Abstract
Lenvatinib is an approved first-line therapy for unresectable hepatocellular carcinoma (HCC), but the effect of dose modification on its efficacy is unclear. We analyzed the relationship between the relative dose intensity during the initial 4 weeks of therapy [4W-relative dose intensity (RDI)] and the efficacy of lenvatinib therapy in the real-world setting. A total of 48 consecutive patients with unresectable HCC who received lenvatinib therapy for more than 4 weeks were included. The 4W-RDI was calculated as the cumulative dose in the initial 4 weeks divided by the weight-based standard dose, and we evaluated its association with overall survival (OS) and best response by modified Response Evaluation Criteria in Solid Tumor (mRECIST). The baseline factors predicting high 4W-RDI were analyzed further. The median durations of follow-up and of therapy among the 48 participants were 7.6 and 6.6 months, respectively. The median OS was not reached. Drug interruption and/or dose reduction were necessary in 30 patients (62.5%) and the median 4W-RDI was 70% (range 22%–100%). Patients with 4W-RDI ≥70% had longer OS [hazard ratio (HR) 0.28, 95% confidential interval (CI):0.09–0.90, p = 0.03], and longer duration of lenvatinib therapy (HR 0.39, 95%CI:0.16–0.92, p = 0.03). Patients with 4W-RDI ≥70% showed higher disease control rate compared to those with 4W-RDI <70% (91.7% vs. 54.2%, p = 0.008). A baseline albumin level >3.4g/dL or ALBI score less than -2.171 were significantly associated with achieving 4W-RDI ≥70%. In conclusion, 4W-RDI of lenvatinib therapy is associated with favorable radiological response and longer OS.
Introduction
Lenvatinib (LEN) is a tyrosine kinase inhibitor (TKI) which has been approved for unresectable hepatocellular carcinoma (HCC) as a 1st line treatment. This was based on confirmation of its non-inferiority versus sorafenib in the phase III REFLECT study [1]. The efficacy of lenvatinib has also been reported in several real-world practices [2–5]. However, an important drawback of TKI therapy is that some cases need drug interruptions or dose reductions at early phase of the treatment because of adverse events [1, 6–8]. Thus, cumulative doses taken by patients often differ from weight-based planned doses, and to date, the relation between dose intensity and treatment effects has not been fully verified. The relative dose intensity (RDI) is a tool for assessing the total dose of chemotherapy. It is defined as the actual dose received divided by the standard calculated dose during a set period [9, 10] and is reported as being associated with favorable treatment response to TKI therapies in various cancers [11–13].
In this study, we analyzed relation between RDI during the initial 4 weeks of therapy (4W-RDI) and the efficacy of LEN for unresectable HCC in a real-world setting.
Methods
Patient
A total of 60 patients with unresectable HCC who received LEN therapy at Musashino Red Cross Hospital from April 2018 to May 2019 were included. To evaluate effects of LEN therapy, 12 patients who could not continue LEN therapy for 4 weeks were excluded. Subsequently, 48 patients were enrolled in this study. Flow chart of the study is shown in S1 Fig. HCC was diagnosed by contrast enhanced computed tomography (CT) or magnetic resonance imaging (MRI) when tumors displayed vascular enhancement in the early phase and washout in the later phase according to guidelines from the American Association for the Study of Liver Diseases [14] and the Japan Society of Hepatology [15]. Tumor biopsy was used to diagnose tumors with nontypical imaging findings.
Protocol of LEN therapy
The standard dose of LEN therapy was determined by body weight: patients weighing <60 kg were given 8 mg/day and those weighing ≥60 kg were given 12 mg/day. Starting with a reduced dose was permitted based on the patient’s condition and the preference of the attending physicians. Tumor assessment was done in accordance with the modified Response Evaluation Criteria in Solid Tumor (mRECIST), using dynamic CT within 4–8 weeks and every 8 weeks thereafter [16, 17]. During follow up, all patients were measured routine blood-chemistry and tumor markers testing every 2–4 weeks.
The albumin-bilirubin (ALBI) grade was used to assess hepatic reserve function. This score was calculated based on serum albumin and total bilirubin (T.Bil) values using the following formula: [ALBI score = (log10 T.Bil(μmol/L) × 0.66) + (albumin (g/L) × −0.085)]. Scores were then interpreted as follows: grade 1, if less than or equal to −2.60; grade 2, if greater than −2.60 but less than or equal to −1.39; and grade 3, if greater than −1.39 [18, 19].
