TEACHING POINTS.
-
•
Treatment with antibiotics shortens the duration of symptoms and reduces the complications of tonsillitis and pharyngitis, otitis media, sinusitis, deep neck abscesses, epiglottitis, and bacterial tracheitis.
-
•
Over-the-counter medicines for colds are not efficacious.
-
•
Penicillin remains the treatment of choice for Streptococcus pyogenes pharyngitis.
-
•
Airway obstruction occurs in deep neck abscesses, viral croup, epiglottitis, bacterial tracheitis, and recurrent respiratory papillomatosis.
-
•
Clinical features and patterns of disease are different from those in adults.
Infections of the upper respiratory tract are very common in children. Their epidemiology is described in Chapter 31. Although infections of the upper respiratory tract often resolve completely without complications, treatment is indicated where it can achieve more rapid resolution of symptoms, prevent the illness becoming more severe, prevent complications, or prevent chronic disease.
The conditions covered in this chapter are the common cold, tonsillitis and pharyngitis, otitis media, sinusitis, deep neck abscesses, viral croup, epiglottitis, bacterial tracheitis, and recurrent respiratory papillomatosis.
THE COMMON COLD
I love the doctors—they are dears;
But must they spend such years and years
Investigating such a lot
Of illnesses which no one's got,
When everybody, young and old,
Is frantic with the common cold?
And I will eat my only hat
If they know anything of that!1
Epidemiology, Risk Factors, and Pathogenesis
The common cold may be caused by one of over 100 different viral types; the major ones are listed in Box 32-1 . The main clinical difference among colds induced by different viruses is in the duration of the incubation period.2 Other types of organisms occasionally cause a syndrome that can overlap with the common cold. Such organisms include Mycoplasma pneumoniae, Bordetella pertussis, Streptococcus pyogenes, Coccidiodes immitis, Histoplasma capsulatum, Chlamydia psittaci, and Coxiella burnetii. 1
Box 32-1. Viral Causes of the Common Cold.
Most Common Cause
Rhinoviruses
Common Causes
Coronaviruses
Influenza viruses*
Parainfluenza viruses*
Respiratory syncytial virus*
Occasional Causes
Adenoviruses*
Enteroviruses*
With the use of polymerase chain reaction (PCR)-based assays, the proportion of common colds for which an etiologic organism is identifiable has increased to 70% to 80%. The discovery of human metapneumovirus as a cause of acute respiratory infections suggests that currently unidentified infectious agents cause at least a proportion of the remainder.1
Common colds vary in frequency with age and season. They are more frequent in autumn and winter in temperate regions and in the rainy season in tropical regions.1 Children have more colds per year than do adults and can be expected to have approximately six colds annually from age 2 to 6 years.3, 4, 5 For children, day care attendance increases the risk of illness, with a dose-response effect evident between the number of children in the day care setting and the number of colds.1, 6, 7, 8 Among adults, psychological stress is associated with an increased risk of having the common cold.9 Colds occur less frequently in women who work outside of the home, suggesting that exposure to children is a risk factor for adults.1, 10
The mode of transmission (hand contact with infected secretions versus small-particle aerosols versus direct hit by large-particle aerosols) varies between viruses. Rhinovirus, the most frequent pathogen, is transmitted mainly by hand contact with infected secretions followed by self-inoculation onto the nasal mucosa or conjunctiva, but it also spreads as an aerosol.11, 12
The pathophysiology of the common cold is understood mainly from studies of adult volunteers infected with rhinoviruses.1, 11, 13 After deposition of the virus on the nasal mucosa or from the conjunctiva via the lacrimal duct, the virus reaches the nasopharynx via mucociliary transport. After binding to specific cell surface receptors, the virus enters the epithelial cell. Once inside the cell, the virus starts to replicate rapidly. The infectious dose is small.14 Up to 95% of people without serotype-specific antibodies become infected, with 75% of these infections resulting in symptomatic colds.15
Infection of nasal mucosal epithelial cells results in vasodilation and increased vascular permeability leading to rhinorrhea and nasal obstruction.1 Sneezing and increased mucus gland secretion occur as a result of increased cholinergic stimulation.1 In contrast with influenza and adenovirus infection, epithelial destruction is not a feature of rhinovirus infection.1, 13
Components of the upper respiratory tract other than the nasal cavity are affected by the common cold. Paranasal sinus abnormalities are evident on radiography.16, 17 These abnormalities resolve spontaneously. Eustachian tube dysfunction occurs frequently in both children and adults and predisposes the susceptible individual to otitis media.18, 19, 20, 21
During the common cold, the greatest concentration of virus is in the nasal secretions, with little found in secretions generated by coughing or talking or in saliva. The greatest amount of virus comes from sneezing, nose blowing, and secretions from the nose transmitted on contaminated hands. Children have a greater concentration of virus in their secretion and tend to shed virus for longer periods than adults. Viral shedding is maximal 2 to 7 days after inoculation, although some shedding may continue for another 2 weeks.
Serum antibody and secretory antibody develop from the infection and appear to be protective against reinfections. Clinically abortive colds may be reinfection colds with early antibody recall.
Impairment of nasal mucociliary transport persists for approximately 1 month after a cold. Children who have four to six colds in a winter may have constantly impaired mucociliary transport.
Clinical Features
The common cold is an acute, highly infectious illness characterized by nasal stuffiness, sneezing, coryza, throat irritation, cough, and little or no fever; it occurs multiple times each year in each person. Although older children have an illness similar to that of adults, in infants, the symptoms and signs may be more varied. The minimal symptoms that define the diagnosis are nasal discharge, nasal obstruction, and throat irritation. At the onset of symptoms, there is a feeling of chilliness on exposure to cold, dryness and irritation in the nose, and a scratchy throat. This progresses rapidly to nasal stuffiness or obstruction, sneezing, watery nasal discharge, throat irritation, watering eyes or eye irritation, coughing, occasional muscular aches, general malaise, anorexia, and, sometimes, low-grade fever.2 After 1 to 3 days, the nasal secretions may become thicker and purulent. Persistent nasal discharge may lead to excoriation around the nose. If nasal obstruction occurs, it leads to mouth-breathing, aggravating the irritation of the throat. The usual duration of the illness is about 7 days, but lingering nasal discharge may persist for 2 weeks or longer.
In infants, the onset is more likely to be associated with a fever of 38° to 39° C (100.4° to 102.2° F).1, 22, 23 The infant may be irritable and restless, and the nasal obstruction may significantly interfere with both feeding and sleeping. Vomiting and diarrhea may also occur.
Diagnosis
If the clinical features and exposure history are specific, then investigations are not indicated. In infants, investigation for alternative diagnoses, including invasive bacterial infections, may be necessary, particularly if fever is a predominant symptom and/or nasal obstruction results in apnea. The early symptoms of many illnesses such as pertussis, epiglottitis, measles, and diphtheria are similar to those of the common cold, but in a short time, the other features of the specific illness appear. Allergic rhinitis may need to be distinguished from the common cold in the child with “recurrent colds.” Assessment of the family history, possible allergic triggers, nasal eosinophilia, and serum immunoglobulin E (IgE) concentration help confirm or exclude this diagnosis. In children, intranasal foreign bodies should be searched for if the nasal discharge is atypical in terms of persistence, blood staining, or malodor.1
The features of the illness caused by different viruses overlap widely, making clinical differentiation unreliable. Laboratory confirmation of the specific viral cause is of little value to clinical practice with the exception of influenza. Several laboratory methods are available for identification of viruses. Isolation by cell culture is too slow to be clinically relevant. Rapid antigen tests are available for some organisms, for example, influenza virus. In comparison with cell culture, influenza antigen detection in respiratory specimens by immunofluorescent staining has a sensitivity of 70% to 100% and specificity of 80% to 100%. PCR assays are more sensitive than culture, but their laboratory requirements limit clinical applicability.24 Near patient tests, which produce results within 30 minutes, have highly variable sensitivities and negative predictive values.25
Treatment and Prevention
The common cold in children usually resolves quickly, and no specific therapy is indicated in the majority of cases. Although the common cold is a relatively mild and self-limiting illness, it is enormously expensive in terms of lost productivity and money spent on various treatments, the majority of which have minimal or no efficacy.
In infants, nasal obstruction may be relieved by isotonic saline nosedrops, which can moisten irritated nasal mucosa, loosen nasal secretions, and induce sneezing. Gentle aspirationof the nasal secretion using a blunt syringe or suction can provide temporary relief for an infant. Use of concentrated capsules of eucalyptus for inhalation to clear the nose is contraindicated in young children; these can be highly dangerous if applied incorrectly to the face.26
Frequent intake of fluid helps relieve the irritated throat. Environmental tobacco smoke aggravates all the symptoms and should be avoided.
The published literature on modalities used for symptomatic relief of cold symptoms has been summarized using systematic reviews as follows:
Antibiotics: In a meta-analysis of six trials that included 1147 patients (children and adults), people receiving antibiotics did no better than did those receiving placebo in terms of cure or persistence of symptoms.27
Vitamin C: In studies of adults, beneficial effects of vitamin C have been shown when it is used prophylactically for 2 to 3 months but not when started at the onset of symptoms. However, fewer trials have examined this later use.
In studies of adults, 200 mg or more per day of vitamin C does not reduce the incidence of colds, except in those exposed to brief periods of extreme physical exercise and/or cold environments.28
Vitamin C given prophylactically results in a small but significant reduction in the duration and severity of cold symptoms. In studies of children, there is an approximately 14% reduction in duration of symptoms. The severity of symptoms, as measured by “days confined to home” or “days off work or school,” is significantly reduced in studies that include both children and adults.28
Zinc lozenges: There are no studies specifically of children, although some older children have been included in predominantly adult studies. There is no evidence that zinc lozenges are effective.
Echinacea: Most studies have examined the use of echinacea for treating rather than preventing colds. The results of placebo-controlled trials have been inconsistent, with some showing effect and others not. This variability is likely to be due at least in part to the large variability in composition of products that are sold as “Echinacea.”29
Nasal decongestants (either oral or intranasal): The efficacy of these in children remains unproved.5 In adults, a single dose of a nasal decongestant is moderately effective for the short-term relief of congestion.5 The potential side effects are a concern, especially in young children. In children, they have a measurable sedating effect.30 Excessive use of sprays and drops with vasoconstrictive medications can lead to rebound obstruction, which prolongs the illness symptoms.
Over-the-counter medicines for acute cough 31: There is no good evidence that over-the-counter medicines provide any benefit greater than that seen with a placebo. These medications confer no protection against the development of otitis media.32
Antitussives: Neither dextromethorphan nor codeine is better than a placebo at reducing cough during the day or night.33
Expectorants (Guaifenesin): This has not been adequately studied in children.
Mucolytics (letosteine): One study involving 40 children showed a statistically but not clinically significant difference in symptom score in comparison with placebo (a difference of 0.2 point on a 4-point scale).34
Antihistamines as monotherapy: In children and adults, antihistamines do not significantly reduce cold symptoms (nasal congestion, rhinorrhea, sneezing) or alter subjective improvement.35, 36 First-generation antihistamines cause more sedation than does placebo.35
Antihistamine decongestant combinations: These are not effective in small children.35 In older children and adults, a small amount of improvement in general and in nasal symptoms specifically may occur.30, 35, 37
Of the numerous etiologic agents that can cause colds, only for influenza virus is there commercially available antiviral therapy. The newer class of antiviral drugs, the neuraminidase inhibitors (zanamivir and oseltamivir), are effective against both influenza A and B viruses.38, 39 Oral oseltamivir, 2 mg/kg/dose, given twice daily, to children 1 to 12 years old shortens illness duration by 26% and reduces cough, coryza, duration of fever, and new diagnoses of otitis media.39 In children 5 to 12 years of age, the nasally administered zanamivir has also been shown to decrease symptom duration and severity.40
Prevention by immunization is currently possible only for influenza. In addition to the present inactivated vaccine that is given intramuscularly, a live attenuated cold adapted intranasal vaccine has been shown to be efficacious in children.41, 42, 43
Clinical Course and Prognosis
The uncomplicated common cold has a uniformly excellent outcome with complete recovery. However, complications are common and include acute otitis media, otitis media with effusion, tonsillitis, sinusitis, lower respiratory tract infections, and acute exacerbations of asthma44 (Box 32-2 ).
Box 32-2. Common Cold Teaching Points.
-
•
Children have more colds than do adults. They have approximately six colds per year from age 2 to 6 years.
-
•
Children are very effective spreaders of colds, having a greater concentration of virus in their secretions and longer duration of viral shedding than do adults.
-
•
In infants, colds are more likely to cause fever, and at initial presentation, the colds may be clinically indistinguishable from serious bacterial infections.
-
•
Vast amounts of money are wasted on over-the-counter products for colds. Meta-analyses of clinical trials have confirmed their lack of efficacy.
PHARYNGITIS AND TONSILLITIS
Pharyngitis is an inflammatory illness of the mucous membranes and underlying structures of the throat; it is invariably associated with the symptom of sore throat. Most cases of pharyngitis in children are caused by viruses and are benign self-limiting illnesses. Group A β-hemolytic streptococcus (S. pyogenes) is the most important etiologic agent because of its potential to cause rheumatic fever. The prevention of rheumatic fever defines the management of pharyngitis.
Epidemiology, Risk Factors, and Pathogenesis
Pharyngitis includes tonsillitis, tonsillopharyngitis, and nasopharyngitis. The inflammation frequently also involves the nasopharynx, uvula, and soft palate. Pharyngitis with nasal symptoms (sometimes called nasopharyngitis) is usually caused by a virus, whereas pharyngitis without nasal symptoms can be caused by a wide variety of infectious agents.
When an infectious agent is inoculated onto the pharyngeal or tonsillar tissue, localized inflammation occurs. This may occur de novo or as a complication of the common cold, when the etiologic agent is more likely to be viral. A list of etiologic agents is presented in Box 32-3, Box 32-4 . S. pyogenes causes 15% to 30% of acute pharyngitis in children.45
Box 32-3. Viral Agents in Pharyngitis and Tonsillitis.
Common Viral Causes
Adenovirus types 1 to 7, 7a, 9, 14 to 16
Coronavirus
Enteroviruses: coxsackievirus types A and B, echovirus type A
Epstein-Barr virus
Influenza virus types A and B
Parainfluenza virus types 1 to 4
Respiratory syncytial virus
Less Common Viral Causes
Cytomegalovirus
Herpes simplex virus
Measles virus
Poliovirus
Reovirus
Rhinoviruses
Rotaviruses
Rubella virus
Box 32-4. Other Agents in Pharyngitis and Tonsillitis.
Common Bacterial Causes
Streptococcus pyogenes
Less Common Bacterial Causes
Actinomyces spp.
Bacteroides melaninogenicus
Bacteroides spp.
Borrelia spp.
Corynebacterium diphtheriae
Corynebacterium pyogenes
Corynebacterium ulcerans
Francisella tularensis
Fusobacterium spp.
Haemophilus influenzae
βHemolytic streptococci B, C, and G
Legionella pneumophila
Leptospira spp.
Neisseria gonorrhoeae
Neisseria meningitidis
Peptostreptococcus spp.
Salmonella typhi
Streptobacillus moniliformis
Streptococcus pneumoniae
Treponema pallidum
Yersinia enterocolitica
Other Organisms
Candida spp.
Chlamydia pneumoniae strain TWAR
Coxiella burnetii
Mycoplasma hominis
Mycoplasma pneumoniae
Toxoplasma gondii
Pharyngitis occurs more frequently during the colder months of the year. In temperate climates, pharyngitis due to S. pyogenes infection usually occurs in the winter and early spring.45 Pharyngitis due to S. pyogenes is primarily a disease of children 5 to 15 years old. Group C streptococci are a common cause of pharyngitis in college students.46 Group C streptococci are also described as the etiologic organism in epidemic pharyngitis spread by contaminated food.47, 48
The inflammation causes erythema of the pharynx, the tonsils, or both structures. Exudate typically occurs with only some organisms, including adenovirus, herpes simplex virus, β-hemolytic streptococci, Corynebacterium diphtheriae, Arcanobacterium haemolyticum, Epstein-Barr virus, and Candida species. Ulceration is usually seen only with herpes simplex virus and enterovirus.
The pharyngeal involvement may be overshadowed by other symptoms, such as cough and coryza, when, for example,the infecting organism is the parainfluenza virus, and fever, exanthem, and meningitis when the infecting organism is an enterovirus.
The tonsillopharyngeal involvement with marked exudate caused by Epstein-Barr virus looks similar to that caused by S. pyogenes. It appears that bacterial adhesion is the cause of the exudate that occurs with this Epstein-Barr virus infection.49
Primary and recurrent herpes simplex virus infection occasionally has associated pharyngitis.50 In almost all instances, there are herpes lesions in the anterior mouth, externally around the mouth, and at the mucocutaneous border.
Clinical Features
Children of any age can develop pharyngitis and tonsillitis. The onset is usually sudden with fever, sore throat, and anorexia. There may be headache, nausea, vomiting, lassitude, and sometimes abdominal pain. With viral infection, there are often other signs of respiratory tract infection, with more or less systemic involvement. The cervical lymph nodes are enlarged and tender. There is moderate to severe pharyngealerythema, and there may be follicles, ulcers, petechiae, and generalized exudate. Petechial lesions on the soft palate may occur with pharyngitis due to S. pyogenes, Epstein-Barr virus, measles virus, and rubella virus.
In all cases of acute pharyngitis, streptococcal disease must be considered. Various clinical factors (exposure, season, incubation period, age of patient, and associated clinical findings) may distinguish among causative organisms in large epidemiologic studies, but in the individual child, the clinical distinction of streptococcal pharyngitis from viral pharyngitis is unreliable. If there is an obvious nasal infection, ulceration, or conjunctivitis, the etiology is most likely viral. In a child under the age of 4 years, pharyngitis with no exudate is almost always viral. In a child older than 4 years of age, pharyngitis with exudate or fever is most likely caused by S. pyogenes, but other bacteria may mimic this condition.11, 12 The clinical features of pharyngitis due to group A, C, and G β-hemolytic streptococci are similar.45 The clinical and epidemiologic features that differ in pharyngitis due to S. pyogenes versus a viral cause are shown in Box 32-5, Box 32-6 (see also Fig. 32-1 ).
Box 32-5. Clinical and Epidemiologic Features Suggesting Streptococcus pyogenes Pharyngitis.
Sudden onset
Sore throat
Fever
Scarlet fever rash
Headache
Nausea, vomiting, and abdominal pain
Inflammation of pharynx and tonsils and uvula
Patchy discrete exudates
Palatal petechiae
Excoriated nares (especially in infants)
Tender, enlarged anterior cervical nodes
Patient age 5 to 15 years
Presentation in winter or early spring
History of exposure
© 2008
Modified from Gerber MA: Diagnosis and treatment of pharyngitis in children. Pediatr Clin North Am 52:729-747, 2005; and Bisno AL, Gerber MA, Gwaltney JM, et al: Practice guidelines for the diagnosis and management of group A streptococcal pharyngitis. Clin Infect Dis 35:113-125, 2002.
Box 32-6. Clinical and Epidemiologic Features Suggesting Viral Pharyngitis.
Conjunctivitis
Coryza
Cough
Hoarseness
Anterior stomatitis
Discrete oral ulcers
Diarrhea
Characteristic exanthems
© 2008
Modified from Gerber MA: Diagnosis and treatment of pharyngitis in children. Pediatr Clin North Am 52:729-747, 2005; and Bisno AL, Gerber MA, Gwaltney JM, et al: Practice guidelines for the diagnosis and management of group A streptococcal pharyngitis. Clin Infect Dis 35:113-125, 2002.
Figure 32-1.
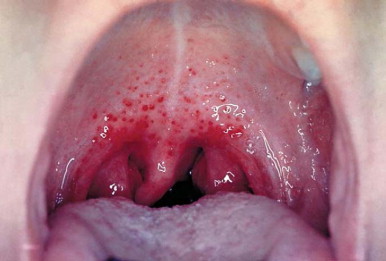
Palatal petechiae in a child with Streptococcus pyogenes pharyngitis.
Diagnosis
A throat swab is necessary to determine the presence of S. pyogenes. Identification can be with either culture or a rapid antigen detection test. For both, an adequate swab of the inflamed tonsillar area is required and the manner in which the swab is obtained is the main determinant of diagnostic accuracy.51 The surfaces of both tonsils and the pharyngeal wall should be swabbed. Other areas of the mouth and pharynx should not be swabbed. If collected in this manner, a single swab has a sensitivity of 90% to 95% for the detection of S. pyogenes in the pharynx.45, 52
The throat swab should be incubated for 18 to 24 hours on a sheep blood agar plate. Agar plates that are negative at 24 hours should be reexamined at 48 hours.45
Rapid antigen detection tests have been developed because of this 24- to 48-hour delay before a throat swab can inform clinical management. The results may be obtained in about 10 minutes. Most available rapid antigen detection tests have specificities of 95% or greater, and thus a positive result is a very good indicator of the need to treat.53 Sensitivities range from 80% to 90%, so a negative antigen test does not exclude S. pyogenes infection.53 When S. pyogenes pharyngitis is suspected clinically but the rapid diagnostic test is negative, a throat swab for culture should be obtained.24 A large proportion of false-negative rapid antigen tests are true infections rather than S. pyogenes carriage.54 Because of the limited number of direct test-to-test comparisons that have been performed, the relative sensitivities of different rapid antigen test have not been established.45
A positive culture or rapid antigen test for S pyogenes cannot differentiate a child with a true infection from another with a symptomatic viral pharyngitis who is a S. pyogenes carrier.
Treatment
Symptomatic relief may be obtained from drinking warm fluids or, in the older child, saltwater gargles. An analgesic such as acetaminophen is appropriate. Simple lemon-based throat lozenges may be soothing, but ones that contain potentially toxic substances should be avoided. Decongestants and antihistamines have no place in the treatment of pharyngitis and tonsillitis.
Antibiotics are used to treat symptomatic pharyngitis caused by infection with S. pyogenes. The aim is to prevent the development of rheumatic fever. If the rapid antigen test and culture are both negative, then antibiotics should be withheld or, if already started, discontinued.
In addition to preventing rheumatic fever, treatment of S. pyogenes pharyngitis reduces the duration of symptoms and the risk of spread and enables quicker return to school and work.55
Several different antibiotics are effective, including penicillin, ampicillin and amoxicillin, many cephalosporins, macrolides, and clindamycin. Antimicrobial therapy options for S. pyogenes pharyngitis are summarized in Table 32-1 . Penicillin remains the recommended treatment because of its proved efficacy, narrow antimicrobial spectrum, low cost, and excellent safety profile.56 S. pyogenes has never developed resistance to penicillins or cephalosporins. The minimum inhibitory concentration of penicillin has not increased over the past 50 years.45, 57
Table 32-1.
Antimicrobial Therapy for Streptococcus pyogenes Pharyngitis
| Route of Administration, Antimicrobial Agent | Dosage | Duration |
|---|---|---|
| Oral | ||
| Penicillin* | Children: 250 mg bid or tid | 10 days |
| Adolescents and adults: 250 mg tid or qid | 10 days | |
| Adolescents and adults: 500 mg bid | 10 days | |
| Intramuscular | ||
| Benzathine penicillin G | 1.2 × 106 U (for patients ≥27 kg) | 1 dose |
| 6.0 × 105 U (for patients <27 kg) | 1 dose | |
| Mixtures of benzathine and procaine penicillin G | Varies with formulation† | 1 dose |
| Oral, for Patients Allergic to Penicillin | ||
| Erythromycin | Varies with formulation | 10 days |
| First-generation cephalosporin‡ | Varies with agent | 10 days |
Amoxicillin is often used in place of oral penicillin V in young children because of the acceptance of the taste of the suspension, not because of any microbiologic advantage.
