Abstract
Neurodevelopmental disorders are defined by highly heritable problems during development and brain growth. Attention-deficit/hyperactivity disorder (ADHD), autism spectrum disorders (ASDs), and intellectual disability (ID) are frequent neurodevelopmental disorders, with common comorbidity among them. Imaging genetics studies on the role of disease-linked genetic variants on brain structure and function have been performed to unravel the etiology of these disorders. Here, we reviewed imaging genetics literature on these disorders attempting to understand the mechanisms of individual disorders and their clinical overlap. For ADHD and ASD, we selected replicated candidate genes implicated through common genetic variants. For ID, which is mainly caused by rare variants, we included genes for relatively frequent forms of ID occurring comorbid with ADHD or ASD. We reviewed case-control studies and studies of risk variants in healthy individuals. Imaging genetics studies for ADHD were retrieved for SLC6A3/DAT1, DRD2, DRD4, NOS1, and SLC6A4/5HTT. For ASD, studies on CNTNAP2, MET, OXTR, and SLC6A4/5HTT were found. For ID, we reviewed the genes FMR1, TSC1 and TSC2, NF1, and MECP2. Alterations in brain volume, activity, and connectivity were observed. Several findings were consistent across studies, implicating e.g. SLC6A4/5HTT in brain activation and functional connectivity related to emotion regulation. However, many studies had small sample sizes, and hypothesis-based, brain region-specific studies were common. Results from available studies confirm that imaging genetics can provide insight into the link between genes, disease-related behavior, and the brain. However, the field is still in its early stages, and conclusions about shared mechanisms cannot yet be drawn.
Keywords: ADHD, ASD, ID, brain imaging genetics, neurodevelopmental disorders
Introduction
Neurodevelopmental disorders are broadly defined as disorders in the development and growth of the brain (Goldstein 1999), but this term is largely used to describe neurological and psychiatric disorders that have their onset prior to adulthood. Most neurodevelopmental disorders are highly heritable, either caused by single genetic defects, like many of the intellectual disability (ID) disorders (Deciphering Developmental Disorders Study 2015), or with a more multifactorial background, in which several to multiple less penetrant genetic variants cause the disease in combination with environmental factors, like in many cases of autism spectrum disorders (ASDs; (Gaugler et al., 2014; Iossifov et al., 2014), as well as in attention-deficit/hyperactivity disorder (ADHD; (Faraone et al., 2015; Franke et al., 2012), oppositional defiant disorder, and conduct disorder (Salvatore and Dick 2016).
While technological advances in the last decade, especially genome-wide association studies (GWASs) and next generation sequencing, have enabled the identification of many genetic factors involved, the biological mechanisms contributing to the neurodevelopmental disorders are still largely unknown. It is thought that gene variation/mutation will alter molecular and cellular processes, which leads to altered brain development, be it structurally and/or functionally, and subsequently to altered behavior and disease symptoms (Franke et al., 2009). Measures that mediate the effects of genes on behavioral/disease phenotypes have been termed endophenotypes or intermediate phenotypes (Gottesman and Gould 2003; Kendler and Neale 2010).
Much research into the consequences of gene aberrations is performed in animal models. However, brain imaging methods like magnetic resonance imaging (MRI), electroencephalography (EEG), and magnetoencephalography (MEG) offer excellent ways to investigate the effects of genetic variation on brain structure, function, and connectivity directly in humans in vivo. Such ‘imaging genetics’ approaches can unveil the brain-biological consequences of molecular changes induced by genetic variants – both common and rare – linked to neurodevelopmental disorders. In that way they can help to understand the mechanisms through which differences in behavior arise. It has been argued that the effects of disease-linked (common) genetic variation on the brain would be larger than those on behavior and clinical phenotypes (Gottesman and Gould 2003; Rose and Donohoe 2013)), although more recent work using hypothesis-free imaging genetics approaches argues against this – at least for brain structural phenotypes (Franke et al., 2016).
Different neuroimaging methods can be used in imaging genetics studies, including different forms of structural and functional MRI as well as EEG and MEG. They have complementary characteristics enabling information to be gathered on different aspects of (gene effects on) brain anatomy and function, like location (especially MRI-based methods) and timing (especially EEG and MEG). In this review, we concentrated on those methods that have most frequently been used in imaging genetics studies of neurodevelopmental disorders, i.e. MRI-based methods evaluating gene effects on brain structure, function, and connectivity.
With structural magnetic resonance imaging (sMRI) it is possible to noninvasively characterize the structure of the human brain. Thereby, the different magnetic properties of brain tissues are used to map the spatial distribution of these structural properties of the brain. In this way, the different brain tissues (grey and white matter) and cortical and subcortical structures of the brain can be mapped. By adapting scanning parameters, different weighting techniques of the signal can be used, such as T1-weighted imaging (used to visualise anatomy) and T2-weighted imaging (which is useful for demonstrating lesions and pathology). Different aspects of brain structure can be used for quantitative analyses. To investigate whether volumetric differences are global or regional, specific brain regions of interest (ROIs) can be selected a priori and studied individually. In contrast, global changes in grey or white matter intensity can be detected by using voxel-based morphometry (VBM) analyses. Next to volumetric differences observed in grey matter, structural differences of white matter connectivity can also be quantified. With the help of diffusion tensor imaging (DTI), it is possible to non-invasively investigate the macrostructural integrity and orientation of white matter fibre bundles. Thereby, the directional diffusion of water molecules along neuronal membranes is measured, allowing to map white matter connection within the brain. Multiple measures can be derived from DTI. A frequently measured parameter is fractional anisotropy (FA). Basically, anisotropy indicates that diffusion takes place in a directional manner, whereas isotropy indicates diffusion in all directions. Additional DTI-derived parameters include mean diffusivity (MD; average of axial diffusivity (AD) and perpendicular diffusivities), and radial diffusivity (RD; average of perpendicular diffusivities), the mode of anisotropy (sensitive to crossing fibres), and the apparent diffusion coefficient (indicating the magnitude of diffusion) (Le Bihan 2003; Le Bihan et al., 2001; Yoncheva et al., 2016).
Resting state functional MRI (rs-fMRI), allows to analyse the temporal correlations of neural activity across anatomically disparate brain regions and thereby to examine the functional connectivity based on spontaneous brain activity, neural organization, and circuit architecture.
To investigate potential changes in brain activity, functional magnetic resonance imaging (fMRI) can be used. Since fMRI is sensitive to the oxygenation of the blood, the so-called blood-oxygen-level-dependent (BOLD) signal can be measured. Thereby brain function is measured, based on the premise that active cells consume oxygen, thus causing changes in blood oxygenation, and subsequently leading to increased blood flow. However, the exact link between cell activation, oxygen saturation, and cerebral blood flow changes is debatable (Hillman 2014). Generally in fMRI, alterations in blood flow after e.g. a task-induced stimulus or during a resting condition are measured.
Here, we systematically reviewed the imaging genetics literature for three frequent neurodevelopmental disorders, ADHD, ASDs, and selected intellectual disability (ID) disorders. The choice for those three neurodevelopmental disorders was based on their frequent comorbidity (Vorstman and Ophoff 2013) and robustly established associations with specific genetic variants. The aim of this work was to extract core brain mechanisms affected by disease-linked genetic factors related to the individual disorders as well as their clinical overlap.
ADHD is one of the most common neurodevelopmental disorders, with a prevalence of 5–6% in childhood (American Psychiatric Association 2013; Polanczyk et al., 2007). ADHD can be clinically characterized by two core symptom domains: inattention and hyperactivity/impulsivity (American Psychiatric Association 2013; Faraone et al., 2015). Up to 60% of all patients diagnosed in childhood show ADHD symptoms and/or meet formal diagnostic criteria for the disorder in adulthood, and prevalence rates of persistent ADHD in adults range between 2.5 and 4.9% (Simon et al., 2009). ASD affects approximately 0.6% to 1% of the children, making it one of the most prevalent disorders in childhood (Elsabbagh et al., 2012). Although there are some important differences in core symptom definition, the co-occurrence between ADHD and ASD is supported by clinical (Craig et al., 2015), common biological (Rommelse et al., 2010), and non-biological risk factors (Kroger et al., 2011). Moreover, several studies identified that symptoms of autism or autistic traits appear in 20% to 30% of children with ADHD (Grzadzinski et al., 2011; Kochhar et al., 2011). Additionally, ADHD is a common comorbid disorder in children with ID, and the risk increases with increasing severity of ID (Voigt et al., 2006). Studies of children with mild and borderline ID have identified ADHD in 8% to 39% of the cases (Baker et al., 2010; Dekker and Koot 2003; Emerson 2003). ADHD is highly heritable (heritability 70–80%) (Burt 2009; Faraone et al., 2005). However, identification of ADHD risk genes has been difficult (Franke et al., 2009; Gizer et al., 2009), mainly due to ADHD’s complex genetic background (Faraone et al., 2015; Franke et al., 2012). Mostly genetic variants, which occur quite frequent in the population and have generally small effects on disease risk have been investigated for their role in ADHD until today, either through candidate gene studies or hypothesis-free GWASs. Only a few of the candidate genes have been confirmed through meta-analysis (Gizer et al., 2009). However, none of the eleven GWAS (Hinney et al., 2011; Lasky-Su et al., 2008a; Lasky-Su et al., 2008b; Lesch et al., 2008; Mick et al., 2010; Neale et al., 2008; Neale et al., 2010a; Sanchez-Mora et al., 2014; Sonuga-Barke et al., 2008; Stergiakouli et al., 2012; Yang et al., 2013) nor a meta-analysis of many of them (Neale et al., 2010b) published to date, reported any genome-wide significant risk variant.
ASDs refer to a heterogeneous group of neurodevelopmental disorders diagnosed in approximately 1 of 88 children (Autism and Developmental Disabilities Monitoring Network Surveillance Year 2008 Principal Incestigators and Centers for Disease Control and Prevention 2012). It is characterized by deficits in social behavior and language development, as well as restricted or stereotypic interests (American Psychiatric Association 2013). About 70% of individuals with ASDs have some level of ID while the remaining 30% have some disability (speech, behavior) other than cognitive dysfunction (Mefford et al., 2012). Whereas early reports estimated ASD heritability to be higher than 90% (Bailey et al., 1995; Folstein and Rutter 1977; Ritvo et al., 1985; Steffenburg et al., 1989), recent population-based studies provided an estimate of ~50% heritability (Gaugler et al., 2014; Sandin et al., 2014). ASDs are genetically highly complex, as part of the cases has oligogenic or even monogenic causes (with an important role for de novo mutations (Iossifov et al., 2014)), whereas the concerted action of common genetic variants of individually small effect sizes and environmental factors is likely to cause most of the disease burden of ASDs (Iossifov et al., 2014) (Gaugler et al., 2014; Zhao et al., 2007). Several of those common variants contributing to ASD risk have been identified through hypothesis-driven studies. Until now, three GWASs have been performed for ASDs (Anney et al., 2010; Wang et al., 2009; Weiss et al., 2009), which identified a single locus on chromosome 5p14, in-between CDH10 and CDH9 (Wang et al., 2009). Association with this locus might be driven by markers located within the MSNP1AS pseudogene (Ma et al., 2009).
ID refers to a highly heterogeneous group of disorders characterized by below average intellectual functioning (IQ < 70) in conjunction with significant limitations in adaptive functioning with onset during development. ID may occur as an isolated phenomenon or accompanied with malformations, neurological signs, impairment of the special senses, seizures and behavioral disturbances (van Bokhoven 2011). ID has an estimated prevalence of approximately 2% to 3%, and approximately 0.3% to 0.5% of the population is severely handicapped (Perou et al., 2013). Comorbidity with ADHD and ASDs is frequently observed (Vorstman and Ophoff 2013). Disease etiology of ID is thought to be largely monogenic, but with many different genetic anomalies implicated (van Bokhoven 2011). Genetic causes of ID range from large cytogenetically visible chromosomal aberrations, such as trisomy 21, to translocations, subchromosomal abnormalities (such as Prader-Willi syndrome (15q11.2-q13)), copy number variations, and to single gene defects. We concentrated only on the latter in our review, based on the assumption that we can learn most from understanding effects of specific genes/variants on brain structure, function and connectivity. While in many ID disorders, a defect in a single gene can be identified as the cause of the disorder, only a few genes are hit more frequently and cause relatively common ID disorders. To prevent bias of our review by single case reports, we concentrated on those common forms of ID, especially selecting those, in which comorbidity with ADHD and ASD is common. This resulted in five ID disorders included in this review: fragile X syndrome, tuberous sclerosis, neurofibromatosis type 1, Rett syndrome, and Timothy syndrome. Fragile X syndrome (FXS), caused by genetic defects in the FMR1 gene, is associated with a variable clinical phenotype, including intellectual disabilities with a broad range of severities. IQ is 40 on average for affected men (Merenstein et al., 1996) and normal or borderline in females (de Vries et al., 1996), who show a milder phenotype because the disorder is X-chromosome-linked. High rates of autism and autistic behaviors are seen in individuals with FXS (Hagerman et al., 2009), and 59% of FXS subjects shows ADHD symptoms (Sullivan et al., 2006). Neurofibromatosis type 1 (NF1), caused by mutations in NF1, is associated with the presence of usually benign neurofibromas. While IQ in general is average to low average, up to 8% of children with NF1 have an IQ below 70. Learning difficulties and neuropsychological deficits are common, and the core cognitive impairments are in visual spatial function, attention, executive function, and language skills. About 38% of children with NF1 meet diagnostic criteria for ADHD, and a substantial proportion of subjects show social deficits related to ASD (Hyman et al., 2005; Walsh et al., 2013). Tuberous sclerosis complex (TSC) is caused primarily by mutations in the genes TSC1 and TSC2 and is characterized by benign hamartomas in multiple organ systems, including the brain. Intellectual ability in TSC ranges from normal to profoundly impaired, and neurobehavioral abnormalities and epilepsy are common. Both ASD and ADHD are reported in about 50% of individuals with TSC, with an even higher number of diagnoses in intellectually impaired individuals (Prather and de Vries 2004). Rett syndrome, caused by mutations in the MECP2 gene, primarily affects females. Language problems and cognitive and motor deficits start to become obvious around the age of 6 months in the patients. Testing of cognitive dysfunction is difficult because of a characteristic absence of speech, but ASD-related features, such as avoidance of eye contact, are common (Armstrong 2005). Timothy syndrome is a multisystem disorder caused by missense mutations in the CACNA1C gene. Neurodevelopmental features include global developmental delays and ASDs. Average age of death is 2.5 years, usually caused by ventricular tachyarrhythmia, infection, or complications of hypoglycemia (Splawski et al., 1993).
With this review, we aimed at providing a comprehensive overview on the imaging genetics literature for the three neurodevelopmental disorders. To prevent bias, we excluded reports including less than 10 cases and focused on specific genetic variants, which for ADHD and ASDs resulted in a focus on genes/loci implicated through variants that are common in the population, and for ID, we restricted the review to the genes causing the single-gene ID disorders described above. While imaging genetics studies have been performed in patients, the underlying candidate genes and their common genetic variants are also frequently studied in healthy individuals. This allows analysis of effects of common genetic variation in candidate genes on imaging correlates in the general population and offers the opportunity to study brains not influenced by chronic disease and medication. Previous studies showed that neuroimaging correlates of common genetic variants are likely to be similar in typical and psychiatric populations (Hibar et al., 2015b). As such studies of healthy individuals may also be informative regarding the biological mechanisms leading to the diseases of interest, they were also included in this review.
Methods
Search terms
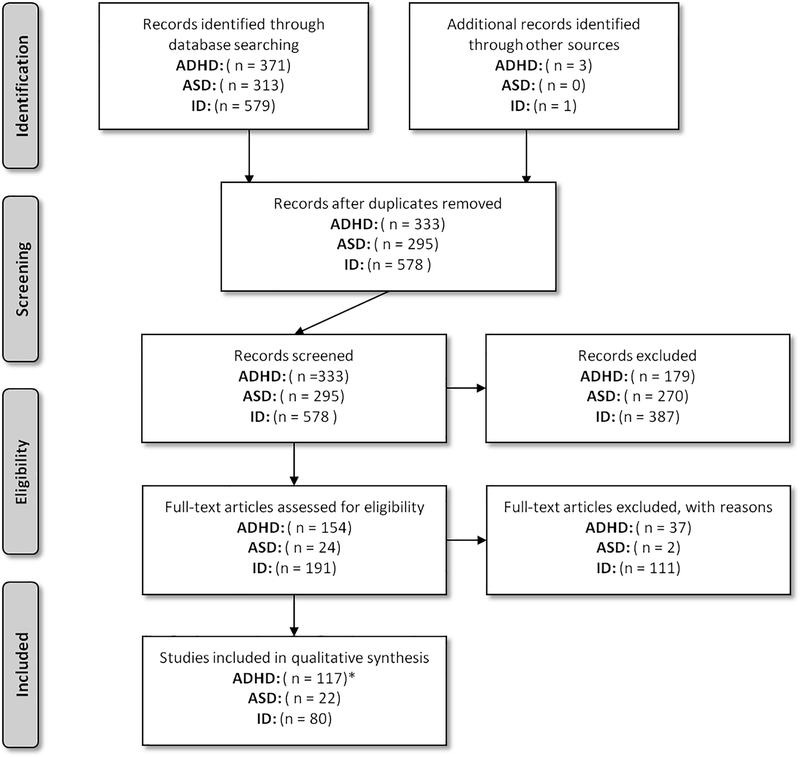
Pubmed was searched for research articles describing imaging genetics studies (April, 14th, 2015; http://www.ncbi.nlm.nih.gov/pubmed). Only studies using magnetic resonance imaging (MRI) were reviewed, specifically structural MRI (sMRI), functional MRI (fMRI), resting-state functional MRI (rs-fMRI), and diffusion tensor imaging (DTI). A general search term was created and was extended by adding the disorder (for ADHD and ASD) or syndrome name and gene (for ID) of interest. The following search term shows an example for ADHD (for [Title/Abstract]): ((((ADHD OR Attention-Deficit Hyperactivity Disorder) AND (gene* OR genetic* OR imaging genetic OR imaging genetics OR genotype OR polymorphism OR SNP OR single nucleotide polymorphism OR meta-analysis OR genome wide association OR GWA OR GWAS)) AND (structural magnetic resonance imaging OR volume OR sMRI OR voxel-based morphometry OR brain morphometry OR brain volumetry OR VBM OR functional magnetic resonance imaging OR fMRI OR diffusion tensor imaging OR diffusion imaging OR connectivity OR tractography OR DTI OR resting-state functional magnetic resonance imaging OR voxel-wise analysis OR rsfMRI)) NOT “review”[Publication Type]). For ID syndromes, the search term did not include (gene* OR genetic* OR imaging genetic OR imaging genetics OR genotype OR polymorphism OR SNP OR single nucleotide polymorphism OR meta-analysis OR genome wide association OR GWA OR GWAS), as the genes of interest were added specifically. Titles and abstracts of the retrieved records were evaluated for relevant publications. Case-reports and reports describing less than 10 cases were excluded to prevent bias, and review articles, medical hypotheses, non-English articles, and studies on animal models were not considered (for a graphical summary of the selection procedure, please see Figure 1).
Figure 1.
Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) flowchart of the literature search and study selection for qualitative analysis. Note: see http://www.prismastatement.org/ for more information in this reporting system. ADHD = Attention-Deficit/Hyperactivity Disorder, ASD = Autism Spectrum Disorder, ID = Intellectual Disability. Records excluded for ID contain unrelated records identified by screening as well as records describing non-ID samples. * The number of studies for ADHD candidate genes also include the records for SLC6A4 (5-HTTLPR), which is also a candidate gene for ASD.
Candidate gene selection for ADHD, ASD, and ID studies
Taking into account the differences in the genetic architecture of the three neurodevelopmental disorders of interest, we defined selection criteria for the genes to be included in this review as similar as possible. The restriction to studies with 10 or more cases and single genetic variants/single-gene mutations largely defined our search strategy, which resulted in a focus on common genetic variants for ADHD and ASDs (minor allele frequency ≥ 1%); for ID disorders, this lead to the selection of relatively common forms of the disorder. For ADHD and ASDs, we selected the most promising genes containing common variants associated with the disorder based on meta-analyses, successful replication studies, and/or significant findings from hypothesis-free (genome-wide) studies.
For ADHD, we included all genes and genetic variants mentioned in Table I of the meta-analytic study by Gizer and coworkers (2009) that had reached a significant result at P ≤ 0.05 for association with ADHD. In addition to this, we also included genes with reported and replicated evidence for association with ADHD from more recent studies. These included two meta-analytic studies (Pan et al., 2015; Wu et al., 2012), a research article (Ribases et al., 2011), and the more recently observed replicated candidate genes NOS1 and SLC9A9 (Stergiakouli et al., 2012; Weber et al., 2015) (total number of candidate genes = 10; Table I). A recent overview of these ADHD candidate genes has been published by Hawi and colleagues (2015).
Table I:
Genes containing common variants most consistently implicated in ADHD, based on (Gizer et al. 2009) and more recent (meta-)analyses.
| Gene | Protein | Associated variant/polymorphism | Risk allele | Location/chr position | References for reports of association with ADHD |
|---|---|---|---|---|---|
| DRD2/ANNK1 | Dopamine receptor D2/ Ankyrin repeat and kinase domain containing 1 | Taq1A (rs1800497) | T allele = A1-allele | Exon 8/ 3’ flanking/11q23 | (Comings et al. 1991)a; (Pan et al. 2015)b |
| DRD4* | Dopamine receptor D4 | 48 bp VNTR | 7 repeat (5 repeat in Asians) | Exon 3/11p15 | (LaHoste et al. 1996)a; (Gizer et al. 2009)b; (Wu et al. 2012)b |
| rs1800955 | T allele | Promoter/11p15 | (Barr et al. 2001)a; (Yang et al. 2008)d; (Gizer et al. 2009)b | ||
| DRD5 | Dopamine receptor D5 | 148 bp dinucleotide repeats | 148 bp allele | 5’ flanking/4p16 | (Daly et al. 1999)a; (Gizer et al. 2009)b; (Wu et al. 2012)b |
| HTR1B | Serotonin receptor 1B, G protein-coupled | rs6296 | G allele | Exon 1/6q14 | (Hawi et al. 2002)a; (Gizer et al. 2009)b |
| LPHN3 | Latrophilin 3 | rs6551665 | G allele | 4q13 | (Arcos-Burgos et al. 2010)a; (Hwang et al. 2015)d; (Ribases et al. 2011)d; (Labbe et al. 2012)a |
| rs6858066 | G allele | ||||
| NOS1* | Nitric oxide synthase 1 | 180-210 bp CA repeat | Short allele | Exon 1/12q24 | (Reif et al. 2009)a; (Franke et al. 2009)c; (Weber et al. 2015)b |
| SLC6A3/DAT1* | Solute Carrier Family 6 (Neurotransmitter Transporter), Member 3; Dopamine transporter 1 | 40 bp VNTR | 10 repeat | 3’ UTR/5p15 | (Cook et al. 1995)a; (Gizer et al. 2009)b |
| rs27072 | G allele | 3’ UTR/5p15 | (Galili-Weisstub and Segman 2003)a; (Gizer et al. 2009)b | ||
| 30 bp VNTR | 6 repeat | Intron 8/5p15 | (Brookes et al. 2006)a; (Gizer et al. 2009)b | ||
| SLC6A4/5HTT* | Solute carrier family 6 (neurotransmitter transporter), member 4; serotonin transporter | 5-HTTLPR | Long allele | Promoter/17q11 | (Manor et al. 2001)a; (Gizer et al. 2009)b; (Landaas et al. 2010)b |
| SLC9A9/NHE9 | Solute Carrier Family 9, Subfamily A, Member 9 | rs9810857 | T allele | Region 3p14-q21 | (de Silva et al. 2003)a; (Stergiakouli et al. 2012)c; (Mick et al. 2010)c |
| SNAP25 | Synaptosomal-associated protein, 25kDa | rs3746544 | T allele | 3’ UTR/20p12 | (Brophy et al. 2002)a; (Gizer et al. 2009)b |
Bold text indicates significant result at P < 0.05 in Gizer et al., 2009.
Association first reported by.
Meta-analysis article.
GWAS finding.
Association in large sample or validation using animal model.
Gene with at least one case-control imaging genetics study
ADHD = Attention deficit/hyperactivity disorder, bp = base pair, chr = chromosome, CNV = copy number variation, UTR = untranslated region, VNTR = variable number tandem repeat; no imaging genetics studies found.
For the ASD genes, we based our selection on the review of the most consistently replicated genes harboring common variants associated with autism by Persico and Napolioni (2013). Additionally, the CDH9/CDH10 locus was included, because it has shown genome-wide significant association with ASD (Prandini et al., 2012; Wang et al., 2009). Selection of the candidate polymorphisms in the selected genes was based on recent research articles, as meta-analyses were only available for the OXTR and RELN gene (total number of candidate genes = 11; Table II).
Table II:
Genes containing common variants most convincingly implicated in ASDs, adapted from Persico and Napolioni (2013). We added CDH9, CDH10, and MSNP1AS, because the locus harbouring these genes has shown genome-wide significant association with ASDs in GWAS (Prandini et al. 2012; Wang et al. 2009). Selection of candidate polymorphisms and risk alleles for ASD was based on recent research articles.
| Gene | Protein | Associated variant/polymorphism | Risk allele | Location/chr position | References for association with ASD |
|---|---|---|---|---|---|
| CDH9 | Cadherin 9 | rs4307059 | C allele | Intergenic/5p14 | (Wang et al. 2009)a,c; (Prandini et al. 2012)d |
| CDH10 | Cadherin 10 | rs4307059 | C allele | Intergenic/5p14 | (Wang et al. 2009)a,c; (Prandini et al. 2012)d |
| MSNP1AS | Moesin pseudogene 1, antisense | rs4307059 | C allele | Intergenic/5p14 | (Wang et al. 2009)a,c; (Prandini et al. 2012)d |
| CNTNAP2* | Contactin associated protein-like 2 | rs7794745 | T allele | Intron 2/7q35 | (Arking et al. 2008)a; (Li et al. 2010)d |
| rs2710102 | C allele | Exon 8/7q35 | (Stein et al. 2011) | ||
| EN2 | Engrailed homeobox 2 | rs1861972 | G allele | Intron/7q36 | (Gharani et al. 2004)a; (Benayed et al. 2005)d |
| rs1861973 | T allele | Intron/7q36 | (Gharani et al. 2004)a; (Benayed et al. 2005)d | ||
| GABRB3 | Gamma-aminobutyric acid (GABA) A receptor, beta 3 | rs7171512 | G allele | Intron/15q12 | (Warrier et al. 2013)a |
| rs7180158 (AS) | G allele | Intron/15q12 | (Warrier et al. 2013)a | ||
| rs7165604 (AS) | T allele | Intron/15q12 | (Warrier et al. 2013)a | ||
| rs12593579 (AS) | C allele | Intron/15q12 | (Warrier et al. 2013)a | ||
| rs9806546 (EQ) | G allele | Intron/15q12 | (Warrier et al. 2013)a | ||
| rs11636966 (EQ) | T allele | Intron/15q12 | (Warrier et al. 2013)a | ||
| ITGB3 | Integrin, beta 3 (platelet glycoprotein IIIa, antigen CD61) | rs12603582 | T allele | Intron 11/17q21.32 | (Napolioni et al. 2011)a; (Schuch et al. 2014)d |
| rs15908 | A allele | Exon 9/17q21.32 | (Schuch et al. 2014)a | ||
| MET* | Met proto-oncogene (hepatocyte growth factor receptor) | rs1858830 | C allele | Promoter/7q31 | (Campbell et al. 2006)a; (Sousa et al. 2009)d; (Thanseem et al. 2010)d; (Zhou et al. 2011)d |
| OXTR | Oxytocin receptor | rs7632287 | A allele | 3’ flanking/3p25 | (Tansey et al. 2010)a; (LoParo and Waldman 2014)b; (Campbell et al. 2011)d |
| rs237887 | A allele | Intron3/3p25 | (Liu et al. 2010)a; (LoParo and Waldman 2014)b | ||
| rs2268491 | T allele | Intron3/3p25 | (Liu et al. 2010)a; (LoParo and Waldman 2014)b | ||
| rs2254298 | A allele | Intron3/3p25 | (Wu et al. 2005)a; (LoParo and Waldman 2014)b; (Liu et al. 2010)d; (Nyffeler et al. 2014)d | ||
| rs2268493 | C allele | Intron3/3p25 | (Yrigollen et al. 2008)a; (Campbell et al. 2011)d; (Di Napoli et al. 2014)d | ||
| rs53576 | A allele | Intron3/3p25 | (Wu et al. 2005)a; (Nyffeler et al. 2014)d | ||
| rs2268494 | T allele | Intron3/3p25 | (Lerer et al. 2008)a | ||
| RELN | Reelin | rs362691 | Population specific? | Exon 22/7q22 | (Wang et al. 2014)b |
| rs362780 | G allele | Intron 41/7q22 | (Holt et al. 2010) a | ||
| rs736707 | Population specific? | Intron 59/7q22 | (Sharma et al. 2013)a | ||
| rs2073559 | T allele | Intron 11/7q22 | (Ashley-Koch et al. 2007) a | ||
| SLC6A4/5HTT* | Serotonin transporter | 5-HTTLPR | Long allele | Promoter/17q11.2 | (Nyffeler et al. 2014)d; (Gadow et al. 2013) |
Association first reported by.
Meta-analysis article.
GWAS finding.
Association in large sample or validation using animal model.
Gene with at least one case-control imaging genetics study
ASD = Autism spectrum disorder, AS = Asperger’s syndrome, chr = chromosome, EQ = empathy quotient); no imaging genetics studies found.
For the ID, the restrictions to relatively common forms of the disorder resulting from single gene mutations (as opposed to structural genetic variants involving several to many genes) as well as our aim to study potential brain mechanisms contributing to comorbidity among the three disorders lead to the inclusion of the following 5 syndromes: fragile X syndrome (FMR1), tuberous sclerosis (TSC1 and TSC2), neurofibromatosis type 1 (NF1), Rett syndrome (MECP2), and Timothy syndrome (CACNA1C) (Table III). For our selection, we used Table I from Vorstman and Ophoff (2013), describing genetic anomalies associated with ID. We included all disorders with known genetic cause including a single gene (FMR1, TSC1 and TSC2, NF1, and CACNA1C). Patients with these disorders also show a high rate of ASD and/or ADHD phenotypes (Vorstman and Ophoff 2013). Additionally, we included the Rett syndrome (MECP2), because of its known ASD- and ADHD-related features (Armstrong 2005; Rose et al., 2016; Suter et al., 2014).
Table III:
Genes causing prevalent and well-studied single-gene ID disorders with behavioral and cognitive overlap with ADHD and/or ASD.
| Gene | Protein | Chr position | Associated ID disorder | Reported rate of ASD-related phenotype | Reported rate of ADHD-related phenotype |
|---|---|---|---|---|---|
| FMR1 | Fragile X mental retardation protein | Xq27 | Fragile X syndrome | 30% [Hagerman and others 2009] | 59% [Sullivan and others 2006] |
| NF1 | Neurofibromin | 17q11 | Neurofibromatosis type 1 | 40% [Walsh and others 2013] | 38% [Hyman and others 2005] |
| TSC1 | Hamartin | 9q34 | Tuberous sclerosis complex | 50% [Prather and de Vries 2004] | 30-60% [D’Agati and others 2009] |
| TSC2 | Tuberin | 16p13 | |||
| MECP2 | Methyl-CpG-binding protein 2 | Xq28 | Rett syndrome | 42-58% [Wulffaert and others 2009] | unknown |
| CACNA1C | Voltage-dependent L-type calcium channel subunit alpha-1C | 12p13 | Timothy syndrome | 60% [Splawski and others 2004] | unknown |
Phenotypic overlap as adapted from [Vorstman and Ophoff 2013]; ID= intellectual disability; ASD= Autism spectrum disorder; ADHD= Attention deficit/hyperactivity disorder; Chr= chromosome; no imaging genetics studies found.
