Abstract
Oxidative stress is one of the main causes of significant severe diseases. The discovery of new potent antioxidants with high efficiency and low toxicity is a great demand in the field of medicinal chemistry. Herein, we report the design, synthesis molecular modelling and biological evaluation of novel hybrids containing pyrazole, naphthalene and pyrazoline/isoxazoline moiety. Chalcones 2a–e were synthesized efficiently and were used as starting materials for synthesis of a variety of heterocycles. A novel series of pyrazoline 3a–e, phenylpyrazoline 4a–e, isoxazoline 5a–e and pyrazoline carbothioamide derivatives 6a–e were synthesized and screened for in vitro antioxidant activity using 2,2-diphenyl-1-picrylhydrazyl (DPPH), nitric oxide (NO) and superoxide radical scavenging assay as well as 15-lipoxygenase (15-LOX) inhibition activity. Compounds 3a, 4e, 5b, 5c, 6a, 6c, and 6e showed excellent radical scavenging activity in all three methods in comparison with ascorbic acid and 15-LOX inhibition potency using quercetin as standard then were subjected to in vivo study. Catalase (CAT) activity, glutathione (GSH) and malondialdehyde (MDA) levels were assayed in liver of treated rats. Compounds 5b, 5c, and 6e showed significant in vivo antioxidant potentials compared to control group at dose of 100 mg/kg B.W. Molecular docking of compound 6a endorsed its proper binding at the active site pocket of the human 15-LOX which explains its potent antioxidant activity in comparison with standard ascorbic acid.
Keywords: Pyrazole, hybrids, antioxidant activity, scavenging activity, 15-lipoxygenase inhibitors
Graphical Abstract
1. Introduction
Oxidative stress is one of the main causes of significant severe diseases, i.e. cancer, aging, atherosclerosis, hypertension, inflammation, renal disorders, liver disorders, rheumatoid arthritis, neurological disorders, cardiovascular, autoimmune diseases and neurodegenerative disorders such as Alzheimer’s, Huntington’s diseases and Parkinson’s diseases1–3. It is caused by the human body excessive production of reactive oxygen species (ROS) and nitrogen reactive species (NRS) such as hydrogen peroxides (H2O2) and free radicals. The balance between the production and neutralization of ROS by antioxidants is very delicate4. Every day a human cell is targeted by ROS, the hydroxyl radical (.OH), and other species inducing oxidative stress5. Free radicals (atoms, molecules or ions contain an unpaired electron) are highly unstable and very reactive species that are able to create ROS such as .OH, hydroperoxyl radical (HO2.), superoxide anion (.O2−), nitric oxide (NO), singlet oxygen (O) and H2O2 as well as nitrogen reactive species (RNS) and reactive sulphur species (RSS). These species are generated either internally from normal metabolic activities or external factors, such as smoking, environmental pollutants and radiation, that promote the production of free radicals. The main human body targets of ROS, RNS and RSS are sugars, proteins, lipids, DNA and RNA molecules6. High concentrations of such species can cause damage to the normal cell structures, embedded proteins, carbohydrates, lipids, and disrupt nitrogen bases of nucleic acids leading to the above-mentioned diseases. The human body creates a primary defence antioxidant mechanism for the detoxification of the formed free radicals. This mechanism involves three enzymes: superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx)7. The action of these enzymes is more prominent in the presence of antioxidant agents. Antioxidants are molecules that delay and prevent oxidative damage to a target molecule. In addition, antioxidants inhibit ROS production and diminish oxidative stress8. The essential defence role of antioxidants in the human body is via scavenging or regulating the production and elimination of ROS and RNS. The presence of favourable balance between ROS and antioxidants is important for healthy tissues and proper physiological function. It is also well known that the balance between free radicals, antioxidants and co-factors can contribute to the delay of the aging process, reduce the incidence of diseases and thus contributing to a better quality of life. Therefore, the discovery of new potent antioxidants with high efficiency and low toxicity is of a great demand in the field of medicinal chemistry.
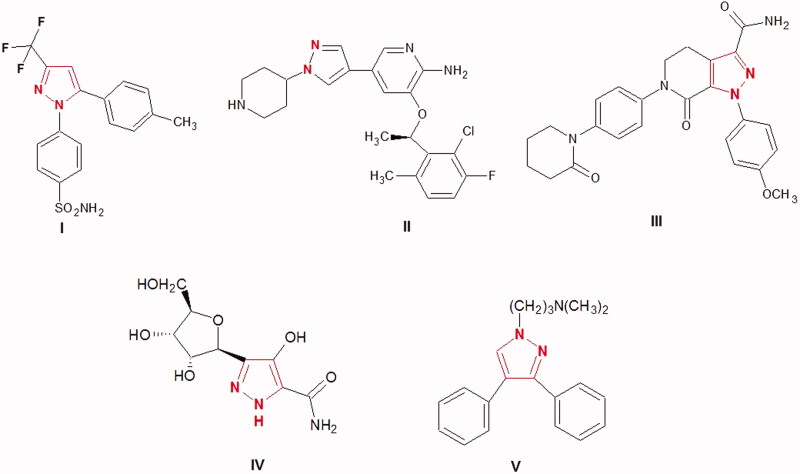
Pyrazole ring is an important scaffold in medicinal chemistry. Pyrazole is a five-membered heterocyclic ring that consists of three carbons and two adjacent nitrogen atoms. Pyrazole derivatives have received considerable attention due to their remarkable broad spectrum of medicinal and pharmacological activities i.e. anticancer9, antiviral10, anti‐tubercular11, anti-microbial12, antimalarial13, anti-inflammatory14, antihypertensive15, anti-Alzheimer’s16, antipsychotic17, and antiparkinsonian18. Various drugs that have pyrazole ring are available in the market with diverse medicinal activities i.e. celecoxib I as anti-inflammatory19, Crizotinib II as anticancer20, Apixaban III as anticoagulant21, Pyrazofurin IV as anticancer, antibiotic22 and Fezolamine V as antidepressant23 (Figure 1).
Figure 1.
Biologically active compounds have pyrazole ring.
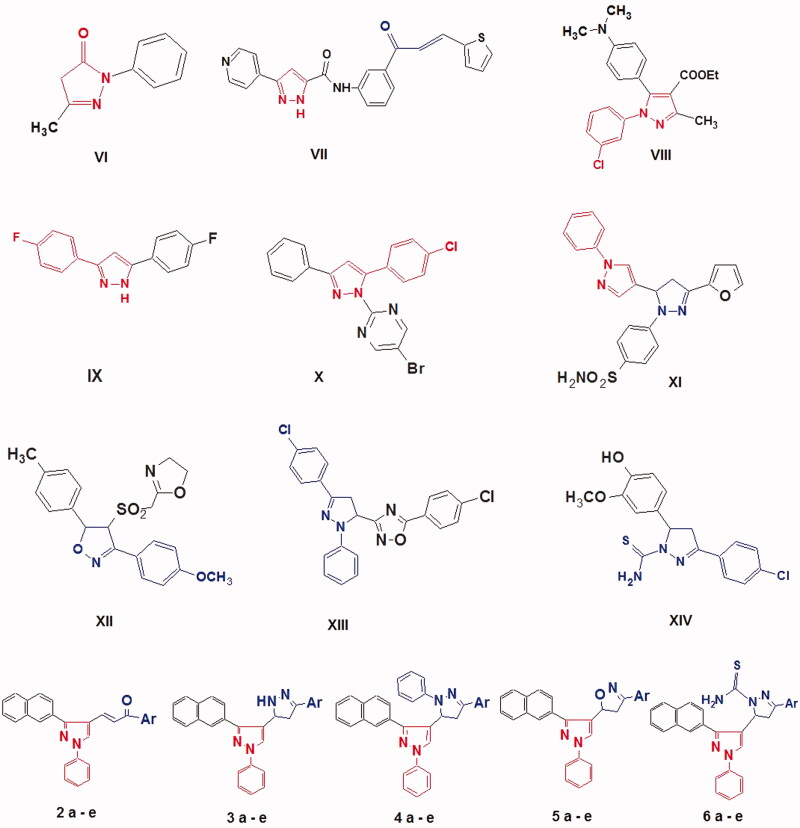
The pyrazole (1,2-diazole) has antioxidant activity and can prevent oxidative stress by increasing antioxidant enzymes, such as GPx, and diminishing the lipid peroxidation process. Examples for the pharmacological effects of 1,2-diazole or its related drugs. 1,2-Diazole was found to be effective in preventing nephrotoxicity caused by the anti-neoplastic drug cisplatin24. Edaravone VI (Figure 2) is a novel antioxidant that has been used for patients in cerebral infarction as support therapy for stroke25,26 and improves ischemia/reperfusion-induced hepatic energy metabolism27.
Figure 2.
Structure of the lead antioxidant pyrazole derivatives and the designed target compounds 2–6.
Recently, the study of pyrazole as a pharmacophore for the development of potential antioxidants has led to the synthesis of several compounds containing pyrazole core in their structures. Among the reported synthetic pyrazoles: 3-(Pyridin-4-yl)-1H-pyrazole-5-carboxamide chalcones VII showed potent radical scavenging activity (RSA) against 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical28, Moreover, in comparison with the standard ascorbic acid, 1,5-diarylpyrazoles VIII showed good DPPH RSA29. It was found that 3,5-diarylpyrazole IX has shown potent RSA as well. The antioxidant activity of pyrazole is attributed to the presence of NH proton of the pyrazole moiety30. In addition, 3,5-diarylpyrazoline derivative X showed excellent RSA using DPPH, .OH, .O2− and NO anion assays, compared to butylated hydroxy toluene (BHT7)31. Derivatives of pyrazole such as bipyrazole XI showed good scavenging activity (19%, BHT7=20%) in the DPPH assay at 10−4 M concentration32. As well as, Bis-isoxazoline XII showed good RSA using DPPH, NO and H2O2 methods in comparison with ascorbic ascid33. Moreover, Pyrazolyl-1,2,4-oxadiazoles XIII possessed potent DPPH RSA34. 4,5-Dihydropyrazole-1-carbothioamide derivative XIV exhibited good antioxidant activity at low concentrations (0.25 mg/mL) in DPPH method35 (Figure 2).
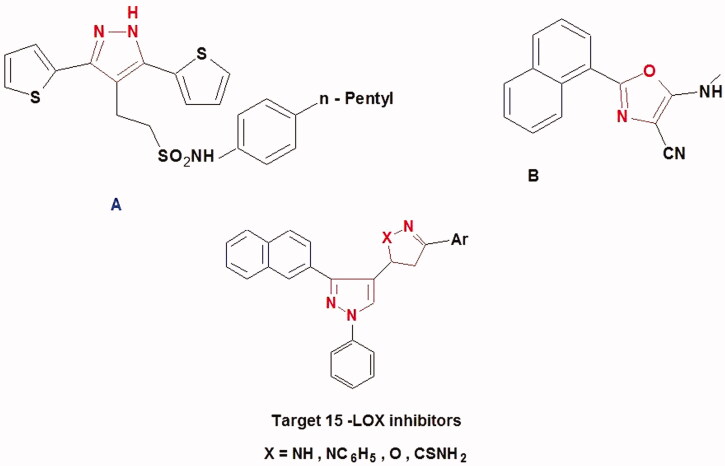
The mechanism of action of antioxidants can be through various pathways such as free radical scavengers (preventive oxidants) and as lipoxygenase inhibitors (pro-oxidative enzymes)36,37. 15-Lipoxygenases (15-LOXes)38,39 are a unique class of non-heme iron containing enzymes that catalyse the peroxidation of polyunsaturated fatty acids such as arachidonic acid (AA) and linoleic acid to their related hydroperoxides. In addition, 15-LOXes are involved in various human diseases. 15-lipoxygenase-1 (15-LOX-1) has been recently documented as a target for reduction of the biosynthesis of eoxines, pro-inflammatory mediator40 and cancer promoter41. Also, it was reported that 15-LOX participates in the oxidative modification of low-density lipoproteins (LDLs) that leads to the progress of atherosclerosis42. Moreover, human 15-LOX-1 is one of the key mediators in neurodegenerative diseases such as Alzheimer’s disease43. There has been some literature work targeting 15-LOX-1. It was reported that 3,4,5-trisubstituted pyrazole (A) was found to work as a potent rabbit 15-LOX-1 inhibitor44. Recently, oxazole derivative (ML351) (B) showed novel 15- LOX inhibition with potent activity against human 15-LOX-1 in both a cellular and an in vivo model of stroke45 (Figure 3).
Figure 3.
Design strategy of new pyrazole hybrid compounds as 15-LOX inhibitors.
In this study, we report the design, synthesis and biological evaluation of a hybrid scaffold in which 3-naphthyl pyrazole is substituted with pyrazoline/isoxazoline ring at position 3 to generate novel and new derivatives of 3-(2-naphthyl)-1-phenyl-1H-pyrazole (Figure 3). These novel hybrid derivatives were tested against 15-LOX enzymatic assay. Moreover, these compounds were evaluated for their potential as antioxidants in DPPH, NO, and superoxide scavenging assays as well as in vivo antioxidant activity using CAT, glutathione (GSH) and lipid peroxidation (MDA) assays. The results of in vitro antioxidant activity of the newly designed hybrids and their 15-LOX inhibitory activity would identify the required antioxidant parameters that are most reliable in the design of 15-LOX inhibitors for the future studies. The structure–activity relationship (SAR) and possible mechanisms of action of these derivatives were also investigated.
2. Materials and methods
2.1. Instruments
Melting points were determined with Electro-thermal IA 9100 apparatus (Shimadzu, Japan) and the values given were uncorrected. Fourier-transform infrared spectroscopy (FT-IR) spectra were recorded as KBr pellets on a Perkin-Elmer 1650 spectrophotometer (USA), Faculty of Science, Cairo University, Cairo, Egypt. Proton nuclear magnetic resonance (1HNMR) and carbon-13 nuclear magnetic resonance (13C-NMR) spectra were recorded in dimethyl sulfoxide-d6 (DMSO-d6) on a Varian Mercury (300 MHz) spectrometer (Varian UK) using TMS as internal standard and chemical shifts were given as ppm (Faculty of Science, Cairo University, Cairo, Egypt). Mass spectra were carried out using 70 eV EI Ms-QP 1000 EX (Shimadzu, Japan), Faculty of Science, Cairo University, and Cairo, Egypt. Microanalyses were performed on Vario, Elementar apparatus (Shimadzu, Japan), Organic Microanalysis Unit, Faculty of Science, Cairo University, Cairo, Egypt and the results were within the accepted range (0.40) of the calculated values. Column Chromatography was performed on (Merck) Silica gel 60 (particle size 0.06–0.20 mm).