Dose should be determined on basis of benzathine component.
These agents should not be used to treat patients with immediate-type hypersensitivity to β-lactam antibiotics.
Modified from Gerber MA: Diagnosis and treatment of pharyngitis in children. Pediatr Clin North Am 52:729-747, 2005; and Bisno AL, Gerber MA, Gwaltney JM, et al: Practice guidelines for the diagnosis and management of group A streptococcal pharyngitis. Clin Infect Dis 35:113-125, 2002.
© 2008
Penicillin can be effective in preventing rheumatic fever even when therapy is started up to 9 days after the onset of the acute illness. Although the conventional oral dosage regimen is penicillin V, 250 mg 3 to 4 times a day,24 a twice-daily dose of 250 mg, if reliably given, is as effective.58 In children over 12 years of age, a higher dose of 500 mg twice a day is recommended.58 Intramuscular benzathine penicillin is very effective and should be considered for children who are particularly unlikely to complete a course of oral treatment.
Although the efficacy of penicillin in eliminating S. pyogenes from the tonsils and pharynx has not diminished after 40 years of use,59 the failure rate in practice may be at least as high as 18% in certain communities. Ampicillin and amoxicillin are associated with a 95% risk of skin rash in infectious mononucleosis24; therefore, they are not recommended in the treatment of pharyngitis.
The course of oral antibiotic must be 10 days; courses of shorter duration are associated with lack of effective treatment. A child must complete a full 24 hours of therapy before returning to school or day care; otherwise, he or she remains infectious to other children.60
Both suppurative and nonsuppurative (acute rheumatic fever, acute post streptococcal glomerulonephritis, and post streptococcal reactive arthritis) complications can develop from pharyngitis.
Scarlet fever is a streptococcal pharyngitis with a characteristic rash. The rash occurs if the S. pyogenes causing the infection produces a pyrogenic (erythrogenic) toxin and infects an individual who does not have antitoxin antibodies. The rash is either the first sign of the illness or occurs within 24 to 48 hours of illness onset. It begins around the neck and chest, spreads downward and is often more intense in the skin creases of the neck, axillae, elbows, groins, and knees (Pastia's lines). The palms and soles are spared as is the face, where there is characteristic circumoral pallor and flushed cheeks. The rash is diffuse, bright red, papular, and rough to the touch. The sandpaper texture is caused by occlusion of sweat glands. The rash fades over a week and is followed by desquamation for several weeks. In addition to palatal petechiae, the tongue has a white strawberry (yellowish white coating through which the red papillae are seen) and then, when the coating disappears, a red strawberry appearance (red swollen papillae).45, 61
Clinical Course and Prognosis
Pharyngitis is self-limited, lasting 4 to 10 days, and it has an excellent prognosis. However, in 0.3% to 3.0% of untreated S. pyogenes throat infections, the serious complication of rheumatic fever results. Suppurative involvement of both adjacent and more distant tissue is a well-recognized complication of S. pyogenes pharyngitis.62
Follow-up cultures should be performed in children who have had rheumatic fever.45 Such testing should also be considered in patients living in communities where there are outbreaks of S. pyogenes infections, post streptococcal glomerulonephritis, or rheumatic fever.63 Follow-up throat cultures are not indicated in patients who have completed an appropriate antibiotic course and are asymptomatic. If tested, most of such children in whom S. pyogenes is identified are carriers.63
Tonsillectomy is sometimes considered in the child with recurrent pharyngitis. The frequency of symptomatic episodes diminishes with time whether or not tonsillectomy is performed. Tonsillectomy results in a small additional reduction in number of symptomatic episodes, days of symptoms, and days of school missed64 (Box 32-7 ).
Box 32-7. Pharyngitis and Tonsillitis Teaching Points.
-
•
Most cases of pharyngitis in children are caused by viruses and are benign self-limiting illnesses.
-
•
The prevention of rheumatic fever defines the management of pharyngitis.
-
•
In the individual child, the clinical distinction of streptococcal pharyngitis from viral pharyngitis is unreliable.
-
•
The manner in which the throat swab is obtained is the main determinant of diagnostic accuracy.
-
•
Penicillin remains the recommended treatment because of its proved efficacy, narrow antimicrobial spectrum, low cost, and excellent safety profile.
-
•
Tonsillectomy results in a further small reduction in number of symptomatic episodes of tonsillitis, in addition to the decrease in frequency that occurs with time without tonsillectomy.
RETROPHARYNGEAL, PARAPHARYNGEAL, AND PERITONSILLAR ABSCESSES
Deep abscesses in the neck may cause serious problems because of local pressure, local destruction, or airway obstruction. They are classified by location into peritonsillar abscess (also known as quinsy), retropharyngeal abscess, and parapharyngeal abscess. Multiple abscess types can coexist. They have become sufficiently uncommon that they can be overlooked in the differential diagnosis when a young child presents with nonspecific symptoms of sepsis or of an acute pharyngeal infection. They have the potential to be catastrophically fatal or to result in significant morbidity if not detected early.65
Epidemiology, Risk Factors, and Pathogenesis
Key features and differences between these abscess types are summarized in Table 32-2 .
Table 32-2.
Clinical Features of Retropharyngeal, Parapharyngeal, and Peritonsillar Abscesses
| Usual Age | Sites of Origin | Location | Clinical Findings | Complications/Extension Site | Management | |
|---|---|---|---|---|---|---|
| Retropharyngeal abscess | < 4 yr | Pharyngitis, dental infection, trauma | Between posterior pharynx and prevertebral fascia | Unilateral posterior pharyngeal bulging; neck hyperextension, drooling, respiratory distress | Spontaneous rupture and aspiration; contiguous spread to posterior mediastinum, parapharyngeal space | Antibiotics, drainage; artificial airway |
| Parapharyngeal abscess | > 8 yr, adolescents, adults | Tonsillitis, otitis media, mastoiditis, parotitis, dental manipulation | Anterior and posterior pharyngomaxillary space | Anterior compartment: swelling of the parotid area; trismus; tonsillar prolapse. | Carotid erosion; airway obstruction; intracranial, lung, contiguous spread to mediastinum; septicemia | Antibiotics, drainage; artificial airway |
| Posterior compartment: septicemia; minimal pain or trismus | ||||||
| Peritonsillar abscess | Adolescents, adults | Tonsillitis | Tonsillar capsule, and space below superior constrictor muscle | Swelling of 1 tonsil, uvular displacement; trismus, muffled voice | Spontaneous rupture and aspiration; contiguous spread to parapharyngeal space | Antibiotics, drainage |
From Brook I: Microbiology and management of peritonsillar, retropharyngeal, and parapharyngeal abscesses. J Oral Maxillofac Surg 62:1545-1550, 2004.
© 2008
RETROPHARYNGEAL ABSCESS
An abscess can form in the retropharyngeal space, which is a potential space immediately anterior to the pre-vertebral fascia. It extends inferiorly from the skull base for the length of the pharynx.66 This space receives lymphatic drainage from many surrounding structures, including the middle ear, pharynx, nasopharynx, nose, and paranasal sinuses.67
The retropharyngeal space is continuous laterally with another potential space, the parapharyngeal space. The fascia that separates these two spaces is an ineffectual barrier to the spread of infection.65 Infection may result from suppurative adenitis of the lymph nodes in the retropharyngeal space, or penetrating trauma, or foreign body aspiration.68, 69, 70
PARAPHARYNGEAL ABSCESS
The parapharyngeal space (or lateral pharyngeal or pharyngomaxillary space) is in the upper neck above the hyoid bone. It is an inverted cone-shaped potential space that extends from the hyoid bone to the base of the skull. Medially, it is bound by the pretracheal fascia, and laterally, by the pterygoid muscles and mandible.71 Anteriorly, it is bound by the submandibular space, and posteriorly, by the retropharyngeal space.72 The clinical manifestations of a parapharyngeal abscess are determined by the structures involved around the abscess cavity. An abscess in the posterior component of the space may result in medial displacement of the lateral pharyngeal wall. Extension can result in serious local nerve and life-threatening vascular complications (the internal carotid artery, internal jugular vein, cranial nerves IX, X, XI, and XII, and the sympathetic chain pass posteriorly through the parapharyngeal space).73 An anterior compartment abscess can cause trismus from irritation of the internal pterygoid muscle. The source of the abscess is often unclear, but it seems likely to result from extension of infection from nearby tissues.
PERITONSILLAR ABSCESS
The peritonsillar space is limited medially by the fibrous wall of the tonsil capsule and laterally by the superior constrictor muscle.74 Pus may be found in a single pocket or in several pockets. The majority occur following tonsillitis, presumably from local extension of the infection through the tonsillar capsule.75 The three types of abscess have similar microbiology. The microbiology reflects the flora of the oropharynx and nasopharynx. Most are polymicrobial infections with an average number of five isolates.71 Anaerobic bacteria can be isolated from most abscesses if appropriate culture techniques are used.71 The predominant anaerobic organisms are Prevotella, Porphyromonas, Fusobacterium, and Peptostreptococcus spp.71 Retropharyngeal abscesses in young children are more likely to have pathogenic aerobic isolates, most frequently, S. pyogenes, Staphylococcus aureus, and Haemophilus species.71, 76
Retropharyngeal Abscess
CLINICAL FEATURES
The clinical presentation of a retropharyngeal abscess can be very nonspecific, particularly in younger children. Torticollis is a key clinical sign, particularly in combination with fever and dysphagia.65, 67, 77 Other clinical manifestations include drooling, airway stridor, dyspnea, tachypnea, stiff neck, and ipsilateral cervical adenopathy. There is sometimes midline or unilateral swelling of the posterior pharynx. Presenting symptoms and signs in infants include neck swelling, fever, dysphagia, and stridor.65
Diagnosis
An acute inflammatory response will be demonstrable with measurement of, for example, the peripheral white blood cell count and C-reactive protein, but radiologic investigation is necessary to confirm the diagnosis.65
A lateral neck radiograph can yield diagnostic information. To prevent the false appearance of a retropharyngeal mass when none exists, it is important that the neck be in true lateral orientation and in extension and the image be obtained on full inspiration.78 The lateral neck radiograph may show an increase in the thickness of the soft tissue space anterior to the cervical spine (>7 mm at the level of the second and >14 mm at the sixth cervical vertebra)* with narrowing of the oropharyngeal aiway65, 79 (Fig. 32-2 ). Other radiographic signs include straightening of the cervical vertebra, reversal of the normal lordotic curve of the cervical spine, and presence of air in the soft tissues.80 A negative ultrasound examination cannot exclude a retropharyngeal abscess.65 A computed tomography (CT) scan is the preferred investigation for distinguishing deep neck abscesses from cellulitis of the neck and for defining any extension into adjacent areas.67 However, even with a CT scan, it is not always possible to differentiate cellulitis from abscess78, 81, 82 (Fig. 32-3 ).
Figure 32-2.
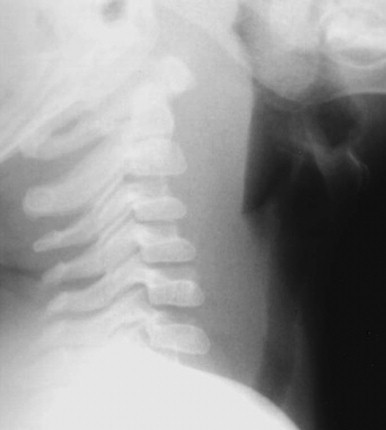
Lateral neck radiograph showing increased thickness of the retropharyngeal space.
Figure 32-3.
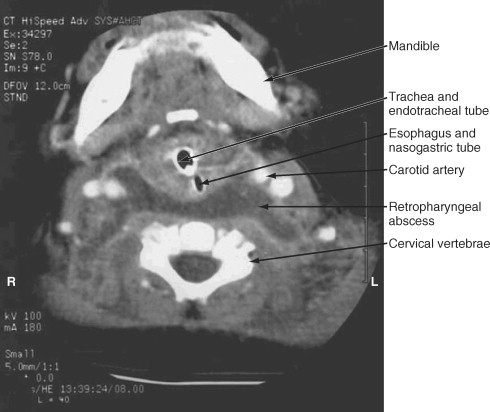
Computed tomography scan of a 6-month-old child at the level of mandible demonstrating a retropharyngeal abscess.
(From Cheema B, Grant CC, Mahadevan M, Beca J: An infant with a persistent empyema. Acta Paediatr 88:1168-1171, 1999.)
© 2008
Magnetic resonance imaging (MRI) has the potential to provide better definition of any complications such as venous thrombosis and impending carotid artery erosion or rupture.65, 72
TREATMENT
Intravenous antibiotics and incision and drainage are the necessary treatments. Intubation and, rarely in severe cases, tracheostomy may be necessary to secure the airway.65, 72, 75, 84
Antibiotic choice needs to acknowledge the polymicrobial nature of the infection and the frequent presence of one or more anaerobes. Appropriate first-line choices include amoxicillin–clavulanic acid, clindamycin+cefuroxime, ceftriaxone plus metronidazole, gentamicin, and ampicillin plus sulbactam.65, 75, 84
The decision to operate should be based on the clinical course, including response to antimicrobial therapy, rather than just on the CT scan findings.65 Antimicrobial therapy alone may be sufficient in children without severe systemic toxicity, who have no respiratory difficulties, who are able to swallow their secretions adequately, and in whom airway examination by indirect mirror or direct flexible endoscopy confirms a lack of airway compromise.73, 82
If the abscess is medial to the great vessels and confined to the retropharyngeal space, the abscess can be drained intraorally.72, 85, 86 Large abscesses, particularly those that extend laterally or that involve other spaces in the neck, mayneed external drainage.75 External drainage in children has the potential to damage important structures such as the great vessels and cranial nerves VII, IX, X, XI, and XII.65 Successful drainage of uniloculated abscesses using ultrasound and CT to guide either needle aspiration or catheter insertion has been reported.87, 88
Abscesses can extend laterally, posteriorly into the posterior mediastinum, and cranially, causing a cerebral abscess or meningitis.71 Abscesses left untreated can rupture into the pharynx, leading to aspiration. Direct pressure, sudden rupture, or hemorrhage can all result in asphyxia. Death can occur from aspiration, airway obstruction, erosion of a major vessel, extension into the posterior mediastinum, or from dissemination and sepsis.71
Other complications include abscess recurrence (1% to 5%), epiglottitis, empyema, pyopneumothorax, pneumomediastinum, and purulent pericarditis.65, 83
Peritonsillar Abscess
CLINICAL FEATURES
Clinical features include sore throat (occasionally with unilateral pain), malaise, low-grade fever, chills, dysphagia, and reduced oral intake. Trismus can result from irritation and reflex spasm of the internal pterygoid muscle. A muffled voice can result from edema, impairing movement of the palate. There may be signs of toxicity, drooling, and sometimes dehydration. The soft palate and uvula are displaced away from the affected side by swelling. The tonsil is displaced medially, and there is ipsilateral tender cervical adenopathy. Untreated peritonsillar abscess may spontaneously rupture into the mouth or extend into the parapharyngeal space with potentially fatal complications.
DIAGNOSIS
Identification of the organisms from aspirated pus is highly desirable. The peripheral white blood cell count is elevated, with a predominance of neutrophils.
TREATMENT
A combination of broad-spectrum parenteral antibiotic therapy that is active against anaerobes and drainage of the abscess is required.75 Needle aspiration is also effective definitive therapy.75, 89 Tonsillectomy can be performed either during the same procedure or after an interval. It is indicated in those presenting with a peritonsillar abscess who have a history of recurrent tonsillitis, who have recurrent peritonsillar abscess, or who have tonsillar hypertrophy causing airway obstruction.75
Parapharyngeal Abscess
CLINICAL FEATURES
Clinical features include tender cervical swelling, induration and erythema of the side of the neck, sore throat, dysphagia, trismus, hoarseness, malaise, chills, and fever, which may be low grade. In addition to evidence of toxicity, there may be respiratory distress, medial displacement of the lateral pharyngeal wall and inferior tonsil pole, and drooling. Sometimes the presentation is of a high cervical mass palpable in the neck that progresses to fluctuance. Other signs arise if there is further extension or complications. Initially, a parapharyngeal abscess may be difficult to differentiate from a peritonsillar abscess, but the child with the latter is usually less toxic and has obvious palatal fluctuance.
DIAGNOSIS
The peripheral white blood cell count is elevated, with a predominance of neutrophils. Radiographs may be helpful. A submental vertex skull radiograph typically shows pharyngeal fullness on the side of the abscess. An anteroposterior view of the upper airway shows ipsilateral edema and obliteration of the pyriform sinus. As with retropharyngeal abscesses, a CT scan is able to localize the inflammatory process to the parapharyngeal space but cannot always differentiate an abscess from cellulitis.82, 90
TREATMENT
The definitive treatment is incision and drainage and intravenous antibiotic therapy. In patients with larger abscesses or an unstable airway, emergency drainage with postoperative airway management, including intubation for several days, is indicated.90 In the stable child, drainage can be deferred for 24 to 48 hours to determine whether intravenous antibiotics alone are sufficient to treat the infection.90 Children with abscesses that are limited to this space and who demonstrate clinical improvement with intravenous antibiotics can be managed without drainage.73 If drainage is required, this can be intraoral rather than external in most, unless the abscess is lateral to the great vessels or involves multiple spaces.91
Parapharyngeal abscesses can cause life-threatening complications, including internal carotid artery pseudoaneurysm or rupture, internal jugular vein thrombophlebitis, mediastinitis,and dysfunction of cranial nerves IX to XII (Box 32-8 ).90, 92, 93, 94
Box 32-8. Retropharyngeal, Parapharyngeal, and Peritonsillar Abscesses Teaching Points.
-
•
Deep neck abscesses are potentially fatal infections that cause significant morbidity.
-
•
Multiple abscess types can coexist.
-
•
The deep neck potential spaces are in close proximity to vital structures, including the airway, carotid arteries, and many cranial nerves.
-
•
Retropharyngeal abscesses are more common in the preschool-age group, and parapharyngeal and peritonsillar abscesses are more common in older children and adolescents.
-
•
The three types of abscess have similar microbiology; most are polymicrobial infections.
-
•
The clinical presentation of a retropharyngeal abscess can be very nonspecific, particularly in younger children. Torticollis is a key clinical sign, particularly in combination with fever and dysphagia. A lateral neck radiograph can be diagnostic, but care needs to be taken to perform it correctly. Even with a CT scan, it can be difficult to differentiate an abscess from cellulitis.
-
•
A peritonsillar abscess usually presents with a sore throat, with systemic signs of infection. Trismus and a muffled voice can be present. The soft palate and tonsil are usually displaced by the abscess.
-
•
A parapharyngeal abscess presents with dysphagia, trismus, and hoarseness and tender cervical swelling, which is indurated. It can sometimes be difficult to differentiate clinically from a peritonsillar abscess. A CT scan is required to define the extent of the abscess and the potential for complications.
-
•
All abscess types require intravenous antibiotics. Anaerobic cover must be provided.
-
•
Drainage is frequently required. The decision to drain and timing of surgical drainage are determined by the clinical course.
OTITIS MEDIA
Otitis media is a very common condition in childhood. There are three categories of otitis media: acute otitis media, otitis media with effusion (secretory otitis media), and chronic suppurative otitis media. The widespread use of antibiotics for acute otitis media in the developed world has drastically reduced the previously fairly common suppurative complications of otitis media, but otitis media with effusion has become more common.
Epidemiology, Risk Factors, and Pathogenesis
A higher rate of acute otitis media and chronic suppurative otitis media is found in children in developing countries95 and in indigenous populations in developing countries, including New Zealand Maori,96 Australian Aborigines, Alaskan Inuit, and North American Indians.
Acute otitis media is an acute infection of the middle ear, and most children have at least one episode by the age of 7 years.97 Acute otitis media is particularly common in the preschool-age child and more common in boys than girls. Exclusive breastfeeding for at least 4 months appears to protect against otitis media in the first 12 months of life.98 The increased environmental exposure to respiratory tract infections in day care centers increases the risk of acute otitis media.99 Side-stream smoking increases the risk of otitis media with effusion and recurrent acute otitis media.100
Acute otitis media may occur de novo; more commonly, it occurs as a complication of the common cold. It may occur in the context of infection with recognized respiratory viruses such as respiratory syncytial virus, influenza viruses, adenoviruses, parainfluenza viruses, enteroviruses (coxsackievirus, echovirus), rhinoviruses,101 and even herpes simplex virus type 1 and cytomegalovirus.102 Viral infection in isolation is a rare cause of otitis media (5%), but up to 20% of cases are combined viral and bacterial infections.101 The remainder are caused by bacteria alone. Bacterial causes of acute otitis media are listed in Box 32-9 .
Box 32-9. Bacterial Causes of Acute Otitis Media and Bacteria Found in Otitis Media With Effusion.
Common Causes
50% Streptococcus pneumoniae serotypes 1, 3, 4, 6, 7, 9, 14, 15, 18, 19, and 23
25% Nontypable Haemophilus influenzae and H. influenzae type b
25% Moraxella catarrhalis
Rare Causes
Mycoplasma pneumoniae
Chlamydia trachomatis
Chlamydia pneumoniae
Enteric bacteria
Staphylococcus aureus
Staphylococcus epidermidis
Streptococcus pyogenes
Pseudomonas aeruginosa
Two or more organisms are found in about 7% of cases. A different organism may be found in each ear in about 20% of children with bilateral otitis media. In neonates, there may be a higher incidence of S. aureus and gram-negative bacilli than in older children.103
The organisms isolated from acute otitis media with tympanostomy tubes are different in prevalence from acute otitis media with an intact tympanic membrane, being mainly Streptococcus pneumoniae, S. aureus, Haemophilus influenzae, Pseudomonas aeruginosa, Moraxella catarrhalis, anaerobes, and fungi.104, 105, 106
Acute otitis media occurs when viral infection causes respiratory epithelial injury in the nasopharynx, which iscolonized with pathogenic bacteria, leading to hyperemia and edema of the eustachian tubes with consequent obstruction. Bacteria may arise in the middle ear by positive or negative forces through the eustachian tube or occasionally through the bloodstream or by direct spread through a damaged tympanic membrane. The inflammation of the tympanic membrane and infected inflammatory exudate in the middle ear are caused primarily by bacteria, polymorphonuclear leukocytes, and edema. The eustachian tubes are believed to play a part in the pathophysiology of this process. The eustachian tubes in a young child are shorter, wider, straighter, and more horizontal and patulous than in the older child, allowing more ready access of organisms to the middle ear.
