KEY POINTS
-
•
The pleural space is a potential space that contains a small amount of fluid and maintains a negative pressure.
-
•
Clinical symptoms in animals with pleural space disease include tachypnea, open-mouth breathing, extended head and neck, crouched sternal recumbency with elbow abduction (orthopnea), cyanosis, and short, shallow breathing with an increased abdominal component. Thoracic auscultation often reveals muffled or absent breath sounds over affected areas of the thorax.
-
•
Abnormalities within the pleural space may include pleural effusion, pneumothorax, or space-occupying soft tissue (diaphragmatic hernia, neoplasia). A diagnostic thoracentesis may also prove therapeutic in severely affected patients.
-
•
Cytologic and fluid analysis should always be performed on aspirate from a newly diagnosed pleural effusion of unconfirmed etiology.
-
•
Aerobic and anaerobic cultures with sensitivity testing of suppurative effusion are imperative.
-
•
Pleural fluid triglyceride levels and cholesterol concentrations are necessary to establish the diagnosis of chylothorax.
-
•
Clinical evidence of cardiovascular shock will often precede dyspnea in patients with hemothorax.
-
•
Tension pneumothorax, regardless of its origin, may be rapidly fatal. Immediate thoracentesis is required before taking thoracic radiographs.
-
•
Clinical signs of a traumatic diaphragmatic hernia may be delayed; however, early detection and correction are important because perioperative outcome is worse in patients with a preexisting hernia.
PLEURAL SPACE
The pleural space is a potential space formed by the parietal and visceral pleura. It normally contains a minimal amount (few milliliters) of serous fluid to facilitate motion of the lungs in relation to the thoracic cavity and to each other.1 The pleura is a thin epithelium formed of mesothelial cells overlying a thin basal membrane. The pleura contain a superficial network of lymphatic ducts, blood vessels, and rare nerves.2 The partition between the right and left hemithoraces is incomplete in small animals, but unilateral or unevenly distributed disease is common.3
Physiologic fluid flux in the pleural space is governed by Starling's law (Box 30-1 ). Hydrostatic pressure favors fluid accumulation within the pleural cavity (where pressure is subatmospheric), and the parietal pleura (systemic circulation) has greater filtration capacity than the visceral pleura (pulmonary circulation). However, oncotic pressure favors reabsorption of fluid from both pleura because the colloid osmotic pressure of the pleural space is 3.2 cm H2O in dogs (compared with 24.5 to 27 cm H2O in the vascular space). The visceral pleura assumes a larger role in determining the net pressure and favors reabsorption of fluid from the pleural space, where a greater vascular supply and lower hydrostatic pressure exist. Pleural lymphatic vessels are also an important component of fluid and blood reabsorption from the thorax.3
Box 30-1. Modified Starling's Law Applied to the Pleural Cavity.
Net filtration = K{[(Pc parietal−Pc visceral)−Pif]−(πc−πif)}
Pcap: capillary hydrostatic pressure of the visceral and parietal pleura
Pif: intrapleural hydrostatic pressure
πcap: plasma oncotic pressure
πif: intrapleural oncotic pressure
Modified from Pleural effusion and diseases of the pleura, Vet Clin North Am Small Anim Pract 15:1069, 1985.
There is an average pleural pressure of −5 cm H2O, representing the difference between the lung recoil and the thoracic cavity expanding forces, at rest.4 Air, fluid, or soft tissue within the pleural space can cause the lungs to collapse and the chest wall to spring out by increasing the subatmospheric pressure within the thorax.5 Pleural pathologies such as these subsequently lead to a decrease in tidal volume, total vital capacity, and functional residual capacity.6 The resulting atelectasis can lead to both hypoxemia and hypoventilation.
CLINICAL EVALUATION
Clinical signs of pleural disease may include tachypnea, open-mouth breathing, extended head and neck, crouched sternal recumbency with elbow abduction (orthopnea), cyanosis, and short, shallow breathing with an increased abdominal component. The degree of dyspnea will vary depending on the amount of fluid, rate of fluid accumulation, and concurrent respiratory and metabolic disturbances. Auscultation reveals muffled breath sounds ventrally (fluid or tissue) or dorsally (air). Thoracic percussion reveals a low-pitched (fluid) or high-pitched (air) resonance of the affected area. The heart sounds may be muffled by fluid or tissue, or abnormally loud or displaced with unilateral or focal disease.