2.2. Chemistry
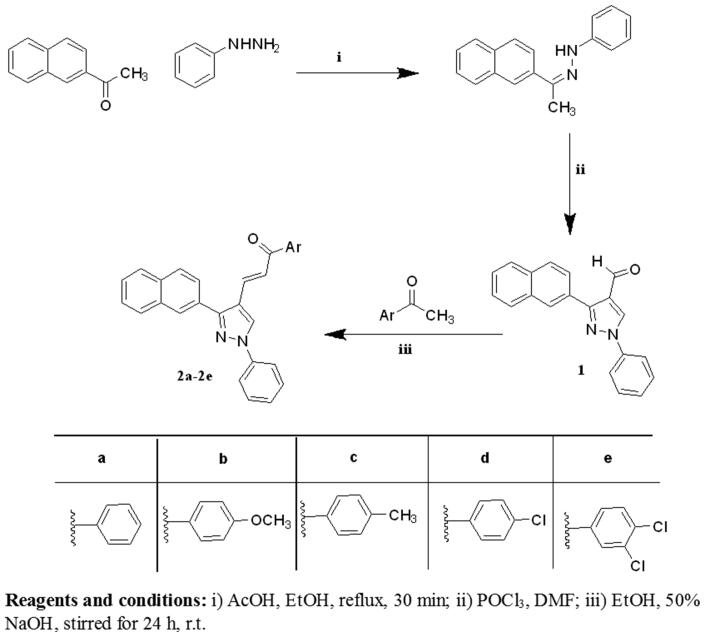
3-(2-Naphthyl)-1-phenyl-1H-pyrazole-4-carbaldehyde (1). The titled compound 1 was synthesized according to the literature procedure46,47. A mixture of β-acetyl naphthalene (0.03 mol) and 0.04 mol of phenyl hydrazine (0.03 mol) in absolute ethanol (50 mL) and few drops of glacial acetic acid were heated on water bath for 30 min. The progress of reaction was monitored by thin-layer chromatography (TLC) using hexane and ethanol (90:10). Cooling the mixture and filtering the formed precipitate that was dried and crystallized from ethanol, a pure phenyl hydrazone was obtained. Pyrazole-4-carbaldehyde was carried out by the application of two moles of cold solution of Vismyeir–Haack (VH) reagent (DMF-POCl3) with the phenyl hydrazone (0.01 mol) in DMF (3 mL). The reaction mixture was stirred at 70–80 °C for 5–6 h. The progress of reaction was monitored by TLC using hexane and ethanol (90:10). The reaction was cooled to room temperature, then poured into cold water and a saturated solution of sodium bicarbonate was added to neutralise the mixture. The white solid obtained was filtered followed by washing with water.
3-(3-Naphthalen-2-yl-1-phenyl-1H-pyrazole-4-yl)-1-aryl propenone (2a–e). A mixture of 4-substituted acetophenone (0.03 mol) and the aldehyde 1 (0.03 mol) in 25 mL 50% alcoholic NaOH solution were stirred at room temperature for 24 h, then the solution was cooled, poured on ice/water acidified with dil. HCl. The produced solid was filtered off, dried and crystallized from ethanol to give compounds 2a–e.
3-(3-Naphthalen-2-yl-1-phenyl-1H-pyrazole-4-yl)-1-phenyl propenone (2a).Yellow solid, yield 81%, m.p.158–159 °C. IR (KBr) vmax (cm−1): 3150 (CH–Ar), 1695 (C=O), 1604 (C=N). 1H NMR (300 MHz, DMSO-d6) δ: 6.5 (d, 1H, J=6.8 Hz, –CH=CH–), 6.6 (d, 1H, J=19.1 Hz, –CH=CH–), 6.8 (s, 1H, pyrazole), 7.1–7.9 (m, 17H, Ar–H).13C NMR (300 MHz, DMSO-d6) δ: 105.0 (pyrazole-C4), 126.0 (pyrazole-C5), 114.7–140.1 (aromatic Cs), 129. 26, 142.8 (CH=CH), 160.0 (pyrazole-C3), 187.0 (C=O). MS (EI): m/z: 400 [M+] (20%). Anal. Calcd for C28H20N2O (400.471): C, 83.98; H, 5.03; N, 7.00; Found: C,83. 77; H, 5.15; N, 6.93.
1-(4-Methoxyphenyl)-3-(3-naphthalen-2-yl-1-phenyl-1H-pyrazole-4-yl)-propenone (2b). Brown solid, yield 85%, m.p.187–188 °C. IR (KBr) vmax (cm−1): 2970 (CH-sp3), 3157 (CH–Ar), 1691 (C=O), 1605 (C=N). 1H NMR (300 MHz, DMSO-d6) δ: 3.3 (s, 3H, OCH3), 7.0 (d, 1H, J=6.5 Hz, –CH=CH–), 7.4 (d, 1H, J=18.1 Hz, –CH=CH–), 6.6 (s, 1H, pyrazole), 7.5-8.55 (m, 16H, Ar–H). 13C NMR (300 MHz, DMSO-d6) δ: 55.87 (OCH3), 105.21 (pyrazole-C4), 126.66 (pyrazole-C5), 113.33–145.0 (aromatic carbons), 129.30, 148.40 (CH=CH), 161.0 (pyrazole-C3), 183.0 (C=O). MS (EI): m/z: 430 [M+] (20%). Anal. Calcd for C29H22N2O2 (430.497): C,80.91; H, 5.15; N, 6.51. Found: C,80.78; H,5.17; N,6.72.
3-(3-Naphthalen-2-yl-1-phenyl-1H-pyrazol-4-yl)-1-p-tolyl propenone (2c). Yellow solid, yield 80%, m.p.146–147 °C. IR (KBr) vmax (cm−1): 2975 (CH-sp3), 3160 (CH–Ar), 1696 (C=O), 1605 (C=N). 1H NMR (300 MHz, DMSO-d6) δ: 2.3 (s, 3H, CH3), 6.5 (d, 1H, J=6.7 Hz, –CH=CH–), 6.7 (d, 1H, J=18.1 Hz, –CH=CH–), 6.8 (s, 1H, pyrazole), 7.0–7.9 (m, 16H, Ar–H). 13C NMR (300 MHz, DMSO-d6) δ: 20.7 (CH3), 105.0 (pyrazole-C4), 126.50 (pyrazole-C5), 112.7–142.1 (aromatic carbons), 126.26, 140.8 (CH=CH), 163.0 (pyrazole-C3), 187.0 (C=O). MS (EI): m/z: 414 [M+] (17.7%). Anal. Calcd for C29H22 N2O (414.49): C,84.03; H, 5.35; N, 6.76; Found: C,84.19; H,5.27; N,6.67.
1-(4-chlorophenyl)-3-(3-naphthalen-2-yl-1-phenyl-1H-pyrazol-4-yl)propenone (2d). Yellow solid, yield 77%, m.p.161–162 °C. IR (KBr) vmax (cm−1): 3157 (CH–Ar), 1692 (C=O), 1655 (C=N). 1H NMR (300 MHz, DMSO-d6) δ: 6.4 (d, 1H, J=6.6 Hz, –CH=CH–), 6.8 (d, 1H, J=17.1 Hz, –CH=CH–), 6.9 (s, 1H, pyrazole), 7.1–7.8 (m, 16H, Ar–H). 13C NMR (300 MHz, DMSO-d6) δ:105.5 (pyrazole-C4), 126.2 (pyrazole-C5), 115.7–145.1 (aromatic carbons), 126.2,141.1 (CH=CH), 160.3 (pyrazole-C3), 187.0 (C=O). MS (EI): m/z: 434 [M+] (20.1%), 436 (M + 2, 6.7%). Anal. Calcd for C28H19ClN2O: (434.916): C, 77.33; H, 4.40; N, 6.44; Found: C,77.29; H, 4.45; N,6.47.
1-(3,4-Dichlorophenyl)-3-(3-naphthalen-2-yl-1-phenyl-1H-pyrazol-4-yl)propenone (2e). Yellow solid, yield 79%, m.p.168-169 °C. IR (KBr) vmax (cm−1): 3156 (CH–Ar), 1691 (C=O), 1603 (C=N). 1H NMR (300 MHz, DMSO-d6) δ: 6.2 (d, 1H, J=6.6 Hz, –CH=CH–), 6.4 (d, 1H, J=18.1 Hz, –CH=CH–), 6.7 (s, 1H, pyrazole), 7.0–7.9 (m,15H,Ar–H). 13 C NMR (300 MHz, DMSO-d6) δ: 105.0 (pyrazole-C4), 126.0 (pyrazole-C5), 113.3–140.4 (aromatic carbons), 126.2,142.2 (CH=CH), 160.2 (pyrazole-C3), 187.0 (C=O). MS(EI): m/z: 469 [M+] (15.3%), 471 (M + 2, 5.1%). Anal. Calcd for C28H18Cl2N2O (469.36): C,71.65; H, 3.87; N, 5.97; Found: C,71.55; H, 3.85; N,5.83.
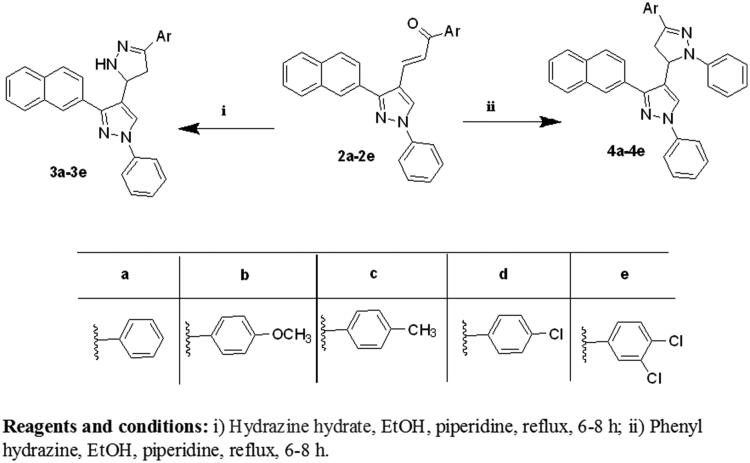
3-Naphthalen-2-yl-5-aryl,1’-phenyl-3,4-dihydro-2H,1H’-[3,4] bipyrazole (3a–e). A solution of (2a–e) (1.0 mmol) and hydrazine hydrate 99% (1.0 mmol) in absolute ethanol (15 mL) was refluxed for 6-8 h. The resulting solution was concentrated, cooled, the solid obtained was filtered off and recrystallized from ethanol to give compounds 3a–e.
3′-Naphthalen-2-yl-5,1′-diphenyl-3,4-dihydro-2H,1’H-[3,4′]bipyrazole (3a). Yellow solid, yield 61%, m.p.172–173 °C. IR (KBr) vmax (cm−1): 2960 (CH-sp3), 3052 (CH–Ar), 3439 (NH), 1593 (C=N). 1H NMR (300 MHz, DMSO-d6) δ: 3.10–3.88 (dd, 2H, pyrazoline -C4-H), 5.26 (t, J = 11.5 Hz, 1H, pyrazoline-C5-H), 6.8 (s,1H,pyrazole),7.0–8.0 (m, 17H, Ar–H), 8.3 (s, 1H, NH-pyrazoline, D2O exchangeable). 13C NMR (300 MHz, DMSO-d6) δ: 39.79 (pyrazoline-C4), 55.87 (pyrazoline-C5), 105.0 (pyrazole-C4), 125.99 (pyrazole-C5), 118.68–145.05 (aromatic carbons), 150.71 (pyrazoline-C3), 158.01 (pyrazole-C3). MS (EI): m/z:414 [M+] (13.7%). Anal. Calcd for C28H22N4 (414.50): C,81.13; H, 5.35; N, 13.52; Found: C,81.17; H,5.23; N,13.43.
5-(4-Methoxyphenyl)-3′-naphthalen-2-yl-1′-phenyl-3,4-dihydro-2H,1’H-[3,4′] bipyrazole (3b). Brown solid, yield 67%, m.p.177–178 °C. IR (KBr) vmax (cm−1): 2965 (CH-sp3), 3163 (CH–Ar), 3356 (NH), 1605 (C=N). 1H NMR (300 MHz, DMSO-d6) δ: 3.22–3.40 (dd, 2H, pyrazoline-C4-H), 3.9 (s, 3H, OCH3), 5.20 (t, J = 11.5 Hz, 1H, pyrazoline-C5-H), 6.7(s, 1H, pyrazole), 6.9–7.9 (m, 16H, Ar–H), 8.0 (s, 1H, NH-pyrazoline, D2O exchangeable). 13C NMR (300 MHz, DMSO-d6) δ: 38.30 (pyrazoline-C4), 55.60 (pyrazoline-C5), 56.0(OCH3), 105.0 (pyrazole-C4), 126.0 (pyrazole-C5), 112.2–141.1 (aromatic carbons), 148.54 (pyrazoline-C3), 159.0 (pyrazole-C3). MS (EI): m/z: 444 [M+] (14.3%). Anal. Calcd for C29H24N4O: (444.53):C,78.36; H, 5.44; N, 12.60; Found: C,78. 33; H,5.43; N,12.70.
3’-Naphthalen-2-yl-1’-phenyl-5-p-tolyl-3,4-dihydro-2H, 1’H -[3,4’]bipyrazole (3c). Yellow crystals, yield 66%, m.p.187-188 °C. IR (KBr) vmax (cm−1): 2963 (CH-sp3), 3164 (CH–Ar), 3357 (NH), 1605 (C=N). 1H NMR (300 MHz, DMSO-d6) δ: 2.5 (s, 3H, CH3), 3.20–3.51 (dd, 2H, pyrazoline-C4-H), 5.3 (t, J = 11.5 Hz, 1H, pyrazoline-C5-H), 6.6 (s, 1H, pyrazole), 7.0-7.9 (m, 16H, Ar–H), 8.0 (s, 1H, NH-pyrazoline, D2O exchangeable). 13C NMR (300 MHz, DMSO-d6) δ: 20.7 (CH3), 36.30 (pyrazoline-C4), 57.50 (pyrazoline-C5), 105.0 (pyrazole-C4), 125.0 (pyrazole-C5), 115.5-145.1 (aromatic carbons), 148.54 (pyrazoline-C3) 160.0 (pyrazole-C3). MS (EI): m/z: 428 [M+] (9.8%). Anal. Calcd for C29H24N4 (428.53): C,81. 28; H, 5.65; N, 13.07; Found: C,81.27; H,5.58; N,13.03.
