Abstract
Preeclampsia is a dangerous hypertensive disorder of pregnancy with known links to negative child health outcomes. Here, we review epidemiological and basic neuroscience work from the past several decades linking prenatal preeclampsia to altered neurodevelopment. This work demonstrates increased rates of neuropsychiatric disorders [e.g., increased autism spectrum disorder, attention deficit hyperactivity disorder (ADHD)] in children of preeclamptic pregnancies, as well as increased rates of cognitive impairments [e.g., decreased intelligence quotient (IQ), academic performance] and neurological disease (e.g., stroke and epilepsy). We also review findings from multiple animal models of preeclampsia. Manipulation of key clinical preeclampsia processes in these models (e.g., placental hypoxia, immune dysfunction, angiogenesis, oxidative stress) causes various disruptions in offspring, including ones in white matter/glia, glucocorticoid receptors, neuroimmune outcomes, cerebrovascular structure, and cognition/behavior. This animal work implicates potentially high-yield targets that may be leveraged in the future for clinical application.
Preeclampsia and Child Health
Preeclampsia is a complex hypertensive disorder of pregnancy, characterized by hypertension (high blood pressure) and proteinuria (elevated urine protein levels). Severe forms of the disease demonstrate significant dysfunction of the liver, kidney, platelets, brain, and lung. Preeclampsia can be deadly and therapy is often limited to preterm induction of labor and antihypertensives [1]. Its growing prevalence and lack of curative measures emphasizes the value of understanding the underlying mechanisms and impacts on offspring. Preeclampsia is linked to increased risk for adverse neonatal health outcomes, including prematurity and low birthweight. As they age, children from preeclamptic pregnancies also have an increased risk for cardiovascular and metabolic disease, as previously reviewed [2].
Multiple poor child neurodevelopmental outcomes are associated with maternal preeclampsia exposure. Increased risk for aberrant brain development, psychopathology, and neurological disease has been found in children prenatally exposed to preeclampsia. In this review, we describe preeclampsia links to clinical neurodevelopmental outcomes in children and how maternal, placental, and fetal mechanisms of preeclampsia are implicated in offspring brain outcomes. Critically reviewing these mechanisms reveals potential novel therapeutic targets and pathways for enhancing offspring resilience during neurodevelopment.
Neuropsychiatric Morbidity
The children of preeclamptic pregnancies face cognitive and psychiatric vulnerabilities (Table 1, Table S1 in the supplemental information online). Data has most frequently demonstrated an increased risk for autism spectrum disorder (referred to as ‘autism’ here) in these children. Autism is diagnosed when symptoms of social impairment and repetitive behaviors arise at an early age; the pathophysiology is poorly understood but may involve multiple neurobiological processes, including altered chromatin modifications affecting offspring neuronal progenitor production and differentiation [3–5]. Interestingly, chromatin modifications also occur in the placenta due to hypoxia, a common component of preeclampsia. Hypoxia decreases global placental histone acetylation and placental acetyl-CoA, sources of acetyl donors in placental trophoblast cells in vitro. Disproportionately high levels of placental genome domains (37%) are enriched for repressive histone modifiers, which are also implicated in synaptic transmission, neurodevelopmental processes, and autism [6]. Increased methylation of oncogenes and decreased repressive histone modifier (h3k9me3 and h3k27me3) levels at the telomerase promoter also occur in human preeclamptic placenta [7]. Future work is needed to identify causal impacts of these placental epigenetic modifications on offspring neuropsychiatric outcomes.
Table 1.
Summary of Clinical Studies of Brain Outcomes Associated with Prenatal Preeclampsia Exposurea
| Category | Relevant findings in offspring | Refs | |
|---|---|---|---|
| Neuropsychiatric outcomes | Autism | ↑ Autism and autism symptoms with PEb and ↑ PE severity, ↑ PE with autism | [8–14] |
| ADHD | ↑ ADHD +/– stimulant treatment with PE | [8,17,18] | |
| Anxiety and mood disorders | ↑ Anxiety and depressive disorders and symptoms +/– autonomic dysfunction with PE or hypertensive pregnancy (females N males) | [19,27,28,30] | |
| Schizophrenia | ↑ Schizophrenia, psychotic disorders with PE or hypertensive pregnancy | [27,29,30,36] | |
| Emotional dysregulation | ↑ Difficult temperament, ↓ or no change in internalizing problems with PE | [20,38,39] | |
| Eating disorders | No change in eating disorder incidence with PE | [14] | |
| Cognitive outcomes | ↓ Or no change in IQ, ↓ development, ↓ working memory, ↓ verbal ability, ↓ math scores, ↑ memory problems with PE or hypertensive pregnancy | [30,39,42–51] | |
| Neurological outcomes | Cerebral palsy | ↑ Cerebral palsy (overall, not subtype-specific) with PE | [14,58,59] |
| Stroke | ↑ Stroke with PE | [56] | |
| Other | ↑ Epilepsy, infantile spasms, sleep apnea, or no motor/neurological problems with PE | [14,39] | |
| Brain abnormalities | Volume | ↑ Cerebellum, temporal lobe, brain stem, amygdala, caudate nucleus, and superior longitudinal fasciculus volume with PE | [16,63] |
| Connectivity | ↑ Fractional anisotropy in caudate; ↑ parallel diffusivity in cingulate; ↑ functional connectivity between amygdalae and frontal poles and medial prefrontal cortex and precuneus; ↓ functional connectivity between medial prefrontal cortex and left occipital fusiform with PE | [63,64] | |
| Vasculature | ↓ Occipital and parietal lobe cerebral vessel radii with PE | [16] | |
See Table S1 in the supplemental information online for details of each study.
Abbreviation: PE, preeclampsia.
A pooled odds ratio estimate (across studies that did and did not control for gestational age and birthweight) of 1.50 [8] or 32% greater risk for autism has been reported in children prenatally exposed to preeclampsia [9]. Increased offspring autism risk after preeclampsia exposure remains even when controlling for birthweight, which is itself negatively associated with both preeclampsia and autism [10]. Commensurately, women with preeclampsia have more than twice the risk of having a child diagnosed with autism (odds ratio: 2.36, not adjusted for birthweight or gestational age) [11]. Autism symptoms (repetitive behaviors and communication deficits) are also more severe in children of preeclamptic pregnancies when gestational age, birthweight, and maternal/gestational health factors are accounted for [12]. Furthermore, disruptions related to autism risk, including theory of mind and sociability, are negatively associated with maternal hypertension during pregnancy in 3- and 4-year-old children after covarying for gestational age and birthweight [13]. These effects may not determine medical risks, however, since one large retrospective study shows no increase in pediatric autism-related hospitalizations due to prenatal preeclampsia exposure [14].
Epidemiological data also demonstrate increased attention-deficit/hyperactivity disorder (ADHD) risk among children of preeclamptic pregnancies. ADHD is diagnosed due to symptoms of inattention, impulsivity, and high activity before 12 years of age. Neurobiologically, ADHD often features early reductions in neocortical and striatal volumes [15], a parallel to the reduced overall growth found in children exposed to preeclampsia [16]. A meta-analysis of nine studies (some controlled for birthweight and gestational age) reveals an odds ratio of 1.31 for childhood ADHD in children prenatally exposed to preeclampsia [8]. This increased odds is found prospectively, when preeclampsia is recorded at birth and ADHD is assessed repeatedly across the first 7–10 years of childhood, even after adjusting for potential contributions by other maternal health factors (e.g., age, prepregnancy body mass index, diabetes status, parity, depression, and smoking and alcohol use), child sex, and gestational age [17]. Girls typically experience lower rates of ADHD but may have increased risk in the context of prenatal preeclampsia exposure [18]. Further analyses of risks to boys and girls is required to better understand these differential impacts.
Notably, psychiatric disruptions after prenatal preeclampsia exposure are not particular to a single age or developmental period, but rather persist across development. How these disruptions present at a particular age, however, may differ. For instance, anxiety and mood disorders, characterized by physical, cognitive, and emotional symptoms that often reflect stress sensitivity, are increased among adults prenatally exposed to preeclampsia and born full term (adjusted for birthweight) [19]. Difficult infant temperament (odds ratio: 2.17), which may capture developmental antecedents of anxiety and mood disorders, is increased (adjusted for maternal age, parity, family income, birthweight, and gestational age) among children of preeclamptic pregnancies [20].
From a neurobiological standpoint, anxiety and mood disorders involve disruptions to multiple systems, including serotonergic ones, potentially during development. For instance, hippocampal and cortical serotonin1A receptor (5-HT1AR) expression during development, but not in adults, rescues anxiety-like behaviors in 5-HT1AR knockout animals [21], while the constitutive loss of Pet1 serotonin-expressing cells leads to anxiety-like behavior [22]. Maternal serotonin sources (including platelets) are critical to normal development of the offspring brain, though the placenta is the primary source of serotonin for the early fetal brain [23]. Disruptions to placental sources and metabolism of serotonin occur in preeclampsia and have functional consequences for placental vascular health and fetal growth [24,25]. Serotonin receptor activation regulates cellular proliferation, migration, and differentiation [26], but it is unclear how placental sources of serotonin specifically modulate developmental processes of neurons and how this may change in preeclampsia.
