [Google Scholar]
- 2.Epler GR, Colby TV, McLoud TC, Carrington CB, Gaensler EA. Bronchiolitis obliterans organizing pneumonia. N Engl J Med. 1985;312(3):152–8. doi: 10.1056/NEJM198501173120304. [DOI] [PubMed] [Google Scholar]
- 3.Barroso E, Hernandez L, Gil J, Garcia R, Aranda I, Romero S. Idiopathic organizing pneumonia: a relapsing disease. 19 years of experience in a hospital setting. Respiration. 2007;74(6):624–31. doi: 10.1159/000103240. [DOI] [PubMed] [Google Scholar]
- 4.Cazzato S, Zompatori M, Baruzzi G, et al. Bronchiolitis obliterans-organizing pneumonia: an Italian experience. Respir Med. 2000;94(7):702–8. doi: 10.1053/rmed.2000.0805. [DOI] [PubMed] [Google Scholar]
- 5.King TE, Jr, Mortenson RL. Cryptogenic organizing pneumonitis. The North American experience. Chest. 1992;102(1 Suppl):8S–13S. [PubMed] [Google Scholar]
- 6.Lohr RH, Boland BJ, Douglas WW, et al. Organizing pneumonia. Features and prognosis of cryptogenic, secondary, and focal variants. Arch Intern Med. 1997;157(12):1323–9. doi: 10.1001/archinte.157.12.1323. [DOI] [PubMed] [Google Scholar]
- 7.Drakopanagiotakis F, Paschalaki K, Abu-Hijleh M, et al. Cryptogenic and secondary organizing pneumonia: clinical presentation, radiographic findings, treatment response, and prognosis. Chest. 2011;139(4):893–900. doi: 10.1378/chest.10-0883. [DOI] [PubMed] [Google Scholar]
- 8.Papakosta D, Manika K, Gounari E, et al. Bronchoalveolar lavage fluid and blood natural killer and natural killer T-like cells in cryptogenic organizing pneumonia. Respirology. 2014;19(5):748–54. doi: 10.1111/resp.12305. [DOI] [PubMed] [Google Scholar]
- 9.Poletti V, Castrilli G, Romagna M, et al. Bronchoalveolar lavage, histological and immunohistochemical features in cryptogenic organizing pneumonia. Monaldi Arch Chest Dis. 1996;51(4):289–95. [PubMed] [Google Scholar]
- 10.Lazor R, Vandevenne A, Pelletier A, Leclerc P, Court-Fortune I, Cordier JF. and the Groupe d’Etudes et de Recherche sur les Maladies “Orphelines” Pulmonaires (GERM”O”P). Cryptogenic organizing pneumonia. Characteristics of relapses in a series of 48 patients. The Groupe d’Etudes et de Recherche sur les Maladies “Orphelines” Pulmonaires (GERM”O”P) Am J Respir Crit Care Med. 2000;162(2 Pt 1):571–7. doi: 10.1164/ajrccm.162.2.9909015. [DOI] [PubMed] [Google Scholar]
- 11.Florescu DF, Murphy PJ, Kalil AC. Effects of prolonged use of azithromycin in patients with cystic fibrosis: a meta-analysis. Pulm Pharmacol Ther. 2009;22(6):467–72. doi: 10.1016/j.pupt.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Haworth CS, Bilton D, Elborn JS. Long-term macrolide maintenance therapy in non-CF bronchiectasis: evidence and questions. Respir Med. 2014;108(10):1397–408. doi: 10.1016/j.rmed.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Kudoh S, Azuma A, Yamamoto M, Izumi T, Ando M. Improvement of survival in patients with diffuse panbronchiolitis treated with low-dose erythromycin. Am J Respir Crit Care Med. 1998;157(6 Pt 1):1829–32. doi: 10.1164/ajrccm.157.6.9710075. [DOI] [PubMed] [Google Scholar]