5-(4-Chlorophenyl)-3′-naphthalen-2-yl-1′-phenyl-3,4-dihydro-2H,1’H-[3,4′] bipyrazole (3d). Yellow crystals, yield 75%, m.p.190–191 °C. IR (KBr) vmax (cm−1): 2979 (CH-sp3), 3173 (CH–Ar), 3354 (NH), 1605 (C=N). 1H NMR (300 MHz, DMSO-d6) δ: 3.2-3.5 (dd, 1H, pyrazoline-C4-H), 5.20 (t, J = 11.6 Hz, 1H, pyrazoline-C5-H), 6.8 (s,1H,pyrazole), 7-7.8 (m, 16H, Ar–H), 8.01 (s, 1H,NH-pyrazoline, D2O exchangeable). 13C NMR (300 MHz, DMSO-d6) δ: 34.30 (pyrazoline-C4), 56.57 (pyrazoline-C5), 105.0 (pyrazole-C4), 127.0 (pyrazole-C5), 117.7–147.1 (aromatic carbons), 148.50 (pyrazoline-C3), 161.0 (pyrazole-C3). MS (EI): m/z: 448 [M+] (11.8%),450(M + 2,4.1%). Anal. Calcd for C28H21ClN4 (448.95):C,74.91; H,4.71; N,12.48; Found: C,74.87; H, 4.80; N,12. 53.
5-(3,4-Dichlorophenyl)-3′-naphthalen-2-yl-1′-phenyl-3,4-dihydro-2H,1’H-[3,4′]-bipyrazole (3e). Yellow solid, yield 77%, m.p.195–196 °C. IR (KBr) vmax (cm−1): 2973 (CH-sp3), 3175 (CH–Ar), 3351 (NH), 1608 (C=N). 1H NMR (300 MHz, DMSO-d6) δ: 3.4 (dd, J=16.5, 11.1 Hz, 1H, pyrazoline-C4-H), 3.2 (dd, J=16.4, 11.2 Hz, 1H, pyrazoline-C4-H), 5.23 (t, J=11.6 Hz, 1H, pyrazoline-C5-H), 6.8 (s, 1H, pyrazole), 7.2–7.8 (m, 15H, Ar–H), 8.0 (s, 1H, NH-pyrazoline, D2Oexchangeable). 13C NMR (300 MHz, DMSO-d6) δ: 38.30 (pyrazoline-C4), 58.50 (pyrazoline-C5), 108.0 (pyrazole-C4), 128.0 (pyrazole-C5), 118.7–140.1 (aromatic carbons), 148.54 (pyrazoline-C3), 158.5 (pyrazole-C3). MS (EI): m/z: 482 [M+] (14.5%), 484 (M + 2, 4.8%). Anal. Calcd for C28H20Cl2N4 (483.39): C,69.57; H, 4.17; N, 11.59; found: C,69.77; H,4.10; N,11.60.
3-Naphthalen-2-yl-5-aryl,2,1’-diphenyl-3,4-dihydro-2H,1H’-[3,4] bipyrazole (4a–e). A solution of (2a–e) (1.0 mmol) and phenyl hydrazine (1.0 mmol) in 25 mL ethanol containing 0.5 mL piperidine was refluxed for 6–8 h. The mixture was cooled, filtered off and recrystallized from ethanol to give compounds 4a–e.
3′-Naphthalen-2-yl-2,5,1′-triphenyl-3,4-dihydro-2H,1’H-[3,4′]bipyrazole (4a). Yellow solid, yield 68%, m.p.187–188 °C. IR (KBr) vmax (cm−1): 2963 (CH-sp3), 3186 (CH–Ar), 1607 (C=N). 1H NMR (300 MHz, DMSO-d6) δ: 3.0–3.4 (dd, 2H, pyrazoline-C4-H), 5.21 (t, J=11.5 Hz, 1H, pyrazoline-C5-H), 6.8 (s, 1H, pyrazole), 6.9-7.7 (m, 22H, Ar–H). 13C NMR (300 MHz, DMSO-d6) δ: 35.2 (pyrazoline-C4), 57.50 (pyrazoline-C5), 106.2 (pyrazole-C4), 129.0 (pyrazole-C5), 116.0-140.0 (aromatic carbons), 148.8 (pyrazoline-C3), 158.8 (pyrazole-C3). MS (EI): m/z: 490 [M+] (9.7%). Anal.Calcd for C34H26N4 (490.6): C, 83.24; H, 5.34; N, 11.42; Found: C,83.47; H,5.33; N,11.49.
5-(4-Methoxyphenyl)-3′-naphthalen-2-yl-2,1′-diphenyl-3,4-dihydro-2H,1’H-[3,4′] bipyrazole (4b). Brown solid, yield 60%, m.p.156–157 °C. IR (KBr) vmax (cm−1): 2967 (CH-sp3), 3187 (CH–Ar), 1600 (C=N). 1H NMR (300 MHz, DMSO-d6) δ: 3.1–3.3 (dd, 2H, pyrazoline-C4-H), 3.8 (s, 3H, OCH3), 5.3 (t, J=11.5 Hz, 1H, pyrazoline-C5-H), 6.6 (s, 1H, pyrazole), 6.8-7.6 (m, 21H, Ar–H). 13C NMR (300 MHz, DMSO-d6) δ: 35.8 (pyrazoline-C4), 57.6 (pyrazoline-C5), 56.0 (OCH3), 106.8 (pyrazole-C4), 129.2 (pyrazole-C5), 116.0 − 140.0 (aromatic carbons), 149.0 (pyrazoline-C3), 159.0 (pyrazole-C3). MS (EI): m/z: 520 [M+] (11.3%). Anal. Calcd for C35H28N4O (520.62): C,80.74; H, 5.42; N, 10.76. Found: C,80.73; H,5.43; N,10.70.
3′-Naphthalen-2-yl-2,1′-diphenyl-5-p-tolyl-3,4-dihydro-2H, 1’H -[3,4′]bipyrazole (4c). Yellow solid, yield 77%, m.p.198–199 °C. IR (KBr) vmax (cm−1): 2923 (CH-sp3), 3052 (CH–Ar), 1595 (C=N). 1H NMR (300 MHz, DMSO-d6) δ: 2.5 (s, 3H, CH3), 3.3–3.6 (dd, 2H, pyrazoline-C4-H), 5.28 (t, J=11.5 Hz, 1H, pyrazoline-C5-H), 6.6 (s, 1H, pyrazole), 6.9–8.2 (m, 21H, Ar–H). 13C NMR (300 MHz, DMSO-d6) δ: 20.3 (CH3), 39.5 (pyrazoline-C4), 55.8 (pyrazoline-C5), 107.1 (pyrazole-C4), 129.5 (pyrazole-C5), 118.6-140.6 (aromatic carbons), 150.0 (pyrazoline-C3), 159.0 (pyrazole-C3). MS(EI): m/z: 504 [M+] (13.5%). Anal. Calcd for C35H28N4(504.62): C,83.30; H, 5.59; N, 11.10; Found: C,83.37; H, 6.00; N,11.01.
5-(4-Chlorophenyl)-3′-naphthalen-2-yl-2,1′-diphenyl-3,4-dihydro-2H,1'H-[3,4′] bipyrazole (4d). Yellow solid, yield 71%, m.p.192-193 °C. IR (KBr) vmax (cm−1): 2978 (CH-sp3), 3174 (CH–Ar), 1605 (C=N). 1H NMR (300 MHz, DMSO-d6) δ: 3.3–3.5 (dd, 1H, pyrazoline-C4-H), 5.27 (t, J=11.6 Hz, 1H, pyrazoline-C5-H), 6.7 (s, 1H, pyrazole), 7-7.8 (m, 21H, Ar–H). 13C NMR (300 MHz, DMSO-d6) δ:35.9 (pyrazoline-C4), 57.8 (pyrazoline-C5), 106.9 (pyrazole-C4), 129.4 (pyrazole-C5), 116.3–141.1 (aromatic carbons), 149.6 (pyrazoline-C3), 159.6 (pyrazole-C3). MS (EI): m/z: 524 [M+] (10.8%), 426 (M + 2, 3.5%). Anal. Calcd for C34H25ClN4(525.04): C,77.78; H, 4.80; N, 10.67; Found: C,77.87; H, 4.82; N,10.53.
5-(3,4-Dichlorophenyl)-3′-naphthalen-2-yl-2,1′-diphenyl-3,4-dihydro-2H,1’H-[3,4′]-bipyrazole (4e). Brown solid, yield 75%, m.p.172–173 °C. IR (KBr) vmax (cm−1): 2974 (CH-sp3), 3176 (CH–Ar), 1605 (C=N). 1H NMR (300 MHz, DMSO-d6) δ: 3.5 (dd, J=16.8, 11.1 Hz, 1H, pyrazoline-C4-H), 3.3 (dd, J=16.6, 11.2 Hz, 1H, pyrazoline-C4-H), 5.26 (t, J=11.6 Hz, 1H, pyrazoline-C5-H), 6.8 (s, 1H, pyrazole), 7–7.8 (m, 20H, Ar–H). 13C NMR (300 MHz, DMSO-d6) δ: 38.80 (pyrazoline-C4), 58.80 (pyrazoline-C5), 108.8 (pyrazole-C4), 128.2(pyrazole-C5), 118.8–140.4 (aromatic carbons), 148.58 (pyrazoline-C3), 158.8 (pyrazole-C3). MS(EI): m/z: 558 [M+] (16.5%), 560 (M + 2, 5.3%). Anal. Calcd for C34H24Cl2N4 (559.48): C,72.99; H, 4.32; N, 10.01; Found: C,72.96; H,4.30; N,10.10.
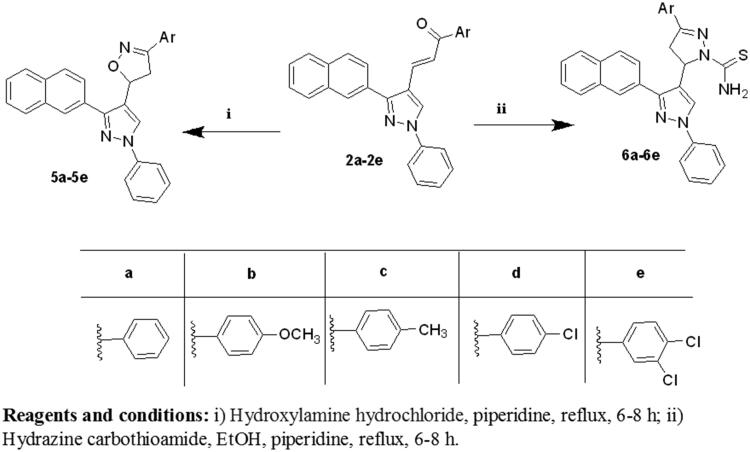
5-(3-Naphthalen-2-yl-1-phenyl-1H-pyrazol-4-yl)-3-aryl-4,5-dihydro-isoxazole (5a–e). A solution (2a–e) (1.0 mmol), and hydroxylamine HCl 99% (1.0 mmol) in absolute ethanol (15 mL) with 0.5 mL piperidine was refluxed for 8–10 h. The resulting solution was concentrated, cooled, the solid obtained was filtered off and recrystallized from ethanol to give compounds 5a–e.
5-(3-Naphthalen-2-yl-1-phenyl-1H-pyrazol-4-yl)-3-phenyl-4,5-dihydro-isoxazole (5a).White solid, yield 81%, m.p.190–191 °C. IR (KBr) vmax (cm−1): 2967 (CH-sp3), 3167 (CH–Ar), 1608 (C=N). 1H NMR (300 MHz, DMSO-d6) δ: 3.60-3.80 (dd, 2H, isoxazoline-C4-H), 5.50 (t, J=11.4 Hz, 1H, isoxazoline-C5-H), 6.5 (s, 1H, pyrazole), 6.8–7.6 (m, 17H, Ar–H). 13C NMR (300 MHz, DMSO-d6) δ: 37.30 (isoxazoline-C4), 56.50 (isoxazoline-C5), 114.0 (pyrazole-C4), 125.0 (pyrazole-C5), 118.0–140.1 (aromatic carbons), 157.0 (pyrazole-C3), 160. 0 (isoxazoline-C3). MS (EI): m/z: 415 [M+] (17.7%). Anal. Calcd for C28H21N3O (415.48): C,80.94; H, 5.09; N, 10.11; Found: C,80.97; H, 5.13; N,10.23.
3-(4-Methoxyphenyl)-5-(3-naphthalen-2-yl-1-phenyl-1H-pyrazol-4-yl)-4,5-dihydro isoxazole (5b). Yellow solid, yield 85%, m.p.197–198 °C. IR (KBr) vmax (cm−1): 2966 (CH-sp3), 3169 (CH–Ar), 1605 (C=N). 1H NMR (300 MHz, DMSO-d6) δ: 3.61-3.90 (dd, 2H, isoxazoline-C4-H), 3.5 (s, 3H, OCH3), 5.40 (t, J=11.5 Hz,1H, isoxazoline-C5-H), 6.6 (s,1H,pyrazole), 7.1-7.9 (m, 16H, Ar–H). 13C NMR (300 MHz, DMSO-d6) δ: 33.30 (isoxazoline-C4), 56.1 (OCH3), 60.0 (isoxazoline-C5), 115.0 (pyrazole-C4), 127.0 (pyrazole-C5), 119.0–141.0 (aromatic carbons), 158.0 (pyrazole-C3), 162.0 (isoxazoline-C3). MS (EI): m/z: 445 [M+] (18.9%). Anal. Calcd for C29H23N3O2: (445.51): C,78.18; H, 5.20; N, 9.43; Found: C,78.27; H,5.14; N,9.33.