Adult children from preeclamptic pregnancies have higher depression symptom scores, even after controlling for age, birthweight, gestational age, and other familial factors [19]. Adult offspring born to mothers who experienced a condition similar to preeclampsia, gestational hypertension without proteinuria, also have 1.44- and 1.39-fold increased rates of severe mood disorder and several anxiety disorders, respectively. Commensurately, preeclampsia is protective against affective disorders in older men [27]. Furthermore, girls born from preeclamptic or growth-restricted pregnancies are more vulnerable to comorbid major depressive disorder and cardiac parasympathetic dysregulation [28]. These results indicate differential vulnerability for affective disorders in male and female offspring, though relevant studies are sparse and further work is necessary to determine developmental and disorder-specific impacts of exposure.
Risk for schizophrenia is also increased among adults born from preeclamptic pregnancies (1.3 adjusted odds ratio, increased to 2.0 among preterm births) [29], as are psychotic symptoms (3.82 adjusted odds ratio from analysis controlled for gestational age and birthweight) [30]. Schizophrenia typically onsets in young adulthood with psychotic and disorganized symptoms. Altered neuroimmune interactions during development and subsequent synaptic plasticity deficits may underlie schizophrenia risk [31,32]. For instance, umbilical and maternal serum levels of tumor necrosis factor (TNF)-α are increased in preeclampsia [33]. TNF-α has known impacts on neuron-microglia crosstalk and glial regulation of synaptic processes relevant to schizophrenia pathophysiology [32,34]. Disrupted TNF-α signaling pathways have also been directly implicated in schizophrenia risk by genome-wide association studies (GWAS) [35].
Schizophrenia-related findings are mixed, however, potentially due to differences in preeclampsia or schizophrenia definitions across studies. For instance, a Danish cohort shows no increased rates of preeclampsia but increased rates of gestational hypertension and diuretic treatment in mothers of individuals who later develop schizophrenia [36]. Assessments of the 1934–1944 Helsinki Birth Cohort revealed that the hypertensive but not the proteinuria component of preeclampsia predicts offspring mental health [27]. This indicates that clinical components of preeclampsia, which differ between mothers and across gestation, may differentially confer offspring risk. It is conceivable that varied molecular subtypes of preeclampsia (placental transcriptome clusters indicate immune dysregulation, protein catabolism, and poor oxygenation and hormone dysregulation subtypes [37]) pose varying pathological risk to developing brain.
Broader behavioral emotional symptom incidences have mixed sensitivities to prenatal preeclampsia exposure. Internalizing and externalizing behaviors are higher in children at ages 8–14 years only after gestational hypertension but not preeclampsia [38]. These behaviors in children with early, managed prenatal preeclampsia exposure are no different than in the general population [39]. Interestingly, preeclampsia severity (cases defined as no preeclampsia, mild preeclampsia, severe preeclampsia, and eclampsia) and offspring neuropsychiatric morbidity (defined as hospitalizations due to neuropsychiatric illness) [14] are positively correlated.
Psychiatric outcomes are broadly linked with preeclampsia exposure. These links may occur through exposure of the developing brain to specific physiological factors or more complex genetic links of psychiatric risk genes and the dysfunction of these same genes in preeclampsia. For instance, some genes which are dysregulated in preeclampsia models and more broadly (EBF3, SEMA3F) are also associated with autism [40]. The contribution of preeclampsia genetics and gene-environment interaction to the neurobiological underpinnings of these disorders has not been examined and is a critical direction for future work.
Cognitive Dysfunction
One of the most highlighted domains of dysfunction in children of preeclamptic mothers is cognitive functioning (Table 1, Table S1). Cognitive and intellectual disabilities often begin early in life and persist throughout adulthood. The preeclampsia literature has likewise demonstrated that impacts to cognition are persistent. In growth-restricted 3-year-old children, lower IQ (average 11 points lower) or lower developmental index scores occur particularly when gestation is complicated by preeclampsia [41], while preeclampsia independently predicts cognitive impairments in preterm 3-year-olds [42]. Further, children from severe but not mild preeclamptic pregnancies do not reach, on average, appropriate cognitive milestones at 3 years of age [43]. Eleven-year-old children prenatally exposed to maternal gestational hypertension have a 2.4-fold increase in mild cognitive limitations, regardless of birthweight [44,45]. Males born prematurely and growth-restricted also have lower IQs at 5–8 years of age if also exposed to preeclampsia [46]. This association has not been universal, however, with another study reporting normal IQ in adolescents exposed to prenatal preeclampsia [47], though differences in child age at assessment likely impact results.
Specific domains of cognition are also disrupted by prenatal preeclampsia exposure. Children (7–10 years old) exposed to prenatal preeclampsia have working memory and oculomotor impairments indicative of deficient executive control (not controlled for birthweight or length of gestation) [48]. Ten-year-old children of preeclamptic pregnancies exhibit deficits in verbal ability but not nonverbal reasoning; these were not significantly associated with birthweight or gestational age [49]. Unsurprisingly, cognitive disruptions have functional consequences for affected children. For instance, school-aged children (9–15 years old) prenatally exposed to preeclampsia or eclampsia perform worse on mathematics exams after controlling for significant confounds (e.g., gestational age, small for gestational age status) [50].
Changes in cognitive abilities after prenatal preeclampsia exposure are likely lasting. Men in the 1978–1983 Danish Medical Birth Registry have reduced cognitive functioning if exposed prenatally to gestational hypertensive disorders, including preeclampsia and eclampsia (controlling for gestational age, birthweight, and parity). Hypertensive disorders in term and preterm pregnancy (controlled for birthweight) predict greater cognitive decline and lower total cognitive abilities, particularly related to mathematics, for men in late adulthood (68 years of age) [51]. Symptomatically, memory impairments are reported by adults with prenatal preeclampsia exposure after controlling for gestational age and birthweight [30]. Neurobiological vulnerability after many years indicates the durability of preeclampsia exposure impacts on the neurocircuitry underlying cognitive functioning.
New insights demonstrate that cognitive disability, even due to genetic syndromes, may involve fetal brain inflammation, which also occurs in modeled preeclampsia [52], as we discuss later (see ‘Inflammation’ section). For instance, in Ts1Cje Down syndrome mouse models, inflammation pathways (i.e., reactome-apoptosis, reactome-cell cycle, and JAK/STAT cascade) are transcriptionally disrupted in both embryonic and adult brain [53]. Maternal immune dysregulation in pregnancy may also underlie cognitive dysfunction in children. More specifically, elevated maternal plasma interleukin (IL)-6, frequently increased in preeclampsia [54], predicts working memory at 2 years old (a core component of executive functioning) and working memory-related brain network connectivity at approximately 4 weeks old [55]. Prenatal inflammatory disruptions (both fetal and maternal) may be a shared pathobiological mechanism for preeclampsia and offspring cognitive development.
Neurological Injury and Disease
Neurological problems in children born to mothers with preeclampsia include a wide range of pathologies, from cerebrovascular-related to those underlying seizure risk and motor dysfunction (Tables 1 and S1). Prenatal preeclampsia increased the hazard ratio (1.9) for all forms of stroke at any age, irrespective of decreased gestational age or birthweight [56]. This increased risk of stroke may result from abnormal angiogenic processes [57], neurovasculature [16], and systemic blood pressure [56] in children exposed to prenatal preeclampsia and severe preeclampsia, respectively.
Recent work has shown that epilepsy and infantile spasms, a particular seizure disorder, are increased in children of preeclamptic mothers (controlled for gestational age but not birthweight) [14]. Prenatal exposure to preeclampsia also increases risk for cerebral palsy, interacting with gestational age and birthweight [58]. Risk for cerebral palsy is increased by nearly twofold (odds ratio: 1.94) in children of preeclamptic women, though only for preeclampsia diagnosed prior to 37 weeks of gestation, indicating that more severe preeclampsia may drive this effect [59]. However, when properly managed, preeclampsia does not lead to increased rates of childhood motor or neurological problems [39].
Epidemiological findings on psychiatric illness, cognitive functioning, and neurological disease suggest early and persistent effects of prenatal preeclampsia on brain function. However, few clinical studies have examined the relationship between neurobiological outcomes in offspring and the prenatal neurodevelopmental, placental, and maternal processes disrupted by preeclampsia. Future research on these outcomes may inform clinical treatments for preeclampsia, which are limited and have not been examined for their role in mitigating neurodevelopmental outcomes. However, new and increasing awareness of the early underpinnings of brain functioning and interactions with maternal factors, which we detail later, as well as improved modeling of preeclampsia in animals, suggests that additional insights are coming.