Results
Imaging genetics of ADHD candidate genes
A total of 76 records were retrieved for the ADHD search term, and a total of 16 research articles describing case-control studies were eligible for review according to our criteria. To those, we added three more recent papers from our own group ((Onnink et al., 2016; Sokolova et al., 2015; van der Meer et al., 2015); Figure 1). Most of the studies investigated a single gene (all in Caucasians), and three studies investigated multiple genes (2 in Caucasians, 1 in Asians). In addition, we obtained 295 records for the ADHD candidate gene studies in healthy population samples, of which 98 were eligible (Figure 1). Of those, 73 studies investigated a single gene (68 in Caucasians, 5 in Asians), and 25 studies tested more than one gene (1 Asian). The ADHD case-control samples consisted of both childhood/adolescent and adult samples, whereas the studies in the healthy population were largely restricted to samples of (young) adults. Single-gene findings of ADHD case-control studies and studies in the healthy population of both Caucasian and Asian ethnicities can be found in Table IV, multi-locus studies are shown in Table VI. Most of the genes investigated in brain imaging genetics studies in ADHD are from the dopaminergic and serotonergic neurotransmitter systems (SLC6A3/DAT1, DRD2, DRD4, SLC6A4/5-HTT/SERT). SNAP25, DRD5, HTR1B, and LPHN3 had also been selected for this study, but for these genes no imaging genetics studies using MRI were found with our search terms.
Table IV:
Imaging genetics studies in ADHD case-control samples and ADHD candidate genes studies in the healthy population (for selection of candidate genes see Table I).
| Gene | Variant | Imaging modality | Imaging/cognitive phenotype | Genotype groups compared | Samples size (mean age in years) | Primary results (main effect of genotype) | Reference |
|---|---|---|---|---|---|---|---|
| DRD2 | DRD2/ANKK1-Taq1a (rs1800497, T allele = A1 allele) | sMRI (VBM) | Global GM volume | A1- carriers vs. A2/A2-carriers | 70 HC (30.7) | A1- carriers: ↓ part of midbrain, encompassing substantia nigra bilaterally | (Cerasa et al. 2009) |
| sMRI (VBM) | GM and WM volume | A1- carriers vs. A2/A2-carriers | 25 HC (25) | A1-carriers: ↓ Volume in cerebellar cluster | (Wiener et al. 2014) | ||
| fMRI | Temporal or color discrimination task | A1-carriers: ↑ Activation in striatum and right dorsolateral PFC | |||||
| Reward anticipation paradigm | A1- carriers vs. A2/A2-carriers | 24 HC (25.7) | ↑ Nucleus accumbens activation in three-way interaction analysis from placebo to bromocriptine (D2 receptor agonist); ↑ performance under bromocriptine in A1- carriers. | (Kirsch et al. 2006) | |||
| Striatal activation in response to receiving palatable food (2 fMRI paradigms) | A1- carriers vs. A2/A2-carriers | fMRI 1: 43 HC (20.4) † fMRI 2: 33 HC (15.7) † |
↑ Negative relation between striatal response to food receipt and BMI. A1-non-carriers : striatal activation in response to food intake was positively related to weight gain (negatively related to weight gain for A1- carriers). | (Stice et al. 2008) | |||
| Emotional face task | A1/A1-carriers vs. A1/A2-carriers vs. A2/A2-carriers | 45 HC (23.2)†§ | TaqIA genotype modifies activations in putamen, ACC, and amygdala in response to negative facial stimuli (higher signal intensity in homozygous groups (A1/A1 + A2/A2) than in heterozygous group (A1/A2)). | (Lee et al. 2011) | |||
| Flanker task with a motivation manipulation | A1- carriers vs. A2/A2-carriers | 32 HC (22.9) | A1- carriers: ↓ Interference effects to reward alone (as compared to reward + punishment) and ↑ anterior insula activation | (Richter et al. 2013) | |||
| Task-switching paradigm | A1-non-carriers vs. A1-carriers | 48 HC (22) | A1 non-carriers: ↑ Task-switching costs, ↑ prefrontal switching activity in inferior frontal junction area, and ↑ functional connectivity in dorsal frontostriatal circuits | (Stelzel et al. 2010) | |||
| Feedback-based reversal learning task | A1- carriers vs. A2/A2-carriers | 22 HC (age range 20-31) | A1- carriers in placebo condition: ↓ neural responses to positive feedback; cabergoline: ↑neural reward responses in medial OFC, cingulate cortex, and striatum, but ↓task performance and frontostriatal functional connectivity | (Cohen et al. 2007) | |||
| Probabilistic reversal learning task | A1- carriers vs. A2/A2-carriers | 28 HC (26.1)‡ | A1- carriers: no graded increase in RCZ activity to preceding negative feedback; ↓ recruitment of right VS and right lateral OFC during reversals. | (Jocham et al. 2009) | |||
| “Wug” test (knowledge of grammar, opposed to vocabulary) | A2/A2-carriers vs. A1- carriers | 22 HC (22) | A2/A2-carriers: ↑ At concatenative (but not analogical) grammar learning; ↑ striatal responses | (Wong et al. 2013) | |||
| DRD4 | exon 3 VNTR | sMRI | Superior frontal, middle frontal, anterior cingulate, and cerebellum cortices volumes | ADHD 7R-carriers vs. non 7R-carriers | 24 ADHD (38.1) 19 ADHD+BPD (35.8) 20 HC (33.2) |
7R- carriers: ↓ volumes of superior frontal cortex and cerebellum cortex compared to non-carriers. No effects in ADHD+BPD or HC. | (Monuteaux et al. 2008) |
| TBV, PFC, cerebellum, CN and pallidum volume | 7R-carriers vs. non-7R-carriers | 41 ADHD (9.7) 56 HC (17.6) |
No volumetric differences between 7R-carriers and non-7R-carriers. No group × genotype interactions. | (Castellanos et al. 1998) ‡ | |||
| DTI | WM integrity | 5R- carriers vs. non 5R-carriers | 765 HC (20.7) § | 5R-carriers : ↑ MD in widespread GM and WM areas of cerebral cortex, and subcortical areas | (Takeuchi et al. 2015) | ||
| fMRI | Activity related to N-back paradigm | 5R-carriers : ↓ Task-induced deactivation in precuneus areas in both attention-demanding working memory task and sensorimotor task; similar patterns were observed in posterior cingulate cortex and areas around midbrain and hippocampus. | |||||
| fMRI | MID task | 7R-carriers vs. non 7R-carriers | 78 HC (16.3) |
DRD4 status moderated relation between Behavioral Inhibition (BI) and activation in CN. 7R-carriers: ↑ striatal response to incentive cues. DRD4 genotype influenced relations among neural response to incentives, early childhood BI and anxiety. |
(Perez-Edgar et al. 2014) | ||
| Emotional rating task | 4R/7R-carriers vs. 4R/4R-carriers | 26 HC (23.3) | 4R/7R-carriers: ↑ activity in response to unpleasant images compared to neutral images in right temporal lobe. | (Gehricke et al. 2015) | |||
| Go/No-go task | 7R-carriers vs. non 7R-carriers | 62 HC (18) | 7R-carriers “No-Go” trials:, ↓ activation in right anterior PFC/IFG, left premotor cortex, and right occipital/ cerebellar areas (7-repeat status accounted for ca. 5-6% of variance in BOLD response during “No-Go” trials). | (Mulligan et al. 2014) | |||
| Combined stimulus-response Incompatibility Task (IC) and Time Discrimination Task (TT) | 7R-non-carriers vs. 7R-carriers | 26 HC (11.4) | 7R-non-carriers: ↑activation of left middle and IFG in IC and ↑cerebellar activation in TT; ↑functional connectivity between left IFG and ACC during IC and between cerebellar activation and IFG and ACC during TT. | (Gilsbach et al. 2012) | |||
| NOS1 | Exon 1f-VNTR | fMRI | Reward anticipation task/ modified MID task | SS-carriers vs. SL/LL-carriers | 63 ADHD (38.3) 41 HC (38.0) |
SS-carriers: ↑ in VS. No group × genotype interactions. | (Hoogman et al. 2011) |
| SLC6A3/DAT1 | 3’UTR and intron 8 VNTR haplotype | sMRI | Bilateral striatal volumes (nucleus accumbens, CN, and putamen) | Three DAT1 alleles (10/10 genotype, and the haplotypes 10-6 and 9-6) | 118 ADHD (35.9) 111 HC (37) 301 ADHD (17.2) 186 HC (16.6) 1718 HC (26.1) |
Adult ADHD 9-6 haplotype carriers ↑ 5.9 % larger striatum volume relative to participants not carrying this haplotype (in adult ADHD patients only). Effect was not replicated in adolescent case-control and adult population-based cohort. |
(Onnink et al. 2016) |
| 3’ UTR VNTR | sMRI | CN volume | 9R-carriers vs. 10R/10R-carriers | 33 ADHD (10.5) 26 HC (10.6) |
9R-carriers: ↑ volumes of CN. | (Shook et al. 2011) | |
| 3’UTR and intron 8 VNTR haplotype | fMRI | VS and CN activity during reward-predicting cues | SLC6A3 10-6 dosage (2 copies vs. <2 copies) | 29 ADHD (combined type; 15.8)‡ 30 HC (15.6)‡ |
ADHD: Activation in CN ↓ as number of copies ↑, but in control group reverse was found. | (Paloyelis et al. 2012) | |
| Striatal activity during reward anticipation task | 9-6 haplotype carriers vs. non 9-6 haplotype carriers | 87 ADHD (38.3) 77 HC (38) |
No differences in striatal activity compared with non 9-6 haplotype carriers nor 9R- and 10R/10R-carriers. | (Hoogman et al. 2013) | |||
| 9-6 haplotype carriers vs. non 9-6 haplotype carriers | 87 ADHD (38.3) 77 HC (38); same as above |
Bayesian Constraint-based Causal Discovery (BCCD) algorithm confirmed that there is no evidence of a direct link between DAT1 genetic variability and brain activation, but suggested an indirect link mediated through inattention symptoms and diagnostic status of ADHD | (Sokolova et al. 2015) | ||||
| 3’ UTR VNTR | fMRI | Working memory task | 9R-carriers vs. 10R/10R-carriers | 53 ADHD (35.7) 38 HC (31.2) |
9R-carriers: ↓ left medial PFC activation compared to 10R/10R-carriers. Group × genotype interaction showed that 10R/10R-ADHD patients had ↑ activity in pre-SMA/dorsal ACC compared to HC. | (Brown et al. 2011) | |
| Go/No-go task | 10R/10R carriers vs. 9R-carriers | 20 ADHD (14.1) 38 HC (13.12) |
10R/10R carriers: ↑ activity in frontal, medial, and parietal regions during response inhibition compared to 9R-carriers; ↓error response in the parahippocampal gyrus | (Braet et al. 2011) | |||
| 10R/10R carriers vs. 9R-carriers | 33 ADHD (11.1) | 10R/10R carriers: ↑ activity in left striatum, right dorsal premotor cortex, and temporoparietal cortical junction compared to 9R-carriers. | (Bedard et al. 2010) | ||||
| 9R-carriers vs. 10R/10R carriers | 10 ADHD (14.6)‡ 10 unaffected siblings (14.8)‡ 9 HC (15.3)‡ |
9R-carriers: ↑ activity in CN and ↓ in cerebellar vermis compared to 10R/10R-carriers. Group × genotype interaction: effect in CN is observed in ADHD and unaffected siblings, but not HC. | (Durston et al. 2008) | ||||
| Multi-source interference task | 10R/10R carriers vs. 9R-carriers | 42 ADHD (35.4) | 9R-carriers: ↓ activity in dorsal ACC compared to 10R/10R-carriers. | (Brown et al. 2010) | |||
| 3’ UTR VNTR | rs-fMRI | Striatal FC | 9R/10R-carriers vs. 10R/10R carriers | 50 HC (20.4) | 9R/10R-carriers: stronger connectivity between dorsal CN and insula, dorsal anterior cingulate, and dorsolateral prefrontal regions, as well as between VS and ventrolateral PFC, compared with 10R/10R-carriers. | (Gordon et al. 2015) | |
| fMRI | Modified version of the MID task | 10R/10R-carriers vs. 9R carriers | 53 HC (29) | 10R/10R-carriers: strong positive correlation between reward sensitivity and reward-related VS activity (relationship is absent in 9R-carriers). | (Hahn et al. 2011) | ||
| Exposure to threatening faces | 10R/10R-carriers vs. 9R-carriers | 85 HC (45.2) | 9R-carriers: ↑ amygdala reactivity compared with 10R/10R-carriers. | (Bergman et al. 2014) | |||
| Go/No-Go task under influence of 40 mg MPH or placebo | 9R-carriers vs. 10R/10R- carriers | 50 HC (23.7)‡ | 9R-carriers: MPH induced ↑ activation during successful no-go trials compared with oddball trials in thalamocortical network 10R/10R-carriers: ↓ activation in thalamocortical network. Same pattern was observed in CN and IFG (successful no-go trials compared with successful go trials). | (Kasparbauer et al. 2015) | |||
| Pre-cued task-switching task | 9R-carriers vs. 10R/10R- carriers | 20 HC (21.6) | 9R-carriers: ↑ventromedial striatum activation during reward anticipation compared with 10R/10R-carriers; ↑ influence of anticipated reward on switch costs, and ↑activity in dorsomedial striatum during task switching in anticipation of high reward relative to low reward in 9R-carriers. | (Aarts et al. 2010) | |||
| Verbal n-back task | 9R/10R-carriers vs. 10R/10R-carriers | 20 HC (10.4) | 9R/10R-carriers: ↑ performance accuracy, ↑ activation in frontalstriatal-parietal regions in high but not low runs compared with 10R/10R-carriers. Genotype × load interaction in right CN. 9R/10R-carriers: ↑ activation in striatal and parietal regions under high compared to low load, and genotype differences (9R/10R>10R/10R) were evident only under high load. 10R/10R-carriers: ↑ activation of substantial nigra/subthalamic nuclei under low than high load and genotype differences (10R/10R>9R/10R) were evident only under low load. |
(Stollstorff et al. 2010) | |||
| SLC6A4/5HTT | 5-HTTLPR | sMRI (VBM) | GM volume | S-carriers vs. LL | 291 ADHD 78 subthreshold ADHD 332 HC; Average age: 17 years |
S-carriers: stress exposure is associated with ↓ GM volume in precentral gyrus, middle and superior frontal gyri, frontal pole, and cingulated gyrus. Association of G × E interaction with ADHD symptom count was mediated by GM volume in frontal pole and anterior cingulated gyrus only. | (van der Meer et al. 2015) |
| 5-HTTLPR | sMRI | Amygdala | SS vs. SL vs. LL | 138 HC (41.2) | SS-carriers × anxiety: ↑ right amygdala volume (only in females) | (Cerasa et al. 2014) | |
| Hippocampus | S-carriers vs. LL | 56 HC (71) | ↓ Hippocampal volume in interaction with increased waking cortisol levels | (O’Hara et al. 2007) | |||
| SS/SL vs. LL | 357 HC (24.3) | S-carriers: ↓ hippocampal volume (females only); ↓ hippocampal volume correlated with severe CA (males only) | (Everaerd et al. 2012) | ||||
| S-carriers vs. LL | 51 HC (∼21) | ↑ Left hippocampal volumes in woman ↓ Left hippocampal volumes in men |
(Price et al. 2013) | ||||
| LL vs. SS/SL | 159 HC (69.5) | LL-carriers × stress: ↓ hippocampal volume | (Zannas et al. 2013) | ||||
| Multiple regions | S-carriers vs. LL | 113 HC (37.6) | ↓ GM volume of right IFG, left anterior cingulate, and superior temporal gyrus | (Selvaraj et al. 2011) | |||
| 5-HTTLPR, rs25531 | sMRI | Total GM volume | SS vs. LL, S’ vs. L’ | 58 HC (18.5) | No significant association with total GM volume | (Walsh et al. 2014) | |
| 5-HTTLPR, rs25531, AluJb methylation of promoter | sMRI (VBM) | Hippocampus, amygdala, insula, anterior cingulated gyrus | S’ vs. L’ quantitative methylation score | Sample 1: 94 HC (36.9) Sample 2: 95 HC (34.2) |
No significant association of genotype. Strong association of methylation and hippocampal GM volume; amygdala, insula, and CN showed similar associations, genotype-independent. |
(Dannlowski et al. 2014) | |
| 5-HTTLPR | sMRI (VBM) | GM volume | S-carriers vs. LL | sMRI: 114 HC (32.8) fMRI: 94 HC (31.3) (26 included in both) |
S-carriers (VBM): ↓GM volume in limbic regions, particularly perigenual ACC and medial amygdala. | (Pezawas et al. 2005) | |
| fMRI | perceptual processing of fearful stimuli | S-carriers (fMRI): ↓ of amygdala- perigenual ACC connectivity, particularly in rostral ACC; ↓ structural covariance between amygdala and rostral ACC | |||||
| GM volume, attentional interference task | S-carriers vs. LL | 41 HC (adults) | S-carriers (VBM): ↑ volume in left cerebellum LL (VBM): ↑ volume in left superior and medial frontal gyri, left anterior cingulated, and right IFG S-carriers (fMRI): ↑ activation in response to negative, relative to neutral, words in right amygdala (driven by ↓ activation to neutral stimuli, rather than ↑ activation to negative stimuli); for negative-neutral contrast ↑ activation most prominent in insula, putamen, and CN |
(Canli et al. 2005) | |||
| sMRI (VBM) | Hippocampus, amygdala | S-carriers vs. LL, interaction with SLEs | 48 HC (24.7); | S-carriers: no correlation of hippocampus and amygdala volume with SLEs. LL-carriers: positive correlation in GM volume with SLEs. |
(Canli et al. 2006) | ||
| fMRI | Face-stimuli | Negative correlation between SLEs and amygdala and hippocampus activation in response to face stimuli in S-carriers; positive correlation in LL-carriers. | |||||
| rs-fMRI | FC between amygdala and hippocampus; absolute CBF at rest | 21 HC for perfusion scan | GxE effect altered FC between hippocampus and putamen. Interaction effect of 5-HTTLPR genotype and life stress on resting level activation in amygdala and hippocampus (positive correlation in S-group and negative correlation in L-group). |
||||
| sMRI | GM volume resting CBF | SS vs. LL | 26 HC (20.3) | SS-carriers: No effect on amygdala and ventromedial PFC volume | (Rao et al. 2007) | ||
| rs-fMRI | SS-carriers: ↑ resting CBF in amygdala and ↓ CBF in ventromedial PFC | ||||||
| DTI | WM integrity | L-carriers vs. SS | 233 HC (22.7) § | L-carriers: ↓ anatomical connectivity between amygdala and PFC through uncinate fasciculus. | (Long et al. 2013) | ||
| rs-fMRI | TC | L-carriers: ↓ FC between right amygdala and right frontal pole. | |||||
| 5-HTTLPR, rs25531 | DTI | Structural connectivity | S’-carriers × SLE vs. L’L’ × SLE | 34 HC (25.6) † | ↑ Structural connectivity between hippocampus and putamen (seed-based). | (Favaro et al. 2014) | |
| rs-fMRI | FC | ↑ Positive correlation of co-activation of right parahippocampus and posterior cingulate cortex with SLEs (seed-based). | |||||
| 5-HTTLPR | rs-fMRI | Task-free activity | SS vs. LL | 30 HC (20.3) | ↑ Negative correlation of right amygdala activity and depressive symptoms | (Gillihan et al. 2011) | |
| FC | SS vs. L-carriers | 200 HC (22.1) ‡§ | SS-carriers: ↑ fractional amplitude of low-frequency fluctuation in amygdala; ↓ rsFC between amygdala and various regions (including insula, Heschl’s gyrus, lateral occipital cortex, superior temporal gyrus, hippocampus) and ↑ rsFC between amygdala and various regions (including supramarginal gyrus and middle frontal gyrus) | (Zhang et al. 2015) | |||
| 5-HTTLPR, rs25531 | rs-fMRI | FC | S’S’ vs. S’L’ vs. L’L’ | 39 HC (14.8) | ↓ Superior medial frontal cortex connectivity ↓ Age-related increase in FC between posterior hub and superior medial frontal cortex |
(Wiggins et al. 2012) | |
| 5-HTTLPR | fMRI | Sadness induction - regulation to normal emotion | SS vs. LL | 30 HC (20.3) | ↑ Amygdala activity during mood recovery. | (Gillihan et al. 2010) | |
| Emotion regulation task | S-carriers vs. LL | 37 HC (22.6) † | ↑ Right amygdala reactivity to fearful faces. ↑ Signal reductions in right amygdala during regulation of fear. ↑ Modulatory influence of cognitive regulation on FC between amygdala and bilateral ventrolateral PFC, left medial OFC, subgenual ACC and rostral ACC. |
(Schardt et al. 2010) | |||
| SS vs. LL | 30 HC (20.3), same sample as above | ↑Anti-correlation between amygdala and posterior cingulate cortex/precuneus during mood recovery. | (Fang et al. 2013) | ||||
| 5-HTTLPR, rs25531 | fMRI | Emotion regulation task | S’S’ vs. L’L’ | 30 HC (20.5) | ↓ Posterior insula and prefrontal brain activation during passive perception of negative emotional information. ↑ Prefrontal activation and anterior insula activation during down- and upregulation of negative emotional responses. |
(Firk et al. 2013) | |
| 5-HTTLPR | fMRI | Mood induction, sadness (film) | S-carriers vs. LL | 48 HC (8.3) | ↑ Right putamen, right CN, right rostro-ventral ACC, left CN, and left putamen in sad mood. | (Fortier et al. 2010) | |
| S-carriers vs. LL | 49 HC (12) † | ↑ Earlier rise of left amygdala activation as sad mood increases. | (Furman et al. 2011) | ||||
| 5-HTTLPR | rs-fMRI | FC | LL vs. SS | 38 HC (20.4) § | ↑ Regional homogeneity in right amygdala; no effects on FC of right amygdala. | (Li et al. 2012) | |
| fMRI | Emotional processing | No difference in amygdala activity in response to negative stimuli. | |||||
| 5-HTTLPR, rs25531 | fMRI | Emotion processing task | S’S’ vs. S’L’ vs. L’L’ (treatment with escitalopram) | 36 HC (25.1) † | ↑Left amygdala activation with escitalopram treatment linearly related to 5-HTTLPR S’ allele load for negative stimuli increased. | (Outhred et al. 2014) | |
| 5-HTTLPR | fMRI | Emotional face task | S-carriers vs. LL | 28 HC | S-carriers: ↑ right amygdala activity | (Hariri et al. 2002) | |
| S-carriers vs. LL | 92 HC (30.5) | S-carriers: ↑ right amygdala activity | (Hariri et al. 2005) | ||||
| S-carriers vs. LL | 29 HC (40) ‡ | S-carriers: ↑ activation of amygdala and ↑ coupling between amygdala and ventromedial PFC. | (Heinz et al. 2005) | ||||
| SS vs. SL vs. LL | 29 HC (37.5) | ↑ Activity in right fusiform gyrus to fearful faces. ↑Positive FC between amygdala and fusiform gyrus and between right fusiform gyrus and right ventrolateral PFC. |
(Surguladze et al. 2008) | ||||
| S-carriers vs. LL | 21 HC (15) | ↑ Left amygdala activation in response to anger. | (Battaglia et al. 2012) | ||||
| 5-HTTLPR, rs25531 | fMRI | Emotional face task | S’-carriers vs. L’L’ | 44 HC (30.3) | ↑ Right amygdala responses to sad faces. | (Dannlowski et al. 2010) | |
| L’L’ vs. S’S’ | 30 HC (26.6) | No association with amygdala reactivity. ↓ Subgenual cingulate cortex activation in response to fearful faces. |
(O’Nions et al. 2011) | ||||
| sMRI | Amygdala volume | S’-carriers vs. L’L’ | 54 HC (41.6) | ↓ Amygdala volume Path analysis suggests effects on left amygdala volume are mediated by right amygdala volume but not through (midbrain) 5-HTT availability. |
(Kobiella et al. 2011) | ||
| PET | 5-HTT availability | No genotype effect on (midbrain) 5-HTT availability. | |||||
| fMRI | Amygdala activation | ↑ Left amygdala activation in response to emotional stimuli. | |||||
| S’S’ vs. L’L | 67 HC (18.6) | ↑ Left amygdala reactivity in multivariate analysis; additive effects of recent SLEs. | (Walsh et al. 2012) | ||||
| S’S’ vs. L’-carriers, interaction with SLEs | 44 HC (26.8) ‡ | ↑ Bilateral amygdala activation in response to fearful faces. Interaction with SLEs: highest activity in S’S with SLEs for fearful faces in bilateral amygdala. |
(Alexander et al. 2012) | ||||
| rs-fMRI | S’S’ vs. L’-carriers | 48 HC (14.8) | ↓ Connectivity between right amygdala and ventromedial PFC with age. | (Wiggins et al. 2014) | |||
| fMRI | ↑ Amygdala activation with age (age range 9-19 years) | ||||||
| S’-carriers vs. L’L’ (bright-light intervention) | 30 HC (24.3)‡ | Bright-light dose positively associated with intra-prefrontal (medial PFC coupling with medial PFC seed) functional coupling only in S’-carriers. | (Fisher et al. 2014) | ||||
| 5-HTTLPR | fMRI | Perceptual task of threatening stimuli | S-carriers vs. LL | 14 HC phobic-prone (32.7) 14 HC eating disorders prone (34.3) |
S-carriers: ↑ activity in right amygdala | (Bertolino et al. 2005) | |
| fMRI | Emotional face task with approach-avoidance | S-carriers vs. LL | 48 HC (22.5) ‡ | ↑ Amygdala activity originating from reduced prefrontal inhibitory regulation. | (Volman et al. 2013) | ||
| Emotional face-emotional word conflict task | S-carriers vs. LL | 26 HC (70.5) | ↓ Connectivity between dorsal ACC and pregenual ACC for incongruent face-word combination. | (Waring et al. 2014) | |||
| 5-HTTLPR, rs25531 | fMRI | Emotional face task with self-referential and emotion labeling conditions | S-carriers vs. LL, SLE interaction | 45 HC (23.3) | ↑ Amygdala activation and ↓ FC of amygdala with subgenual ACC in self-referential processing vs. emotion labeling. Negative correlation of bilateral amygdala activation during self-referential with SLEs in S-carriers; positive correlation in LL; pattern opposite during emotion labeling. |
(Lemogne et al. 2011) | |
| Emotional face- word conflict task (Stroop-like task) | S’-carriers vs. L’L’ | 42 HC (∼20) | ↓ Recruitment of prefrontal control regions and superior temporal sulcus during conflict when task-irrelevant information was positively-valenced. ↑ Recruitment of these regions during conflict when task-irrelevant information was negatively-valenced. |
(Stollstorff et al. 2013) | |||
| 5-HTTLPR | fMRI | Pain rating task | LL vs. SS | 50 HC (24.9) † | ↑ Positive linear effect of target pain in posterior cerebellum. | (Laursen et al. 2014) | |
| (un)predictable electric shocks | SS vs. L-carriers | 51 HC (22) † | ↑ Activity of amygdala, hippocampus, anterior insula, thalamus, pulvinar, CN, precuneus, ACC, and mPFC during threat anticipation. ↑ Positive coupling between mPFC activation and anxiety experience; L-carriers show ↑ negative coupling between insula and success of regulating anxiety. |
(Drabant et al. 2012) | |||
| S- carriers vs. LL | 99 HC (21.9)‡ 69 HC (33.4) |
S-carriers: ↑ dorsomedial PFC, anterior insula, bed nucleus of stria terminalis, thalamus and midbrain activation with increasing threat conditions across both samples. | (Klumpers et al. 2014) | ||||
| 5-HTTLPR, rs25531 | fMRI | Modified Flanker task | S’-carriers vs. L’L’ | 33 HC (23.4) | ↑ Error-related rostral ACC activation. ↓ Conflict-related dorsal ACC activation. |
(Holmes et al. 2010) | |
| Decision making task | S’S’ vs. L’L’ | 30 HC (26.6) | ↑ Amygdala activation during decisions made counter to, relative to decisions made in accord with, the frame effect (gain or loss). Anterior cingulate-amygdala coupling during choices to made in counter to, relative to those made in accord with, the frame effect only observed in L’L’. |
(Roiser et al. 2009) | |||
| n-back task | S’S’ vs. S’L’ vs. L’L’ | 33 HC (37) † | ↑ Bilateral prefrontal activation in right and left IFG pars triangularis with increasing S-allele count. | (Jonassen et al. 2012) | |||
| 5-HTTLPR | fMRI | Source memory task | S-carriers vs. LL | 23 HC (66.8) [17 (23.3), not analyzed for genotype effects in fMRI] | ↓ Activity in left IFG, middle frontal gyrus and anterior paracingulate cortex. | (Pacheco et al. 2012) | |
| Food / non-food pictures | LL vs. S-carriers | 28 HC (25.5) | ↑ Left posterior cingulate cortex activity for food pictures. | (Kaurijoki et al. 2008) | |||
| 5-HTTLPR, rs25531 | fMRI | Differential fear conditioning | S’S’ vs. L’-carriers | 47 HC (26.8) ‡ | ↑ Activity in fear network: amygdala (right), insula, thalamus (left) and occipital cortex for conditioned stimulus. Interaction with SLEs: ↑ activity in right insula and left occipital cortex in S’S’. |
(Klucken et al. 2013) | |
ACC = anterior cingulated cortex, ADHD = attention-deficit/hyperactivity disorder, BCCD = Bayesian Constraint-based Causal Discovery, BI = Behavioral Inhibition, BMI = Body mass index, BOLD = blood oxygen level–dependent, BPD = bipolar disorder, CA = childhood adversity, CBF = cerebral blood flow, CN = caudate nucleus, FC = functional connectivity, fMRI = functional magnetic resonance imaging, GM = gray matter, HC = healthy control, IC = Incompatibility Task, IFG = inferior frontal gyrus, MD = mean diffusivity, MID task = monetary incentive delay task, MPH = methylphenidate, OFC = orbitofrontal cortex, PET = positron emission tomography, PFC = prefrontal cortex, RCZ = rostral cingulate zone, rsFC = resting-state functional connectivity, SLE = stressful life events, SMA = supplementary motor area, sMRI = structural magnetic resonance imaging, TBV = total brain volume, TT = Time Discrimination Task, VBM = voxel-based morphometry, VS = ventral striatum, WM = white matter
only females
only males
Asian sample
S’= functional S-allele (S or LG), L’= functional L’-allele (LA); in gray case-control studies
Table VI:
Imaging genetics studies in ADHD and ASDs case-control samples and candidate genes studies in the healthy population studying more than one single gene.