5-(3-Naphthalen-2-yl-1-phenyl-1H-pyrazol-4-yl)-3-p-tolyl-4,5-dihydroisoxazole (5c). Yellowish brown solid, yield 77%, m.p.181–182 °C. IR (KBr) vmax (cm−1): 2967 (CH-sp3), 3170 (CH–Ar), 1600 (C=N). 1H NMR (300 MHz, DMSO-d6) δ: 2.5 (s, 3H, CH3), 3.66-3.82 (dd, 2H, isoxazoline -C4-H), 5.55 (t, J=11.4 Hz, 1H, isoxazoline-C5-H), 6.7 (s, 1H, pyrazole), 7.0-7.9 (m, 16H, Ar–H). 13C NMR (300 MHz, DMSO-d6) δ: 20.5 (CH3), 32.30 (isoxazoline-C4), 60.6 (isoxazoline-C5), 115.5 (pyrazole-C4), 127.2 (pyrazole-C5), 118.0-144.0 (aromatic carbons), 157.5 (pyrazole-C3), 160.6 (isoxazoline C3). MS (EI): m/z: 429 [M+] (11.8%). Anal. Calcd for C29H23N3O (429.51): C,81.09; H, 5.40; N, 9.78; Found: C,81.17; H, 5.43; N,9.76.
3-(4-Chlorophenyl)-5-(3-naphthalen-2-yl-1-phenyl-1H-pyrazol-4-yl)-4,5-dihydro isoxazole (5d). White solid, yield 79%, m.p.185–186 °C. IR (KBr) vmax (cm−1): 2916 (CH-sp3), 3046 (CH–Ar), 1594 (C=N). 1H NMR (300 MHz, DMSO-d6) δ: 3.31–3.50 (dd, 1H, isoxazoline-C4-H), 5.56 (t, J=11.6 Hz, 1H, isoxazoline-C5-H), 6.60(s, 1H, pyrazole),7-8 (m, 16H, Ar–H). 13C NMR (300 MHz, DMSO-d6) δ:32.5 (isoxazoline-C4), 60.8 (isoxazoline-C5), 115.6 (pyrazole-C4), 127.1 (pyrazole-C5), 117.6-140.2 (aromatic carbons), 159.7 (pyrazole-C3), 162.8(isoxazoline-C3). MS (EI): m/z: 449 [M+] (12.6%), 451 (M + 2, 4.2%). Anal. Calcd for C28H20ClN3 (449.93): C,74.74; H, 4,48; N, 9.34; Found: C,74.77; H,4.43; N,9.36.
3-(3,4-Dichlorophenyl)-5-(3-naphthalen-2-yl-1-phenyl-1H-pyrazol-4-yl)-4,5-dihydro isoxazole (5e). White solid, yield 79%, m.p.187–189 °C. IR (KBr) vmax (cm−1): 2963 (CH-sp3), 3166 (CH–Ar), 1604 (C=N). 1H NMR (300 MHz, DMSO-d6) δ: 3.69 (dd, J=16.6,11.1 Hz, 1H, isoxazoline-C4-H), 3.82 (dd, J=16.7, 11.2 Hz, 1H, isoxazoline-C4-H), 6.06(t, J=11.6 Hz, 1H, isoxazoline-C5-H), 6.6 (s, 1H, pyrazole), 7.0-7.8 (m, 15H, Ar–H). 13C NMR (300 MHz, DMSO-d6) δ: 32.5 (isoxazoline-C4), 60.3 (isoxazoline-C5), 118.0 (pyrazole-C4), 128.8 (pyrazole-C5), 119.9-143.0 (aromatic carbons), 159.5 (pyrazole-C3), 162.0 (isoxazoline-C3). MS (EI): m/z: 484 [M+] (20.7%),486 (M + 2, 6.9%). Anal. Calcd for C28H19Cl2N3O (484.3): C,69.43; H,3,95; N,8.68. Found:C,69. 47; H,3.93; N,8.66.
3’-Naphthalen-2-yl-5-aryl,1’-phenyl-3,4-dihydro-1’H-[3,4’]bipyrazolyl-2-carbo thioic acid amide (6a–e). To a solution of chalcones 2a–e (1.6 mmol) in absolute ethanol (25 mL), semicarbazide hydrochloride (3.66 mmol) and piperidine (0.5 mL) were added and the solution was refluxed for 9–12 h. The reaction mixture was poured on ice water. The obtained solid was filtered off and recrystallized from ethanol to give compounds 6 a–e.
3′-Naphthalen-2-yl-5,1′-diphenyl-3,4-dihydro-1’H-[3,4′]bipyrazolyl-2-carbothioic acid amide (6a). Yellow solid, yield 65%, m.p.198–199 °C. IR (KBr) vmax (cm−1): 2966 (CH-sp3), 3166 (CH–Ar), 3350 (NH2), 1603 (C=N). 1H NMR (300 MHz, DMSO-d6) δ: 2.9–3.3 (dd, 2H, pyrazoline-C4-H), 5.40 (t, J=11.7 Hz, 1H, pyrazoline-C5-H), 6.7(s,1H, pyrazole), 7–7.7 (m, 17H, Ar–H), 10.1 (s, 2H, NH2, D2O exchangeable). 13C NMR (300 MHz, DMSO-d6) δ: 45.1 (pyrazoline-C4), 55.5 (pyrazoline-C5), 117.1 (pyrazole-C4), 125.1 (pyrazole-C5), 118.5–145.0 (aromatic carbons), 155.0 (pyrazoline-C3), 157.0 (pyrazole-C3), 180.0 (C=S). MS (EI): m/z:473 [M+] (16.7%). Anal. Calcd for C29 H23N5S (473.59): C,73.55; H, 4.90; N, 14.79. Found: C,73.47; H,4.87; N,14.73.
5-(4-Methoxyphenyl)-3′-naphthalen-2-yl-1′-phenyl-3,4-dihydro-1’H-[3,4′] bipyrazolyl-2-carbothioic acid (6b). Yellow solid, yield 67%, m.p.193-194 °C. IR (KBr) vmax (cm−1): 2956 (CH-sp3), 3049 (CH–Ar), 3255 (NH2), 1597 (C=N). 1H NMR (300 MHz, DMSO-d6) δ: 2.8-3.03 (dd, 2H, pyrazoline-C4-H), 3.36 (s, 3H, OCH3), 5.66 (t, J=11.7 Hz, 1H, pyrazoline-C5-H), 6.0 (s, 1H, pyrazole), 6.7–7.6 (m, 16H, Ar–H), 9.66 (s, 2H, NH2, D2Oexchangeable). 13C NMR (300 MHz, DMSO-d6) δ:40.0 (pyrazoline-C4), 55.0 (pyrazoline-C5), 56.0 (OCH3), 113.9 (pyrazole-C4), 126.9 (pyrazole-C5), 118.6–148.4 (aromatic carbons), 155.6 (pyrazoline-C3), 158.3 (pyrazole-C3), 180.0 (C=S). MS(EI): m/z: 503 [M+] (12.8%). Anal. Calcd for C30H25N5OS: (503.62): C,71.55; H, 5.00; N, 13.91. Found: C,71.47; H,4.97; N,13.90.
3′-Naphthalen-2-yl-1′-phenyl-5-p-tolyl-3,4-dihydro-1’H-[3,4′]bipyrazolyl-2-carbo thioic acid amide (6c). Yellow solid, yield 63%, m.p.184–185 °C. IR (KBr) vmax (cm−1): 2968 (CH-sp3), 3161 (CH–Ar), 3307 (NH2), 1606 (C=N). 1H NMR (300 MHz, DMSO-d6) δ: 2.51(s, 3H, CH3), 3.25–3.51 (dd, 2H, pyrazoline-C4-H), 5.43 (t, J=11.8 Hz, 1H, pyrazoline-C5-H), 6.7(s, 1H, pyrazole), 6.9–7.9(m, 16H, Ar–H), 10.5 (s,1H, NH2, D2Oexchangeable). 13C NMR (300 MHz, DMSO-d6) δ: 20.6 (CH3), 45.5 (pyrazoline-C4), 56.6 (pyrazoline-C5), 117.5 (pyrazole-C4), 125.5 (pyrazole-C5), 118.7–144.0 (aromatic carbons), 155.6 (pyrazoline-C3), 157.50 (pyrazole-C3), 180.2 (C=S). MS(EI): m/z: 487 [M+] (16.3%). Anal. Calcd for C30H25N5S (487.62): C,73.89; H, 5.17; N, 14.36. Found: C,73.87; H,5.13; N,14.43.
5-(4-Chlorophenyl)-3′-naphthalen-2-yl-1′-phenyl-3,4-dihydro-1’H-[3,4′] bipyrazo lyl-2-carbothioic acid (6d). Yellow solid, yield 69%, m.p.186–187 °C. IR (KBr) vmax (cm−1): 2963 (CH-sp3), 3162 (CH–Ar), 3310 (NH2), 1606 (C=N). 1H NMR (300 MHz, DMSO-d6) δ: 2.90–3.37 (dd, 1H, pyrazoline-C4-H), 5.62 (t, J=11.4 Hz, 1H, pyrazoline-C5-H), 6.6 (s, 1H, pyrazole), 7.0–7.8 (m, 16H, Ar–H), 10.1 (s, 1H, NH2, D2O exchangeable). 13C NMR (300 MHz, DMSO-d6) δ: 45.1 (pyrazoline-C4), 56.6 (pyrazoline-C5), 117.6.0 (pyrazole-C4), 125.3 (pyrazole-C5), 118.9–143.0 (aromatic carbons), 157.7 (pyrazoline-C3), 160.0 (pyrazole-C3), 180.0 (C=S). MS (EI): m/z: 507 [M+] (20%), 509 (M + 2, 6.7%). Anal. Calcd for C29H22ClN5S (508.3): C,68.56; H, 4.36; N, 13.79. Found: C, 68.57; H,4.33; N,13.63.
5-(3,4-Dichlorophenyl)-3′-naphthalen-2-yl-1′-phenyl-3,4-dihydro-1’H-[3,4′]bi pyrazolyl-2-carbothioic acid (6e). Yellow solid, yield 69%, m.p. 180–181 °C. IR (KBr) vmax (cm−1): 2967 (CH–sp3), 3163 (CH–Ar), 3317 (NH2), 1600 (C=N). 1H NMR (300 MHz, DMSO-d6) δ: 3.01 (dd, J=16.1, 11.2 Hz, 1H, pyrazoline-C4-H), 3.6 (dd, J=16.2, 11.2 Hz, 1H, pyrazoline-C4-H), 5.25 (t, J = 11.5 Hz, 1H, pyrazoline-C5-H), 6.8 (s, 1H, pyrazole), 7.0–7.8 (m, 15H, Ar–H), 10.2 (s, 1H, NH2, D2O exchangeable). 13C NMR (300 MHz, DMSO-d6) δ: 45.3 (pyrazoline-C4), 56.3 (pyrazoline-C5), 117.3.0 (pyrazole-C4), 125.4 (pyrazole-C5), 118.1–142.0 (aromatic carbons), 155.3 (pyrazoline-C3), 157.1 (pyrazole-C3), 180.0 (C=S). MS (EI): m/z: 541 [M+] (22.1%), 543 (M + 2, 7.3%). Anal. Calcd for C29H21Cl2N5S (542.48):C,64.21; H, 3.90; N,12.91. Found: C,64.27; H,3.83; N,12.83.
2.3. In vitro assays for biological antioxidant Activity
Chemicals: All chemicals required for all assays were used as analytical grade, and were purchased from Sigma-Aldrich Chemicals Co., St. Louis, MO, USA.
2.3.1. DPPH scavenging method
The DPPH scavenging activity of all synthesized compounds was measured as previously described by Nahar et al.48 with some modifications. Briefly, 100 µL of different concentrations of the tested compounds (12.5, 25, 50, 100, and 200 µg/mL) were pipetted into a 96-well plate. Then, 100 µL of 100-µM DPPH methanolic solution were added to each well and the plate was incubated protected from light at room temperature for 30 min. The absorbance of the solution was measured at λ517 nm.49 Ascorbic acid was used as the positive control while DMSO was the negative control. The percentage of DPPH scavenging activity was calculated according to the following equation:
where Acontrol is the absorbance of the control reaction (with all reagents except the test compound), and Asample is the absorbance of the test sample. Linear regression analysis was performed to calculate drug concentration showing 50% free radical inhibition activity (IC50). All tests were performed in triplicates.
2.3.2. No scavenging method
The NO scavenging activity of all synthesized compounds was measured as previously described by Ho et al.50 Briefly, 50 µL of different concentrations of the tested compounds (12.5, 25, 50,100, and 200 µg/mL) were pipetted into a 96-well plate. Then, 50 µL of 10-mM sodium nitroprussides dissolved in phosphate-buffered saline PBS (pH 7.4) were added to each well and the plate was incubated for 90 min at room temperature. Next, an equal volume of Griess reagent (1% of sulphanilamide and 0.1% of naphthyl ethylene diamine in 2.5% H3PO3) was added to each well to measure the nitrite content. The absorbance of the formed pink-coloured chromophore was measured at λ 546 nm. DMSO and ascorbic acid were used as the negative and positive control, respectively. All tests were performed in triplicate. The percentage of NO scavenging activity was calculated according to the following equation:
where Acontrol is the absorbance of the control reaction (with all reagents except the test compound), and Asample is the absorbance of the test sample. Linear regression analysis was performed to calculate drug concentration showing 50% free radical inhibition activity (IC50).
2.3.3. Superoxide scavenging assay (O2−.)
The improved pyrogallol autoxidation method was used to determine O2−. RSA of all synthesized compounds as previously described51. Briefly, 50 µL of test compounds (12.5, 25, 50,100, and 200 µg/mL) was added to 2900 µl of 5-mM Tris HCl buffer (0.05 M, pH 7.4) containing 1-mM Na2EDTA. Next, 50 µL of 60-mM pyrogallol in1mM HCl had been thoroughly mixed with the mixture. The absorbance of the reaction mixture was measured at A325 nm every 30 s for 5 min. O2− RSA was expressed by the oxidation degree of a test group in comparison to that of the control. The absorbance at 325 nm was measured against the Tris-HCl buffer every 30 s for 5 min. The percentage of scavenging effect was calculated using the following equation:
where ΔA325 nm, control is the increase in A325 nm of the reaction mixture without the sample and ΔA325 nm, sample is that for the mixture with the sample; T=5 min. The experiments were performed in triplicate. The IC50 value was defined as the concentration for 50% superoxide free radical inhibition and was calculated by linear regression.