Brain Volume, Connectivity, and Growth Abnormalities
In addition to altered behavior, psychiatric, and neurological risk in the children of preeclamptic pregnancies, structural brain changes have also been revealed by neuroimaging. In 7–10-year-old children of mothers with and without preeclampsia (n = 10 each), multiple brain regions (the cerebellum, temporal lobe, brainstem, and bilateral amygdalae) are enlarged relative to total intracranial volume in preeclampsia-exposed children. This occurs despite no differences in total intracranial volume or head circumference between groups but reduced birth weight and trend-wise reduced gestational age and abdominal girth among children of preeclamptics [16]. Of note, serum taken from women with preeclampsia increases neuronal growth and branching in rat fetal cortical neurons in vitro [60]. These findings suggest that maternal serum-secreted factors may play a role in enlargement of some brain regions via neuronal growth mechanisms.
Despite some evidence for relative brain enlargements in this small cohort, the brain/head growth impacts of preeclampsia exposure in larger populations have been mixed. In a study of the Helsinki Birth Cohort, severe preeclampsia predicts reduced offspring normalized head circumference relative to body length at birth, while gestational hypertension predicts increased length-normalized head circumference [56]. Animal models of preeclampsia exhibit both offspring brain-sparing (asymmetrical, e.g., with infection [61]) and nonsparing (symmetrical, e.g., with impaired placental blood flow [62]) growth restriction. Brain and/or head size findings may diverge according to maternal disease severity, with more severe disease restricting growth and less severe disease either not impacting or promoting growth.
In addition to gray matter changes, tractographic white matter alterations have also been noted with brain disruptions after prenatal preeclampsia exposure. Specifically, superior longitudinal fasciculus volume is increased in children of preeclamptic pregnancies [63]. While from a limited cohort and only from a single point in development, these data suggest broad neuroanatomical impacts, particularly to later-developing structures such as white matter tracts and the cerebellum. These neuroanatomical changes are accompanied by increased functional connectivity (via resting state functional magnetic resonance imaging) between the amygdalae and frontal poles and between the medial prefrontal cortex (mPFC) and precuneus, but decreased connectivity between the mPFC and left occipital fusiform gyrus [64]. Collectively, alterations to these areas and their connectivity indicate impairments to social brain structures (mPFC, precuneus) and those involved in threat detection and appraisal (amygdala), as well as goal-oriented behavior (frontal pole) [65,66].
Given the known impacts of preeclampsia on maternal vascular function, it is notable that prenatal preeclampsia exposure also decreases offspring parietal and occipital cerebral vessel radii [16]. These changes are associated with decreased placental growth factor (PlGF) levels in maternal plasma, a potential diagnostic marker for preeclampsia. Decreased PlGF may drive angiogenic and microvascular deficits in offspring (reviewed elsewhere [57]), which are also linked to autism [67]. However, no longitudinal studies have yet examined blood–brain barrier or cerebrovasculature changes over time with prenatal preeclampsia exposure, nor the associations between these changes and concurrent angiogenic factor disruptions.
All imaging data evidencing brain abnormalities in children after preeclampsia are derived from the same small cohort of children, a cohort that differs from age-matched controls in birth outcomes aside from preeclampsia (trend lower gestational age and significantly lower birthweight, maternal age unchanged between groups). This highlights the need for additional studies with greater numbers of children to more rigorously determine associations. Alternate clinical and translational study methods can also add to a deeper understanding of the links between preeclampsia and altered neurodevelopment and which maternal, placental, and fetal brain molecular and cellular changes during preeclampsia are most closely related to larger-scale changes. The same questions can be asked in the context of preeclampsia’s association with clinical outcomes, as we reviewed in the preceding sections.
Animal Models and Mechanisms
While preeclampsia in clinical populations is complex, animal models of preeclampsia may be leveraged to determine specific underlying mechanisms. Multiple such models exist, including reduced uterine perfusion pressure (RUPP) [68]; angiogenic and growth factor mutants [61,69,70]; the Nω-nitro-L-arginine methyl ester (L-NAME) mutant [62]; and the arginine vasopressin (AVP) infusion model [71] (Table 2, Table S2 in the supplemental information online). These mammalian models are characterized by hypoxia/placental ischemia, oxidative stress, angiogenic and growth factor changes, inflammation, and interactions between these mechanisms (Figure 1), which we detail later.
Table 2.
Summary of Animal Models for the Study of Preeclampsia and Associated Offspring Phenotypea
| Model | General findings | Offspring neurobiology | Refs |
|---|---|---|---|
| AVP infusion in mice | Dams: gestation-specific hypertension, renal dysfunction, proteinuria; ↑ interferon (IFN)g, IL-17; ↓ IL-4, IL-10 Placentas: ↓ PlGF, altered morphology, blood flow, oxidative stress, and immune dysregulation; ↑ IL-17 Fetuses: growth restriction; ↑ IL-17; ↓ IL-4, IL-10 |
n/a | [40,89] |
| IL-17 manipulation in rats | Dams: hypertension, oxidative stress; ↑ IL-6, TNFα, IL-17 Placentas: oxidative stress |
n/a | [96,97] |
| L-NAME treatment in rats | Dams: hypertension, renal dysfunction, proteinuria, oxidative stress; angiogenic dysfunction; stillbirths Placentas: inflammation, structural changes Fetuses: growth restriction |
↓ Neonatal brain weight, neurogenesis; ↑ adult gliogenesis; ↓ learning, memory, hippocampal neurogenesis and glucocorticoid receptor expression; neonatal and adult brain ↑ microglial protein, and ↓ glial glutamate transporter protein | [62b,99,100b,102b,117] |
| PlGF deficiency in mice | Impaired adult angiogenesis and revascularization, abnormal vascular permeability/growth, and inflammation | Impaired memory, locomotor, anxiety- and depressive-like behavior; ↓ regional brain volumes; cerebral blood vessel changes | [69b,70b,105b,106] |
| RUPP in rats | Dams: hypertension, ↑ sFlt-1, renal dysfunction, proteinuria, oxidative stress; ↑ cortical water; neuroinflammation; altered astrocyte, microglia numbers; ↓ litter size Placentas: hypoxia, inflammation, oxidative stress Fetuses: ↑ hematocrit |
↓ fetal brain microglia, ↑ cerebral micro-bleeds, neuroinflammation; cortical thickness unchanged | [52b,76,77,91,114–116,118] |
| Angiogenic dysregulation and systemic inflammation in mice | Dams: hypertension and albuminuria Fetuses: growth restriction; altered metabolism |
n/a | [61] |
| Impaired umbilical circulation in sheep | Fetuses: ↑ hypoxemia | In fetal cortex: gliosis, myelination, neuronal process defects; white matter lesions; abnormal cerebrovasculature; ↓ Purkinje neurons | [78] |
See Table S2 in the supplemental information online for details of each study.
Reference contains neurobiological offspring outcomes.
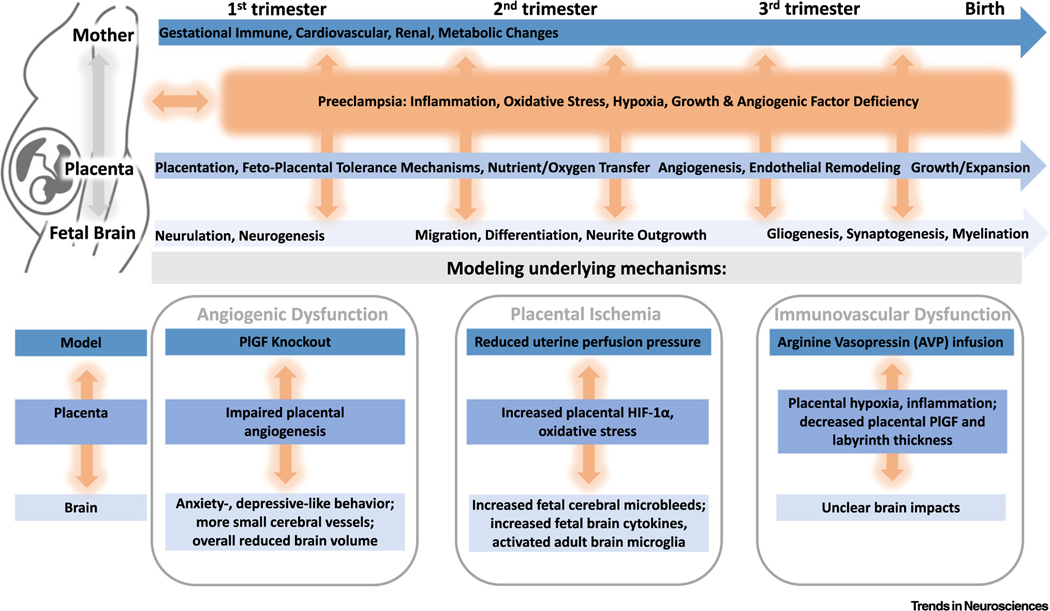
Figure 1. Interactions of Preeclampsia Mechanisms with Maternal and Placental Physiology and Implications for Key Neurodevelopmental Processes.