| ADHD/ASD candidate gene (polymorphism) | Additional gene(s) studied | Imaging modality | Imaging/cognitive phenotype | Genotype groups compared (candidate genes or interaction) | Samples size (mean age in years) | Primary results (main effect of candidate gene genotype or interaction) | Reference |
|---|---|---|---|---|---|---|---|
| SLC6A3 (3’ UTR VNTR), DRD4 (exon 3 VNTR) | --- | sMRI | PFC gray matter and CN volume | 9R-carriers vs. 10R/10R-carriers, 4R/4R-carriers vs. rest | 26 ADHD (12.1) 26 unaffected siblings (11.6) 20 HC (10.7); all ‡ |
SLC6A3 9R-carriers: ↑ CN volumes DRD4 4R/4R-carriers: ↓ prefrontal GM volume. No effects on CN, or TBV. No interactions between ADHD status and genotype. |
(Durston et al. 2005) |
| DRD1 | sMRI; longitudinal study (mean follow-up, 6 years) | Cortical thickness | 9R-carriers vs. 10R/10R-carriers, 7R-carriers vs. non-7R-carriers | 105 ADHD (10.1; 13.1; 15.9) 103 HC (10.0; 12.4; 14.4) |
SLC6A3 9R-carriers: No effect on cortical development. DRD4 7R-carriers: thinner right orbitofrontal/inferior prefrontal and posterior parietal cortex. ADHD 7R-carriers: distinct trajectory of cortical development; normalization of right parietal cortical region. |
(Shaw et al. 2007) | |
| COMT | DTI | WM integrity, FA values | 9R-carriers vs. 10R/10R-carriers; 4R/4R-carriers vs. rest | 58 stimulant- and atomoxetine-naïve ADHD (8.7) § |
SLC6A3 9R-carriers: no effect on WM integrity DRD4 4R/4R-carriers: no effect on WM integrity. |
(Hong et al. 2015) | |
| SLC6A3 (3’ UTR VNTR) | COMT | fMRI | Episodic memory task | 9R-carriers vs. 10R/10R-carriers | 49 HC (22.7) | 9R-carriers: ↑ midbrain activation (right substantia nigra and the ventral tegmental area) | (Schott et al. 2006) |
| N-back task | 9R/9R-carriers × val/val-carriers | 75 HC (19.6) | No effects on brain activation were found for each genotype independently. Val/val and 9R/9R subjects show highest activation dorsolateral prefrontal region. |
(Caldu et al. 2007) | |||
| Response inhibition (stop-signal) task | 9R-carriers vs. 10R/10R-carriers | 43 HC (22.7) | SLC6A3 9-allele carriers: ↑ activation during inhibition in subthalamic nucleus and (pre-Supplementary motor area | (Congdon et al. 2009) | |||
| Reward anticipation task | 9R-carriers vs. 10R/10R-carriers; val-carriers vs. met/met-carriers | 22 HC (27.9) |
SLC6A3 9R- carriers: highest activity in CN and VS during reward anticipation and in lateral PFC and midbrain at time of reward delivery. Interaction SLC6A3 and COMT: DAT1 9R-allele carriers and COMT met/met-allele carriers showing highest activation in VS and lateral PFC during reward anticipation and in lateral prefrontal and orbitofrontal cortices, and in midbrain at time of reward delivery. |
(Dreher et al. 2009) | |||
| TREK, COMT | fMRI | MID task | 9R-carriers vs. 10R/10R-carriers | 32 HC (21.7) | TREK1 and SLC6A3 /COMT genotypes were independently related to basal ganglia responses to gains. | (Dillon et al. 2010) | |
| COMT | fMRI | Fear conditioning, extinction and reacquisition task | 9R- carriers vs. 10R/10R-carriers | 69 HC ‡ | 9R- carriers: ↑ learning rates and stronger hemodynamic appetitive prediction error signals in VS. | (Raczka et al. 2011) | |
| SLC6A4 (5-HTTLPR) | BDNF | sMRI | Global GM volume | S-carriers × val/val | 111 HC (32.60) | ↓ ACC volume | (Pezawas et al. 2008) |
| SLC6A4 (5-HTTLPR, rs25531, STin2) | OXTR, STMN1 | sMRI | Amygdala volume | SS vs. SL vs. LL. | 139 HC (22)‡ | SLC6A4 risk alleles are associated with ↓ amygdala volumes. | (Stjepanovic et al. 2013) |
| SLC6A4 (5-HTTLPR) | COMT | sMRI (VBM) | Global GM volume | S-carriers vs. LL-carriers × met-carriers vs. val/val-carriers | 91 HC (33) | Interaction: ↓GM volume of bilateral parahippocampal gyrus, amygdala, hippocampus, vermis of cerebellum and right putamen/insula | (Radua et al. 2014) |
| SLC6A4 (5-HTTLPR, rs25531) | TPH2 | fMRI | MID task | L’L’ vs. S’-carriers | 89 HC (27.8) | L’L’-carriers: positive association with amygdala-hippocampus activity and trait anxiety score. | (Hahn et al. 2013) |
| SLC6A4 (5-HTTLPR) | MAOA | fMRI | Response inhibition task | S-carriers vs. LL | 35 HC (32.1) ‡ | S-carriers: ↑ activation in ACC Allele–allele interaction: ↑ BOLD activity in ACC. |
(Passamonti et al. 2008) |
| SLC6A4 (5-HTTLPR, rs25531) | COMT | fMRI | Emotion processing task | S’S’-carriers and L’L’-carriers × val/val –carriers and met/met-carriers | 48 HC (41.2) ‡ | Interaction effects in amygdala, hippocampal and limbic cortical regions elected by unpleasant stimuli. No additive or interaction effects. | (Smolka et al. 2007) |
| Emotional face task | S’-carriers vs. L’L’ met/met vs. val-carrier s | 54 HC (24.1) | S’-carriers : ↑right amygdala activity in response to angry stimuli. | (Lonsdorf et al. 2011) | |||
| SLC6A4 (5-HTTLPR) | TPH2, HTR1A, HTR2A | fMRI | Emotional face task | L-carriers vs. SS | 55 HC (23.3) †§ | L-carriers: ↑Bilateral amygdala activation in response to angry faces | (Lee and Ham 2008) |
| SLC6A4 (5-HTTLPR, rs25531) | COMT | fMRI | Emotional face task | S’S’-carriers and L’L’-carriers × met/met-carriers and val/val-carriers | 91 HC (32.5) | Interaction: ↓Reciprocal connectivity within bilateral fusiform and inferior occipital regions, right superior temporal gyrus and superior temporal sulcus, bilateral inferior and middle PFC and right amygdala, in fear processing conditions. | (Surguladze et al. 2012) |
| SLC6A4 (5-HTTLPR) | TPH2 | fMRI | Emotional face task | S-carriers and LL-carriers × TPH2 | 49 HC (24.0) | Interaction: ↑ activation of putamen and amygdala, most robust for visuospatial and negatively valenced stimuli | (Canli et al. 2008) |
| SLC6A4 (5-HTTLPR, rs25531) | BDNF | fMRI | Emotion processing | S-carriers vs. LL; interaction val/met | 28 HC (24.49)† | S-carriers: ↑rostral ACC and amygdala activation during presentation of emotional images. S-carriers and met-carriers: ↑ activation in rostral ACC and amygdala. |
(Outhred et al. 2012) |
| DRD2 (A1 allele) | BDNF | sMRI | Global GMV | A1-carriers × met-carriers | 161 HC (27.29) | Interaction: ↓GM volume of ACC | (Montag et al. 2010) |
| DRD4 (rs1800955) | COMT | fMRI | Gambling paradigm featuring unexpectedly high monetary gains and losses | CC-carriers vs. TT-carriers | 53 HC (21.2) | CC-carriers: ↑ responses in anterior insula and cingulate cortex. | (Camara et al. 2010) |
| DRD2 (rs1800497), DRD4 (exon 3 VNTR) | --- | fMRI | Imagined intake of palatable foods, unpalatable foods, glasses of water (pictures). | A1-carriers and 7R- carriers | 44 HC (15.6) † | ↓ Activation of frontal operculum, lateral OFC, and striatum in response to imagined intake of palatable foods (vs. unpalatable food or water), predicted future ↑ in body mass for those with A1 or 7R-allele. | (Stice et al. 2010) |
| SLC6A3 (3’ UTR VNTR), DRD2 (rs1800497) | COMT | fMRI | Cue-target reading paradigm | A1-carriers vs. A2/A2, 9R-carriers vs. 10R/10R, met/met vs. val/met vs. val/val | 71 HC (27.6) ‡ |
DRD2 polymorphism did not affect results. 10R-carriers: ↑ dorsal IFG activation. Linear effect of COMT val/met and DAT1 9R/10R on preparatory activity in left IFG pointed to negative interaction between tonic lateral prefrontal and phasic subcortical DA. |
(Arnold et al. 2015) |
| DRD2 (rs1800497), DRD4 (exon 3 VNTR), SLC6A3 (3’ UTR VNTR and intron 8 VNTR) | ADRA1A, ADRA1B, ADRA1D, ADRA2A, ADRA2B, ADRB1, ADRB2, ADRB3, COMT, DBH, DDC, DRD1, DRD3, DRD5, SLC6A2, TH | fMRI | Stop-signal task | SLC6A3 rs37020 (T-carriers vs. GG-carriers) | 50 HC (22.1) | Activity in frontal regions (anterior frontal, superior frontal and superior medial gyri) and CN varied additively with T-allele of rs37020. | (Cummins et al. 2012) |
| DRD2 (rs1800497, rs1799732), DRD4 (exon 3 VNTR) SLC6A3 (3’ UTR VNTR) | COMT | fMRI | Receipt and anticipated receipt of palatable food and monetary reward | Individual risk genotypes and multilocus score | 160 HC (15.3) | Individuals with 5 ‘risk’ genotypes: did not show ↓ activation of DA-based reward regions. DRD4-L vs. DRD4-S genotype: ↓ middle occipital gyrus activation in response to monetary reward. Multilocus composite score: ↑ number of ‘risk’ genotypes ↓ activation in putamen, CN, and insula in response to monetary reward. |
(Stice et al. 2012) |
| Card guessing game task | Multilocus DA profile | 69 HC (44.5) | ↑ Reactivity correlated with ↑ number of risk factors. Multilocus DA profile scores accounted for 10.9% of inter-individual variability in reward-related VS reactivity. None of individual polymorphisms accounted for significant variability. | (Nikolova et al. 2011) | |||
| SLC6A4 (5-HTTLPR, rs25531), OXTR (rs2268498 and rs53576) | --- | fMRI | Empathic performance task (facial responses of target person to electric stimulation) | SS-carriers vs. LL-carriers; rs2268498: CC- vs. CT- vs. TT-carriers; rs53576: AA-vs. AG- vs. GG-carriers | 50 HC (24.9) † | rs2268498 CC-carriers: high empathic accuracy was associated with ↑ responsiveness of right STS to observed pain. | (Laursen et al. 2014) |
ACC = anterior cingulate cortex, ADHD = Attention deficit/hyperactivity disorder, BOLD = blood oxygen level–dependent, CN = caudate nucleus, DA = dopamine, DTI = diffusion tensor imaging, FA = fractional anisotropy, fMRI = functional magnetic resonance imaging, GMV = gray matter volume, HC = healthy control, MID task = monetary incentive delay task, OFC = orbitofrontal cortex, PFC = prefrontal cortex, sMRI = structural magnetic resonance imaging, UTR = untranslated region, TBV = total brain volume, VAC task = variable attentional control task, VNTR = variable number tandem repeat, VS = ventral striatum, VSWM = visuospatial working memory, WM = white matter
only females
only males
Asian sample
in gray only case-control studies
The dopamine transporter gene DAT1 (official name SLC6A3) codes for a solute carrier protein, responsible for the reuptake of dopamine from the synaptic cleft into the presynaptic neuron, representing a primary mechanism of dopamine regulation in the striatum (Ciliax et al., 1999). The most widely studied polymorphism in SLC6A3/DAT1 is a variable number of tandem repeat (VNTR) sequence in the 3’ untranslated region (3’UTR) that is 40 base pairs (bp) in length. Most common alleles are those with 9 and 10 repeats. Additionally, a 30 bp VNTR in intron 8 of the gene (most common alleles with 5 and 6 repeats), is sometimes studied together with the 3’UTR VNTR as a haplotype. The 10R/10R genotype of the 3’UTR VNTR and the 10–6 haplotype of the two VNTRs are thought to be risk factors for ADHD in children (Asherson et al., 2007; Brookes et al., 2006; Faraone et al., 2005). In contrast, the 9R/9R genotype and the 9–6 haplotype are associated with persistent ADHD (Franke et al., 2010). The sMRI and fMRI studies for SLC6A3/DAT1, the latter investigating several cognitive domains known to be impaired in ADHD, i.e. reward processing, working memory, and response inhibition, are summarized in Table IV and VI. The main focus of the studies for this gene has clearly been on the striatum, which shows highest gene expression.
The two sMRI case-control studies were performed in children, and both reported a smaller volume of the caudate nucleus in homozygotes for the 10R allele as compared to children with the 9R/10R genotype (Durston et al., 2005; Shook et al., 2011). A third study, including a large sample of children and adults with and without ADHD, showed that only in the adult ADHD case-control cohort, carriers of the DAT1 adult ADHD risk haplotype 9–6 had a 5.9% larger striatum volume relative to participants not carrying this haplotype. The effect was depended on diagnostic status, since the risk haplotype affected striatal volume only in patients with ADHD (Onnink et al., 2016).
Two fMRI studies in case-control design investigated the SLC6A3/DAT1 haplotype using reward paradigms. Independent of the genotype, a recent meta-analysis has shown that in reward-processing paradigms, most studies report lower activation of the ventral striatum in patients with ADHD in anticipation of reward than controls (Plichta and Scheres 2014). Consistent with this, a study in adolescents (including only males) found the activation of the caudate nucleus to be reduced in the ADHD group as the number of 10–6-haplotype copies increased (Paloyelis et al., 2012). The other study, in adult ADHD cases and controls (in whom the 9–6 allele is the ADHD risk allele), found no effect of DAT1 haplotype on striatal activity (Hoogman et al., 2013). Studies in healthy adult individuals point in different directions. One found higher activation during reward anticipation in 9R-carriers (Dreher et al., 2009). Another also found increased striatal activation in 9R-carriers in a rewarded task-switching task, especially in high reward conditions (Aarts et al., 2010). A third study in healthy adults suggested that a link between reward sensitivity and striatal activation during reward anticipation is only present in 10R/10R individuals, and is lost in 9R-carriers (Hahn et al., 2011). In studies of response inhibition in children/adolescents, the 10R/10R genotype was found linked to lower (Durston et al., 2008) but also higher (Bedard et al., 2010) striatal activation. Methylphenidate was able to increase activity in the caudate nucleus (as well as a thalamocortical network and inferior frontal gyrus) during successful inhibition in healthy adult male 9R-carriers, but decreased activity in 10R/10R individuals (Kasparbauer et al., 2015). A working memory task in healthy adults elicited more activation in fronto-striatal-parietal regions in 9R/10R individuals under high memory load (Stollstorff et al., 2010). Additionally, a resting-state fMRI study in healthy adults showed stronger connectivity between midbrain (mainly striatal) and prefrontal regions in 9R/10R heterozygotes compared with 10R/10R homozygotes (Gordon et al., 2015).
Beyond striatum, SLC6A3/DAT1 genotype effects have also been observed in fMRI studies of cortical regions, especially (pre)frontal, medial (pre-SMA, dorsal ACC), and (temporo)parietal regions (Bedard et al., 2010; Braet et al., 2011) (Table IV and VI). As expression of DAT is limited outside of striatum and cerebellum, these effects are likely due to direct or indirect connections between the regions of gene expression and the rest of the brain. This is in line with the fact that no effect of SLC6A3/DAT1 genotype on cortical development has been observed in a longitudinal study (Shaw et al., 2007). Of particular interest might be studies showing effects of SLC6A3/DAT1 genotype on amygdala reactivity upon exposure to threatening faces (Bergman et al., 2014) as well as on cerebellar activation during response inhibition (Durston et al., 2008). These regions are currently understudied in ADHD. A first study using DTI did not suggest a strong effect of SLC6A3/DAT1 genotype on structural brain connectivity (Hong et al., 2015) (Table IV).
In summary, although SLC6A3/DAT1 is one of the best-studied genes in imaging genetics literature covered in this review, existing studies do not yet clarify sufficiently the role of ADHD-linked genetic variation in brain activity and connectivity related to symptoms/cognitive deficits or their structural brain correlates. A complicating matter for this gene is the switch in ADHD risk allele from childhood to adulthood. Furthermore, interactions between genotype and diagnosis are observed in some studies, which suggest that studying effects of SLC6A3/DAT1 in healthy individuals will not suffice to fully understand the brain mechanisms linking this gene to ADHD.
The dopamine D2 receptor gene (DRD2) codes for a G protein-coupled receptor, which inhibits adenylate cyclase (Andersen et al., 1990). Consistent with its broad expression in the brain being highest in striatum, DRD2 plays a key role in regulating mesolimbic reward processing pathways (Usiello et al., 2000) and is also implicated in other cognitive domains, such as cognitive flexibility and learning (Puig et al., 2014). The gene has been implicated in many different psychiatric disorders, including schizophrenia and substance use disorders (Patriquin et al., 2015; Schizophrenia Working Group of the Psychiatric Genomics 2014) and is the target of several antipsychotics (Moore et al., 2014). The risk factor for ADHD is the most frequently investigated common genetic variant of DRD2 rs1800497 (also known as Taq1A restriction fragment length polymorphism). This SNP actually lies downstream of DRD2 in an exon of a neighboring gene, ANKK1 (Neville et al., 2004). It affects dopamine D2 receptor expression and striatal dopamine metabolism, with the A1-allele (the ADHD risk allele) reducing the number of DRD2 receptors (Laakso et al., 2005). No studies in ADHD case-control design are yet available for DRD2. The risk SNP has, however, been investigated in healthy individuals using structural and functional MRI covering the cognitive domains of reward processing, task-switching and reversal learning, working memory, emotion recognition, and language (Table IV and VI).
Structural MRI showed that the SNP affects the volume of midbrain structures, with A1-allele carriers having smaller volumes of substantia nigra (Cerasa et al., 2009), cerebellum (Wiener et al., 2014), and ACC (in interaction with BDNF; (Montag et al., 2010)).
Functional MRI during reversal learning tasks revealed that A1-allele carriers showed reduced response of the rostral cingulate to negative feedback and had a reduced recruitment of the right ventral striatum and right lateral occipital frontal cortex (OFC) during reversals (Jocham et al., 2009). Pharmacological fMRI in a reversal learning task showed that cabergoline (D2 receptor agonist) administration induced an allele-specific response, where A1-allele carriers showed increased neural reward responses in medial OFC, cingulate cortex, and striatum (consistent with increased D2-mediated dopamine signaling); this was coupled, however, to worse task performance and lower fronto-striatal functional connectivity (Cohen et al., 2007). The reward-related paradigms showed that A1-allele carriers exhibited increased anterior insula (Richter et al., 2013) and increased nucleus accumbens activation, the latter observed only in a three-way interaction analysis looking for differences between a placebo and bromocriptine (D2 receptor agonist) administration condition (Kirsch et al., 2006). Two multi-locus studies including the DRD2 Taq1A variant suggested higher activation during reward anticipation, but blunted activity during reward receipt with increasing number of risk factors (Table VI).
In summary, the effects of the ADHD risk factor in DRD2 in fMRI appear to be relatively consistent across most of the studies currently available, with stronger brain activity in parts of the wider reward processing and memory/learning circuits. It seems that this stronger activity is linked to worse functional connectivity and/or performance, thus potentially reflecting compensatory processes. Currently, no data from patients with ADHD are available.
The dopamine D4 receptor (encoded by the DRD4 gene) is another G protein-coupled receptor and belongs to the dopamine D2-like receptor family (Oldenhof et al., 1998). The most widely studied DRD4 polymorphism in ADHD has been the 48 bp VNTR in exon 3, with the 2-, 4-, and 7-repeat alleles being the most common alleles. Allele frequencies vary significantly across ethnic groups (Chang et al., 1996; Van Tol et al., 1992), and the ADHD risk allele in the Caucasian population (7R) seems to be a different one from that in Asians (Nikolaidis and Gray 2010; Wang et al., 2004).
Structural MRI suggested that patients with ADHD carrying the 7R-allele have smaller volumes of the superior frontal and cerebellar cortex (Monuteaux et al., 2008), while no differences were found in another study (Castellanos et al., 1998) (Table IV). Interestingly, carriership of the DRD4 7R-allele seemed to affect cortical development in a longitudinal study, with 7R-carriers showing thinner prefrontal and parietal cortex and ADHD patients with this allele having a distinct trajectory of cortical development characterized by normalization of parietal cortical regions (Shaw et al., 2007) (Table VI). Structural connectivity was investigated in two studies in Asians using DTI, and while one did not find effects for 4R homozygotes (Hong et al., 2015), a very large recent study reported widespread increases in mean diffusivity in 5R-carriers (Takeuchi et al., 2015) (Table IV).
With the role of the D4 dopamine receptor in cognition not sufficiently characterized yet, and DRD4 being expressed in large parts of the cortex (predominantly in frontal lobe regions, such as the OFC and ACC (Floresco and Tse 2007; Noain et al., 2006)), fMRI studies have investigated the DRD4 gene in healthy Caucasians covering different cognitive domains, i.e. emotion processing, response inhibition, reward, stimulus-response incompatibility, and time discrimination tasks, as summarized in Table IV. Depending on the type of paradigm used in the fMRI studies, DRD4 genotype was found to modulate brain activity in prefrontal and temporal, but also in striatal and cerebellar brain regions in the healthy adults (Table IV).
Thus, though existing evidence does not support firm conclusions, DRD4 may mark a particular developmental trajectory in cortical brain structure related to adult outcome of ADHD, and plays a role in structural connectivity. With only one fMRI study per cognitive domain published to date, no clear picture of DRD4 action on brain activity emerges, but those studies do clearly indicate that DRD4 (like DAT1) influences brain activity beyond its regions of expression, possibly due to its effects on white matter connectivity (Takeuchi et al., 2015).
The serotonin transporter gene (SLC6A4, 5HTT, SERT) codes for a solute carrier protein responsible for the reuptake of serotonin from the synaptic cleft back into the presynaptic neuron, which is the primary mechanism for regulation of serotonergic activity in the brain (Lesch et al., 1996). A functional polymorphism in the promoter region of the gene (referred to as 5HTTLPR) is a 44-bp insertion/deletion yielding short (S) and long (L) alleles. The long variant is associated with more rapid serotonin reuptake, resulting in lower levels of active serotonin (Lesch et al., 1996). However, allele frequencies vary across different ethnic groups (Haberstick et al., 2015). A SNP in the long allele, rs25531, can modify the activity of this allele (Lesch et al., 1996). SLC6A4/5HTT has been implicated in emotion regulation as well as (emotional) memory and learning processes (Araragi and Lesch 2013; Barzman et al., 2015; Meneses and Liy-Salmeron 2012). Expression of the transporter is observed in regions implicated in attention, memory, and motor activities, such as the amygdala, hippocampus, thalamus, putamen, and ACC (Frankle et al., 2004; Oquendo et al., 2007).
Only one recent imaging genetics study in patients with ADHD has been performed for the 5HTTLPR, showing that stress exposure, which is associated with increased ADHD severity in S-allele carriers, was associated with reduced cortical gray matter volume in precentral gyrus, middle and superior frontal gyri, frontal pole, and cingulate gyrus in these individuals. Interestingly, this paper showed that only some of these regions, the frontal pole and the ACC, actually mediated the effects of the gene-environment interaction on ADHD severity. In sMRI studies in healthy individuals, the 5HTTLPR has been associated with volume of the ACC and amygdala as well as hippocampus, though the direction of effect seemed to differ with gender and/or in interaction with environmental factors (Table IV). Few studies have looked at effects of the 5HTTLPR on structural connectivity (Table IV). A large study observed reduced connectivity of amygdala with PFC in S-allele carriers (Long et al., 2013), while another reported increased hippocampus-putamen connectivity for this genotype group (Favaro et al., 2014).
Brain activation patterns in task-based fMRI have been studied extensively for the 5HTTLPR following hallmark studies by the Weinberger lab (Hariri et al., 2005; Hariri et al., 2002). They were the first to report increased activation of the amygdala in S-allele carriers in response to negative-emotional faces. Since then, increased amygdala activation has been observed in S-allele carriers in many tasks activating the amygdala (Table IV and VI). In 2013, 34 studies investigating effects of the 5HTTLPR on amygdala activation were meta-analyzed, confirming the increased activation in S-allele carriers (although only borderline significant) (Murphy et al., 2013). However, this meta-analysis also showed strong heterogeneity between studies and a potential publication bias (towards studies reporting significant associations). Linked to the increased activation seems to be a reduced functional connectivity of the amygdala, as first observed by Pezawas and colleagues (2005) and subsequently also seen in additional studies (Table IV). Not only the amygdala, but also other cortical and subcortical brain regions (forming the ‘threat circuit’) seem to be influenced by 5HTTLPR genotype. A recent, replicated fMRI study, for example, also showed stronger activity in dorsomedial prefrontal cortex (dmPFC), insula, thalamus, and regions of the midbrain, in reaction to threat in S-allele carriers (Klumpers et al., 2014); interestingly, also in this study (like in the one by van der Meer and coworkers (2015)) only some of the activated regions actually mediated the genotype effects on psychophysiological responsivity to pending threats (in this case the dmPFC activation, Table IV).
Increasing evidence suggests that S-allele carriers are hypervigilant to environmental stimuli (Homberg and Lesch 2011). Potential sustained effects of environmental factors have not sufficiently been addressed in imaging genetics studies published to date. Several studies have taken stressful life events into account, and these studies suggested effects on both brain volume and activation. Only one study to date has directly looked at methylation of the promoter of the SLC6A4/5HTT gene, and found correlations with the volume of several regions in the ‘threat circuit’ of the brain, though these appeared genotype-independent (Dannlowski et al., 2014). Also a combined PET, sMRI plus fMRI study indicated that 5HTTLPR genotype did not influence current (midbrain) serotonin transporter availability (Kobiella et al., 2011), suggesting that other factors (like environmental ones) might overrule this effect. Taking into account epigenetic effects on the SLC6A4/5HTT gene might thus help explain the strong heterogeneity observed in the meta-analysis of amygdala reactivity studies (Murphy et al., 2013).
In summary, functional genetic variation in the SLC6A4/5HTT gene is clearly linked to emotion regulation through effects on brain activation in the amygdala and the wider ‘threat circuit’, with those carrying the risk factor for emotional dysregulation showing increased activation in tasks related to emotion processing and learning. Those experiments link reduced availability of the transporter (at some point in development) - and thus increased serotonin signaling capacity - to increased brain activation. This increased activation seems to be linked to functional dysconnectivity, however. Whether brain volume and structural integrity are influenced by the 5HTTLPR, remains to be clarified. Importantly, genotype effects are likely to be sensitive to environmental factors.
The nitric oxide synthase 1 (encoded by the NOS1 gene) is an enzyme which synthesizes nitric oxide from L-arginine. Nitric oxide is a reactive free radical, which acts as a biological mediator in several processes, including dopaminergic and serotonergic neurotransmission (Kiss and Vizi 2001). The NOS1 gene has a complex structure, including 12 alternative untranslated first exons (exon 1a-1l). In exon 1f, a functional VNTR that affects gene expression has been linked to hyperactive and impulsive behavior in humans (Reif et al., 2009; Weber et al., 2015), with the short allele being the risk factor for ADHD. In addition, a recent Nos1 knock-out mouse model showed dysregulation of rhythmic activities mimicking ADHD-like behaviors (Gao and Heldt 2015).
So far, only one case-control study investigated the effect of the VNTR polymorphism on the brain, in his case on reward-related ventral striatal activity (Hoogman et al., 2011) (Table IV). The study revealed that homozygous carriers of the short allele of NOS1 demonstrated higher ventral striatal activity than carriers of the other NOS1 VNTR genotypes (Hoogman et al., 2011). This effect was comparable for both patients and healthy individuals. Similar effects of the genotype were also observed for behavioral impulsivity, with those carrying the ADHD risk factor acting more impulsive than other participants.
Imaging genetics of candidate genes for autism spectrum disorders
A total of 193 records were retrieved for the ASD search terms, and a total of six research articles were eligible for review according to our criteria. All studies investigated a single gene and were performed in Caucasian populations. For studies in the healthy population, we obtained 120 records, and 17 were included in the review (Figure 1). Twelve of those investigated a single gene in a Caucasian study sample, and five studies used Asian samples (studies for SLC6A4/5HTT are included in the ADHD section above). Generally, the ASD case/control samples included mainly childhood and adolescent study samples, whereas the studies in healthy population samples mostly used samples of (young) adults. From the eleven genes selected and listed in Table V, imaging genetics studies could only be retrieved for genetic variants in CNTNAP2, MET, OXTR, and the SLC6A4/5HTT gene.