2.4. In-vitro lipoxygenase inhibition activity
The assay was performed using Cayman’s lipoxygenase inhibitor screening assay kit (Catalog No. 760700, Cayman Chemical, USA) according to the manufacturer’s instructions. Briefly, 90 µL of 15-LOX was pipetted into a 96-well plate. Next, 10 µL of test compound at concentrations (2.5, 5.0 and 10 µM) dissolved in DMSO were added to each well. The reaction was initiated by adding 10 µL substrate (AA) and the plate was placed on a shaker for at least 5 minutes. Finally, 100 µL of chromogen (prepared according to manufacturer’s instructions) was added to each well to stop enzyme catalysis and develop the reaction. 100 µL of Assay buffer (0.1 M Tris-HCl, pH7.4) was used in blank wells. Quercetin and DMSO were used as the positive control and 100% initial activity, respectively. The absorbance of the solution was measured at λ 490–500 nm. The percentage inhibition was calculated according to the following equation:
where (IA) is the 100% initial activity and (Ainhibitor sample) is the absorbance of the test sample. Dose–response curve was plotted between % inhibition and the drug concentration. The non-linear dose–response curve was used for calculating drug concentration showing 50% enzyme inhibition.
2.5. In vivo biological antioxidant assays
To determine in vivo antioxidant potentials of the test compounds which showed promising in vitro antioxidant activities, CAT activity, GSH and MDA levels were assayed in liver of treated animals.
2.5.1. Animals
The complete progress of the experiment was conducted using male Wistar albino rats (200–250 g), delivered by the Institutional Breeding House, Egypt, reared and maintained in the animal house of the institution. The animals had free access to food and water ad libitum and maintained in a controlled environment under standard conditions of temperature and humidity with an alternating 12 h light and dark cycle for about a week for acclimatization. The protocol of the study was approved by the Animal Ethics Committee of the Faculty of Pharmacy, Helwan University (ethical code number: 05A2019; date: October 2019). The study was conducted in accordance with the EC, directive 86/609/EEC for animal experiments.
2.5.2. Acute oral toxicity study
The acute toxicity study of the selected compounds was performed on albino rats according Organization for Economic Co-operation and development guidelines-42552. The animals were fasted overnight prior to the experiment with free access to water. Selected drugs were administered at doses equal to and half of Ascorbic Acid dose (50 and 100 mg/kg/p.o.), and the behavioural change was observed up to 24 h. The selected compounds were found to be non-toxic in the selected doses. Dose selected for in vivo antioxidant study was 100 mg/kg B.W.
2.5.3. Animal treatment
Rats are weighed at the beginning and at the end of experiment. Fifty-four male albino rats (n=6) were divided into nine different groups. Group I served as a control group and treated with the same volume vehicle only. Group II treated with 100 mg/kg of Ascorbic acid as standard antioxidant drug. Groups (III–IX) orally administered 100 mg/kg of compounds 3a, 4e, 5b, 5c, 6a, 6c, 6e, respectively, for 3 days. The animals were sacrificed by cervical dislocation 24 h after the last dose. Sacrificing is carried out at the same time of the day, to avoid the circadian variation in the level of tissue GSH53. Each liver was excised, weighed, rinsed in ice-cold normal saline and frozen for not more than 72 h await analysis of endogenous antioxidant status (GSH levels and CAT activity) and lipid peroxide concentrations. For performing biochemical assays, a 10% liver homogenate in 10 mM phosphate buffer was prepared using tissue homogeniser (Glas-Col®, Cat no.099C K6424, TERRE HAUTE, USA).
2.5.3.1. CAT activity
Catalase activity in 10% liver homogenates was determined spectrophotometrically according to Sinha AK54. The decrease in absorbance at 240 nm due to H2O2 decomposition was measured and the results were expressed in U/mg tissue.
2.5.3.2. Determination of reduced glutathione
Levels of glutathione (GSH) in liver homogenates were assayed by the deproteinization of tissue homogenate55. Then 200 mL supernatant was mixed with di-potassium hydrogen phosphate buffer (pH 8) and 0.4% 5,5′-dithiobis-2-nitrobenzoic acid (Ellman’s reagent). The yellow-coloured substance formed was measured at 412 nm. The results were expressed as GSH mg/g tissue.
2.5.3.3. Determination of lipid peroxide level
Lipid peroxidation level in the liver homogenates was determined as thiobarbituric acid reactive substances (TBARS) by measuring malondialdehyde ‘MDA’ level according to Mihara and Uchiyama56. Briefly, 0.5 mL supernatant of tissue homogenate was mixed with 0.6% thiobarbituric acid (TBA) and 1% orthophosphoric acid solution, and heated in a boiling water bath for 45 min. The pink-coloured chromogen formed by the reaction of TBA with MDA was extracted by n-butanol and measured at 535 nm. The results were expressed as nmol/g tissue.
2.6. Data presentation and statistical analysis
The data were represented as mean ± SEM. Significant differences between groups were tested by using GraphPad InStat software version 3.05 (GraphPad Inc., La Jolla, CA, USA). Appropriate graphs were plotted when needed using GraphPad Prism version 5 for Windows (GraphPad Inc., USA). The results were analysed using one-way analysis of variance (ANOVA) with post hoc Scheffe’s test. A value of p < 0.05 was considered statistically significant.
2.7. Molecular modelling procedure
The modelling experiment described in this study was performed by using the Discovery Studio (DS) version 4.5 (Accelrys Inc., San Diego, CA, USA) software57. The required pdb coordinates were downloaded from the Brookhaven website (www.rcsb.org). The hydrogen atoms were then added to both the small molecule and the 15-LOX enzyme structure. The atom and bond types as well as the protonation state for the small molecule and the binding site were checked and corrected when needed. Water molecules were deleted. This was followed by minimising the complex with the DS force field using the default parameters. The resulted binding mode of the designed compound in bound to catalytic active site of 15-LOX will be discussed later.
3. Results and discussion
3.1. Chemistry
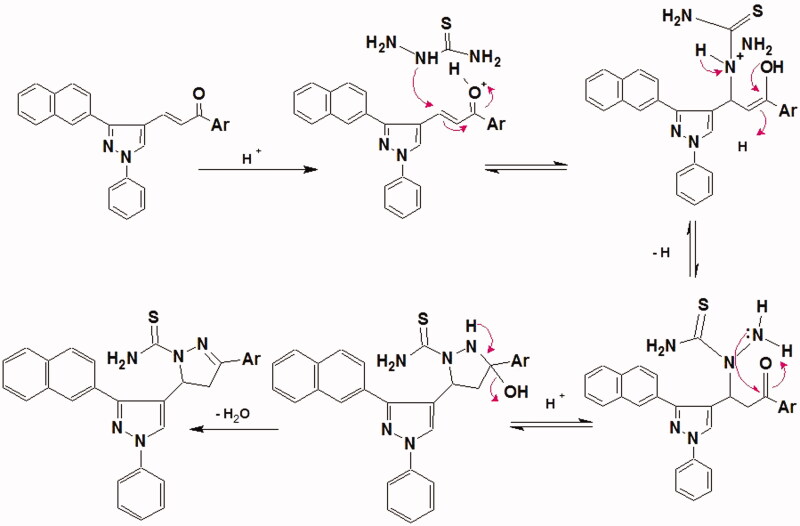
The synthesis of the target compounds (2-6) was depicted in Schemes (1-3). The key starting derivative 3-(2-naphthyl)-1-phenyl-1H-pyrazole-4-carbaldehyde (1) was prepared via Vilsmeier-Haack reaction14 of a phenyl hydrazone, derived from the reaction of β-acetyl naphthalene with phenyl hydrazine, in refluxing absolute ethanol containing few glacial acetic acid followed by the addition of two equivalents of dimethyl formamide and POCl3. Claisen-Schmidt condensation58 of (1) with different aromatic ketones such as 1-phenylethanone, 1-(4-methoxyphenyl)ethanone, 1-(4-methylphenyl)ethanone, 1-(4-chlorophenyl)ethanone and 1-(3,4-dichloro phenyl)ethanone was performed in 30% ethanolic sodium hydroxide solution at room temperature to afford the corresponding chalcones (2a–e), respectively, as outlined in (Scheme 1) . The formed chalcone derivatives (2a–e) was used as key intermediates for synthesizing the target pyrazole-pyrazolines (3) and (4) through 1,4-addition of hydrazine hydrate or phenyl hydrazine to the α, β-unsaturated carbonyl system of the precursor chalcones 2a–e, followed by dehydration and rearrangement. Cyclocondensation of the chalcones (2a–e) with hydrazine hydrate or phenyl hydrazine59–61 in absolute ethanol with catalytic amount of piperidine gave pyrazolines (3a–e) and phenylpyrazolines (4a–e), respectively (Scheme 2). Cyclization of chalcones (2a–e) into the corresponding isoxazolines (5a–e) was conducted by condensation of the chalcones with hydroxylamine hydrochloride62 in ethanol containing a catalytic amount of piperidine to give the target derivatives. In addition, pyrazoline-1-carboxamides (6a–e) were prepared by base-catalysed cyclization of chalcones 2a–e through reaction with semicarbazide HCl63 in absolute ethanol and piperidine (Scheme 3). The reaction mechanism for formation of carbothioamide is via the nucleophilic attack of thiosemicarbazide at β-carbon of the α-β unsaturated C=O of chalcone followed by the proton transfer and intramolecular cyclization of molecule by the nucleophilic attack of NH2 to carbonyl carbon which is stabilized by the proton transfer and further dehydration leads to the formation of pyrazoline (Figure 4).
Scheme 1.
Synthesis of the designed compounds 1 and 2 (a–e).
Scheme 2.
Synthesis of the designed compounds 3 (a–e) and 4 (a–e).
Scheme 3.
Synthesis of the designed compounds 5 (a–e) and 6 (a–e).
Figure 4.
Proposed reaction mechanism for the formation of pyrazole-carbothioamide.
The formed new compounds (2-6) were confirmed by IR, 1HNMR, 13C NMR, mass spectroscopy and microanalysis. The IR spectrum of compounds 2a–e exhibited characteristic bands at around 1690 cm−1 for C=O. 1H NMR spectra showed two doublets signals at δ 6.2–7.4 ppm for CH=CH protons of chalcones. The 1H NMR spectra of compounds 3-6 showed signal doublet of doublet at δ 2.80–3.90 ppm and triplet at δ 5.20-5.66 ppm corresponding to the protons at C-4 and C-5 of the pyrazoline ring in addition to the signals of pyrazole and other protons (CH3, OCH3, and aromatic Hs) . Moreover, the 1H NMR spectra of compounds 3 and 6 showed singlet exchangeable signal at around δ 8 ppm or δ 10 ppm corresponding to (NH) of the pyrazoline or (NH2) of the carboxamide, respectively. 13C NMR showed the characteristic signals at d δ 32.1-38.3 and 55.1-60.6 ppm corresponding to C-4 and C-5 carbon of the pyrazoline ring, respectively. In addition to the other signals for the carbons of the target compounds, The 13C NMR spectra of compounds 2 and 6 showed the presence of signals corresponding to C=O around at δ 187 ppm and C=S at around δ 180 ppm, respectively (cf. experimental part).
3.2. Biological antioxidant studies
This study presents the synthesis and biological evaluation of antioxidant activity of compounds having pyrazole, naphthalene and pyrazoline/isoxazoline pharmacophore. Most of LOX inhibitors show antioxidant or free radical scavenging activities as lipoxygenation occurs via a carbon centred radical64. Thus, all compounds 2–6 (a–e) were investigated for their RSA by DPPH, NO and superoxide assays while Ascorbic Acid (AA) was used as antioxidant reference standard. The in vitro antioxidant activities of tested compounds were expressed as IC50 values (Table 1).
Table 1.
In vitro antioxidant potential and 15-LOX inhibition activity of compounds (2–6).
| Compounds | DPPH IC50a (µg/mL) | NO IC50a (µg/mL) | Superoxide IC50a (µg/mL) | 15-LOX IC50a (µM) |
|---|---|---|---|---|
| 2a | >200 | >200 | >200 | NDb |
| 2b | 182.17 ± 0.99 | 78.25 ± 1.25 | >200 | 3.13 ± 0.09 |
| 2c | 192.70 ± 1.63 | >200 | >200 | 2.80 ± 0.06 |
| 2d | >200 | >200 | >200 | ND |
| 2e | 118.99 ± 1.78 | 11.04 ± 0.72 | >200 | 4.63 ± 0.09 |
| 3a | 13.99 ± 0.78 | 27.65 ± 1.53 | 50.42 ± 1.45 | 2.23 ± 0.07 |
| 3b | 11.70 ± 0.29 | 147.95 ± 1.32 | 118.65 ± 1.03 | 4.60 ± 0.06 |
| 3c | 12.06 ± 1.17 | >200 | >200 | 3.77 ± 0.07 |
| 3d | >200 | >200 | >200 | ND |
| 3e | 9.63 ± 0.55 | 175.72 ± 1.41 | 129.12 ± 0.82 | 2.53 ± 0.07 |
| 4a | 34.39 ± 1.03 | >200 | 176.14 ± 1.63 | 2.53 ± 0.09 |
| 4b | 21.28 ± 1.14 | >200 | 65.63 ± 1.46 | 4.00 ± 0.06 |
| 4c | 24.42 ± 0.9 | 179.9 ± 1.31 | 145.7 ± 1.42 | 1.83 ± 0.07 |
| 4d | >200 | >200 | >200 | ND |
| 4e | 19.56 ± 1.06 | 57.01 ± 1.29 | 130.19 ± 1.1 | 3.53 ± 0.07 |
| 5a | 97.17 ± 1.4 | 37.5 ± 1.36 | 192.37 ± 1.74 | 5.53 ± 0.07 |
| 5b | 46.62 ± 1.63 | 75.53 ± 1.43 | 101.8 ± 1.39 | 4.37 ± 0.09 |
| 5c | 47.27 ± 1.13 | 38.99 ± 1.31 | 44.54 ± 1.44 | 4.23 ± 0.07 |
| 5d | >200 | >200 | >200 | ND |
| 5e | 38.44 ± 1.28 | 96.56 ± 1.4 | 168.77 ± 1.42 | 3.00 ± 0.06 |
| 6a | 20.47 ± 1.43 | 36.37 ± 0.75 | 127.25 ± 1.47 | 1.50 ± 0.06 |
| 6b | 12.02 ± 0.63 | 198.08 ± 1.28 | >200 | 1.90 ± 0.06 |
| 6c | 18.98 ± 1.73 | 23.79 ± 0.83 | 140.17 ± 1.52 | 2.10 ± 0.06 |
| 6d | >200 | 148.08 ± 1.36 | >200 | 1.67 ± 0.03 |
| 6e | 9.66 ± 0.34 | 71.39 ± 1.25 | 87.31 ± 1.58 | 1.57 ± 0.03 |
| Ascorbic acid | 13.67 ± 0.97 | 37.9 ± 1.31 | 124.99 ± 1.32 | 2.5 |
| Quercetin | ND | ND | ND | 3.34 |
IC50 values are expressed as a mean ± SEM of three experiments.