Gestational disruptions (including immune, cardiovascular, renal, and metabolic) across all three trimesters influence placental processes (placentation, immune tolerance, nutrient and oxygen transfer, endothelial remodeling, and growth), which may also alter neurodevelopmental processes that proceed in turn (neurulation and neurogenesis followed by neuronal migration, differentiation, and neurite outgrown, and finally gliogenesis, synaptogenesis, and myelination). The interactions between pregnancy physiology, placental processes, and neurodevelopment are likely impacted at all levels by preeclampsia mechanisms of disruption (inflammation, oxidative stress, hypoxia, abnormal growth, and angiogenic factor levels). These disruptions may be modeled preclinically, for example, in the placental growth factor (PlGF) knockout, reduced uterine perfusion pressure, and arginine vasopressin infusion mouse models, which model preeclamptic angiogenic dysfunction, placental ischemia, and immunovascular dysfunction, respectively. Each of these preclinical models has impacts on the placenta and offspring neurodevelopment (e.g., impaired angiogenesis and cerebrovascular disruptions, respectively, in the PlGF knockout). Abbreviation: HIF-1α, hypoxia-inducible factor 1α.
Mammalian models allow for critical mechanistic studies of placenta-mediated pathologies, such as preeclampsia [72]. The placenta is a critical mediator of inflammatory and oxidative stress cascades extending to the fetal compartment. For instance, maternal immune activation influences the outgrowth of serotonin axons in fetal forebrain, effects mediated by increased placental tryptophan conversion to serotonin [73]. The placenta also serves as a substrate for sex-specific impacts on neurodevelopment. In one notable example, early prenatal stress sex-specifically reduces placental expression of O-GlcNAc transferase, an x-linked gene, leading to hypothalamic–pituitary–adrenal stress axis dysregulation in male offspring [74].
Placental development processes, including implantation, vascular remodeling, and morphogenesis, as well as efficient nutrient transfer/oxygenation, are all impacted by preeclampsia [75]. When overlaid onto the metabolically demanding processes of prenatal neurodevelopment, it becomes clear that deficits in placental growth and/or function may have lasting impacts on the developing brain (Figure 1). Further study is required, however, to determine how these deficits vary in their impact across the phases of pregnancy and neurodevelopment.
Placental Ischemia
Placental ischemia is recapitulated in the RUPP model by clipping the uterine arteries and abdominal aorta to reduce blood flow in a pregnant mouse or rat. This results in fetal growth restriction, maternal hypertension, and renal pathology [68], key preeclampsia phenotypes. This model also directly causes placental hypoxia, with in vivo imaging revealing 12% decreased placental oxygen saturation in late gestation while hypoxia-inducible factor 1α is upregulated [76].
Findings in the RUPP model echo clinical cerebrovascular findings in children of preeclamptic pregnancies [16] and further implicate angiogenic dysfunction as a mechanism for preeclampsia-mediated disruption of neurodevelopment. Cerebral micro-bleeds, a form of micro-hemorrhages, in the cortical parenchyma and ventricles of late-gestation rat offspring are increased (in anterior cortex by nearly double and in posterior cortex by more than double) in this model [52]. RUPP also results in changed postpartum cortical astrocyte density in dams, though protein levels of glial fibrillary acidic protein (GFAP) are unchanged and offspring glial and brain changes more broadly remain unexplored [77]. Impaired umbilical circulation and placental hypoxemia in other models cause a number of white matter disruptions in offspring, including lesions, cortical gliosis, and reduced subcortical myelination, as well as dysregulated neuronal process and cerebral vessel formation [78]. Important parallels exist here with the white matter and glial disruptions that occur in psychiatric, cognitive, and neurological disorders in humans [79] and which could be prime therapeutic targets.
Multiple mechanisms are implicated in white matter vulnerability to hypoxia. Under hypoxic conditions like those of preeclampsia, undifferentiated oligodendrocytes downregulate production of critical proteolipids and glycoproteins, accumulate cytotoxic levels of iron, and generate reactive oxygen species (ROS), all responses that ultimately impair myelination [80]. Oligodendrocyte precursors and myelin basic protein (MBP) synthesis are particularly susceptible to hypoxia-induced ROS, which can cause global hypomyelination and synaptic loss. In fact, synaptic and behavioral deficits following hypoxia are mediated by hypomyelination mechanisms [81].
Inflammation
Proinflammatory dysregulation occurs in clinical preeclampsia [82–84] and in a number of animal models of preeclampsia. The RUPP model results in increased fetal brain levels of IL-1β, IL-6, and IL-18, eotaxin (CCL11), LIX (CXCL5), and MIP-2 (CXCL2) [52]. Microglia, the immune cells of the brain, decrease, however, in a proliferative region of the fetal RUPP brain [52], though microglia exhibit more activated states in postnatal RUPP cortex [77]. Maternal immune activation may be necessary for these impacts since maternal hypertension does not occur in athymic nude RUPP rats lacking T cells [85]; similarly, normal regulatory T cells or anti-inflammatory IL-10 or IL-4 in maternal circulation block key components of RUPP pathophysiology [86–88].
The AVP infusion model of preeclampsia also involves proinflammatory changes across maternal, fetal, and placental tissues [40,89]. More specifically, chronic gestational AVP leads to increased maternal and fetal IL-17 and decreased maternal and fetal IL-4 and IL-10. The neurodevelopmental impacts of these immune perturbations in the context of the AVP model remain unclear. However, dysregulation of brain cytokines and microglia can have long-term impacts, as microglia are critical regulators of neural progenitor populations [90]. Brain proinflammation in modeled preeclampsia may also occur secondary to placental inflammation [91], though this requires further targeted study. This proinflammatory AVP model may also highlight other mechanisms at work in human preeclampsia. Elevating AVP to model preeclampsia has good construct validity, dysregulating the osmotic and volemic mechanisms regulated by AVP [40,92], which may also have independent impacts on offspring brain development. Hyponatremia and low extracellular osmolarity can impair neuronal differentiation, activate cell stress and death responses [93], increase neuronal excitability [94], and may have additional adverse effects when combined with inflammation.
A point of convergence is IL-17, which is increased in the AVP [89] and RUPP models [95] and in clinical preeclampsia [83]. Maternal IL-17 infusion in pregnant rats replicates multiple pathological features of preeclampsia, including maternal hypertension and maternal and placental oxidative stress [96]. T helper (Th)17 cells taken from RUPP dams induce intrauterine growth restriction, hypertension, and oxidative stress in control dams, demonstrating the centrality of IL-17 mechanisms [97]. Maternal immune activation drives autism-like deficits (e.g., embryonic cortical dysplasia and laminar organization defects, autism-relevant behavior changes) in mice via IL-17dependent mechanisms [98], suggesting that IL-17 dysregulation in preeclampsia may underlie this risk in clinical populations.
Maternal IL-17 also increases with L-NAME, as does MCP-1, a key monocyte-regulating chemokine [99]. Maternal L-NAME, a nitric oxide synthase inhibitor, causes intrauterine growth restriction, proteinuria, inflammation, and hypertension and is thus administered to animals to model preeclampsia [99]. L-NAME administration increases pre- and postnatal microglial activation in offspring [100], with increased numbers of rod-shaped and large amoeboid-like microglial in the cortex, indicating increased reactivity [101]. Prenatal maternal manipulation of L-NAME and thus inflammatory processes may lead to offspring cognitive deficits, as demonstrated by longer escape latencies on the Morris water maze test. This was further associated with increased glucocorticoid receptor expression in L-NAME offspring hippocampus [102], which may indicate hypothalamic–pituitary–adrenal axis hyperexcitability and increased stress reactivity. Dysregulation of these processes creates vulnerability for later psychiatric and cognitive pathology, including anxiety, mood, and learning disorders [103].
Angiogenic and Growth Factors
PlGF is a member of the vascular endothelial growth factor (VEGF) family that plays critical roles in angiogenesis and placental trophoblast growth and differentiation and is decreased in clinical preeclampsia starting in the first trimester [104]. Recombinant PlGF can reverse hypertension and renal pathology but not intrauterine growth restriction [69] in pregnant PlGF constitutive knockout mice (PlGF−/−), a preeclampsia mouse model. Angiogenesis is subtly, if at all, impaired in early gestation PlGF−/− embryos and placenta, perhaps due to compensatory increases in VEGF. Placenta-specific knockdown of PlGF leads to cerebrovascular deficits in late-gestation embryonic offspring, including decreased VEGF receptor 1 (R1) protein levels and radial distribution of cortical microvessels, while overexpression rescues offspring cerebrovascular deficits following in utero alcohol exposure [105].
