Abstract
There is limited understanding of adverse health effect associations with chemical constituents of fine particulate matter (PM2.5) as well as the underlying mechanisms. We outlined a workflow to assess metrics, beyond concentration, using household and personal PM2.5 filter samples collected in India as a proof of concept for future large-scale studies. Oxidative potential, chemical composition (polycyclic aromatic hydrocarbons and elements), and bioactivity (developmental exposures in zebrafish) were determined. Significant differences were observed in all metrics between personal and household PM2.5 samples. This work established methods to characterize multiple metrics of PM2.5 to ultimately support the identification of more health-relevant metrics than concentration.
Keywords: Indoor PM2.5, Household air pollution, zebrafish, oxidative potential, PM2.5 composition, PAH diagnostic ratios
Graphical abstract
1. INTRODUCTION
Annually, 4.3 million premature deaths occur from illnesses attributed to household air pollution (HAP).1 Sources vary regionally but most HAP across the globe comes from home heating and cooking with solid fuel sources,2,3 an exposure that disproportionately affects women and children.4 Fine particulate matter (PM2.5) is a component of HAP with 48 well-established health effects,5–7 although far less is understood about the chemical constituents of PM2.5 that result from HAP.
Identifying the chemical composition and toxicological effects of PM2.5 from HAPwill lead to a more robust characterization of these exposures with the potential for PM2.5 hazard prediction. Instead of using PM2.5 mass as an indicator of health outcomes, composition and oxidative potential have been proposed as more health relevant metrics.8,9 Stronger associations have been observed between human health impacts and oxidative potential10 or composition11 compared to associations with outdoor PM2.5 concentrations. There is currently limited data available for composition and oxidative potential in comparison to the more extensive reports for PM2.5 concentration, especially when considering HAP and personal PM2.5 exposures. With even less data generated thus far measuring both metrics in the same PM2.5 sample.12
Oxidative potential and other commonly used in vitro assays of PM2.5 toxicity lack the complexity of in vivo systems, but can quickly assess many PM2.5 samples. While whole animal models are often the opposite, offering systems level complexity, but low throughput. Recently, the developmental zebrafish (Danio rerio) was established as an in vivo platform for rapid testing of PM2.5.13,14 This highly sensitive vertebrate model offers a systems level approach complemented by many well-defined developmental toxicity endpoints15,16 and an extensive molecular toolkit.17 Integrating this high-throughput screening model will provide a rapid sensor for assessing the bioactivity of HAP and personal PM2.5.
Quantifying the chemical composition, oxidative potential, and bioactivity in zebrafish for the same PM2.5 sample will lead to a better understanding of the connection between exposure sources, chemical composition, and health impacts. Additionally, these metrics have not been assessed for paired kitchen and personal samples, in the same households. Identifying differences in PM2.5 metrics based on household vs personal samples may elucidate possible limitations of current HAP PM2.5 exposure assessments.
We sought to outline a workflow to collect oxidative potential, chemical composition, and bioactivity data for the same PM2.5 sample. As a proof of concept, PM2.5 collected with kitchen and personal monitors from six households in India, which used the same primary fuel source for cooking, underwent the proposed workflow. Notably, this workflow enables multiple analysis methods including oxidative potential assessment utilizing a 96-well plate format. Our findings demonstrate the feasibility of a workflow for chemical characterization and biological response assessment of a single PM2.5 sample and the ability to identify differences in these metrics based on the type of monitor used for collection.
2. MATERIAL AND METHODS
2.1. Chemicals
Information for PAHs and deuterated or isotopically labelled standards, including abbreviations, is provided in the Supporting Information (Table A1). Solvents including: acetonitrile (ACN), acetone (Ace), ethyl acetate (EA), dichloromethane (DCM), hexane, and methanol (MetOH); all optima grade were purchased from Thermo Fisher Scientific (Santa Clara, CA). Toluene, dimethylsulfoxide (DMSO), N-methyl-N-(tert-butyldimethylsilyl) trifluoroacetamide (MTBSTFA), dithiobis-2-nitrobenzoic acid (DTNB), dithiothreitol (DTT), and 1,4-naphtoquinone were purchased from Sigma-Aldrich (Milwaukee, WI).
2.2. PM2.5 Samples
Kitchen and personal PM2.5 samples were collected as part of the previously described Prospective Urban and Rural Epidemiological (PURE)-AIR pilot study between July 13th and September 2nd, 2015 in Kheri, India.18 Ultrasonic personal aerosol samplers (UPAS) were used with Pallflex fiberfilm filters (37 mm) to collect time-integrated PM2.5 over a 48 hour period for both kitchen and personal monitors. Kitchen monitors were placed in the kitchen area of the household approximately 1 m from the primary cook stove and 1 m above the floor. Personal monitors were worn over the shoulder or on the upper arm with custom built harnesses. After the 48-hour monitoring period participants completed a survey to document household characteristics, cooking and heating practices and personal behaviours. Following collection, pre-weighed filters were post-weighed to determine PM2.5 mass loadings using an automated filter weighing system.18 Households (n=6) from the PURE-AIR pilot study were selected that had both kitchen PM2.5 monitor filters and PM2.5 personal filters worn by female participants living in the selected households. All households reported liquified petroleum gas (LPG) as their primary fuel source.
2.3. Filter Extraction
Extraction of PM2.5 from filters occurred via sonication in methanol for 60 min in a waterbath sonicator (40 Hz, Bransonic). Following sonication, filters were rinsed with methanol to remove any residual particles, the extract was pooled with all other filter extracts from the corresponding group, and concentrated under a steady stream of N2 to dryness. Dried PM2.5 samples were re-suspended in DMSO through 30 min sonication (40 Hz, Bransonic) and then centrifuged (5 min, 13 g). The supernatant was collected as the DMSO soluble fraction of the PM2.5 samples.13 Aliquots of this fraction were used for chemical and toxicological analyses (Fig 1). Blank filters (filters without PM2.5 collected) underwent the same procedures to serve as a method controls throughout the subsequent analyses.
Figure 1:
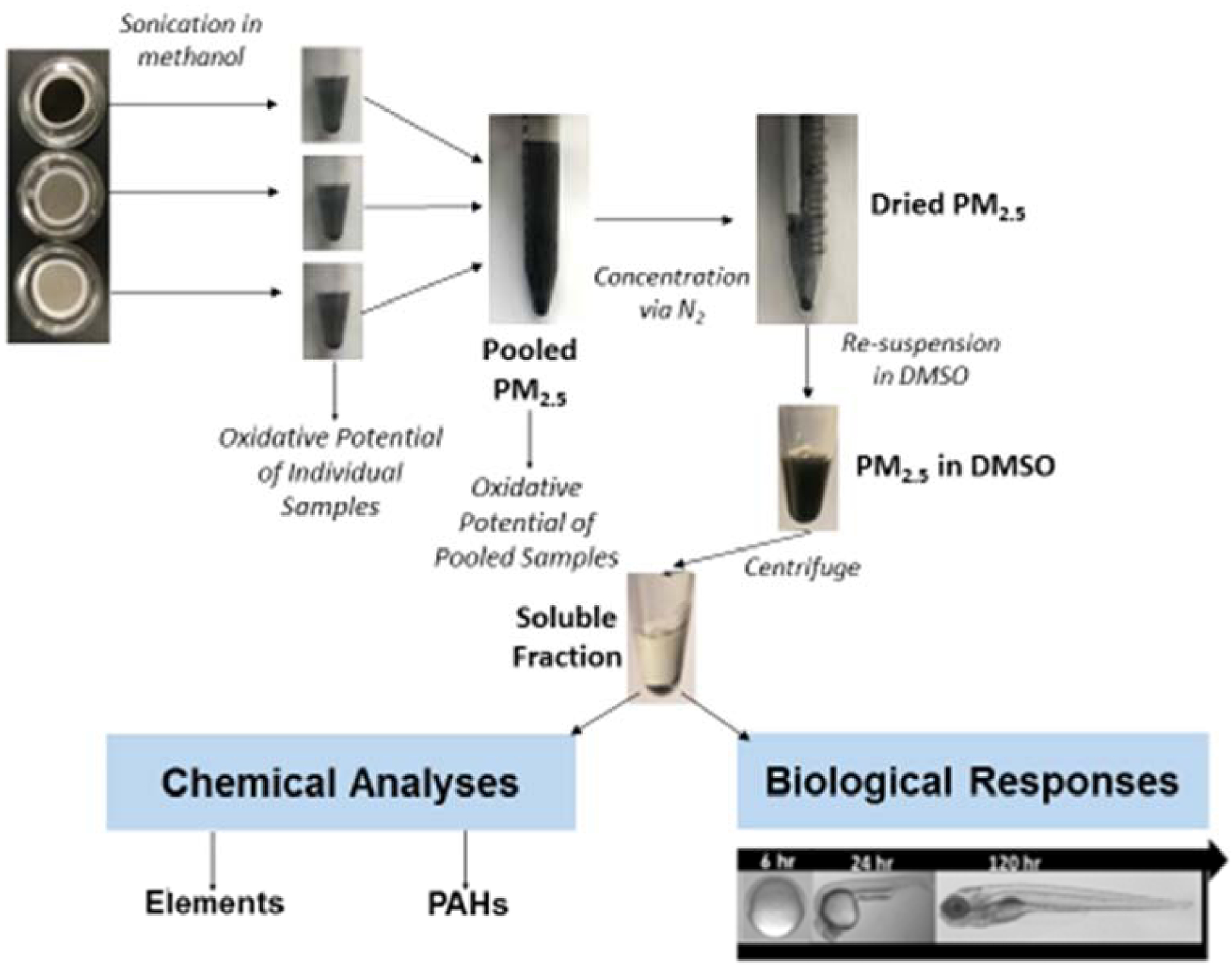
Experimental Design. Schematic of the preparation of PM2.5 following collection from kitchen or personal monitors from households in Kheri, India. PM2.5 was used for chemical and biological response data.