Not determined.
3.2.1. DPPH scavenging activity
Currently, antioxidants showing DPPH scavenging activity are receiving attention due to their role as anticancer, anti-inflammatory and antiaging agents65. Therefore, the antioxidant potential of all novel molecules (2–6) was determined using DPPH radical scavenging assay in comparison with ascorbic acid (AA) as control treatment. The mechanism of radical scavenging is based on acidic H-atom transfer from the compound to the DPPH to form DPPH-H. The results are presented in Table 1. Out of the twenty-five tested pyrazole derivatives, sixteen showed moderate to potent activity, which indicates their radical scavenging and their reducing activities. The rest being less active derivatives .The pyrazolyl pyrazolines (3a-d) and pyrazoline carbothioamides (6a-d) have potent antioxidant activities while pyrazolyl isoxazolines (5a-d) have demonstrated moderate RSA. The most active compounds were 3b, 3c, 3e, 6b and 6e (IC50=11.70 ± 0.29, 12.06 ± 1.17, 9.63 ± 0.55, 12.02 ± 0.63, and 9.66 ± 0.34 μg/mL, respectively). They exhibited potent RSA than ascorbic acid (IC50= 13.67 ± 0.97 μg/mL). Suggesting that, the presence of free NH of pyrazoline enhances the antioxidant activity by increasing their hydrogen donor capacity. Moreover, good radical scavenger property of S atom C=S and free NH2 that act as hydrogen donor in pyrazoline carbothioamides possessed potent DPPH RSA. Compounds 3a, 6a, and 6c (IC50=13.99 ± 0.78, 20.47 ± 1.43 and 18.98 ± 1.73 μg/mL, respectively) displayed good RSA but lower than ascorbic acid. In addition, N-phenyl pyrazolyl pyrazolines 4b (IC50=21.28 ± 1.14 μg/mL), 4c (IC50=24.42 ± 0.9 μg/mL) and 4e (IC50= 19.56 ± 1.06 μg/mL) showed good activity in comparison to standard treatment AA. The lower DPPH RSA of N-phenyl pyrazolyl pyrazolines (4a–e) and pyrazolyl isoxazolines (5a–e) prove the significant role of NH of pyrazoline in antioxidant activity. All compounds (3–6) showed higher antioxidant RSA than their precursors, chalcones (2a–e) thus indicating that pyrazoline and isoxazoline rings enhance RSA of these compounds. SAR studies showed that antioxidant activity of compounds tested by DPPH assay depends not only on the type of heterocyclic pharmacophore but also on substituents R on the aromatic ring of pyrazoline/isooxazoline since antioxidant activity is related to electron or hydrogen donation capacity to DPPH.Radicals (Figure 5). Regarding heterocyclic pharmacophore, the order of free radical scavenging activity (FRSA) were found to be: (3e > 6e> 4e> 5e). Pyrazolines 3 and pyrazoline carbothioamides 6 have higher FRSA than N-phenyl pyrazolines 4 and isoxazolines 5. For compounds 3e and 6e their potent antioxidant activity is due to the presence of pyrazoline and carbothioamide moiety30,66. While the replacement of N atom by O atom gives lower antioxidant activity as shown in isoxazolines 5. Concerning substitution patterns of pyrazolineAQ4/isoxazoline. On the other hand, the order of antioxidant activity of pyrazoline and isoxazoline compounds was found to be: 3,4-(Cl) 2 (3e) > 4-OCH3 (3b) > 4-CH3 (3c) > H (3a) > 4-Cl (3d), in descending order.
Figure 5.
Structure activity relationship of the pyrazole derivatives against DPPH radical scavenging assay.
The di-halogenated compounds exhibited significant DPPH RSA than the corresponding chloro-substituted compounds. Pyrazole derivative 3e and 6e show the most potent antioxidant activity, both having 3,4 (di-Cl) substituents on phenyl ring, which is in accordance with the reported results67. Presence of electron donating groups such as OCH3 and CH3 are more beneficial than unsubstituted or mono chloro-substituted phenyl ring, which may be due to + I and mesomeric effects68. This all indicate that the physicochemical properties of the designed compound impose an important role in the extent of its antioxidant activity. It is notable that the calculated cLog P for these derivatives are high (more lipophilic) compared to the standard treatment. In addition, these derivatives are cyclized heterocyclic analogues with fewer rotatable bonds that make them more favourable for cellular permeability compared to the standard treatment. It was found that the more lipophilic is the compound, the more active it is as 15 LOX-inhibitor. Also, the more electron withdrawal substitutions on the aromatic side chain of the heterocyclic ring, the more antioxidant activity was observed.
3.2.2. No scavenging activity
NO assay was used to determine the scavenging power of the target compounds (2–6) to NO radical. The results are presented in Table 1. Sodium nitroprusside in aqueous solution at physiological pH spontaneously generates Nitrite oxide which interacts with oxygen to produce Nitrite ions, which can be measured at 550 nm by spectrophotometer in the presence of Griess reagent69. Compounds 2e, 6c, 3a and 6a showed NO scavenging activity through competing with oxygen to scavenge for the nitrite radical; higher than that of ascorbic acid (IC50=11.04 ± 0.72, 23.79 ± 0.83, 27.65 ± 1.53 and 36.37 ± 0.75 μg/mL, respectively). Pyrazole 2e was the most potent antioxidant derivative via reducing nitrite production with 3.4 folds that of ascorbic acid, Pyrazolyl isoxazolines 5a, 5c (IC50=37.5 ± 1.36 and 38.99 ± 1.31 μg/mL, respectively) displayed comparable potency to ascorbic acid. Moreover, N-phenyl pyrazolyl pyrazoline 4e displayed good antioxidant activity (IC50=57.01 ± 1.29 μg/mL). SAR studies showed that pyrazoline carbothioamide 6c and pyrazolyl pyrazoline 3a exhibited higher NO scavenging activity than pyrazolyl isoxazoline 5a and N-phenyl pyrazolyl pyrazoline 4e. This confirms that pyrazoline and carbothioamide rings were favourable substitutions over isoxazoline and N-phenyl pyrazoline for the antioxidant activity of the tested compounds against NO assay.
3.2.3. Superoxide scavenging activity
All target compounds (2–6) were evaluated for their antioxidant activity via superoxide scavenging assay to estimate their capability to scavenge O2−. and so, preventing the formation of elemental oxygen. The resulted IC50 values were measured in μg/mL as shown in Table 1. Compounds 5c, 3a, 4b, 6e and 5b (IC50=44.54 ± 1.44, 50.42 ± 1.45, 65.63 ± 1.46, 87.31 ± 1.58 and 101.8 ± 1.39 μg/mL, respectively) possessed pronouncing superoxide scavenging activity than ascorbic acid (IC50=124.99 ± 1.32 μg/mL). Pyrazolyl isoxazoline 5c and pyrazolyl pyrazoline 3a were the most potent derivatives with 2.8 and 2.5 folds of ascorbic acid, respectively. In addition, pyrazolyl pyrazolines 3b and 3e (IC50=118.65 ± 1.03 and 129.12 ± 0.82 μg/mL, respectively), pyrazoline carbothioamide 6a (IC50= 127.25 ± 1.47 μg/mL) displayed comparable O2−. scavenging potency to ascorbic acid. Moreover, pyrazoline carbothioamide 6c (IC50 = 140.17 ± 1.52 μg/mL), N-phenylpyrazoline 4c, 4e (IC50= 145.7 ± 1.42 and 130.19 ± 1.1 μg/mL, respectively) showed good superoxide scavenging activity. SAR studies showed that most of synthesized compounds revealed higher activity than the parent chalcones in superoxide scavenging assay. Pyrazoline 3a, isoxazoline 5c, pyrazoline carbothioamide 6e and N-phenylpyrazoline 4b rings showed a significant RSA towards superoxide radical anion. Di-halogenated compounds displayed good superoxide RSA while, mono halogenated ones didn’t.
3.3. In vitro 15-lipoxygenase inhibition activity
All target compounds (2–6) were tested against Soybean 15-LOX enzyme. The results are expressed as IC50 values (µM) as shown in Table 1. Compounds 2b, 2c, 3a, 3e, 4a, 4c, 5e, 6a, 6b, 6c and 6d showed potential 15-LOX inhibition activity when compared to quercetin (IC50=3.34 µM) as reference inhibitor. Carbothioamides 6a, 6e in which the pyrazoline ring is substituted with phenyl moiety and 3,4-di-Cl phenyl, were the most potent compounds (IC50=1.50 ± 0.06 and 1.57 ± 0.03 µM, respectively) with 2.2 and 2.1 folds that of quercetin, respectively. Pyrazoline 3c and N-phenyl pyrazoline 4e showed comparable potency to that of quercetin (IC50= 3.77 ± 0.07 and 3.53 ± 0.07 µM, respectively). Moreover, pyrazole 2e (IC50=4.63 ± 0.09 µM), pyrazoline 3b (IC50=4.60 ± 0.06 µM), isoxazolines 5a, 5b, and 5c (IC50= 5.53 ± 0.07, 4.37 ± 0.09 and 4.23 ± 0.07 µM, respectively) and N-phenyl pyrazoline 4b (IC50=4.00 ± 0.06 µM) displayed good 15-LOX inhibitory activity but lower than quercetin. The results of the tested compounds (2–6) as 15-LOX inhibitors emphasized the important role of 3-naphthylpyarazole in this enzymatic assay regardless the derivative was either α, β-unsaturated ketone 2c, pyrazoline 3a, N-phenylpyrazoline 4c, isoxazoline 5e or pyrazoline carbothioamide counterpart 6a. Those derivatives were superior to quercetin in 15-LOX inhibition. 15-LOX inhibition appeared to be (6a> 4c >3a> 2c> 5e) to confirm the excel pyrazoline carbothioamide over pyrazoline, N-phenyl pyrazoline and isoxazoline for antioxidant activity of the tested compounds against Soybean 15-LOX enzyme. It was also noticed that di-halogenated derivatives showed significant 15-LOX inhibition activity. This might be due to better fitting of derivative into the catalytic pocket of 15-LOX enzyme. In summary, compounds 3a, 4e, 5b, 5c, 6a, 6c and 6e showed significant RSA in all three methods in comparison with ascorbic acid and 15-LOX inhibition potency using quercetin as standard. This suggests an important influence of EDGs (CH3, OCH3) and di-halogen (di-Cl) in benzene ring. Regarding heterocyclic pharmacophore, pyrazoline carbothioamide and pyrazoline showed higher RSA and 15-LOX inhibition potency than N-phenyl pyrazoline and isoxazoline and these observations should be regarded in the future on the designed LOX inhibitors.
3.4. In vivo estimation of antioxidant activity
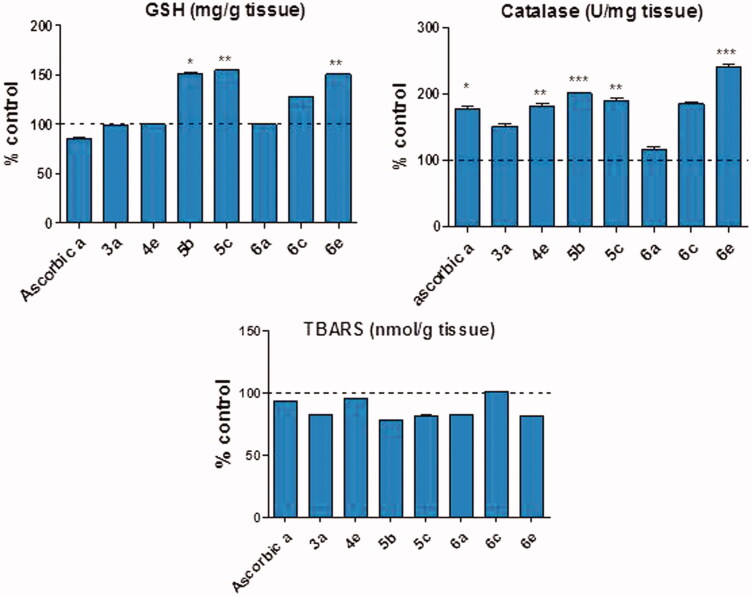
Measuring the in vitro antioxidant ability of the synthetic compounds was not enough to estimate their antioxidant effects in biological systems. In vivo antioxidant assays could reflect the related biological implications of dietary consumption, such as effects on antioxidant enzymes and oxidation-related metabolic pathways. It is well known that lipid peroxidation is a complex process which occurs as a result of the interaction between molecular oxygen and polyunsaturated fatty acids. These free radicals can cause the oxidation of biomolecules (e.g. protein, lipid, and DNA) leading to cell injury and death70. Lipid peroxidation in biological systems can lead to various pathological consequences71. The end products of lipid peroxidation are reactive aldehydes, such as MDA, which are highly toxic to cells72. In addition, the MDA can react with biomolecules and exert cytotoxic, genotoxic, and neurodegenerative disorders. Since, MDA is one of the end products of lipid peroxidation, thus the level of MDA can indicate the degree of lipid peroxidation in the body. In Fact, GSH provides the first line of body defence by scavenging ROS or by acting as a co substrate in the GPx-catalysed reduction of H2O2 and lipid peroxides. Oxidative stress readily oxidises GSH to glutathione disulphide by free radicals and ROS causing depletion of GSH level73. Moreover, the endogenous antioxidant enzymes such as SOD removes the superoxide anion74, while CAT catalyses the reduction of H2O2 and protects the tissues from highly reactive stabilization that may be produced from H2O2. Pyrazole derivatives (3a, 4e, 5b, 5c, 6a, 6c, 6e) that showed promising in vitro antioxidant activities were subjected to in vivo study. In the acute toxicity study, the orally administered compounds did not show toxic effects in doses up to 100 mg/kg B.W. Oral administration of test compounds for 3 days increased CAT activity and GSH level and decreased the MDA concentration in the liver, which indicated that they could enhance the antioxidant status as presented in Table 2 and Figure 6. This validates the potent in vitro antioxidant activity shown by these compounds. However, only compounds (5b, 5c, and 6e) showed significant potent antioxidant activity compared to control group at dose of 100 mg/kg. This may be attributed to the short treatment period of the animals. The results of our study showed that treatment of animals by those compounds significantly increased the level of CAT enzyme by about 101, 89, and 141%, respectively, compared to control group. The increased level of CAT leads to break down of H2O2 and prevent further generation of free radicals. The increase in intracellular thiol-based antioxidant GSH was by about 52, 55, 51%, shown by compounds (5b, 5c, and 6e). The antioxidant activity may be due to potent radical-scavenging activity of isoxazoline and carbothioamide pyrazoline.