Table V:
Imaging genetics studies in ASDs case-control samples and ASDs candidate genes studies in the healthy population (for selection of candidate genes see Table II).
| Gene | Polymorphism | Imaging modality | Imaging/cognitive phenotype | Genotype groups compared | Samples size (mean age in years) | Primary results (main effect of genotype) | Reference |
|---|---|---|---|---|---|---|---|
| CNTNAP2 | rs2710102 | fMRI | Reward-guided implicit learning task (fronto-striatal circuits) | C-allele carriers vs. non-risk-carriers | Discovery sample: 16 ASD (12.4) ‡ 16 HC (12.3) ‡ |
Non-risk group (collapsed across patients and controls): ↓ Activity in medial PFC during reward feedback processing; Risk group: ↑ long-range anterior-posterior connectivity between medial PFC, medial occipital, and ventral temporal cortices. | (Scott-Van Zeeland et al. 2010) |
| C-allele carriers vs. non-risk-carriers | Replication sample: 39 HC (13) | Non-risk-group: ↑ long-range anterior-posterior functional connectivity between mPFC, medial occipital, and ventral temporal cortices. | |||||
| rs2710102 | DTI | Whole-brain fiber tractography (graph theory analyses) | CC-carriers vs. CT/TT-carriers | 328 HC (23.4); twins from 189 families | CC-carriers: ↓ path length, ↑ small-worldness and global efficiency in whole-brain analyses, and ↑ eccentricity (maximum path length) in 60 of the 70 nodes in regional analyses. | (Dennis et al. 2011) | |
| rs7794745 | sMRI | WM and GM morphology | TT-carriers vs. AT/AA-carriers | 314 HC | TT-carriers: ↓ GM and WM volume in cerebellum, fusiform gyrus, occipital and frontal cortices. Male TT-carriers: ↓ GM in right frontal pole in right rostral fronto-occipital fasciculus. |
(Tan et al. 2010) | |
| DTI | WM integrity | TT-carriers: ↓ FA in cerebellum, fusiform gyrus, occipital and frontal cortices. Male TT-carriers: ↓FA in right rostral fronto-occipital fasciculus. Female TT-carriers: ↓ FA of anterior thalamic radiation. |
|||||
| rs7794745, rs2710102 | fMRI | Language task | Risk group (T- and C-allele) vs. non-risk group | 66 HC (20.54) | Risk group: ↑ activation in right IFG (Broca’s area homologue) and right lateral temporal cortex. | (Whalley et al. 2011) | |
| MET | rs1858830 | fMRI | Emotional faces task (n = 144), DMN functional connectivity (n = 71), WM structural connectivity (n = 84). | CC-carriers vs. CG-carriers vs. GG-carriers (non-risk) | 75 ASD (13.1) 87 HC (12.5) |
Risk genotype predicted wide-spread atypical fMRI activation (↑ amygdala and striatum) and deactivation patterns (↓ mainly posterior cingulate cortex) to social stimuli. Effects were more pronounced ASD group, especially within heterozygous risk group. | (Rudie et al. 2012) |
| rs-fMRI | Risk genotype: ↓ Functional and structural connectivity in temporo-parietal regions (within DMN) | ||||||
| DTI | Risk genotype: Altered WM integrity | ||||||
| rs1858830 | sMRI | Measures of cortical thickness (CT) development | CC-carriers vs. CG-carriers vs. GG-carriers | 222 HC (9-22) | C-carriers: ↓ CT (lowest in CC group) in superior and middle temporal gyri, ventral precentral and postcentral gyri, and anterior cingulate bilaterally, and in right frontopolar cortex. | (Hedrick et al. 2012) | |
| OXTR | rs2254298 | sMRI | Amygdala volume, TBV | GG-carriers vs. GA-carriers | 51 HC (13) † | GG-carriers: ↑ GM volume, ↓ amygdala volumes. VBM analysis revealed ↑ volume in region of dorsomedial ACC in GG-carriers and ↑ in posterior brainstem in G/A-carriers | (Furman et al. 2011) |
| sMRI (VBM) | Global brain measures (GM, WM, TBV) | AA- carriers vs. AG-carriers vs. GG-carriers | 135 HC (28.8)§ | Male A-allele carrier: ↓ GM volume in right insula (neuroanatomical correlate of ALTs). | (Saito et al. 2014) | ||
| rs1042778, rs2254298, rs237887, rs918316, rs2268493, rs53576, rs2268495 | sMRI | Amygdala and hippocampus volume, TBV | rs2254298: AA- carriers vs. AG-carriers vs. GG-carriers | 208 HC (33.9)§ | rs2254298: A-allele carriers: ↑ bilateral amygdala volume. Two 3-SNP haplotypes (including rs2254298 G-allele), showed associations with ↓ bilateral amygdala volume. | (Inoue et al. 2010) | |
| rs53576 | sMRI (VBM) | Global brain measures (GM, WM, TBV) | AA-carriers vs. GG/GA- carriers | 290 HC (23.7)§ | Female AA-carriers: ↓ amygdala volumes bilaterally (especially centromedial subregion, with a trend of allele-load-dependence) | (Wang et al. 2014) | |
| rs-fMRI | rsFC | ↓ Resting-state functional coupling between PFC and amygdala bilaterally (allele-load-dependent trend). | |||||
| rs-fMRI | Functional connectivity density (FCD) using a voxel-wise, data-driven approach | Male AA-carriers vs. male G-allele carriers | 270 HC (24.2)§ | FCD of hypothalamus exhibited main effect of genotype (↓FCD in male AA homozygotes). Gender-by-genotype interaction in resting-state FC (rsFC) between hypothalamic region and left dorsolateral PFC, but no main effect of genotype (↓ rsFC in male AA homozygotes). | (Wang et al. 2013) | ||
| sMRI (VBM) | Regional alterations in GM volume | GG-carriers vs. GA-carriers vs. AA-carriers | VBM: 212 HC (29.9) fMRI: 228 HC (31.9) (98 overlap) |
A-allele carriers: ↓ hypothalamus GM volume | (Tost et al. 2010) | ||
| fMRI | Face-matching task | A-allele carriers: ↓ amygdala activation, ↑ functional correlation of hypothalamus and amygdala during perceptual processing of facial emotion (specifically in male risk allele carriers lower levels of reward dependence predicted). | |||||
| 23-tagging SNPs (including rs7632287, rs237887, rs2268491, rs2254298, rs2268494) | fMRI | Animated angry faces task | rs237915: CC-carriers vs. CT/TT-carriers | 1445 HC (14.4) | CC-carriers: ↓ VS activity (related to more peer problems). | (Loth et al. 2014) | |
| rs53576 | fMRI | Others’ suffering task | GG-carriers vs. AA-carriers | 60 HC (20.2)§ | GG-carriers: hierarchical regression analyses revealed ↑ associations between interdependence and empathic neural responses in insula, amygdala, and superior temporal gyrus. | (Luo et al. 2015) | |
| fMRI | Emotional-valenced stimuli task | GG-carriers vs. AG/AA-carriers | 21 HC (34) | GG-carriers: ↑ functional connectivity between regions of interest. Bilateral amygdala and medial PFC show ↑ influence on other brain regions; bilateral pars opercularis, left amygdala, and left medial PFC are more receptive to activity in other brain regions. | (Verbeke et al. 2013) | ||
| rs1042778, rs2268493, rs237887 | fMRI | MID task | rs2268493: TT-carriers vs. CT/CC-carriers | 31 HC (23.6) | rs2268493 TT-carriers: ↓ Activation in mesolimbic reward circuitry (nucleus accumbens, amygdala, insula, thalamus and prefrontal cortical regions) during anticipation of rewards but not during outcome phase. | (Damiano et al. 2014) | |
| rs53576, rs1042778 | fMRI | Mother-child interaction task | 3 genotype groups per SNP | 40 HC † | Both rs53576 and rs1042778 were associated with both positive parenting and hemodynamic responses to child stimuli in OFC, ACC, and hippocampus (rs53576 GG group showed lowest hemodynamic response). | (Michalska et al. 2014) | |
| rs2268498, rs180789, rs401015 | fMRI, doubleblind placebo-controlled crossover study | Social-emotional and gaze processing task; amygdala activation after intranasal oxytocin self-administration | rs401015: CT-carriers vs. TT-carriers | 55 HC (24.9) ‡ | rs401015 modulated right amygdala activity under influence of oxytocin (CT-carriers: ↑ amygdala activity). | (Montag et al. 2013) | |
| SLC6A4/5HTT | 5-HTTLPR | sMRI (VBM) | Total GM and WM volume | LL vs. LS vs. SS | 43 ASD (30) | No associations between total GM or WM volume and genotype. | (Raznahan et al. 2009) |
| sMRI (longitudinal) | Cerebral cortical and cerebellar GM and WM volume | SS vs. SL vs. LL | 44 ASD (3.4) ‡ | S-carriers: ↑ cortical and frontal lobe GM | (Wassink et al. 2007) | ||
| 5-HTTLPR, rs25531 | rs-fMRI | Functional connectivity | Low vs. high expressing | 54 ASD (13.7) 66 HC (14.5) |
Low expressing genotypes (SS, SLG, LGLG): ↑ posterior-anterior connectivity in ASD group (converse for HC). | (Wiggins et al. 2012) | |
| fMRI | Emotional faces task | Low vs. high expressing | 44 ASD (13.5) 65 HC (14.7) |
Low expressing genotypes (SS, SLG, LGLG): ↑ amygdala activation in ASD group. | (Wiggins et al. 2014) | ||
ACC = anterior cingulate cortex, ALT = autistic-like traits, CA = childhood adversity, CN = caudate nucleus, CT = cortical thickness, CV = cortical volume, DMN = default mode network, DTI = diffusion tensor imaging, GM = gray matter, FC = functional connectivity, FCD = functional connectivity density, HC = healthy control, IFG = inferior frontal gyrus, MID = monetary incentive delay task, mPFC = medial prefrontal cortex, OFC = orbitofrontal cortex, PFC = prefrontal cortex, ROI = region of interest, rsFC = resting-state functional connectivity, SA = surface area, SLE = stressful life events, sMRI = structural magnetic resonance imaging, STS = superior temporal sulcus, VBM = voxel-based morphometry, VS = ventral striatum, WM = white matter, TBV = total brain volume
only females
only males
Asian sample
in gray only case-control studies; for SLC6A4 studies in healthy individuals see Tables IV and VI (ADHD).
The contactin-associated protein-like 2 (CASPR2), encoded by the gene CNTNAP2 (the largest gene in the human genome), is a neural transmembrane protein involved in neuronal-glial interactions and in clustering K+-channels in myelinated axons; as such, it is involved in neuronal cell adhesion, migration, and the formation of neuronal networks (Rodenas-Cuadrado et al., 2014). Several single nucleotide polymorphisms (SNPs) in CNTNAP2 have been associated with ASDs. During human brain development, CNTNAP2 expression is broad, with highest levels in frontal and anterior lobes, striatum, and dorsal thalamus. This cortico-striato-thalamic circuitry is important for higher order cognitive functions, including speech and language, reward, and frontal executive function (Rodenas-Cuadrado et al., 2014). This is reflected in the imaging genetics studies having been performed for CNTNAP2, which cover studies of brain volume and structural connectivity as well as brain activity and functional connectivity during tasks related to rewarded learning and language (Table V).
Two studies performed DTI in healthy individuals. For the SNP rs2710102 it was found that carriers of the CC risk genotype showed reduced overall path length and increased small-worldness of brain-wide structural connectivity, which appeared to be a general phenomenon rather than being localized to individual tracts (Dennis et al., 2011). A large study in healthy individuals combining sMRI with DTI for the SNP rs7794745 showed that carriers of the ASD risk genotype exhibited reduced gray and white matter volume as well as reduced white matter integrity in the cerebellum, fusiform gyrus, occipital and frontal cortices; distribution of reductions was found to be sex-specific (Tan et al., 2010).
In a case-control study, an association between the SNP rs2710102 and medial prefrontal cortex activation during a rewarded implicit learning task was found, when collapsing patients and controls into one group. The non-risk allele was linked to reduced activation. Furthermore, the risk carriers had more widespread and bilateral connectivity throughout the frontal cortex and anterior temporal poles. The latter finding was confirmed in an independent healthy sample (Scott-Van Zeeland et al., 2010). An additional fMRI study using a sentence completion paradigm showed that carriers of the risk genotype for one of two SNPs had increased activation of the IFG (Broca’s area), the lateral temporal cortex, or right middle temporal gyrus (Whalley et al., 2011).
The Met proto-oncogene encoded by the MET gene is a cell surface receptor with tyrosine-kinase activity. In the forebrain, MET gene and protein expression is regulated in excitatory projection neurons during synaptogenesis (Judson et al., 2011) and is restricted to regions of temporal, occipital, and medial parietal cortex in humans. These regions are known to be of relevance to the processing of socially relevant information (Rudie et al., 2012). The effects of the ASD risk variant rs1858830 have been studied in two imaging genetics studies (Table V).
A case-control study combining fMRI (emotional face task), resting-state fMRI, and DTI modalities showed that the ASD risk genotype predicted wide-spread atypical brain activity patterns to social stimuli, with increased activation in amygdala and striatum, and impaired deactivation patterns in part of the default mode network (DMN) in the posterior cingulate cortex. In addition, reduced functional and structural connectivity was observed in temporo-parietal regions belonging to the DMN suggesting altered white matter integrity. In general, the effects were more pronounced in the ASD group (Rudie et al., 2012). An sMRI study in a large sample of healthy individuals revealed that cortical thickness in temporal, pre- and postcentral gyri, anterior cingulate, and frontopolar cortex was reduced in risk-allele carriers, with reductions increasing with increasing number of risk alleles (Hedrick et al., 2012).
The oxytocin receptor (OXTR) gene encodes the receptor protein for oxytocin, which has an important role in the regulation of social cognition and behavior (Meyer-Lindenberg et al., 2011). So far, no imaging genetic studies were performed for risk variants in the OXTR gene in ASD case-control samples, but twelve studies in healthy samples were found (Table V). Various different SNPs and combinations of those were investigated, not all related to ASD risk.
Two sMRI studies showed that adolescents homozygous for the rs2254298 risk factor for psychopathology displayed an overall increased gray matter volume, but a decreased amygdala volume (Furman et al., 2011); for carriers of the rs53576 SNP, a risk factor for disorders associated with social impairment, a smaller hypothalamus gray matter volume was reported in healthy adults (Tost et al., 2010).
Functional MRI paradigms used to study OXTR all covered the cognitive domains of emotion processing and reward (Table V). In a face matching task, adult carriers of the rs53576 risk allele showed increased functional correlation of hypothalamus and amygdala during perceptual processing of facial emotion (Tost et al., 2010). Investigating a large group of 1445 healthy adolescents in a passive face viewing task for effects of 23 SNPs across OXTR, the IMAGEN Consortium found significant effects of one SNP on ventral striatal activity in a region of interest analysis. In the presence of stressful life events, this SNP modulated the occurrence of emotional problems in the participants, linking more emotional problems to reduced striatal activation; no effects of the risk variants for ASD were observed (Loth et al., 2014). A study of brain regions related to processing of social stimuli observed increased functional connectivity between such regions in adult carriers of the risk genotype for rs53576 (Verbeke et al., 2013). Functional MRI of mesolimbic structures during reward processing was modulated by the rs2268493 risk factor for ASD: young adult carriers of the risk genotype showed reduced activation in mesolimbic reward circuitry (nucleus accumbens, amygdala, insula, thalamus, and prefrontal cortical regions) during the anticipation of rewards but not during reward receipt (Damiano et al., 2014). Using a mother-child interaction task, Michalska and coworkers (2014) showed that females carrying the ASD risk genotypes for rs53576 or rs1042778 had lower brain activity in OFC, ACC, and hippocampus in response to child stimuli. When healthy adult females were tested for empathic response and associated brain activation, carriers of the rs2254298 risk factor for psychopathology showed increased responsiveness of the superior temporal sulcus to observed pain (Laursen et al., 2014). In a pharmacologic imaging genetics study in adult males, one of three SNPs modulated the response of the amygdala (only) after oxytocin inhalation, with increased activation to directed gaze and decreased activation to averted gaze under oxytocin in the carriers of the variant allele (Montag et al., 2013). This study did not find any effects of rs2254298 on brain activation.
In summary, genetic variation in the OXTR gene has been linked to brain activation during emotional processing. Risk factors for ASD/psychopathology appear to reduce activation during most relevant paradigms, but may increase functional connectivity during those tasks.
Four ASD case-control imaging genetics studies investigated the gene encoding the serotonin transporter gene (SLC6A4, 5HTT) in addition to those in healthy individuals (and ADHD case-control samples) described in the section on ADHD candidate genes. Structural MRI, fMRI, and rs-fMRI were used to study the effect of either only the 5HTTLPR or the combination of this variant with rs25531 (Table V).
Whereas a VBM study did not reveal an association between total gray or white matter volume and genotype in adult patients (Raznahan et al., 2009), another sMRI study showed that in 2–4 year old boys with ASD, carriers of the 5HTTLPR S-allele had increased total cortical and frontal lobe gray matter volume (Wassink et al., 2007), suggesting an age-dependent effect of the variant.
The fMRI and rs-fMRI study, performed in overlapping samples of adolescent patients and controls, showed that carriers of alleles that mark low gene expression had increased amygdala activation during an emotional face task, an effect that was observed only in the patients (Wiggins et al., 2014b), and increased posterior-anterior connectivity during a resting-state condition in patients, where the converse was observed in the healthy group (Wiggins et al., 2012).
The findings of those case-control studies are not easily reconciled with those observed in healthy individuals (Table IV and VI), and indeed the latter two studies suggest the existence of differential effects in patients and healthy individuals.
Imaging genetics in selected intellectual disability disorders
A total of 579 records were retrieved for the ID syndromes of interest. Eighty research articles were eligible for review according to our criteria, 30 for fragile X syndrome, 24 for neurofibromatosis type 1, 22 for tuberous sclerosis complex, and four for Rett syndrome (Figure 1). No imaging studies of Timothy syndrome patients were uncovered by our search term. The reviewed imaging genetics studies in ID syndromes are presented in Table VII.
Table VII:
Imaging genetics studies in intellectual disability syndromes (fragile X syndrome (FMR1), tuberous sclerosis (TSC1 and TSC2), neurofibromatosis type 1 (NF1), and Rett syndrome (MECP2)). No studies for Timothy syndrome (CACNA1C) were retrieved in our search of the literature.
| Disorder | Gene | Imaging modality | Imaging phenotype | Samples size (mean age in years) | Primary results (main effect of genotype) | Reference |
|---|---|---|---|---|---|---|
| Fragile X syndrome | FMR1 full mutation | sMRI | Quantitative morphometry | Subgroups of 51 FXS; 120 HC | ↑ CN volume, and lateral ventricle (in males). | (Reiss et al. 1995) |
| Hippocampus and amygdala volume | 10 FXS (5 ‡ (6.3); 5 † (9.0)); 10 HC (5 ‡ (6.4); 5 † (8.7)) | ↑ Right hippocampal volume. | (Kates et al. 1997) | |||
| Regional brain volumes | 10 FXS (9.0); 10 HC (8.5) | ↑ CN and ventricular volumes. | (Kaplan et al. 1997) | |||
| Tissue volumes | 10 FXS (9.1); 10 HC (8.5) | ↑ CN GM volume. | (Reiss et al. 1998) | |||
| TBM | 36 FXS (14.66); 33 HC (14.67) | ↑ CN and lateral ventricle volumes, and trend-level parietal and temporal WM ↑. | (Lee et al. 2007) | |||
| GM VBM and manual tracing; multivariate pattern classification | 51 FXS (35 months); 32 HC (29.7 months); 18 DD (34.8 months) ‡ | ↓ GM volumes in regions including hypothalamus, insula, medial and lateral PFC. Spatial patterns that discriminated FXS from other groups included a medial to lateral gradient of increased and decreased regional brain volumes in posterior vermis, amygdala, and hippocampus. |
(Hoeft et al. 2008) | |||
| CN, hippocampus, putamen, amygdala volume | 52 FXS (2.9); 63 ASD (2.8); 19 DD (3,0); 31 HC (2.6) ‡ | ↑ CN volume compared to all control groups. FXS: ↓ amygdala volume. ASD:↑ amygdala volume. |
(Hazlett et al. 2009) | |||
| VBM of regional GM | 10 FXS (28.9); 10 ASD (30.1); 10 HC (29.4) | FXS: ↑ GM volumes within frontal, parietal, temporal, and cingulate gyri, and in CN and cerebellum compared to ASD. FXS: ↑ GM volumes in frontal gyri and CN and ↓ GM volumes in cerebellar, parietal and temporal regions compared to HC. ASD: ↑ GM volumes in frontal and temporal gyri compared to FXS and ↓ GM cerebellar volumes compared to HC. |
(Wilson et al. 2009) | |||
| Total and regional insular volumes | 11 FXS (5 † (15.3); 6 ‡ (16.3)); 8 HC ((5 † (16.5); 3 ‡ (13.3)); 11 DD (6 † (16.4); 5 ‡ (16.0)) | ↓ Total, anterior and posterior insular volumes compared to HC and DD. | (Cohen et al. 2011) | |||
| Univariate VBM; multivariate pattern classification and clustering. | 52 FXS (2.90); 63 ASD (2.77); 31 HC (2.55); 19 DD (2.96) ‡ | ↓ (for FXS) and ↑ (for ASD) volumes of frontal and temporal GM and WM regions (including medial PFC, OFC, superior temporal region, temporal pole, amygdala, insula, and dorsal cingulum) compared to HC. Overall pattern of brain structure in ASD resembles that of HC more than FXS. |
(Hoeft et al. 2011) | |||
| Regional brain bulk volumes (stereology) and GM and WM volume (VBM) | 17 FXS (30); 18 HC (35) ‡ | ↑ CN, parietal lobes and right brainstem bulk volume. ↓ Left frontal lobe volume. ↑ GM volumes of fronto-striatal regions including CN. ↑ WM in regions extending from brainstem to parahippocampal gyrus, and from left cingulate cortex to CC. |
(Hallahan et al. 2011) | |||
| Age-related change in regional brain volumes | 59 FXS (36 † (16.0); 23 ‡ (15.2)) (19 with longitudinal data); 83 HC (47 † (15.8); 36 ‡ (15.5)) (17 with longitudinal data) | Consistent FXS related volume differences in CN compared to HC across adolescence. Aberrant maturation of PFC gyri. | (Bray et al. 2011) | |||
| Cortical volume, thickness, complexity, surface area and gyrification index | 11 FXS (9.16) (6 FXS; 5 FXS+ASD); 10 HC (8.25) ‡ | FXS: ↑Cortical volume, thickness and complexity compared to HC. FXS+ASD: ↑ Left parietal lobe volume, ↓ gyrification specifically in the left temporal and a trend for ↓ right frontal surface area compared to FXS. |
(Meguid et al. 2012) | |||
| Total brain, regional (lobar) and subcortical volumes; brain growth | 53 FXS (2.9); 68 ASD (2.8); 19 DD (3.0); 31 HC (2.6) ‡ | FXS: ↑ Global brain volumes compared to HC but not ASD. ↑ Temporal lobe WM, cerebellar GM, and CN volume compared to ASD. ↓ Amygdala volume compared to ASD. Rate of brain growth from 2 to 5 years similar to HC. |
(Hazlett et al. 2012) | |||
| Relationship repetitive behaviors and CN volume | 41 FXS (4.6) (16 FXS+ASD (4.8)); 30 ASD (4.7) ‡ | FXS: Positive correlation of self-injury with CN volume. ASD: Positive correlation of compulsive behaviors with CN volume. |
(Wolff et al. 2013) | |||
| CN volume and topography | 48 FXS (21.3); 28 IQ-matched controls (19.5); 36 HC (19.7) | ↑ CN compared to both control groups, with ↑ bilateral CN radial distance, ↑ dorsolateral CN head and ventromedial CN body radial distances. | (Peng et al. 2014) | |||
| CN and hippocampal volume | 14 FXS+ASD (22.6); 17 HI (22.0); 25 HC (21.6) ‡ | FXS: ↑ Hippocampus and CN volume compared to HC. HI: ↓ Hippocampal volumes. |
(Molnar and Keri 2014) | |||
| DTI | Whole-brain, frontal-caudate, and sensory-motor tract FA | 10 FXS (16.7); 10 HC (17.1) † | ↓ FA in WM in fronto-striatal pathways and parietal sensory-motor tracts. | (Barnea-Goraly et al. 2003) | ||
| Ventral frontostriatal WM | 17 FXS (2.8); 13 HC (2.3); 8 DD (3.0) ‡ | ↑ Density of fibers localized in left ventral frontostriatal pathway. | (Haas et al. 2009) | |||
| Voxel-based comparison of anisotropy and diffusivity | 18 FXS (11.01); 25 22q11.2DS (10.75); 17 TS (10.56); 41 HC (10.6) † | FXS: ↓ FA in posterior limbs of internal capsule, posterior thalami, and precentral gyrus. | (Villalon-Reina et al. 2013) | |||
| sMRI | GM density (VBM) | 17 FXS (17.5); 16 HC (16.3) | ↑ GM density in bilateral caudate head, left hippocampus, left planum temporale, left angular gyrus, and left superior parietal lobule. ↓ GM density in bilateral insular cortex, precuneus cortex, thalamus, and subgenual cingulate cortex. |
(Hall et al. 2013) | ||
| rs-fMRI | Fractional Amplitude (fALFF); functional connectivity (group ICA and dual regression) | ↓ Functional connectivity in salience, precuneus, left executive control, language, and visuospatial networks. ↓fALFF in bilateral insular, precuneus, and ACC. |
||||
| fMRI | ROI activation during 1-back and 2-back visuospatial working memory tasks | 10 FXS; 15 HC † | No change in activation between 1-back and 2-back tasks in IFG, middle frontal gyrus, superior parietal lobule, and supramarginal gyrus, while HCs showed ↑ activation. | (Kwon et al. 2001) | ||
| Activation during a counting Stroop task | 14 FXS; 14 HC † (15.4) † | ↓ Activation in orbitofrontal gyrus, insular cortex, superior temporal gyrus. No activation in inferior/superior parietal lobe as seen in HC. |
(Tamm et al. 2002) | |||
| FG and STS activation in response to face and gaze stimuli | 11 FXS (16.4); 11 HC (15.5) † | ↓ Left STS activation to all stimuli. No greater FG activation to forward faces compared to angled faces as seen in HC. |
(Garrett et al. 2004) | |||
| Go/nogo task | 10 FXS (15.4); 10 DD (14.6); 10 HC (16.7) ‡ | ↓ Activation in right ventrolateral PFC and right caudate head, and ↑ left ventrolateral PFC activation compared with both control groups. Positive correlation between task performance and activation in left ventrolateral PFC. |
(Hoeft et al. 2007) | |||
| Emotional attribution task | 10 FXS (16.4); 10 HC (15.6) † | ↓ ACC activation for neutral compared to scrambled faces. ↓ CN activation for sad compared to scrambled faces. FXS: ↑ Negative correlation between IQ and insula activation for neutral compared to scrambled faces. HC: ↑ Positive correlation between IQ and ACC activation for neutral compared to scrambled faces. |
(Hagan et al. 2008) | |||
| Activation during face encoding | 11 FXS (18.5); 11 HC (18.7) | ↓ Activation of prefrontal regions including medial and superior frontal cortex during successful face encoding. Negative correlation social anxiety and brain activity during face encoding. | (Holsen et al. 2008) | |||
| Whole-brain and ROI activation during directed or averted eye gaze stimuli | 13 FXS (15.5); 10 DD (16.1); 13 HC (15.0) ‡ | ↓ PFC activation and ↑ left insula activation to direct eye gaze stimuli. ↑ Sensitization in left amygdala with successive exposure to direct gaze compared to controls. |
(Watson et al. 2008) | |||
| Auditory temporal discrimination task | 10 FXS (18.7); 10 HC (14.7) † | ↑ Activation in a left-lateralized network including left medial frontal gyrus, left superior and middle temporal gyrus, left cerebellum, and left brainstem (pons). | (Hall et al. 2009) | |||
| Brain activity during a gaze habituation task | 30 FXS (20.9); 25 HC (19.0) | ↓ Neural habituation and significant sensitization in cingulated gyrus, fusiform gyrus and frontal cortex in response to gaze stimuli. | (Bruno et al. 2014) | |||
| Neurofibromatosis type 1 | NF1 | sMRI | Cerebral GM and WM | 22 NF1; 20 HC | ↑ Brain volume, especially WM. | (Said et al. 1996) |
| Number, volume, distribution and change in time of UBOs | 46 NF1 (7.8) (28‡; 18†) | UBOs found in 93% of subjects, localized most commonly in GP (30.4%), cerebellum (23.5%), and midbrain (16.2%). ↑ UBO number and volume between 4 to 10 years with a reduction in subjects aged 10+ years. |
(Griffiths et al. 1999) | |||
| 24 ventricular and parenchymal dimensions and area calculations | 27 NF1 (8.8) (20‡; 7†); 43 HC (5.9) (22‡;21†) | ↑ Bicaudate width, biatrial width, and biparietal diameter, but not hemispheric length. ↑ Iter measures, descending sigmoid sinus, and ↑ brainstem height (age-specific). |
(DiMario et al. 1999) | |||
| TBV, GM, WM, CSF, CC regions and hyperintensities | 52 NF1 (10.9); 19 HC (9.8) | ↑ TBV due to ↑ GM volume. ↑ CC size. ↑ Group differences in GM to WM ratio in younger compared to older subjects. |
(Moore et al. 2000) | |||
| Morphometric and volumetric measures of (midline) structures; GM and WM volume | 18 NF1 (range 6.2-14.7); 60 HC (range 4.5-16.1) | ↑ Bilateral hyperintensities and ↑ midline structure size in macrocephalic compared to normocephalic NF1. ↑ Brain volume and WM volume but not GM or ventricular volume in macrocephalic subjects compared to HC. |
(Steen et al. 2001) | |||
| Surface area, GM volume, and asymmetry of the PT and PP | 24 NF1 (11.1); 24HC (11.8) | ↓ Left PT surface area and GM volume and ↑ symmetry between left and right PT in NF1 boys compared to NF1 girls and HC. | (Billingsley et al. 2002) | |||
| Number of affected regions, UBO volume and number | 12 NF1 (13.0) | UBO prevalent in GP/internal capsule. ↓UBO locations, number and/or volume for all regions except cerebellar hemispheres between ages 7 to 12 years and ↑ during adolescence. |
(Kraut et al. 2004) | |||
| GM and WM volumes | 36 NF1 (9.3); 39 HC (9.5) | ↑ GM volumes predominantly in temporal, parietal and occipital regions and WM volumes predominantly in frontal lobe. | (Greenwood et al. 2005) | |||
| Frequency, signal characteristics and localization of T2 hyperintensities at different ages | 103 NF1 (13.9) | ↓ Frequency, size, and intensity of T2 hyperintensities in BG and cerebellum/brainstem with age. No differences in hemispheric lesions with age. |
(Gill et al. 2006) | |||
| Regional subcortical volumes; cortical volume, thickness, surface area and gyrification | 14 NF1 (11.3); 14 HC (11.9) | ↑ Volume of thalami, right CN and middle CC. ↓ Gyrification indices in frontal and temporal lobes, insula, cingulate cortex, parietal and occipital regions. No differences in cortical volume, thickness and surface area. |
(Violante et al. 2013) | |||
| SVM; VBM | 21 NF1 (11.1); 29 HC; 18 NF1 (33.1); 31 HC (35.0) | SVM classifiers correctly classified 94% of cases (sensitivity 92%; specificity 96%). | (Duarte et al. 2014) | |||
| GM and WM volume | 16 NF1 (29.8); 16 HC (33.1) | ↓ GM volume of superior frontal gyrus, orbital gyrus and right STG ↑ GM volume in frontal, temporal,,parietal and limbic lobes |
(Pride et al. 2014) | |||
| sMRI | TBV; CC morphology | 10 NF1 (range 20-68): 10 HC (range 21-64) | No differences in TBV. ↑ CC length (10%), CC area (20%). |
(Wignall et al. 2010) | ||
| DTI | CC diffusion characteristics | ↑ Minor eigenvalues at genu of CC. | ||||
| GM and WM volume | 14 NF1 (24); 12 HC (22.7) | ↑ GM and WM volume. | (Karlsgodt et al. 2012) | |||
| TBSS | ↓ FA and radial diffusion and ↑ ADC with greatest magnitude in frontal lobe. | |||||
| DTI | FA and ADC brainstem, basal ganglia, thalamus, CC, and frontal and parietooccipital WM regions | 10 NF1 (25.8); 10 HC (26.3) | ↑ ADC and ↓ FA in all regions of interest. | (Zamboni et al. 2007) | ||
| Diffusion characteristics (ADC, FA, A(m), eigenvalues) healthy and disordered brain matter | 50 NF1 ((21 female (12.2); 29 male (12.3)); 8 HC | ↑ ADC and eigenvalues in UBO compared to normal-appearing sites ↑ ADC in normal-appearing sites compared to HC. No differences in FA or A(m) in most regions. |
(van Engelen et al. 2008) | |||
| FA BG, cerebellum, pons, thalamus | 44 NF1(12.8); 20 HC (14.1) | ↓ Bilateral cerebellar and thalamic FA. | (Ferraz-Filho et al. 2012) | |||
| FA, ADC CN, putamen, GP, thalamus | 14 NF1 (16.3) (8 with UBOs; 6 without UBOs and 9 <18 years;5 > 18 years); 8 HC (16.1) | ↑ ADC in CN, putamen, GP, thalamus. | (Nicita et al. 2014) | |||
| ADC,FA, RD, eigenvalues for 7 GM and 8WM ROIs; WM trajectories for adjacent WM tracts of NBOs | 14 NF1 (7.2); HC | ↑ ADC and eigenvalues in GM and WM UBOs compared to contralateral normal-appearing sites and HC and ↓ FA compared to HC. Three out of 18 UBOs disrupt WM tracts. ↑ADC, lambda(2) and radial diffusivity of WM UBOs in patients with neurological symptoms compared to patients without. |
(Ertan et al. 2014) | |||
| fMRI | Activity in ten ROIs during phonologic processing | 15 NF1 (14.4); 15 HC (15.3) | Inferior and dorsolateral PFC activation relative to posterior activation ↑ during auditory phonologic processing and ↓ during orthographic processing. | (Billingsley et al. 2003) | ||
| Occipital and parietal cortex activity during visual-spatial processing | 15 NF1 (14.4); 15 HC (15.3) | ↑ Posterior cortex activation relative to lateral and inferior frontal activation. | (Billingsley et al. 2004) | |||
| Activation in frontal, temporal, parietal, and occipital regions during visuospatial processing | 13 NF1 (9.8); 13 HC (9.8) | ↑ Left instead of right hemisphere activation. ↓ Activation in primary visual cortex. |
(Clements-Stephens et al. 2008) | |||
| Early cortical visual pathway and DN activation during visual stimuli activating magnocellular and parvocellular pathways | 15 NF1 (11.7); 24 HC (12.0); 13 NF1 (33.1)†; 15 HC (32.7)† | ↓ Activation of low-level visual cortex. ↓ Deactivation or ↑ activation of midline regions of DN during magnocellular- biased stimulation. |
(Violante et al. 2012) | |||