Thoracic radiographs are extremely helpful in diagnosing and quantifying pleural space disease and other intrathoracic pathology. Repeat radiographs after thoracentesis can be of diagnostic utility but were found to rarely be beneficial in providing additional diagnostic information in one human study.7 Routine radiographs after thoracentesis are considered unnecessary for stable patients in the absence of suspicion, clinical indication, or risk factors for complications (mostly pneumothorax)7,8 (Figure 30-1 ). Because the shape of the canine and feline chest is much different from that of humans, it is possible that dorsoventral radiographs in animals with pleural effusion (following thoracentesis) may still have improved diagnostic utility for evaluating the dorsal lung fields. Ultrasonographic examination is very helpful for rapid identification of pleural fluid in the emergency setting. In human medicine, indications for use of ultrasonographically guided thoracentesis include a small-volume effusion, inability to properly position the patient, failure of fluid to layer out on radiographs, and coagulopathy.9 “In veterinary medicine, ultrasound guidance is used routinely to confirm the ideal point of needle insertion for thoracentesis, mostly in patients with a small volume of effusion, fluid pockets or those at increased risk for complications.”
Figure 30-1.
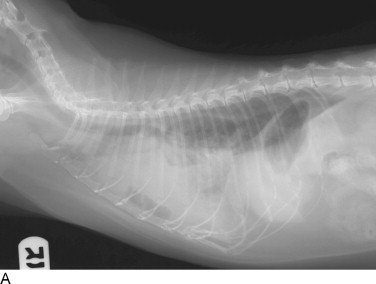
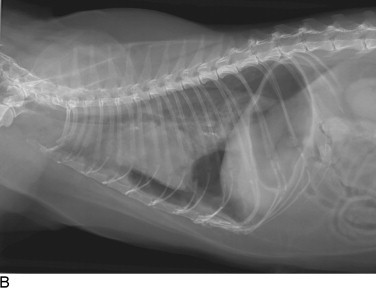


Cats with pleural space disease. A, Moderate volume of malignant effusion secondary to bronchogenic adenocarcinoma. B, Pneumothorax after thoracentesis in the patient shown in A.C, Traumatic pneumothorax from high-rise syndrome. D, Spontaneous pneumothorax from diffuse pulmonary metastasis of salivary gland adenocarcinoma.
Thoracic ultrasonography may reveal underlying pathology such as a diaphragmatic hernia, neoplastic process, or lung lobe torsion.10,11 Echocardiography will permit a diagnosis of cardiac disease, heart base tumor, and pericardial disease. Ultrasonography may also identify a pneumothorax by identifying the presence or absence of “lung sliding” (normal respiratory movement) at the lung surface.12 Computed tomography is commonly used to characterize pleural and pulmonary lesions.10 Thoracic scintigraphy is used mostly in small animal practice to identify pulmonary thromboembolism.10 Thoracoscopy is another useful diagnostic and therapeutic tool in patients with pleural effusion and other intrathoracic pathology.13
Thoracentesis is an invaluable diagnostic, and often therapeutic, tool (see Chapter 31, Thoracentesis). Its indications include (1) the presence of any undiagnosed pleural effusion and (2) therapeutic thoracentesis to relieve respiratory signs caused by large amounts of air or fluid. However, if the etiology of the effusion is known and the patient is not dyspneic, the procedure may be delayed and the clinical signs followed.14 Fluid analysis has great diagnostic utility in patients with pleural effusion of an undetermined etiology.14,15
PLEURAL EFFUSION
Pure Transudate and Modified Transudate
Transudative pleural effusion, or hydrothorax, is the result of variations in the Starling forces that govern pleural fluid flux (see Box 30-1). Pure transudates are characterized by a low total protein and total nucleated cell count (Table 30-1 ). Hydrothoraces generally develop secondary to decreased oncotic pressure within the vasculature and are commonly associated with hypoalbuminemia, although it also may be secondary to an increased hydrostatic pressure or neoplasia. Modified transudates are associated with an increased hydrostatic pressure (i.e., heart failure) or vascular permeability (e.g., vasculitis, lung lobe torsion, diaphragmatic hernia) causing leakage of a higher protein ultrafiltrate.16 However, in animals with chronic effusion, irritation of the pleura may cause an increased nucleated cell count and water can be reabsorbed in excess of protein and cells.6 Translocation of abdominal effusion, neoplastic effusion, and chylothorax are other causes of transudates.