Adverse events (AEs) were graded according to the CTCAE version 5.0. The LEN dose was reduced or treatment was interrupted for AEs of at least grade 2 or for AEs considered otherwise unacceptable. LEN treatment was interrupted until adverse events were improved to less than grade1 or got acceptable. Doses were reduced from 12mg to 8mg and 8mg to 4mg. Alternatively, maintenance of the same dose with planned drug withdrawal for 2 days/week, such as weekday (5days)-on and weekend (2days)-off schedule, were permitted based on the patient’s condition and the preference of the attending physicians.
Calculation of RDI
RDI was defined as the actual dose divided by the standard dose [9–11]. To evaluate RDI of early phase of LEN therapy, the 4-week RDI (4W-RDI) was calculated as the cumulative dose within the initial 4 weeks of starting LEN treatment divided by the standard dose.
Statistical analysis
Overall survival (OS) was calculated using the Kaplan–Meier method and difference was analyzed by the log-rank test. Statistical significance was defined as a p value of <0.05. The factors associated with OS were analyzed using the Cox-proportional hazard model, and the backward stepwise selection method was used for multivariate analysis. Associations between 4W-RDI and factors were evaluated with Fisher’s exact test and Mann–Whitney U-test. Correlations between two variables were tested using Pearson’s correlation analysis. All statistical analyses were performed with EZR (Saitama Medical Centre, Jichi Medical University, Shimotsuke, Japan), a graphical user interface for R version 3.2.2 (The R Foundation for Statistical Computing, Vienna, Austria) [20].
This study protocol conformed to the ethical guidelines in the Declaration of Helsinki and was approved by Musashino Red Cross Hospital Clinical Research Ethics Committee. Written informed consent was obtained from every patient.
Results
Baseline characteristics
Baseline characteristics of patients are shown in Table 1. Median age was 74 years. All patients had an Eastern Cooperative Oncology Group Performance Status Scale (ECOG PS) score of 0 (30 patients) or 1 (18 patients). 44 patients (91.7%) were Child Pugh grade A and 4 patients (8.3%) were Child Pugh grade B. 12 patients (25.5%) had macrovascular invasion (MVI). 30 patients (62.5%) were treated as 1st line tyrosine kinase inhibitor. Patients treated as 2nd line and 3rd line were 4 (8.3%) and 14 (29%), respectively.
Table 1. Baseline characteristics.
| Age, years median (range) | 74(52–92) |
| Male gender, n(%) | 42(87.5) |
| Body weight(Kg), median (range) | 60.0(30.3–101) |
| Etiology: HCV/HBV/Alcohol /Others, n(%) | 26(54.2)/9(18.8)/5(10.4)/8(16.7) |
| PS (ECOG) 0/1, n(%) | 30(62.5)/18(37.5) |
| Child Pugh Grade A/B, n(%) | 44(91.7)/4(8.3) |
| ALBI Grade: 1 / 2 /3, n(%) | 13(27.0)/34(70.9)/1(2.1) |
| AFP(ng/ml), n(%) | 104.9(1.60–115112) |
| BCLC Stage B/C, n(%) | 17(35.4)/31(64.6) |
| MVI, n(%) | 12(25.5) |
| Extrahepatic spread, n(%) | 21(43.8) |
| Clinical course: 1st line/ 2nd line/ 3rd line, n(%) | 30(62.5)/4(8.3)/14(29.2) |
| Initial dose: 4mg/8mg/12mg, n(%) | 9(18.8)/21(43.8)/18(37.5) |
| Follow-up duration (Month), median (range) | 7.6(1.8–15.7) |
| 4W-RDI(%), median (range) | 70(22–100) |
AFP: α-Fetoprotein; BCLC: Barcelona Clinical Liver Cancer; HCV: hepatitis C virus; HBV: hepatitis B virus; MVI: macrovascular invasion; PS: performance status; 4W-RDI: 4 week relative dose intensity.
Efficacy of LEN therapy
OS and duration of LEN therapy were shown in Figs 1A and 2A. Median OS was not reached and median duration of LEN therapy was 6.6 months. Median progression free survival (PFS) was 6.0 months. Radiological best response rates for complete response (CR), partial response (PR), stable disease (SD) and progression disease (PD) were 6.3%, 33.3%, 35.4% and 25.0%, respectively. Overall response rate (ORR) and disease control rate (DCR) were 39.6% and 75.0%, respectively.