Otitis media with effusion is the presence of fluid in the middle ear without signs or symptoms of acute ear infection. It can result from prolonged negative pressure in the middle ear after viral infection, and stimulation of inflammatory mediators can promote fluid leakage from the mucous membrane. Persistent middle ear fluid results in decreased mobility of the tympanic membrane and serves as a barrier to sound transmission.95 Recurrence of bilateral otitis media with effusion after tympanostomy tube placement was more likely in children with a combination of low IgA or IgG2 levels with poor eustachian tube functioning and decreased levels of mannose-binding lectin.107 However, eustachian tube functioning is not predictive of risk of recurrence of otitis media with effusion.108 Eustachian opening and closing functions are dynamic and highly variable in ears with otitis media with effusion.109
Chronic suppurative otitis media is a stage of ear disease in which there is ongoing chronic infection of the middle ear without an intact tympanic membrane (presence of a perforation or tympanostomy tube).95 It is one of the most common infectious diseases of childhood and is most common in developing countries, in certain high-risk groups in developed nations, and among children who have had tympanostomy tubes inserted.95 Risk factors that have been attributed to the high rates of chronic suppurative otitis media are lack of breastfeeding, overcrowding, poor hygiene, poor nutrition, passive smoking, high rates of nasopharyngeal colonization with potentially pathogenic bacteria, and inadequate health care.95 Bacteria isolated in chronic suppurative otitis media are listed in Box 32-10 .
Box 32-10. Bacterial Isolates in Chronic Suppurative Otitis Media.
Enteric gram-negative bacilli
Mixed aerobic and anaerobic bacteria
Mycobacterium tuberculosis
Pseudomonas aeruginosa
Staphylococcus aureus
Clinical Features
Acute otitis media typically presents with generalized symptoms of malaise, earache, and often fever. An older child complains of muffled hearing, a sense of fullness, and discomfort of the ear. In a younger child, there are more likely to be systemic signs such as high fever, nausea, vomiting, loss of appetite, malaise, generalized muscle pain, nasal congestion, flushed face, and, occasionally, diarrhea and restlessness. The pain may be severe and accentuated by swallowing, and occasionally there may be throbbing tinnitus. The fever, pain, deafness, and tinnitus may worsen, and there may be tenderness over the mastoids, but there is immediate relief of pain and systemic symptoms if the drum ruptures and the pus drains.
The clinical suspicion of otitis media is confirmed by appropriate otoscopic examination. Typical signs of acute otitis media are retraction, diminished light reflex, and poor mobility of the drum. The light reflex may completely disappear, and the drum becomes opaque. There is injection of vessels around the margin of the tympanic membrane and adjoining external auditory canal skin. The tympanic membrane moves but less freely with insufflation, and such movement is painful. The drum becomes red, and the pars tensa becomes thick and convex and bulges, with loss of landmarks. In young children, there may be swelling of the posterosuperior aspect of the adjacent external auditory canal skin. As the condition progresses, the drum becomes convex, tense, and whitish, and it bulges, with no mobility and hyperemic vessels on the periphery. There may be yellowish necrotic areas. The drum may rupture in the pars tensa, causing a gush of purulent material, blood, or serosanguineous fluid. Drainage usually stops after 1 to 2 days and the perforation becomes dry. The perforation is generally small and does not enlarge, and after the infection subsides, it usually heals completely (Fig. 32-4 ).
Figure 32-4.
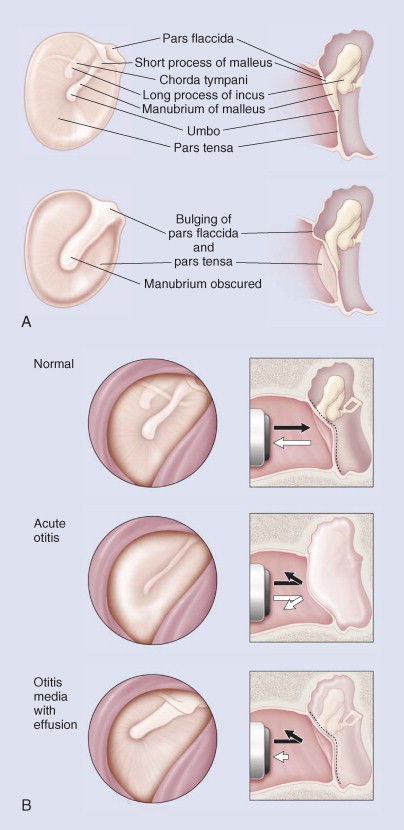
A, Visual assessment of middle ear status: normal and bulging. B, Visual assessment of tympanic membrane: normal, acute otitis media, otitis media with effusion.
(From Pelton SI: Otoscopy for the diagnosis of otitis media. Pediatr Infect Dis J 17:540-543; discussion 580, 1998.)
© 2008
Although viral infection may be associated with otitis media, there is no clinical way of distinguishing between viral and bacterial otitis media. If purulent conjunctivitis is also present, acute otitis media is most likely due to nontypable H. influenzae, and these clinical signs in combination should influence antibiotic choice. M. pneumoniae is a more likely cause if pneumonia is present.
Otitis media with effusion causes fluctuating hearing loss, which may have an adverse effect on speech, language, and cognitive development, although there seems to be a catch-up to normal by age 7 years. The clinical suspicion is confirmed by persistence of middle ear effusion without signs of inflammation. Cases may occur with infection, tubal obstruction, allergic or immunologic disorders, enlarged adenoids, or, rarely, nasopharyngeal tumors.
In chronic suppurative otitis media, the cardinal feature is chronic otorrhea, which is often smelly. Hearing loss occurs in 96% and is more severe than in otitis media with effusion.95
Diagnosis
When acute otitis media has classic symptoms and signs, making the diagnosis from clinical features is not difficult. However, uncommonly, acute otitis media may have no localizing symptoms or less impressive signs of inflammation. Sometimes, the tympanic membrane is difficult to visualize. In this situation, the clinical distinction between acute otitismedia and otitis media with effusion is difficult. The critical distinguishing factors are signs of acute inflammation versus otoscopic evidence of middle ear effusion. If otoscopic examination cannot be satisfactorily completed, tympanometry is indicated.
A strong light source and adequate magnification are necessary. Where possible, debris in the canal is removed. The mobility of the drum should be tested, by occluding the external canal completely with a large ear speculum and using pneumatic otoscopy, permitting the application of positive and negative pressure. Visualization of the tympanic membrane and assessment of mobility are the standard for the diagnosis of otitis media.110
There is a poor correlation between qualitative and semiquantitative cultures of the nose and throat and those of the middle ear. Tympanocentesis is the only reliable way of detecting middle ear pathogens, but it is primarily a research tool and is seldom done in clinical practice.
Tympanometry gives an objective, reproducible measure of middle ear function. It is particularly useful in situations in which otoscopy is difficult or unreliable. In the infant under 6 months of age, it can be unreliable because of collapsing ear canals. Normative values have been established for 7- to 24-month-old children.111 The findings of whether there are signs of acute inflammation are of a type B (flat) tympanogram or C2 (peak at less than -200 mm H2O). Tympanometry is at least as sensitive in detecting middle ear fluid as pneumatic otoscopy112 (Fig. 32-5 ).
Figure 32-5.
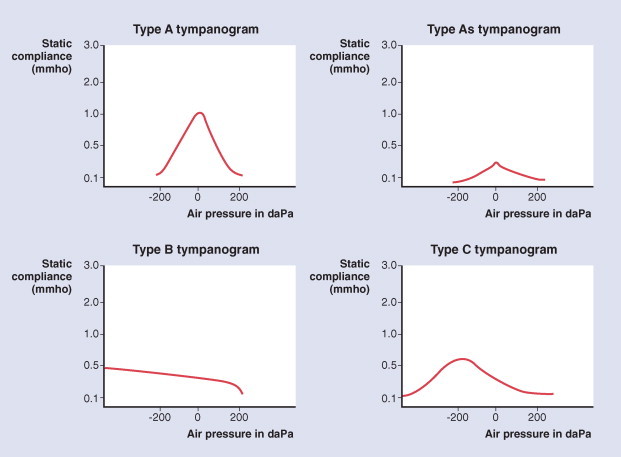
Tympanogram: classification system for low-frequency tympanograms. Based on Jerger (1970).
(From Harris PK, Hutchinson KM, Moravec J: The use of tympanometry and pneumatic otoscopy for predicting middle ear disease. Am J Audiol 14:3-13, 2005.)
© 2008
Clinicians should document the laterality, duration of effusion, and presence and severity of associated symptoms in the child with otitis media with effusion at each assessment.113 Children with otitis media with effusion who are at risk for speech, language, or learning problems need to be distinguished from all other children with otitis media with effusion. Those at risk include children with permanent hearing loss independent of otitis media with effusion, suspected or diagnosed speech and language delay or disorder, autism-spectrum disorder, syndromes such as Down syndrome that include cognitive, speech, and language delays, uncorrectable visual impairment, cleft palate with or without associated syndrome, and developmental delay.113 In such children intervention may be required more promptly. In healthy children not at risk, watchful waiting for 3 months from the date of effusion onset (if known) or diagnosis (if onset is unknown) is recommended.113
The symptoms of acute otitis media need to be distinguished from those of acute systemic illness. The specific diagnosis can usually be made by noting the general symptoms and performing an adequate and complete inspection of the tympanic membrane. There can be difficulties when the external canal or debris within it does not allow adequate visualization.
Ear pulling in the absence of other symptoms is not related to ear infection.114 Hyperemia of the tympanic membrane can occur with crying, trauma to the external auditory canal, or mild upper respiratory tract infections. These situations can be distinguished from acute otitis media because other abnormal features of the drum, in particular reduced mobility, would be lacking in them. Acute bullous myringitis can occur with acute otitis media. It causes more severe symptoms with blisters on the tympanic membrane, but it has a good clinical outcome.115 Otalgia may be caused by referred pain from infections in the adenoids, tonsils, teeth, nasopharynx, hypopharynx, or larynx through the tenth cranial nerve. Tumors of the palate, nasopharynx, or base of the skull eventually occlude one or both eustachian tubes.
Treatment
The goals of treatment are to shorten the duration of symptoms ofacute otitis media, to prevent complications, to prevent progression to chronic suppurative otitis media, and to prevent long-term hearing loss.
ACUTE OTITIS MEDIA
The management of acute otitis media varies within the Western world,116 but the development of evidence-based guidelines may lead to a more standardized approach. In the past decade, several randomized controlled trials and meta-analyses have advanced knowledge about treatment strategies for this condition and otitis media with effusion.
All randomized clinical trials of antibiotic use for acute otitis media are from developed countries. They have shown that about 15 children needed to be treated to prevent one child from having pain on days 2 to 7 (no benefit on day 1). The effect on hearing is inconclusive.117 Antibiotic use in groups where mastoiditis is common may reduce the risk of its development.118
Patients with acute otitis media are treated as outpatients if there is no systemic infection, unless there is frequent vomiting requiring hospital care. Children should be allowed to rest until the fever has resolved for 24 hours. Pain relief with acetaminophen is indicated. The complications of bacterial otitis media can be so serious that every child with acute inflammation should be seriously considered for antibiotics. The only indication for withholding antibiotics is a situation in which there is redness and no other sign of inflammation and the child can be reliably monitored every 1 to 2 days by otoscopy, with the parent bringing the child between visits if the condition deteriorates. The indications for antibiotics are absolute in children under the age of 6 months, regardless of symptoms, and in children of any age who have severe symptoms such as fever or vomiting. Observation with monitoring is allowable for nonsevere illness.
The choice of antibiotics for acute otitis media is determined by the known likely pathogens and local sensitivity patterns. Other factors influencing choice of therapy include the age of the patient, likelihood of compliance with the dosing frequencies, hypersensitivity to antibiotics, the cost of the antibiotics, and the patient's previous experience with the medication.119 As illustrated in Figure 32-6 , amoxicillin, 80 mg/kg/day in three divided doses, is the usual first choice of treatment.120 If the patient is allergic to penicillin, trimethoprim-sulfamethoxazole is the usual alternative (8 mg of trimethoprim and 40 mg of sulfamethoxazole per kilogram per day in two doses). If the child has had no symptomatic response within 3 days, a change of an antibiotic is indicated. Alternatives are amoxicillin-clavulanate potassium, 40 mg/kg/day in three doses; cefixime, 8 mg/kg/day in one or two doses121; and erythromycin-sulfisoxazole, 50 mg/kg/day in four doses. Cefaclor (40 mg/kg/day in two or three doses) is less efficacious. If the child is vomiting, a single intramuscular dose of ceftriaxone, 50 mg/kg/day, is as effective as 10 days of oral amoxicillin.122 All these antibiotic regimens seem to have comparable efficacy in resolving the clinical features of acute otitis media. Paradoxically, there can be a good clinical response to amoxicillin despite the presence of middle ear pathogens that produce β-lactamase. In acute otitis media in the neonate, special vigilance is required because infection may progress. If there is accompanying systemic infection, hospital admission with parenteral therapy covering S. aureus and gram-negative bacilli is indicated.
Figure 32-6.
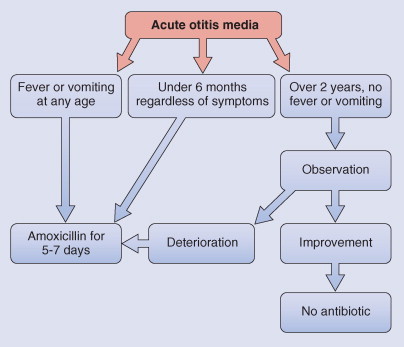
Antibiotics for acute otitis media.
When antibiotics are given early, the length of the symptomatic period may be reduced, and the infection is usually arrested before the drum ruptures. Immediate antibiotic treatment brings more rapid relief of pain and malaise compared with delaying the onset of treatment by 48 hours.123 Early eradication of pathogens from middle ear fluid during antibiotic treatment of acute otitis media is associated with improved clinical outcome.124 However, improved clinical outcome does not necessarily predict bacteriologic outcome. Bacteriologic failure occurs most often in children under 18 months of age.125 Incomplete eradication could be one reason that otitis media with effusion may develop.
There is no consistent evidence from randomized, controlled trials that nasal decongestants, mucolytic agents, or antihistamines help prevent or treat any form of otitis media.126 Intranasal steroids given to children with viral upper respiratory infections do not provide symptomatic relief or decrease episodes of acute otitis media and may even increase this undesired outcome.127
Myringotomy is indicated when there is severe, persistent pain and failure to respond to initial antibiotic therapy or when there is a complication of otitis media with an intact drum or persistent conductive hearing loss. In clinical practice, myringotomy is seldom performed despite these indications.
The role of adenoidectomy and adenotonsillectomy in reducing recurrence of acute otitis media is not established. Coyte and colleagues128 found that among children over 2 years old, adenoidectomy and adenotonsillectomy at the time of insertion of tympanostomy tubes reduced the likelihood of additional hospitalizations and operations related to otitis media. However, Hammaren-Malmi and colleagues129 found in children 1 to 4 years of age that adenoidectomy did not reduce the incidence of acute otitis media in children who have recurrent acute otitis media, or otitis media with effusion with tympanostomy tubes. Paradise and colleagues130 found that in children with recurrent acute otitis media with or without otitis media with effusion, that adenoidectomy and adenotonsillectomy showed limited short-term benefit but did not recommend it as a first intervention because of adverse events and cost.
Following interventions by an otolaryngologist, there was a significant disease-specific improvement in quality of life, physical suffering, emotional distress, and caregiver concerns, regardless of the treatment given.131, 132
Adjuvant treatment with prednisone 2 mg/kg/day versus placebo for 3 days has been shown in a randomized controlled trial to reduce the duration of otorrhea from 3 days to 1 day in children also treated with amoxicillin/clavulanate who have acute otitis media with tympanostomy tubes.133
Myringotomy with insertion of tympanostomy tubes is indicated for persistent otitis media with effusion with hearing loss, taking into account that in an otherwise healthy child 50% of cases resolve spontaneously. The timing of tympanostomy tube insertion depends on the clinical risk of the child (Fig. 32-7 ). Tympanostomy tube insertion has become very common and is the main reason a child in the United States receives a general anesthetic. Waiting for spontaneous resolution in otherwise healthy children may reduce the number of children receiving this operation. If otitis media with effusion has been present for 9 to 12 months with decreased hearing, tympanostomy tubes are indicated. This can result in improvement in hearing (average, 12 decibels [dB]) in the short term, but there is no evidence that there is a beneficial effect on development or behavior. At the time of insertion of tympanostomy tubes, the clinician should conduct a preoperative assessment, including a developmental assessment, history of hearing difficulty or speech or learning problems, documentation of actual hearing impairment, and pneumatic otoscopy and tympanometry.
Figure 32-7.
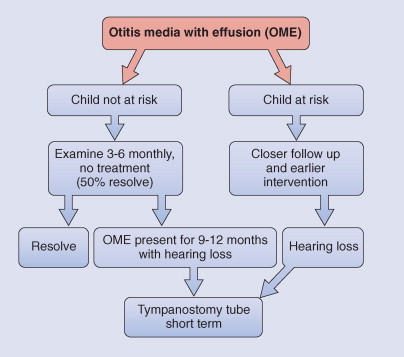
Management of otitis media with effusion (OME).
Anesthetic complications of bilateral tympanostomy tube placement in a tertiary care children's hospital are infrequent, and all can be successfully treated. A minor adverse event occurred in 9% (agitation or prolonged recovery) and a major event occurred in 1.9% (upper airway obstruction or laryngospasm), most commonly in a child with an acute or a chronic illness.134
In otherwise healthy children younger than 3 years of age who have persistent otitis media with effusion, prompt insertion of tympanostomy tubes does not improve developmental outcomes at 3 and 6 years of age.135, 136
Sequelae of tympanostomy tubes are common but are generally transient (otorrhea) or cosmetic (tympanoslerosis or focal atrophy). Nonetheless, the high incidence suggests the need for ongoing surveillance of all patients with indwelling tubes and for a reasonable time period after tube extrusions. Long-term tubes should be used rarely for unusually severe cases.137 Acute otitis media with tympanostomy tubes responds better to topical antibiotics than oral antibiotics138, 139 (Box 32-11 ).
Box 32-11. Tympanostomy Tubes.
This is the most common operation in childhood in developed countries.
Complications include
Anesthetic risks
Purulent discharge
Granulation tissue
Chronic perforation
Retraction pockets
Atrophic scars
Tympanosclerosis
Eustachian tube inflation by mechanical means, using nasal balloons (Otovent), appears to be associated with short-term improvement in otoscopic findings in 3- to 10-year-old children compared with findings in controls.140 Its place in the treatment of otitis media with effusion has yet to be established.
The components of treatment of chronic suppurative otitis media include aural toilet, topical antibiotics, and closure of prolonged tympanic perforation. There are few randomized controlled trials for this condition. Treatment of chronic suppurative otitis media with aural toilet and topical antibiotics, particularly quinolones, is effective in resolving otorrhea and eradicating bacteria from the middle ear.141 Aural toilet is ideally performed using the microscope and microinstruments to mechanically remove debris. Topical quinolone antibiotics can clear aural discharge better than no drug treatment or topical antiseptics. Studies are inconclusive regarding any differences between quinolone and nonquinolone antibiotics.142 Topical antibiotics are more effective than oral antibiotics, probably because a higher local concentration of antibiotic is achieved.
Clinical Course and Prognosis
Chronic perforation without otorrhea can occur as a complication of acute otitis media or after tympanostomy extrusion.95
Eighty percent of children with acute otitis media settle spontaneously in 7 days.118 Intracranial or intratemporal complications can occur with acute otitis media. Antibiotic therapy is indicated to prevent these serious bacterial complications. Extension of inflammation and infection beyond the mucoperiosteal lining of the middle ear may result in mastoiditis or meningitis (especially with H. influenzae). The symptoms and signs of acute mastoiditis may be subtle, especially if they are partially treated by antibiotics or if the tympanic membrane is ruptured. The recurrence of pain and the presence of copious purulent discharge associated with low-grade fever suggest mastoiditis. Usually, there is tenderness over the mastoid process, and there may be edema of the mastoid periosteum, sometimes with postauricular pitting. In the external auditory canal, there is a sagging bulge in the posterior superior wall.
After acute otitis media, up to 20% of cases progress to otitis media with effusion. Some 50% of such patients recover after 3 months, but in about 5% the condition persists after 12 months. A large number of cases are transient, with episodes varying in duration and severity. Sometimes, there is recurrence of otitis media with effusion when acute otitis media does not recur.
In children with tympanostomy tubes for otitis media with effusion, 50% to 83% have episodes of otorrhea,106 mostly nonsevere and self-limited, reflecting secondary inflammation of the eustachian tube and middle ear during viral upper respiratory infection.137 These simple cases of otorrhea are inevitable and cannot be prevented by water precautions, prophylactic drops, or changes in surgical technique. Management with ototopical antibiotic drops such as ofloxacin alone is sufficient to treat purulent otorrhea.138
The hearing level at 14 years of age of healed ears after tympanostomy tubes is normal. In ears showing abnormalities (perforation, pars tensa retraction, otitis media with effusion) 25% have varying degrees of hearing loss.143
Prevention
The advent of pneumococcal vaccines has led to investigation of their role in the prevention of acute otitis media where 50% of bacterial causes are due to S. pneumoniae. A small benefit has been shown for pneumococcal polysaccharide vaccine in children over 2 years of age who have had previous episodes of acute otitis media.144 There was also a small reduction in risk of recurrent disease with pneumococcal conjugate vaccine. An 11-valent pneumococcal vaccine conjugated with H. influenzae–derived protein D, given at 3, 4, 5, and 12 to 15 months of age, has been shown to have an efficacy of 34% against first episodes of otitis media in the first 2 years of life (95% confidence interval, 21% to 44%).145
Efforts to prevent chronic suppurative otitis media should be directed toward improvement in health care and living conditions in all populations that have a high prevalence of chronic suppurative otitis media, encouragement of breastfeeding, and reduction of cigarette smoking exposure.95
Pitfalls and Controversies
In neonates, otitis media may be overdiagnosed. The tympanic membrane often appears thickened and opaque duringthe first few weeks of life and lies in an extremely oblique position, making it difficult to distinguish it from the canal wall. The ear canal is particularly compliant, with positive pressure simulating the movement of the tympanic membrane.
There is frequently a gap in training in pneumatic otoscopy for most clinicians due to the lack of easy-to-use dual-headed equipment to enable training and evaluation of medical personnel. These skills can be learnt and result in high sensitivity and specificity.110
The method of use of antibiotics for acute otitis media and tympanostomy tubes for otitis media with effusion used to be highly controversial. However, many well-conducted randomized controlled trials and evidence-based Cochrane reviews have clarified most of the contentious issues. The challenge is to disseminate this knowledge to achieve evidence-based practice (Box 32-12 ).
Box 32-12. Otitis Media Teaching Points.
-
•
In acute otitis media, there is acute inflammation with 5% viral only, 75% bacterial only, and 20% both bacterial and viral. The most common organism is Streptococcus pneumoniae, and the first-line antibiotic is amoxicillin.
-
•
In otitis media with effusion, there is middle ear effusion, and up to 20% of cases of acute otitis media progress to otitis media with effusion.
-
•
In chronic suppurative otitis media including perforation, there is otorrhea and deafness. This is an important public health problem.
ADENOIDECTOMY AND TONSILLECTOMY
Elective surgical removal of the tonsils and adenoids was once widely performed, usually with the hope of reducing the frequency of recurrent sore throats, but the rate for this surgery has fallen over the past three decades. However, it remains the most common major operation in children in the United States, although the scientific basis of this practice is not well established.146 Children aged 3 to 8 years normally have up to nine respiratory tract infections a year. Prospective objective monitoring of symptoms demonstrates lower rates than frequency of symptoms recalled. In severely affected children with frequent, well-documented episodes of sore throat,147 tonsillectomy reduces the occurrence of throat infection.147 In children moderately affected with recurrent throat infection, however, the modest benefit conferred by tonsillectomy or adenotonsillectomy does not justify the inherent risks, morbidity, and cost of the operations.148 Adenotonsillectomy was no more efficacious than tonsillectomy alone.148
The most important indication for adenotonsillectomy is obstructive sleep apnea, which can be serious and even life threatening. Removal of both tonsils and adenoids is usually of marked clinical benefit.149, 150 Tonsillar or adenoidal size is not always a reliable indicator of the potential benefit of tonsillar removal.