Table 30-1.
Fluid Type and Characteristics16
| Fluid Type | Fluid Characteristics |
|---|---|
| Pure transudate | TP <2.5 g/dl TNCC <1500/μl |
| Modified transudate | TP 2.5 to 7.5 g/dl TNCC 1000 to 7000/μl |
| Exudate | TP >3.0 g/dl TNCC >7000/μl |
TP, Total protein; TNCC, total nucleated cell count.
Exudates
Exudative effusions are the result of chemotactant (causing white blood cell accumulation) and vasoactive substances (causing high-protein fluid efflux) within the pleural cavity secondary to an inflammatory process. Degenerate neutrophils usually will predominate with a bacterial infection.16 Bacteria may originate from hematogenous or lymphatic spread, penetrating insults (iatrogenic, inhaled or external foreign body, bite wound, trauma), or spread from infected organs (lung, gastrointestinal).16 Aerobic and anaerobic cultures are recommended for all exudates. Nocardia spp, Actinomyces spp, and Fusobacterium spp are filamentous rods that are difficult to grow on culture media or identify with culture, cytologic, or histologic examination.16,17 Other types of organisms, such as fungi, protozoa, and rickettsiae, may also cause septic pleural exudates.16
In aseptic exudates, the predominant cell type may vary to include nondegenerate neutrophils (inflammation), small lymphocytes (chylothorax), or neoplastic cells. Potential causes of an aseptic exudate include pneumonia and other well-circumscribed infections (e.g., abscess), generalized sepsis, pancreatitis, or necrosis of intracavitary neoplasia.16
Feline Infectious Peritonitis
Feline infectious peritonitis (FIP), caused by a coronavirus (feline immunodeficiency virus [FIPV] or feline coronavirus [FCoV]), is a common cause of aseptic pleural exudative effusion in cats, but it may also cause a modified transudate. Abdominal and pericardial effusion can be concomitant. The effusive form is a more acute disease process but may be present terminally in noneffusive FIP.17 The effusive form results from either a greater viral load causing a larger quantity of circulating immune complexes or an ineffective cell-mediated immune response.17,18 Deposition of infected macrophages and immune complexes on the endothelium result in complement-mediated, severe pyogranulomatous vasculitis.18
FIP is most often found in young, purebred, intact males.19 Clinical signs will vary depending on the form and organ systems affected. Pleural or peritoneal fluid typically will be viscous, straw-colored, and have a high protein concentration (>3.5 g/dl) with a relatively low nucleated cell count (<5000 cells/ml).17 Nondegenerate neutrophils predominate in the fluid, with or without macrophages and lymphocytes.17 Immunofluorescent staining of intracellular FCoV antigen has high specificity, and anti-FCoV antibody testing has both high positive and negative predictive values.20 The highest serum antibody titer (1:1600) is an excellent predictor of disease, and reverse transcriptase polymerase chain reaction on the effusion has shown promising results although false results are possible.20 Histopathology remains the gold standard for diagnosing this disease.18 The prognosis for recovery is grave; however, a new treatment protocol including feline recombinant interferon-ω and glucocorticoids has been described in a small group of cats, with more promising results.21
Pyothorax
A pyothorax is defined as an accumulation of purulent exudate within the thoracic cavity. Bacterial infection within a feline thorax is most often the result of bite wounds in young cats from multicat households.22 Migrating inhaled foreign bodies, and traumatic thoracic penetration are more frequent in dogs.23,24 Young adult hunting or working breeds are over-represented.25 Other bacterial sources reported include pneumonia, pleuropneumonia, lung abscess, aberrant migration of Cuterebra larvae or grass awns, hematogenous or lymphatic dissemination, esophageal or tracheal perforations, lung parasites, diskospondylitis, neoplasia with abscess formation, and iatrogenic causes.22-24 Septic suppurative effusion typically is diagnosed when intracellular organisms are present on cytologic examination and the presence of intracellular organisms. Culture and sensitivity testing should be performed on the fluid and antibiotic therapy initiated. Ancillary tests such as glucose and lactate levels and pH may also be helpful to diagnose the presence of sepsis.25,28 Anaerobic bacteria are found most commonly,25,26 and infections with multiple organisms are highly prevalent.22,25 In cats, nonenteric bacteria are most common and Pasteurella spp is most frequently isolated.22,26 In dogs, Escherichia coli and other members of the family Enterobacteriaceae are isolated most often.26,27