2.4. Oxidative Potential
Aliquots of the PM2.5 or blank filter extracts were taken from the individual filter samples (n=12) and the pooled kitchen or personal groups and used to measure oxidative potential with the dithiothreitol (DTT) assay. The assay was performed following standard procedures19 but with volume modifications to allow for measurement in 96-well plates containing 100 μL of 0.05 M potassium phosphate monobasic sodium hydroxide, 5 μL of 0.5 mM DTT, 10 μL of sample, and 10 μL of 1 mM dithiobis-2-nitrobenzoic acid (DTNB). Controls included: a blank (phosphate buffer solution), a blank filter (blank filter undergoing extraction procedures performed on the PM2.5 filters), and a positive control (1,4-naphtoquinone). The rate of DTT loss was determined via quantification of 2-nitro-5-thiobenzoic acid (TNB) by fluorescence readings with comparison to a linear regression of known DTT standards.20 TNB is formed from the reaction of DTNB with the remaining DTT, not reacted with the sample, and allows for quantification of DTT consumption during the reaction.
2.5. Bioactivity Assessment
The well-established, semi-automated high-throughput developmental toxicity screening procedures at the Sinnhuber Aquatic Research Laboratory (SARL)15,21,22 were utilized for all PM2.5 exposures. Following zebrafish embryo static exposure starting at 6 hours post fertilization (hpf) to the DMSO soluble fraction of PM2.5, 96-well plates were sealed with parafilm to prevent evaporation, wrapped in aluminium foil to prevent photodegradation, and placed on an orbital shaker at 235 rpm overnight; plates were stored at 28 °C throughout the experiment.15 The personal monitor sample was set to a final concentration of 200 μg/mL in 1% DMSO, a concentration previously established through use of a standard reference material to induce developmental toxicity.13 The same volume of DMSO was used to re-suspend the pooled kitchen monitor sample, resulting in a final concentration of 409 μg/mL. This maintained the variability between samples based on the volume of air collected from all monitors. Developmental toxicity was assessed at 24 and 120 hpf as morphological changes and mortality in all treatments (DMSO soluble fraction from extraction of kitchen monitor or personal monitor PM2.5 filters) and controls (1% DMSO vehicle control; blank filter control, n=32 embryos/group). The soluble fraction was tested based on previous studies identifying this portion to be bioactive in the model.13 All data was processed using custom designed software called the Zebrafish Acquisition and Analysis Program (ZAAP) with statistical significance computed as previously reported.15
2.6. Chemical Analysis
2.6.1. Polycyclic Aromatic Hydrocarbon (PAH) Analysis.
Aliquots of the DMSO soluble fraction of PM2.5 from pooled filters of kitchen or personal monitors and blank filter extracts were solvent exchanged to hexane via a TurboVap evaporation system (N2 gas, 30 °C) followed by solid phase extraction (SPE) clean-up (Appendix A). Samples were then solvent exchanged to EA and concentrated to 300 μL under a stream of N2. Samples were spiked with isotopically labelled internal standards. Hydroxy-PAH analysis was performed with an aliquot of the concentrated sample that was derivatized following addition of internal standards (Appendix A). Organic compounds, specifically parent/methyl PAHs (n=19), nitro-(n=22), oxy-(n=23), hydroxy-(n=36), and high molecular weight (MW≥ 278, HMW, n=14) PAHs, were quantitatively measured using Agilent 6890 gas chromatography (GC) coupled with an Agilent 5973N mass spectrometer (MS). Selected ion monitoring (SIM) was utilized with spectral data analysis performed with ChemStation software (V. E.02.02.1431, Agilent Technologies). Commercially available standards were used for all measured compounds and all samples and controls were run in triplicate with laboratory blanks and blank filters throughout.
2.6.2. Elemental Analysis.
Aliquots of the DMSO soluble fraction of PM2.5 from pooled kitchen and personal sampling and blank filter extracts were added to ultrapure water, resulting in a 0.1 % DMSO concentration. Elements (n=14), were quantitatively measured using an Agilent 5110 inductively coupled plasma optical emission spectrometry (ICP-OES) system in axial view mode at the Central Analytical Laboratory at Oregon State University. Commercially available standards were utilized for all measured compounds and all samples and controls were run in triplicate.
2.7. Statistical Analysis
Data was blank corrected by laboratory blank and blank filter extracts where noted. All figures were generated with SigmaPlot 14.0 (San Jose, CA). For oxidative potential and chemical characterization data, histograms and statistical significance calculations (one- or two-way analysis of variance (ANOVA) tests and pairwise multiple comparison procedures (Hom-Sidak method) with significance set at p<0.05) were completed in SigmaPlot 14.0. For developmental toxicity screening in zebrafish, statistical significance was computed as previously reported.15
3. RESULTS AND DISCUSSION
3.1. PM2.5
PM2.5 concentrations for the six households were calculated for both the kitchen and personal monitors (Figure 2). Variability was observed between the households for the kitchen (189.7 ± 127.5 μg/m3) and personal monitors (100.2 ± 41.1 μg/m3). In 75% of the households that had kitchen monitors, PM2.5 concentrations below the mean (households A, B, D, and F), the calculated PM2.5 concentrations from the personal monitors were higher than those from the kitchen monitors. This suggested that while the overall mean concentrations were elevated in the kitchen monitors, the trend was heavily influenced by two households (C and E). Based on questionnaire data collected in all households, these two households did not report using outdoor cooking sources, a factor that may have contributed to the elevated concentrations. The kitchen monitors had elevated PM2.5 concentrations over 3 times higher than the corresponding personal monitors, which aligns with previous research23 but also contradicts several studies that found increased concentrations with personal monitoring.24–26 Potential reasons for these findings include: geographic community differences between previous studies (housing characteristics, social practices), participant activities, number of residents in the household, cooking practices, and skewed averages due to high concentrations of kitchen monitors in two of the households. The kitchen/personal differential in PM2.5 concentrations here occurred between households from the same geographic community using the same fuel source. There were large differences between kitchen and personal monitors (average difference of 100.8 ± 122.8 μg/m3). Differences in personal activities and factors within each home are likely driving these differences, which will be explored in future research using all measurements (~4,000) collected in the PURE study and detailed survey data on activities during monitoring. Notably, the measured concentrations from all household and personal monitors far exceed the available outdoor concentration guidelines from the World Health Organization for annual and 24-hour mean concentrations (10 and 25 μg/m3, respectively).27 The kitchen monitors in this study had similar concentrations to previous indoor air studies in India (averages of 135–173 μg/m3)28 and were elevated compared to previous studies of indoor PM2.5 throughout Europe29–31 and the United States.24,32,33 We found personal PM2.5 exposures to be comparable to those measured in a previous study in India with female participants in households using LPG as their primary fuel source. Similarity between studies from different regions of India after a 13-year sampling gap, suggest that personal PM2.5 exposures of female LPG users across India have changed little. Further research into PM2.5 exposures is critical to better understand concentration differences due to geographic, sociocultural, and temporal variation.