Postnatal repair processes in brain conditions of ischemia, inflammation, traumatic injury, and cancer are impaired in PlGF−/– mice [106]. This implicates PlGF in adult angiogenic and other processes that may directly impact mature brain structure and function; preeclampsia effects on fetal brain may also involve similar PlGF-dependent processes. In adult rats, PlGF is detected primarily in cortical interstitium and microvessels during hypoxia. PlGF blunts primary mature cortical neuron death in an in vitro oxygen- and glucose-deprived model [107], likely via a cell-autonomous rescue of mitochondrial activity deficits in these neurons. Adult PlGF−/− mice also have increased small-diameter cerebrovasculature; smaller whole brain volumes [70]; and altered locomotor, anxiety-like, and depressive-like behaviors (but unchanged spatial learning), which are partially rescued by postnatal PlGF supplementation [69]. Notably, clinical preeclampsia leads to increased volume in specific brain regions [16], rather than decreased whole brain volume as reported in PlGF−/−mice. As discussed earlier (‘Brain Volume, Connectivity, and Growth Abnormalities’ section), these differences in brain size are likely due to diverging mechanisms and severities of maternal disease. Further work is needed to determine impacts on particular brain regions and circuits in PlGF–/– animals, as in children of preeclamptic pregnancies [16].
The developmental and pathological roles of PlGF may also be mediated by immune mechanisms. PlGF stimulates macrophage proliferation and the release of angiogenic factors [108] and binds VEGF-R1 on monocytes to activate calcineurin-dependent production of TNF-α and IL-6 [109]. PlGF-overexpressing mice have increased IL-6, IL-17, and TNF-α; their CD4TA+ T cells preferentially differentiate into Th1 and Th17 cells [110]. In fact, normal bone marrow transplantation into PlGF−/− mice rescues components of angiogenic repair deficiencies, indicating that immune cells mediate these effects [106].
Given these findings, maternal PlGF is a promising potential early treatment target and/or biomarker for offspring neurodevelopmental risk.
Oxidative Stress
Placental and maternal endothelial dysfunction characteristic of preeclampsia may be the result of dysregulation in redox processes or oxidative stress [111]. Closely related heat shock mechanisms have also been implicated in preeclampsia and other prenatal insults [112]. Placental ROS and antioxidants are dysregulated in preeclampsia [113]. Multiple preeclampsia model phenotypes are also mediated by placental oxidative stress mechanisms. For instance, placental levels of 8-isoprostane and malondialdehyde are increased in RUPP rats, while the broad antioxidant tempol [114] or the mitochondrial-specific antioxidant MitoQ [115] attenuate hypertension in this model. Placental superoxide dismutase and glutathione peroxidase, endogenous antioxidants, are also decreased in the RUPP model [116]. Similarly, placenta transcriptomics in the AVP model of preeclampsia reveal altered oxidative stress pathways [40]. L-NAME-induced phenotypes, including placental proinflammation, maternal hypertension, and placental pathology, are prevented by maternal treatment with the antioxidant astaxanthin, implicating oxidative stress mechanisms in these endophenotypes [117]. PlGF infusion also decreases oxidative stress systemically and rescues the hypertension and increased plasma soluble fms-like tyrosine kinase-1 in the RUPP model, therefore blunting RUPP model angiogenic dysfunction and other phenotypes [118].
Oxidative stress mechanisms in the placenta likely play critical roles in clinical preeclampsia. Incomplete placentation and hypoxia due to spiral artery insufficiency and extravillous trophoblast cell accumulation causes increased endothelial free radical production in preeclampsia. These free radicals act as secondary messengers to alter transcriptomic and inflammatory processes [111]. Redox dysregulation, due to unbalanced reductive and oxidative metabolic molecules, in placenta and brain, has been implicated in key prenatal and postnatal developmental processes in neurons, glia, and their progenitors. For example, redox impairs migration of cortical inhibitory interneurons, potentially via transcriptional and mitochondrial function processes [119]. Reduced extracellular conditions promote progenitor proliferation, while oxidation promotes differentiation and, at extreme levels, cell death [120]. Cellular fate determination may be related to ROS levels, which are high in neuronal progenitors and low in glial progenitors [121]. Oxidative stress occurs normally during neuronal migration and intracellular repair mechanisms are necessary to maintain cell survival. Potential changes in oxidative stress from preeclampsia could interact with these processes. Additionally, redox abnormalities due to lower levels of the antioxidant glutathione impair oligodendrocyte proliferation and maturation, implicated in white matter changes in schizophrenia. Mechanisms by which preeclampsia may influence redox processes in developing neurons include ischemia, reduced nutrient supply essential for reducing enzymes, and epigenetic modifications of redox pathways [112]. Further work is needed to determine whether placenta and/or brain oxidative stress is responsible for the neurodevelopmental impacts of clinical preeclampsia.
Other Considerations and Future Perspectives
Studies on the impacts of preeclampsia on neurodevelopmental outcomes have the clear limitation of potential interactions between preeclampsia and prematurity, and intrauterine growth restriction, which are all associated with neurodevelopmental defects [122]. Maternal anxiety and depression, themselves significant risk factors for offspring psychopathology [123], are also potential confounds due to their association with an increased risk of preeclampsia [124].
Shared maternal–offspring genetics may also play a role. For instance, maternal variants in or near the RGS2, CTLA4, SERPINE1, PLEKHG1, and other genes involved in cardiovascular, cell signaling, metabolic, immune, and neurological functioning have been associated with preeclampsia [82,125]. Maternal polymorphisms in RGS2, which encodes a modulator of signaling via G protein-coupled receptors, are implicated in both preeclampsia and severity of baseline symptoms in schizophrenia (per the Positive and Negative Symptoms Scale) [126,127]. Furthermore, the SERPINE1 gene product PAI-1 regulates neurite outgrowth in neuronal-astrocyte cocultures via the extracellular matrix proteins fibronectin and laminin [128], indicating a role for mutations in this preeclampsia-related gene in neurodevelopmental processes. These gene variants may also have impacts in mothers, placenta, and offspring brain without invoking environmental mechanisms, though further work (e.g., trio analyses) is required to identify these variants and their precise roles.
Future work is also needed to determine sex-specific mechanisms. Very few studies specifically evaluate the critical role of offspring sex for preeclampsia-associated neurodevelopmental risk. Increased early-life neurodevelopmental vulnerability has been suggested in males [129], and male-specific vulnerability to preeclampsia appears in animal work [130] and clinical populations [27]. Effects are not uniform, however, as females may be more vulnerable to cardiovascular impacts [28]. Fortunately, the role of sex as a biological variable in the outcomes of preeclampsia-affected births is an active area of investigation. Similarly, temporal impacts of preeclampsia and the existence of particular neurodevelopmental periods of increased vulnerability to preeclampsia require future investigation (see Outstanding Questions).
Outstanding Questions.
Preeclampsia is associated with known genetic variants in women. Do these variants also mediate offspring neurodevelopmental risk, such that there is a role for shared maternal and offspring genetic risk in preeclampsia and neurodevelopmental disorders?
How do the multiple preeclampsia subtypes and severities differentially impact offspring neurodevelopment?
Are there differences in the vulnerabilities of male and female offspring of preeclamptic pregnancies to neurological and psychiatric disease? And if so, what mechanisms mediate these sex-specific differences?
Do the mechanisms by which preeclampsia may alter neurodevelopment (e.g., inflammation, oxidative stress, angiogenic dysfunction) differentially impact the fetal brain through the phases of gestation?
What particular cellular and molecular targets mediate neurodevelopmental risk in children after preeclampsia exposure and are specific pharmacotherapies possible?
Concluding Remarks
Collectively, the findings discussed here reveal central mechanisms that may underlie neurodevelopmental risk in the context of preeclampsia. These mechanisms involve interactions between maternal physiology and placental health and have the potential to impact neurodevelopment (e.g., regional brain structure, cerebral vasculature, and connectivity) in lasting and clinically relevant ways. The existing body of work on this topic also points to potential molecular targets for intervention, including PlGF and immune and redox molecules, which are the focus of interventions already in the treatment pipeline for preeclamptic mothers [e.g., non-steroidal anti-inflammatory drugs (NSAIDS)] that could also benefit children. At a minimum, this work strongly suggests that early screening for neuropsychiatric and neurological disorders and other developmental delays is warranted in children of preeclamptic pregnancies.
Supplementary Material
Highlights.
Preeclampsia exposure in utero increases later risk for psychiatric and neurological problems and is associated with brain morphological, white matter, and vascular abnormalities.
Neurodevelopmental processes are likely affected by dysregulation of immune, oxidative stress, growth factor, and angiogenic processes in maternal, placental, and fetal physiology during preeclampsia.
Animal models have highlighted multiple mechanisms underlying this risk and potential therapeutic/intervention targets.