Hospitalization for appropriate supportive care and intravenous antibiotics is indicated. Pending culture and sensitivity results, broad spectrum intravenous antibiotic therapy, such as enrofloxacin for gram-negative bacteria and ampicillin with sulbactam or ticarcillin with clavulanate for gram-positive and anaerobic infections,29 should be instituted as soon as possible. However, an increasing resistance of E. coli to enrofloxacin has been documented, and amikacin and ceftizoxime have shown to have better efficacy against this organism.26 Clindamycin is also effective against many of the offending organisms in cats. Medical management with thoracostomy tubes (bilateral in most cases) is recommended, and sterile lavage with physiologic saline (10 to 20 ml/kg q6-12h daily) may be used initially if the effusion is thick and flocculent (see Chapter 32, Thoracostomy Tube Placement and Drainage). Absorbed lavage solution by the inflamed pleura can lead to fluid overload, so close monitoring of fluid “ins and outs” is recommended. Intermittent thoracentesis is not a recommended means of drainage and is associated with increased mortality.17 Tubes will often be necessary for 4 to 6 days,22,25,27 and removal is based on daily fluid reevaluation and the quantity of fluid produced (<2.2 ml/kg per tube q24h, although this can vary depending on the severity of pleuritis).30 Thoracic radiographs or ultrasonographic examination should be used to monitor the efficacy of drainage. A thoracotomy should be performed if pocketed fluid is persistently not drained by the thoracostomy tubes; if lung or pleural abscess, foreign body, or neoplasia is suspected; or if medical management is failing.
In cats with a pyothorax, reported survival is 66.1% overall, 77.6% after the first 24 hours of hospitalization. A lower heart rate and hypersalivation were associated with death, and a higher white blood cell count was associated with survival. The need for surgical exploration has not been associated with poorer outcome, and recurrence is rare (5.9%).22 In dogs, surgical treatment is associated with a better outcome: 78% were disease free after 1 year versus 25% with medical treatment.27
Chylothorax
Chylous effusion is opaque and white or pink. Small lymphocytes usually predominate; however, nondegenerate neutrophils may become predominant after repeated thoracocenteses or with chronic disease.16 The triglyceride concentration within the effusion is higher than the concentration in the serum, while the cholesterol level is equal to or lower than that of the serum. Causes of chylothorax include heart disease (cardiomyopathy, congestive heart failure, pericardial disease), thoracic duct obstruction (intraluminal neoplasia or granuloma or extraluminal), traumatic rupture of the thoracic duct, cranial mediastinal mass (thymoma, lymphosarcoma, aortic body tumor), lung lobe torsion, diaphragmatic or peritoneopericardial hernia, post–pacemaker implantation in cats, heartworm disease, congenital malformations, cranial vena caval thromboembolism, ligation of the left brachiocephalic vein, and idiopathic contributors.31,32
Idiopathic chylous effusion is diagnosed by exclusion in most animals with true chylothorax.33 Medical management consists of intermittent thoracentesis, a reduced-fat diet, and rutin (a benzopyrone). Rutin is a nutraceutical that stimulates macrophage breakdown of protein in lymph, accelerating its reabsorption.31,34 Thoracostomy tubes are indicated only in animals with a traumatic chylothorax, if thoracentesis is required several times weekly, or following surgery.6 Surgical intervention is recommended if the medical management is unsuccessful at providing good quality of life to the animal. Multiple interventions have been described, however a recent study has shown improved success rates with a combination of thoracic duct ligation and subtotal pericardectomy (100% of dogs and 80% of cats had resolution of pleural fluid accumulation for at least 60 days following the procedure).33 Other surgical interventions reported include omentalization, passive pleuroperitoneal shunt, active pleuroperitoneal or pleurovenous shunt, and pleurodesis, but these procedures have shown a worse outcome.31,33 Thoracoscopic ligation of the thoracic duct has been described in an experimental study.35
Complications of chylous effusion and its drainage include weight loss, electrolyte abnormalities (pseudoaddisonian), lymphopenia, hypoproteinemia, dehydration, and fibrosing pleuritis.31 Rarely, spontaneous resolution of idiopathic effusion occurs. This is expected in most animals suffering from traumatic thoracic duct rupture.