Figure 2:
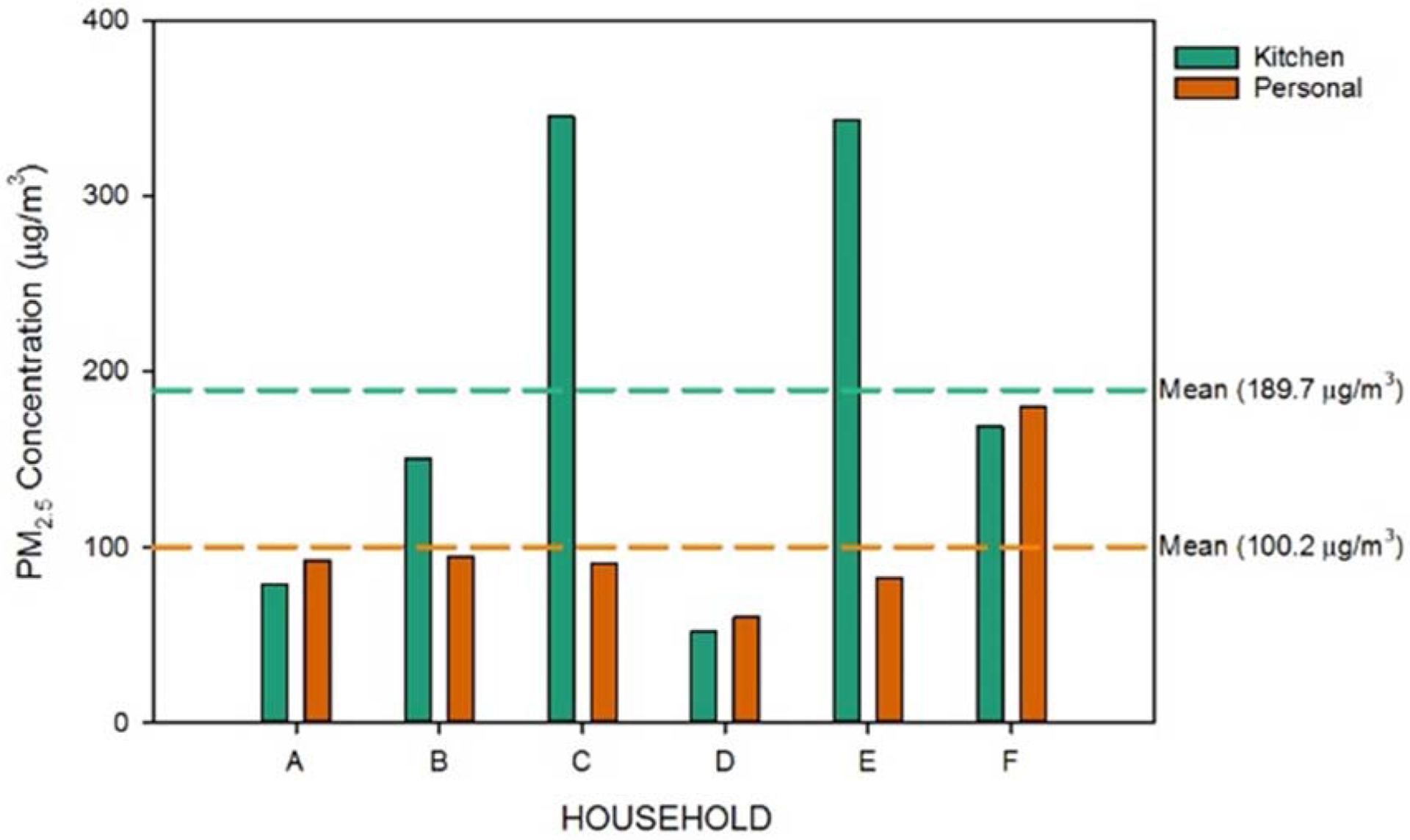
PM2.5 Concentrations from Households with Kitchen and Personal Monitors. Concentrations (μg/m3) are reported for each individual filter collected from either a kitchen or personal monitor worn by a female participant in six households (A-F). Dashed lines indicate the mean concentration for the particular monitor type across all sampled households.
3.2. Oxidative Potential
Oxidative potential was assessed for all samples from individual households and from the pooled samples (Figure 3) using a 96-well plate to reduce time, costs, and solvent use. Pooled samples were comprised of all filter extracts for a monitor type (i.e., personal extracts from all six households were combined and referred to as the pooled personal sample). In the oxidative potential assay, the amount of DTT consumed would be proportional to the amount of redox-active species and thus the ability of the PM2.5 sample to induce oxidative stress.
Figure 3:
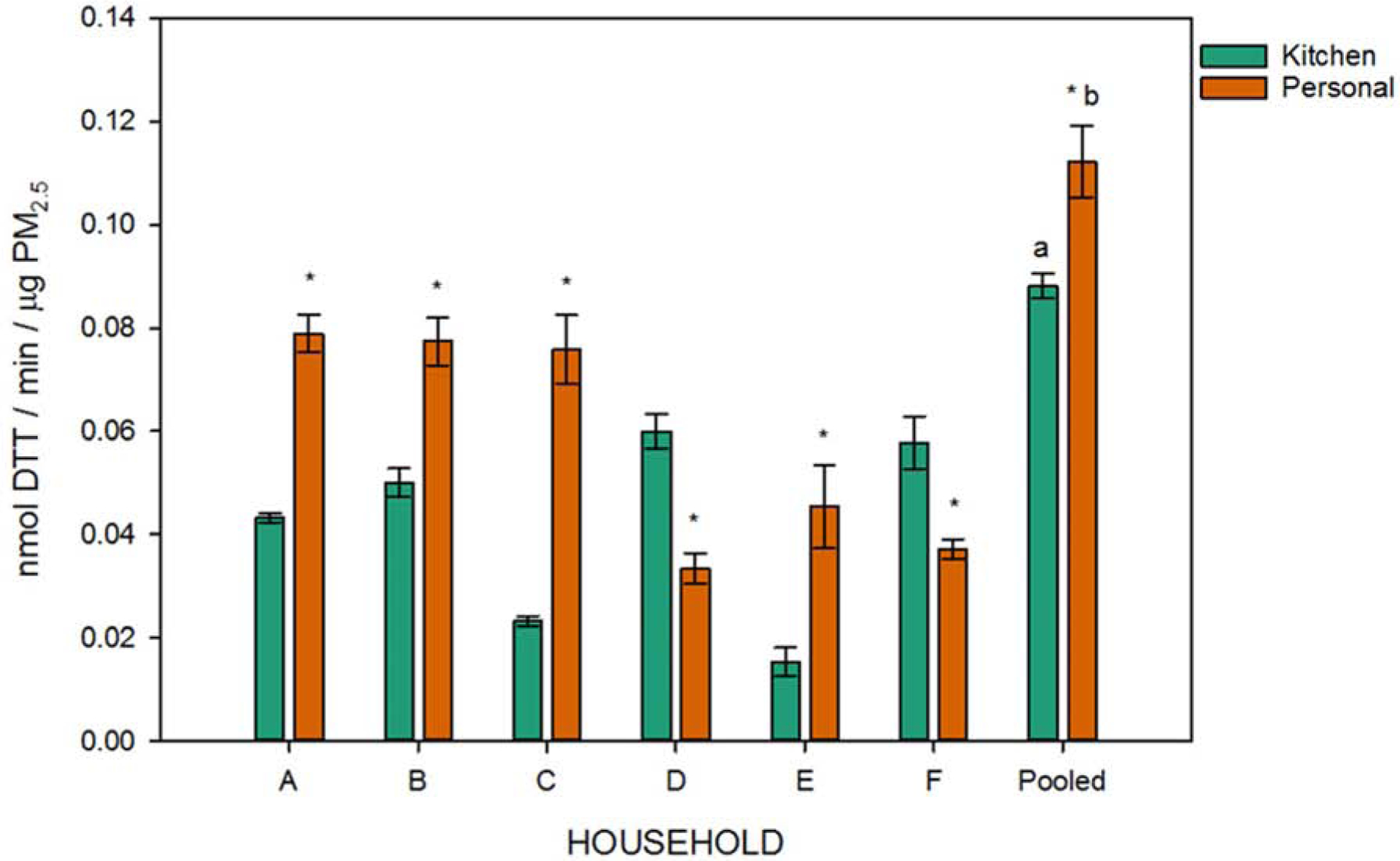
Oxidative potential for PM2.5 from kitchen and personal monitors with individual household and pooled samples. Data are reported as mean ± standard deviation of DTT consumption (nmol) per minute per μg PM2.5. All analyses were run in triplicate and statistical significance of a p-value ≤ 0.05 was determined by two-way ANOVA with * denoting significant differences between monitor types from individual households (A-F) and a,b denoting significance between pooled and individual samples for kitchen or personal monitors, respectively.