Acknowledgments
The authors would like to thank sources of funding support, including the University of Iowa Hypertension grant program (H. E.S.), the Iowa Center for Research by Undergraduates (A.S.S.C.), the Development and Learning from Theory to Application Research Grant (S.B.G.), the National Institute of Neurological Disorders and Stroke (Predoctoral Training Grant T32NS007421 to S.B.G.), and the Roy J. Carver Charitable Trust (H.E.S.). This work was also supported by the NIH [HD089940 (M.K.S.) and 3UL1TR002537 (M.K.S. and D.A.S.)] and the American Heart Association [18SCG34350001 (M.K.S.) and 19IPLOI34760288 (M.K.S.)]. The authors are grateful to the Stevens and Santillan labs for helpful discussion and to Melis Gumusoglu for assistance with graphical design.
Footnotes
Disclaimer Statement
M.K.S and D.A.S. hold patents related to vasopressin (AVP) for the prediction and treatment of preeclampsia: US 293 #9,937,182 (April 10, 2018), EU #2,954,324 (July 31, 2019), and PCT/US2018/027152.
Supplemental Information
Supplemental information associated with this article can be found online https://doi.org/10.1016/j.tins.2020.02.003.
References
- 1.Lo JO et al. (2013) Hypertensive disease of pregnancy and maternal mortality. Curr. Opin. Obstet. Gynecol. 25, 124–132 [DOI] [PubMed] [Google Scholar]
- 2.Anderson CM (2007) Preeclampsia: exposing future cardiovascular risk in mothers and their children. J. Obstet. Gynecol. Neonatal. Nurs. 36, 3–8 [DOI] [PubMed] [Google Scholar]
- 3.Sanders SJ (2015) First glimpses of the neurobiology of autism spectrum disorder. Curr. Opin. Genet. Dev. 33, 80–92 [DOI] [PubMed] [Google Scholar]
- 4.Sahin M and Sur M (2015) Genes, circuits, and precision therapies for autism and related neurodevelopmental disorders. Science 350, aab3897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rashid B et al. (2018) Connectivity dynamics in typical development and its relationship to autistic traits and autism spectrum disorder. Hum. Brain Mapp. 39, 3127–3142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lasalle JM (2013) Autism genes keep turning up chromatin. OA Autism 1, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rahat B et al. (2014) Epigenetic mechanisms regulate placental c-myc and hTERT in normal and pathological pregnancies; c-myc as a novel fetal DNA epigenetic marker for pre-eclampsia. Mol. Hum. Reprod. 20, 1026–1040 [DOI] [PubMed] [Google Scholar]
- 8.Maher GM et al. (2018) Association of hypertensive disorders of pregnancy with risk of neurodevelopmental disorders in offspring: a systematic review and meta-analysis. JAMA Psychiatry 75, 809–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dachew BA et al. (2018) Pre-eclampsia and the risk of autism-spectrum disorder in offspring: meta-analysis. Br. J. Psychiatry 212, 142–147 [DOI] [PubMed] [Google Scholar]
- 10.Mann JR et al. (2010) Pre-eclampsia, birth weight, and autism spectrum disorders. J. Autism Dev. Disord. 40, 548–554 [DOI] [PubMed] [Google Scholar]
- 11.Walker CK et al. (2015) Preeclampsia, placental insufficiency, and autism spectrum disorder or developmental delay. JAMA Pediatr. 169, 154–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wallace AE et al. (2008) Obstetric and parental psychiatric variables as potential predictors of autism severity. J. Autism Dev. Disord. 38, 1542–1554 [DOI] [PubMed] [Google Scholar]
- 13.Wade M and Jenkins JM (2016) Pregnancy hypertension and the risk for neuropsychological difficulties across early development: a brief report. Child Neuropsychol. 22, 247–254 [DOI] [PubMed] [Google Scholar]
- 14.Nahum Sacks K et al. (2019) Long-term neuropsychiatric morbidity in children exposed prenatally to preeclampsia. Early Hum. Dev. 130, 96–100 [DOI] [PubMed] [Google Scholar]
- 15.Kasparek T et al. (2015) Neurobiology of ADHD from childhood to adulthood: findings of imaging methods. J. Atten. Disord. 19, 931–943 [DOI] [PubMed] [Google Scholar]
- 16.Rätsep MT et al. (2016) Brain structural and vascular anatomy is altered in offspring of pre-eclamptic pregnancies: a pilot study. AJNR Am. J. Neuroradiol. 37, 939–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dachew BA et al. (2019) Pre-eclampsia and the risk of attention-deficit/hyperactivity disorder in offspring: findings from the ALSPAC birth cohort study. Psychiatry Res. 272,392–397 [DOI] [PubMed] [Google Scholar]
- 18.Silva D et al. (2014) Environmental risk factors by gender associated with attention-deficit/hyperactivity disorder. Pediatrics 133, e14–e22 [DOI] [PubMed] [Google Scholar]
- 19.Tuovinen S et al. (2010) Depressive symptoms in adulthood and intrauterine exposure to pre-eclampsia: the Helsinki Birth Cohort Study. BJOG 117, 1236–1242 [DOI] [PubMed] [Google Scholar]
- 20.Robinson M et al. (2013) Hypertensive diseases of pregnancy predict parent-reported difficult temperament in infancy. J. Dev. Behav. Pediatr. 34, 174–180 [DOI] [PubMed] [Google Scholar]
- 21.Gross C et al. (2002) Serotonin1A receptor acts during development to establish normal anxiety-like behaviour in the adult. Nature 416, 396–400 [DOI] [PubMed] [Google Scholar]
- 22.Hendricks TJ et al. (2003) Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron 37, 233–247 [DOI] [PubMed] [Google Scholar]
- 23.Rosenfeld CS (2019) Placental serotonin signaling, pregnancy outcomes, and regulation of fetal brain development. Biol. Reprod. Published online November 11, 2019. 10.1093/biolre/ioz204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gujrati VR et al. (1996) Novel appearance of placental nuclear monoamine oxidase: biochemical and histochemical evidence for hyperserotonomic state in preeclampsia-eclampsia. Am. J. Obstet. Gynecol. 175, 1543–1550 [DOI] [PubMed] [Google Scholar]
- 25.Bertrand C and St-Louis J (1999) Reactivities to serotonin and histamine in umbilical and placental vessels during the third trimester after normotensive pregnancies and pregnancies complicated by preeclampsia. Am. J. Obstet. Gynecol. 180, 650–659 [DOI] [PubMed] [Google Scholar]
- 26.Gaspar P et al. (2003) The developmental role of serotonin: news from mouse molecular genetics. Nat. Rev. Neurosci. 4, 1002–1012 [DOI] [PubMed] [Google Scholar]
- 27.Tuovinen S et al. (2012) Hypertensive disorders in pregnancy and risk of severe mental disorders in the offspring in adulthood: the Helsinki Birth Cohort Study. J. Psychiatr. Res. 46, 303–310 [DOI] [PubMed] [Google Scholar]
- 28.Goldstein JM et al. (2011) Sex-specific impact of maternalfetal risk factors on depression and cardiovascular risk 40 years later. J. Dev. Orig. Health Dis. 2, 353–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eide MG et al. (2013) Degree of fetal growth restriction associated with schizophrenia risk in a national cohort. Psychol. Med. 43, 2057–2066 [DOI] [PubMed] [Google Scholar]
- 30.Tuovinen S et al. (2014) Maternal hypertensive disorders during pregnancy: adaptive functioning and psychiatric and psychological problems of the older offspring. BJOG 121, 1482–1491 [DOI] [PubMed] [Google Scholar]
- 31.Schork AJ et al. (2016) New statistical approaches exploit the polygenic architecture of schizophrenia–implications for the underlying neurobiology. Curr. Opin. Neurobiol. 36, 89–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smyth AM and Lawrie SM (2013) The neuroimmunology of schizophrenia. Clin. Psychopharmacol. Neurosci. 11, 107–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laskowska M (2006) Evaluation of maternal and umbilical serum TNFalpha levels in preeclamptic pregnancies in the intrauterine normal and growth-restricted fetus. J. Matern. Fetal Neonatal Med. 19, 347–351 [DOI] [PubMed] [Google Scholar]
- 34.Stellwagen D and Malenka RC (2006) Synaptic scaling mediated by glial TNF-alpha. Nature 440, 1054–1059 [DOI] [PubMed] [Google Scholar]
- 35.Jia P et al. (2010) Common variants conferring risk of schizophrenia: a pathway analysis of GWAS data. Schizophr. Res. 122, 38–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sørensen HJ et al. (2003) Do hypertension and diuretic treatment in pregnancy increase the risk of schizophrenia in offspring? Am. J. Psychiatry 160, 464–468 [DOI] [PubMed] [Google Scholar]
- 37.Leavey K et al. (2015) Large scale aggregate microarray analysis reveals three distinct molecular subclasses of human preeclampsia. PLoS One 10, e0116508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robinson M et al. (2009) Hypertensive diseases of pregnancy and the development of behavioral problems in childhood and adolescence: the Western Australian Pregnancy Cohort Study. J. Pediatr. 154, 218–224 [DOI] [PubMed] [Google Scholar]
- 39.van Wassenaer AG et al. (2011) Outcome at 4.5 years of children born after expectant management of early-onset hypertensive disorders of pregnancy. Am. J. Obstet. Gynecol. 204, 510. [DOI] [PubMed] [Google Scholar]