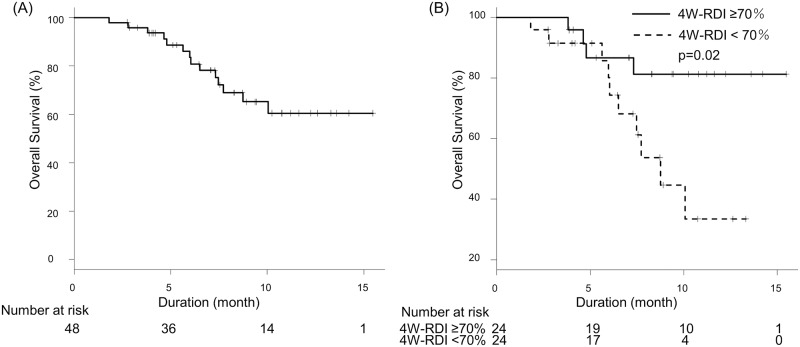
Fig 1. Overall survival.
(A) OS of all patients. Median duration of follow up was 7.6 months, and median OS was not reached. (B) OS stratified by 4W-RDI. Patients with 4W-RDI ≥70% showed significantly longer OS compared to those with 4W-RDI <70% (p = 0.02). Median OS was not reached in patients with 4W-RDI ≥70% and 8.7 months in 4W-RDI <70%.
Fig 2. Time to discontinuation of lenvatinib therapy.
(A) Time to discontinuation of lenvatinib therapy of all patients. Median duration of LEN therapy was 6.6 months. (B) Time to discontinuation of lenvatinib therapy stratified by 4W-RDI. Patients with 4W-RDI ≥70% were able to continue LEN treatment longer (p = 0.03). Median duration of LEN therapy was 9.5 months in patients with 4W-RDI ≥70% and 5.6 months in 4W-RDI <70%.
Distribution of 4W-RDI
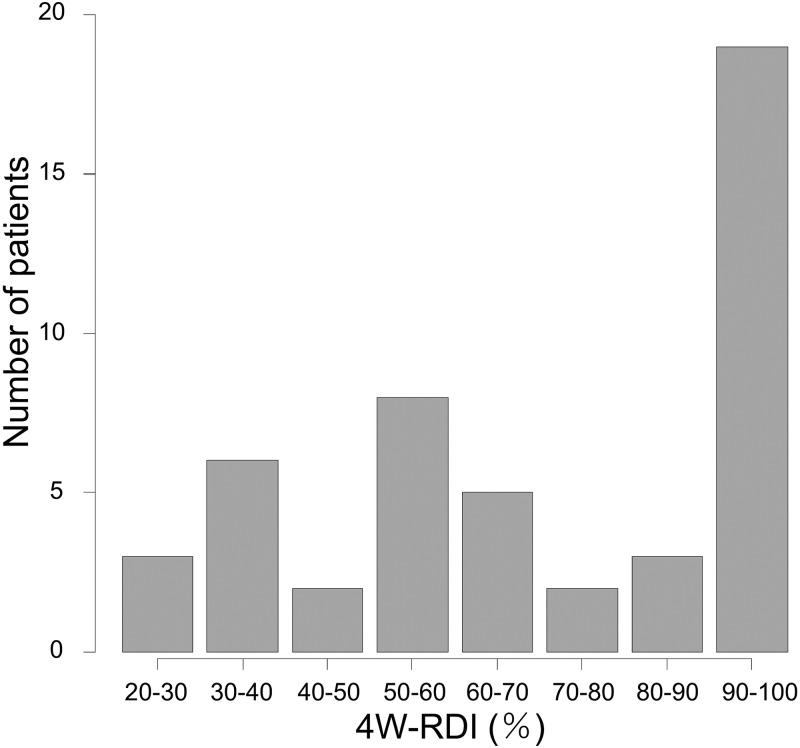
There were variations of 4W-RDI among cases, as shown by the distribution in Fig 3. Only 18 patients (37.5%) maintained 4W-RDI of 100%, and 30 patients (62.5%) required drug interruption and/or dose reduction in the 4weeks after administration. 16 patients (33.3%) had initial dose reduction. The reasons of initial dose reduction were impaired renal function (37.5%), Child Pugh B (18.8%), 40kg or lower body weight (12.5%), under anticoagulation therapy for pulmonary thrombosis (12.5%), decrease of appetite and depression (6.2%), past history of severe hand-foot syndrome by sorafenib treatment (6.2%), 50% or higher liver occupation of tumor (6.2%), respectively. Among them no patient was able to increase the dose. 15 patients (31.3%) needed drug interruption, and reasons of initial dose reductions were old age (37.5%), low renal function (37.5%), after treatments of infection (12.5%), thrombosis (6.2%), low platelet counts (6.2%), decrease of appetite (6.2%), past history of thyroid dysfunction (6.2%) and low body weight (6.2%), respectively. Reasons of dose reductions were fatigue (25%), proteinuria (20%), AST/ALT increase (15%), NH3 increase (10%), renal dysfunction (10%), hand-foot-syndrome (5%), nausea (5%), decreased appetite (15%), body weight decrease (5%), respectively. 20 patients (41.7%) needed dose reduction and reasons of dose interruptions were fatigue (40%), AST/ALT increase (26.7%), renal dysfunction (20%), NH3 increase (6.7%), nausea (6.7%), decreased appetite (6.7%), infection (6.7%), respectively. Median duration of dose interruptions was 6 (2–17) days Median 4W-RDI was 70% because of these modifications.