Significant differences were observed between monitor types in all of the individual households sampled, as well as between households when assessing the same monitor type (Table A2). The DTT consumption rates of the pooled samples for both monitor types were significantly different from all of the individual household samples. However, these findings were not additive, suggesting that specific components in each sample were responsible for the observed effects and that chemical interactions between the combined samples prevented additive DTT consumption. Notably, the trends observed for PM2.5 concentration (Figure 1) differed from those of oxidative potential (Figure 2), with increased oxidative potential in the personal samples which overall had lower PM2.5 concentration.
We saw elevated oxidative potential compared to a previous study of water soluble fraction personal PM2.5. A potential reason for more oxidative potential in our study is that our samples contained many hydrophobic compounds with significant oxidative potential.34 Also, the household fuel sources, and thus the PM2.5 chemical composition, differed between the previous study (using solid fuels) and ours (using LPG). The DTT consumption rates observed here were comparable to work measuring the DTT activity of cooking organic aerosols,35 further supporting the conclusion that the sources of PM2.5 play a role in oxidative potential.
3.3. Bioactivity Assessment
Pooled samples from the six households were used to identify differences in bioactivity between monitor types. Samples were pooled to ensure adequate mass for both bioactivity assessments and subsequent chemical analyses. Developmental exposure to the DMSO soluble extracts of each PM2.5 monitor sample was associated with significant mortality (Table 1). By the end of the exposure (120 hpf) mortality was over 90 % for extracts of both monitor types. These responses were significantly different from the blank filter and DMSO vehicle controls. Temporal differences were observed between the monitor samples, with significant 24 hpf mortality associated with the personal samples and 120 hpf mortality associated with the kitchen monitor monitor concentrations being nearly double to reflect the observed concentrations in the households. This was achieved by re-suspending the extracted PM2.5 in the same volume of DMSO for each group for the zebrafish exposures. The DMSO re-suspension volume used for the personal monitor samples was intended to equal previous concentration-response tests in zebrafish using a particulate matter standard reference material (200 μg/mL).13 This concentration impacted mortality, morphological, and behavioral endpoints,13,36 however, with exposure to the current PM2.5 samples, insufficient animals survived to evaluate other endpoints. The striking differential in timing of mortality onset between kitchen and personal samples alone would suggest that the chemical constituency can serve as a driver of PM2.5 bioactivity.
Table 1.
Percent Incidence of Mortality following Developmental Exposure to PM2.5
| PM2.5 Collection Method | 24 hpf Mortality (%) | 120 hpf Mortality (%) |
|---|---|---|
| Kitchen Monitors | 28.12 | 93.75* |
| Personal Monitors | 43.75* | 100.00* |
indicates statistical significance (above threshold – Fisher’s Exact test) from vehicle and blank 279 filter controls (n=32/group)
3.4. Chemical Analysis
Differences between monitor types in oxidative potential and bioactivity of the PM2.5 samples, independent of concentration, prompted investigation into the chemical constituents to identify components that were driving the observed effects. Total summed classes of PAHs by μg PM2.5 (Fig 4a) indicated significant monitor-associated differences in oxy-PAHs and individual parent PAHs (Table A3). This agrees with previous findings of higher PAH concentrations on personal versus kitchen indoor monitors in Poland.37 However, a similar study in China found kitchen monitors collected more PAHs than personal monitors.9 Variability in these studies is likely due to a range of factors in the participants: home fuel sources, daily activities, kitchen monitor placement, regional outdoor factors, season of sampling, and methods of filter extraction. Higher prevalence of oxy-PAHs in the personal monitor PM2.5 samples coincided with the greater oxidative potential and bioactivity of the personal samples. Oxygenated organic compounds were previously correlated with the oxidative potential of particulate matter samples34 and exposure to individual oxy-PAHs has been shown to induce developmental toxicity in zebrafish.21
Figure 4:
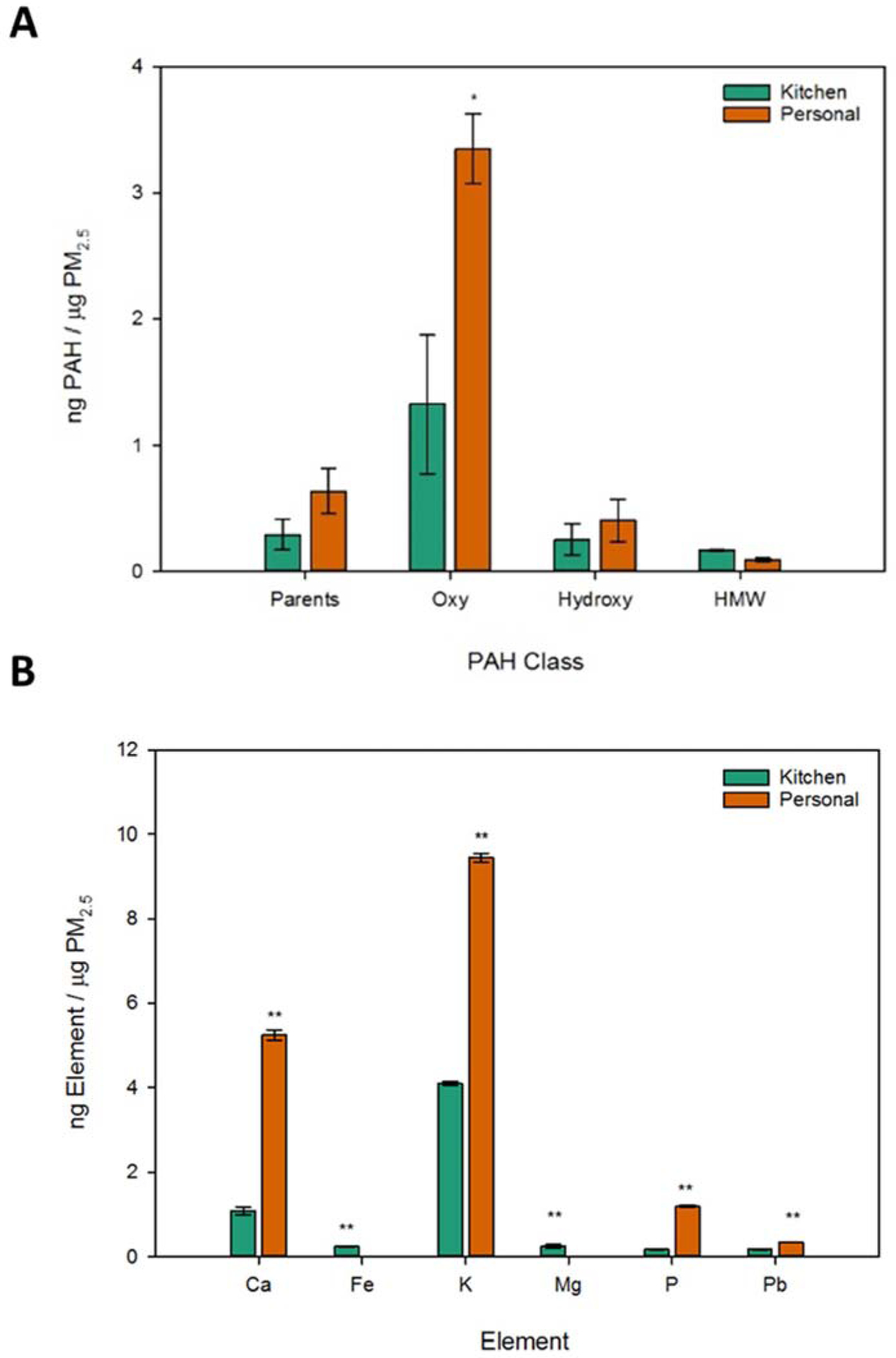
PAH and element concentrations in PM2.5 from kitchen or 309 personal monitors. Following pooling of household samples by monitor type (each group comprised of 6 filter extracts), aliquots of the PM2.5 kitchen and personal samples were analyzed for PAHs (n=114, a) via GC-MS and elements (n=14, b) via ICP-OES. Data is reported as concentration ± standard error mean (SEM). Data was blank corrected (laboratory and filter blank) and normalized by μg of PM2.5 with all samples run in triplicate. Statistical significance between monitor types was determined by one-way ANOVA, with p ≤ 0.05 designated as * and p ≤ 0.001 as **.