- 40.Sandgren JA et al. (2018) Arginine vasopressin infusion is sufficient to model clinical features of preeclampsia in mice. JCI Insight 3, 99403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Many A et al. (2003) Neurodevelopmental and cognitive assessment of children born growth restricted to mothers with and without preeclampsia. Hypertens. Pregnancy 22, 25–29 [DOI] [PubMed] [Google Scholar]
- 42.Johnson S et al. (2015) Neurodevelopmental outcomes following late and moderate prematurity: a population-based cohort study. Arch. Dis. Child. Fetal Neonatal Ed. 100, F301–F308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Warshafsky C et al. (2016) Prospective assessment of neurodevelopment in children following a pregnancy complicated by severe pre-eclampsia. BMJ Open 6, e010884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heikura U et al. (2013) Maternal hypertensive disorders during pregnancy and mild cognitive limitations in the offspring. Paediatr. Perinat. Epidemiol. 27, 188–198 [DOI] [PubMed] [Google Scholar]
- 45.Ehrenstein V et al. (2009) Pregnancy-associated hypertensive disorders and adult cognitive function among Danish conscripts. Am. J. Epidemiol. 170, 1025–1031 [DOI] [PubMed] [Google Scholar]
- 46.Morsing E and Maršál K (2014) Pre-eclampsia- an additional risk factor for cognitive impairment at school age after intrauterine growth restriction and very preterm birth. Early Hum. Dev. 90, 99–101 [DOI] [PubMed] [Google Scholar]
- 47.Seidman DS et al. (1991) Pre-eclampsia and offspring’s blood pressure, cognitive ability and physical development at 17-years-of-age. Br. J. Obstet. Gynaecol. 98, 1009–1014 [DOI] [PubMed] [Google Scholar]
- 48.Rätsep MT et al. (2016) Impact of preeclampsia on cognitive function in the offspring. Behav. Brain Res. 302, 175–181 [DOI] [PubMed] [Google Scholar]
- 49.Whitehouse AJ et al. (2012) Do hypertensive diseases of pregnancy disrupt neurocognitive development in offspring? Paediatr. Perinat. Epidemiol. 26, 101–108 [DOI] [PubMed] [Google Scholar]
- 50.Sverrisson FA et al. (2018) Preeclampsia and academic performance in children: a nationwide study from Iceland. PLoS One 13, e0207884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tuovinen S et al. (2012) Hypertensive disorders in pregnancy and cognitive decline in the offspring up to old age. Neurology 79, 1578–1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Giambrone AB et al. (2019) Perinatal micro-bleeds and neuroinflammation in E19 rat fetuses exposed to utero-placental ischemia. Int. J. Mol. Sci. 20, E4051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guedj F et al. (2015) The fetal brain transcriptome and neonatal behavioral phenotype in the Ts1Cje mouse model of Down syndrome. Am. J. Med. Genet. A 167A, 1993–2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lockwood CJ et al. (2008) Preeclampsia-related inflammatory cytokines regulate interleukin-6 expression in human decidual cells. Am. J. Pathol. 172, 1571–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rudolph MD et al. (2018) Maternal IL-6 during pregnancy can be estimated from newborn brain connectivity and predicts future working memory in offspring. Nat. Neurosci. 21, 765–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kajantie E et al. (2009) Pre-eclampsia is associated with increased risk of stroke in the adult offspring: the Helsinki birth cohort study. Stroke 40, 1176–1180 [DOI] [PubMed] [Google Scholar]
- 57.Wang A et al. (2009) Preeclampsia: the role of angiogenic factors in its pathogenesis. Physiology (Bethesda) 24, 147–158 [DOI] [PubMed] [Google Scholar]
- 58.Strand KM et al. (2013) Mediators of the association between pre-eclampsia and cerebral palsy: population based cohort study. BMJ 347, f4089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mann JR et al. (2011) Uncovering the complex relationship between pre-eclampsia, preterm birth and cerebral palsy. Paediatr. Perinat. Epidemiol. 25, 100–110 [DOI] [PubMed] [Google Scholar]
- 60.Curran EA et al. (2018) Exposure to hypertensive disorders of pregnancy increases the risk of autism spectrum disorder in affected offspring. Mol. Neurobiol. 55, 5557–5564 [DOI] [PubMed] [Google Scholar]
- 61.Stojanovska V et al. (2019) A double-hit pre-eclampsia model results in sex-specific growth restriction patterns. Dis. Model. Mech. 12, dmm035980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu X et al. (2016) Developmental and functional brain impairment in offspring from preeclampsia-like rats. Mol. Neurobiol. 53, 1009–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Figueiro-Filho EA et al. (2017) Diffusion tensor imaging of white matter in children born from preeclamptic gestations. AJNR Am. J. Neuroradiol. 38, 801–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mak LE et al. (2018) Resting-state functional connectivity in children born from gestations complicated by preeclampsia:a pilot study cohort. Pregnancy Hypertens. 12, 23–28 [DOI] [PubMed] [Google Scholar]
- 65.Amodio DM and Frith CD (2006) Meeting of minds: the medial frontal cortex and social cognition. Nat. Rev. Neurosci. 7, 268–277 [DOI] [PubMed] [Google Scholar]
- 66.Cavanna AE and Trimble MR (2006) The precuneus: a review of its functional anatomy and behavioural correlates. Brain 129, 564–583 [DOI] [PubMed] [Google Scholar]
- 67.Azmitia EC et al. (2016) Persistent angiogenesis in the autism brain: an immunocytochemical study of postmortem cortex, brainstem and cerebellum. J. Autism Dev. Disord. 46, 1307–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li J et al. (2012) A model of preeclampsia in rats: the reduced uterine perfusion pressure (RUPP) model. Am. J. Physiol. Heart Circ. Physiol. 303, H1–H8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kay VR et al. (2019) Adult Pgf(−/−) mice behaviour and neuroanatomy are altered by neonatal treatment with recombinant placental growth factor. Sci. Rep. 9, 9285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kay VR et al. (2018) Effects of placental growth factor deficiency on behavior, neuroanatomy, and cerebrovasculature of mice. Physiol. Genomics 50, 862–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Santillan MK et al. (2014) Vasopressin in preeclampsia: a novel very early human pregnancy biomarker and clinically relevant mouse model. Hypertension 64, 852–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shallie PD and Naicker T (2019) The placenta as a window to the brain: a review on the role of placental markers in prenatal programming of neurodevelopment. Int. J. Dev. Neurosci. 73, 41–49 [DOI] [PubMed] [Google Scholar]
- 73.Goeden N et al. (2016) Maternal inflammation disrupts fetal neurodevelopment via increased placental output of serotonin to the fetal brain. J. Neurosci. 36, 6041–6049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Howerton CL and Bale TL (2014) Targeted placental deletion of OGT recapitulates the prenatal stress phenotype including hypothalamic mitochondrial dysfunction. Proc. Natl. Acad. Sci. U. S. A. 111, 9639–9644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Roberts JM and Escudero C (2012) The placenta in preeclampsia. Pregnancy Hypertens. 2, 72–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lawrence DJ et al. (2019) Spectral photoacoustic imaging to estimate in vivo placental oxygenation during preeclampsia. Sci. Rep. 9, 558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Clayton AM et al. (2018) Postpartum increases in cerebral edema and inflammation in response to placental ischemia during pregnancy. Brain Behav. Immun. 70, 376–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mallard EC et al. (1998) Effects of chronic placental insufficiency on brain development in fetal sheep. Pediatr. Res. 43, 262–270 [DOI] [PubMed] [Google Scholar]
- 79.Bennett MR and Lagopoulos J (2015) Neurodevelopmental sequelae associated with gray and white matter changes and their cellular basis: a comparison between autism spectrum disorder, ADHD and dyslexia. Int. J. Dev. Neurosci. 46, 132–143 [DOI] [PubMed] [Google Scholar]
- 80.Singh DK et al. (2018) Hypoxia and myelination deficits in the developing brain. Int. J. Dev. Neurosci. 70, 3–11 [DOI] [PubMed] [Google Scholar]
- 81.Wang F et al. (2018) Enhancing oligodendrocyte myelination rescues synaptic loss and improves functional recovery after chronic hypoxia. Neuron 99, 689–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fong FM (2014) Maternal genotype and severe preeclampsia: a HuGE review. Am. J. Epidemiol. 180, 335–345 [DOI] [PubMed] [Google Scholar]
- 83.Saito S (2010) Th17 cells and regulatory T cells: new light on pathophysiology of preeclampsia. Immunol. Cell Biol. 88, 615–617 [DOI] [PubMed] [Google Scholar]