Table 2.
In vivo antioxidant potential of compounds.
| Compounds | CAT (U/mg tissue) | GSH (mg/g tissue) | TBARS (nmol/g tissue) |
|---|---|---|---|
| Control | 23.99 ± 3.83 | 7.07 ± 0.50 | 0.422 ± 0.04 |
| Ascorbic a | 42.56 ± 3.76* | 8.25 ± 0.45 | 0.392 ± 0.07 |
| 3a | 36.21 ± 3.49 | 7.00 ± 0.27 | 0.350 ± 0.05 |
| 4e | 43.48 ± 4.51** | 7.05 ± 0.56 | 0.402 ± 0.04 |
| 5b | 48.19 ± 1.24*** | 10.71 ± 0.86* | 0.330 ± 0.02 |
| 5c | 45.42 ± 4.30** | 10.92 ± 0.96** | 0.346 ± 0.01 |
| 6a | 27.97 ± 3.46 | 7.07 ± 0.35 | 0.348 ± 0.03 |
| 6c | 44.38 ± 2.81 | 9.01 ± 0.72 | 0.428 ± 0.02 |
| 6e | 57.84 ± 3.50*** | 10.65 ± 0.97** | 0.344 ± 0.05 |
CAT: catalase; GSH: reduced glutathione; TBARS: thiobarbituric acid reactive substances values are mean ± SEM. *, ** and *** p < 0.05, p < 0.01, and p < 0.001 as compared to control (n = 6).
Figure 6.
Effect of compounds (3a, 4e, 5 b, 5c, 6a, 6c, 6e) and ascorbic acid on the endogenous antioxidant status of rats. GSH: reduced glutathione; TBARS: thiobarbituric acid reactive substances. Data are expressed as mean ± SEM% control. (n = 6). *, **, and *** p < 0.05, p < 0.01, and p < 0.001 compared to control group.
3.5. Molecular modelling
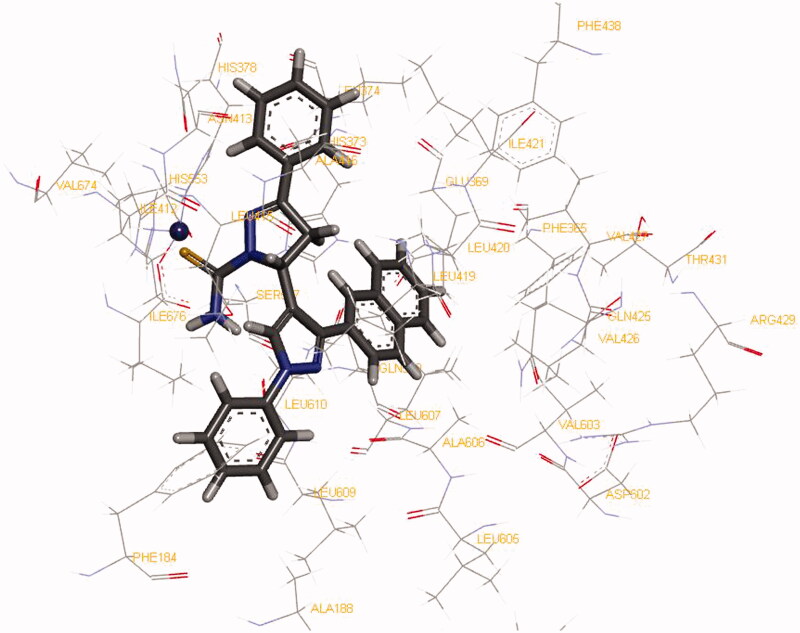
To investigate the orientation of the most potent compound 6a into the active binding site pocket of the human 15-LOX (PDB: 4NRE)75 and to view the inhibitor-receptor interactions, molecular modelling study was performed. All the modelling experiments described here were performed by using the DS version 4.5 (Accelrys Inc., San Diego, CA, USA). The required pdb coordinates were downloaded from the Brookhaven website (www.rcsb.org). The hydrogen atoms were then added to both the small molecule and the protein. The atom and bond types as well as the protonation state for the small molecule and the binding site were checked and corrected when needed. Water molecules were deleted. This was followed by minimizing the complex with the DS force field by using the default parameters. Analysis of the proposed binding of inhibitor 6a (Figure 7) into the catalytic binding site revealed that both naphthalene ring and the close by phenyl ring on the pyrazole group was directed towards the hydrophobic cavity of the active binding pocket making hydrophobic-hydrophobic interactions. In addition, on the other pyrazole group, the S-atom was found to be directed towards the catalytic Fe3+ of the active site and the phenyl ring is making π–cationic interactions with the catalytic Fe3+ of the active site as well as π–π interactions with the His378 amino acid residue. It also was observed that the terminal NH2 group is able is make a weak H-bond (3.7 Å) with the carbonyl of the Ile 676 amino acid residue.
Figure 7.
Molecular modelling of 15-LOX inhibitors 6a (coloured by element), into the active binding site of human 15-LOX (PDB: 4NRE), tagging the protein residues that coordinate with Fe3+ catalytic metal (blue ball) and that interacted with the inhibitors.
4. Conclusions
In summary, novel hybrids containing pyrazole, naphthalene and pyrazoline/isoxazoline pharmacophore were synthesized and investigated for their in vitro antioxidant activity using DPPH, NO and Superoxide radical scavenging assays as well as 15-LOX inhibition activity. One important pathway for antioxidant agents is through inhibiting lipid peroxidation that is mainly catalyzed by 15-LOX. The activity of the compound 6a was assed towards both antioxidant and anti-LOX activities. It was found that compound 6a showed NO scavenging activity higher than that of ascorbic acid. In addition, it showed potent anti-LOX activity by 2.2 folds compared to that of quercetin. Furthermore, compounds 5a and 5c showed comparable potency to ascorbic acid. Moreover, compound 4e displayed good antioxidant activity. SAR studies showed that pyrazoline carbothioamide 6c and pyrazolyl pyrazoline 3a exhibited higher NO scavenging activity than pyrazolyl isoxazoline 5a and N-phenyl pyrazolyl pyrazoline 4e. In addition, compounds 2c, 3a, 3e, 4a, 4c, 5e, 6a, 6b, 6c, 6d, and 6e showed potential 15-LOX inhibition activity which was almost aligned with DPPH assay results only but was conflicted for some compounds such as 2c, 3e, 4a, 4c, 5e, 6b, and 6d with the other antioxidant assays. Interestingly, carbothioamides 6a and 6e were the most potent compounds with 2.2 and 2.1 folds that of quercetin, respectively, and almost showed similar potential antioxidant activity in all three assays. Furthermore, compounds 3a, 4e, 5b, 5c, 6a, 6c, and 6e showed significant RSA in all three in vitro assays in comparison with ascorbic acid along with 15-LOX inhibition potency using quercetin as standard suggesting an important influence of EDGs (CH3, OCH3) and di-halogen (di-Cl) on the benzene ring. Regarding heterocyclic pharmacophore, pyrazoline carbothioamide and pyrazoline showed higher RSA and 15-LOX inhibition potency than N-phenyl pyrazoline and isoxazoline and these observations should be taken in consideration for future designed LOX inhibitors. Furthermore, the in vivo results supported the in vitro data, i.e. compounds 5b, 5c and 6e at dose of 100 mg/kg B.W showed significant in vivo antioxidant activity through increased CAT activity, GSH level and decreased lipid peroxidation in the treated rat liver compared to control treatment. This indicates their role in enhancing the antioxidant status. These data validate the potent in vitro antioxidant activity shown by those derivatives. Docking study of the most potent candidate 6a revealed that stabilization of the ligand inhibitor through hydrophobic-hydrophobic interactions. In addition, π–cationic interactions with the catalytic Fe3+ of the active site as well as π–π interactions with the His378 amino acid residue might be required for the potential activity of 15-LOX inhibitor. In conclusion, the obtained results suggest that these potent compounds may serve as lead candidates for 15-LOX inhibitors. Furthermore, the designed pyrazole hybrid scaffold is an interesting antioxidant pharmacophore and considered as novel lead scaffold for any future optimization.
Acknowledgements
The first author (S.A.A) and the second author (S.M.A) should both be considered as first author for this manuscript. The design, synthesis and molecular modelling parts of this manuscript were performed by S.M.A, A.M.S, and N.M.A. The biology part was performed by S.A.A, S.M and H.T. The authors would like to thank for Mossad Sayed Mohamed (Professor of Pharmaceutical Organic Chemistry) for his inspiration and support.
Disclosure statement
No conflict of interest was reported by the author(s).
References
- 1.Lobo V, Patil A, Phatak A, Chandra N.. Free radicals, antioxidants and functional foods: impact on human health. Pharm Rev 2010;4:118–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu JM, Lin PH, Yao Q, Chen C.. Chemical and molecular mechanisms of antioxidants: experimental approaches and model systems. J Cellular Mol Med 2010;14:840–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh PP, Chandra A, Mahdi F, et al. Reconvene and reconnect the antioxidant hypothesis in human health and disease. Indian J Clin Biochem 2010;25:225–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiernsperger NF. Oxidative stress as a therapeutic target in diabetes: revisiting the controversy. Diabet Metabol 2003;29:579–85. [DOI] [PubMed] [Google Scholar]
- 5.Valko M, Izakovic M, Mazur M, et al. Role of oxygen radicals in DNA damage and cancer incidence. Mol Cellular Biochem 2004;266:37–56. [DOI] [PubMed] [Google Scholar]
- 6.Craft BD, Kerrihard AL, Amarowicz R, Pegg RB.. phenol-based antioxidants and the in vitro methods used for their assessment. Comprehensive Rev Food Sci Food Safety 2012;11:148–73. [Google Scholar]
- 7.Rahman K. Studies on free radicals, antioxidants, and co-factors. Clin Intervent Aging 2007;2:219–36. [PMC free article] [PubMed] [Google Scholar]
- 8.Halliwell B. Biochemistry of oxidative stress. Biochem Soc Trans 2007;35:1147–50. [DOI] [PubMed] [Google Scholar]
- 9.Akhtar MJ, Khan AA, Ali Z, et al. Synthesis of stable benzimidazole derivatives bearing pyrazole as anticancer and EGFR receptor inhibitors. Bioorgan Chem 2018;78:158–69. [DOI] [PubMed] [Google Scholar]
- 10.Farghaly TA, Abdel Hafez NA, Ragab EA, et al. Synthesis, anti-HCV, antioxidant, and peroxynitrite inhibitory activity of fused benzosuberone derivatives. Eur J Med Chem 2010;45:492–500. [DOI] [PubMed] [Google Scholar]
- 11.Maurya HK, Verma R, Alam S, et al. Studies on substituted benzo[h]quinazolines, benzo[g]indazoles, pyrazoles, 2,6-diarylpyridines as anti-tubercular agents. Bioorgan Med Chem Lett 2013;23:5844–9. [DOI] [PubMed] [Google Scholar]
- 12.Sayed GH, Azab ME, Anwer KE, et al. Pyrazole, pyrazolone and enaminonitrile pyrazole derivatives: Synthesis, characterization and potential in corrosion inhibition and antimicrobial applications. J Mol Liquids 2018;252:329–38. [Google Scholar]
- 13.Bekhit AA, Hassan AM, Abd El Razik HA, et al. New heterocyclic hybrids of pyrazole and its bioisosteres: design, synthesis and biological evaluation as dual acting antimalarial-antileishmanial agents. Eur J Med Chem 2015;94:30–44. [DOI] [PubMed] [Google Scholar]
- 14.Taher AT, Mostafa Sarg MT, El-Sayed Ali NR, Hilmy Elnagdi N.. Hilmy Elnagdi N. Design, synthesis, modeling studies and biological screening of novel pyrazole derivatives as potential analgesic and anti-inflammatory agents. Bioorgan Chem 2019;89:103023. [DOI] [PubMed] [Google Scholar]
- 15.Hasui T, Ohyabu N, Ohra T.. Discovery of 6-[5-(4-fluorophenyl)-3-methyl-pyrazol-4-yl]-benzoxazin-3-one derivatives as novel selective nonsteroidal mineralocorticoid receptor antagonists. Bioorgan Med Chem 2014;22:5428–45. [DOI] [PubMed] [Google Scholar]
- 16.Xu Y, Zhang Z, Jiang X, et al. Discovery of delta-sultone-fused pyrazoles for treating Alzheimer’s disease: design, synthesis, biological evaluation and SAR studies. Eur J Med Chem 2019;181:111598. [DOI] [PubMed] [Google Scholar]
- 17.Hoveyda HR, Roy MO, Blanc S, et al. Discovery of 3-aryl-5-acylpiperazinyl-pyrazoles as antagonists to the NK3 receptor. Bioorgan Med Chem Lett 2011;21:1991–6. [DOI] [PubMed] [Google Scholar]
- 18.Jimenez HN, Liu KG, Hong SP, et al. 4-(1-Phenyl-1H-pyrazol-4-yl)quinolines as novel, selective and brain penetrant metabotropic glutamate receptor 4 positive allosteric modulators. Bioorgan Med Chem Lett 2012;22:3235–9. [DOI] [PubMed] [Google Scholar]
- 19.Hassan GS, Abou-Seri SM, Kamel G, Ali MM.. Celecoxib analogs bearing benzofuran moiety as cyclooxygenase-2 inhibitors: design, synthesis and evaluation as potential anti-inflammatory agents. Eur J Med Chem 2014;76:482–93. [DOI] [PubMed] [Google Scholar]
- 20.Sun HY, Ji FQ.. A molecular dynamics investigation on the crizotinib resistance mechanism of C1156Y mutation in ALK. Biochem Biophys Res Commun 2012;423:319–24. [DOI] [PubMed] [Google Scholar]
- 21.Tripodi A, Padovan L, Veena C, et al. How the direct oral anticoagulant apixaban affects thrombin generation parameters. Thrombosis Res 2015;135:1186–90. [DOI] [PubMed] [Google Scholar]