| rs-fMRI | Ventral ACC, amygdala, OFC, PCC RSFC | 14 NF1 (12.5); 30 HC (12.3) | ↑ Connectivity between: left ventral ACC and frontal cortex, insula, and subcortical areas (CN, putamen); left amygdala and frontal cortex, insula, supramarginal gyrus, and PCC/precuneus; left OFC and frontal and subcortical areas (CN, pallidum). | (Loitfelder et al. 2015) | ||
| Tuberous sclerosis complex | TSC1/TSC2 | sMRI | Number and location of cerebellar tubers and volumes of underlying parenchyma | 34 TSC (8.9) | Mean tuber number was 14.3 and 44.1% of subjects showed both cerebral and cerebellar tubers and had more global cortical lesions than subjects with cerebral tubers only. ↓ Focal volume associated with tubers in cerebellum. |
(Marti-Bonmati et al. 2000) |
| GM, WM and CSF volume | 10 TSC (41.5); 8 HC (40.0) | ↓ GM volume in medial temporal lobes, posterior cingulate gyrus, thalamus, BG and right fronto-parietal cortex. ↓ Of limbic and subcortical GM volume negatively correlated with tuber count. ↓ WM of longitudinal fasciculi and other major intrahemispheric tracts. ↑ Cerebellar WM. |
(Ridler et al. 2001) | |||
| Tuber distribution and lesion load | 25 TSC (39.0) | Highest tuber frequency in frontal lobes and highest tuber density in parietal regions with variation in tuber density but no lateralization of tubers. Nodules were located predominantly in CN. Tuber and nodule volumes positively correlated. ↑ Tuber volume in subjects with a history of epilepsy. |
(Ridler et al. 2004) | |||
| Characteristics of cerebellar lesions | 73 TSC (range 0-28 years) | 16.4% of TSC subjects showed cerebellar lesions. Six subjects showed atrophy of cerebellar parenchyma around tubers. | (Jurkiewicz et al. 2006) | |||
| GM, WM and CSF volume and lesion load | 25 TSC (39.3); 25 HC (34.3) | ↓ Subcortical GM volume in regions including thalamus, BG, insula, and cerebellum. ↓ WM in intrahemispheric tracts. |
(Ridler et al. 2007) | |||
| Tuber number and tuber/brain proportion | 58 TSC (20.6) (19 TSC1 (25.0); 34 TSC2 (19.0)) | ↑ Tubers and tuber/brain proportion in TSC2 compared to TSC1 subjects and in subjects with a mutation deleting or directly inactivating tuberin GAP domain compared to subjects with an intact GAP domain. | (Jansen et al. 2008a) | |||
| Tuber number and tuber/brain proportion as determinants of seizures and cognitive function | 61 TSC (17.9) (14 TSC1; 30 TSC2) | Tuber/brain proportion was inversely related to age at seizure onset and intelligence. | (Jansen et al. 2008b) | |||
| Presence of SENs and SGCTs | 81 TSC (28) | 15% of TSC subjects showed SGCTs. 62% showed SENs, 24% of which also showed SGCTs. ↑ SGCT volume at follow-up. |
(Michelozzi et al. 2013) | |||
| Cerebellar volume | 36 TSC (9.7)(19 TSC2; 7 TSC1); HC (9.7) | ↓ Cerebellar volume, with strongest effect in subjects with TSC2 mutations. | (Weisenfeld et al. 2013) | |||
| (Cyst-like) tuber/ brain proportion and tuber number in relation to age at seizure onset | 23 TSC (12.4) | Tuber/brain proportion and number of tubers, but not cyst-like tuber/brain proportion and number of cyst-like tubers, were negatively correlated with age at seizure onset. | (Nakata et al. 2013) | |||
| DTI | ADC, FA of epileptogenic tubers | 15 TSC | ↑ ADC values in subtuber WM in epileptogenic tubers compared to nonepileptogenic tubers. | (Chandra et al. 2006) | ||
| ADC of NAWM in frontal, parietal and occipital lobes, and pons | 23 TSC (12); 18 HC | ↑ ADC values in frontal WM and pons for age group between 96 and 144 months and in right parietal and occipital WM for subjects older than 144 months. | (Arulrajah et al. 2009) | |||
| ADC, FA in tubers and WM lesions | 14 TSC (15.1) | ↑ ADC values in cortical tubers. ↑ ADC values and ↓ FA values in WM lesions compared with contralateral regions. |
(Piao et al. 2009) | |||
| FA, diffusion characteristics in ROIs in or adjacent to cortical tubers in epileptogenic and non-epileptogenic zones | 12 TSC (8.2) | ↓ FA of cortical tubers in epileptogenic compared to non-epileptogenic zones. ↑ Radial diffusivity and ↓ FA in NAWM in epileptogenic zones compared to non-epileptogenic zones. |
(Widjaja et al. 2010) | |||
| FA, trace, eigenvalues CC and internal capsules, in relation to tuber load | 12 TSC (9.2); 23 HC (11.1) | Tubers were found in frontal lobes (144), parietal lobes (64), temporal lobes (42), occipital lobes (57) and insular cortex (7). ↓ FA, ↑ trace and average lambda(3) in CC and ↑ trace in internal capsules. Tuber volume correlated with multiple DTI characteristics in CC and internal capsules. |
(Simao et al. 2010) | |||
| Diffusion characteristics geniculocalcarine tract, internal capsule, temporal gyri and splenium of the CC | 10 TSC (range 1.5-25 years); 6 HC (range 1.1-25 years) | ↓ FA in geniculocalcarine tracts and splenium of CC. ↓ Axial diffusivity in internal capsule, STG, and geniculocalcarine tracts. ↑ Mean and radial diffusivity in splenium of CC. |
(Krishnan et al. 2010) | |||
| FA, mean radial and axial diffusivities of CC | 40 TSC (7.2) (12 with ASD); 29 HC (7.7) | ↓ Average FA and ↑ diffusivity values in CC. ↓ Average FA in TSC +ASD subjects compared to HC and TSC −ASD subjects (who showed no differences). |
(Peters et al. 2012) | |||
| Diffusion characteristics in major tracts | 16 TSC (13.0); 12 HC (15.3) | ↓ FA and axial diffusivity in wide-spread WM regions. ↓ Number of fibers and number of tract points of commissural fibers, projection fibers and major WM tracts. |
(Wong et al. 2013) | |||
| Diffusion characteristics of RMLs, tubers, SENs, cerebellar lesions and SGCT and NAWM | 30 TSC (15.5); 16 HC (7 children (9); 9 adults (36)) | Mean of 47 RMLs, 27 tubers, and 10 SENs per TSC subject. Inverse correlation of RML FA and MD. No differences NAWM FA and MD. |
(van Eeghen et al. 2013) | |||
| FA dorsal language circuit tract | 38 TSC (10 TSC + ASD; 17 TSC − ASD); 24 HC | ↓ FA values in dorsal language circuit tract. ↓ FA in WM close to Geschwind’s territory and WM close to Broca’s area in TSC +ASD compared to TSC −ASD subjects. |
(Taquet et al. 2014) | |||
| FA, ADC, axial and radial diffusivity of tubers and WM lesions | 18 TSC (9.3) | ↓ FA and ↑ ADC and axial and radial diffusivity values in tubers compared to contralateral normal regions. ↑ Radial diffusivity and ↓ FA in WM lesions. |
(Dogan et al. 2015) | |||
| Global and regional WM connectivity | 20 TSC (range 3-24 years)(11 TSC+ DD; 9 TSC − DD; 20 HC (range 2-23 years) | ↓ Interhemispheric connectivity. ↑ MD, positively correlated with tuber load severity. ↑ MD in TSC + DD subjects compared to TSC – DD subjects. |
(Im et al. 2015) | |||
| Rett syndrome | MECP | sMRI | TBV, cortical GM and WM, subcortical gray nuclei, CSF volumes | 11 RTT (10.1); 15 HC (11.2) † | ↓ Cerebral volume ↑ Loss of GM in comparison to WM, with largest decrease in frontal regions and CN and midbrain volume. |
(Reiss et al. 1993) |
| TBV, cortical GM and WM, subcortical GM, CSF and posterior fossa volumes | 20 RTT (9.8); 20 HC (9.0) † | ↓ GM volume most pronounced in prefrontal, posterior-frontal, and anterior-temporal regions. ↓ WM volume uniformly throughout brain. ↓ CN volume. No differences in midbrain volumes. |
(Subramaniam et al. 1997) | |||
| Absolute and relative changes in GM and WM volumes | 23 RTT (8.6) (12 more severe (8.8); 10 less severe (8.3)); 25 HC (8.9)† | ↓ Absolute volume throughout the brain ↓ Relative parietal lobe GM volume, particularly dorsal. ↓ Cortical WM volume. ↓ Anterior frontal lobe volumes in more severely affected subjects. |
(Carter et al. 2008) | |||
| DTI | Regional FA | 32 RTT (5.5); 37 HC (6.1)† | ↓ FA in genu and splenium of CC and external capsule, and regions of cingulate, internal capsule, posterior thalamic radiation, and frontal WM. No differences in visual pathways. ↓ FA in superior longitudinal fasciculus in patients who were nonverbal or speaking only single words. |
(Mahmood et al. 2010) | ||
22q11.2DS= 22.q11.2 deletion syndrome, ACC= anterior cingulate cortex, ADC= apparent diffusion coefficient, A(m)= axial anisotropy, ASD= autism spectrum disorder, BG=basal gangli, CC= corpus callosum, CN= caudate nucleus, CSF= cerebrospinal fluid, DD= developmental delay, DN= default network, DTI = diffusion tensor imaging, FA= fractional anisotropy, fALFF = fractional amplitude of low-frequency fluctuations, FG= fusiform gyrus, fMRI= functional MRI, FXS= fragile X syndrome, GAP= GTPase activating protein, GM= grey matter, GP= globus pallidum, HI= hypoxic injury, IFG= inferior frontal gyrus, IPL= inferior parietal lobule, IPS= intraparietal sulcus, MCP= middle cerebellar peduncle, MD= mean diffusivity, MTI=magnetization transfer imaging, NAWM= normal-appearing white matter, NF1= neurofibromatosis 1, OFC= orbitofrontal cortex, PCC= posterior cingulate cortex, PFC= prefrontal cortex, PP= planum parietale, PT= planum temporale, RML= radial migration lines, ROI= region of interest, RSFC= resting state functional connectivity; RTT= Rett syndrome, SCP= superior cerebellar peduncle, SEN= subependymal nodule, SGCT= subependymal giant cell tumour, sMRI= structural MRI, STG= superior temporal gyrus, STS= superior temporal sulcus, SVM= support vector machines, SWM= spatial working memory, T1= timepoint 1, T2= timepoint 2, TBM = tensor-based morphometry, TBV= total brain volume, TPJ= temporoparietal junction, TS= turner syndrome, TSC= tuberous sclerosis, TWM = temporal working memory, UBO = unidentified bright objects, VBM= voxel based morphometry, WM= white matter
female
male
The fragile X mental retardation 1 gene (FMR1) is located on the X chromosome and codes for fragile X mental retardation protein. Large expansions of a CGG repeat (>200 repeats) in the 5’- untranslated (5’UTR) region of the gene, leading to protein deficiency, are the cause of fragile X syndrome (FXS). FMR1 has a prominent role in synaptic plasticity and maturation (Saldarriaga et al., 2014). In studies including participants with the FMR1 full mutation, brain structure was most often investigated, followed by task-based brain activation (Table VII). A few studies investigated brain structural integrity and resting-state functional connectivity. Several studies compared individuals with FXS with and without ASD or included an idiopathic autism or IQ-matched group (Table VII).
The most robust finding in investigations of brain structure in FXS is an increased caudate nucleus volume. This enlargement was observed early in development (Hazlett et al., 2009), throughout adolescence (Bray et al., 2011; Hall et al., 2013; Lee et al., 2007) as well as in adult samples (Hallahan et al., 2011; Molnar and Keri 2014; Wilson et al., 2009). Studies comparing individuals with FXS and with ASD found increased caudate volumes in children and adults with FXS compared to children/adults with idiopathic autism (Hazlett et al., 2009; Wilson et al., 2009). Consistent volumetric abnormalities have also been found for cerebellar regions in FXS; a reduction in the volume was observed in both children and adults with FXS (Hazlett et al., 2012; Hoeft et al., 2008; Wilson et al., 2009). Several studies found cerebellar volumes to be larger in children and adults with FXS relative to individuals with autism, in whom reduced volume of cerebellar regions compared to control subjects is often seen as well (Hazlett et al., 2012; Wilson et al., 2009). Few studies have investigated white matter integrity in people with the full FMR1 mutation, and deficits seem most prominent in fronto-striatal connections. Increased density of fibers was found in the left ventral fronto-striatal pathway in boys with FXS compared to typically developing and developmentally delayed controls (Haas et al., 2009), and differences in white matter in frontal-caudate circuits were found in females with FXS compared to controls (Barnea-Goraly et al., 2003). More widespread reductions in white matter integrity have also been observed (Villalon-Reina et al., 2013).
Cognitive and psychiatric characteristics associated with FXS include poor eye contact, repetitive motor behavior, language deficits, inattention, hyperactivity, inhibition, and anxiety (Saldarriaga et al., 2014). Functional neuroimaging studies have focused on these deficits, with a main focus on poor eye contact and behavioral inhibition. Several fMRI studies have investigated the circuitry underlying face/gaze processing in subjects with FXS, as eye-gaze avoidance is common in this population. Abnormal activation was found in several regions, including superior temporal gyrus and fusiform gyrus (Garrett et al., 2004), amygdala and insula (Watson et al., 2008), regions within the ventrolateral prefrontal cortex (vlPFC) (Holsen et al., 2008), and frontal cortex and cingulate and fusiform gyri (Bruno et al., 2014). These regions are associated with visual processing, social cognition, emotion processing, and executive functioning, indicating that eye-gaze avoidance in FXS may be linked to social anxiety. Investigating attention and inhibition, a study using a Go/No-go task found that boys with FXS show reduced activation in the right vlPFC and caudate head. The authors suggested that defective fronto-striatal signaling is a key feature of FXS, leading to impairments in executive functioning (Hoeft et al., 2007), which is in line with the altered white matter connectivity in fronto-striatal connections, described above.
The neurofibromin 1 gene (NF1) located on chromosome 17q11.2 codes for neurofibromin, a protein which is thought to be a regulator of the RAS signal transduction pathway and necessary for embryonic development. Neurofibromatosis type 1 (NF1) is caused by mutations in the gene, often leading to the synthesis of truncated or otherwise non-functional proteins. We found 14 studies investigating effects of NF1 on brain structure and four investigating brain function. Additional studies of brain structural and functional connectivity have been conducted. While most studies included children and adolescents, a few studies have included adults as well (Duarte et al., 2014; Karlsgodt et al., 2012; Pride et al., 2014; Violante et al., 2012; Wignall et al., 2010; Zamboni et al., 2007) (Table VII).
The structural brain abnormalities most commonly seen in subjects with NF1 are T2 hyperintensities and an increased brain volume. T2 hyperintensities are areas of high signal intensity on T2-weighted MR images also referred to as ‘unidentified bright objects’ (UBOs). Although their association with cognitive and intellectual deficits remains controversial, thalamic hyperintensities have repeatedly been associated with cognitive impairments (Payne et al., 2010). Multiple studies have investigated the characteristics of UBOs. UBOs are found in almost all children with NF1, but reports on whether their volume and number increases or decreases with age are inconsistent (Gill et al., 2006; Griffiths et al., 1999; Kraut et al., 2004). A few studies have used diffusion tensor imaging (DTI) to characterize white matter microstructure and integrity of UBOs by measuring the degree and directionality of diffusivity. Higher apparent diffusion coefficient (ADC) and (radial) diffusivity values and lower fractional anisotropy (FA) values have been found in UBOs compared to normal appearing white matter (Ertan et al., 2014; van Engelen et al., 2008). These findings can be explained by myelin deficiency and axonal damage. An increase in brain volume is observed in children with NF1, which was found to be due to increases in white matter volume (Said et al., 1996; Steen et al., 2001), gray matter volume (with an increased gray to white matter ratio especially in younger subjects (Moore et al., 2000)), or both gray and white matter volume (Karlsgodt et al., 2012). These volume increases involve temporal, parietal, occipital, and frontal regions (Duarte et al., 2014; Greenwood et al., 2005; Pride et al., 2014). In addition, the corpus callosum seems larger in cases compared to controls, which has been found in children with NF1 as well as adults, marking it as a robust finding for NF1 (Duarte et al., 2014; Moore et al., 2000; Violante et al., 2013; Wignall et al., 2010). In addition to the investigation of UBOs, DTI studies have been used to study microstructural integrity in NF1 more broadly. Increased ADC values (Ertan et al., 2014; Nicita et al., 2014; van Engelen et al., 2008) and decreased FA values (Ertan et al., 2014; Ferraz-Filho et al., 2012) are found widespread across the brain. Karlsgodt et al. also found increased radial diffusion, which may be explained by decreased myelination or axonal packing density (2012). Differences in radial diffusivity have also been observed at the genu and anterior body of the corpus callosum (Wignall et al., 2010). The change in corpus callosum size and connectivity observed in NF1 may have functional importance, as they have been associated with academic achievement and visual-spatial and motor skills (Moore et al., 2000).
Three fMRI studies have investigated visual-spatial processing in subjects with NF1, and one study investigated phonologic processing (Table VII). During visual-spatial processing, decreased activation in the primary visual cortex was found for individuals with NF1 compared to controls (Clements-Stephens et al., 2008), although an earlier study reported contrasting findings of increased posterior (occipital) cortex activation relative to lateral/inferior frontal activation (Billingsley et al., 2004). A later study did confirm that both children and adults with NF1 showed deficient activation of the low-level visual cortex during tasks specifically designed to activate magnocellular and parvocellular pathways (Violante et al., 2012). During such magnocellular-biased stimulation, NF1 patients did not deactivate regions belonging to the brain default-mode network as would be expected during cognitively demanding tasks (Violante et al., 2012).
The tumor growth suppressor genes tuberous sclerosis 1 (TSC1) and tuberous sclerosis 2 (TSC2) code for the hamartin and tuberin proteins, respectively. Mutations in either TSC1 or TSC2 disrupt the function of the GTPase-activating protein (GAP) complex formed by these proteins that regulates mTOR signaling. The neurocutaneous syndrome tuberous sclerosis complex (TSC), characterized by benign hamartomas in multiple organ systems, is caused primarily by these mutations. In the brain, the hamartomas manifest as subendymal giant cell astrocytomas, subendymal nodules (SEN), and tubers. Tubers show disrupted cortical architecture and contain a number of atypical cells. For TSC, structural MRI and DTI studies have been conducted investigating both typical neuropathological lesions, especially tubers, and normal-appearing brain matter (Table VII). A consistent imaging determinant of the cognitive phenotype in TSC has not been established. Findings of an inverse correlation of tuber number and cognitive functioning have not been consistent (Ridler et al., 2004). Tuber/brain proportion may be a better predictor of IQ than tuber load, although the age of seizure onset in patients seemed to predict cognitive functioning best (Jansen et al., 2008). However, abnormal brain structure and connectivity unrelated to tubers are likely also important factors contributing to the neurobehavioral abnormalities in TSC. Decreased white matter volume of major intrahemispheric tracts has been found in adults with TSC compared to age-matched controls, as has a decrease of gray matter volume in several cortical and subcortical structures (Ridler et al., 2001; Ridler et al., 2007). Reduced volume in the cerebellum has been associated with tuber-associated loss of the underlying parenchyma (Jurkiewicz et al., 2006; Marti-Bonmati et al., 2000). Reduced cerebellar volume was observed in all cerebellar regions in a more recent study, with strongest volume reductions in patients with a mutation in TSC2 (Weisenfeld et al., 2013). The finding of reduced cerebellar volume is in line with mouse models showing cerebellar involvement in TSC (Reith et al., 2011). White matter abnormalities are another typical finding in TSC. DTI studies generally report increased ADC values and decreased FA values in individuals with TSC compared to controls, in tubers and white matter lesions, but also in other white matter portions (Table VII). Compared to contralateral white matter or white matter in control subjects, increased ADC values were found in cortical tubers, and higher ADC and lower FA values were found in white matter lesions (Piao et al., 2009). A recent study also found increased radial diffusivity values and decreased FA values in cortical tubers and white matter lesions (Dogan et al., 2015). Hypomyelination, gliosis, and heterotopic cells may lead to ADC and FA changes observed in such lesions (Alexander et al., 2007). Abnormalities have also been reported in normal-appearing white matter in individuals with TSC compared to control groups. Decreased FA and increased ADC, especially in corpus callosum and internal and external capsules, have been reported repeatedly (Krishnan et al., 2010; Peters et al., 2012; Simao et al., 2010). A recent whole-brain analysis of white matter connectivity showed that increased radial diffusivity exists throughout the brains of TSC patients and that interhemispheric connectivity is decreased (Im et al., 2015).
The methyl CpG binding protein 2 gene (MECP2) is located on the short arm of chromosome X (Xq28) and codes for the protein MECP2. MECP2 acts as a modifier of gene expression and is highly expressed in the brain. Mutations in MECP2 are the cause of Rett syndrome, a disorder primarily affecting female patients. Brain weight is reduced in Rett syndrome, particularly that of cerebral hemispheres. Although the anatomical basis for this reduction is not completely clear, it has been suggested that it is caused by defective neuronal maturation for which MECP2 is essential, rather than by atrophy (Armstrong 2005). Only few imaging studies have been conducted in series of patients with Rett syndrome (Table VII). All investigated brain structure in girls. These studies confirmed a wide-spread reduction in cerebral white and gray matter volumes, the latter most pronounced in subcortical nuclei including the caudate nucleus and in prefrontal, posterior-frontal, and anterior-temporal (Reiss et al., 1993; Subramaniam et al., 1997) and parietal regions (Carter et al., 2008). Using DTI, evidence of reduced white matter integrity was found in frontal regions, corpus callosum, and internal capsule. FA was also reduced in the superior longitudinal fasciculus, but only in patients who had little or no ability to speak (Mahmood et al., 2010).
Discussion
In this review, we set out to summarize the literature on imaging genetics studies in neurodevelopmental disorders. This being a very broad field, we focused on three most frequent and often comorbid disorder spectra, ADHD, ASDs, and selected forms of ID, and we only considered MRI-based imaging genetics studies. Further restriction of the search space was achieved by focusing on genes harboring common genetic variants with the most consistent evidence for association with ADHD and ASDs, and by selecting five relatively common ID disorders with frequent ADHD/ASDs comorbidity implicating single genes. The review was driven by the wish to learn more about the mechanisms by which genetic factors influence disease-related behavior specific to the individual disorders and their clinical overlap.
At the level of the individual genes, the most extensively studied candidate gene is the SLC6A4 (5HTT) gene encoding the serotonin transporter (associated with both ADHD and ASDs). Limitations regarding power of individual studies and hypothesis-driven designs aside, the fMRI-based imaging genetics literature on this gene does show a remarkably coherent picture of functional genetic variation leading to hyperactivation of the amygdala and connected areas in conjunction with functional dysconnectivity amongst those areas. However, since much of this research has been performed in healthy individuals only, the link to cognition in ADHD and ASD patients needs further investigation. Findings for SLC6A3 (DAT1) and DRD4, which have also been studied quite often already, still lack the consistency observed for SLC6A4 (5HTT), partly due to the much less restricted focus on a particular cognitive domain, and thus more ‘patchy’ literature.
The most consistent findings observed in all of the imaging genetics literature reviewed here are for the different genetic variants for ID. This is likely linked to the severity of the variants present in the patients, with those for ID being rare and most damaging. Consistent are finding for increased caudate volume and reduced cerebellum due to FMR1 mutations, and for T2 hyperintensities and increased brain volume in patients carrying NF1 mutations. However, in terms of finding overlap between different forms of ID, we find that conclusiveness of studies still is limited, as most concentrated on a limited set of (often non-overlapping) features. Tubers and T2 hyperintensities have received a lot of attention in studies of TSC and NF1, for example, although reports on their contribution to cognitive deficits are inconsistent. In recent years, DTI studies have produced evidence that tissue microstructure and white matter connectivity patterns are affected in all ID disorders, and often in widespread brain areas. Effects on brain volumes are also often widespread, but can go in opposite directions, with reductions in total brain volume in Rett, but increases in NF1. One may conclude that while altered (structural) connectivity is likely to play a role in ID etiology, MRI at its current resolution (1.5 – 4 Tesla), does not allow a sufficiently detailed view on the brain to understand the neuroanatomical overlap between disorders (Williams and Casanova 2011).
Similar to the situation amongst the ID disorders, there seems to be little overlap between the findings for different genes in ADHD or ASD. This is likely to be heavily influenced by the strong focus on regions and cognitive domains of interest (consistent with the limited power of many of the studies published to date). Some overlap is seen, e.g., for DAT1 and DRD2, both of which have been studied for their effects on striatal phenotypes. (Appropriately powered) brain-wide studies and phenome-wide association study (PheWAS)/RDoc-like approaches (Cuthbert and Insel 2013; Pendergrass et al., 2011) would help to determine, whether the apparent specificity of brain phenotypes for individual genes is real. An important observation is that gene expression does not predict/limit the location of effects of a genetic factor ( e.g., SLC6A3/DAT1 shows effects outside of its region of gene expression), most likely through effects on structural and/or functional connectivity.
Did the reported imaging genetics findings help us understand the comorbidity between different neurodevelopmental disorders? This would be expected, since several of the genes implicated in ID, ASD, and ADHD function in the same or overlapping molecular networks (Poelmans et al., 2011; Rudie et al., 2012; van Bokhoven 2011). However, the limited availability of genes investigated through imaging genetics to date might bias our interpretation of the data. In ID, the genes studied thus far are related to mTOR signaling, RAS signaling, and translation repression/regulation, thus functioning in very ‘basal’ cell signaling pathways in comparison to the genes investigated for ADHD, which regulate the dopamine and serotonin neurotransmitter systems specifically. This could explain the much more widespread cell proliferation/migration defects observed in ID, whereas in ADHD defects seem more specific, e.g. limited to individual neurotransmitter systems and or affecting cell-cell communication more acutely. ASD seems to be intermediate between the other two disorder spectra, but more studies are necessary to substantiate this view. What is already very clear from the available studies, is that the associations of genetic factors are with behavioral traits, and not with the disorders directly (e.g., (Hoogman et al., 2011). Some level of pleiotropy is highly likely, which may also form the basis of comorbidity between the neurodevelopmental disorders.
In general, we found the existing imaging genetics literature for the three neurodevelopmental disorders of our interest lacking in several aspects. Firstly, despite our focus on well-supported candidate genes, several of the selected genes had not been studied at all with MRI in humans. In several additional cases, only single studies were available for different MRI modalities (sMRI, DTI, fMRI), thus limiting the conclusiveness of the reported findings. Secondly, most imaging genetics studies, especially the earlier ones, suffer from being underpowered. The small sample sizes are severely hampering the generalization of findings to the population the samples are meant to represent (Button et al., 2013). Although the endophenotype concept postulates that measures, which mediate a genetic effect on behavior (including some of those investigated in the imaging genetics studies), should have stronger effect sizes for gene effects than the behavioral/disease measures (Gottesman and Gould 2003), the sample size of most studies would still have to be considered too small. The problem of limited number of samples becomes evident from e.g. a recent review by Strike and coworkers. They showed that at the most lenient threshold for significance (α = 0.05) studies with at least 1,566 participants would be needed to achieve the canonical 80% power threshold to detect a reasonable effect size (0.5% of the phenotypic variance explained) (Strike et al., 2015). Furthermore, recent work raises doubts about whether larger effect sizes can really be expected for neuroimaging (endo)phenotypes, at least for volumetric MRI measures (Franke et al., 2016; Hibar et al., 2015b). Major challenges are the large inconsistency across genetic variants tested and genotype groups compared, differences in study designs and imaging modalities, and the fact that data acquisition and analysis protocols usually were not standardized across studies. Additionally, we observed large inconsistency across studies in the way how genotypic effects were reported and recommend a standardized way of reporting results, e.g. including at least effect estimates and standard errors. Nevertheless, meta-analyses are strongly needed in order to enable definition of robust findings and realistic effect estimates. Therefore, meta-analytic studies would be beneficial for those brain measures covered by multiple studies, as it was shown for the effect of the serotonin transporter 5HTTLPR on amygdala activation (Murphy et al., 2013). Thirdly, to interpret observed links between genes, brain, and behavior properly, one needs to determine, whether a brain (endo)phenotype is really intermediate between a genetic factor and a behavioral outcome, or if it is only an epiphenomenon unrelated to the behavior of interest (Kendler and Neale 2010; Preacher and Hayes 2008). Only few studies have really studied this, e.g. by mediation analysis including environmental, behavioral, and/or physiological variables (Klumpers et al., 2014; van der Meer et al., 2015), by applying combinations of different imaging modalities (Kobiella et al., 2011; Zhang et al., 2015), or by using causal modeling (Sokolova et al., 2015). The results of those studies show that only part of the brain regions showing genotype effects actually do mediate between genetics and behavior, proving the importance of such multilevel investigations. Fourthly, age effects might also be of importance, but have been neglected in most studies. Our own work has shown, for example, that the risk factor for ADHD in DAT1 differs between children and adults, which resulted in effects of the 9–6 VNTR haplotype on caudate nucleus volume only in adult patients (Onnink et al., 2016). Age effects have also been observed for the 5-HTTLPR variant (Wiggins et al., 2014a). Fifthly, current brain imaging genetics studies often suffer from additional limitations, such as the low ethnic diversity, as most studies included cohorts of only Caucasian origin, and gender imbalance, especially in studies of childhood ADHD and ASD that showed an over-representation of males.
An important additional aspect is that this review enabled us to look at the overlap between studies in healthy individuals and those in patients (case-control designs). An interaction between genetic variant and diagnosis was indeed observed in some studies (e.g. (Durston et al., 2008; Monuteaux et al., 2008; Wiggins et al., 2012; Wiggins et al., 2014b). With the available limited amount of evidence it is hard to judge though, whether this is a true difference between patients and healthy individuals, or whether it is simply due to power restrictions in the samples investigated. Recent genome-wide studies investigating the genetics of brain structure as part of the ENIGMA Consortium (Thompson et al., 2014) suggest that effects are largely similar for healthy individuals and those with a psychiatric disorder (Hibar et al., 2015b; Stein et al., 2012). This means, that brain imaging genetics studies with healthy participants can be very informative in discovering related brain correlates and in understanding the biological mechanisms leading to diseases of interest.