Fig 3. Distributions of patients by 4W-RDI.
Patient distribution from low to high 4W-RDI categorized in 10% increment (median = 70%).
Relation between 4W-RDI and OS
The relation between 4W-RDI and OS is shown with various cutoff values of 4W-RDI at 10% intervals in Table 2. When cutoff value was 70%, there was a statistically significant difference in OS [hazard ratio (HR) 0.28, 95% confidential interval (CI):0.09–0.90, p = 0.03]. Patients with 4W-RDI ≥70% showed significantly longer OS compared to those with 4W-RDI <70% (Fig 1B, p = 0.02). Median OS was not reached in patients with 4W-RDI ≥70% and 8.7 months in 4W-RDI <70%.
Table 2. Comparison of OS according to 4W-RDI cutoff value.
| 4W-RDI cutoff | Patient number | OS | ||
|---|---|---|---|---|
| HR | 95%CI | p Value | ||
| ≥40%/<40% | 39/9 | 1.03 | 0.23–4.64 | 0.96 |
| ≥50%/<50% | 34/14 | 0.91 | 0.25–3.30 | 0.89 |
| ≥60%/<60% | 29/19 | 0.61 | 0.21–1.78 | 0.37 |
| ≥70%/<70% | 24/24 | 0.28 | 0.09–0.90 | 0.03 |
| ≥80%/<80% | 22/26 | 0.32 | 0.10–1.06 | 0.06 |
| ≥90%/<90% | 19/29 | 0.49 | 0.15–1.57 | 0.23 |
CI: confidence interval; HR: hazard ratio; OS: overall survival; 4W-RDI: 4 week relative dose intensity.
In the univariable analysis, 4W-RDI ≥70% was significantly associated with longer survival than 4W-RDI <70%, whereas there were no significant differences by age (HR 0.99, 95%CI:0.94–1.04, p = 0.66), gender (HR 0.63, 95%CI:0.08–5.07, p = 0.67), previous TKI experience (HR 0.58, 95%CI:0.20–1.65, p = 0.30), α-Fetoprotein (AFP) level (HR 1.00, 95%CI:0.99–1.00, p = 0.61), ALBI score (HR 1.80, 95%CI:0.56–5.81, p = 0.33) and BCLC Stage C (HR 1.34, 95%CI:0.42–4.30, p = 0.62) (S1 Table).
In the multivariable analysis, the 4W-RDI ≥70% remained an independent prognostic factor of lenvatinib therapy (HR 0.3, 95%CI:0.09–0.96, p = 0.04) after adjusting for variables based on existing knowledge of prognostic factors of unresectable HCC such as age, gender, ALBI score, and BCLC stage (S1 Table).
4W-RDI and duration of LEN therapy
The relation between 4W-RDI and duration of LEN therapy is shown in Fig 2B. Patients with 4W-RDI ≥70% were able to continue LEN treatment longer beyond the initial 4 weeks (p = 0.03). In the univariable analysis, 4W-RDI ≥70% was a factor of long duration of lenvatinib therapy (HR 0.39, 95%CI:0.16–0.92, p = 0.03). Median duration of LEN therapy was 9.5 and 5.6 months in patients with 4W-RDIs of ≥70% and <70%, respectively.