Hemothorax
A hemothorax is defined as a pleural space effusion with a hematocrit that is 25% greater than that of the peripheral blood.6 Evidence of erythrophagocytosis and absence of clotting or platelets on cytologic examination differentiate iatrogenic hemorrhage from a true hemorrhagic effusion (unless peracute). Hemorrhage within the pleural cavity can be caused by a severe coagulopathy, often associated with ingestion of an anticoagulant rodenticide that causes vitamin K epoxide reductase inhibition. Blunt or penetrating trauma, diaphragmatic hernia, thymic hemorrhage, neoplasia, pulmonary thromboembolism, lung lobe torsion, and dirofilariasis are other reported causes. Finally, iatrogenic hemorrhage may be caused by venipuncture, jugular catheter placement, Swan-Ganz catheter placement, thoracentesis, intrathoracic biopsy, intrathoracic fine-needle aspiration, and following thoracostomy or herniorrhaphy.
Cardiovascular shock often precedes respiratory compromise because as much as 30 to 60 ml/kg (dogs) or 20 ml/kg (cats) of pleural effusion is required to impair ventilation in those with normal pulmonary parenchyma.36,37 Therefore treatment includes appropriate fluid resuscitation and blood transfusions as needed. Only sufficient blood should be retrieved from the pleural space to relieve dyspnea and allow adequate oxygenation, because the red blood cells that remain will be reabsorbed over the ensuing several days. Autotransfusion should be considered in trauma patients if more than 10 ml/kg of effusion is present.36 Thoracostomy tube placement should be considered if the animal cannot be stabilized with thoracentesis and the hemorrhage is ongoing (see Chapter 32, Thoracostomy Tube Placement and Drainage). Surgery is rarely indicated with traumatic hemothorax unless a penetrating injury or uncontrollable hemorrhage is present.
Neoplastic Effusions and Pleural Neoplasia
Intrathoracic neoplasia may result in transudates or exudates by causing increased vascular permeability, obstruction of pleural and pulmonary lymphatic vessels or veins, shedding of necrotic material at the pleural surface (increasing oncotic pressure within pleural space), and obstruction or perforation of the thoracic duct.38 Hemorrhage and pneumothorax may also result from neoplasia. Common primary thoracic cancers include mesothelioma, pulmonary carcinomas, and lymphosarcoma, but metastatic disease can also result in pleural abnormalities. Fluid analysis and cytologic studies are informative, but thoracic ultrasonography and computed tomography with fine-needle aspiration or biopsy will often be necessary to obtain a definitive diagnosis.
Fibrosing Pleuritis
Fibrosing pleuritis is a chronic condition in which the visceral pleura becomes thickened and restricts lung expansion as a result of inflammation within the thoracic cavity. Causes of this condition in humans include chylothorax, hemothorax, pleural infection, drugs, neoplasia, asbestosis, rheumatoid pleurisy, coronary bypass surgery, and uremia.38 In veterinary medicine, this pathology is most frequently associated with chylous effusion.39 Development of fibrosis depends on the degree of mesothelial cell and basement membrane damage and regeneration. A disorder of fibrin turnover is thought to lead to deposition of the intrapleural fibrin matrix.39 Radiographs will show rounded, retracted lung lobe(s) that will not expand following thoracentesis. Pulmonary edema and interstitial fibrosis may contribute to dyspnea.40 Decortication is the only successful therapy in humans and should be considered early for better outcome, while pulmonary changes are minimal. Pneumothorax is a common complication and reexpansion pulmonary edema is also possible. The prognosis is guarded with diffuse disease.40
PNEUMOTHORAX
A pneumothorax is open if it results from an insult to the thoracic wall, such as a penetrating thoracic trauma. In patients with a closed pneumothorax, the thoracic cavity is intact and the air originates from a lesion within the lung parenchyma, trachea, airways, esophagus, mediastinum, or diaphragm. A tension pneumothorax develops if the site of air leakage creates a one-way valve during inspiration and results in a rapidly increasing pleural pressure that exceeds atmospheric pressure.