Did we overlook important literature through the choices made in our review? We did restrict our selection of genes to study. For ASD, we did not include genes harboring rare genetic variants, while those might result in stronger effect sizes, as observed for the ID genes. However, most of the rare variants linked to ASD have only recently been identified, making the availability of imaging genetics studies (with 10 or more cases) unlikely. A similar argument holds true for our selection of ID genes, where the imaging genetics literature is largely focused on the relatively common disorder subtypes we included in our study. We also restricted our search to MRI-based studies, following a first screen of the literature showing that this was the predominant method used for imaging genetics studies of the neurodevelopmental disorders. Nevertheless, for several genes/variants, also other imaging modalities have been employed, which may provide additional insights. EEG and MEG offer a much higher time resolution than MRI, and may allow investigation of genetic influences on neuronal functioning and oscillation patterns. PET can provide information on (acute) protein availability. Especially the integration of modalities in the study of individual participants can provide deeper insights into mechanisms (e.g. (Kobiella et al., 2011)). Moreover, future studies might want to investigate additional comorbid neurodevelopmental disorders, such as conduct disorder (CD) or obsessive-compulsive disorder (OCD), once robust association of genetic variants with these disorders has been established and investigated in imaging genetics studies.
To summarize, despite the considerable numbers of imaging genetics studies in neurodevelopmental disorders available for review, this field of research should still be considered in its early stages. More genes need to be studied, and individual genes need to be investigated in larger samples, with more hypothesis-generating brain- and phenome-wide methods. Gene-environment interactions and age effects should be taken into account. While we see consistent findings for single genes and variants, gene-wide and gene-set analyses, with polygenic scores explaining more phenotypic variance and thus improving study power (Bralten et al., 2011), are likely to take the stage in the future. Several early examples reviewed here already show the promise of this work (e.g. (Nikolova et al., 2011; Passamonti et al., 2008; Stice et al., 2012). As the genes in such sets often show different gene expression patterns, (structural and functional) connectivity patterns are likely the best brain phenotypes to be studied with such approaches (see above). In the future, we are also likely to see studies approaching imaging genetics in a different way, by asking the question, whether genes contributing to brain structure/function observed in hypothesis-free, genome-wide approaches also contribute to disease-related phenotypes (Franke et al., 2016). First studies of this kind have been published for schizophrenia (Franke et al., 2016) and obsessive compulsive disorder (OCD) (Hibar et al., 2015a), based on results of findings from the ENIGMA GWAS of brain structure (Hibar et al., 2015b; Stein et al., 2012). To successfully map the biological pathways from gene to disease, imaging genetics studies need to be combined with complementary approaches (Klein et al., in press). Recent examples for this are provided by studies by our own group, in which we investigated effects of ADHD-associated genes for their effects in the fruit fly Drosophila melanogaster (Klein et al., 2015; van der Voet et al., 2016), as well as the study by Jia and coworkers, in which the authors identified a genetic variant significantly associated with dysfunctional reward, a cognitive and affective deficit frequently observed in ADHD, then verified gene function in locomotion in the fruit fly model (Jia et al., 2016). In conclusion, although still in its early stages, results from studies available thus far already confirm that the imaging genetics approach is suitable to provide more insight into the link between genes, the brain, and behavior in neurodevelopmental disorders.
Acknowledgements
The authors would like to acknowledge grants supporting their work from the Netherlands Organization for Scientific Research (NWO), i.e. the NWO Brain & Cognition Excellence Program (grant 433-09-229) and the Vici Innovation Program (grant 016-130-669 to BF). Additional support is received from the European Community’s Seventh Framework Programme (FP7/2007 – 2013) under grant agreements n° 602805 (Aggressotype), n° 602450 (IMAGEMEND), and n° 278948 (TACTICS), and from the European Community’s Horizon 2020 Programme (H2020/2014 – 2020) under grant agreements n° 643051 (MiND) and n° 667302 (CoCA). The work was also supported by grants for the ENIGMA Consortium (grant number U54 EB020403) from the BD2K Initiative of a cross-NIH partnership.
Footnotes
CONFLICT OF INTEREST
None of the authors report conflicts of interest. Barbara Franke discloses having received educational speaking fees from Merz and Shire.
References
- Aarts E, Roelofs A, Franke B, Rijpkema M, Fernandez G, Helmich RC, Cools R. 2010. Striatal dopamine mediates the interface between motivational and cognitive control in humans: evidence from genetic imaging. Neuropsychopharmacology 35(9):1943–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander AL, Lee JE, Lazar M, Field AS. 2007. Diffusion tensor imaging of the brain. Neurotherapeutics 4(3):316–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. 2013. Diagnostic and statistical manual of mental disorders: DSM-V. Washington, DC: American Psychiatric Press. [Google Scholar]
- Andersen PH, Gingrich JA, Bates MD, Dearry A, Falardeau P, Senogles SE, Caron MG. 1990. Dopamine receptor subtypes: beyond the D1/D2 classification. Trends Pharmacol Sci 11(6):231–6. [DOI] [PubMed] [Google Scholar]
- Anney R, Klei L, Pinto D, Regan R, Conroy J, Magalhaes TR, Correia C, Abrahams BS, Sykes N, Pagnamenta AT, Almeida J, Bacchelli E, Bailey AJ, Baird G, Battaglia A, Berney T, Bolshakova N, Bolte S, Bolton PF, Bourgeron T, Brennan S, Brian J, Carson AR, Casallo G, Casey J, Chu SH, Cochrane L, Corsello C, Crawford EL, Crossett A, Dawson G, de Jonge M, Delorme R, Drmic I, Duketis E, Duque F, Estes A, Farrar P, Fernandez BA, Folstein SE, Fombonne E, Freitag CM, Gilbert J, Gillberg C, Glessner JT, Goldberg J, Green J, Guter SJ, Hakonarson H, Heron EA, Hill M, Holt R, Howe JL, Hughes G, Hus V, Igliozzi R, Kim C, Klauck SM, Kolevzon A, Korvatska O, Kustanovich V, Lajonchere CM, Lamb JA, Laskawiec M, Leboyer M, Le Couteur A, Leventhal BL, Lionel AC, Liu XQ, Lord C, Lotspeich L, Lund SC, Maestrini E, Mahoney W, Mantoulan C, Marshall CR, McConachie H, McDougle CJ, McGrath J, McMahon WM, Melhem NM, Merikangas A, Migita O, Minshew NJ, Mirza GK, Munson J, Nelson SF, Noakes C, Noor A, Nygren G, Oliveira G, Papanikolaou K, Parr JR, Parrini B, Paton T, Pickles A, Piven J, Posey DJ, Poustka A, Poustka F, Prasad A, Ragoussis J, Renshaw K, Rickaby J, Roberts W, Roeder K, Roge B, Rutter ML, Bierut LJ, Rice JP, Salt J, Sansom K, Sato D, Segurado R, Senman L, Shah N, Sheffield VC, Soorya L, Sousa I, Stoppioni V, Strawbridge C, Tancredi R, Tansey K, Thiruvahindrapduram B, Thompson AP, Thomson S, Tryfon A, Tsiantis J, Van Engeland H, Vincent JB, Volkmar F, Wallace S, Wang K, Wang Z, Wassink TH, Wing K, Wittemeyer K, Wood S, Yaspan BL, Zurawiecki D, Zwaigenbaum L, Betancur C, Buxbaum JD, Cantor RM, Cook EH, Coon H, Cuccaro ML, Gallagher L, Geschwind DH, Gill M, Haines JL, Miller J, Monaco AP, Nurnberger JI, Jr., Paterson AD, Pericak-Vance MA, Schellenberg GD, Scherer SW, Sutcliffe JS, Szatmari P, Vicente AM, Vieland VJ, Wijsman EM, Devlin B, Ennis S, Hallmayer J. 2010. A genome-wide scan for common alleles affecting risk for autism. Hum Mol Genet 19(20):4072–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araragi N, Lesch KP. 2013. Serotonin (5-HT) in the regulation of depression-related emotionality: insight from 5-HT transporter and tryptophan hydroxylase-2 knockout mouse models. Curr Drug Targets 14(5):549–70. [DOI] [PubMed] [Google Scholar]
- Armstrong DD. 2005. Neuropathology of Rett syndrome. J Child Neurol 20(9):747–53. [DOI] [PubMed] [Google Scholar]
- Asherson P, Brookes K, Franke B, Chen W, Gill M, Ebstein RP, Buitelaar J, Banaschewski T, Sonuga-Barke E, Eisenberg J, Manor I, Miranda A, Oades RD, Roeyers H, Rothenberger A, Sergeant J, Steinhausen HC, Faraone SV. 2007. Confirmation that a specific haplotype of the dopamine transporter gene is associated with combined-type ADHD. Am J Psychiatry 164(4):674–7. [DOI] [PubMed] [Google Scholar]
- Autism and Developmental Disabilities Monitoring Network Surveillance Year 2008 Principal Incestigators and Centers for Disease Control and Prevention. 2012. Prevalence of autism spectrum disorders - Autism and Developmental Disabilities Monitoring Network, 14 sites, United States, 2008. MMWR Sruveill Summ 61:1–19. [PubMed] [Google Scholar]
- Bailey A, Le Couteur A, Gottesman I, Bolton P, Simonoff E, Yuzda E, Rutter M. 1995. Autism as a strongly genetic disorder: evidence from a British twin study. Psychol Med 25(1):63–77. [DOI] [PubMed] [Google Scholar]
- Baker BL, Neece CL, Fenning RM, Crnic KA, Blacher J. 2010. Mental disorders in five-year-old children with or without developmental delay: focus on ADHD. J Clin Child Adolesc Psychol 39(4):492–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnea-Goraly N, Eliez S, Hedeus M, Menon V, White CD, Moseley M, Reiss AL. 2003. White matter tract alterations in fragile X syndrome: preliminary evidence from diffusion tensor imaging. Am J Med Genet B Neuropsychiatr Genet 118b(1):81–8. [DOI] [PubMed] [Google Scholar]
- Barzman D, Geise C, Lin PI. 2015. Review of the genetic basis of emotion dysregulation in children and adolescents. World J Psychiatry 5(1):112–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard AC, Schulz KP, Cook EH, Jr., Fan J, Clerkin SM,Ivanov I, Halperin JM, Newcorn JH. 2010. Dopamine transporter gene variation modulates activation of striatum in youth with ADHD. Neuroimage 53(3):935–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman O, Ahs F, Furmark T, Appel L, Linnman C, Faria V, Bani M, Pich EM, Bettica P, Henningsson S, Manuck SB, Ferrell RE, Nikolova YS, Hariri AR, Fredrikson M, Westberg L, Eriksson E. 2014. Association between amygdala reactivity and a dopamine transporter gene polymorphism. Transl Psychiatry 5(4):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billingsley RL, Jackson EF, Slopis JM, Swank PR, Mahankali S, Moore BD. 2004. Functional MRI of visual-spatial processing in neurofibromatosis, type I. Neuropsychologia 42(3):395–404. [DOI] [PubMed] [Google Scholar]
- Braet W, Johnson KA, Tobin CT, Acheson R, McDonnell C, Hawi Z, Barry E, Mulligan A, Gill M, Bellgrove MA, Robertson IH, Garavan H. 2011. fMRI activation during response inhibition and error processing: the role of the DAT1 gene in typically developing adolescents and those diagnosed with ADHD. Neuropsychologia 49(7):1641–50. [DOI] [PubMed] [Google Scholar]
- Bralten J, Arias-Vasquez A, Makkinje R, Veltman JA, Brunner HG, Fernandez G, Rijpkema M, Franke B. 2011. Association of the Alzheimer’s gene SORL1 with hippocampal volume in young, healthy adults. Am J Psychiatry 168(10):1083–9. [DOI] [PubMed] [Google Scholar]
- Bray S, Hirt M, Jo B, Hall SS, Lightbody AA, Walter E, Chen K, Patnaik S, Reiss AL. 2011. Aberrant frontal lobe maturation in adolescents with fragile X syndrome is related to delayed cognitive maturation. Biol Psychiatry 70(9):852–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes KJ, Mill J, Guindalini C, Curran S, Xu X, Knight J, Chen CK, Huang YS, Sethna V, Taylor E, Chen W, Breen G, Asherson P. 2006. A common haplotype of the dopamine transporter gene associated with attention-deficit/hyperactivity disorder and interacting with maternal use of alcohol during pregnancy. Arch Gen Psychiatry 63(1):74–81. [DOI] [PubMed] [Google Scholar]
- Bruno JL, Garrett AS, Quintin EM, Mazaika PK, Reiss AL. 2014. Aberrant face and gaze habituation in fragile x syndrome. Am J Psychiatry 171(10):1099–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt SA. 2009. Rethinking environmental contributions to child and adolescent psychopathology: a meta-analysis of shared environmental influences. Psychol Bull 135(4):608–37. [DOI] [PubMed] [Google Scholar]
- Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, Munafo MR. 2013. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci 14(5):365–76. [DOI] [PubMed] [Google Scholar]
- Carter JC, Lanham DC, Pham D, Bibat G, Naidu S, Kaufmann WE. 2008. Selective cerebral volume reduction in Rett syndrome: a multiple-approach MR imaging study. AJNR Am J Neuroradiol 29(3):436–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Lau E, Tayebi N, Lee P, Long RE, Giedd JN, Sharp W, Marsh WL, Walter JM, Hamburger SD, Ginns EI, Rapoport JL, Sidransky E. 1998. Lack of an association between a dopamine-4 receptor polymorphism and attention-deficit/hyperactivity disorder: genetic and brain morphometric analyses. Mol Psychiatry 3(5):431–4. [DOI] [PubMed] [Google Scholar]
- Cerasa A, Gioia MC, Tarantino P, Labate A, Arabia G, Annesi G, Lanza P, Di Palma G, Blasi V, Quattrone A. 2009. The DRD2 TaqIA polymorphism associated with changed midbrain volumes in healthy individuals. Genes Brain Behav 8(4):459–63. [DOI] [PubMed] [Google Scholar]
- Chang FM, Kidd JR, Livak KJ, Pakstis AJ, Kidd KK. 1996. The world-wide distribution of allele frequencies at the human dopamine D4 receptor locus. Hum Genet 98(1):91–101. [DOI] [PubMed] [Google Scholar]
- Ciliax BJ, Drash GW, Staley JK, Haber S, Mobley CJ, Miller GW, Mufson EJ, Mash DC, Levey AI. 1999. Immunocytochemical localization of the dopamine transporter in human brain. J Comp Neurol 409(1):38–56. [DOI] [PubMed] [Google Scholar]
- Clements-Stephens AM, Rimrodt SL, Gaur P, Cutting LE. 2008. Visuospatial processing in children with neurofibromatosis type 1. Neuropsychologia 46(2):690–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX, Krohn-Grimberghe A, Elger CE, Weber B. 2007. Dopamine gene predicts the brain’s response to dopaminergic drug. Eur J Neurosci 26(12):3652–60. [DOI] [PubMed] [Google Scholar]
- Craig F, Lamanna AL, Margari F, Matera E, Simone M, Margari L. 2015. Overlap Between Autism Spectrum Disorders and Attention Deficit Hyperactivity Disorder: Searching for Distinctive/Common Clinical Features. Autism Res 8(3):328–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert BN, Insel TR. 2013. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med 11:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiano CR, Aloi J, Dunlap K, Burrus CJ, Mosner MG, Kozink RV, McLaurin RE, Mullette-Gillman OA, Carter RM, Huettel SA, McClernon FJ, Ashley-Koch A, Dichter GS. 2014. Association between the oxytocin receptor (OXTR) gene and mesolimbic responses to rewards. Mol Autism 5(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannlowski U, Kugel H, Redlich R, Halik A, Schneider I, Opel N, Grotegerd D, Schwarte K, Schettler C, Ambree O, Rust S, Domschke K, Arolt V, Heindel W, Baune BT, Suslow T, Zhang W, Hohoff C. 2014. Serotonin transporter gene methylation is associated with hippocampal gray matter volume. Hum Brain Mapp 35(11):5356–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries BB, Wiegers AM, Smits AP, Mohkamsing S, Duivenvoorden HJ, Fryns JP, Curfs LM, Halley DJ, Oostra BA, van den Ouweland AM, Niermeijer MF. 1996. Mental status of females with an FMR1 gene full mutation. Am J Hum Genet 58(5):1025–32. [PMC free article] [PubMed] [Google Scholar]
- Deciphering Developmental Disorders Study. 2015. Large-scale discovery of novel genetic causes of developmental disorders. Nature 519(7542):223–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker MC, Koot HM. 2003. DSM-IV disorders in children with borderline to moderate intellectual disability. I: prevalence and impact. J Am Acad Child Adolesc Psychiatry 42(8):915–22. [DOI] [PubMed] [Google Scholar]
- Dennis EL, Jahanshad N, Rudie JD, Brown JA, Johnson K, McMahon KL, de Zubicaray GI, Montgomery G, Martin NG, Wright MJ, Bookheimer SY, Dapretto M, Toga AW, Thompson PM. 2011. Altered structural brain connectivity in healthy carriers of the autism risk gene, CNTNAP2. Brain Connect 1(6):447–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogan MS, Gumus K, Koc G, Doganay S, Per H, Gorkem SB, Canpolat M, Bayram AK, Coskun A. 2015. Brain diffusion tensor imaging in children with tuberous sclerosis. Diagn Interv Imaging. [DOI] [PubMed] [Google Scholar]
- Dreher JC, Kohn P, Kolachana B, Weinberger DR, Berman KF. 2009. Variation in dopamine genes influences responsivity of the human reward system. Proc Natl Acad Sci U S A 106(2):617–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte JV, Ribeiro MJ, Violante IR, Cunha G, Silva E, Castelo-Branco M. 2014. Multivariate pattern analysis reveals subtle brain anomalies relevant to the cognitive phenotype in neurofibromatosis type 1. Hum Brain Mapp 35(1):89–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston S, Fossella JA, Casey BJ, Hulshoff Pol HE, Galvan A, Schnack HG, Steenhuis MP, Minderaa RB, Buitelaar JK, Kahn RS, van Engeland H. 2005. Differential effects of DRD4 and DAT1 genotype on fronto-striatal gray matter volumes in a sample of subjects with attention deficit hyperactivity disorder, their unaffected siblings, and controls. Mol Psychiatry 10(7):678–85. [DOI] [PubMed] [Google Scholar]
- Durston S, Fossella JA, Mulder MJ, Casey BJ, Ziermans TB, Vessaz MN, Van Engeland H. 2008. Dopamine transporter genotype conveys familial risk of attention-deficit/hyperactivity disorder through striatal activation. J Am Acad Child Adolesc Psychiatry 47(1):61–7. [DOI] [PubMed] [Google Scholar]
- Elsabbagh M, Divan G, Koh YJ, Kim YS, Kauchali S, Marcin C, Montiel-Nava C, Patel V, Paula CS, Wang C, Yasamy MT, Fombonne E. 2012. Global prevalence of autism and other pervasive developmental disorders. Autism Res 5(3):160–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson E 2003. Prevalence of psychiatric disorders in children and adolescents with and without intellectual disability. J Intellect Disabil Res 47(Pt 1):51–8. [DOI] [PubMed] [Google Scholar]
- Ertan G, Zan E, Yousem DM, Ceritoglu C, Tekes A, Poretti A, Huisman TA. 2014. Diffusion tensor imaging of neurofibromatosis bright objects in children with neurofibromatosis type 1. Neuroradiol J 27(5):616–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone SV, Asherson P, Banaschewski T, Biederman J, Buitelaar JK, Ramos-Quiroga JA, Rohde LA, Sonuga-Barke EJS, Tannock R, Franke B. 2015. Attention-deficit/hyperactivity disorder. Nature Reviews Disease Primers:15020. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, Sklar P. 2005. Molecular genetics of attention-deficit/hyperactivity disorder. Biol Psychiatry 57(11):1313–23. [DOI] [PubMed] [Google Scholar]
- Favaro A, Manara R, Pievani M, Clementi M, Forzan M, Bruson A, Tenconi E, Degortes D, Pinato C, Giannunzio V, Battista Frisoni G, Santonastaso P. 2014. Neural signatures of the interaction between the 5-HTTLPR genotype and stressful life events in healthy women. Psychiatry Res 223(2):157–63. [DOI] [PubMed] [Google Scholar]
- Ferraz-Filho JR, da Rocha AJ, Muniz MP, Souza AS, Goloni-Bertollo EM, Pavarino-Bertelli EC. 2012. Diffusion tensor MR imaging in neurofibromatosis type 1: expanding the knowledge of microstructural brain abnormalities. Pediatr Radiol 42(4):449–54. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Tse MT. 2007. Dopaminergic regulation of inhibitory and excitatory transmission in the basolateral amygdala-prefrontal cortical pathway. J Neurosci 27(8):2045–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein S, Rutter M. 1977. Infantile autism: a genetic study of 21 twin pairs. J Child Psychol Psychiatry 18(4):297–321. [DOI] [PubMed] [Google Scholar]
- Franke B, Faraone SV, Asherson P, Buitelaar J, Bau CH, Ramos-Quiroga JA, Mick E, Grevet EH, Johansson S, Haavik J, Lesch KP, Cormand B, Reif A, International Multicentre persistent AC. 2012. The genetics of attention deficit/hyperactivity disorder in adults, a review. Mol Psychiatry 17(10):960–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke B, Neale BM, Faraone SV. 2009. Genome-wide association studies in ADHD. Hum Genet 126(1):13–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke B, Stein JL, Ripke S, Anttila V, Hibar DP, van Hulzen KJ, Arias-Vasquez A, Smoller JW, Nichols TE, Neale MC, McIntosh AM, Lee P, McMahon FJ, Meyer-Lindenberg A, Mattheisen M, Andreassen OA, Gruber O, Sachdev PS, Roiz-Santianez R, Saykin AJ, Ehrlich S, Mather KA, Turner JA, Schwarz E, Thalamuthu A, Yao Y, Ho YY, Martin NG, Wright MJ, Schizophrenia Working Group of the Psychiatric Genomics C, Psychosis Endophenotypes International C, Wellcome Trust Case Control C, Enigma C, O’Donovan MC, Thompson PM, Neale BM, Medland SE,Sullivan PF. 2016. Genetic influences on schizophrenia and subcortical brain volumes: large-scale proof of concept. Nat Neurosci 19(3):420–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke B, Vasquez AA, Johansson S, Hoogman M, Romanos J, Boreatti-Hummer A, Heine M, Jacob CP, Lesch KP, Casas M, Ribases M, Bosch R, Sanchez-Mora C, Gomez-Barros N, Fernandez-Castillo N, Bayes M, Halmoy A, Halleland H, Landaas ET, Fasmer OB, Knappskog PM, Heister AJ, Kiemeney LA, Kooij JJ, Boonstra AM, Kan CC, Asherson P, Faraone SV, Buitelaar JK, Haavik J, Cormand B, Ramos-Quiroga JA, Reif A. 2010. Multicenter analysis of the SLC6A3/DAT1 VNTR haplotype in persistent ADHD suggests differential involvement of the gene in childhood and persistent ADHD. Neuropsychopharmacology 35(3):656–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankle WG, Huang Y, Hwang DR, Talbot PS, Slifstein M, Van Heertum R, Abi-Dargham A, Laruelle M. 2004. Comparative evaluation of serotonin transporter radioligands 11C-DASB and 11C-McN 5652 in healthy humans. J Nucl Med 45(4):682–94. [PubMed] [Google Scholar]
- Furman DJ, Chen MC, Gotlib IH. 2011. Variant in oxytocin receptor gene is associated with amygdala volume. Psychoneuroendocrinology 36(6):891–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Heldt SA. 2015. Lack of neuronal nitric oxide synthase results in attention deficit hyperactivity disorder-like behaviors in mice. Behav Neurosci 129(1):50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett AS, Menon V, MacKenzie K, Reiss AL. 2004. Here’s looking at you, kid: neural systems underlying face and gaze processing in fragile X syndrome. Arch Gen Psychiatry 61(3):281–8. [DOI] [PubMed] [Google Scholar]
- Gaugler T, Klei L, Sanders SJ, Bodea CA, Goldberg AP, Lee AB, Mahajan M, Manaa D, Pawitan Y, Reichert J, Ripke S, Sandin S, Sklar P, Svantesson O, Reichenberg A, Hultman CM, Devlin B, Roeder K, Buxbaum JD. 2014. Most genetic risk for autism resides with common variation. Nat Genet 46(8):881–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill DS, Hyman SL, Steinberg A, North KN. 2006. Age-related findings on MRI in neurofibromatosis type 1. Pediatr Radiol 36(10):1048–56. [DOI] [PubMed] [Google Scholar]
- Gizer IR, Ficks C, Waldman ID. 2009. Candidate gene studies of ADHD: a meta-analytic review. Hum Genet 126(1):51–90. [DOI] [PubMed] [Google Scholar]
- Goldstein SRCR 1999. Handbook of neurodevelopmental and genetic disorders in children. New York: Guilford Press. [Google Scholar]
- Gordon EM, Devaney JM, Bean S, Vaidya CJ. 2015. Resting-state striato-frontal functional connectivity is sensitive to DAT1 genotype and predicts executive function. Cereb Cortex 25(2):336–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. 2003. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry 160(4):636–45. [DOI] [PubMed] [Google Scholar]
- Greenwood RS, Tupler LA, Whitt JK, Buu A, Dombeck CB, Harp AG, Payne ME, Eastwood JD, Krishnan KR, MacFall JR. 2005. Brain morphometry, T2-weighted hyperintensities, and IQ in children with neurofibromatosis type 1. Arch Neurol 62(12):1904–8. [DOI] [PubMed] [Google Scholar]
- Griffiths PD, Blaser S, Mukonoweshuro W, Armstrong D, Milo-Mason G, Cheung S. 1999. Neurofibromatosis bright objects in children with neurofibromatosis type 1: a proliferative potential? Pediatrics 104(4):e49. [DOI] [PubMed] [Google Scholar]
- Grzadzinski R, Di Martino A, Brady E, Mairena MA, O’Neale M, Petkova E, Lord C, Castellanos FX. 2011. Examining autistic traits in children with ADHD: does the autism spectrum extend to ADHD? J Autism Dev Disord 41(9):1178–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas BW, Barnea-Goraly N, Lightbody AA, Patnaik SS, Hoeft F, Hazlett H, Piven J, Reiss AL. 2009. Early white-matter abnormalities of the ventral frontostriatal pathway in fragile X syndrome. Dev Med Child Neurol 51(8):593–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberstick BC, Smolen A, Williams RB, Bishop GD, Foshee VA, Thornberry TP, Conger R, Siegler IC, Zhang X, Boardman JD, Frajzyngier Z, Stallings MC, Brent Donnellan M, Halpern CT, Harris KM. 2015. Population frequencies of the Triallelic 5HTTLPR in six Ethnicially diverse samples from North America, Southeast Asia, and Africa. Behav Genet 45(2):255–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman RJ, Berry-Kravis E, Kaufmann WE, Ono MY, Tartaglia N, Lachiewicz A, Kronk R, Delahunty C, Hessl D, Visootsak J, Picker J, Gane L, Tranfaglia M. 2009. Advances in the treatment of fragile X syndrome. Pediatrics 123(1):378–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn T, Heinzel S, Dresler T, Plichta MM, Renner TJ, Markulin F, Jakob PM, Lesch KP, Fallgatter AJ. 2011. Association between reward-related activation in the ventral striatum and trait reward sensitivity is moderated by dopamine transporter genotype. Hum Brain Mapp 32(10):1557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SS, Jiang H, Reiss AL, Greicius MD. 2013. Identifying large-scale brain networks in fragile X syndrome. JAMA Psychiatry 70(11):1215–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallahan BP, Craig MC, Toal F, Daly EM, Moore CJ, Ambikapathy A, Robertson D, Murphy KC, Murphy DG. 2011. In vivo brain anatomy of adult males with Fragile X syndrome: an MRI study. Neuroimage 54(1):16–24. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Drabant EM, Munoz KE, Kolachana BS, Mattay VS, Egan MF, Weinberger DR. 2005. A susceptibility gene for affective disorders and the response of the human amygdala. Arch Gen Psychiatry 62(2):146–52. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Egan MF, Weinberger DR. 2002. Serotonin transporter genetic variation and the response of the human amygdala. Science 297(5580):400–3. [DOI] [PubMed] [Google Scholar]
- Hawi Z, Cummins TD, Tong J, Johnson B, Lau R, Samarrai W, Bellgrove MA. 2015. The molecular genetic architecture of attention deficit hyperactivity disorder. Mol Psychiatry 20(3):289–97. [DOI] [PubMed] [Google Scholar]