Adenoidectomy has been used in children with otitis media, in an attempt to reduce recurrence of acute otitis media and persistence or recurrence of otitis media with effusion, but the indications remain unclear. Although adenoidectomy and bilateral myringotomy (without tympanostomy tubes) were beneficial in children 4 to 8 years old who were severely affected by otitis media with effusion,151 adenoidectomy did not reduce the occurrence of acute otitis media in children who have recurrent acute otitis media, or otitis media with effusion with tympanostomy tubes.129 Among children over 2 years old, adenoidectomy and adenotonsillectomy at the time of insertion of tympanostomy tubes for otitis media with effusion reduced the likelihood of additional hospitalizations and operations related to otitis media.128 In a further study of children with recurrent acute otitis media, with or without otitis media with effusion, adenoidectomy and adenotonsillectomy showed limited short-term benefit and could not be recommended as a first intervention because of adverse events and cost130 (Box 32-13 ).
Box 32-13. Adenoidectomy and Tonsillectomy Teaching Points.
-
•
The main indication for adenoidectomy and tonsillectomy is obstructive sleep apnea.
-
•
Adenoidectomy and adenotonsillectomy are not recommended for prevention of recurrent otitis media.
-
•
Tonsillectomy may be beneficial in children with recurrent severe tonsillitis.
SINUSITIS
Sinusitis is a bacterial infection of the paranasal sinuses that uncommonly complicates the common cold. The use of antibiotics dramatically reduces the occurrence of complications of sinusitis.
Epidemiology, Risk Factors, and Pathogenesis
In children, sinusitis almost always occurs as a complication of the common cold in developmentally aerated sinuses. It is more common in boys than in girls. The viral infection inflames the mucosa and causes damage to nasal ciliated epithelial cells, encouraging infection with bacteria colonizing the upper respiratory tract. Infection can occur in any of the paranasal sinuses as they develop (Box 32-14 ). Although the full development of the frontal sinuses may take 20 years, sinusitis can occur at any age.
Box 32-14. Development of Sinuses.
Birth: ethmoid sinuses aerated but small
Birth: maxillary sinuses aerated but small
1 to 2 years: sphenoidal sinus aerated
5 to 7 years: frontal sinuses aerated
Developmentally poorly aerated sinuses appear radiologically opaque
The four-paired paranasal sinuses communicate with the anterior nose, with which they form a system of narrow channels. The mucosa of the sinuses, like that of the nose, is a continuous ciliated columnar epithelium with goblet cells and is covered in part by a mucous blanket. A continual flow of mucus from the frontal, maxillary, and anterior ethmoid sinuses is propelled toward the ostia and then posteriorly to the nasopharynx. The mucus contains IgA, IgG, IgM, and lysozyme, and the paranasal sinuses are usually sterile. Damage to mucociliary function allows the inoculation oflarge numbers of pathogens into the sinuses, which causes infection. Once started, sinus infection is aggravated by further inflammation of the ostia of the sinuses, resulting in progressive obstruction. Irritants, such as swimming underwater and drying of the mucosa during winter in cold climates, can set the stage for sinus infection. Host factors predisposing to sinusitis are listed in Box 32-15 .
Box 32-15. Host Factors in Sinusitis.
Common cold
Respiratory allergies
Defects of ciliary function
Cystic fibrosis
Immunodeficiency
Anatomic abnormalities
Gastroesophageal reflux
The organisms causing sinusitis are listed in Box 32-16 . The most common causes are S. pneumoniae, H. influenzae, and M. catarrhalis. These organisms, along with S. aureus and S. pyogenes, account for over 90% of cases of sinusitis in children. In adolescents, penicillin-sensitive anaerobes become more common. There may be a vast array of enteric gram-negative and other bacilli recovered, mostly from those who have had antibiotic therapy before culture.152, 153
Box 32-16. Organisms Causing Sinusitis.
Most Common
Haemophilus influenzae
Moraxella catarrhalis
Streptococcus pneumoniae
Less Common
Acinetobacter spp.
Alcaligenes spp.
Citrobacter spp.
Diphtheroids
Eikenella corrodens
Enterococci
Escherichia coli
Haemophilus spp.
Klebsiella pneumoniae
Neisseria spp.
Proteus spp.
Pseudomonas aeruginosa
Rare
Serratia spp.
Staphylococcus aureus
Staphylococcus epidermidis
Streptococcus pyogenes
αHemolytic and nonhemolytic streptococci
Anaerobic Bacteria
Bacteroides spp.
Bifidobacterium spp.
Fusobacterium spp.
Peptococcus spp.
Peptostreptococcus spp.
Propionibacterium spp.
Veillonella spp.
Other organisms
l-forms
Mixed: aerobes and anaerobes
Mixed: Haemophilus influenzae with other organisms
Mycoplasma pneumoniae
Other (rhinovirus, adenovirus, Aspergillus spp., other fungi)
Fungi
Aspergillus spp.
Bipolaris spp.
Curvularia lunata
Drechslera spicifera
Zygomycetes
Clinical Features
Acute sinusitis is heralded by failure of common cold symptoms to resolve after 10 days.153, 154 Older children have more specific complaints than younger children. Sometimes, the sinusitis can be more acute with severe initial symptoms: fever greater than 39° C and purulent nasal discharge. Acute sinusitis involves symptoms persisting from 10 to 30 days, and after this time, it is usually categorized as chronic. However, an international consensus panel has suggested that the term rhinosinusitis be used instead of sinusitis because of the coexistence of rhinitis and that the term chronic be reserved for those with symptoms persisting beyond 12 weeks.155
The main symptom is rhinorrhea (80%), which is frequently purulent but can be serous or watery. In a minority of patients, there is fever, cough (especially at night), pain, headache, sore throat, periorbital swelling, vomiting, and, occasionally, malodorous breath. Sinus tenderness is uncommon in children. Posterior pharyngeal pus is not usually seen in acute sinusitis and is very uncommon in chronic sinusitis. Periorbital swelling is usually a sign of acute ethmoid sinusitis. Acute sinusitis is more frequently unilateral, and chronic sinusitis is usually bilateral. In chronic sinusitis, the symptoms may be minimal: vague unwellness with some persistent signs of upper respiratory tract infection.
Diagnosis
The diagnosis is usually made on clinical grounds alone. In acute sinusitis, clinicians seldom undertake investigations. If investigations are undertaken, nasal culture reveals the organism in the majority of cases, but it should be obtained with careful technique. A vasoconstrictor such as phenylephrine hydrochloride 0.25% should be applied to the anterior nose, and bilateral cultures should be obtained under direct vision using a wire cotton swab touching material as it comes from the sinus ostium. In children who have neurologic complications or in whom treatment fails, antral puncture for aerobic and anaerobic culture can identify the organism.
In acute sinusitis, the number of band neutrophils may be increased. Some cases of sinusitis have an elevated erythrocyte sedimentation rate.
The differential diagnosis includes chronic nasal allergy, foreign bodies in the nose, cysts in the maxillary antra, nasal structural defects, palatal defects, dental infections, and infection of the adenoids.
Sinus radiographs are not sensitive or specific in diagnosing sinusitis.156 CT scans are more sensitive than radiographs in chronic sinusitis but have low specificity. CT scans are indicated when there are complications or surgery is contemplated.155, 157, 158 Ultrasonography is of doubtful value unless there is one normal air-filled maxillary sinus for comparison.
In chronic sinusitis poorly responsive to treatment, clinicians should consider investigation for underlying abnormalities of host defense.
Treatment
The goals of treatment are to reduce symptoms rapidly and to reduce the possibility of persistence of symptoms. Antibiotics should not be started if symptoms are improving.159 Analgesics such as acetaminophen may be useful for controlling headache, pain, or fever. The primary treatment is antibiotics. Amoxicillin, 40 mg/kg/day in three doses, is adequate for the majority of S. pneumoniae, H. influenzae, and M. catarrhalis infections. A brisk response to treatment is expected in 3 to 4 days, in which case a 10-day course of treatment is satisfactory.160, 161 The benefits are modest: eight children must be treated to achieve one additional cure.160 In communities with β-lactamase–producing H. influenzae and M. catarrhalis, the following antibiotics should be considered: amoxicillin–clavulanate potassium (40 mg/kg/day in three doses), trimethoprim-sulfamethoxazole (8 mg of trimethoprim and 40 mg of sulfamethoxazole per kilogram per day in two or three doses), and cefaclor (40 mg/kg/day in three doses).
Vasoconstrictive drugs are often used locally or systemically in an attempt to relieve obstruction at the sinus ostia to help establish drainage, but there is no evidence supporting their effectiveness.161 Because topical medications can cause rebound vasodilation, these medications should be used only when there is considerable pain and then for no longer than 3 days.
Saline irrigation may have a role in the management of symptoms of chronic sinusitis,162 but further studies are required.
Clinical Course and Prognosis
The outlook of sinusitis in otherwise healthy children receiving adequate treatment is excellent. Untreated sinusitis can progress to orbital infection, meningitis, osteomyelitis, cavernous sinus thrombosis, and abscesses of the epidura, subdura, or brain.163
If the sinusitis is recurrent, fails to improve, or has more serious clinical features with high fever or periorbital swelling, consider amoxicillin–clavulanate potassium (40 mg/kg/day in three doses), trimethoprim-sulfamethoxazole (8 mg of trimethoprim and 40 mg of sulfamethoxazole per kilogram per day in two or three doses), and cefaclor (40 mg/kg/day in three doses).
If there are bacterial complications of sinusitis, the child should be hospitalized and given parenteral antibiotics. Initially, intravenous cefuroxime (100 mg/kg/day in three divided doses) is recommended; however, if S. aureus is a major concern, oxacillin or nafcillin should be added. Therapy should be adjusted on the basis of response to treatment and the results from culture.
Surgical drainage is rarely necessary in children. Fiberoptic endoscopic sinus surgery has replaced more invasive approaches. It is indicated only if there is lack of response to maximal medical therapy and continuing symptoms or in specific situations (Box 32-17 ). Optimal medical management, including 2 to 6 weeks of adequate antibiotics (intravenous or oral) and treatment of concomitant diseases, is indicated for uncomplicated sinusitis before medical treatment is regarded as failed, and surgery is recommended.
Box 32-17. Indications for Surgery.
Complete nasal obstruction in cystic fibrosis due to massive polyposis or closure of the nose by medialization of the lateral nasal wall
Antrochoanal polyp
Intracranial complications
Mucoceles and mucopyoceles
Fungal sinusitis
Dacryocystorhinitis due to sinusitis and resistant to appropriate medical treatment
Chronic rhinosinusitis that persists despite optimal medical management and after exclusion of any systemic disease; endoscopic sinus surgery is a reasonable alternative to continuous medical treatment
© 2008
From Clement PA, Bluestone CD, Gordts F, et al: Management of rhinosinusitis in children: Consensus meeting, Brussels, Belgium, September 13, 1996. Arch Otolaryngol Head Neck Surg 124:31-34, 1998.
Pitfalls and Controversies
The relationship between sinus disease and lower respiratory tract infections and asthma has caused debate because of the lack of controlled studies. Nevertheless, treating sinus disease may be associated with improvement in concurrent lower respiratory tract symptoms through improved nasal airway or the direct effects of antibiotics on the lower airways156 (Box 32-18 ).
Box 32-18. Sinusitis in Children Teaching Points.
-
•
Acute sinusitis symptoms are present 10 to 30 days after common cold.
-
•
Chronic sinusitis symptoms persist past 30 days.
-
•
Main symptom is rhinorrhea.
-
•
Sinus tenderness is uncommon in children.
-
•
Postpharyngeal pus is seldom seen.
-
•
First-line antibiotic is amoxicillin.
-
•
Outlook is excellent in healthy children.
VIRAL CROUP
Viral croup is common, affecting approximately 15% of children. Although epiglottitis is less common, it is still necessary to consider this and other alternative diagnoses such as congenital airway abnormalities, foreign body, and bacterial tracheitis.
Many children with croup require no specific treatment. Oxygen, single-dose glucocorticoids, and inhaled epinephrine are effective therapies for those who have more significant airways obstruction.
Epidemiology, Risk Factors, and Pathogenesis
Acute upper airway obstruction in children is most commonly due to a viral infection causing laryngotracheitis or spasmodic croup. The term acute laryngotracheitis defines the site of inflammation, which always involves the larynx and trachea; if it is believed to extend to the bronchi, the name laryngotracheobronchitis is used. Spasmodic croup and recurrent croup are often regarded as separate diagnoses but may be part of the spectrum of the same condition.
Viral croup (which when used in this chapter will refer to both acute laryngotracheitis and spasmodic croup) is uncommon in the first 6 months of life. Under this age, preexisting abnormalities of the upper airway such as subglottic stenosis or hemangioma should be considered. These lesions may also be the cause of prolonged stridor because viral croup rarely lasts longer than 10 to 14 days.
Viral croup is a very common condition, affecting about 15% of children. The annual incidence of croup in children younger than 6 years old is between 1.5% and 6%.164 It is most common between 6 months and 5 years of age, with a peak prevalence in the second year of life; the youngest reported patient is 3 months old.165 The full picture of viral croup is rare over the age of 10 years. Boys are affected more often than are girls.
The symptoms and signs result from inflammation in the larynx, trachea, and sometimes the bronchi. They are almost always caused by viral infection. The causative viruses are listed in Box 32-19 .
Box 32-19. Viral Causes of Acute Laryngotracheitis.
Common Causes
Influenza virus types A and B
Parainfluenza virus types 1 to 3
Respiratory syncytial virus
Uncommon Causes
Adenoviruses
Enteroviruses
Herpes simplex virus
Morbilli (measles) virus
Reovirus
Rhinoviruses
Viral, Bacterial, and Fungal Causes in an Immunodeficient Host
Candida albicans
Candida spp.
Herpes simplex virus type 2 (neonate)
Pseudomonas aeruginosa
The parainfluenza viruses cause most cases of croup, with type 1 being most common, type 3 less common, and type 2 infrequent. Respiratory syncytial virus and several of the adenoviruses infrequently cause croup, as does influenza virus type A, which induces a particularly severe form.166 Rhinoviruses, enteroviruses, herpes simplex virus, and reovirus have been associated with mild cases of croup. Morbilli (measles) virus may cause upper airway obstruction resulting from laryngotracheitis, sometimes severe enough to require intubation, and there may be complicating bacterial tracheitis. Rarely, the vesicular eruption of varicella may involve the larynx. Mild “viral” croup may also be caused by M. pneumoniae infection.
Primary bacterial croup is now uncommon. Immunization resulted in a rapid decline in croup due to C. diphtheriae infection. Bacterial croup became uncommon with the introduction of antibiotics.167 Diphtheria must be considered if the child has not been immunized against C. diphtheriae. This organisms may cause a membranous obstructive laryngitis. In most cases now where bacterial infection occurs, this is secondary to a preceding viral infection. S. aureus is the most common bacteria implicated in this way. Others include S. pyogenes, S. pneumoniae, H. influenzae, and M. catarrhalis.167
Seasonal variability in croup reflects the epidemiology of the different etiologic viruses. Parainfluenza type 1 causes outbreaks of croup in autumn and croup due to parainfluenza type 3 occurs in spring. The seasonality of croup due to parainfluenza type 2 is more variable.167, 168
After inhalation of the virus, the cells of the local respiratory epithelium become infected. There is marked edema of the lamina propria, submucosa, and adventitia accompanied by cellular infiltration with histiocytes, lymphocytes, plasma cells, and polymorphonuclear leukocytes.
There is redness and swelling of the involved airway, most marked in the lateral walls of the trachea just below the vocal cords. The subglottic trachea is surrounded by the fixed cricoid cartilage, forcing the inflammatory swelling to encroach on the internal airway lumen, narrowing it or reducing it to a slit (Fig. 32-8 ). The infant's glottis and subglottic region are normally narrow, and a small decrease in diameter results in a large increase in airway resistance and a decreasein airflow. As the airway diameter enlarges with growth, the impact of the subglottic airway swelling is reduced.
Figure 32-8.
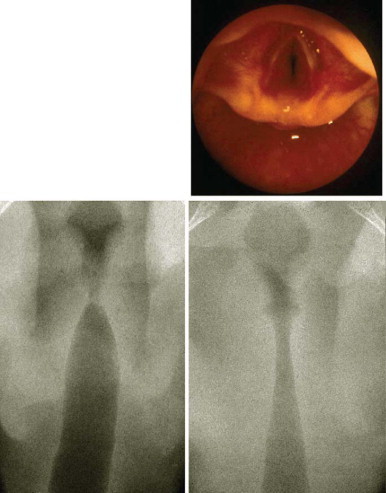
Endoscopic view of subglottic edema in viral croup (top). Radiologic presentation of subglottic edema in viral croup (bottom right) compared with normal trachea (bottom left).
(From Hammer J: Acquired upper airway obstruction. Paediatr Respir Rev 5:25-33, 2004.)
© 2008
In spasmodic croup, the direct laryngoscopic appearance shows pale, watery edema of subglottic tissues. There is an association with the same viruses that cause acute laryngotracheitis, but evidence suggests that the response to these viruses may be atopic rather than cytopathic.167 Spasmodic croup occurs more frequently in children who are atopic.
Clinical Features
Typically, the illness starts with rhinorrhea, sore throat, and mild fever for a few days. Then the child develops a characteristic barking cough, hoarseness, and inspiratory stridor with or without low-grade fever.
An increasing severity of obstruction is evident with increasing heart and respiratory rate, flaring of alar nasi, and indrawing, especially suprasternal, intercostal, and sternal, in the younger child or infant. Increasing chest wall retractions occur as the intrathoracic pressure becomes increasingly negative and correlates with the severity of the upper airway obstruction.169 Ribcage and abdominal asynchrony occurs as the condition deteriorates.170
Obstruction to airflow through the upper airway results in stridor and difficulty breathing and progresses to hypoxia when the obstruction is severe. Hypoxia with mild obstruction indicates lower airway involvement and ventilation-perfusion mismatch resulting from lower airway obstruction or lung parenchymal infection or even fluid.171 Hypercapnia occurs as a late change as hypoventilation progresses with obstruction.
As progressive hypoxia develops, the child is anxious or restless or may have depressed consciousness or cyanosis. Death can occur either from asphyxia or from respiratory fatigue.
On auscultation, breath sounds are normal with no added sounds except transmission of the stridor. Occasionally, there may be wheezing, indicating severe narrowing, bronchitis, or possibly coexistent asthma.
Becoming upset or worried may decrease the child's ability to manage the airway obstruction. Therefore, physical examination should be limited to the respiratory tract and reasonable exclusion of other diagnostic possibilities. Investigations are intrusive and should be avoided when possible.
The term spasmodic croup has been used to define a sudden onset of symptoms at night in a child who has been well. The symptoms are identical to viral croup but without fever, last for hours rather than days, and are seldom life threatening. The child may be well during the day and have attacks on three or four successive evenings. During the first attack, it is difficult to make an accurate diagnostic distinction from viral croup.
Diagnosis
The differential diagnosis includes any condition that causes obstruction in the region of the larynx. The most important are epiglottitis and laryngeal foreign body aspiration, both of which require emergency treatment. Acute angioedema usually presents with other evidence of swelling of the face and neck. Other conditions to be considered are retropharyngeal and peritonsillar abscess, bacterial tracheitis, subglottic stenosis, infectious mononucleosis, laryngeal diphtheria, and paraquat poisoning.
Investigations are seldom necessary in straightforward viral croup. When there is severe obstruction, neck radiographs or blood tests cause anxiety in the child, which may precipitate critically poor gas exchange. Pulse oximetry may support the clinical suspicion of hypoxia but should not be used as the only means of clinical assessment.
If radiography is deemed necessary to exclude epiglottitis or foreign body inhalation in a child whose airway is severely obstructed, it should be done in the presence of medical staff able to resuscitate and intubate a child with upper airway obstruction. However, epiglottitis is usually diagnosed clinically and confirmed under direct vision in the intensive care unit.
When radiography has been done, specific abnormalities are seen in 40% to 50% of cases (see Chapter 11). Posteroanterior neck radiographs may show a very narrowed subglottic region. In lateral neck radiographs, there may be widening of the hypopharynx and haziness in the subglottic region. Radiographic changes do not reliably reflect the severity of airway obstruction.
Treatment
Airway obstruction in croup can worsen rapidly; hence, repeated careful clinical assessment is a key component of management.172, 173
Most children with viral croup have only mild airway obstruction that spontaneously settles; therefore, no specific treatment is indicated. Most of these children can be managed at home. By the time medical attention is sought, the airway obstruction often does not progress but usually lasts 4 additional days.
A commonly used home treatment is to sit the child in the bathroom with a parent with a hot shower running, in the belief that the warm mist will help the breathing. Trials of mist therapy have shown no evidence of efficacy. A single-blinded study of blow-by humidity showed it to result in identical changes in croup scores to those seen in children receiving no therapy.174 A study of children with moderate croup that compared blow-by humidity with either 100% humidity or 40% humidity with smaller water particles generated by a nebulizer showed no significant difference between treatment groups in the change in croup score.175
In the child with obvious indrawing, anxiety, or other evidence of moderate or severe airway obstruction, it is vital to use a careful nonintrusive approach. A parent should stay with the child, and all interactions with the child should appear calm and reassuring. Mist treatment in the hospital is no longer recommended, and it may increase the child's anxiety.