Traumatic pneumothorax is a common sequela of motor vehicular accidents and was found concurrently in 47% of dogs with pulmonary contusions.41 It has also been reported in most (63%) cats with high-rise syndrome.42 External wounds, such as a projectile injury, bite wounds, and penetrating sharp objects to the thorax and cervical spine, are also frequent causes. Iatrogenic pneumothorax following thoracentesis is common, with an incidence of 3% to 20% in humans, with approximately 20% of those patients requiring a thoracostomy tube(s) placement.14 Other common iatrogenic causes include leakage following lung lobectomy or respiratory tract surgery, thoracostomy tubes, fine-needle lung aspiration, barotrauma during positive-pressure ventilation, and tracheal tears. Spontaneous pneumothorax is most often associated with pulmonary bullous emphysema in dogs, with the Siberian Husky being overrepresented.43 Multiple other pathologic conditions can lead to a spontaneous pneumothorax: neoplasia, feline asthma, pulmonary abscess, heartworm disease and other parasitic infections, foreign body migration, subpleural blebs, and pneumonia.6 Finally, an infectious pneumothorax can be created by gas-forming bacteria within the thoracic cavity.
A tension pneumothorax can rapidly become life threatening, and immediate thoracentesis is indicated in animals suspected to have this condition. If the pneumothorax is not easily relieved with thoracentesis, an emergency minithoracostomy, with intubation and mechanical ventilation may prove lifesaving. Decreased venous return to the thorax in animals with a tension pneumothorax can be associated with cardiovascular collapse and shock. The thorax may become barrel shaped, and limited chest expansion is noted despite significant respiratory effort. However, animals with subclinical air accumulation may not require thoracentesis and the animal's progression should be followed closely because the air will be reabsorbed over days to weeks. A small amount of air in animals with severe pulmonary pathology may contribute significantly to dyspnea and should be relieved. Most patients with a closed traumatic or iatrogenic pneumothorax require thoracentesis only once or twice.
Animals should be monitored closely after thoracentesis for return of dyspnea, and cage rest is recommended for 2 weeks. The indications for a thoracostomy tube vary according to the clinical situation, but a tube should be placed in patients requiring more than two thoracocenteses within 6 to 12 hours (see Chapter 32, Thoracostomy Tube Placement and Drainage). Other indications include patients with a tension pneumothorax and those with a pneumothorax that require mechanical ventilation.
Constant negative pressure applied within the pleural cavity is recommended using a two-chambered or three-chambered continuous suction device, or commercially available Pleur-evac. Alternatively, a Heimlich valve may be used in medium and large breed dogs (although caution should be exercised if fluid accumulation is also present within the pleural space).
An exploratory thoracostomy is indicated if a closed traumatic pneumothorax does not resolve after 3 to 5 days of drainage. If an open pneumothorax is caused by a penetrating injury, the injury should be covered with an occlusive bandage, thoracentesis performed, and surgical repair is required as soon as the patient is stable. A spontaneous pneumothorax in dogs is best treated with surgical exploration, leading to a higher survival rate and decreased recurrence.43 Thoracoscopic lobectomy has also been described in these patients.44 Overall prognosis is good, with an 86% survival rate for treated dogs and cats with various causes of air accumulation. Favorable prognostic factors included absence of dyspnea, no need for thoracentesis, longer intensive care stay for dogs, and normal body temperature on admission in cats.45
SPACE-OCCUPYING LESIONS
Space-occupying lesions within the pleural space may occur secondary to benign or malignant masses within the mediastinum or chest wall. These typically are diagnosed with thoracic radiographs or computed tomography. Further details on these diseases are beyond the scope of this chapter.
DIAPHRAGMATIC HERNIA
Acquired diaphragmatic hernias are usually the result of blunt trauma associated with vehicular trauma, high-rise syndrome, or dog fighting or attacks, but may also be iatrogenic. Congenital diaphragmatic hernias are a result of aberrant embryogenesis and may be pleuroperitoneal, peritoneopericardial, or hiatal. These hernias are rare and beyond the scope of this chapter.