- 14.Hui D, Yan F, Chen RH. The effects of azithromycin on patients with diffuse panbronchiolitis: a retrospective study of 29 cases. J Thorac Dis. 2013;5(5):613–7. doi: 10.3978/j.issn.2072-1439.2013.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vos R, Vanaudenaerde BM, Verleden SE, Van Raemdonck DE, Dupont LJ, Verleden GM. Azithromycin in posttransplant bronchiolitis obliterans syndrome. Chest. 2011;139(5):1246. doi: 10.1378/chest.10-2944. author reply 7. [DOI] [PubMed] [Google Scholar]
- 16.Yates B, Murphy DM, Forrest IA, et al. Azithromycin reverses airflow obstruction in established bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 2005;172(6):772–5. doi: 10.1164/rccm.200411-1537OC. [DOI] [PubMed] [Google Scholar]
- 17.Albert RK, Connett J, Bailey WC, et al. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365(8):689–98. doi: 10.1056/NEJMoa1104623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yao GY, Ma YL, Zhang MQ, Gao ZC. Macrolide therapy decreases chronic obstructive pulmonary disease exacerbation: a meta-analysis. Respiration. 2013;86(3):254–60. doi: 10.1159/000350828. [DOI] [PubMed] [Google Scholar]
- 19.Uzun S, Djamin RS, Kluytmans JA, et al. Azithromycin maintenance treatment in patients with frequent exacerbations of chronic obstructive pulmonary disease (COLUMBUS): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2014;2(5):361–8. doi: 10.1016/S2213-2600(14)70019-0. [DOI] [PubMed] [Google Scholar]
- 20.Stover DE, Mangino D. Macrolides: a treatment alternative for bronchiolitis obliterans organizing pneumonia. Chest. 2005;128(5):3611–7. doi: 10.1378/chest.128.5.3611. [DOI] [PubMed] [Google Scholar]
- 21.Kastelik JA, Greenstone M, McGivern DV, Morice AH. Cryptogenic organising pneumonia. Eur Respir J. 2006;28(6):1291. doi: 10.1183/09031936.00100106. [DOI] [PubMed] [Google Scholar]
- 22.Radzikowska E, Wiatr E, Gawryluk D, et al. [Organizing pneumonia--clarithromycin treatment] Pneumonol Alergol Pol. 2008;76(5):334–9. [PubMed] [Google Scholar]
- 23.Pathak V, Kuhn JM, Durham C, Funkhouser WK, Henke DC. Macrolide use leads to clinical and radiological improvement in patients with cryptogenic organizing pneumonia. Ann Am Thorac Soc. 2014;11(1):87–91. doi: 10.1513/AnnalsATS.201308-261CR. [DOI] [PubMed] [Google Scholar]
- 24.Radzikowska E, Rozy A, Jagus P, et al. Clarithromycin Decreases IL-6 Concentration in Serum and BAL Fluid in Patients with Cryptogenic Organizing Pneumonia. Adv Clin Exp Med. 2016;25(5):871–8. doi: 10.17219/acem/61953. [DOI] [PubMed] [Google Scholar]
- 25.Hotta M. Neutrophil chemotactic activity in cryptogenic organizing pneumonia and the response to erythromycin. Kurume Med J. 1996;43(3):207–17. doi: 10.2739/kurumemedj.43.207. [DOI] [PubMed] [Google Scholar]
- 26.Cai M, Bonella F, Dai H, Sarria R, Guzman J, Costabel U. Macrolides inhibit cytokine production by alveolar macrophages in bronchiolitis obliterans organizing pneumonia. Immunobiology. 2013;218(6):930–7. doi: 10.1016/j.imbio.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 27.Ichikawa Y, Ninomiya H, Katsuki M, Hotta M, Tanaka M, Oizumi K. Low-dose/long-term erythromycin for treatment of bronchiolitis obliterans organizing pneumonia (BOOP) Kurume Med J. 1993;40(2):65–7. doi: 10.2739/kurumemedj.40.65. [DOI] [PubMed] [Google Scholar]
- 28.Ding QL, Lv D, Wang BJ, et al. Macrolide therapy in cryptogenic organizing pneumonia: A case report and literature review. Exp Ther Med. 2015;9(3):829–34. doi: 10.3892/etm.2015.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]