Mild hypoxia with a hemoglobin oxygen saturation lower than 93% is common and closely correlated to the respiratory rate. When there are clinical signs of hypoxia such as restlessness, marked tachycardia, and cyanosis, or when there is significant oxygen desaturation (Sao2<90%) as measured by pulse oximetry, oxygen should be administered.172
At the same time, treatment to relieve the obstruction is needed. In a minority of children, the airway obstruction progresses to become severe. Admission to the pediatric intensive care unit is indicated for children with signs of hypoxia or progressive severity of obstruction. Among children hospitalized for viral croup, less than 1% require intubation. Rarely, idiopathic pulmonary edema occurs in severe obstruction.176
GLUCOCORTICOIDS
The theoretical mechanism of action of the steroids is suppression of local inflammatory reaction, shrinkage of lymphoid swelling, and reduction in capillary permeability. The mechanism by which glucocorticoids exert their effect in croup is unknown.172 The place of steroids in the management of viral croup has been debated for 30 years. It was subjected to meta-analysis.177
In the large number of studies of corticosteroids for croup, treatment efficacy has been measured using clinical symptom severity scores and the need for return visits and hospital admission, length of hospital or emergency department stay, and the need for other additional therapy.177 The validated scoring system that is most frequently used is the Westley score. This uses a 17-point scale to assess air entry (2 points), stridor (2 points), intercostal retractions (3 points), cyanosis (5 points), and level of consciousness (5 points).178
Efficacy of Glucocorticoids
As measured by improvement in Westley symptom scores: In placebo-controlled studies, glucocorticoids have been shown to result in significant improvement in symptom scores at 6 and 12 hours but not at 24 hours after administration. The lack of significance of the effect at 24 hours may be due to low study power. The effect size seen at 24 hours is similar to that at 12 hours but has been examined in fewer studies. The number needed to treat at 6, 12, and 24 hours was five, with this considered sufficient to support the use of glucocorticoids over placebo.177 *
As measured by return visits, length of stay, and use of additional therapy: In comparison with placebo, the rates of return visits and hospital admission are both significantly reduced (number needed to treat to prevent one return visit=17). Length of stay in the emergency department or inpatient ward are both reduced, with the mean difference being approximately 12 hours. Children treated with glucocorticoids are approximately 10% less likely to be also treated with epinephrine.177
Different doses and routes of administration of glucocorticoids: Using symptom scores and return visits/readmissions as outcome measures, the efficacy of glucocorticoids versus placebo given by different routes of administration (oral, spray, intramuscular, subcutaneous) does not differ. Combinations of budesonide and dexamethasone versus either alone do not result in any increased effect.179 Different doses of dexamethasone (0.15, 0.3, or 0.6 mg/kg) have similar efficacy.177, 180 The onset of action of nebulized budesonide is faster than that of oral or intramuscular glucocorticoids but slower than that of nebulized epinephrine.172
Efficacy of glucocorticoids for croup of varying severity: A single dose of glucocorticoid has been shown to be effective in croup of all grades of severity including mild croup defined as a score of 2 or less (of 17) on the Westley scoring system.181
Comparison with epinephrine: There is no significant difference in improvement in croup scores in studies that have compared glucocorticoids (dexamethasone or budesonide) with epinephrine.
Adverse effects of glucocorticoids: There is no evidence of any ill effects of a single dose of glucocorticoids given to a child with viral croup. The efficacy and safety of repeated doses of glucocorticoids in children with severe croup have not been established. However, there may be complications with steroids used inadvertently for diagnoses mimicking viral croup, such as epiglottitis or bacterial tracheitis, so the clinician must be certain of the diagnosis before administering them.167 Both bacterial tracheitis and Candida laryngotracheitis have been reported to occur in children with croup being treated with glucocorticoids.182, 183
EPINEPHRINE
Epinephrine (Adrenalin) was first introduced for viral croup in 1971. It is thought to stimulate αadrenergic receptors in subglottic mucosa, producing vasoconstriction, resulting in less hyperemia and edema of the larynx and subglottic region. This results in increased airway diameter within 30 minutes. However, the effect is short lived, lasting about 2 hours because of dispersion of the epinephrine.184 The first use of this treatment was with racemic epinephrine hydrochloride (Vaponefrin, equivalent to 2.25% epinephrine base), a mixture of equal parts of the inactive d-isomer and the active l-isomer. In some early studies,185 this treatment was given with intermittent positive-pressure breathing, but a similar effect is seen without it.186 Racemic epinephrine is not readily available in some countries, and it has been replaced by the use of l-epinephrine solution, which is cheaper.184
Epinephrine does not alter the natural history of the airway obstruction. Therefore, when the effects wear off, rebound may occur. The obstruction may be either as bad as before or worse if the overall condition is deteriorating. It is dangerous to discharge a child with croup who has been given nebulized epinephrine before ensuring that there is no rebound. The child should be observed for 6 hours after the dose. Epinephrine is used to provide immediate symptomatic relief in patients with moderate and severe croup and in those admitted to the intensive care unit in an attempt to avert the need for intubation.
Epinephrine is given via a nebulizer with a face mask and is driven with oxygen. The usual dose in infants weighing 10 kg is 5 mg, which may be given as 5 mL of 1:1000 solution of l-epinephrine or as 0.5 mL of 2.25% solution (22.5 mg/mL) of racemic epinephrine solution, which contains 5 mg of l-isomer. The latter is diluted with isotonic saline to a 3- to 5-mL volume. In young infants, graded doses based on body weight are appropriate: 0.5 mL/kg concentration of 1:1000 l-epinephrine to a maximum of 5 mL or 0.05 mL/kg of 2.25% solution to a maximum of 0.5 mL of racemic epinephrine. Doses may be repeated every 2 hours or even more often. Adverse reactions have not been reported. Nebulized epinephrine is relatively contraindicated in children with ventricular outflow tract obstruction, for example, tetralogy of Fallot.172
OTHER TREATMENTS
Intravenous fluids are not usually required in viral croup, but if a child is unable to drink, they may become necessary.
Admission to the intensive care unit is indicated when there is restlessness, anxiety, marked tachycardia, or cyanosis or when the child is tiring. In this situation, epinephrine and corticosteroids should be administered.
Helium and oxygen mixtures (helium:oxygen 80:20 or 70:30) improve gas flow when there is turbulent flow through high-resistance airways. This is because helium is one seventh the density of air, thereby increasing flow as well as allowing carbon dioxide to diffuse through it 4 to 5 times faster than it does through air.187 Helium is an inert, nonflammable gas with no known pharmacologic effects.172 Heliox (helium:oxygen 70:30) has been shown to be of similar efficacy to nebulized epinephrine in one small double-blind, randomized trial of children with croup who were receiving oxygen and glucocorticoids.188
Despite vigorous treatment with epinephrine and steroids, a child occasionally progresses to critical airway obstruction necessitating endotracheal intubation. This should be performed by a pediatric anesthetist or intensive care pediatricianexperienced in endotracheal intubation using inhalational anesthesia. Intubation should be maintained until an air leak develops, indicating a reduction of airway edema, or until a maximum of 5 days passes, at which time a trial of extubation is attempted. Rarely, tracheostomy may be the only method of providing an alternative airway.
Clinical Course and Prognosis
About half the cases of croup progress to recurrent croup (at least two episodes). In a few individuals, numerous episodes occur. More of these children are boys; more have asthma, hay fever, eczema, and positive allergy prick tests; and more come from families with a history of atopy or croup than do children with nonrecurrent croup.189 Pulmonary function studies have demonstrated lower expiratory flow rates, and increased airway responsiveness to histamine has been documented on inspiratory and expiratory flow-volume loops (Box 32-20 ).
Box 32-20. Viral Croup Teaching Points.
-
•
Viral croup includes acute laryngotracheitis and spasmodic croup.
-
•
Parainfluenza virus infections are the most frequent cause. Seasonal variability in croup reflects the epidemiology of the different etiologic viruses.
-
•
Viral croup is common, affecting 15% of children, most commonly between 6 months and 5 years of age.
-
•
Viral croup is uncommon in the first 6 months of life. Presentation in this age group necessitates consideration of alternative diagnoses such as congenital or vascular airway abnormalities.
-
•
Characteristic clinical features are a barking cough, hoarseness, and inspiratory stridor with an absence of symptoms and signs of systemic toxicity.
-
•
Investigations are seldom necessary in straightforward viral croup.
-
•
Airway obstruction can progress rapidly; therefore, it is necessary to clinically reassess at frequent intervals.
-
•
A single dose of a glucocorticoid has been shown to reduce clinical severity and decrease the need for hospital or emergency department care. Inhaled, oral, and parenteral routes of administration are all effective but do not have an additive effect. Beneficial effect has been shown for mild, moderate, and severe croup.
-
•
Epinephrine provides rapid-onset, short-term relief of airway obstruction but does not alter the natural history of the disease.
-
•
Some children get recurrent croup. Most of them are atopic.
EPIGLOTTITIS
Epiglottitis is a very serious infection of the epiglottis and supraglottic structures that results in acute airway obstruction and high risk of death if untreated. It is rare but must be considered in a child with dyspnea and stridor. Ifsuspected, management must be initially focused on securing the airway.
Epidemiology, Risk Factors, and Pathogenesis
Since the introduction of vaccines that protect against H. influenzae type B (Hib) infection, epiglottitis has become a rare disease. Most cases now occur in adolescents and adults.190, 191 However, cases of epiglottitis due to Hib continue to be reported.192 Other causative organisms include nontypable H. influenzae, H. parainfluenzae, S. aureus, and S. pneumoniae. 74 Occasionally, viruses193, 194, 195 or Candida organisms are also causative.196
Direct bacterial invasion causes cellulitis with marked edema of the epiglottis, aryepiglottic folds, ventricular bands, and arytenoids. There is a large potential space for the accumulation of inflammatory cells and edema fluid where the stratified squamous epithelium is loosely adherent to the anterior surface and the superior third of the posterior portion of the epiglottis. There is diffuse infiltration with polymorphonuclear leukocytes, hemorrhage, edema, and fibrin deposition, and microabscesses may form. Infection of the supraglottic larynx may extend but does not usually reach the subglottis or the laryngeal lymphatic system.
Clinical Features
Up to half of the children have preceding upper respiratory tract symptoms. The onset of epiglottitis is typically abrupt, with early toxicity. The duration of symptoms before presentation to the hospital is usually less than 24 hours. Localizing symptoms are caused by supraglottic swelling and airway obstruction.
There is a very sore throat, difficulty swallowing because of pain, respiratory distress, drooling, a choking sensation, irritability, restlessness, and anxiety. The temperature is high, usually between 38.8° and 40° C (101.8° to 104° F). Sighing respirations, mild stridor, retractions, and mild tachypnea occur. Less common symptoms and signs are cough, which may be harsh, and occasionally barking, delirium, lethargy, hoarseness or aphonia, vomiting, chills, anorexia, cervical adenopathy, wheezing, and hypotonia.
The child naturally assumes a posture that maximizes the diameter of the obstructed airway: sitting and leaning forward with hyperextension of the neck and protrusion of the chin. A few may have shock with cyanosis, prostration, and loss of consciousness.
Children with epiglottitis are at risk for total airway obstruction. The enlarged, inflamed supraglottic ring can progress to respiratory obstruction with unexpected suddenness. Epiglottitis progresses to death in about 7% of children who do not have a secured airway. With accurate early recognition and elective intubation, the mortality rate should approach zero. Most deaths occur in the community, during transit to hospital or in the first few hours after arrival.
Chronic epiglottic enlargement may be seen with neck radiotherapy for cancer, granulomatous lymphangitis, or lymphangiectasis and in infection with the human immunodeficiency virus.197 The chronicity of symptoms makes these conditions easily distinguishable from acute epiglottitis. Similarly, congenital anomalies of the airway and laryngeal papillomatosis are usually quite distinct.
Diagnosis
Investigations should be left until the airway is secured. The diagnosis is confirmed under direct visualization. Detection of the responsible organism is important for guiding antibiotic management. Direct culture of supraglottic tissues reveals the causative organism in the majority of patients. The blood culture may also be positive. Blood leukocyte counts, mainly polymorphonuclear leukocytes, are increased. The numbers of immature neutrophils are increased in most cases. The level of C-reactive protein is usually raised.
The diagnosis is often clear from the specific clinical signs. However, it is sometimes difficult to differentiate epiglottitis from severe viral croup of a more rapid onset. Distinguishing features include the absence of spontaneous cough and the presence of drooling and agitation.198 Toxicity, high fever, and sore throat may also occur with bacterial tracheitis, uvulitis, and retropharyngeal or parapharyngeal abscess. Nasopharyngeal diphtheria is now rare but may mimic acute epiglottitis and is associated with serosanguineous discharge. Noninfectious causes mimicking epiglottitis include angioedema, a pharyngeal burn, and a foreign body that is in the valleculae or larynx or that penetrates the posterior pharyngeal tissues.
Lateral radiographic views of the soft tissues of the neck may be needed if a laryngeal foreign body is suspected, but the patient's airway must be carefully monitored throughout the procedure. The best view of the anatomic structures of the upper airway is obtained with the patient upright. The hypopharynx is dilated, and the normal cervical lordosis may be replaced by a straight or kyphotic contour. The valleculae are narrowed and may be obliterated. A thickened mass of tissue extends from the valleculae to the arytenoid muscles (Fig. 32-9 ).
Figure 32-9.
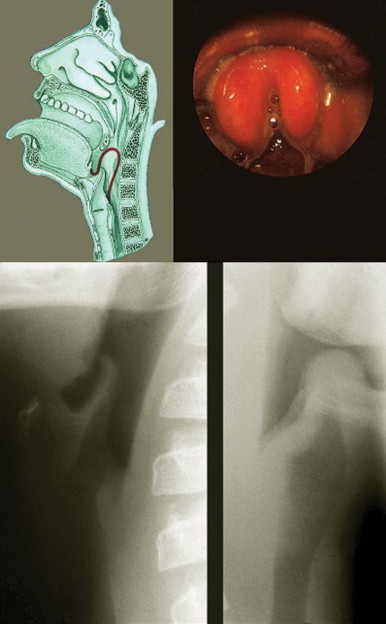
Schematic (top left) and endoscopic (top right) views of epiglottis. Bottom, Lateral neck radiographs of a normal child (left) and a child with the typical thumb sign (right).
(From Hammer J: Acquired upper airway obstruction. Paediatr Respir Rev 5:25-33, 2004.)
© 2008
Treatment
Because of the high risk of complete airway obstruction, great care should be taken in treating epiglottitis. Once a physician suspects this diagnosis, the child should be constantly attended by an individual skilled in resuscitation using the appropriate equipment for airway stabilization and ventilatory support. Delays of 2 to 3 hours have proved fatal. Every effort should be made to reduce the time needed to secure a patient's airway and initiate antibiotic therapy. During this waiting interval, unnecessary stress for the child should be prevented; the throat should not be examined. Extensive clinical assessment, transport delay, and blood tests should be avoided.
The airways should be secured as early as possible after diagnosis. A large body of literature attests to the safety and efficacy of elective nasotracheal intubation, which is the treatment of choice. A short period of airway maintenance is usually all that is required. A nasotracheal tube that is 0.5 mm smaller than that predicted by the patient's age is recommended. Expert nursing care is essential to prevent inadvertent extubation, particularly in the first 12 to 18 hours. The criteria for extubation include being afebrile and swallowingcomfortably. Repeat examination of the epiglottis and supraglottic structures by direct laryngoscopy or fiberoptic bronchoscopy is not normally necessary.
Until the results of sensitivity tests are known, the child should be treated with a broad-spectrum intravenous antibiotic to cover the majority of possible isolates. Initial treatment is usually a second-generation cephalosporin such as cefuroxime (if meningitis is not present) or a third-generation cephalosporin such as cefotaxime or ceftriaxone. If the isolate is proved to be susceptible, ampicillin, a cheaper agent, may be substituted. If S. pyogenes is isolated from the airway, penicillin is the drug of choice. When S. aureus is isolated, a semisynthetic penicillinase-resistant penicillin or glycopeptide such as vancomycin should be used depending on sensitivity patterns. Erythromycin should be used for C. diphtheriae.
No controlled studies address the duration of antibiotic treatment, but a course of 7 days of intravenous administration (until the child is afebrile for 48 hours) followed by oral therapy is commonly used. Ceftriaxone in a single daily dose of 100 mg/kg for 5 days is effective.199
Although there have been some recommendations to use corticosteroids, no controlled data support their use; in fact, they may be hazardous because of the side effects. Therapy with inhaled epinephrine is of no benefit.
Clinical Course and Prognosis
Complications are uncommon.200 Evidence of pneumonia or atelectasis is sometimes seen on the chest radiograph. Other findings may include exudative tonsillitis, cervical lymphadenitis, and otitis media. Meningitis, septic arthritis, and pericarditis occurring with epiglottitis are rare; routine lumbar puncture is unnecessary.
In about 10% of children with epiglottitis in whom there is severe airway obstruction, idiopathic pulmonary edema may occur before or after insertion of endotracheal tubes.176 The hypothetical mechanism is an increased pulmonary blood flow secondary to airway obstruction, causing markedly negative intrapleural pressure with increased venous return to the right side of the heart and decreased left ventricular output. These changes increase the pulmonary microvascular pressure and produce pulmonary hyperemia and edema. Endotoxemia may play a role in altering vascular permeability, but it is not a necessary prerequisite. Continuous positive airway pressure in intubated patients may decrease the occurrence of pulmonary edema.
Complications occurring after extubation include laryngeal edema and subglottic granulations. Long-term complications of nasotracheal intubation are rare. Tracheostomy is lifesaving but has been replaced by safer nasotracheal intubation.
Secondary disease can occur in household contacts of epiglottitis due to Hib. Epiglottitis has also occurred in household contacts of meningitis resulting from Hib. Rifampin (Rifampicin) prophylaxis eradicates nasopharyngeal carriage and is recommended as follows: a dosage of 20 mg/kg/day (600 mg maximum per dose) for 4 days for all members of a patient contact group when the index case has invasive Hib and there is at least one contact who is 4 years of age or younger. For patients younger than 2 years of age, prophylaxis is required for the child and all household contacts.201
Prevention of invasive Hib infection is now universally recommended using one of the approved polysaccharide conjugate vaccine regimens for children up to 5 years of age. These vaccines are highly effective in lowering the incidence of invasive epiglottitis resulting from Hib202, 203 (Box 32-21 ).
Box 32-21. Epiglottitis Teaching Points.
-
•
Since the introduction of vaccines that protect against H. influenzae type B infection, epiglottitis has become a rare disease.
-
•
Most cases now occur in adolescents and adults.
-
•
The onset is typically abrupt, with duration of symptoms before presentation to the hospital usually less than 24 hours.
-
•
Children with epiglottitis are at risk of total airway obstruction and can progress to respiratory obstruction with unexpected suddenness.
-
•
The diagnosis is confirmed under direct visualization. Other investigations are deferred until the airway is secured.
BACTERIAL TRACHEITIS
Bacterial tracheitis is uncommon but potentially life threatening. It is characterized by thick membranous tracheal secretions. These do not clear with coughing and can occlude the airway and cause death.204
Epidemiology, Risk Factors, and Pathogenesis
The age group most commonly affected is similar to that for viral croup with a mean age of 4 years.204
Direct bacterial infection of the tracheal mucosa is caused by the organisms listed in Box 32-22 . S. aureus is the most common bacteria reported.205 A significant proportion of infections are polymicrobial.204 Moraxella catarrhalis is described to be more frequent in younger children and to be associated with a more severe course, although this may be due to its association with younger age.204
Box 32-22. Causes of Bacterial Tracheitis.
Common Causes
Haemophilus influenzae type b and nontypable Haemophilus influenzae
Klebsiella pneumoniae
Staphylococcus aureus
Streptococcus pneumoniae
Streptococcus pyogenes group A
Rare Causes
Moraxella catarrhalis
Pseudomonas spp.
Influenza virus, parainfluenza virus, and enterovirus have been isolated in children with bacterial tracheitis, suggesting that bacterial invasion may occur in an airway already inflamed by viral infection. Bacterial tracheitis is a recognized complication of measles.206
The bacterial infection causes a diffuse inflammatory process of the larynx, trachea, and bronchi with mucopurulent exudate and semiadherent “membranes” within the trachea. These membranes contain numerous neutrophils and cellular debris and cause major obstruction.
Clinical Features
In most children, there are prodromal upper respiratory tract symptoms. Bacterial tracheitis usually presents as severe upper airway obstruction, most often in a child who has had viral croup for several days. Not all children present with high fever, systemic toxicity, and severe airway obstruction. In some, particularly those who are older, the illness can remain localized to the trachea.204
The differential diagnosis includes severe viral croup, laryngeal or tracheal foreign body aspiration, or epiglottitis. Bacterial tracheitis has a longer duration, a more typical barking cough than epiglottitis, and no drooling. Diphtheria was once a serious consideration as the most common cause of “membranous croup” that produced severe airway obstruction because of adherent membranes that separate from the airway wall with difficulty, causing bleeding.
Diagnosis
The definitive diagnosis requires direct laryngoscopy and tracheoscopy.207 Bacterial cultures of tracheal secretions are required to isolate causative organisms. The results from blood cultures are usually negative. White blood cell counts may be high or normal.
Endoscopy reveals thick mucopus and sloughed epithelium. The epithelium forms a sheet-like pseudomembrane that separates easily from the airway wall without hemorrhage and sometimes extends from the trachea to the major bronchi.
A lateral neck radiograph shows subglottic narrowing and often reveals findings of radiopaque material in the airway lumen (pseudomembrane) and tracheal irregularities.204, 208 Plain radiographic abnormalities can be confused with those due to a foreign body.
Treatment
Specific diagnosis and treatment are secondary to definitive treatment of impending airway obstruction.207 In a child suspected of having bacterial tracheitis, management should occur in a pediatric intensive care unit. At least half of children with bacterial tracheitis will need to be intubated.204 Intermittent positive-pressure breathing is sometimes needed. Repeated suctioning is usually required because of the thick secretions and their tendency to form crusts, with intubation lasting 3 to 11 days. Sometimes, repeat endoscopic removal of the pseudomembrane is required. Occasionally, tracheostomy is needed if endotracheal tube management of secretions proves too difficult.
Empirical antibiotic choice should provide broad-spectrum gram-positive and gram-negative cover. Appropriate first-line choices include amoxicillin/clavulanic acid, cefuroxime, and ampicillin+sulbactam.
Nebulized epinephrine or corticosteroids do not relieve the acute airway obstruction.
Clinical Course and Prognosis
With effective early management, children should make a complete recovery from this severe illness. Reported complications include toxic shock syndrome, septic shock, pulmonary edema, and acute respiratory distress syndrome (Box 32-23 ).
Box 32-23. Bacterial Tracheitis Teaching Points.
-
•
Bacterial tracheitis is characterized by thick tracheal membranes, which can occlude the airway and cause death.
-
•
It occurs in both the preschool- and school-age groups and can present either with fever and systemic toxicity or as more localized disease.
-
•
Definitive diagnosis requires laryngoscopy and tracheoscopy.
-
•
The etiology can be polymicrobial.
-
•
At least half of children with bacterial tracheitis will need to be intubated.
-
•
Children with this condition should be managed initially in the intensive care unit.
-
•
Broad-spectrum intravenous antibiotics are required.
RECURRENT RESPIRATORY PAPILLOMATOSIS
Juvenile recurrent respiratory papillomatosis (RRP; also known as laryngeal papillomatosis) is a rare condition with benign, wart-like tumors in the respiratory tract, especiallythe larynx, usually associated with upper airway obstruction, which can become life threatening.209
Epidemiology, Risk Factors, and Pathogenesis
The incidence of recurrent respiratory papillomatosis has been estimated as 4.3 per 100,000 children.210 It occurs at all ages, with about half of all cases appearing in children and the youngest reported patient being 1 month of age.211
Human papillomavirus (HPV) types 6 and 11 cause about 90% of cases of recurrent respiratory papillomatosis.212, 213 Type II is more virulent, associated with earlier presentation and more surgical procedures.212 Occasional coinfection with other viruses has been demonstrated (e.g., herpes simplex, cytomegalovirus, Epstein-Barr virus) and is associated with a more aggressive course.214 The replicating virus may cause overgrowth of squamous epithelial cells. The papillomata are multiple projections, each with a connective tissue stalk covered by well-differentiated stratified squamous epithelium. The viral antigen is localized in the nuclei of cells in the very superficial layers. Papilloma occur in the larynx, trachea, bronchi, and lung parenchyma.