- Hazlett HC, Poe MD, Lightbody AA, Gerig G, Macfall JR, Ross AK, Provenzale J, Martin A, Reiss AL, Piven J. 2009. Teasing apart the heterogeneity of autism: Same behavior, different brains in toddlers with fragile X syndrome and autism. J Neurodev Disord 1(1):81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett HC, Poe MD, Lightbody AA, Styner M, MacFall JR, Reiss AL, Piven J. 2012. Trajectories of early brain volume development in fragile X syndrome and autism. J Am Acad Child Adolesc Psychiatry 51(9):921–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick A, Lee Y, Wallace GL, Greenstein D, Clasen L, Giedd JN, Raznahan A. 2012. Autism risk gene MET variation and cortical thickness in typically developing children and adolescents. Autism Res 5(6):434–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibar DP, Consortium I-G, Consortium E, Stewart E, van den Heuvel OA, Pauls DL, Knowles JA, Stein J, Thompson PM. 2015a. Significant concordance of the genetic variation that increases both the risk for OCD and the volumes of the nucleus accumbens and putamen. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibar DP, Stein JL, Renteria ME, Arias-Vasquez A, Desrivieres S, Jahanshad N, Toro R, Wittfeld K, Abramovic L, Andersson M, Aribisala BS, Armstrong NJ, Bernard M, Bohlken MM, Boks MP, Bralten J, Brown AA, Chakravarty MM, Chen Q, Ching CR, Cuellar-Partida G, den Braber A, Giddaluru S, Goldman AL, Grimm O, Guadalupe T, Hass J, Woldehawariat G, Holmes AJ, Hoogman M, Janowitz D, Jia T, Kim S, Klein M, Kraemer B, Lee PH, Olde Loohuis LM, Luciano M, Macare C, Mather KA, Mattheisen M, Milaneschi Y, Nho K, Papmeyer M, Ramasamy A, Risacher SL, Roiz-Santianez R, Rose EJ, Salami A, Samann PG, Schmaal L, Schork AJ, Shin J, Strike LT, Teumer A, van Donkelaar MM, van Eijk KR, Walters RK, Westlye LT, Whelan CD, Winkler AM, Zwiers MP, Alhusaini S, Athanasiu L, Ehrlich S, Hakobjan MM, Hartberg CB, Haukvik UK, Heister AJ, Hoehn D, Kasperaviciute D, Liewald DC, Lopez LM, Makkinje RR, Matarin M, Naber MA, McKay DR, Needham M, Nugent AC, Putz B, Royle NA, Shen L, Sprooten E, Trabzuni D, van der Marel SS, van Hulzen KJ, Walton E, Wolf C, Almasy L, Ames D, Arepalli S, Assareh AA, Bastin ME, Brodaty H, Bulayeva KB, Carless MA, Cichon S, Corvin A, Curran JE, Czisch M, de Zubicaray GI, Dillman A, Duggirala R, Dyer TD, Erk S, Fedko IO, Ferrucci L, Foroud TM, Fox PT, Fukunaga M, Gibbs JR, Goring HH, Green RC, Guelfi S, Hansell NK, Hartman CA, Hegenscheid K, Heinz A, Hernandez DG, Heslenfeld DJ, Hoekstra PJ, Holsboer F, Homuth G, Hottenga JJ, Ikeda M, Jack CR Jr., Jenkinson M, Johnson R, Kanai R, Keil M, Kent JW Jr., Kochunov P, Kwok JB, Lawrie SM, Liu X, Longo DL, McMahon KL, Meisenzahl E, Melle I, Mohnke S, Montgomery GW, Mostert JC, Muhleisen TW, Nalls MA, Nichols TE, Nilsson LG, Nothen MM, Ohi K, Olvera RL, Perez-Iglesias R, Pike GB, Potkin SG, Reinvang I, Reppermund S, Rietschel M, Romanczuk-Seiferth N, Rosen GD, Rujescu D, Schnell K, Schofield PR, Smith C, Steen VM, Sussmann JE, Thalamuthu A, Toga AW, Traynor BJ, Troncoso J Turner JA, Valdes Hernandez MC, van ‘t Ent D, van der Brug M, van der Wee NJ, van Tol MJ, Veltman DJ, Wassink TH, Westman E, Zielke RH, Zonderman AB, Ashbrook DG, Hager R, Lu L, McMahon FJ, Morris DW, Williams RW, Brunner HG, Buckner RL, Buitelaar JK, Cahn W, Calhoun VD, Cavalleri GL, Crespo-Facorro B, Dale AM, Davies GE, Delanty N, Depondt C, Djurovic S, Drevets WC, Espeseth T, Gollub RL, Ho BC, Hoffmann W, Hosten N, Kahn RS, Le Hellard S, Meyer-Lindenberg A, Muller-Myhsok B, Nauck M, Nyberg L, Pandolfo M, Penninx BW, Roffman JL, Sisodiya SM, Smoller JW, van Bokhoven H, van Haren NE, Volzke H, Walter H, Weiner MW, Wen W, White T, Agartz I, Andreassen OA, Blangero J, Boomsma DI, Brouwer RM, Cannon DM, Cookson MR, de Geus EJ, Deary IJ, Donohoe G, Fernandez G, Fisher SE, Francks C, Glahn DC, Grabe HJ, Gruber O, Hardy J, Hashimoto R, Hulshoff Pol HE, Jonsson EG, Kloszewska I, Lovestone S, Mattay VS, Mecocci P, McDonald C, McIntosh AM, Ophoff RA, Paus T, Pausova Z, Ryten M, Sachdev PS, Saykin AJ, Simmons A, Singleton A, Soininen H, Wardlaw JM, Weale ME, Weinberger DR, Adams HH, Launer LJ, Seiler S, Schmidt R, Chauhan G, Satizabal CL, Becker JT, Yanek L, van der Lee SJ, Ebling M, Fischl B, Longstreth WT Jr, Greve D, Schmidt H, Nyquist P, Vinke LN, van Duijn CM, Xue L, Mazoyer B, Bis JC, Gudnason V, Seshadri S, Ikram MA, Alzheimer’s Disease Neuroimaging I, Consortium C Epigen, Imagen, Sys, Martin NG, Wright MJ, Schumann G, Franke B, Thompson PM, Medland SE. 2015b. Common genetic variants influence human subcortical brain structures. Nature 520(7546):224–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman EM. 2014. Coupling mechanism and significance of the BOLD signal: a status report. Annu Rev Neurosci 37:161–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinney A, Scherag A, Jarick I, Albayrak O, Putter C, Pechlivanis S, Dauvermann MR, Beck S, Weber H, Scherag S, Nguyen TT, Volckmar AL, Knoll N, Faraone SV, Neale BM, Franke B, Cichon S, Hoffmann P, Nothen MM, Schreiber S, Jockel KH, Wichmann HE, Freitag C, Lempp T, Meyer J, Gilsbach S, Herpertz-Dahlmann B, Sinzig J, Lehmkuhl G, Renner TJ, Warnke A, Romanos M, Lesch KP, Reif A, Schimmelmann BG, Hebebrand J, Psychiatric GCAs. 2011. Genome-wide association study in German patients with attention deficit/hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet 156B(8):888–97. [DOI] [PubMed] [Google Scholar]
- Hoeft F, Hernandez A, Parthasarathy S, Watson CL, Hall SS, Reiss AL. 2007. Fronto-striatal dysfunction and potential compensatory mechanisms in male adolescents with fragile X syndrome. Hum Brain Mapp 28(6):543–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F, Lightbody AA, Hazlett HC, Patnaik S, Piven J, Reiss AL. 2008. Morphometric spatial patterns differentiating boys with fragile X syndrome, typically developing boys, and developmentally delayed boys aged 1 to 3 years. Arch Gen Psychiatry 65(9):1087–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsen LM, Dalton KM, Johnstone T, Davidson RJ. 2008. Prefrontal social cognition network dysfunction underlying face encoding and social anxiety in fragile X syndrome. Neuroimage 43(3):592–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homberg JR, Lesch KP. 2011. Looking on the bright side of serotonin transporter gene variation. Biol Psychiatry 69(6):513–9. [DOI] [PubMed] [Google Scholar]
- Hong SB, Zalesky A, Park S, Yang YH, Park MH, Kim B, Song IC, Sohn CH, Shin MS, Kim BN, Cho SC, Kim JW. 2015. COMT genotype affects brain white matter pathways in attention-deficit/hyperactivity disorder. Hum Brain Mapp 36(1):367–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogman M, Aarts E, Zwiers M, Slaats-Willemse D, Naber M, Onnink M, Cools R, Kan C, Buitelaar J, Franke B. 2011. Nitric oxide synthase genotype modulation of impulsivity and ventral striatal activity in adult ADHD patients and healthy comparison subjects. Am J Psychiatry 168(10):1099–106. [DOI] [PubMed] [Google Scholar]
- Hoogman M, Onnink M, Cools R, Aarts E, Kan C, Arias Vasquez A, Buitelaar J, Franke B. 2013. The dopamine transporter haplotype and reward-related striatal responses in adult ADHD. Eur Neuropsychopharmacol 23(6):469–78. [DOI] [PubMed] [Google Scholar]
- Hyman SL, Shores A, North KN. 2005. The nature and frequency of cognitive deficits in children with neurofibromatosis type 1. Neurology 65(7):1037–44. [DOI] [PubMed] [Google Scholar]
- Im K, Ahtam B, Haehn D, Peters JM, Warfield SK, Sahin M, Ellen Grant P. 2015. Altered Structural Brain Networks in Tuberous Sclerosis Complex. Cereb Cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iossifov I, O’Roak BJ, Sanders SJ, Ronemus M, Krumm N, Levy D, Stessman HA, Witherspoon KT, Vives L, Patterson KE, Smith JD, Paeper B, Nickerson DA, Dea J, Dong S, Gonzalez LE, Mandell JD, Mane SM, Murtha MT, Sullivan CA, Walker MF, Waqar Z, Wei L, Willsey AJ, Yamrom B, Lee YH, Grabowska E, Dalkic E, Wang Z, Marks S, Andrews P, Leotta A, Kendall J, Hakker I, Rosenbaum J, Ma B, Rodgers L, Troge J, Narzisi G, Yoon S, Schatz MC, Ye K, McCombie WR, Shendure J, Eichler EE, State MW, Wigler M. 2014. The contribution of de novo coding mutations to autism spectrum disorder. Nature 515(7526):216–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen FE, Vincken KL, Algra A, Anbeek P, Braams O, Nellist M, Zonnenberg BA, Jennekens-Schinkel A, van den Ouweland A, Halley D, van Huffelen AC, van Nieuwenhuizen O. 2008. Cognitive impairment in tuberous sclerosis complex is a multifactorial condition. Neurology 70(12):916–23. [DOI] [PubMed] [Google Scholar]
- Jia T, Macare C, Desrivieres S, Gonzalez DA, Tao C, Ji X, Ruggeri B, Nees F, Banaschewski T, Barker GJ, Bokde AL, Bromberg U, Buchel C, Conrod PJ, Dove R, Frouin V, Gallinat J, Garavan H, Gowland PA, Heinz A, Ittermann B, Lathrop M, Lemaitre H, Martinot JL, Paus T, Pausova Z, Poline JB, Rietschel M, Robbins T, Smolka MN, Muller CP, Feng J, Rothenfluh A, Flor H, Schumann G, Consortium I. 2016. Neural basis of reward anticipation and its genetic determinants. Proc Natl Acad Sci U S A 113(14):3879–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jocham G, Klein TA, Neumann J, von Cramon DY, Reuter M, Ullsperger M. 2009. Dopamine DRD2 polymorphism alters reversal learning and associated neural activity. J Neurosci 29(12):3695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judson MC, Amaral DG, Levitt P. 2011. Conserved subcortical and divergent cortical expression of proteins encoded by orthologs of the autism risk gene MET. Cereb Cortex 21(7):1613–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurkiewicz E, Jozwiak S, Bekiesinska-Figatowska M, Pakiela-Domanska D, Pakula-Kosciesza I, Walecki J. 2006. Cerebellar lesions in children with tuberous sclerosis complex. Neuroradiol J 19(5):577–82. [DOI] [PubMed] [Google Scholar]
- Karlsgodt KH, Rosser T, Lutkenhoff ES, Cannon TD, Silva A, Bearden CE. 2012. Alterations in white matter microstructure in neurofibromatosis-1. PLoS One 7(10):e47854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasparbauer AM, Rujescu D, Riedel M, Pogarell O, Costa A, Meindl T, la Fougere C, Ettinger U. 2015. Methylphenidate effects on brain activity as a function of SLC6A3 genotype and striatal dopamine transporter availability. Neuropsychopharmacology 40(3):736–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Neale MC. 2010. Endophenotype: a conceptual analysis. Mol Psychiatry 15(8):789–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch P, Reuter M, Mier D, Lonsdorf T, Stark R, Gallhofer B, Vaitl D, Hennig J. 2006. Imaging gene-substance interactions: the effect of the DRD2 TaqIA polymorphism and the dopamine agonist bromocriptine on the brain activation during the anticipation of reward. Neurosci Lett 405(3):196–201. [DOI] [PubMed] [Google Scholar]
- Kiss JP, Vizi ES. 2001. Nitric oxide: a novel link between synaptic and nonsynaptic transmission. Trends Neurosci 24(4):211–5. [DOI] [PubMed] [Google Scholar]
- Klein M, Onnink M, van Donkelaar M, Wolfers T, Harich B, Shi Y, Dammers J, Arias-Vásquez A, Hoogman M, Franke B. in press. Brain imaging genetics in ADHD and beyond − mapping pathways from gene to disorder at different levels of complexity. Neuroscience & Biobehavioral Reviews. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M, van der Voet M, Harich B, van Hulzen KJ, Onnink AM, Hoogman M, Guadalupe T, Zwiers M, Groothuismink JM, Verberkt A, Nijhof B, Castells-Nobau A, Faraone SV, Buitelaar JK, Schenck A, Arias-Vasquez A, Franke B, Psychiatric Genomics Consortium AWG. 2015. Converging evidence does not support GIT1 as an ADHD risk gene. Am J Med Genet B Neuropsychiatr Genet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpers F, Kroes MC, Heitland I, Everaerd D, Akkermans SE, Oosting RS, van Wingen G, Franke B, Kenemans JL, Fernandez G, Baas JM. 2014. Dorsomedial Prefrontal Cortex Mediates the Impact of Serotonin Transporter Linked Polymorphic Region Genotype on Anticipatory Threat Reactions. Biol Psychiatry. [DOI] [PubMed] [Google Scholar]
- Kobiella A, Reimold M, Ulshofer DE, Ikonomidou VN, Vollmert C, Vollstadt-Klein S, Rietschel M, Reischl G, Heinz A, Smolka MN. 2011. How the serotonin transporter 5-HTTLPR polymorphism influences amygdala function: the roles of in vivo serotonin transporter expression and amygdala structure. Transl Psychiatry 1:e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochhar P, Batty MJ, Liddle EB, Groom MJ, Scerif G, Liddle PF, Hollis CP. 2011. Autistic spectrum disorder traits in children with attention deficit hyperactivity disorder. Child Care Health Dev 37(1):103–10. [DOI] [PubMed] [Google Scholar]
- Kraut MA, Gerring JP, Cooper KL, Thompson RE, Denckla MB, Kaufmann WE. 2004. Longitudinal evolution of unidentified bright objects in children with neurofibromatosis-1. Am J Med Genet A 129a(2):113–9. [DOI] [PubMed] [Google Scholar]
- Krishnan ML, Commowick O, Jeste SS, Weisenfeld N, Hans A, Gregas MC, Sahin M, Warfield SK. 2010. Diffusion features of white matter in tuberous sclerosis with tractography. Pediatr Neurol 42(2):101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroger A, Hanig S, Seitz C, Palmason H, Meyer J, Freitag CM. 2011. Risk factors of autistic symptoms in children with ADHD. Eur Child Adolesc Psychiatry 20(11–12):561–70. [DOI] [PubMed] [Google Scholar]
- Laakso A, Pohjalainen T, Bergman J, Kajander J, Haaparanta M, Solin O, Syvalahti E, Hietala J. 2005. The A1 allele of the human D2 dopamine receptor gene is associated with increased activity of striatal L-amino acid decarboxylase in healthy subjects. Pharmacogenet Genomics 15(6):387–91. [DOI] [PubMed] [Google Scholar]
- Lasky-Su J, Anney RJ, Neale BM, Franke B, Zhou K, Maller JB, Vasquez AA, Chen W, Asherson P, Buitelaar J, Banaschewski T, Ebstein R, Gill M, Miranda A, Mulas F, Oades RD, Roeyers H, Rothenberger A, Sergeant J, Sonuga-Barke E, Steinhausen HC, Taylor E, Daly M, Laird N, Lange C, Faraone SV. 2008a. Genome-wide association scan of the time to onset of attention deficit hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet 147B(8):1355–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasky-Su J, Neale BM, Franke B, Anney RJ, Zhou K, Maller JB, Vasquez AA, Chen W, Asherson P, Buitelaar J, Banaschewski T, Ebstein R, Gill M, Miranda A, Mulas F, Oades RD, Roeyers H, Rothenberger A, Sergeant J, Sonuga-Barke E, Steinhausen HC, Taylor E, Daly M, Laird N, Lange C, Faraone SV. 2008b. Genome-wide association scan of quantitative traits for attention deficit hyperactivity disorder identifies novel associations and confirms candidate gene associations. Am J Med Genet B Neuropsychiatr Genet 147B(8):1345–54. [DOI] [PubMed] [Google Scholar]
- Laursen HR, Siebner HR, Haren T, Madsen K, Gronlund R, Hulme O, Henningsson S. 2014. Variation in the oxytocin receptor gene is associated with behavioral and neural correlates of empathic accuracy. Front Behav Neurosci 8:423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bihan D 2003. Looking into the functional architecture of the brain with diffusion MRI. Nat Rev Neurosci 4(6):469–80. [DOI] [PubMed] [Google Scholar]
- Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, Chabriat H. 2001. Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging 13(4):534–46. [DOI] [PubMed] [Google Scholar]
- Lee AD, Leow AD, Lu A, Reiss AL, Hall S, Chiang MC, Toga AW, Thompson PM. 2007. 3D pattern of brain abnormalities in Fragile X syndrome visualized using tensor-based morphometry. Neuroimage 34(3):924–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. 1996. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 274(5292):1527–31. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Timmesfeld N, Renner TJ, Halperin R, Roser C, Nguyen TT, Craig DW, Romanos J, Heine M, Meyer J, Freitag C, Warnke A, Romanos M, Schafer H, Walitza S, Reif A, Stephan DA, Jacob C. 2008. Molecular genetics of adult ADHD: converging evidence from genome-wide association and extended pedigree linkage studies. J Neural Transm 115(11):1573–85. [DOI] [PubMed] [Google Scholar]
- Long H, Liu B, Hou B, Wang C, Li J, Qin W, Wang D, Zhou Y, Kendrick KM, Yu C, Jiang T. 2013. The long rather than the short allele of 5-HTTLPR predisposes Han Chinese to anxiety and reduced connectivity between prefrontal cortex and amygdala. Neurosci Bull 29(1):4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loth E, Poline JB, Thyreau B, Jia T, Tao C, Lourdusamy A, Stacey D, Cattrell A, Desrivieres S, Ruggeri B, Fritsch V, Banaschewski T, Barker GJ, Bokde AL, Buchel C, Carvalho FM, Conrod PJ, Fauth-Buehler M, Flor H, Gallinat J, Garavan H, Heinz A, Bruehl R, Lawrence C, Mann K, Martinot JL, Nees F, Paus T, Pausova Z, Poustka L, Rietschel M, Smolka M, Struve M, Feng J, Schumann G, Consortium I. 2014. Oxytocin receptor genotype modulates ventral striatal activity to social cues and response to stressful life events. Biol Psychiatry 76(5):367–76. [DOI] [PubMed] [Google Scholar]
- Ma D, Salyakina D, Jaworski JM, Konidari I, Whitehead PL, Andersen AN, Hoffman JD, Slifer SH, Hedges DJ, Cukier HN, Griswold AJ, McCauley JL, Beecham GW, Wright HH, Abramson RK, Martin ER, Hussman JP, Gilbert JR, Cuccaro ML, Haines JL, Pericak-Vance MA. 2009. A genome-wide association study of autism reveals a common novel risk locus at 5p14.1. Ann Hum Genet 73(Pt 3):263–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood A, Bibat G, Zhan AL, Izbudak I, Farage L, Horska A, Mori S, Naidu S. 2010. White matter impairment in Rett syndrome: diffusion tensor imaging study with clinical correlations. AJNR Am J Neuroradiol 31(2):295–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti-Bonmati L, Menor F, Dosda R. 2000. Tuberous sclerosis: differences between cerebral and cerebellar cortical tubers in a pediatric population. AJNR Am J Neuroradiol 21(3):557–60. [PMC free article] [PubMed] [Google Scholar]
- Mefford HC, Batshaw ML, Hoffman EP. 2012. Genomics, intellectual disability, and autism. N Engl J Med 366(8):733–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneses A, Liy-Salmeron G. 2012. Serotonin and emotion, learning and memory. Rev Neurosci 23(5–6):543–53. [DOI] [PubMed] [Google Scholar]
- Merenstein SA, Sobesky WE, Taylor AK, Riddle JE, Tran HX, Hagerman RJ. 1996. Molecular-clinical correlations in males with an expanded FMR1 mutation. Am J Med Genet 64(2):388–94. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. 2011. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci 12(9):524–38. [DOI] [PubMed] [Google Scholar]
- Michalska KJ, Decety J, Liu C, Chen Q, Martz ME, Jacob S, Hipwell AE, Lee SS, Chronis-Tuscano A, Waldman ID, Lahey BB. 2014. Genetic imaging of the association of oxytocin receptor gene (OXTR) polymorphisms with positive maternal parenting. Front Behav Neurosci 8:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mick E, Todorov A, Smalley S, Hu X, Loo S, Todd RD, Biederman J, Byrne D, Dechairo B, Guiney A, McCracken J, McGough J, Nelson SF, Reiersen AM, Wilens TE, Wozniak J, Neale BM, Faraone SV. 2010. Family-based genome-wide association scan of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 49(9):898–905 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar K, Keri S. 2014. Bigger is better and worse: on the intricate relationship between hippocampal size and memory. Neuropsychologia 56:73–8. [DOI] [PubMed] [Google Scholar]
- Montag C, Sauer C, Reuter M, Kirsch P. 2013. An interaction between oxytocin and a genetic variation of the oxytocin receptor modulates amygdala activity toward direct gaze: evidence from a pharmacological imaging genetics study. Eur Arch Psychiatry Clin Neurosci 263 Suppl 2:S169–75. [DOI] [PubMed] [Google Scholar]
- Montag C, Weber B, Jentgens E, Elger C, Reuter M. 2010. An epistasis effect of functional variants on the BDNF and DRD2 genes modulates gray matter volume of the anterior cingulate cortex in healthy humans. Neuropsychologia 48(4):1016–21. [DOI] [PubMed] [Google Scholar]
- Monuteaux MC, Seidman LJ, Faraone SV, Makris N, Spencer T, Valera E, Brown A, Bush G, Doyle AE, Hughes S, Helliesen M, Mick E, Biederman J. 2008. A preliminary study of dopamine D4 receptor genotype and structural brain alterations in adults with ADHD. Am J Med Genet B Neuropsychiatr Genet 147B(8):1436–41. [DOI] [PubMed] [Google Scholar]
- Moore BD, 3rd, Slopis JM, Jackson EF, De Winter AE, Leeds NE. 2000. Brain volume in children with neurofibromatosis type 1: relation to neuropsychological status. Neurology 54(4):914–20. [DOI] [PubMed] [Google Scholar]
- Moore TR, Hill AM, Panguluri SK. 2014. Pharmacogenomics in psychiatry: implications for practice. Recent Pat Biotechnol 8(2):152–9. [DOI] [PubMed] [Google Scholar]
- Murphy SE, Norbury R, Godlewska BR, Cowen PJ, Mannie ZM, Harmer CJ, Munafo MR. 2013. The effect of the serotonin transporter polymorphism (5-HTTLPR) on amygdala function: a meta-analysis. Mol Psychiatry 18(4):512–20. [DOI] [PubMed] [Google Scholar]
- Neale BM, Lasky-Su J, Anney R, Franke B, Zhou K, Maller JB, Vasquez AA, Asherson P, Chen W, Banaschewski T. 2008. Genome-wide association scan of attention deficit hyperactivity disorder. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics 147(8):1337–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale BM, Medland S, Ripke S, Anney RJ, Asherson P, Buitelaar J, Franke B, Gill M, Kent L, Holmans P. 2010a. Case-control genome-wide association study of attention-deficit/hyperactivity disorder. Journal of the American Academy of Child & Adolescent Psychiatry 49(9):906–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale BM, Medland SE, Ripke S, Asherson P, Franke B, Lesch KP, Faraone SV, Nguyen TT, Schafer H, Holmans P, Daly M, Steinhausen HC, Freitag C, Reif A, Renner TJ, Romanos M, Romanos J, Walitza S, Warnke A, Meyer J, Palmason H, Buitelaar J, Vasquez AA, Lambregts-Rommelse N, Gill M, Anney RJ, Langely K, O’Donovan M, Williams N, Owen M, Thapar A, Kent L, Sergeant J, Roeyers H, Mick E, Biederman J, Doyle A, Smalley S, Loo S, Hakonarson H, Elia J, Todorov A, Miranda A, Mulas F, Ebstein RP, Rothenberger A, Banaschewski T, Oades RD, Sonuga-Barke E, McGough J, Nisenbaum L, Middleton F, Hu X, Nelson S, Psychiatric GCAS. 2010b. Meta-analysis of genome-wide association studies of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 49(9):884–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville MJ, Johnstone EC, Walton RT. 2004. Identification and characterization of ANKK1: a novel kinase gene closely linked to DRD2 on chromosome band 11q23.1. Hum Mutat 23(6):540–5. [DOI] [PubMed] [Google Scholar]
- Nicita F, Di Biasi C, Sollaku S, Cecchini S, Salpietro V, Pittalis A, Papetti L, Ursitti F, Ulgiati F, Zicari AM, Gualdi GF, Properzi E, Duse M, Ruggieri M, Spalice A. 2014. Evaluation of the basal ganglia in neurofibromatosis type 1. Childs Nerv Syst 30(2):319–25. [DOI] [PubMed] [Google Scholar]
- Nikolaidis A, Gray JR. 2010. ADHD and the DRD4 exon III 7-repeat polymorphism: an international meta-analysis. Soc Cogn Affect Neurosci 5(2–3):188–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolova YS, Ferrell RE, Manuck SB, Hariri AR. 2011. Multilocus genetic profile for dopamine signaling predicts ventral striatum reactivity. Neuropsychopharmacology 36(9):1940–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noain D, Avale ME, Wedemeyer C, Calvo D, Peper M, Rubinstein M. 2006. Identification of brain neurons expressing the dopamine D4 receptor gene using BAC transgenic mice. Eur J Neurosci 24(9):2429–38. [DOI] [PubMed] [Google Scholar]
- Oldenhof J, Vickery R, Anafi M, Oak J, Ray A, Schoots O, Pawson T, von Zastrow M, Van Tol HH. 1998. SH3 binding domains in the dopamine D4 receptor. Biochemistry 37(45):15726–36. [DOI] [PubMed] [Google Scholar]
- Onnink AM, Franke B, van Hulzen K, Zwiers MP, Mostert JC, Schene AH, Heslenfeld DJ, Oosterlaan J, Hoekstra PJ, Hartman CA, Vasquez AA, Kan CC, Buitelaar J, Hoogman M. 2016. Enlarged striatal volume in adults with ADHD carrying the 9–6 haplotype of the dopamine transporter gene DAT1. J Neural Transm (Vienna). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oquendo MA, Hastings RS, Huang YY, Simpson N, Ogden RT, Hu XZ, Goldman D, Arango V, Van Heertum RL, Mann JJ, Parsey RV. 2007. Brain serotonin transporter binding in depressed patients with bipolar disorder using positron emission tomography. Arch Gen Psychiatry 64(2):201–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paloyelis Y, Mehta MA, Faraone SV, Asherson P, Kuntsi J. 2012. Striatal sensitivity during reward processing in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 51(7):722–732 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan YQ, Qiao L, Xue XD, Fu JH. 2015. Association between ANKK1 (rs1800497) polymorphism of DRD2 gene and attention deficit hyperactivity disorder: a meta-analysis. Neurosci Lett 590:101–5. [DOI] [PubMed] [Google Scholar]
- Passamonti L, Cerasa A, Gioia MC, Magariello A, Muglia M, Quattrone A, Fera F. 2008. Genetically dependent modulation of serotonergic inactivation in the human prefrontal cortex. Neuroimage 40(3):1264–73. [DOI] [PubMed] [Google Scholar]
- Patriquin MA, Bauer IE, Soares JC, Graham DP, Nielsen DA. 2015. Addiction pharmacogenetics: a systematic review of the genetic variation of the dopaminergic system. Psychiatr Genet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne JM, Moharir MD, Webster R, North KN. 2010. Brain structure and function in neurofibromatosis type 1: current concepts and future directions. J Neurol Neurosurg Psychiatry 81(3):304–9. [DOI] [PubMed] [Google Scholar]
- Pendergrass SA, Brown-Gentry K, Dudek SM, Torstenson ES, Ambite JL, Avery CL, Buyske S, Cai C, Fesinmeyer MD, Haiman C, Heiss G, Hindorff LA, Hsu CN, Jackson RD, Kooperberg C, Le Marchand L, Lin Y, Matise TC, Moreland L, Monroe K, Reiner AP, Wallace R, Wilkens LR, Crawford DC, Ritchie MD. 2011. The use of phenome-wide association studies (PheWAS) for exploration of novel genotype-phenotype relationships and pleiotropy discovery. Genet Epidemiol 35(5):410–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perou R, Bitsko RH, Blumberg SJ, Pastor P, Ghandour RM, Gfroerer JC, Hedden SL, Crosby AE, Visser SN, Schieve LA, Parks SE, Hall JE, Brody D, Simile CM, Thompson WW, Baio J, Avenevoli S, Kogan MD, Huang LN, Centers for Disease C, Prevention. 2013. Mental health surveillance among children--United States, 2005–2011. MMWR Suppl 62(2):1–35. [PubMed] [Google Scholar]
- Persico AM, Napolioni V. 2013. Autism genetics. Behav Brain Res 251:95–112. [DOI] [PubMed] [Google Scholar]
- Peters JM, Sahin M, Vogel-Farley VK, Jeste SS, Nelson CA, 3rd, Gregas MC, Prabhu SP, Scherrer B, Warfield SK. 2012. Loss of white matter microstructural integrity is associated with adverse neurological outcome in tuberous sclerosis complex. Acad Radiol 19(1):17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR. 2005. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci 8(6):828–34. [DOI] [PubMed] [Google Scholar]
- Piao C, Yu A, Li K, Wang Y, Qin W, Xue S. 2009. Cerebral diffusion tensor imaging in tuberous sclerosis. Eur J Radiol 71(2):249–52. [DOI] [PubMed] [Google Scholar]
- Plichta MM, Scheres A. 2014. Ventral-striatal responsiveness during reward anticipation in ADHD and its relation to trait impulsivity in the healthy population: a meta-analytic review of the fMRI literature. Neurosci Biobehav Rev 38:125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poelmans G, Pauls DL, Buitelaar JK, Franke B. 2011. Integrated genome-wide association study findings: identification of a neurodevelopmental network for attention deficit hyperactivity disorder. Am J Psychiatry 168(4):365–77. [DOI] [PubMed] [Google Scholar]
- Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. 2007. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry 164(6):942–8. [DOI] [PubMed] [Google Scholar]
- Prandini P, Pasquali A, Malerba G, Marostica A, Zusi C, Xumerle L, Muglia P, Da Ros L, Ratti E, Trabetti E, Pignatti PF, Italian Autism N. 2012. The association of rs4307059 and rs35678 markers with autism spectrum disorders is replicated in Italian families. Psychiatr Genet 22(4):177–81. [DOI] [PubMed] [Google Scholar]
- Prather P, de Vries PJ. 2004. Behavioral and cognitive aspects of tuberous sclerosis complex. J Child Neurol 19(9):666–74. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. 2008. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods 40(3):879–91. [DOI] [PubMed] [Google Scholar]