Relation between 4W-RDI and radiological best response
Radiological best response rates stratified by 4W-RDI are shown in Table 3. DCR of patients with 4W-RDI ≥70% was significantly high compared to 4W-RDI <70% (95.8% vs 54.2% p = 0.002). Overall response rate (ORR) of 4W-RDI ≥70% patients and 4W-RDI <70% patients were 54.2% and 25.0%, respectively (p = 0.08).
Table 3. Radiological responses to lenvatinib therapy by 4W-RDI.
| 4W-RDI ≥ 70% | 4W-RDI <70% | p value | |
|---|---|---|---|
| CR | 2(8.3%) | 1(4.2%) | |
| PR | 11(45.8%) | 5(20.8%) | |
| SD | 10(41.7%) | 7(29.2%) | |
| PD | 1(4.2%) | 11(45.8%) | |
| ORR | 13(54.2%) | 6(25.0%) | 0.08 |
| DCR | 23(95.8%) | 13(54.2%) | 0.002 |
CR: complete response; DCR: disease control rate; ORR: overall response rate; PD: progressive disease; PR: partial response; SD: stable disease; 4W-RDI: 4 week relative dose intensity.
Baseline factors associated with high 4W-RDI
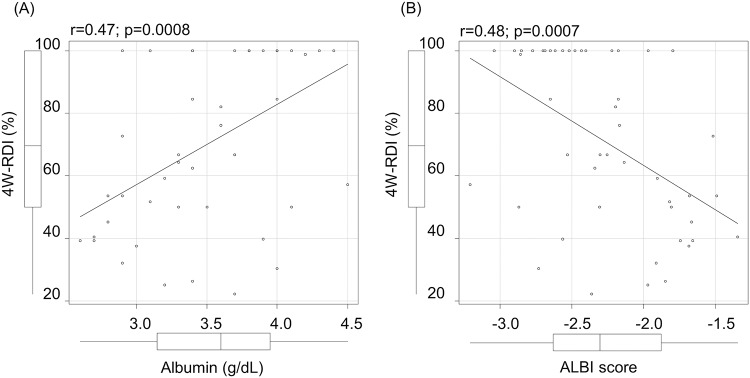
Baseline characteristics of patients with 4W-RDI ≥70% and <70% are shown in Table 4. Mean ALBI scores of patients with 4W-RDI ≥70% and those with 4W-RDI <70% were -2.52 and -1.94, respectively and showed statistically significant difference (p = 0.006). Mean albumin level, which is a component of ALBI score were 3.8g/dL and 3.3g/dL, respectively (p = 0.003). A Pearson correlation analysis between 4W-RDI and ALBI score or 4W-RDI and albumin level revealed a significant correlation (ALBI score r = 0.48; p = 0.0007, albumin r = 0.47; p = 0.0008, respectively) (Fig 4).
Table 4. Factors associated with 4W-RDI≥70%.
| 4W-RDI ≥ 70% | 4W-RDI <70% | p value | |
|---|---|---|---|
| Age, years, median (range) | 75(54–92) | 76(52–87) | 0.77 |
| Male gender, n(%) | 23 (95.8) | 19 (79.2) | 0.19 |
| Body weight(Kg), median (range) | 65.1 (49.3–94.1) | 58.2 (30.3–101.0) | 0.05 |
| ALBI score, median (range) | -2.52 (-1.52 - -3.04) | -1.94 (-1.35 - -3.21) | 0.006 |
| Albumin (g/dL), median (range) | 3.8 (2.9–4.4) | 3.3 (2.6–4.5) | 0.003 |
| T.Bil (mg/dL), median (range) | 0.7 (0.4–1.9) | 0.75 (0.4–2.2) | 0.90 |
| AFP (ng/ml), n (%) | 38.4(1.6–37334) | 227.1(2.1–115112) | 0.61 |
| TKI naïve, n (%) | 17 (70.8) | 13 (54.2) | 0.37 |
| MVI, n (%) | 5(20.8) | 7(30.4) | 0.52 |
| BCLC stage C, n (%) | 17 (70.8) | 14 (58.3) | 0.55 |
| Total AEs of grade 3/4, n (%) | 4 (16.7) | 14 (58.3) | 0.01 |
AEs: adverse events; BCLC: Barcelona Clinical Liver Cancer; MVI: macrovascular invasion; TKI: tyrosin kinase inhibitor; 4W-RDI: 4 week relative dose intensity.
Fig 4. Correlation between 4W-RDI and baseline albumin or ALBI.
(A) Correlation between 4W-RDI and baseline albumin level. (B) Correlation between 4W-RDI and baseline ALBI score.