There is debate about the mechanism of infection with HPV. The same types of HPV that cause perineal condylomata in women also cause juvenile recurrent respiratory papillomatosis. Children can acquire the infection during the birth process from the mother with perineal condylomata.215, 216, 217 However, there are many children in whom there is no evidence of maternal HPV infection in whom the source of infection remains obscure. Host susceptibility has been investigated in a limited number of patients, and only one child has been found to have IgG2 subclass deficiency.218
Clinical Features
In the pediatric age group, about half of patients have symptoms within the first year of life, although clinical recognition of the disease is often delayed. Patients usually come to medical attention late, with respiratory distress due to airway obstruction and stridor, together with hoarseness or a weak cry. Life-threatening upper airway obstruction may occur and, less commonly, chronic cough, recurrent pneumonia, failure to thrive, dyspnea, and dysphagia.209 Although the lesions are usually localized within the larynx, spread to other areas (pharynx, esophagus, trachea, and lung parenchyma) may occur and indicates a more pessimistic outlook.211 When the lung parenchyma is involved, lung tissue may be destroyed with multiple nodular and cystic lesions (Figure 32-10, Figure 32-11 ). Pneumothorax can occur after the development of cystic pneumatoceles, presumably from the ball-valve effect of a nodular lesion.219
Figure 32-10.
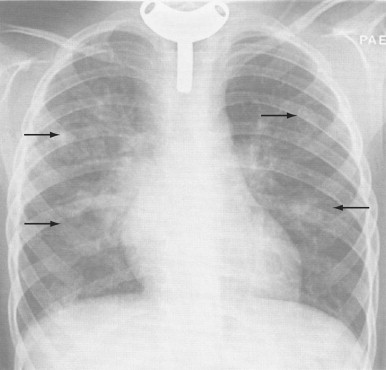
In a 13-year-old boy with a tracheostomy tube placed due to laryngeal papillomatosis, chest radiograph shows multiple nodular processes (arrows), some of which are cavitating, caused by parenchymal dissemination of the papillomatosis.
(From Diagnostic imaging of the respiratory tract. In Chernick V, Boat TF, Wilmott RW, Bush A [eds]: Kendig's Disorders of the Respiratory Tract. Philadelphia, WB Saunders, 2006.)
Figure 32-11.
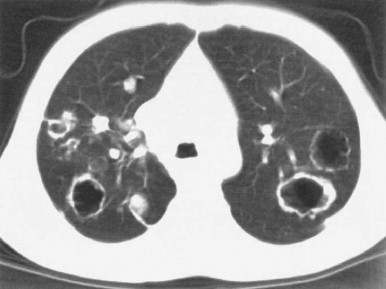
Chest computed tomography scan of an 8-year-old girl with laryngotracheal papillomatosis shows multiple peripheral nodules and cavities with posterior dominance, in keeping with pulmonary dissemination.
(From Diagnostic imaging of the respiratory tract. In Chernick V, Boat TF, Wilmott RW, Bush A [eds]: Kendig's Disorders of the Respiratory Tract. Philadelphia, WB Saunders, 2006.)
The tumors are benign but present obstructive problems because of their localization in the vocal cords or other sites. At presentation, papillomas are usually present on one or both vocal cords with the anterior commissure, supraglottis, or subglottis also commonly affected.
Diagnosis
The condition is diagnosed by inspection of the larynx, either by indirect means such as fiberoptic laryngoscopy or by formal laryngoscopy and bronchoscopy when tissue biopsy samples can be taken for histologic confirmation. The virus signal can be identified in the tissue biopsy, but its intensity does not generally correlate with the clinical behavior of the disease.
Multiple endoscopies are usually required for further investigation and management, and flexible bronchoscopy is the method of choice for surveillance.
A staging system has been developed by Derkay and colleagues209 with good interobserver reliability.220 It includes area and severity of involvement, characteristics of voice, and respiratory distress.
Treatment
PREVENTION
Vaccines against HPV types 6, 11, 16, and 18 have been developed to prevent cervical cancer. Vaccination against HPV is likely to be recommended for use in 11- to 12-year-olds.213 Because of the timing of the vaccination, it is unlikely that it will have an impact on the prevalence of RRP in childhood but could potentially reduce early childhood disease in the next generation.
TREATMENT OF DISEASE
The goals of treatment are to relieve airway obstruction, improve voice quality, and minimize the risk of recurrence. Recurrent respiratory papillomatosis is frustrating to treat because lesions are often recurrent after excision and sometimes locally aggressive. The focus of management is to debulk the papilloma and ensure a safe airway without causing irreversible long-term scarring, especially affecting the voice. Total surgical removal of the disease is impossible in most cases because subclinical viral infection occurs in apparently normal adjacent areas and the degree of destruction necessary to clear the field would require too great a degree of tissue damage.
The carbon dioxide laser is the most widely used surgical tool for removing recurrent respiratory papilloma, vaporizing the papilloma. However, it does not prevent regrowth any better than the older surgical methods, such as direct removal or suction diathermy. Photodynamic therapy has been used, pretreating the patient with a hematoporphyrin derivative and then subjecting the lesion to an argon dye laser beam at a 630-nm wavelength.221 If possible, tracheotomy should be avoided because of seeding of the disease to the tracheotomy site.
Historically, clinicians have used numerous other therapeutic modalities, including cryotherapy, painting of the lesions with podophyllin, indole-3-carbinol, photodynamic therapy and antimetabolites, vaccines, and immunotherapy, but the results have been generally poor.
Adjuvant antiviral therapy with acyclovir or ribavirin, or cidovir injected into the lesions has been considered, but there are no controlled clinical trials.222
Interferon-a used with surgery has disappointing results. Although most studies have shown a dramatic decrease in the frequency of regrowth immediately after beginning such treatment, regrowth gradually occurs. In one large multicenter, randomized study,223 interferon was neither of curative nor of substantial value as an adjunctive agent after 1 year of treatment. In another study of 66 patients,224 there was a 33% sustained complex remission rate, leading the authors to suggest a 6-month trial of interferon-a in children requiring surgery at 2- to 3-month intervals. Although this agent is moderately well tolerated, the almost universal side effects of mild influenza-like symptoms are unpleasant, and the frequent parenteral mode of delivery is disliked, particularly in the younger age group. Interferon therapy may be used in particularly aggressive disease, but it should not be continued beyond 12 months unless the disease responds.225 In less severe cases, the indication is not absolute.
Clinical Course and Prognosis
The most usual course of the disease is for the papilloma to continue to grow locally despite surgical removal and without significant spread. Over time, the majority of cases in children undergo spontaneous remission (analogous to skin warts). Death is rare. Malignant change to squamous cell carcinoma has been reported in 3% to 5%, mainly in adults.226 The now-abandoned treatment of these lesions with radiotherapy has been implicated in ensuing malignancies in the pediatric age group (Box 32-24 ).
Box 32-24. Recurrent Respiratory Papillomatosis Teaching Points.
-
•
Papillomas are projections with squamous epithelium infected with human papillomavirus on a connective tissue stalk.
-
•
They cause airway obstruction, especially around the larynx.
-
•
Diagnosis is often delayed.
-
•
Interferon is considered for aggressive cases.
In the studies included in the meta-analysis, improvement in the Westley score was defined in a number of ways.
SUGGESTED READINGS
Common Cold
- Herbert AP. Look Back and Laugh. Methuen; London: 1960. The common cold; pp. 115–117. (cited in. [Google Scholar]; Heikkinen T, Jarvinen A. The common cold. Lancet. 2003;361:51–59. doi: 10.1016/S0140-6736(03)12162-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pharyngitis and Tonsillitis
- Gerber MA. Diagnosis and treatment of pharyngitis in children. Pediatr Clin North Am. 2005;52:729–747. doi: 10.1016/j.pcl.2005.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Retropharyngeal, Parapharyngeal, and Peritonsillar Abscess
- Brook I. Microbiology and management of peritonsillar, retropharyngeal, and parapharyngeal abscesses. J Oral Maxillofac Surg. 2004;62:1545–1550. doi: 10.1016/j.joms.2003.12.043. [DOI] [PubMed] [Google Scholar]
- Philpott CM, Selvadurai D, Banerjee AR. Paediatric retropharyngeal abscess. J Laryngol Otol. 2004;118:919–926. doi: 10.1258/0022215042790538. [DOI] [PubMed] [Google Scholar]
- Sichel JY, Dano I, Hocwald E. Nonsurgical management of parapharyngeal space infections: A prospective study. Laryngoscope. 2002;112:906–910. doi: 10.1097/00005537-200205000-00023. [DOI] [PubMed] [Google Scholar]
Otitis Media
- American Academy of Family Physicians, American Academy of Otolaryngology–Head and Neck Surgery, American Academy of Pediatrics Subcommittee on Otitis Media With Effusion Otitis media with effusion. Pediatrics. 2004;113:1412–1429. doi: 10.1542/peds.113.5.1412. [DOI] [PubMed] [Google Scholar]
- American Academy of Pediatrics Subcommittee on Management of Acute Otitis Media Diagnosis and management of acute otitis media. Pediatrics. 2004;113:1451–1465. doi: 10.1542/peds.113.5.1451. [DOI] [PubMed] [Google Scholar]
- Bluestone CD. Epidemiology and pathogenesis of chronic suppurative otitis media: Implications for prevention and treatment. Int J Pediatr Otorhinolaryngol. 1998;42:207–223. doi: 10.1016/s0165-5876(97)00147-x. [DOI] [PubMed] [Google Scholar]
Adenoidectomy and Tonsillectomy
- Burton MJ, Towler B, Glasziou P. Tonsillectomy versus non-surgical treatment for chronic/recurrent acute tonsillitis. Cochrane Database of Syst Rev. 2000;(2):CD001802. doi: 10.1002/14651858.CD001802. [DOI] [PubMed] [Google Scholar]
Sinusitis
- Morris P, Leach A. Antibiotics for persistent nasal discharge (rhinosinusitis) in children. Cochrane Database of Syst Rev. 2002;(4):CD001094. doi: 10.1002/14651858.CD001094. [DOI] [PubMed] [Google Scholar]
Viral Croup
- Fitzgerald DA. The assessment and management of croup. Paediatr Respir Rev. 2006;7:73–81. doi: 10.1016/j.prrv.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Russell K, Wiebe N, Saenz A. Glucocorticoids for croup. Cochrane Database Syst Rev. 2004;(1):CD001955. doi: 10.1002/14651858.CD001955.pub2. [DOI] [PubMed] [Google Scholar]
Epiglottitis
- Hammer J. Acquired upper airway obstruction. Paediatr Respir Rev. 2004;5:25–33. doi: 10.1016/j.prrv.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Loftis L. Acute infectious upper airway obstructions in children. Semin Pediatr Infect Dis. 2006;17:5–10. doi: 10.1053/j.spid.2005.11.003. [DOI] [PubMed] [Google Scholar]
Bacterial Tracheitis
- Graf J, Stein F. Tracheitis in pediatric patients. Semin Pediatr Infect Dis. 2006;17:11–13. doi: 10.1053/j.spid.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Salamone FN, Bobbitt DB, Myer CM. Bacterial tracheitis reexamined: Is there a less severe manifestation? Otolaryngol Head Neck Surg. 2004;131:871–876. doi: 10.1016/j.otohns.2004.06.708. [DOI] [PubMed] [Google Scholar]
Recurrent Respiratory Papillomatosis
- Derkay CS, Darrow DH. Recurrent respiratory papillomatosis. Ann Otol Rhinol Laryngol. 2006;115:1–11. doi: 10.1177/000348940611500101. [DOI] [PubMed] [Google Scholar]
REFERENCES
- 1.Herbert AP. Look Back and Laugh. Methuen; London: 1960. The common cold; pp. 115–117. [Google Scholar]; Heikkinen T, Jarvinen A. The common cold. Lancet. 2003;361:51–59. doi: 10.1016/S0140-6736(03)12162-9. cited in. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tyrrell DA, Cohen S, Schlarb JE. Signs and symptoms in common colds. Epidemiol Infect. 1993;111:143–156. doi: 10.1017/s0950268800056764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monto AS, Ullman BM. Acute respiratory illness in an American community. The Tecumseh study. JAMA. 1974;227:164–169. [PubMed] [Google Scholar]
- 4.Monto AS. Studies of the community and family: Acute respiratory illness and infection. Epidemiol Rev. 1994;16:351–373. doi: 10.1093/oxfordjournals.epirev.a036158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taverner D, Latte J, Draper M. Nasal decongestants for the common cold. Cochrane Database Syst Rev. 2006:1. doi: 10.1002/14651858.CD001953.pub2. [DOI] [PubMed] [Google Scholar]
- 6.Wald ER, Dashefsky B, Byers C. Frequency and severity of infections in day care. J Pediatr. 1988;112:540–546. doi: 10.1016/s0022-3476(88)80164-1. [DOI] [PubMed] [Google Scholar]
- 7.Alho OP, Koivu M, Sorri M. Risk factors for recurrent acute otitis media and respiratory infection in infancy. Int J Pediatr Otorhinolaryngol. 1990;19:151–161. doi: 10.1016/0165-5876(90)90221-c. [DOI] [PubMed] [Google Scholar]
- 8.Ball TM, Holberg CJ, Aldous MB. Influence of attendance at day care on the common cold from birth through 13 years of age. Arch Pediatr Adolesc Med. 2002;156:121–126. doi: 10.1001/archpedi.156.2.121. [DOI] [PubMed] [Google Scholar]
- 9.Cohen S, Tyrrell DA, Smith AP. Psychological stress and susceptibility to the common cold. N Engl J Med. 1991;325:606–612. doi: 10.1056/NEJM199108293250903. [DOI] [PubMed] [Google Scholar]
- 10.Monto AS, Sullivan KM. Acute respiratory illness in the community. Frequency of illness and the agents involved. Epidemiol Infect. 1993;110:145–160. doi: 10.1017/s0950268800050779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gwaltney JM, Jr, Moskalski PB, Hendley JO. Hand-to-hand transmission of rhinovirus colds. Ann Intern Med. 1978;88:463–467. doi: 10.7326/0003-4819-88-4-463. [DOI] [PubMed] [Google Scholar]
- 12.Dick EC, Jennings LC, Mink KA. Aerosol transmission of rhinovirus colds. J Infect Dis. 1987;156:442–448. doi: 10.1093/infdis/156.3.442. [DOI] [PubMed] [Google Scholar]
- 13.Winther B, Gwaltney JM, Jr, Mygind N. Sites of rhinovirus recovery after point inoculation of the upper airway. JAMA. 1986;256:1763–1767. [PubMed] [Google Scholar]
- 14.Hendley JO, Gwaltney JM., Jr Mechanisms of transmission of rhinovirus infections. Epidemiol Rev. 1988;10:243–258. [PubMed] [Google Scholar]
- 15.Gwaltney JM, Jr, Hayden FG. Psychological stress and the common cold. N Engl J Med. 1992;326:644–645. doi: 10.1056/NEJM199202273260915. author reply 645-646. [DOI] [PubMed] [Google Scholar]
- 16.Gwaltney JM, Jr, Phillips CD, Miller RD. Computed tomographic study of the common cold. N Engl J Med. 1994;330:25–30. doi: 10.1056/NEJM199401063300105. [DOI] [PubMed] [Google Scholar]
- 17.Puhakka T, Makela MJ, Alanen A. Sinusitis in the common cold. J Allergy Clin Immunol. 1998;102:403–408. doi: 10.1016/S0091-6749(98)70127-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heikkinen T. The role of respiratory viruses in otitis media. Vaccine. 2000;19(suppl 1):S51–S55. doi: 10.1016/s0264-410x(00)00278-4. [DOI] [PubMed] [Google Scholar]
- 19.Sanyal MA, Henderson FW, Stempel EC. Effect of upper respiratory tract infection on eustachian tube ventilatory function in the preschool child. J Pediatr. 1980;97:11–15. doi: 10.1016/s0022-3476(80)80121-1. [DOI] [PubMed] [Google Scholar]
- 20.Winther B, Hayden FG, Arruda E. Viral respiratory infection in schoolchildren: Effects on middle ear pressure. Pediatrics. 2002;109:826–832. doi: 10.1542/peds.109.5.826. [DOI] [PubMed] [Google Scholar]
- 21.McBride TP, Doyle WJ, Hayden FG. Alterations of the eustachian tube, middle ear, and nose in rhinovirus infection. Arch OtolaryngolHead Neck Surg. 1989;115:1054–1059. doi: 10.1001/archotol.1989.01860330044014. [DOI] [PubMed] [Google Scholar]
- 22.Putto A, Ruuskanen O, Meurman O. Fever in respiratory virus infections. Am J Dis Child. 1986;140:1159–1163. doi: 10.1001/archpedi.1986.02140250085040. [DOI] [PubMed] [Google Scholar]
- 23.Atmar RL. Rhinoviruses. In: Feigin RD, Cherry JD, Demmler GJ, Kaplan SL, editors. Textbook of Pediatric Infectious Diseases. 5th ed. WB Saunders; Philadelphia: 2004. pp. 2042–2068. [Google Scholar]
- 24.Committee on Infectious Diseases, American Academy of Pediatrics . Report of the Committee on Infectious Diseases. 26th ed. American Academy of Pediatrics; Elk Grove Village, IL: 2003. [Google Scholar]
- 25.Templeton KE, Scheltinga SA, Beersma MF. Rapid and sensitive method using multiplex real-time PCR for diagnosis of infections by influenza A and influenza B viruses, respiratory syncytial virus, and parainfluenza viruses 1, 2, 3, and 4. J Clin Microbiol. 2004;42:1564–1569. doi: 10.1128/JCM.42.4.1564-1569.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blake KD, Fertleman CR, Meates MA. Dangers of common cold treatments in children. Lancet. 1993;341:640. doi: 10.1016/0140-6736(93)90410-i. [DOI] [PubMed] [Google Scholar]
- 27.Arroll B, Kenealy T. Antibiotics for the common cold and acute purulent rhinitis. Cochrane Database Syst Rev. 2005;(3):CD000247. doi: 10.1002/14651858.CD000247.pub2. [DOI] [PubMed] [Google Scholar]
- 28.Douglas RM, Hemila H, Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev. 2004;(4):CD000980. doi: 10.1002/14651858.CD000980.pub2. [DOI] [PubMed] [Google Scholar]
- 29.Linde K, Barrett B, Wolkart K. Echinacea for preventing and treating the common cold. Cochrane Database Syst Rev. 2006;(1):CD000530. doi: 10.1002/14651858.CD000530.pub2. [DOI] [PubMed] [Google Scholar]
- 30.Clemens CJ, Taylor JA, Almquist JR. Is an antihistamine-decongestant combination effective in temporarily relieving symptoms of the common cold in preschool children? J Pediatr. 1997;130:463–466. doi: 10.1016/s0022-3476(97)70211-7. [DOI] [PubMed] [Google Scholar]
- 31.Schroeder K, Fahey T. Over-the-counter medications for acute cough in children and adults in ambulatory settings. Cochrane Database of Syst Rev. 2006:1. doi: 10.1002/14651858.CD001831. [DOI] [PubMed] [Google Scholar]
- 32.Bluestone CD, Connell JT, Doyle WJ. Symposium: Questioning the efficacy and safety of antihistamines in the treatment of upper respiratory infection. Consensus. Pediatr Infect Dis J. 1988;7:241–242. [PubMed] [Google Scholar]
- 33.Taylor JA, Novack AH, Almquist JR. Efficacy of cough suppressants in children. J Pediatr. 1993;122(5 Pt 1):799–802. doi: 10.1016/s0022-3476(06)80031-4. [DOI] [PubMed] [Google Scholar]
- 34.Nespoli L, Monafo V, Bonetti F. [Clinical evaluation of letosteine activity in the treatment of acute febrile bronchitis in children. Double-blind controlled study versus placebo] Minerva Pediatr. 1989;41:515–520. [PubMed] [Google Scholar]
- 35.De Sutter AIM, Lemiengre M, Campbell H. Antihistamines for the common cold. Cochrane Database Syst Rev. 2003;(3):CD001267. doi: 10.1002/14651858.CD001267. [DOI] [PubMed] [Google Scholar]
- 36.Sakchainanont B, Ruangkanchanasetr S, Chantarojanasiri T. Effectiveness of antihistamines in common cold. J Med Assoc Thailand. 1990;73:96–101. [PubMed] [Google Scholar]
- 37.Hutton N, Wilson MH, Mellits ED. Effectiveness of an antihistamine-decongestant combination for young children with the common cold: A randomized, controlled clinical trial. J Pediatr. 1991;118:125–130. doi: 10.1016/s0022-3476(05)81865-7. [DOI] [PubMed] [Google Scholar]
- 38.Nicholson KG, Wood JM, Zambon M. Influenza. Lancet. 2003;362:1733–1745. doi: 10.1016/S0140-6736(03)14854-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whitley RJ, Hayden FG, Reisinger KS. Oral oseltamivir treatment of influenza in children. Pediatr Infect Dis J. 2001;20:127–133. doi: 10.1097/00006454-200102000-00002. [DOI] [PubMed] [Google Scholar]
- 40.Hedrick JA, Barzilai A, Behre U. Zanamivir for treatment of symptomatic influenza A and B infection in children five to twelve years of age: A randomized controlled trial. Pediatr Infect Dise J. 2000;19:410–417. doi: 10.1097/00006454-200005000-00005. [DOI] [PubMed] [Google Scholar]
- 41.Belshe RB, Mendelman PM, Treanor J. The efficacy of live attenuated, cold-adapted, trivalent, intranasal influenzavirus vaccine in children. N Engl J Med. 1998;338:1405–1412. doi: 10.1056/NEJM199805143382002. [DOI] [PubMed] [Google Scholar]
- 42.Belshe RB, Gruber WC, Mendelman PM. Efficacy of vaccination with live attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine against a variant (A/Sydney) not contained in the vaccine. J Pediatr. 2000;136:168–175. doi: 10.1016/s0022-3476(00)70097-7. [DOI] [PubMed] [Google Scholar]