Clinical signs may occur immediately after the traumatic event, but are considered chronic if present for more than 2 weeks.46,47 Dyspnea varies from none to severe according to the organ herniated, resulting pleural effusion, and concomitant thoracic injuries. The organs most frequently involved are the liver, stomach, and small intestine; the omentum and spleen are also frequently herniated.46-48 On physical examination, borborygmus over the chest or asymmetrically quiet heart and/or lung sounds may be auscultated. The abdomen may be further tucked in or palpated “empty,” with failure to distinguish certain organs. Thoracic radiographs may reveal gas-filled abdominal organs within the thorax, an incomplete diaphragmatic border, pleural effusion, and/or cranially displaced abdominal organs. Additional radiographic views, ultrasonography, positive contrast celiography, and an upper gastrointestinal contrast study may aid in the diagnosis.
Thoracentesis and gastrocentesis may relieve the dyspnea prior to surgery. Cardiovascular stabilization prior to surgery is also important. Indications for immediate surgical intervention include herniated stomach, strangulated bowel or organs, inability to oxygenate properly after medical intervention, and ruptured viscera. Most data suggest that early surgical intervention (within 24 hours of admission) provides an excellent prognosis for acute cases.47
Postoperative complications include pneumothorax, hemorrhage, aspiration pneumonia, sepsis, arrhythmias, and death.46-48 Reexpansion pulmonary edema (RPE) is a rare complication following surgery. It results from release of endotoxins and oxygen free radicals released, decreased surfactant concentrations, negative interstitial pressures, and/or chronic hypoxia causing increased vascular permeability and protein-rich pulmonary edema. Increased incidence of RPE has been associated with a longer duration of collapsed lung (≥72 hours). Care should be given to keep peak airway pressure below 20 cm H2O to avoid positive end-expiratory pressure, and pleural air should be slowly evacuated postoperatively (>12 hours).49 Prognosis for full recovery is excellent for acute cases (survival rate 94%).47 Perioperative survival rate is lower (82% to 89%) when chronic acquired cases are included in the statistical analysis.46-48 In some studies, dyspnea did not affect prognosis,47 but older age, lower respiratory rate, and concurrent multiple injuries were associated with higher mortality in cats.48
Footnotes
See the CD-ROM for a complete list of references.
SUGGESTED FURTHER READING*
- American Thoracic Society Guidelines for thoracocentesis and needle biopsy of the pleura. Am Rev Respir Dis. 1989;140:257. doi: 10.1164/ajrccm/140.1.257. [DOI] [PubMed] [Google Scholar]; Position of the Board of Directors of the American Thoracic Society (June 1988)
- Fossum TW, Mertens MM, Miller MW. Thoracic duct ligation and pericardectomy for treatment of idiopathic chylothorax. J Vet Intern Med. 2004;18:307. doi: 10.1892/0891-6640(2004)18<307:tdlapf>2.0.co;2. [DOI] [PubMed] [Google Scholar]; Prospective study of 20 animals (10 dogs and 10 cats) with idiopathic chylous effusion
- Gibson TWG, Brisson BA, Sears W. Perioperative survival rates after surgery for diaphragmatic hernia in dogs and cats: 92 cases (1990-2002) J Am Vet Med Assoc. 2005;227:105. doi: 10.2460/javma.2005.227.105. [DOI] [PubMed] [Google Scholar]; Retrospective study evaluating survival rate in patients with traumatic diaphragmatic hernia when surgical correction occurred within 24 hours of admission
- Puerto DA, Brockman DJ, Lindquist C. Surgical and nonsurgical management of and selected risk factors for spontaneous pneumothorax in dogs: 64 cases (1986-1999) J Am Vet Med Assoc. 2002;220:1670. doi: 10.2460/javma.2002.220.1670. [DOI] [PubMed] [Google Scholar]; Retrospective study
- Waddell LS, Brady CA, Drobatz KJ. Risk factors, prognostic indicators, and outcome of pyothorax in cats: 80 cases (1986-1999) J Am Vet Med Assoc. 2002;221:819. doi: 10.2460/javma.2002.221.819. [DOI] [PubMed] [Google Scholar]; Retrospective study