For prediction of 4W-RDI ≥70% by baseline albumin, the AUROC (area under the receiver operating characteristic) was 0.76 (95%CI:0.61–0.90) and the best cutoff values was 3.4g/dL [sensitivity:83.3%, specificity:58.3%, PPV (positive predictive):66.7%, NPV (negative predictive value):77.8%]. By baseline ALBI score, AUROC was 0.73 (95%CI:0.58–0.88) and the best cutoff values was -2.171 (sensitivity:83.3%, specificity:58.3%, PPV66.7%, NPV:77.8%). Baseline albumin and ALBI score both showed high predictability for 4W-RDI ≥70%.
Adverse events
Adverse events (AEs) that occurred in this study are shown in Table 5. Any grades of total AEs were observed in 47 patients (97.9%). The most common AEs were hypertension (64.6%) and fatigue (64.6%). The most common grade 3/4 AEs were Aspartate transaminase (AST) and/or Alanine transaminase (ALT) increase (8.3%), fatigue (6.2%) and proteinuria (6.2%). No grade 5 treatment-related AEs were reported. The total number of grade 3/4 AEs was higher among patients with a 4W-RDI <70% (p = 0.01).
Table 5. Adverse events.
| All patients | 4W-RDI≥70% | 4W-RDI<70% | p value* | |||||
|---|---|---|---|---|---|---|---|---|
| Any grade | Grade 3/4 | Any grade | Grade 3/4 | Any grade | Grade 3/4 | Any grade | Grade 3/4 | |
| Total AEs | 47(97.9%) | 18(37.5%) | 23(95.8%) | 4(16.7%) | 24(100.0%) | 14(58.3%) | 1.00 | 0.01 |
| Hand-foot-syndrome | 22(45.8%) | 0(0.0%) | 12(50.0%) | 0(0.0%) | 10(41.7%) | 0(0%) | 0.77 | - |
| Diarrhea | 10(20.8%) | 0(0.0%) | 5(20.8%) | 0(0.0%) | 5(20.8%) | 0(0.0%) | 1.00 | - |
| Hypertension | 31(64.6%) | 1(2.1%) | 15(62.5%) | 0(0.0%) | 16(66.7%) | 1(4.2%) | 1.00 | 1.00 |
| Decreased appetite | 24(50.0%) | 2(4.2%) | 9(37.5%) | 1(4.2%) | 15(62.5%) | 1(4.2%) | 0.15 | 1.00 |
| Fatigue | 31(64.6%) | 3(6.2%) | 13(54.2%) | 0(0.0%) | 18(75.0%) | 3(12.5%) | 0.23 | 0.23 |
| Proteinuria | 19(39.6%) | 3(6.2%) | 8(33.3%) | 0(0.0%) | 11(45.8%) | 3(12.5%) | 0.56 | 0.23 |
| Nausea | 14(29.2%) | 2(4.2%) | 5(20.8%) | 1(4.2%) | 9(37.5%) | 1(4.2%) | 0.34 | 1.00 |
| Hypothyroidism | 16(33.3%) | 0(0.0%) | 12(50.0%) | 0(0.0%) | 4(16.7%) | 0(0.0%) | 0.03 | - |
| AST/ALT increase | 20(41.7%) | 4(8.3%) | 7(29.2%) | 0(0.0%) | 13(54.2%) | 4(16.7%) | 0.14 | 0.11 |
| Others | 9(18.8%) | 5(10.4%) | 3(12.5%) | 2(8.3%) | 6(25.0%) | 3(12.5%) | 0.46 | 1.00 |
AEs: adverse events; ALT: alanine transaminase; AST: aspartate transaminase; 4W-RDI: 4 week relative dose intensity;
*comparison between 4W-RDI≥70% and 4W-RDI<70%.
Discussion
This study has shown the importance of maintaining high dose intensity within the initial 4 weeks of LEN therapy to obtain the radiological best response and longer OS. The RDI has been reported to be associated with favorable treatment response to chemotherapy in various cancers, including the use of sorafenib for renal cell carcinoma [11] and regorafenib for colorectal cancer [13]. In this study, we focused on dose intensity of early phase of LEN therapy and found that patients with 4W-RDI ≥70% had better radiological response and longer OS. Although drug interruption or dose modification is often necessary during TKI therapy, a 4W-RDI ≥70% in lenvatinib may serve as a benchmark for obtaining favorable treatment effects.