- 43.Longini IM, Halloran ME, Nizam A. Estimation of the efficacy of live, attenuated influenza vaccine from a two-year, multi-center vaccine trial: Implications for influenza epidemic control. Vaccine. 2000;18:1902–1909. doi: 10.1016/s0264-410x(99)00419-3. [DOI] [PubMed] [Google Scholar]
- 44.Johnston SL, Pattemore PK, Sanderson G. Community study of the role of viral infections in exacerbations of asthma in 9-11 year old children. BMJ. 1995;310:1225–1229. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gerber MA. Diagnosis and treatment of pharyngitis in children. Pediatr Clin North Am. 2005;52:729–747. doi: 10.1016/j.pcl.2005.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Turner JC, Hayden FG, Lobo MC. Epidemiologic evidence for Lancefield group C beta-hemolytic streptococci as a cause of exudative pharyngitis in college students. J Clin Microbiol. 1997;35:1–4. doi: 10.1128/jcm.35.1.1-4.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duca E, Teodorovici G, Radu C. A new nephritogenic streptococcus. J Hygiene. 1969;67:691–698. doi: 10.1017/s0022172400042145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Centers for Disease Control and Prevention Group C streptococcal infections associated with eating homemade cheese—New Mexico. MMWR Morb Mortal Wkly Rep. 1983;32:510. [PubMed] [Google Scholar]
- 49.Stenfors LE, Raisanen S. The membranous tonsillitis during infectious mononucleosis is nevertheless of bacterial origin. Int J Pediatr Otorhinolaryngol. 1993;26:149–155. doi: 10.1016/0165-5876(93)90020-4. [DOI] [PubMed] [Google Scholar]
- 50.McMillan JA, Weiner LB, Higgins AM. Pharyngitis associated with herpes simplex virus in college students. Pediatr Infect Dis J. 1993;12:280–284. doi: 10.1097/00006454-199304000-00004. [DOI] [PubMed] [Google Scholar]
- 51.Brien JH, Bass JW. Streptococcal pharyngitis: Optimal site for throat culture. J Pediatr. 1985;106:781–783. doi: 10.1016/s0022-3476(85)80354-1. [DOI] [PubMed] [Google Scholar]
- 52.Gerber MA. Comparison of throat cultures and rapid strep tests for diagnosis of streptococcal pharyngitis. Pediatr Infect Dis J. 1989;8:820–824. doi: 10.1097/00006454-198911000-00032. [DOI] [PubMed] [Google Scholar]
- 53.Gerber MA, Shulman ST. Rapid diagnosis of pharyngitis caused by group A streptococci. Clin Microbiol Rev. 2004;17:571–580. doi: 10.1128/CMR.17.3.571-580.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gerber MA, Randolph MF, Chanatry J. Antigen detection test for streptococcal pharyngitis: Evaluation of sensitivity with respect to true infections. J Pediatr. 1986;108(5 Pt 1):654–658. doi: 10.1016/s0022-3476(86)81036-8. [DOI] [PubMed] [Google Scholar]
- 55.Randolph MF, Gerber MA, DeMeo KK. Effect of antibiotic therapy on the clinical course of streptococcal pharyngitis. J Pediatr. 1985;106:870–875. doi: 10.1016/s0022-3476(85)80228-6. [DOI] [PubMed] [Google Scholar]
- 56.Bisno AL, Gerber MA, Gwaltney JM., Jr Infectious Diseases Society of America: Practice guidelines for the diagnosis and management of group A streptococcal pharyngitis. Infectious Diseases Society of America. Clin Infect Dis. 2002;35:113–125. doi: 10.1086/340949. [DOI] [PubMed] [Google Scholar]
- 57.Kaplan EL, Johnson DR, Del Rosario MC. Susceptibility of group A beta-hemolytic streptococci to thirteen antibiotics: Examination of 301 strains isolated in the United States between 1994 and 1997. Pediatr Infect Dis J. 1999;18:1069–1072. doi: 10.1097/00006454-199912000-00008. [DOI] [PubMed] [Google Scholar]
- 58.Bass JW. Antibiotic management of group A streptococcal pharyngotonsillitis. Pediatr Infect Dis J. 1991;10(10 suppl):S43–S49. doi: 10.1097/00006454-199110001-00010. [DOI] [PubMed] [Google Scholar]
- 59.Markowitz M, Gerber MA, Kaplan EL. Treatment of streptococcal pharyngotonsillitis: Reports of penicillin's demise are premature. J Pediatrics. 1993;123:679–685. doi: 10.1016/s0022-3476(05)80840-6. [DOI] [PubMed] [Google Scholar]
- 60.Snellman LW, Stang HJ, Stang JM. Duration of positive throat cultures for group A streptococci after initiation of antibiotic therapy. Pediatrics. 1993;91:1166–1170. [PubMed] [Google Scholar]
- 61.Bisno AL, Stevens DL, editors. Streptococcus pyogenes (Including Streptococcal Toxic Shock Syndrome and Necrotizing Fasciitis) Churchill Livingstone; Philadelphia: 2000. [Google Scholar]
- 62.Harnden A, Lennon D. Serious suppurative group A streptococcal infections in previously well children. Pediatr Infect Dis J. 1988;7:714–718. doi: 10.1097/00006454-198810000-00010. [DOI] [PubMed] [Google Scholar]
- 63.Gerber MA. Treatment failures and carriers: Perception or problems? Pediatr Infect Dis J. 1994;13:576–579. doi: 10.1097/00006454-199406000-00036. [DOI] [PubMed] [Google Scholar]
- 64.van Staaij BK, van den Akker EH, van der Heijden GJ. Adenotonsillectomy for upper respiratory infections: Evidence based? Arch Dis Child. 2005;90:19–25. doi: 10.1136/adc.2003.047530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Philpott CM, Selvadurai D, Banerjee AR. Paediatric retropharyngeal abscess. J Laryngol Otol. 2004;118:919–926. doi: 10.1258/0022215042790538. [DOI] [PubMed] [Google Scholar]
- 66.Chong VF, Fan YF. Radiology of the retropharyngeal space. Clin Radiol. 2000;55:740–748. doi: 10.1053/crad.2000.0510. [DOI] [PubMed] [Google Scholar]
- 67.Harries PG. Retropharyngeal abscess and acute torticollis. J Laryngol Otol. 1997;111:1183–1185. doi: 10.1017/s0022215100139672. [DOI] [PubMed] [Google Scholar]
- 68.Buchwald C, Nissen F, Thomsen J. Parapharyngeal abscess and torticollis. J Laryngol Otol. 1990;104:829–830. doi: 10.1017/s0022215100114033. [DOI] [PubMed] [Google Scholar]
- 69.Coulthard M, Isaacs D. Retropharyngeal abscess. Arch Dis Child. 1991;66:1227–1230. doi: 10.1136/adc.66.10.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Luqman Z, Khan MA, Nazir Z. Penetrating pharyngeal injuries in children: Trivial trauma leading to devastating complications. Pediatr Surg Int. 2005;21:432–435. doi: 10.1007/s00383-005-1447-0. [DOI] [PubMed] [Google Scholar]
- 71.Brook I. Microbiology and management of peritonsillar, retropharyngeal, and parapharyngeal abscesses. J Oral Maxillofac Surg. 2004;62:1545–1550. doi: 10.1016/j.joms.2003.12.043. [DOI] [PubMed] [Google Scholar]
- 72.Gidley PW, Ghorayeb BY, Stiernberg CM. Contemporary management of deep neck space infections. Otolaryngol Head Neck Surg. 1997;116:16–22. doi: 10.1016/s0194-5998(97)70345-0. [DOI] [PubMed] [Google Scholar]
- 73.Sichel JY, Dano I, Hocwald E. Nonsurgical management of parapharyngeal space infections: A prospective study. Laryngoscope. 2002;112:906–910. doi: 10.1097/00005537-200205000-00023. [DOI] [PubMed] [Google Scholar]
- 74.Loftis L. Acute infectious upper airway obstructions in children. Semin Pediatr Infects Dis. 2006;17:5–10. doi: 10.1053/j.spid.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 75.Ungkanont K, Yellon RF, Weissman JL. Head and neck space infections in infants and children. Otolaryngol Head Neck Surg. 1995;112:375–382. doi: 10.1016/S0194-59989570270-9. [DOI] [PubMed] [Google Scholar]
- 76.Asmar BI. Bacteriology of retropharyngeal abscess in children. Pediatr Infect Dis J. 1990;9:595–597. [PubMed] [Google Scholar]
- 77.Croskell SE, McElligott KM, Young PC. Irritability and neck stiffness in a five-month-old infant. Pediatr Infect Dis J. 2001;20:724. doi: 10.1097/00006454-200107000-00022. [DOI] [PubMed] [Google Scholar]
- 78.Brechtelsbauer PB, Garetz SL, Gebarski SS. Retropharyngeal abscess: Pitfalls of plain films and computed tomography. Am J Otolaryngol. 1997;18:258–262. doi: 10.1016/s0196-0709(97)90006-5. [DOI] [PubMed] [Google Scholar]
- 79.Wholey MH, Bruwer AJ, Baker HL., Jr The lateral roentgenogram of the neck; with comments on the atlanto-odontoid-basion relationship. Radiology. 1958;71:350–356. doi: 10.1148/71.3.350. [DOI] [PubMed] [Google Scholar]
- 80.Welsh LW, Welsh JJ, Gregor FA. Radiographic analysis of deep cervical abscesses. Ann Otol Rhinol Laryngol. 1992;101:854–860. doi: 10.1177/000348949210101009. [DOI] [PubMed] [Google Scholar]
- 81.Boucher C, Dorion D, Fisch C. Retropharyngeal abscesses: A clinical and radiologic correlation. J Otolaryngol. 1999;28:134–137. [PubMed] [Google Scholar]
- 82.Lazor JB, Cunningham MJ, Eavey RD. Comparison of computed tomography and surgical findings in deep neck infections. Otolaryngol Head Neck Surg. 1994;111:746–750. doi: 10.1177/019459989411100608. [DOI] [PubMed] [Google Scholar]
- 83.Cheema B, Grant CC, Mahadevan M. An infant with a persistent empyema. Acta Paediatr. 1999;88:1168–1171. [PubMed] [Google Scholar]
- 84.Lalakea M, Messner AH. Retropharyngeal abscess management in children: Current practices. Otolaryngol Head Neck Surg. 1999;121:398–405. doi: 10.1016/S0194-5998(99)70228-7. [DOI] [PubMed] [Google Scholar]
- 85.Daya H, Lo S, Papsin BC. Retropharyngeal and parapharyngeal infections in children: the Toronto experience. Int J Pediatr Otorhinolaryngol. 2005;69:81–86. doi: 10.1016/j.ijporl.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 86.Kirse DJ, Roberson DW. Surgical management of retropharyngeal space infections in children. Laryngoscope. 2001;111:1413–1422. doi: 10.1097/00005537-200108000-00018. [DOI] [PubMed] [Google Scholar]
- 87.Yeow KM, Liao CT, Hao SP. US-guided needle aspiration and catheter drainage as an alternative to open surgical drainage for uniloculated neck abscesses. J Vasc Intervent Radiol. 2001;12:589–594. doi: 10.1016/s1051-0443(07)61481-x. [DOI] [PubMed] [Google Scholar]
- 88.Adelson RT, Murray AD. Minimally invasive transoral catheter-assisted drainage of a danger-space infection. Ear Nose Throat J. 2005;84:785–786. [PubMed] [Google Scholar]
- 89.Weinberg E, Brodsky L, Stanievich J. Needle aspiration of peritonsillar abscess in children. Arch Otolaryngol Head Neck Surg. 1993;119:169–172. doi: 10.1001/archotol.1993.01880140051009. [DOI] [PubMed] [Google Scholar]
- 90.Sichel JY, Gomori JM, Saah D. Parapharyngeal abscess in children: The role of CT for diagnosis and treatment. Int J Pediatr Otorhinolaryngol. 1996;35:213–222. doi: 10.1016/0165-5876(95)01307-5. [DOI] [PubMed] [Google Scholar]
- 91.Choi SS, Vezina LG, Grundfast KM. Relative incidence and alternative approaches for surgical drainage of different types of deep neck abscesses in children. Arch Otolaryngol Head Neck Surg. 1997;123:1271–1275. doi: 10.1001/archotol.1997.01900120015002. [DOI] [PubMed] [Google Scholar]
- 92.Mordekar SR, Bradley PJ, Whitehouse WP. Occult carotid pseudoaneurysm following streptococcal throat infection. J Paediatr Child Health. 2005;41:682–684. doi: 10.1111/j.1440-1754.2005.00759.x. [DOI] [PubMed] [Google Scholar]
- 93.Eliachar I, Peleg H, Joachims HZ. Mediastinitis and bilateral pyopneumothorax complicating a parapharyngeal abscess. Head Neck Surg. 1981;3:438–442. doi: 10.1002/hed.2890030513. [DOI] [PubMed] [Google Scholar]
- 94.Sethi DS, Stanley RE. Deep neck abscesses—Changing trends. J Laryngol Otol. 1994;108:138–143. doi: 10.1017/s0022215100126106. [DOI] [PubMed] [Google Scholar]
- 95.Bluestone CD. Epidemiology and pathogenesis of chronic suppurative otitis media: implications for prevention and treatment. Int J Pediatr Otorhinolaryngol. 1998;42:207–223. doi: 10.1016/s0165-5876(97)00147-x. [DOI] [PubMed] [Google Scholar]
- 96.Giles M, Asher I. Prevalence and natural history of otitis media with perforation in Maori school children. J Laryngol Otol. 1991;105:257–260. doi: 10.1017/s0022215100115555. [DOI] [PubMed] [Google Scholar]
- 97.Klein JO, Teele DW, Rosner BA, the Greater Boston Otitis Media Study Group . Epidemiology of acute otitis media in Boston children from birth to seven years of age. In: Lim DJ, Bluestone CD, Klein JO, Nelson JD, editors. The Fourth International Symposium—Recent Advances in Otitis Media with Effusion, June 1-4, 1987. BC Decker; Toronto: 1988. pp. 14–15. [Google Scholar]
- 98.Duncan B, Ey J, Holberg CJ. Exclusive breast-feeding for at least 4 months protects against otitis media. Pediatrics. 1993;91:867–872. [PubMed] [Google Scholar]
- 99.Bellanti JA. Recurrent respiratory tract infections in paediatric patients. Drugs. 1997;54(suppl 1):1–4. doi: 10.2165/00003495-199700541-00003. [DOI] [PubMed] [Google Scholar]
- 100.Ilicali OC, Keles N, De er K. Evaluation of the effect of passive smoking on otitis media in children by an objective method: Urinary cotinine analysis. Laryngoscope. 2001;111:163–167. doi: 10.1097/00005537-200101000-00028. [DOI] [PubMed] [Google Scholar]
- 101.Sung BS, Chonmaitree T, Broemeling LD. Association of rhinovirus infection with poor bacteriologic outcome of bacterial-viral otitis media. Clin Infect Dis. 1993;17:38–42. doi: 10.1093/clinids/17.1.38. [DOI] [PubMed] [Google Scholar]
- 102.Chonmaitree T, Owen MJ, Patel JA. Presence of cytomegalovirus and herpes simplex virus in middle ear fluids from children with acute otitis media. Clin Infect Dis. 1992;15:650–653. doi: 10.1093/clind/15.4.650. [DOI] [PubMed] [Google Scholar]
- 103.Burton DM, Seid AB, Kearns DB. Neonatal otitis media. An update. Arch Otolaryngol Head Neck Surg. 1993;119:672–675. doi: 10.1001/archotol.1993.01880180092017. [DOI] [PubMed] [Google Scholar]
- 104.Brook I, Yocum P, Shah K. Aerobic and anaerobic bacteriology of otorrhea associated with tympanostomy tubes in children. Acta Otolaryngol. 1998;118:206–210. doi: 10.1080/00016489850154919. [DOI] [PubMed] [Google Scholar]
- 105.Dohar J. Microbiology of otorrhea in children with tympanostomy tubes: implications for therapy. Int J Pediatr Otorhinolaryngol. 2003;67:1317–1323. doi: 10.1016/j.ijporl.2003.08.043. [DOI] [PubMed] [Google Scholar]
- 106.Roland PS, Parry DA, Stroman DW. Microbiology of acute otitis media with tympanostomy tubes. Otolaryngol Head Neck Surg. 2005;133:585–595. doi: 10.1016/j.otohns.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 107.Straetemans M, van Heerbeek N, Sanders EA. Immune status and eustachian tube function in recurrence of otitis media with effusion. Arch Otolaryngol Head Neck Surg. 2005;131:771–776. doi: 10.1001/archotol.131.9.771. [DOI] [PubMed] [Google Scholar]
- 108.Straetemans M, van Heerbeek N, Schilder AG. Eustachian tube function before recurrence of otitis media with effusion. Arch Otolaryngol Head Neck Surg. 2005;131:118–123. doi: 10.1001/archotol.131.2.118. [DOI] [PubMed] [Google Scholar]
- 109.Bunne M, Magnuson B, Falk B. Eustachian tube function varies over time in children with secretory otitis media. Acta Otolaryngol. 2000;120:716–723. doi: 10.1080/000164800750000234. [DOI] [PubMed] [Google Scholar]
- 110.Pelton SI. Otoscopy for the diagnosis of otitis media. Pediatr Infect Dis J. 1998;17:540–543. doi: 10.1097/00006454-199806000-00032. discussion 580. [DOI] [PubMed] [Google Scholar]
- 111.Palmu A, Puhakka H, Huhtala H. Normative values for tympanometry in 7- and 24-month-old children. Audiology. 2001;40:178–184. [PubMed] [Google Scholar]
- 112.Harris PK, Hutchinson KM, Moravec J. The use of tympanometry and pneumatic otoscopy for predicting middle ear disease. Am J Audiol. 2005;14:3–13. doi: 10.1044/1059-0889(2005/002). [DOI] [PubMed] [Google Scholar]
- 113.American Academy of Family Physicians, American Academy of Otolaryngology-Head and Neck Surgery, American Academy of Pediatrics Subcommittee on Otitis Media With Effusion Otitis media with effusion. Pediatrics. 2004;113:1412–1429. doi: 10.1542/peds.113.5.1412. [DOI] [PubMed] [Google Scholar]
- 114.Baker RB. Is ear pulling associated with ear infection? Pediatrics. 1992;90:1006–1007. [PubMed] [Google Scholar]
- 115.Kotikoski MJ, Palmu AA, Puhakka HJ. The symptoms and clinical course of acute bullous myringitis in children less than two years of age. Int J Pediatr Otorhinolaryngol. 2003;67:165–172. doi: 10.1016/s0165-5876(02)00366-x. [DOI] [PubMed] [Google Scholar]
- 116.Schilder AG, Lok W, Rovers MM. International perspectives on management of acute otitis media: A qualitative review. Int J Pediatr Otorhinolaryngol. 2004;68:29–36. doi: 10.1016/j.ijporl.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 117.Arroll B. Antibiotics for upper respiratory tract infections: An overview of Cochrane reviews. Respir Med. 2005;99:255–261. doi: 10.1016/j.rmed.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 118.Glasziou PP, Del Mar CB, Sanders SL. Antibiotics for acute otitis media in children. Cochrane Database Syst Rev. 2004;(1):CD000219. doi: 10.1002/14651858.CD000219.pub2. [DOI] [PubMed] [Google Scholar]
- 119.Giebink GS, Canafax DM, Kempthorne J. Antimicrobial treatment of acute otitis media. J Pediatr. 1991;119:495–500. doi: 10.1016/s0022-3476(05)82074-8. [DOI] [PubMed] [Google Scholar]
- 120.American Academy of Pediatrics Subcommittee on Management of Acute Otitis Media Diagnosis and management of acute otitis media. Pediatrics. 2004;113:1451–1465. doi: 10.1542/peds.113.5.1451. [DOI] [PubMed] [Google Scholar]
- 121.Bluestone CD. Review of cefixime in the treatment of otitis media in infants and children. Pediatr Infect Dis J. 1993;12:75–82. doi: 10.1097/00006454-199301000-00016. [DOI] [PubMed] [Google Scholar]
- 122.Green SM, Rothrock SG. Single-dose intramuscular ceftriaxone for acute otitis media in children. Pediatrics. 1993;91:23–30. [PubMed] [Google Scholar]
- 123.Little P, Gould C, Williamson I. Pragmatic randomised controlled trial of two prescribing strategies for childhood acute otitis media. Br Med J. 2001;322:336–342. doi: 10.1136/bmj.322.7282.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dagan R, Leibovitz E, Greenberg D. Early eradication of pathogens from middle ear fluid during antibiotic treatment of acute otitis media is associated with improved clinical outcome. Pediatr Infect Dis J. 1998;17:776–782. doi: 10.1097/00006454-199809000-00005. [DOI] [PubMed] [Google Scholar]
- 125.Carlin SA, Marchant CD, Shurin PA. Host factors and early therapeutic response in acute otitis media. J Pediatr. 1991;118:178–183. doi: 10.1016/s0022-3476(05)80479-2. [DOI] [PubMed] [Google Scholar]
- 126.Cantekin EI, Mandel EM, Bluestone CD. Lack of efficacy of a decongestant-antihistamine combination for otitis media with effusion (“secretory” otitis media) in children. Results of a double-blind, randomized trial. N Engl J Med. 1983;308:297–301. doi: 10.1056/NEJM198302103080601. [DOI] [PubMed] [Google Scholar]
- 127.Smalley C. Can intranasal corticosteroids prevent acute otitis media (AOM) in children with viral upper respiratory infections (URIs)? J Fam Pract. 2000;49:1075. [PubMed] [Google Scholar]
- 128.Coyte PC, Croxford R, McIsaac W. The role of adjuvant adenoidectomy and tonsillectomy in the outcome of the insertion of tympanostomy tubes. N Engl J Med. 2001;344:1188–1195. doi: 10.1056/NEJM200104193441602. [DOI] [PubMed] [Google Scholar]
- 129.Hammaren-Malmi S, Saxen H, Tarkkanen J. Adenoidectomy does not significantly reduce the incidence of otitis media in conjunction with the insertion of tympanostomy tubes in children who are younger than 4 years: A randomized trial. Pediatrics. 2005;116:185–189. doi: 10.1542/peds.2004-2253. [DOI] [PubMed] [Google Scholar]
- 130.Paradise JL, Bluestone CD, Colborn DK. Adenoidectomy and adenotonsillectomy for recurrent acute otitis media: Parallel randomized clinical trials in children not previously treated with tympanostomy tubes. JAMA. 1999;282:945–953. doi: 10.1001/jama.282.10.945. [DOI] [PubMed] [Google Scholar]
- 131.Witsell DL, Stewart MG, Monsell EM. The Cooperative Outcomes Group for ENT: A multicenter prospective cohort study on the outcomes of tympanostomy tubes for children with otitis media. Otolaryngol Head Neck Surg. 2005;132:180–188. doi: 10.1016/j.otohns.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 132.Rosenfeld RM, Bhaya MH, Bower CM. Impact of tympanostomy tubes on child quality of life. Arch Otolaryngol Head Neck Surg. 2000;126:585–592. doi: 10.1001/archotol.126.5.585. [DOI] [PubMed] [Google Scholar]
- 133.Ruohola A, Heikkinen T, Jero J. Oral prednisolone is an effective adjuvant therapy for acute otitis media with discharge through tympanostomy tubes. J Pediatr. 1999;134:459–463. doi: 10.1016/s0022-3476(99)70204-0. [DOI] [PubMed] [Google Scholar]
- 134.Hoffmann KK, Thompson GK, Burke BL. Anesthetic complications of tympanostomy tube placement in children. Arch Otolaryngol Head Neck Surg. 2002;128:1040–1043. doi: 10.1001/archotol.128.9.1040. [DOI] [PubMed] [Google Scholar]