- 84.Kelly RS et al. (2017) Integration of metabolomic and transcriptomic networks in pregnant women reveals biological pathways and predictive signatures associated with preeclampsia. Metabolomics 13, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Novotny S et al. (2013) CD4(+) T cells play a critical role in mediating hypertension in response to placental ischemia. J. Hypertens. (Los Angel) 2, 14873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cornelius DC et al. (2015) An increased population of regulatory T cells improves the pathophysiology of placental ischemia in a rat model of preeclampsia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 309, R884–R891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Harmon A et al. (2015) IL-10 supplementation increases Tregs and decreases hypertension in the RUPP rat model of preeclampsia. Hypertens. Pregnancy 34, 291–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cottrell JN et al. (2019) Interleukin-4 supplementation improves the pathophysiology of hypertension in response to placental ischemia in RUPP rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 316, R165–R171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Scroggins SM et al. (2018) Elevated vasopressin in pregnant mice induces T-helper subset alterations consistent with human preeclampsia. Clin. Sci. (Lond.) 132, 419–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cunningham CL et al. (2013) Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J. Neurosci. 33, 4216–4233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.George EM et al. (2014) Placental ischemia induces changes in gene expression in chorionic tissue. Mamm. Genome 25, 253–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sandgren JA et al. (2018) Angiotensin AT1A receptors expressed in vasopressin-producing cells of the supraoptic nucleus contribute to osmotic control of vasopressin. Am. J. Physiol. Regul. Integr. Comp. Physiol. 314, R770–R780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Benvenuti S et al. (2013) Low extracellular sodium causes neuronal distress independently of reduced osmolality in an experimental model of chronic hyponatremia. Neuromolecular Med. 15, 493–503 [DOI] [PubMed] [Google Scholar]
- 94.Lauderdale K et al. (2015) Osmotic edema rapidly increases neuronal excitability through activation of NMDA receptor-dependent slow inward currents in juvenile and adult hippocampus. ASN Neuro 7 1759091415605115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cornelius DC and Lamarca B (2014) TH17- and IL-17- mediated autoantibodies and placental oxidative stress play a role in the pathophysiology of pre-eclampsia. Minerva Ginecol. 66, 243–249 [PMC free article] [PubMed] [Google Scholar]
- 96.Dhillion P et al. (2012) IL-17-mediated oxidative stress is an important stimulator of AT1-AA and hypertension during pregnancy. Am. J. Physiol. Regul. Integr. Comp. Physiol. 303, R353–R358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cornelius DC et al. (2016) Reduced uterine perfusion pressure T-helper 17 cells cause pathophysiology associated with preeclampsia during pregnancy. Am. J. Physiol. Regul. Integr. Comp. Physiol. 311, R1192–R1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Choi GB et al. (2016) The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science 351, 933–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shu W et al. (2018) Evaluation of blood vessel injury, oxidative stress and circulating inflammatory factors in an L-NAME-induced preeclampsia-like rat model. Exp. Ther. Med. 16, 585–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ijomone OK et al. (2019) N-nitro-l-arginine methyl model of pre-eclampsia elicits differential IBA1 and EAAT1 expressions in brain. J. Chem. Neuroanat. 100, 101660 [DOI] [PubMed] [Google Scholar]
- 101.Davis EJ et al. (1994) Cellular forms and functions of brain microglia. Brain Res. Bull. 34, 73–78 [DOI] [PubMed] [Google Scholar]
- 102.Zhu H et al. (2017) The effect of pre-eclampsia-like syndrome induced by L-NAME on learning and memory and hippocampal glucocorticoid receptor expression: a rat model. Hypertens. Pregnancy 36, 36–43 [DOI] [PubMed] [Google Scholar]
- 103.Korte SM (2001) Corticosteroids in relation to fear, anxiety and psychopathology. Neurosci. Biobehav. Rev. 25, 117–142 [DOI] [PubMed] [Google Scholar]
- 104.Bian Z et al. (2015) First-trimester maternal serum levels of sFLT1, PGF and ADMA predict preeclampsia. PLoS One 10, e0124684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lecuyer M et al. (2017) PLGF, a placental marker of fetal brain defects after in utero alcohol exposure. Acta Neuropathol. Commun. 5, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Carmeliet P et al. (2001) Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat. Med. 7, 575–583 [DOI] [PubMed] [Google Scholar]
- 107.Du H et al. (2010) Vascular endothelial growth factor signaling implicated in neuroprotective effects of placental growth factor in an in vitro ischemic model. Brain Res. 1357, 1–8 [DOI] [PubMed] [Google Scholar]
- 108.Albonici L et al. (2019) Multifaceted role of the placental growth factor (PlGF) in the antitumor immune response and cancer progression. Int. J. Mol. Sci. 20, E2970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Selvaraj SK et al. (2003) Mechanism of monocyte activation and expression of proinflammatory cytochemokines by placenta growth factor. Blood 102, 1515–1524 [DOI] [PubMed] [Google Scholar]
- 110.Kang M et al. (2018) Placental growth factor (PlGF) is linked to inflammation and metabolic disorders in mice with diet-induced obesity. Endocr. J. 65, 437–447 [DOI] [PubMed] [Google Scholar]
- 111.Aouache R et al. (2018) Oxidative stress in preeclampsia and placental diseases. Int. J. Mol. Sci. 19, E1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dowell J et al. (2019) Cellular stress mechanisms of prenatal maternal stress: heat shock factors and oxidative stress. Neurosci. Lett. 709, 134368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fisher JJ et al. (2020) Placental mitochondria and reactive oxygen species in the physiology and pathophysiology of pregnancy. Clin. Exp. Pharmacol. Physiol. 47, 176–184 [DOI] [PubMed] [Google Scholar]
- 114.Sedeek M et al. (2008) Role of reactive oxygen species in hypertension produced by reduced uterine perfusion in pregnant rats. Am. J. Hypertens. 21, 1152–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vaka VR et al. (2018) Role of mitochondrial dysfunction and reactive oxygen species in mediating hypertension in the reduced uterine perfusion pressure rat model of preeclampsia. Hypertension 72, 703–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Balta O et al. (2011) Reduced uterine perfusion pressure model is not successful to mimic severe preeclampsia. Placenta 32, 675–680 [DOI] [PubMed] [Google Scholar]
- 117.Xuan RR et al. (2016) Astaxanthin blocks preeclampsia progression by suppressing oxidative stress and inflammation. Mol. Med. Rep. 14, 2697–2704 [DOI] [PubMed] [Google Scholar]
- 118.Spradley FT et al. (2016) Placental growth factor administration abolishes placental ischemia-induced hypertension. Hypertension 67, 740–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bittle J et al. (2019) The role of redox dysregulation in the effects of prenatal stress on embryonic interneuron migration. Cereb. Cortex 29, 5116–5130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schafer FQ and Buettner GR (2001) Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 30, 1191–1212 [DOI] [PubMed] [Google Scholar]
- 121.Tsatmali M et al. (2006) Reactive oxygen species modulate the differentiation of neurons in clonal cortical cultures. Mol. Cell. Neurosci. 33, 345–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Miller SL et al. (2016) The consequences of fetal growth restriction on brain structure and neurodevelopmental outcome. J. Physiol. 594, 807–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Goodman SH et al. (2011) Maternal depression and child psychopathology: a meta-analytic review. Clin. Child. Fam. Psychol. Rev. 14, 1–27 [DOI] [PubMed] [Google Scholar]
- 124.Kurki T et al. (2000) Depression and anxiety in early pregnancy and risk for preeclampsia. Obstet. Gynecol. 95, 487–490 [DOI] [PubMed] [Google Scholar]
- 125.Gray KJ et al. (2018) Gene-centric analysis of preeclampsia identifies maternal association at PLEKHG1. Hypertension 72, 408–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Karppanen T et al. (2016) An RGS2 3′UTR polymorphism is associated with preeclampsia in overweight women. BMC Genet. 17, 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Campbell DB et al. (2008) Association of RGS2 and RGS5 variants with schizophrenia symptom severity. Schizophr. Res. 101, 67–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Guizzetti M et al. (2008) Modulation of neuritogenesis by astrocyte muscarinic receptors. J. Biol. Chem. 283, 41–49 31884–31897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nagy E et al. (2001) Gender-related physiologic differences in human neonates and the greater vulnerability of males to developmental brain disorders. J. Gend. Specif. Med. 4, 41–49 [PubMed] [Google Scholar]
- 130.Ojeda NB et al. (2012) Oxidative stress contributes to sex differences in blood pressure in adult growth-restricted offspring. Hypertension 60, 114–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.