The relation between radiographic best response and LEN dose was also shown in this study. Patients with 4W-RDI ≥70% showed superior radiological best response rate, that response according to the mRECIST criteria may be dose-dependent. Given that sub-analysis of a phase 3 trial revealed that patients with radiological response have a better OS [21], this association between the 4W-RDI and radiographic response may explain the superior OS in patients with a 4W-RDI ≥70%. The role of 4W-RDI ≥70% as a prognostic factor was also clearly shown by its association with patients being able to continue LEN therapy for longer. Patients with a 4W-RDI ≥70% also had fewer grade 3/4 AEs, indicating that a high 4W-RDI may reflect better tolerability beyond 4 weeks. Longer durations of LEN therapy are expected to maintain the anti-tumor effects for longer and to contribute to longer OS. Finally, it has been reported that the modified ALBI grade is a useful prognostic factor in lenvatinib therapy [22]. In the present study, we revealed that the 4W-RDI was associated with the ALBI score. The higher dose intensity in patients with favorable ALBI grade may be one mechanism underlining the ALBI grade as a prognostic factor of LEN therapy. Also we revealed that 4W-RDI <70% were associated with lower ALBI score and higher rate of severe AEs. Previous reports showed PK of LEN were affected by liver functions [6, 7, 23]. Patients with 4W-RDI <70% and low liver function may be exposed high blood concentration of LEN and suffered severe AEs. Further study is necessary in this point.
This study has some limitations. This study is single center retrospective study and the number of patients was relatively small. Validation in a larger population may be necessary to confirm these findings in the future.
In conclusion, the RDI ≥70% during the initial 4 weeks of lenvatinib therapy is associated with a favorable radiological response and longer OS. Therefore, adequate management of AEs and encouragement of patients to continue therapy at levels exceeding the RDI of ≥70% in the initial 4 weeks of therapy may improve outcomes.
Supporting information
(DOCX)
(TIF)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The authors' financial disclosure is as follows: NI received a grant-in-aid from the Japan Agency for Medical Research and Development (grant number: JP19fk0210025h0003, URL: https://www.amed.go.jp/). The funder had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. The Lancet. 2018;391(10126):1163–73. [DOI] [PubMed] [Google Scholar]
- 2.Hiraoka A, Kumada T, Kariyama K, Takaguchi K, Itobayashi E, Shimada N, et al. Therapeutic potential of lenvatinib for unresectable hepatocellular carcinoma in clinical practice: Multicenter analysis. Hepatology Research. 2018;49(1):hepr.13243-hepr. [DOI] [PubMed] [Google Scholar]
- 3.Hiraoka A, Kumada T, Kariyama K, Takaguchi K, Atsukawa M, Itobayashi E, et al. Clinical features of lenvatinib for unresectable hepatocellular carcinoma in real-world conditions: Multicenter analysis. Cancer Medicine. 2019;8(1):137–46. 10.1002/cam4.1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kodama K, Kawaoka T, Namba M, Uchikawa S, Ohya K, Morio K, et al. Correlation between Early Tumor Marker Response and Imaging Response in Patients with Advanced Hepatocellular Carcinoma Treated with Lenvatinib. Oncology. 2019:1–7. [DOI] [PubMed] [Google Scholar]
- 5.Hiraoka A, Kumada T, Kariyama K, Takaguchi K, Itobayashi E, Shimada N, et al. Therapeutic potential of lenvatinib for unresectable hepatocellular carcinoma in clinical practice: Multicenter analysis. Hepatol Res. 2019;49(1):111–7. 10.1111/hepr.13243 [DOI] [PubMed] [Google Scholar]
- 6.Ikeda M, Okusaka T, Mitsunaga S, Ueno H, Tamai T, Suzuki T, et al. Safety and Pharmacokinetics of Lenvatinib in Patients with Advanced Hepatocellular Carcinoma. Clinical Cancer Research. 2016;22(6):1385–94. 10.1158/1078-0432.CCR-15-1354 [DOI] [PubMed] [Google Scholar]