Elemental concentrations in PM2.5 (Fig 4b) showed significant differences between monitor types for all quantifiable elements. Normalized concentrations were over double for Ca, K, P, and Pb in the personal monitor samples compared to the kitchen monitor samples. These elements have previously been associated with increased DTT consumption.38,39 Elevated elemental concentrations from personal monitors compared to indoor kitchen monitors was previously reported for households in urban areas across the United States.40 The normalized concentrations of Fe and Mg were elevated in the kitchen monitors compared to the personal monitor samples which had concentrations below the limit of detection. Sources of indoor Fe have previously been associated with re-suspension of crustal elements in households,41 a factor that would potentially be elevated with the kitchen monitor compared to the personal monitors as the kitchen monitors were placed in an area of high activity for all members of the household, not just the individual wearing the personal monitor.
When assessing the composition of pooled kitchen versus personal monitor PM2.5 samples, the class profiles were similar, but more PAH compounds were detected in each class from the kitchen monitors (Fig 5a). Fifty compounds were common to the kitchen and personal PM2.5 samples, 16 PAHs were unique to the kitchen monitor samples and 3 PAHs and one metal were unique to the personal monitor samples (Fig 5b). While more compounds were detected in the kitchen monitor PM2.5 this did not translate to increased chemical contribution per μg of PM2.5 (Fig 4). This is likely due to the contributions of individual oxy-PAHs (1,4-BQ, CdefPhen, 2-M-9,10-AntQ, AceQ, and BcdPyrn) that were at least two-fold in the personal samples versus the kitchen (Table A3).
Figure 5:
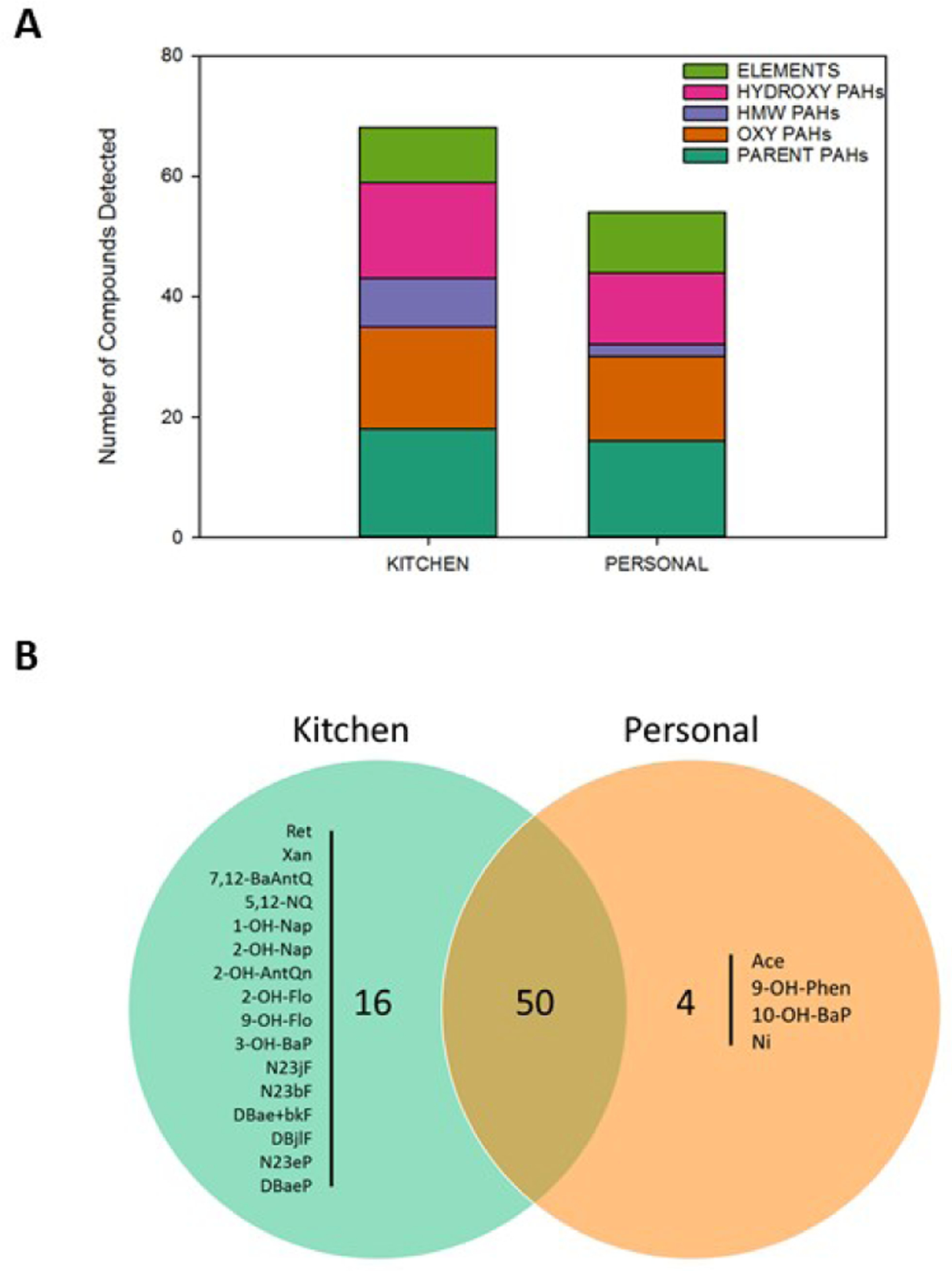
Detected constituents in kitchen and personal PM2.5 samples. Following pooling of household samples by monitor type (each group comprised of 6 filter extracts), aliquots of the PM2.5 kitchen and personal samples were analyzed for PAHs (n=114) and elements (n=14). A) Total number of detected compounds are reported for classes of PAHs and elements. B) Venn diagram of overlap of detected compounds between the monitor types.