- 22.Wyde PR, Gilbert BE, Ambrose MW.. Comparison of the anti-respiratory syncytial virus activity and toxicity of papaverine hydrochloride and pyrazofurin in vitro and in vivo. Antiviral Res 1989;11:15–26. [DOI] [PubMed] [Google Scholar]
- 23.Luttinger DHD. Antidepressant agents. Annu Rep Med Chem 1987; 22:21–30. [Google Scholar]
- 24.Prabhu VV, Kannan N, Guruvayoorappan C.. 1,2-Diazole prevents cisplatin-induced nephrotoxicity in experimental rats. Pharmacol Rep 2013;65:980–90. [DOI] [PubMed] [Google Scholar]
- 25.Watanabe T, Egawa M.. Effects of an antistroke agent MCl-186 on cerebral arachidonate cascade. J Pharmacol Exp Ther 1994;271:1624–9. [PubMed] [Google Scholar]
- 26.Watanabe T, Yuki S, Egawa M, Nishi H.. Protective effects of MCI-186 on cerebral ischemia: possible involvement of free radical scavenging and antioxidant actions. J Pharmacol Exp Ther 1994;268:1597–604. [PubMed] [Google Scholar]
- 27.Okatani Y, Wakatsuki A, Enzan H, Miyahara Y.. Edaravone protects against ischemia/reperfusion-induced oxidative damage to mitochondria in rat liver. Eur J Pharmacol 2003;465:163–70. [DOI] [PubMed] [Google Scholar]
- 28.Sribalan R, Banuppriya G, Kirubavathi M, et al. Multiple biological activities and molecular docking studies of newly synthesized 3-(pyridin-4-yl)-1H-pyrazole-5-carboxamide chalcone hybrids. Bioorgan Med Chem Lett 2016;26:5624–30. [DOI] [PubMed] [Google Scholar]
- 29.Achutha D, Hamse V, Ningappa M, et al. Synthesis and in vitro biological evaluation for antioxidant, anti-inflammatory activities and molecular docking studies of novel pyrazole derivatives. Biointer Res Appl Chem 2017;7:2040–7. [Google Scholar]
- 30.Samshuddin S, Narayana B, Sarojini BK, et al. Synthesis, characterization and biological evaluation of some pyrazoles derived from α, β-dibromo 4, 4’-difluoro chalcone. Der Pharm Chem 2012;4:587–92. [Google Scholar]
- 31.Adhikari A, Kalluraya B, Sujith KV, et al. Synthesis, characterization and pharmacological study of 4,5-dihydropyrazolines carrying pyrimidine moiety. Eur J Med Chem 2012;55:467–74. [DOI] [PubMed] [Google Scholar]
- 32.Faidallah HM, Rostom SA, Khan KA.. Synthesis and biological evaluation of pyrazole chalcones and derived bipyrazoles as anti-inflammatory and antioxidant agents. Arch Pharm Res 2015;38:203–15. [DOI] [PubMed] [Google Scholar]
- 33.Mallikarjuna Reddy G, Muralikrishna A, Padmavathi V, et al. Synthesis and antioxidant activity of styrylsulfonylmethyl 1,3,4-oxadiazoles, pyrazolyl/isoxazolyl-1,3,4-oxadiazoles. Chem Pharm Bull 2013;61:1291–7. [DOI] [PubMed] [Google Scholar]
- 34.Ningaiah S, Bhadraiah UK, Keshavamurthy S, Javarasetty C.. Novel pyrazoline amidoxime and their 1,2,4-oxadiazole analogues: synthesis and pharmacological screening. Bioorgan Med Chem Lett 2013;23:4532–9. [DOI] [PubMed] [Google Scholar]
- 35.Hamada NM, Abdo NY.. Synthesis, characterization, antimicrobial screening and free-radical scavenging activity of some novel substituted pyrazoles. Molecules 2015;20:10468–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heim KE, Tagliaferro AR, Bobilya DJ.. Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J Nutr Biochem 2002;13:572–84. [DOI] [PubMed] [Google Scholar]
- 37.Pokorný J. Are natural antioxidants better – and safer – than synthetic antioxidants?. Eur J Lipid Sci Technol 2007;109:883. [Google Scholar]
- 38.Brash AR. Lipoxygenases: occurrence, functions, catalysis, and acquisition of substrate. J Biol Chem 1999;274:23679–23682. [DOI] [PubMed] [Google Scholar]
- 39.Pontiki E, Hadjipavlou-Litina D.. Lipoxygenases (LOs): an heterogenous family of lipid peroxidizing enzymes implicated in cell differentiation, inflammation, asthma, carcinogenesis, atherogenesis-an interesting target for the development of promising drugs. Curr Enzyme Inhibit 2005;1:309–327. [Google Scholar]
- 40.Feltenmark S, Gautam N, Brunnstrom A, et al. Eoxins are proinflammatory arachidonic acid metabolites produced via the 15-lipoxygenase-1 pathway in human eosinophils and mast cells. Proc Natl Acad Sci USA 2008;105:680–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kelavkar U, Lin Y, Landsittel D, et al. The yin and yang of 15-lipoxygenase-1 and delta-desaturases: dietary omega-6 linoleic acid metabolic pathway in prostate. J Carcinogenesis 2006;5:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bocan TMA, Rosebury WS, Mueller SB, et al. A specific 15-lipoxygenase inhibitor limits the progression and monocyte-macrophage enrichment of hypercholesterolemia-induced atherosclerosis in the rabbit. Atherosclerosis 1998;136:203–216. [DOI] [PubMed] [Google Scholar]
- 43.Pratico D, Zhukareva V, Yao Y, et al. 12/15-lipoxygenase is increased in Alzheimer’s disease: possible involvement in brain oxidative stress. Am J Pathol 2004;164:1655–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ngu K, Weinstein DS, Liu W, et al. Pyrazole-based sulfonamide and sulfamides as potent inhibitors of mammalian 15-lipoxygenase. Bioorgan Med Chem Lett 2011;21:4141–4145. [DOI] [PubMed] [Google Scholar]
- 45.Rai G, Joshi N, Jung JE, et al. Potent and selective inhibitors of human reticulocyte 12/15-lipoxygenase as anti-stroke therapies. J Med Chem 2014;57:4035–4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arbačiauskienė E, Martynaitis V, Krikštolaitytė S, et al. Synthesis of 3-substituted 1-phenyl-1H-pyrazole-4-carbaldehydes and the corresponding ethanones by Pd-catalysed cross-coupling reactions. Arkivoc 2011;11:1–21. [Google Scholar]
- 47.Vovk MV, Mel'nichenko NV, Chornous VA, Bratenko MK.. Intramolecular cyclization of 4-isocyanato-3-(2-naphthyl)-1-phenylpyrazole under Friedel-crafts reaction conditions. Chem Heterocyc Comp 2002;38:1096–1097. [Google Scholar]
- 48.Nahar L, Russell WR, Middleton M, et al. Antioxidant phenylacetic acid derivatives from the seeds of Ilex aquifolium. Acta Pharm 2005;55:187–193. [PubMed] [Google Scholar]
- 49.Padmaja A, Rajasekhar C, Muralikrishna A, Padmavathi V.. Synthesis and antioxidant activity of oxazolyl/thiazolylsulfonylmethyl pyrazoles and isoxazoles. Eur J Med Chem 2011;46:5034–5038. [DOI] [PubMed] [Google Scholar]
- 50.Ho S-C, Tang Y-L, Lin S-M, Liew Y-F.. Evaluation of peroxynitrite-scavenging capacities of several commonly used fresh spices. Food Chem 2010;119:1102–1107. [Google Scholar]
- 51.Li X. Improved pyrogallol autoxidation method: a reliable and cheap superoxide-scavenging assay suitable for all antioxidants. J Agric Food Chem 2012;60:6418–6424. [DOI] [PubMed] [Google Scholar]
- 52.OECD , Test No. 425; Acute Oral Toxicity:Up- and Down-Procedure, OECD Guidelines for the Testing of Chemicals, Section 4, Paris: OECD Publishing, 2008. 10.1787/9789264071049-en [DOI]
- 53.Alam MN, Bristi NJ, Rafiquzzaman M.. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm J 2013;21:143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sinha AK. Colorimetric assay of catalase. Anal Biochem 1972;47:389–394. [DOI] [PubMed] [Google Scholar]
- 55.Beutler E, Duron O, Kelly BM.. Improved method for the determination of blood glutathione. J Labor Clin Med 1963;61:882–888. [PubMed] [Google Scholar]
- 56.Mihara M, Uchiyama M.. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem 1978;86:271–278. [DOI] [PubMed] [Google Scholar]
- 57.Awad SM, Zohny YM, Ali SA, Mahgoub S, et al. Design, synthesis, molecular modeling, and biological evaluation of novel thiouracil derivatives as potential antithyroid agents. Molecules 2018;23:2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kucerova-Chlupacova M, Dosedel M, Kunes J, et al. Chalcones and their pyrazine analogs: synthesis, inhibition of aldose reductase, antioxidant activity, and molecular docking study. Monat Für Chem 2018;149:921–929. [Google Scholar]
- 59.Ahmed NM, Youns M, Soltan MK, Said AM.. Design, synthesis, molecular modelling, and biological evaluation of novel substituted pyrimidine derivatives as potential anticancer agents for hepatocellular carcinoma. J Enzyme Inhibit Med Chem 2019;34:1110–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abd-Rabou AA, Abdel-Wahab BF, Bekheit MS.. Synthesis, molecular docking, and evaluation of novel bivalent pyrazolinyl-1,2,3-triazoles as potential VEGFR TK inhibitors and anti-cancer agents. Chem Papers 2018;72:2225–2237. [Google Scholar]
- 61.Wei Q, Ning J-Y, Dai X, et al. Discovery of novel HSP90 inhibitors that induced apoptosis and impaired autophagic flux in A549 lung cancer cells. Eur J Med Chem 2018;145:551–558. [DOI] [PubMed] [Google Scholar]
- 62.El-Desoky EI, Keshk EM, El-Sawi AA, et al. Synthesis, biological evaluation and in silico molecular docking of novel 1-hydroxy-naphthyl substituted heterocycles. Saudi Pharmaceutical J 2018;26:852–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Saueressig S, Tessmann J, Mastelari R, et al. Synergistic effect of pyrazoles derivatives and doxorubicin in claudin-low breast cancer subtype. Biomed Pharmacother 2018;98:390–398. [DOI] [PubMed] [Google Scholar]
- 64.Muller K. 5-Lipoxygenase and 12-lipoxygenase: attractive targets for the development of novel antipsoriatic drugs. Arch Der Pharm 1994;327:3–19. [DOI] [PubMed] [Google Scholar]
- 65.Kontogiorgis C, Litinas KE, Makri A, et al. Synthesis and biological evaluation of novel angular fused pyrrolocoumarins. J Enzyme Inhibit Med Chem 2008;23:43–49. [DOI] [PubMed] [Google Scholar]
- 66.Khalil NA, Ahmed EM, El-Nassan HB, et al. Synthesis and biological evaluation of novel pyrazoline derivatives as anti-inflammatory and antioxidant agents. Arch Pharm Res 2012;35:995–1002. [DOI] [PubMed] [Google Scholar]
- 67.Lobo PL, Poojary B, Kumsi M, et al. Synthesis, antimicrobial and antioxidant activities of 2-[1-{3,5-diaryl-4,5-dihydro-1H-pyrazolenyl}]-4-(4-nitrophenyl)-[1,3]-thiazoles. Med Chem Res 2013;22:1689–1699. [Google Scholar]
- 68.Lavanya G, Prakash TB, Sravya G, et al. Synthesis and antioxidant activity of bis unsaturated sulfones, bis pyrroles, and bis pyrazoles. Res Chem Interm 2015;41:8815–8828. [Google Scholar]
- 69.Kumar S, Kumar D, Manjusha Saroha K, et al. Antioxidant and free radical scavenging potential of Citrullus colocynthis (L.) Schrad. methanolic fruit extract. Acta Pharm 2008;58:215–220. [DOI] [PubMed] [Google Scholar]
- 70.McCord JM. The evolution of free radicals and oxidative stress. Am J Med 2000;108:652–659. [DOI] [PubMed] [Google Scholar]
- 71.Hochstein P, Atallah AS.. The nature of oxidants and antioxidant systems in the inhibition of mutation and cancer. Mut Res 1988;202:363–375. [DOI] [PubMed] [Google Scholar]
- 72.Yu BP, Yang R.. Critical evaluation of the free radical theory of aging. A proposal for the oxidative stress hypothesis. Ann New York Acad Sci 1996;786:1–11. [DOI] [PubMed] [Google Scholar]
- 73.Wu G, Fang YZ, Yang S, et al. Glutathione metabolism and its implications for health. J Nutr 2004;134:489–492. [DOI] [PubMed] [Google Scholar]
- 74.Teixeira HD, Schumacher RI, Meneghini R.. Lower intracellular hydrogen peroxide levels in cells overexpressing CuZn-superoxide dismutase. Proc Natl Acad Sci U.S.A. 1998;95:7872–7875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kobe MJ, Neau DB, Mitchell CE, et al. The structure of human 15-lipoxygenase-2 with a substrate mimic. The J Biol Chem 2014;289:8562–8569. [DOI] [PMC free article] [PubMed] [Google Scholar]