- 135.Paradise JL, Feldman HM, Campbell TF. Effect of early or delayed insertion of tympanostomy tubes for persistent otitis media on developmental outcomes at the age of three years. N Engl J Med. 2001;344:1179–1187. doi: 10.1056/NEJM200104193441601. [DOI] [PubMed] [Google Scholar]
- 136.Paradise JL, Campbell TF, Dollaghan CA. Developmental outcomes after early or delayed insertion of tympanostomy tubes. N Engl J Med. 2005;353:576–586. doi: 10.1056/NEJMoa050406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kay DJ, Nelson M, Rosenfeld RM. Meta-analysis of tympanostomy tube sequelae. Otolaryngol Head Neck Surg. 2001;124:374–380. doi: 10.1067/mhn.2001.113941. [DOI] [PubMed] [Google Scholar]
- 138.Goldblatt EL, Dohar J, Nozza RJ. Topical ofloxacin versus systemic amoxicillin/clavulanate in purulent otorrhea in children with tympanostomy tubes. Int J Pediatr Otorhinolaryngol. 1998;46(1-2):91–101. doi: 10.1016/s0165-5876(98)00150-5. [DOI] [PubMed] [Google Scholar]
- 139.Roland PS, Anon JB, Moe RD. Topical ciprofloxacin/dexamethasone is superior to ciprofloxacin alone in pediatric patients with acute otitis media and otorrhea through tympanostomy tubes. Laryngoscope. 2003;113:2116–2122. doi: 10.1097/00005537-200312000-00011. [DOI] [PubMed] [Google Scholar]
- 140.Blanshard JD, Maw AR, Bawden R. Conservative treatment of otitis media with effusion by autoinflation of the middle ear. Clin Otolaryngol Allied Sci. 1993;18:188–192. doi: 10.1111/j.1365-2273.1993.tb00827.x. [DOI] [PubMed] [Google Scholar]
- 141.Acuin J, Smith A, Mackenzie I. Interventions for chronic suppurative otitis media. Cochrane Database Syst Rev. 2000;(2):CD000473. doi: 10.1002/14651858.CD000473. [DOI] [PubMed] [Google Scholar]
- 142.Macfadyen CA, Acuin JM, Gamble C. Topical antibiotics without steroids for chronically discharging ears with underlying eardrum perforations. Cochrane Database Syst Rev. 2005;(4):CD004618. doi: 10.1002/14651858.CD004618.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Valtonen H, Tuomilehto H, Qvarnberg Y. A 14-year prospective follow-up study of children treated early in life with tympanostomy tubes: Part 2: Hearing outcomes. Arch Otolaryngol Head Neck Surg. 2005;131:299–303. doi: 10.1001/archotol.131.4.299. [DOI] [PubMed] [Google Scholar]
- 144.Straetemans M, Sanders EA, Veenhoven RH. Pneumococcal vaccines for preventing otitis media. Cochrane Database Syst Rev. 2004;(1):CD001480. doi: 10.1002/14651858.CD001480.pub2. [DOI] [PubMed] [Google Scholar]
- 145.Prymula R, Peeters P, Chrobok V. Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both Streptococcus pneumoniae and non-typable Haemophilus influenzae: A randomised double-blind efficacy study. Lancet. 2006;367:740–748. doi: 10.1016/S0140-6736(06)68304-9. [DOI] [PubMed] [Google Scholar]
- 146.Burton MJ, Towler B, Glasziou P. Tonsillectomy versus non-surgical treatment for chronic/recurrent acute tonsillitis. Cochrane Database Syst Rev. 2000;(2):CD001802. doi: 10.1002/14651858.CD001802. [DOI] [PubMed] [Google Scholar]
- 147.Paradise JL, Bluestone CD, Bachman RZ. Efficacy of tonsillectomy for recurrent throat infection in severely affected children. Results of parallel randomized and nonrandomized clinical trials. N Engl J Med. 1984;310:674–683. doi: 10.1056/NEJM198403153101102. [DOI] [PubMed] [Google Scholar]
- 148.Paradise JL, Bluestone CD, Colborn DK. Tonsillectomy and adenotonsillectomy for recurrent throat infection in moderately affected children. Pediatrics. 2002;110(1 Pt 1):7–15. doi: 10.1542/peds.110.1.7. [DOI] [PubMed] [Google Scholar]
- 149.Brouillette RT, Fernbach SK, Hunt CE. Obstructive sleep apnea in infants and children. J Pediatr. 1982;100:31–40. doi: 10.1016/s0022-3476(82)80231-x. [DOI] [PubMed] [Google Scholar]
- 150.Lim J, McKean M. Adenotonsillectomy for obstructive sleep apnoea in children. Cochrane Database Syst Rev. 2003;(1):CD003136. doi: 10.1002/14651858.CD003136. [DOI] [PubMed] [Google Scholar]
- 151.Gates GA, Avery CA, Prihoda TJ. Effectiveness of adenoidectomy and tympanostomy tubes in the treatment of chronic otitis media with effusion. N Engl J Med. 1987;317:1444–1451. doi: 10.1056/NEJM198712033172305. [DOI] [PubMed] [Google Scholar]
- 152.Tinkelman DG, Silk HJ. Clinical and bacteriologic features of chronic sinusitis in children. Am J Dis Child. 1989;143:938–941. doi: 10.1001/archpedi.1989.02150200098025. [DOI] [PubMed] [Google Scholar]
- 153.Wald ER. Sinusitis in children. N Engl J Med. 1992;326:319–323. doi: 10.1056/NEJM199201303260507. [DOI] [PubMed] [Google Scholar]
- 154.Wald ER, Guerra N, Byers C. Upper respiratory tract infections in young children: Duration of and frequency of complications. Pediatrics. 1991;87:129–133. [PubMed] [Google Scholar]
- 155.Clement PA, Bluestone CD, Gordts F. Management of rhinosinusitis in children: Consensus meeting, Brussels, Belgium, September 13, 1996. Arch Otolaryngol Head Neck Surg. 1998;124:31–34. doi: 10.1001/archotol.124.1.31. [DOI] [PubMed] [Google Scholar]
- 156.Campanella SG, Asher MI. Current controversies: Sinus disease and the lower airways. Pediatr Pulmonol. 2001;31:165–172. doi: 10.1002/1099-0496(200102)31:2<165::aid-ppul1025>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 157.Barnes PD, Wilkinson RH. Radiographic diagnosis of sinusitis in children. Pediatr Infect Dis J. 1991;10:628–629. doi: 10.1097/00006454-199108000-00022. [DOI] [PubMed] [Google Scholar]
- 158.Lusk RP. Pediatric Sinusitis. Raven Press; New York: 1992. [Google Scholar]
- 159.Dowell SF, Schwartz B, Phillips WR: Appropriate use of antibiotics for URIs in children: Part I. Otitis media and acute sinusitis. The Pediatric URI Consensus Team. Am Fam Physician 1123;58:1113-1118. [PubMed]
- 160.Morris P, Leach A. Antibiotics for persistent nasal discharge (rhinosinusitis) in children. Cochrane Database Syst Rev. 2002;(4):CD001094. doi: 10.1002/14651858.CD001094. [DOI] [PubMed] [Google Scholar]
- 161.Williams JW, Jr, Aguilar C, Cornell J. Antibiotics for acute maxillary sinusitis. Cochrane Database Syst Rev. 2003;(2):CD000243. doi: 10.1002/14651858.CD000243. [DOI] [PubMed] [Google Scholar]
- 162.Shoseyov D, Bibi H, Shai P. Treatment with hypertonic saline versus normal saline nasal wash of pediatric chronic sinusitis. J Allergy Clin Immunol. 1998;101:602–605. doi: 10.1016/S0091-6749(98)70166-6. [DOI] [PubMed] [Google Scholar]
- 163.Rosenfeld EA, Rowley AH. Infectious intracranial complications of sinusitis, other than meningitis, in children: 12-Year review. Clin Infect Dis. 1994;18:750–754. doi: 10.1093/clinids/18.5.750. [DOI] [PubMed] [Google Scholar]
- 164.Denny FW, Murphy TF, Clyde WA., Jr Croup: An 11-year study in a pediatric practice. Pediatrics. 1983;71:871–876. [PubMed] [Google Scholar]
- 165.Davison FW. Acute laryngeal obstruction in children. JAMA. 1959;171:1301–1305. doi: 10.1001/jama.1959.03010280025006. [DOI] [PubMed] [Google Scholar]
- 166.Henrickson KJ, Kuhn SM, Savatski LL. Epidemiology and cost of infection with human parainfluenza virus types 1 and 2 in young children. Clin Infect Dis. 1994;18:770–779. doi: 10.1093/clinids/18.5.770. [DOI] [PubMed] [Google Scholar]
- 167.Cherry JD. State of the evidence for standard-of-care treatments for croup: Are we where we need to be? Pediatr Infect Dis J. 2005;24(11 suppl):S198–S202. doi: 10.1097/01.inf.0000188156.23182.eb. [DOI] [PubMed] [Google Scholar]
- 168.Marx A, Torok TJ, Holman RC. Pediatric hospitalizations for croup (laryngotracheobronchitis): Biennial increases associated with human parainfluenza virus 1 epidemics. J Infect Dis. 1997;176:1423–1427. doi: 10.1086/514137. [DOI] [PubMed] [Google Scholar]
- 169.Wagener JS, Landau LI, Olinsky A. Management of children hospitalized for laryngotracheobronchitis. Pediatr Pulmonol. 1986;2:159–162. doi: 10.1002/ppul.1950020308. [DOI] [PubMed] [Google Scholar]
- 170.Sivan Y, Deakers TW, Newth CJ. Thoracoabdominal asynchrony in acute upper airway obstruction in small children. Am Rev Respir Dis. 1990;142:540–544. doi: 10.1164/ajrccm/142.3.540. [DOI] [PubMed] [Google Scholar]
- 171.Newth CJ, Levison H, Bryan AC. The respiratory status of children with croup. J Pediatr. 1972;81:1068–1073. doi: 10.1016/s0022-3476(72)80233-6. [DOI] [PubMed] [Google Scholar]
- 172.Fitzgerald DA. The assessment and management of croup. Paediatr Respir Rev. 2006;7:73–81. doi: 10.1016/j.prrv.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 173.Klassen TP. Croup. A current perspective. Pediatr Clin North Am. 1999;46:1167–1178. doi: 10.1016/s0031-3955(05)70180-2. [DOI] [PubMed] [Google Scholar]
- 174.Neto GM, Kentab O, Klassen TP. A randomized controlled trial of mist in the acute treatment of moderate croup. Acad Emerg Med. 2002;9:873–879. doi: 10.1111/j.1553-2712.2002.tb02187.x. [DOI] [PubMed] [Google Scholar]
- 175.Scolnik D, Coates AL, Stephens D. Controlled delivery of high vs low humidity vs mist therapy for croup in emergency departments: A randomized controlled trial. JAMA. 2006;295:1274–1280. doi: 10.1001/jama.295.11.1274. [DOI] [PubMed] [Google Scholar]
- 176.Kanter RK, Watchko JF. Pulmonary edema associated with upper airway obstruction. Am J Dis Child. 1984;138:356–358. doi: 10.1001/archpedi.1984.02140420022009. [DOI] [PubMed] [Google Scholar]
- 177.Russell K, Wiebe N, Saenz A. Glucocorticoids for croup. Cochrane Database Syst Rev. 2004;(1):CD001955. doi: 10.1002/14651858.CD001955.pub2. [DOI] [PubMed] [Google Scholar]
- 178.Westley CR, Cotton EK, Brooks JG. Nebulized racemic epinephrine by IPPB for the treatment of croup: A double-blind study. Am J Dis Child. 1978;132:484–487. doi: 10.1001/archpedi.1978.02120300044008. [DOI] [PubMed] [Google Scholar]
- 179.Klassen TP, Craig WR, Moher D. Nebulized budesonide and oral dexamethasone for treatment of croup: A randomized controlled trial. JAMA. 1998;279:1629–1632. doi: 10.1001/jama.279.20.1629. [DOI] [PubMed] [Google Scholar]
- 180.Geelhoed GC, Macdonald WB. Oral dexamethasone in the treatment of croup: 0.15 mg/kg versus 0.3 mg/kg versus 0.6 mg/kg. Pediatr Pulmonol. 1995;20:362–368. doi: 10.1002/ppul.1950200605. [DOI] [PubMed] [Google Scholar]
- 181.Bjornson CL, Klassen TP, Williamson J. A randomized trial of a single dose of oral dexamethasone for mild croup. N Engl J Med. 2004;351:1306–1313. doi: 10.1056/NEJMoa033534. [DOI] [PubMed] [Google Scholar]
- 182.Johnson DW, Schuh S, Koren G. Outpatient treatment of croup with nebulized dexamethasone. Arch Pediatr Adolesc Med. 1996;150:349–355. doi: 10.1001/archpedi.1996.02170290015002. [DOI] [PubMed] [Google Scholar]
- 183.Burton DM, Seid AB, Kearns DB. Candida laryngotracheitis: A complication of combined steroid and antibiotic usage in croup. Int J Pediatr Otorhinolaryngol. 1992;23:171–175. doi: 10.1016/0165-5876(92)90053-r. [DOI] [PubMed] [Google Scholar]
- 184.Waisman Y, Klein BL, Boenning DA. Prospective randomized double-blind study comparing L-epinephrine and racemic epinephrine aerosols in the treatment of laryngotracheitis (croup) Pediatrics. 1992;89:302–306. [PubMed] [Google Scholar]
- 185.Taussig LM, Castro O, Beaudry PH. Treatment of laryngotracheobronchitis (croup). Use of intermittent positive-pressure breathing and racemic epinephrine. Am J Dis Child. 1975;129:790–793. doi: 10.1001/archpedi.1975.02120440016004. [DOI] [PubMed] [Google Scholar]
- 186.Fogel JM, Berg IJ, Gerber MA. Racemic epinephrine in the treatment of croup: Nebulization alone versus nebulization with intermittent positive pressure breathing. J Pediatr. 1982;101:1028–1031. doi: 10.1016/s0022-3476(82)80039-5. [DOI] [PubMed] [Google Scholar]
- 187.Gupta VK, Cheifetz IM. Heliox administration in the pediatric intensive care unit: An evidence-based review. Pediatr Crit Care Med. 2005;6:204–211. doi: 10.1097/01.PCC.0000154946.62733.94. [DOI] [PubMed] [Google Scholar]
- 188.Weber JE, Chudnofsky CR, Younger JG. A randomized comparison of helium-oxygen mixture (Heliox) and racemic epinephrine for the treatment of moderate to severe croup. Pediatrics. 2001;107:E96. doi: 10.1542/peds.107.6.e96. [DOI] [PubMed] [Google Scholar]
- 189.Cohen B, Dunt D. Recurrent and non-recurrent croup: An epidemiological study. Austral Paediatr J. 1988;24:339–342. doi: 10.1111/j.1440-1754.1988.tb01384.x. [DOI] [PubMed] [Google Scholar]
- 190.Wood N, Menzies R, McIntyre P. Epiglottitis in Sydney before and after the introduction of vaccination against Haemophilus influenzae type b disease. Intern Med J. 2005;35:530–535. doi: 10.1111/j.1445-5994.2005.00909.x. [DOI] [PubMed] [Google Scholar]
- 191.Shah RK, Roberson DW, Jones DT. Epiglottitis in the Hemophilus influenzae type B vaccine era: Changing trends. Laryngoscope. 2004;114:557–560. doi: 10.1097/00005537-200403000-00031. [DOI] [PubMed] [Google Scholar]
- 192.Tanner K, Fitzsimmons G, Carrol ED. Haemophilus influenzae type b epiglottitis as a cause of acute upper airways obstruction in children. BMJ. 2002;325:1099–1100. doi: 10.1136/bmj.325.7372.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Grattan-Smith T, Forer M, Kilham H. Viral supraglottitis. J Pediatr. 1987;110:434–435. doi: 10.1016/s0022-3476(87)80512-7. [DOI] [PubMed] [Google Scholar]
- 194.Nadel S, Offit PA, Hodinka RL. Upper airway obstruction in association with perinatally acquired herpes simplex virus infection. J Pediatr. 1992;120:127–129. doi: 10.1016/s0022-3476(05)80616-x. [DOI] [PubMed] [Google Scholar]
- 195.Narasimhan N, van Stralen DW, Perkin RM. Acute supraglottitis caused by varicella. Pediatr Infect Dis J. 1993;12:619–620. doi: 10.1097/00006454-199307000-00017. [DOI] [PubMed] [Google Scholar]
- 196.Balsam D, Sorrano D, Barax C. Candida epiglottitis presenting as stridor in a child with HIV infection. Pediatr Radiol. 1992;22:235–236. doi: 10.1007/BF02012509. [DOI] [PubMed] [Google Scholar]
- 197.Diamant EP, Dische RM, Barzilai A. Chronic epiglottitis in a child with acquired immunodeficiency syndrome. Pediatr Infect Dis J. 1992;11:770–771. [PubMed] [Google Scholar]
- 198.Mauro RD, Poole SR, Lockhart CH. Differentiation of epiglottitis from laryngotracheitis in the child with stridor. Am J Dis Child. 1988;142:679–682. doi: 10.1001/archpedi.1988.02150060113044. [DOI] [PubMed] [Google Scholar]
- 199.Knight GJ, Harris MA, Parbari M. Single daily dose ceftriaxone therapy in epiglottitis. J Paediatr Child Health. 1992;28:220–222. doi: 10.1111/j.1440-1754.1992.tb02649.x. [DOI] [PubMed] [Google Scholar]
- 200.Molteni RA. Epiglottitis: incidence of extraepiglottic infection: Report of 72 cases and review of the literature. Pediatrics. 1976;58:526–531. [PubMed] [Google Scholar]
- 201.Gilbert GL, MacInnes SJ, Guise IA. Rifampicin prophylaxis for throat carriage of Haemophilus influenzae type b in patients with invasive disease and their contacts. BMJ. 1991;302:1432–1435. doi: 10.1136/bmj.302.6790.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Wenger JD, Pierce R, Deaver KA. Efficacy of Haemophilus influenzae type b polysaccharide-diphtheria toxoid conjugate vaccine in US children aged 18-59 months. Haemophilus Influenzae Vaccine Efficacy Study Group. Lancet. 1991;338:395–398. [PubMed] [Google Scholar]
- 203.Broadhurst LE, Erickson RL, Kelley PW. Decreases in invasive Haemophilus influenzae diseases in US Army children 1984 through 1991. JAMA. 1993;269:227–231. [PubMed] [Google Scholar]
- 204.Salamone FN, Bobbitt DB, Myer CM. Bacterial tracheitis reexamined: Is there a less severe manifestation? Otolaryngol Head Neck Surg. 2004;131:871–876. doi: 10.1016/j.otohns.2004.06.708. [DOI] [PubMed] [Google Scholar]
- 205.Henry RL, Mellis CM, Benjamin B. Pseudomembranous croup. Arch Dis Child. 1983;58:180–183. doi: 10.1136/adc.58.3.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Manning SC, Ridenour B, Brown OE. Measles: An epidemic of upper airway obstruction. Otolaryngol Head Neck Surg. 1991;105:415–418. doi: 10.1177/019459989110500311. [DOI] [PubMed] [Google Scholar]
- 207.Graf J, Stein F. Tracheitis in pediatric patients. Semin Pediatr Infect Dis. 2006;17:11–13. doi: 10.1053/j.spid.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 208.Han BK, Dunbar JS, Striker TW. Membranous laryngotracheobronchitis (membranous croup) AJR Am J Roentgenol. 1979;133:53–58. doi: 10.2214/ajr.133.1.53. [DOI] [PubMed] [Google Scholar]
- 209.Derkay CS, Darrow DH. Recurrent respiratory papillomatosis. Ann Otol Rhinol Laryngol. 2006;115:1–11. doi: 10.1177/000348940611500101. [DOI] [PubMed] [Google Scholar]
- 210.Shykhon M, Kuo M, Pearman K. Recurrent respiratory papillomatosis. Clin Otolaryngol Allied Sci. 2002;27:237–243. doi: 10.1046/j.1365-2273.2002.00555.x. [DOI] [PubMed] [Google Scholar]
- 211.Quiney RE, Hall D, Croft CB. Laryngeal papillomatosis: Analysis of 113 patients. Clinical Otolaryngol Allied Sci. 1989;14:217–225. doi: 10.1111/j.1365-2273.1989.tb00364.x. [DOI] [PubMed] [Google Scholar]
- 212.Corbitt G, Zarod AP, Arrand JR. Human papillomavirus (HPV) genotypes associated with laryngeal papilloma. J Clin Pathol. 1988;41:284–288. doi: 10.1136/jcp.41.3.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Steinbrook R. The potential of human papillomavirus vaccines. N Engl J Med. 2006;354:1109–1112. doi: 10.1056/NEJMp058305. [DOI] [PubMed] [Google Scholar]
- 214.Pou AM, Rimell FL, Jordan JA. Adult respiratory papillomatosis: Human papillomavirus type and viral coinfections as predictors of prognosis. Ann Otol Rhinol Laryngol. 1995;104(10 Pt 1):758–762. doi: 10.1177/000348949510401002. [DOI] [PubMed] [Google Scholar]
- 215.Dillner J, Andersson-Ellstrom A, Hagmar B. High risk genital papillomavirus infections are not spread vertically. Rev Med Virol. 1999;9:23–29. doi: 10.1002/(sici)1099-1654(199901/03)9:1<23::aid-rmv233>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 216.Rice PS, Cason J, Best JM. High risk genital papillomavirus infections are spread vertically. Rev Med Virol. 1999;9:15–21. doi: 10.1002/(sici)1099-1654(199901/03)9:1<15::aid-rmv232>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 217.Silverberg MJ, Thorsen P, Lindeberg H. Condyloma in pregnancy is strongly predictive of juvenile-onset recurrent respiratory papillomatosis. Obstet Gynecol. 2003;101:645–646. doi: 10.1016/s0029-7844(02)03081-8. [DOI] [PubMed] [Google Scholar]
- 218.Perrick D, Wray BB, Leffell MS. Evaluation of immunocompetency in juvenile laryngeal papillomatosis. Ann Allergy. 1990;65:69–72. [PubMed] [Google Scholar]
- 219.Anderson KC, Roy TM, Fields CL. Juvenile laryngeal papillomatosis: A new complication. South Med J. 1993;86:447–449. doi: 10.1097/00007611-199304000-00015. [DOI] [PubMed] [Google Scholar]
- 220.Hester RP, Derkay CS, Burke BL. Reliability of a staging assessment system for recurrent respiratory papillomatosis. Int J Pediatr Otorhinolaryngol. 2003;67:505–509. doi: 10.1016/s0165-5876(03)00007-7. [DOI] [PubMed] [Google Scholar]
- 221.Abramson AL, Shikowitz MJ, Mullooly VM. Clinical effects of photodynamic therapy on recurrent laryngeal papillomas. Arch Otolaryngol Head Neck Surg. 1992;118:25–29. doi: 10.1001/archotol.1992.01880010029011. [DOI] [PubMed] [Google Scholar]
- 222.Chadha NK, James AL. Adjuvant antiviral therapy for recurrent respiratory papillomatosis. Cochrane Database Syst Rev. 2005;(4):CD005053. doi: 10.1002/14651858.CD005053.pub2. [DOI] [PubMed] [Google Scholar]
- 223.Healy GB, Gelber RD, Trowbridge AL. Treatment of recurrent respiratory papillomatosis with human leukocyte interferon. Results of a multicenter randomized clinical trial. N Engl J Med. 1988;319:401–407. doi: 10.1056/NEJM198808183190704. [DOI] [PubMed] [Google Scholar]
- 224.Leventhal BG, Kashima HK, Mounts P. Long-term response of recurrent respiratory papillomatosis to treatment with lymphoblastoid interferon alfa-N1. Papilloma Study Group. N Engl J Med. 1991;325:613–617. doi: 10.1056/NEJM199108293250904. [DOI] [PubMed] [Google Scholar]
- 225.Tolaymat A, Leventhal B, Sakarcan A. Systemic lupus erythematosus in a child receiving long-term interferon therapy. J Pediatr. 1992;120:429–432. doi: 10.1016/s0022-3476(05)80913-8. [DOI] [PubMed] [Google Scholar]
- 226.Kimberlin DW. Current status of antiviral therapy for juvenile-onset recurrent respiratory papillomatosis. Antiviral Res. 2004;63:141–151. doi: 10.1016/j.antiviral.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 227.Hammer J. Acquired upper airway obstruction. Paediatr Respir Rev. 2004;5:25–33. doi: 10.1016/j.prrv.2003.09.007. [DOI] [PubMed] [Google Scholar]