The PAHs unique to the kitchen monitors included several HMW PAHs. The low-temperature combustion sources and carcinogenicity of HMW PAHs are generally understood, though air concentration data for HMW PAHs is less abundant than for lower MW PAHs.42–44 Nickel (Ni) was unique to the personal sample pool and has been shown to consume DTT39 which may explain the higher oxidative potential of the personal samples. We note that Acenaphthene and 10-Hydroxybenzo(a)pyrene were unique to the personal monitor sample pool. They were previously inactive when tested alone,21,45 but here were indirectly associated with mortality. The other PAH unique to the personal PM2.5 sample, 9-Hydroxyphenanthrene,45 was previously described as bioactive, inducing mortality at comparatively low concentrations to other PAHs. Possible inconsistencies between previous studies and the present, and the larger number of total and unique compounds in the kitchen monitor sample, suggests the need for deeper investigation into the effects of mixtures in these assays.
Diagnostic ratios of PAHs were calculated to identify differences in the sources of PM2.5 for each sample (Table 2). This commonly used technique allows for analysis of the sources present from a mixture (i.e. PM2.5) by identifying ratios between PAHs that have similar environmental fate processes.46 Based on the ratios from the kitchen and personal monitor samples, it is clear that the primary sources were from combustion of fuel sources, likely natural gas or coal. Participants in these homes reported using LPG as their primary fuel source however secondary fuel sources such as coal may have also been used but not reported. Differences in the sources were not observed between the two monitoring types analysed based on the PAH diagnostic ratio cut-offs. Therefore, it is unlikely that kitchen versus personal monitor differences in oxidative potential, bioactivity, and chemical constituents was due to exposure differences in these specific sources. The observed differences in the metrics may be due to other sources or exposures not captured by the PAH diagnostic ratios.
Table 2:
PAH Diagnostic Ratios
| Ratio | Kitchen | Personal | Potential Sources/Characteristics | References |
|---|---|---|---|---|
| BaA/(BaA+CHR) | 0.30 | 0.27 | 0.24–0.54: Natural gas combustion | (Galarneau 2008)47 |
| IcdP/(IcdP+BghiP) | 0.92 | 0.85 | >0.50: Grass, wood, and coal combustion | (Ravindra et al. 2008, Yunker et al. 2002)48,49 |
| FLA/(FLA+Pyr) | 0.58 | 0.54 | >0.50: Grass, wood, and coal combustion | (Contini et al. 2011, Yunker et al. 2002)48,50 |
| Fl/(FL+PYR) | 0.21 | 0.21 | <0.50: Petrol emissions | (Ravindra et al. 2008)49 |
| Ant/(Ant+PHE) | 0.72 | 0.19 | >0.10: Pyrogenic | (Pies et al. 2008)51 |
| Ret/(Ret+CHR) | 0.01 | 0 | Wood burning ~1 | (Tobiszewski and Namiesnik 2012)46 |
| BaP/BghiP | 1.70 | 0.94 | >0.90: Coal | (Wu et al. 2007)52 |
3.5. Associations to PM2.5 and Chemical Constituents
Significant associations between PM2.5 and oxidative potential (Pearson correlations) or zebrafish mortality (odds ratios) are reported in Table A4. PM2.5 mass did not have a significant association to either endpoint, but 9-Flon and 2-M-9,10-AntQwere significantly associated with the endpoints. Both of these compounds are oxy-PAHs, a class of compounds that have previously been associated with increased concentration in homes,9 toxicity53 and zebrafish developmental toxicity54 compared to their parent PAHs. Oxy-PAHs were significantly elevated in the personal monitor samples consistent with the elevated oxidative potential and zebrafish mortality. Our data from these specific samples suggest that oxy-PAH concentration may be more predictive of oxidative potential and toxicity than PM2.5 mass or other chemical components. Importantly, this work highlights the potential association between oxidative potential and biological responses, a recent study observed positive correlations between these two factors55 but further investigation is needed.
4. CONCLUSIONS
We demonstrate a comprehensive workflow to examine the oxidative potential, bioactivity and chemical composition of PM2.5. This workflow was applied to kitchen and personal PM2.5 to characterize exposures occurring in households with the same fuel source, LPG. In this proof of concept study, we demonstrated that there were significant differences in the oxidative potential, bioactivity, and chemical constituents compared to PM2.5 mass, as well as between households and between personal and household exposures. Personal monitor samples, while lower in PM2.5 concentration, were more bioactive, possibly due to a larger mass of oxy-PAH or even a single dominant oxy-PAH species present in the pooled personal sample. Our work suggests the importance of chemical composition in understanding and eventually predicting PM2.5 exposure hazard. Further research is needed to replicate this method in a larger sample of indoor and personal PM2.5 samples.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank members of SARL for assistance with fish husbandry and developmental screening. This work is supported by the National Institutes of Health (NIH) Grants [P42 ES016465, P30 ES000210, T32 ES007060, and DP5OD019850]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Appendix A (Supplementary Information)
Appendix B (Raw Zebrafish Data)
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
REFERENCES
- (1).Household air pollution and health https://www.who.int/news-room/fact-sheets/detail/household-air-pollution-and-health (accessed Oct 30, 2019).
- (2).Chafe ZA; Brauer M; Klimont Z; Van Dingenen R; Mehta S; Rao S; Riahi K; Dentener F; Smith KR Household Cooking with Solid Fuels Contributes to Ambient PM2.5 Air Pollution and the Burden of Disease. Environ. Health Perspect 2014, 122 (12), 1314–1320. 10.1289/ehp.1206340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Liao J; Zimmermann Jin A; Chafe ZA; Pillarisetti A; Yu T; Shan M; Yang X; Li H; Liu G; Smith KR The Impact of Household Cooking and Heating with Solid Fuels on Ambient PM 2.5 in Peri-Urban Beijing. Atmos. Environ 2017, 165, 62–72. 10.1016/j.atmosenv.2017.05.053. [DOI] [Google Scholar]
- (4).Martin WJ; Glass RI; Balbus JM; Collins FS A Major Environmental Cause of Death. Science 2011, 334 (6053), 180–181. 10.1126/science.1213088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Pope CA; Thun MJ; Namboodiri MM; Dockery DW; Evans JS; Speizer FE; Heath CW Particulate Air Pollution as a Predictor of Mortality in a Prospective Study of U.S. Adults. Am. J. Respir. Crit. Care Med 1995, 151 (3 Pt 1), 669–674. 10.1164/ajrccm/151.3_Pt_1.669. [DOI] [PubMed] [Google Scholar]