- Pride NA, Korgaonkar MS, Barton B, Payne JM, Vucic S, North KN. 2014. The genetic and neuroanatomical basis of social dysfunction: lessons from neurofibromatosis type 1. Hum Brain Mapp 35(5):2372–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig MV, Antzoulatos EG, Miller EK. 2014. Prefrontal dopamine in associative learning and memory. Neuroscience 282C:217–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Pugliese L, Barker GJ, Daly E, Powell J, Bolton PF, Murphy DG. 2009. Serotonin transporter genotype and neuroanatomy in autism spectrum disorders. Psychiatr Genet 19(3):147–50. [DOI] [PubMed] [Google Scholar]
- Reif A, Jacob CP, Rujescu D, Herterich S, Lang S, Gutknecht L, Baehne CG, Strobel A, Freitag CM, Giegling I, Romanos M, Hartmann A, Rosler M, Renner TJ, Fallgatter AJ, Retz W, Ehlis AC, Lesch KP. 2009. Influence of functional variant of neuronal nitric oxide synthase on impulsive behaviors in humans. Arch Gen Psychiatry 66(1):41–50. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Faruque F, Naidu S, Abrams M, Beaty T, Bryan RN, Moser H. 1993. Neuroanatomy of Rett syndrome: a volumetric imaging study. Ann Neurol 34(2):227–34. [DOI] [PubMed] [Google Scholar]
- Reith RM, Way S, McKenna J, 3rd, Haines K, Gambello MJ. 2011. Loss of the tuberous sclerosis complex protein tuberin causes Purkinje cell degeneration. Neurobiol Dis 43(1):113–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribases M, Ramos-Quiroga JA, Sanchez-Mora C, Bosch R, Richarte V, Palomar G, Gastaminza X, Bielsa A, Arcos-Burgos M, Muenke M, Castellanos FX, Cormand B, Bayes M, Casas M. 2011. Contribution of LPHN3 to the genetic susceptibility to ADHD in adulthood: a replication study. Genes Brain Behav 10(2):149–57. [DOI] [PubMed] [Google Scholar]
- Richter A, Richter S, Barman A, Soch J, Klein M, Assmann A, Libeau C, Behnisch G, Wustenberg T, Seidenbecher CI, Schott BH. 2013. Motivational salience and genetic variability of dopamine D2 receptor expression interact in the modulation of interference processing. Front Hum Neurosci 7:250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridler K, Bullmore ET, De Vries PJ, Suckling J, Barker GJ, Meara SJ, Williams SC, Bolton PF. 2001. Widespread anatomical abnormalities of grey and white matter structure in tuberous sclerosis. Psychol Med 31(8):1437–46. [DOI] [PubMed] [Google Scholar]
- Ridler K, Suckling J, Higgins N, Bolton P, Bullmore E. 2004. Standardized whole brain mapping of tubers and subependymal nodules in tuberous sclerosis complex. J Child Neurol 19(9):658–65. [DOI] [PubMed] [Google Scholar]
- Ridler K, Suckling J, Higgins NJ, de Vries PJ, Stephenson CM, Bolton PF, Bullmore ET. 2007. Neuroanatomical correlates of memory deficits in tuberous sclerosis complex. Cereb Cortex 17(2):261–71. [DOI] [PubMed] [Google Scholar]
- Ritvo ER, Freeman BJ, Mason-Brothers A, Mo A, Ritvo AM. 1985. Concordance for the syndrome of autism in 40 pairs of afflicted twins. Am J Psychiatry 142(1):74–7. [DOI] [PubMed] [Google Scholar]
- Rodenas-Cuadrado P, Ho J, Vernes SC. 2014. Shining a light on CNTNAP2: complex functions to complex disorders. Eur J Hum Genet 22(2):171–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommelse NN, Franke B, Geurts HM, Hartman CA, Buitelaar JK. 2010. Shared heritability of attention-deficit/hyperactivity disorder and autism spectrum disorder. Eur Child Adolesc Psychiatry 19(3):281–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose EJ, Donohoe G. 2013. Brain vs behavior: an effect size comparison of neuroimaging and cognitive studies of genetic risk for schizophrenia. Schizophr Bull 39(3):518–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose SA, Djukic A, Jankowski JJ, Feldman JF, Rimler M. 2016. Aspects of Attention in Rett Syndrome. Pediatr Neurol 57:22–8. [DOI] [PubMed] [Google Scholar]
- Rudie JD, Hernandez LM, Brown JA, Beck-Pancer D, Colich NL, Gorrindo P, Thompson PM, Geschwind DH, Bookheimer SY, Levitt P, Dapretto M. 2012. Autism-associated promoter variant in MET impacts functional and structural brain networks. Neuron 75(5):904–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said SM, Yeh TL, Greenwood RS, Whitt JK, Tupler LA, Krishnan KR. 1996. MRI morphometric analysis and neuropsychological function in patients with neurofibromatosis. Neuroreport 7(12):1941–4. [DOI] [PubMed] [Google Scholar]
- Saldarriaga W, Tassone F, Gonzalez-Teshima LY, Forero-Forero JV, Ayala-Zapata S, Hagerman R. 2014. Fragile X syndrome. Colomb Med (Cali) 45(4):190–8. [PMC free article] [PubMed] [Google Scholar]
- Salvatore JE, Dick DM. 2016. Genetic influences on conduct disorder. Neurosci Biobehav Rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Mora C, Ramos-Quiroga JA, Bosch R, Corrales M, Garcia-Martinez I, Nogueira M, Pagerols M, Palomar G, Richarte V, Vidal R, Arias-Vasquez A, Bustamante M, Forns J, Gross-Lesch S, Guxens M, Hinney A, Hoogman M, Jacob C, Jacobsen KK, Kan CC, Kiemeney L, Kittel-Schneider S, Klein M, Onnink M, Rivero O, Zayats T, Buitelaar J, Faraone SV, Franke B, Haavik J, Johansson S, Lesch KP, Reif A, Sunyer J, Bayes M, Casas M, Cormand B, Ribases M. 2014. Case-Control Genome-Wide Association Study of Persistent Attention-Deficit Hyperactivity Disorder Identifies FBXO33 as a Novel Susceptibility Gene for the Disorder. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandin S, Lichtenstein P, Kuja-Halkola R, Larsson H, Hultman CM, Reichenberg A. 2014. The familial risk of autism. JAMA 311(17):1770–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schizophrenia Working Group of the Psychiatric Genomics C. 2014. Biological insights from 108 schizophrenia-associated genetic loci. Nature 511(7510):421–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott-Van Zeeland AA, Abrahams BS, Alvarez-Retuerto AI, Sonnenblick LI, Rudie JD, Ghahremani D, Mumford JA, Poldrack RA, Dapretto M, Geschwind DH, Bookheimer SY. 2010. Altered functional connectivity in frontal lobe circuits is associated with variation in the autism risk gene CNTNAP2. Sci Transl Med 2(56):56ra80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Gornick M, Lerch J, Addington A, Seal J, Greenstein D, Sharp W, Evans A, Giedd JN, Castellanos FX, Rapoport JL. 2007. Polymorphisms of the dopamine D4 receptor, clinical outcome, and cortical structure in attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 64(8):921–31. [DOI] [PubMed] [Google Scholar]
- Shook D, Brady C, Lee PS, Kenealy L, Murphy ER, Gaillard WD, VanMeter JW, Cook EH Jr, Stein M, Vaidya CJ. 2011. Effect of dopamine transporter genotype on caudate volume in childhood ADHD and controls. Am J Med Genet B Neuropsychiatr Genet 156B(1):28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simao G, Raybaud C, Chuang S, Go C, Snead OC, Widjaja E. 2010. Diffusion tensor imaging of commissural and projection white matter in tuberous sclerosis complex and correlation with tuber load. AJNR Am J Neuroradiol 31(7):1273–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon V, Czobor P, Balint S, Meszaros A, Bitter I. 2009. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br J Psychiatry 194(3):204–11. [DOI] [PubMed] [Google Scholar]
- Sokolova E, Hoogman M, Groot P, Claassen T, Vasquez AA, Buitelaar JK, Franke B, Heskes T. 2015. Causal discovery in an adult ADHD data set suggests indirect link between DAT1 genetic variants and striatal brain activation during reward processing. Am J Med Genet B Neuropsychiatr Genet. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Lasky-Su J, Neale BM, Oades R, Chen W, Franke B, Buitelaar J, Banaschewski T, Ebstein R, Gill M, Anney R, Miranda A, Mulas F, Roeyers H, Rothenberger A, Sergeant J, Steinhausen HC, Thompson M, Asherson P, Faraone SV. 2008. Does parental expressed emotion moderate genetic effects in ADHD? An exploration using a genome wide association scan. Am J Med Genet B Neuropsychiatr Genet 147B(8):1359–68. [DOI] [PubMed] [Google Scholar]
- Splawski I, Timothy KW, Priori SG, Napolitano C, Bloise R. 1993. Timothy Syndrome. In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, Bird TD, Dolan CR, Fong CT, Smith RJH et al. editors. GeneReviews(R). Seattle (WA): University of Washington, Seattle: University of Washington, Seattle. All rights reserved. [PubMed] [Google Scholar]
- Steen RG, Taylor JS, Langston JW, Glass JO, Brewer VR, Reddick WE, Mages R, Pivnick EK. 2001. Prospective evaluation of the brain in asymptomatic children with neurofibromatosis type 1: relationship of macrocephaly to T1 relaxation changes and structural brain abnormalities. AJNR Am J Neuroradiol 22(5):810–7. [PMC free article] [PubMed] [Google Scholar]
- Steffenburg S, Gillberg C, Hellgren L, Andersson L, Gillberg IC, Jakobsson G, Bohman M. 1989. A twin study of autism in Denmark, Finland, Iceland, Norway and Sweden. J Child Psychol Psychiatry 30(3):405–16. [DOI] [PubMed] [Google Scholar]
- Stein JL, Medland SE, Vasquez AA, Hibar DP, Senstad RE, Winkler AM, Toro R, Appel K, Bartecek R, Bergmann O, Bernard M, Brown AA, Cannon DM, Chakravarty MM, Christoforou A, Domin M, Grimm O, Hollinshead M, Holmes AJ, Homuth G, Hottenga JJ, Langan C, Lopez LM, Hansell NK, Hwang KS, Kim S, Laje G, Lee PH, Liu X, Loth E, Lourdusamy A, Mattingsdal M, Mohnke S, Maniega SM, Nho K, Nugent AC, O’Brien C, Papmeyer M, Putz B, Ramasamy A, Rasmussen J, Rijpkema M, Risacher SL, Roddey JC, Rose EJ, Ryten M, Shen L, Sprooten E, Strengman E, Teumer A, Trabzuni D, Turner J, van Eijk K, van Erp TG, van Tol MJ, Wittfeld K, Wolf C, Woudstra S, Aleman A, Alhusaini S, Almasy L, Binder EB, Brohawn DG, Cantor RM, Carless MA, Corvin A, Czisch M, Curran JE, Davies G, de Almeida MA, Delanty N, Depondt C, Duggirala R, Dyer TD, Erk S, Fagerness J, Fox PT, Freimer NB, Gill M, Goring HH, Hagler DJ, Hoehn D, Holsboer F, Hoogman M, Hosten N, Jahanshad N, Johnson MP, Kasperaviciute D, Kent JW, Jr, Kochunov P, Lancaster JL, Lawrie SM, Liewald DC, Mandl R, Matarin M, Mattheisen M, Meisenzahl E, Melle, Moses EK, Muhleisen TW, Nauck, Nothen MM, Olvera RL, Pandolfo M, Pike GB, Puls R, Reinvang I, Renteria ME, Rietschel M, Roffman JL, Royle NA, Rujescu D, Savitz J, Schnack HG, Schnell K, Seiferth N, Smith C, Steen VM, Valdes Hernandez MC, Van den Heuvel M, van der Wee NJ, Van Haren NE, Veltman JA, Volzke H, Walker R, Westlye LT, Whelan CD, Agartz I, Boomsma DI, Cavalleri GL, Dale AM, Djurovic S, Drevets WC, Hagoort P, Hall J, Heinz A, Jack CR Jr, Foroud TM, Le Hellard S, Macciardi F, Montgomery GW, Poline JB, Porteous DJ, Sisodiya SM, Starr JM, Sussmann J, Toga AW, Veltman DJ, Walter H, Weiner MW, Alzheimer’s Disease Neuroimaging I, Consortium E, Consortium I, Saguenay Youth Study G, Bis JC, Ikram MA, Smith AV, Gudnason V, Tzourio C, Vernooij MW, Launer LJ, DeCarli C, Seshadri S, Cohorts for H, Aging Research in Genomic Epidemiology C, Andreassen OA Apostolova LG, Bastin ME, Blangero J, Brunner HG, Buckner RL, Cichon S, Coppola G, de Zubicaray GI, Deary IJ, Donohoe G, de Geus EJ, Espeseth T, Fernandez G, Glahn DC, Grabe HJ, Hardy J, Hulshoff Pol HE, Jenkinson M, Kahn RS, McDonald C, McIntosh AM, McMahon FJ, McMahon, Meyer-Lindenberg A, Morris DW, Muller-Myhsok B, Nichols TE, Ophoff RA, Paus T, Pausova Z, Penninx BW, Potkin SG, Samann PG, Saykin AJ, Schumann G, Smoller JW, Wardlaw JM, Weale ME, Martin NG, Franke B, Wright MJ, Thompson PM, Enhancing Neuro Imaging Genetics through Meta-Analysis C. 2012. Identification of common variants associated with human hippocampal and intracranial volumes. Nat Genet 44(5):552–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stergiakouli E, Hamshere M, Holmans P, Langley K, Zaharieva I, de CG, Psychiatric GC, Hawi Z, Kent L, Gill M, Williams N, Owen MJ, O’Donovan M, Thapar A. 2012. Investigating the contribution of common genetic variants to the risk and pathogenesis of ADHD. Am J Psychiatry 169(2):186–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Yokum S, Burger K, Epstein L, Smolen A. 2012. Multilocus genetic composite reflecting dopamine signaling capacity predicts reward circuitry responsivity. J Neurosci 32(29):10093–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stollstorff M, Foss-Feig J, Cook EH Jr, Stein MA, Gaillard WD, Vaidya CJ. 2010. Neural response to working memory load varies by dopamine transporter genotype in children. Neuroimage 53(3):970–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strike LT, Couvy-Duchesne B, Hansell NK, Cuellar-Partida G, Medland SE, Wright MJ. 2015. Genetics and brain morphology. Neuropsychol Rev 25(1):63–96. [DOI] [PubMed] [Google Scholar]
- Subramaniam B, Naidu S, Reiss AL. 1997. Neuroanatomy in Rett syndrome: cerebral cortex and posterior fossa. Neurology 48(2):399–407. [DOI] [PubMed] [Google Scholar]
- Sullivan K, Hatton D, Hammer J, Sideris J, Hooper S, Ornstein P, Bailey D, Jr, 2006. ADHD symptoms in children with FXS. Am J Med Genet A 140(21):2275–88. [DOI] [PubMed] [Google Scholar]
- Suter B, Treadwell-Deering D, Zoghbi HY, Glaze DG, Neul JL. 2014. Brief report: MECP2 mutations in people without Rett syndrome. J Autism Dev Disord 44(3):703–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Tomita H, Taki Y, Kikuchi Y, Ono C, Yu Z, Sekiguchi A, Nouchi R, Kotozaki Y, Nakagawa S, Miyauchi CM, Iizuka K, Yokoyama R, Shinada T, Yamamoto Y, Hanawa S, Araki T, Hashizume H, Kunitoki K, Sassa Y, Kawashima R. 2015. Cognitive and neural correlates of the 5-repeat allele of the dopamine D4 receptor gene in a population lacking the 7-repeat allele. Neuroimage 110:124–35. [DOI] [PubMed] [Google Scholar]
- Tan GC, Doke TF, Ashburner J, Wood NW, Frackowiak RS. 2010. Normal variation in fronto-occipital circuitry and cerebellar structure with an autism-associated polymorphism of CNTNAP2. Neuroimage 53(3):1030–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Stein JL, Medland SE, Hibar DP, Vasquez AA, Renteria ME, Toro R, Jahanshad N, Schumann G, Franke B, Wright MJ, Martin NG, Agartz I, Alda M, Alhusaini S, Almasy L, Almeida J, Alpert K, Andreasen NC, Andreassen OA, Apostolova LG, Appel K, Armstrong NJ, Aribisala B, Bastin ME, Bauer M, Bearden CE, Bergmann O, Binder EB, Blangero J, Bockholt HJ, Boen E, Bois C, Boomsma DI, Booth T, Bowman IJ, Bralten J, Brouwer RM, Brunner HG, Brohawn DG, Buckner RL, Buitelaar J, Bulayeva K, Bustillo JR, Calhoun VD, Cannon DM, Cantor RM, Carless MA, Caseras X, Cavalleri GL, Chakravarty MM, Chang KD, Ching CR, Christoforou A, Cichon S, Clark VP, Conrod P, Coppola G, Crespo-Facorro B, Curran JE, Czisch M, Deary IJ, de Geus EJ, den Braber A, Delvecchio G, Depondt C, de Haan L, de Zubicaray GI, Dima D, Dimitrova R, Djurovic S, Dong H, Donohoe G, Duggirala R, Dyer TD, Ehrlich S, Ekman CJ, Elvsashagen T, Emsell L, Erk S, Espeseth T, Fagerness J, Fears S, Fedko I, Fernandez G, Fisher SE, Foroud T, Fox PT, Francks C, Frangou S, Frey EM, Frodl T, Frouin V, Garavan H, Giddaluru S, Glahn DC, Godlewska B, Goldstein RZ, Gollub RL, Grabe HJ, Grimm O, Gruber O, Guadalupe T, Gur RE, Gur RC, Goring HH, Hagenaars S, Hajek T, Hall GB, Hall J, Hardy J, Hartman CA, Hass J, Hatton SN, Haukvik UK, Hegenscheid K, Heinz A, Hickie IB, Ho BC, Hoehn D, Hoekstra PJ, Hollinshead M, Holmes AJ, Homuth G, Hoogman M, Hong LE, Hosten N, Hottenga JJ, Hulshoff Pol HE, Hwang KS, Jack CR, Jr., Jenkinson M, Johnston C, Jonsson EG, Kahn RS, Kasperaviciute D, Kelly S, Kim S, Kochunov P, Koenders L, Kramer B, Kwok JB, Lagopoulos J, Laje G, Landen M, Landman BA, Lauriello J, Lawrie SM, Lee PH, Le Hellard S, Lemaitre H, Leonardo CD, Li CS, Liberg B, Liewald DC, Liu X, Lopez LM, Loth E, Lourdusamy A, Luciano M, Macciardi F, Machielsen MW, Macqueen GM, Malt UF, Mandl R, Manoach DS, Martinot JL, Matarin M, Mather KA, Mattheisen M, Mattingsdal M, Meyer-Lindenberg A, McDonald C, McIntosh AM, McMahon FJ, McMahon KL, Meisenzahl E, Melle I, Milaneschi Y, Mohnke S, Montgomery GW, Morris DW, Moses EK, Mueller BA, Munoz Maniega S, Muhleisen TW, Muller-Myhsok B, Mwangi B, Nauck M, Nho K, Nichols TE, Nilsson LG, Nugent AC, Nyberg L, Olvera RL, Oosterlaan J, Ophoff RA, Pandolfo M, Papalampropoulou-Tsiridou M, Papmeyer M, Paus T, Pausova Z, Pearlson GD, Penninx BW, Peterson CP, Pfennig A, Phillips M, Pike GB, Poline JB, Potkin SG, Putz B, Ramasamy A, Rasmussen J, Rietschel M, Rijpkema M, Risacher SL, Roffman JL, Roiz-Santianez R, Romanczuk-Seiferth N, Rose EJ, Royle NA, Rujescu D, Ryten M, Sachdev PS, Salami A, Satterthwaite TD, Savitz J, Saykin AJ, Scanlon C, Schmaal L, Schnack HG, Schork AJ, Schulz SC, Schur R, Seidman L, Shen L, Shoemaker JM, Simmons A, Sisodiya SM, Smith C, Smoller JW, Soares JC, Sponheim SR, Sprooten E, Starr JM, Steen VM, Strakowski S, Strike L, Sussmann J, Samann PG, Teumer A, Toga AW, Tordesillas-Gutierrez D, Trabzuni D, Trost S, Turner J, Van den Heuve M, van der Wee NJ, van Eijk K, van Erp TG, van Haren NE, van ‘t Ent D, van Tol MJ, Valdes Hernandez MC, Veltman DJ, Versace A, Volzke H, Walker R, Walter H, Wang L, Wardlaw JM, Weale ME, Weiner MW, Wen W, Westlye LT, Whalley HC, Whelan CD, White T, Winkler AM, Wittfeld K, Woldehawariat G, Wolf C, Zilles D, Zwiers MP, Thalamuthu A, Schofield PR, Freimer NB, Lawrence NS, Drevets W, Alzheimer’s Disease Neuroimaging Initiative ECICSYSG. 2014. The ENIGMA Consortium: large-scale collaborative analyses of neuroimaging and genetic data. Brain Imaging Behav 8(2):153–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tost H, Kolachana B, Hakimi S, Lemaitre H, Verchinski BA, Mattay VS, Weinberger DR, Meyer-Lindenberg A. 2010. A common allele in the oxytocin receptor gene (OXTR) impacts prosocial temperament and human hypothalamic-limbic structure and function. Proc Natl Acad Sci U S A 107(31):13936–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usiello A, Baik JH, Rouge-Pont F, Picetti R, Dierich A, LeMeur M, Piazza PV, Borrelli E. 2000. Distinct functions of the two isoforms of dopamine D2 receptors. Nature 408(6809):199–203. [DOI] [PubMed] [Google Scholar]
- van Bokhoven H 2011. Genetic and epigenetic networks in intellectual disabilities. Annu Rev Genet 45:81–104. [DOI] [PubMed] [Google Scholar]
- van der Meer D, Hoekstra PJ, Zwiers M, Mennes M, Schweren LJ, Franke B, Heslenfeld DJ, Oosterlaan J, Faraone SV, Buitelaar JK, Hartman CA. 2015. Brain Correlates of the Interaction Between 5-HTTLPR and Psychosocial Stress Mediating Attention Deficit Hyperactivity Disorder Severity. Am J Psychiatry:appiajp201514081035. [DOI] [PubMed] [Google Scholar]
- van der Voet M, Harich B, Franke B, Schenck A. 2016. ADHD-associated dopamine transporter, latrophilin and neurofibromin share a dopamine-related locomotor signature in Drosophila. Mol Psychiatry 21(4):565–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Engelen SJ, Krab LC, Moll HA, de Goede-Bolder A, Pluijm SM, Catsman-Berrevoets CE, Elgersma Y, Lequin MH. 2008. Quantitative differentiation between healthy and disordered brain matter in patients with neurofibromatosis type I using diffusion tensor imaging. AJNR Am J Neuroradiol 29(4):816–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Tol HH, Wu CM, Guan HC, Ohara K, Bunzow JR, Civelli O, Kennedy J, Seeman P, Niznik HB, Jovanovic V. 1992. Multiple dopamine D4 receptor variants in the human population. Nature 358(6382):149–52. [DOI] [PubMed] [Google Scholar]
- Verbeke W, Bagozzi RP, van den Berg WE, Lemmens A. 2013. Polymorphisms of the OXTR gene explain why sales professionals love to help customers. Front Behav Neurosci 7:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalon-Reina J, Jahanshad N, Beaton E, Toga AW, Thompson PM, Simon TJ. 2013. White matter microstructural abnormalities in girls with chromosome 22q11.2 deletion syndrome, Fragile X or Turner syndrome as evidenced by diffusion tensor imaging. Neuroimage 81:441–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Violante IR, Ribeiro MJ, Cunha G, Bernardino I, Duarte JV, Ramos F, Saraiva J, Silva E, Castelo-Branco M. 2012. Abnormal brain activation in neurofibromatosis type 1: a link between visual processing and the default mode network. PLoS One 7(6):e38785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Violante IR, Ribeiro MJ, Silva ED, Castelo-Branco M. 2013. Gyrification, cortical and subcortical morphometry in neurofibromatosis type 1: an uneven profile of developmental abnormalities. J Neurodev Disord 5(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt RG, Barbaresi WJ, Colligan RC, Weaver AL, Katusic SK. 2006. Developmental dissociation, deviance, and delay: Occurrence of attention-deficit-hyperactivity disorder in individuals with and without borderline-to-mild intellectual disability. Dev Med Child Neurol 48(10):831–5. [DOI] [PubMed] [Google Scholar]
- Vorstman JA, Ophoff RA. 2013. Genetic causes of developmental disorders. Curr Opin Neurol 26(2):128–36. [DOI] [PubMed] [Google Scholar]
- Walsh KS, Velez JI, Kardel PG, Imas DM, Muenke M, Packer RJ, Castellanos FX, Acosta MT. 2013. Symptomatology of autism spectrum disorder in a population with neurofibromatosis type 1. Dev Med Child Neurol 55(2):131–8. [DOI] [PubMed] [Google Scholar]
- Wang E, Ding YC, Flodman P, Kidd JR, Kidd KK, Grady DL, Ryder OA, Spence MA, Swanson JM, Moyzis RK. 2004. The genetic architecture of selection at the human dopamine receptor D4 (DRD4) gene locus. Am J Hum Genet 74(5):931–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Zhang H, Ma D, Bucan M, Glessner JT, Abrahams BS, Salyakina D, Imielinski M, Bradfield JP, Sleiman PM, Kim CE, Hou C, Frackelton E, Chiavacci R, Takahashi N, Sakurai T, Rappaport E, Lajonchere CM, Munson J, Estes A, Korvatska O, Piven J, Sonnenblick LI, Alvarez Retuerto AI, Herman EI, Dong H, Hutman T, Sigman M, Ozonoff S, Klin A, Owley T, Sweeney JA, Brune CW, Cantor RM, Bernier R, Gilbert JR, Cuccaro ML, McMahon WM, Miller J, State MW, Wassink TH, Coon H, Levy SE, Schultz RT, Nurnberger JI, Haines JL, Sutcliffe JS, Cook EH, Minshew NJ, Buxbaum JD, Dawson G, Grant SF, Geschwind DH, Pericak-Vance MA, Schellenberg GD, Hakonarson H. 2009. Common genetic variants on 5p14.1 associate with autism spectrum disorders. Nature 459(7246):528–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassink TH, Hazlett HC, Epping EA, Arndt S, Dager SR, Schellenberg GD, Dawson G, Piven J. 2007. Cerebral cortical gray matter overgrowth and functional variation of the serotonin transporter gene in autism. Arch Gen Psychiatry 64(6):709–17. [DOI] [PubMed] [Google Scholar]
- Watson C, Hoeft F, Garrett AS, Hall SS, Reiss AL. 2008. Aberrant brain activation during gaze processing in boys with fragile X syndrome. Arch Gen Psychiatry 65(11):1315–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber H, Kittel-Schneider S, Heupel J, Weissflog L, Kent L, Freudenberg F, Alttoa A, Post A, Herterich S, Haavik J, Halmoy A, Fasmer OB, Landaas ET, Johansson S, Cormand B, Ribases M, Sanchez-Mora C, Ramos-Quiroga JA, Franke B, Lesch KP, Reif A. 2015. On the role of NOS1 ex1f-VNTR in ADHD-allelic, subgroup, and meta-analysis. Am J Med Genet B Neuropsychiatr Genet. [DOI] [PubMed] [Google Scholar]
- Weisenfeld NI, Peters JM, Tsai PT, Prabhu SP, Dies KA, Sahin M, Warfield SK. 2013. A magnetic resonance imaging study of cerebellar volume in tuberous sclerosis complex. Pediatr Neurol 48(2):105–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss LA, Arking DE, Gene Discovery Project of Johns H, the Autism C, Daly MJ, Chakravarti A. 2009. A genome-wide linkage and association scan reveals novel loci for autism. Nature 461(7265):802–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalley HC, O’Connell G, Sussmann JE, Peel A, Stanfield AC, Hayiou-Thomas ME, Johnstone EC, Lawrie SM, McIntosh AM, Hall J. 2011. Genetic variation in CNTNAP2 alters brain function during linguistic processing in healthy individuals. Am J Med Genet B Neuropsychiatr Genet 156B(8):941–8. [DOI] [PubMed] [Google Scholar]
- Wiener M, Lee YS, Lohoff FW, Coslett HB. 2014. Individual differences in the morphometry and activation of time perception networks are influenced by dopamine genotype. Neuroimage 89:10–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins JL, Bedoyan JK, Carrasco M, Swartz JR, Martin DM, Monk CS. 2014a. Age-related effect of serotonin transporter genotype on amygdala and prefrontal cortex function in adolescence. Hum Brain Mapp 35(2):646–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins JL, Peltier SJ, Bedoyan JK, Carrasco M, Welsh RC, Martin DM, Lord C, Monk CS. 2012. The impact of serotonin transporter genotype on default network connectivity in children and adolescents with autism spectrum disorders. Neuroimage Clin 2:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins JL, Swartz JR, Martin DM, Lord C, Monk CS. 2014b. Serotonin transporter genotype impacts amygdala habituation in youth with autism spectrum disorders. Soc Cogn Affect Neurosci 9(6):832–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wignall EL, Griffiths PD, Papadakis NG, Wilkinson ID, Wallis LI, Bandmann O, Cowell PE, Hoggard N. 2010. Corpus callosum morphology and microstructure assessed using structural MR imaging and diffusion tensor imaging: initial findings in adults with neurofibromatosis type 1. AJNR Am J Neuroradiol 31(5):856–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams EL, Casanova MF. 2011. Above genetics: lessons from cerebral development in autism. Transl Neurosci 2(2):106–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson LB, Tregellas JR, Hagerman RJ, Rogers SJ, Rojas DC. 2009. A voxel-based morphometry comparison of regional gray matter between fragile X syndrome and autism. Psychiatry Res 174(2):138–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Xiao H, Sun H, Zou L, Zhu LQ. 2012. Role of dopamine receptors in ADHD: a systematic meta-analysis. Mol Neurobiol 45(3):605–20. [DOI] [PubMed] [Google Scholar]
- Yang L, Neale BM, Liu L, Lee SH, Wray NR, Ji N, Li H, Qian Q, Wang D, Li J, Faraone SV, Wang Y, Psychiatric GCAS, Doyle AE, Reif A, Rothenberger A, Franke B, Sonuga-Barke EJ, Steinhausen HC, Buitelaar JK, Kuntsi J, Biederman J, Lesch KP, Kent L, Asherson P, Oades RD, Loo SK, Nelson SF, Faraone SV, Smalley SL, Banaschewski T, Arias Vasquez A, Todorov A, Charach A, Miranda A, Warnke A, Thapar A, Neale BM, Cormand B, Freitag C, Mick E, Mulas F, Middleton F, HakonarsonHakonarson H, Palmason H, Schafer H, Roeyers H, McGough JJ, Romanos J, Crosbie J, Meyer J, Ramos-Quiroga JA, Sergeant J, Elia J, Langely K, Nisenbaum L, Romanos M, Daly MJ, Ribases M, Gill M, O’Donovan M, Owen M, Casas M, Bayes M, Lambregts-Rommelse N, Williams N, Holmans P, Anney RJ, Ebstein RP, Schachar R, Medland SE, Ripke S, Walitza S, Nguyen TT, Renner TJ, Hu X. 2013. Polygenic transmission and complex neuro developmental network for attention deficit hyperactivity disorder: genome-wide association study of both common and rare variants. Am J Med Genet B Neuropsychiatr Genet 162B(5):419–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoncheva YN, Somandepalli K, Reiss PT, Kelly C, Di Martino A, Lazar M, Zhou J, Milham MP, Castellanos FX. 2016. Mode of Anisotropy Reveals Global Diffusion Alterations in Attention-Deficit/Hyperactivity Disorder. J Am Acad Child Adolesc Psychiatry 55(2):137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamboni SL, Loenneker T, Boltshauser E, Martin E, Il’yasov KA. 2007. Contribution of diffusion tensor MR imaging in detecting cerebral microstructural changes in adults with neurofibromatosis type 1. AJNR Am J Neuroradiol 28(4):773–6. [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Chen X, Yu P, Zhang Q, Sun X, Gu H, Zhang H, Zhai J, Chen M, Du B, Deng X, Ji F, Wang C, Xiang Y, Li D, Wu H, Li J, Dong Q, Chen C. 2015. Evidence for the Contribution of NOS1 Gene Polymorphism (rs3782206) to Prefrontal Function in Schizophrenia Patients and Healthy Controls. Neuropsychopharmacology 40(6):1383–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Leotta A, Kustanovich V, Lajonchere C, Geschwind DH, Law K, Law P, Qiu S, Lord C, Sebat J, Ye K, Wigler M. 2007. A unified genetic theory for sporadic and inherited autism. Proc Natl Acad Sci U S A 104(31):12831–6. [DOI] [PMC free article] [PubMed] [Google Scholar]