- 7.Ikeda K, Kudo M, Kawazoe S, Osaki Y, Ikeda M, Okusaka T, et al. Phase 2 study of lenvatinib in patients with advanced hepatocellular carcinoma. Journal of Gastroenterology. 2017;52(4):512–9. 10.1007/s00535-016-1263-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuzuya T, Ishigami M, Ito T, Ishizu Y, Honda T, Ishikawa T, et al. Clinical characteristics and outcomes of candidates for second-line therapy, including regorafenib and ramucirumab, for advanced hepatocellular carcinoma after sorafenib treatment. Hepatol Res. 2019. [DOI] [PubMed] [Google Scholar]
- 9.Hryniuk W, Bush H. The importance of dose intensity in chemotherapy of metastatic breast cancer. J Clin Oncol. 1984;2(11):1281–8. 10.1200/JCO.1984.2.11.1281 [DOI] [PubMed] [Google Scholar]
- 10.Levin L, Hryniuk WM. Dose intensity analysis of chemotherapy regimens in ovarian carcinoma. Journal of Clinical Oncology. 1987;5(5):756–67. 10.1200/JCO.1987.5.5.756 [DOI] [PubMed] [Google Scholar]
- 11.Kawashima A, Takayama H, Arai Y, Nin M, Kajikawa J, Imazu T, et al. One-month relative dose intensity of not less than 50% predicts favourable progression-free survival in sorafenib therapy for advanced renal cell carcinoma in Japanese patients. European Journal of Cancer. 2011;47:1521–6. 10.1016/j.ejca.2011.04.001 [DOI] [PubMed] [Google Scholar]
- 12.Nakano K, Funauchi Y, Hayakawa K, Tanizawa T, Ae K, Matsumoto S, et al. Relative Dose Intensity of Induction-Phase Pazopanib Treatment of Soft Tissue Sarcoma: Its Relationship with Prognoses of Pazopanib Responders. Journal of Clinical Medicine. 2019;8(1):60-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirano G, Makiyama A, Makiyama C, Esaki T, Oda H, Uchino K, et al. Reduced dose of salvage-line regorafenib monotherapy for metastatic colorectal cancer in Japan. Anticancer Research. 2015;35(1):371–8. [PubMed] [Google Scholar]
- 14.Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–80. 10.1002/hep.29086 [DOI] [PubMed] [Google Scholar]
- 15.Kokudo N, Hasegawa K, Akahane M, Igaki H, Izumi N, Ichida T, et al. Evidence-based Clinical Practice Guidelines for Hepatocellular Carcinoma: The Japan Society of Hepatology 2013 update (3rd JSH-HCC Guidelines). Hepatology Research. 2015;45(2). [DOI] [PubMed] [Google Scholar]
- 16.Lencioni R, Montal R, Torres F, Park J-W, Decaens T, Raoul J-L, et al. Objective response by mRECIST as a predictor and potential surrogate end-point of overall survival in advanced HCC. Journal of Hepatology. 2017;66(6):1166–72. 10.1016/j.jhep.2017.01.012 [DOI] [PubMed] [Google Scholar]
- 17.Kaneko S, Tsuchiya K, Kurosaki M, Kirino S, Inada K, Yamashita K, et al. Three criteria for radiological response on survival in patients with hepatocellular carcinoma treated with lenvatinib. Hepatol Res. 2019. [DOI] [PubMed] [Google Scholar]
- 18.Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2015;33(6):550–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hiraoka A, Michitaka K, Kumada T, Izumi N, Kadoya M, Kokudo N, et al. Validation and Potential of Albumin-Bilirubin Grade and Prognostication in a Nationwide Survey of 46,681 Hepatocellular Carcinoma Patients in Japan: The Need for a More Detailed Evaluation of Hepatic Function. Liver Cancer. 2017;6(4):325–36. 10.1159/000479984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplantation. 2013;48(3):452–8. 10.1038/bmt.2012.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kudo M, Finn RS, Qin S, Han K-H, Ikeda K, Cheng A-L, et al. Analysis of survival and objective response (OR) in patients with hepatocellular carcinoma in a phase III study of lenvatinib (REFLECT). Journal of Clinical Oncology. 2019;37(4_suppl):186-. [Google Scholar]
- 22.Hiraoka A, Kumada T, Atsukawa M, Hirooka M, Tsuji K, Ishikawa T, et al. Prognostic factor of lenvatinib for unresectable hepatocellular carcinoma in real-world conditions—Multicenter analysis. Cancer Medicine. 2019(March):3719–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tamai T, Hayato S, Hojo S, Suzuki T, Okusaka T, Ikeda K, et al. Dose Finding of Lenvatinib in Subjects With Advanced Hepatocellular Carcinoma Based on Population Pharmacokinetic and Exposure–Response Analyses. The Journal of Clinical Pharmacology. 2017;57(9):1138–47. 10.1002/jcph.917 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(TIF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.