- (6).Ostro B; Roth L; Malig B; Marty M The Effects of Fine Particle Components on Respiratory Hospital Admissions in Children. Environ. Health Perspect 2009, 117 (3), 475–480. 10.1289/ehp.11848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Brook RD; Rajagopalan S; Pope CA; Brook JR; Bhatnagar A; Diez-Roux AV; Holguin F; Hong Y; Luepker RV; Mittleman MA; et al. Particulate Matter Air Pollution and Cardiovascular Disease: An Update to the Scientific Statement from the American Heart Association. Circulation 2010, 121 (21), 2331–2378. 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- (8).Janssen NAH; Yang A; Strak M; Steenhof M; Hellack B; Gerlofs-Nijland ME; Kuhlbusch T; Kelly F; Harrison R; Brunekreef B; et al. Oxidative Potential of Particulate Matter Collected at Sites with Different Source Characteristics. Sci. Total Environ 2014, 472, 572–581. 10.1016/j.scitotenv.2013.11.099. [DOI] [PubMed] [Google Scholar]
- (9).Ding J; Zhong J; Yang Y; Li B; Shen G; Su Y; Wang C; Li W; Shen H; Wang B; et al. Occurrence and Exposure to Polycyclic Aromatic Hydrocarbons and Their Derivatives in a Rural Chinese Home through Biomass Fuelled Cooking. Environ. Pollut. Barking Essex 1987 2012, 169, 160–166. 10.1016/j.envpol.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Strak M; Janssen N; Beelen R; Schmitz O; Vaartjes I; Karssenberg D; van den Brink C; Bots ML; Dijst M; Brunekreef B; et al. Long-Term Exposure to Particulate Matter, NO2 and the Oxidative Potential of Particulates and Diabetes Prevalence in a Large National Health Survey. Environ. Int 2017, 108, 228–236. 10.1016/j.envint.2017.08.017. [DOI] [PubMed] [Google Scholar]
- (11).Dominici F; Wang Y; Correia AW; Ezzati M; Pope CA; Dockery DW Chemical Composition of Fine Particulate Matter and Life Expectancy: In 95 US Counties Between 2002 and 2007. Epidemiol. Camb. Mass 2015, 26 (4), 556–564. 10.1097/EDE.0000000000000297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Strak M; Janssen NAH; Godri KJ; Gosens I; Mudway IS; Cassee FR; Lebret E; Kelly FJ; Harrison RM; Brunekreef B; et al. Respiratory Health Effects of Airborne Particulate Matter: The Role of Particle Size, Composition, and Oxidative Potential-the RAPTES Project. Environ. Health Perspect 2012, 120 (8), 1183–1189. 10.1289/ehp.1104389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Roper C; Simonich SLM; Tanguay RL Development of a High-Throughput in Vivo Screening Platform for Particulate Matter Exposures. Environ. Pollut 2018, 235, 993–1005. 10.1016/j.envpol.2018.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Stevens JS; Padilla S; DeMarini DM; Hunter DL; Martin WK; Thompson LC; Gilmour MI; Hazari MS; Farraj AK Zebrafish Locomotor Responses Reveal Irritant Effects of Fine Particulate Matter Extracts and a Role for TRPA1. Toxicol. Sci. Off. J. Soc. Toxicol 2018, 161 (2), 290–299. 10.1093/toxsci/kfx217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Truong L; Gonnerman G; Simonich MT; Tanguay RL Assessment of the Developmental and Neurotoxicity of the Mosquito Control Larvicide, Pyriproxyfen, Using Embryonic Zebrafish. Environ. Pollut. Barking Essex 1987 2016, 218, 1089–1093. 10.1016/j.envpol.2016.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Mandrell D; Truong L; Jephson C; Sarker MR; Moore A; Lang C; Simonich MT; Tanguay RL Automated Zebrafish Chorion Removal and Single Embryo Placement: Optimizing Throughput of Zebrafish Developmental Toxicity Screens. J. Lab. Autom 2012, 17 (1), 66–74. 10.1177/2211068211432197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Phillips JB; Westerfield M Zebrafish Models in Translational Research: Tipping the Scales toward Advancements in Human Health. Dis. Model. Mech 2014, 7 (7), 739–743. 10.1242/dmm.015545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Arku RE; Birch A; Shupler M; Yusuf S; Hystad P; Brauer M Characterizing Exposure to Household Air Pollution within the Prospective Urban Rural Epidemiology (PURE) Study. Environ. Int 2018, 114, 307–317. 10.1016/j.envint.2018.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Perrone MG; Zhou J; Malandrino M; Sangiorgi G; Rizzi C; Ferrero L; Dommen J; Bolzacchini E PM Chemical Composition and Oxidative Potential of the Soluble Fraction of Particles at Two Sites in the Urban Area of Milan, Northern Italy. Atmos. Environ 2016, 128, 104–113. 10.1016/j.atmosenv.2015.12.040. [DOI] [Google Scholar]
- (20).Kramer AJ; Rattanavaraha W; Zhang Z; Gold A; Surratt JD; Lin Y-H Assessing the Oxidative Potential of Isoprene-Derived Epoxides and Secondary Organic Aerosol. Atmos. Environ 2016, 130, 211–218. 10.1016/j.atmosenv.2015.10.018. [DOI] [Google Scholar]
- (21).Knecht AL; Goodale BC; Truong L; Simonich MT; Swanson AJ; Matzke MM; Anderson KA; Waters KM; Tanguay RL Comparative Developmental Toxicity of Environmentally Relevant Oxygenated PAHs. Toxicol. Appl. Pharmacol 2013, 271 (2), 266–275. 10.1016/j.taap.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Noyes PD; Haggard DE; Gonnerman GD; Tanguay RL Advanced Morphological - Behavioral Test Platform Reveals Neurodevelopmental Defects in Embryonic Zebrafish Exposed to Comprehensive Suite of Halogenated and Organophosphate Flame Retardants. Toxicol. Sci. Off. J. Soc. Toxicol 2015, 145 (1), 177–195. 10.1093/toxsci/kfv044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Lai AM; Carter E; Shan M; Ni K; Clark S; Ezzati M; Wiedinmyer C; Yang X; Baumgartner J; Schauer JJ Chemical Composition and Source Apportionment of Ambient, Household, and Personal Exposures to PM2.5 in Communities Using Biomass Stoves in Rural China. Sci. Total Environ 2019, 646, 309–319. 10.1016/j.scitotenv.2018.07.322. [DOI] [PubMed] [Google Scholar]
- (24).Adgate JL; Ramachandran G; Pratt GC; Waller LA; Sexton K Spatial and Temporal Variability in Outdoor, Indoor, and Personal PM2.5 Exposure. Atmos. Environ 2002, 36 (20), 3255–3265. 10.1016/S1352-2310(02)00326-6. [DOI] [Google Scholar]
- (25).Rodes CE; Lawless PA; Evans GF; Sheldon LS; Williams RW; Vette AF; Creason JP; Walsh D The Relationships between Personal PM Exposures for Elderly Populations and Indoor and Outdoor Concentrations for Three Retirement Center Scenarios. J. Expo. Sci. Environ. Epidemiol 2001, 11 (2), 103–115. 10.1038/sj.jea.7500155. [DOI] [PubMed] [Google Scholar]
- (26).Habil M; Massey DD; Taneja A Personal and Ambient PM2.5 Exposure Assessment in the City of Agra. Data Brief 2016, 6, 495–502. 10.1016/j.dib.2015.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).WHO | Air pollution http://www.who.int/airpollution/en/ (accessed Sep 27, 2019).
- (28).Massey D; Masih J; Kulshrestha A; Habil M; Taneja A Indoor/Outdoor Relationship of Fine Particles Less than 2.5μm (PM2.5) in Residential Homes Locations in Central Indian Region. Build. Environ 2009, 44 (10), 2037–2045. 10.1016/j.buildenv.2009.02.010. [DOI] [Google Scholar]
- (29).Pekey B; Bozkurt ZB; Pekey H; Doğan G; Zararsiz A; Efe N; Tuncel G Indoor/Outdoor Concentrations and Elemental Composition of PM10/PM2.5 in Urban/Industrial Areas of Kocaeli City, Turkey. Indoor Air 2010, 20 (2), 112–125. 10.1111/j.1600-0668.2009.00628.x. [DOI] [PubMed] [Google Scholar]
- (30).Monn C; Fuchs A; Högger D; Junker M; Kogelschatz D; Roth N; Wanner HU Particulate Matter Less than 10 Microns (PM10) and Fine Particles Less than 2.5 Microns (PM2.5): Relationships between Indoor, Outdoor and Personal Concentrations. Sci. Total Environ 1997, 208 (1–2), 15–21. 10.1016/s0048-9697(97)00271-4. [DOI] [PubMed] [Google Scholar]
- (31).Perrino C; Tofful L; Canepari S Chemical Characterization of Indoor and Outdoor Fine Particulate Matter in an Occupied Apartment in Rome, Italy. Indoor Air 2016, 26 (4), 558–570. 10.1111/ina.12235. [DOI] [PubMed] [Google Scholar]
- (32).Holm SM; Balmes J; Gillette D; Hartin K; Seto E; Lindeman D; Polanco D; Fong E Cooking Behaviors Are Related to Household Particulate Matter Exposure in Children with Asthma in the Urban East Bay Area of Northern California. PloS One 2018, 13 (6), e0197199 10.1371/journal.pone.0197199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Levy JI; Dumyahn T; Spengler JD Particulate Matter and Polycyclic Aromatic Hydrocarbon Concentrations in Indoor and Outdoor Microenvironments in Boston, Massachusetts. J. Expo. Anal. Environ. Epidemiol 2002, 12 (2), 104–114. 10.1038/sj.jea.7500203. [DOI] [PubMed] [Google Scholar]
- (34).Stevanovic S; Miljevic B; Surawski NC; Fairfull-Smith KE; Bottle SE; Brown R; Ristovski ZD Influence of Oxygenated Organic Aerosols (OOAs) on the Oxidative Potential of Diesel and Biodiesel Particulate Matter. Environ. Sci. Technol 2013, 47 (14), 7655–7662. 10.1021/es4007433. [DOI] [PubMed] [Google Scholar]
- (35).Verma V; Fang T; Xu L; Peltier RE; Russell AG; Ng NL; Weber RJ Organic Aerosols Associated with the Generation of Reactive Oxygen Species (ROS) by Water-Soluble PM 2.5. Environ. Sci. Technol 2015, 49 (7), 4646–4656. 10.1021/es505577w. [DOI] [PubMed] [Google Scholar]
- (36).Duan J; Hu H; Zhang Y; Feng L; Shi Y; Miller MR; Sun Z Multi-Organ Toxicity Induced by Fine Particulate Matter PM2.5 in Zebrafish (Danio Rerio) Model. Chemosphere 2017, 180, 24–32. 10.1016/j.chemosphere.2017.04.013. [DOI] [PubMed] [Google Scholar]
- (37).Choi H; Spengler J Source Attribution of Personal Exposure to Airborne Polycyclic Aromatic Hydrocarbon Mixture Using Concurrent Personal, Indoor, and Outdoor Measurements. Environ. Int 2014, 63, 173–181. 10.1016/j.envint.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Cheung KL; Ntziachristos L; Tzamkiozis T; Schauer JJ; Samaras Z; Moore KF; Sioutas C Emissions of Particulate Trace Elements, Metals and Organic Species from Gasoline, Diesel, and Biodiesel Passenger Vehicles and Their Relation to Oxidative Potential. Aerosol Sci. Technol 2010, 44 (7), 500–513. 10.1080/02786821003758294. [DOI] [Google Scholar]
- (39).Charrier JG; Anastasio C On Dithiothreitol (DTT) as a Measure of Oxidative Potential for Ambient Particles: Evidence for the Importance of Soluble Transition Metals. Atmospheric Chem. Phys. Print 2012, 12 (5), 11317–11350. 10.5194/acpd-12-11317-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Brokamp C; Rao MB; Fan Z. (Tina); Ryan PH. Does the Elemental Composition of Indoor and Outdoor PM2.5 Accurately Represent the Elemental Composition of Personal PM2.5? Atmos. Environ 2015, 101, 226–234. 10.1016/j.atmosenv.2014.11.022. [DOI] [Google Scholar]
- (41).Habre R; Coull B; Moshier E; Godbold J; Grunin A; Nath A; Castro W; Schachter N; Rohr A; Kattan M; et al. Sources of Indoor Air Pollution in New York City Residences of Asthmatic Children. J. Expo. Sci. Environ. Epidemiol 2014, 24 (3), 269–278. 10.1038/jes.2013.74. [DOI] [PubMed] [Google Scholar]
- (42).Wei S; Liu M; Huang B; Bi X; Sheng G; Fu J Polycyclic Aromatic Hydrocarbons with Molecular Weight 302 in PM2.5 at Two Industrial Sites in South China. J. Environ. Monit 2011, 13 (9), 2568 10.1039/c1em10320b. [DOI] [PubMed] [Google Scholar]
- (43).Bian Q; Alharbi B; Collett J; Kreidenweis S; Pasha MJ Measurements and Source Apportionment of Particle-Associated Polycyclic Aromatic Hydrocarbons in Ambient Air in Riyadh, Saudi Arabia. Atmos. Environ 2016, 137, 186–198. 10.1016/j.atmosenv.2016.04.025. [DOI] [Google Scholar]
- (44).Chen S-J; Wang J; Wang T; Wang T; Mai B-X; Simonich SLM Seasonal Variations and Source Apportionment of Complex Polycyclic Aromatic Hydrocarbon Mixtures in Particulate Matter in an Electronic Waste and Urban Area in South China. Sci. Total Environ 2016, 573, 115–122. 10.1016/j.scitotenv.2016.08.101. [DOI] [PubMed] [Google Scholar]
- (45).Geier MC; Chlebowski AC; Truong L; Massey Simonich SL; Anderson KA; Tanguay RL Comparative Developmental Toxicity of a Comprehensive Suite of Polycyclic Aromatic Hydrocarbons. Arch. Toxicol 2018, 92 (2), 571–586. 10.1007/s00204-017-2068-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Tobiszewski M; Namieśnik J PAH Diagnostic Ratios for the Identification of Pollution Emission Sources. Environ. Pollut 2012, 162, 110–119. 10.1016/j.envpol.2011.10.025. [DOI] [PubMed] [Google Scholar]
- (47).Galarneau E Source Specificity and Atmospheric Processing of Airborne PAHs: Implications for Source Apportionment. Atmos. Environ 2008, 42 (35), 8139–8149. 10.1016/j.atmosenv.2008.07.025. [DOI] [Google Scholar]
- (48).Yunker MB; Macdonald RW; Vingarzan R; Mitchell RH; Goyette D; Sylvestre S PAHs in the Fraser River Basin: A Critical Appraisal of PAH Ratios as Indicators of PAH Source and Composition. Org. Geochem 2002, 33 (4), 489–515. 10.1016/S0146-6380(02)00002-5. [DOI] [Google Scholar]
- (49).Ravindra K; Sokhi R; Vangrieken R Atmospheric Polycyclic Aromatic Hydrocarbons: Source Attribution, Emission Factors and Regulation. Atmos. Environ 2008, 42 (13), 2895–2921. 10.1016/j.atmosenv.2007.12.010. [DOI] [Google Scholar]
- (50).Contini D; Gambaro A; Belosi F; De Pieri S; Cairns WRL; Donateo A; Zanotto E; Citron M The Direct Influence of Ship Traffic on Atmospheric PM2.5, PM10 and PAH in Venice. J. Environ. Manage 2011, 92 (9), 2119–2129. 10.1016/j.jenvman.2011.01.016. [DOI] [PubMed] [Google Scholar]
- (51).Pies C; Hoffmann B; Petrowsky J; Yang Y; Ternes TA; Hofmann T Characterization and Source Identification of Polycyclic Aromatic Hydrocarbons (PAHs) in River Bank Soils. Chemosphere 2008, 72 (10), 1594–1601. 10.1016/j.chemosphere.2008.04.021. [DOI] [PubMed] [Google Scholar]
- (52).Wu S-P; Tao S; Zhang Z-H; Lan T; Zuo Q Characterization of TSP-Bound n-Alkanes and Polycyclic Aromatic Hydrocarbons at Rural and Urban Sites of Tianjin, China. Environ. Pollut. Barking Essex 1987 2007, 147 (1), 203–210. 10.1016/j.envpol.2006.08.020. [DOI] [PubMed] [Google Scholar]
- (53).Lundstedt S; White PA; Lemieux CL; Lynes KD; Lambert IB; Öberg L; Haglund P; Tysklind M Sources, Fate, and Toxic Hazards of Oxygenated Polycyclic Aromatic Hydrocarbons (PAHs) at PAH- Contaminated Sites. AMBIO J. Hum. Environ 2007, 36 (6), 475–485. 10.1579/0044-7447(2007)36[475:SFATHO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- (54).Trine LSD; Davis EL; Roper C; Truong L; Tanguay RL; Simonich SLM Formation of PAH Derivatives and Increased Developmental Toxicity during Steam Enhanced Extraction Remediation of Creosote Contaminated Superfund Soil. Environ. Sci. Technol 2019, 53 (8), 4460–4469. 10.1021/acs.est.8b07231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Romano S; Perrone MR; Becagli S; Pietrogrande MC; Russo M; Caricato R; Lionetto MG Ecotoxicity, Genotoxicity, and Oxidative Potential Tests of Atmospheric PM10 Particles. Atmos. Environ 2020, 221, 117085 10.1016/j.atmosenv.2019.117085. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


