ABSTRACT
Fish-borne liver and intestinal flukes are helminth pathogens that have a negative impact on public health worldwide. We herein investigated the status of infection by the metacercariae (MC) of fish-borne trematodes (FBTs) in randomly selected freshwater wild fishes. Five species of fishes were collected and digested artificially using digestion fluid to recover MC. All fish species, namely, ticto barb (Puntius ticto) (14/16, 87.5%), banded gourami (Colisa fasciata) (8/12, 66.7%), freshwater garfish (Xenentodon cancila) (9/14, 64.3%), flying barb (Esomus danricus) (5/12, 41.7%), and reba carp (Cirrhinus reba) (7/11, 63.7%), were infected with FBTs. The overall infection rate was 66.2% and the mean intensity was 748.3 ± 2947.5 MC/100 g of fishes. The loads of MC in ticto barb, reba carp, freshwater garfish, banded gourami, and flying barb per 100 g of fishes were 1978.8 ± 5053.7, 268.3 ± 440.7, 140 ± 105.4, 134.3 ± 109.2, and 117.6 ± 102.3, respectively. The infection rate was significantly higher (P < 0.05) in the body (55.4%) than in the head (40%) of fishes. Morphological and morphometrical analyzes identified the MC of Clonorchis spp., Opisthorchis spp., Metorchis spp., Metagonimus spp., and Echinostoma spp. Collectively, the present results suggest that wild freshwater fishes are important intermediate hosts for FBTs, and play a critical role in the transmission cycle of these parasites in Bangladesh. The results also indicate that people of the country are at risk of these infections.
KEYWORDS: Fish-borne trematodes, wild fishes, metacercariae, Clonorchis, Opisthorchis, Metagonimus, Metorchis, Echinostoma
1. Introduction
Infections by fish-borne trematodes (FBTs) affect human and animal health worldwide, particularly in Asian countries [1]. FBTs are an emerging and rapidly growing concern in developing and developed countries due to the expanding international trade in fishes and fish products, as well as human demographic diversity, especially mass refugee movement and global settlement, including in Bangladesh [2,3]. Recently, a total of 59 species of FBTs of public health importance have been identified. These trematodes have been divided into two groups: liver flukes (Opisthorchidae: 12 species) and intestinal flukes (Heterophyidae: 36 species, Echonostomatidae: 10 species and Nanophyetidae: 1 species) [4]. FBTs have a complex lifecycle, with a series of developmental changes in freshwater snails in which they produce cercariae, the freely swimming larval stage of trematodes. These cercariae emerge from snails into water and encyst in freshwater fishes, notably cyprinoid fishes, in which they develop into metacercariae (MC), the infective stage of trematodes for mammals and birds [5]. FBT infections are more common in wild fishes, which have a great public health concern [4]. Humans become infected through the consumption of raw or improperly cooked fishes containing viable MC [4,6].
More than 45 million people are affected globally only with opisthorchiasis, diseases caused by opisthorchid flukes. Of the opisthorchid flukes, Clonorchis sinensis, the most important liver fluke, affects 15 million people only in East Asia, including 13 million in China and 1 million in northern Vietnam [7,8]. Historically, although C. sinensis infection was highly endemic in Japan, only a few cases have recently been reported; however, approximately 1.2 million individuals are infected in South Korea [8]. On the other hand, 8.6 million people have been infected with Opisthorchis viverrini in Southeast Asian countries, approximately 6 million of whom were in Thailand [9]. Additionally, 1.6 million individuals are infected with O. felineus globally, including 1.5 million in the former Union of Soviet Socialist Republics [10]. According to Nguyen et al. [4], more than 750 million individuals are at risk to fish borne liver flukes throughout Asia. Furthermore, 40–50 million individuals have been affected with one or more species of fish-borne intestinal flukes and approximately half a billion individuals are at risk globally [11]. In a recent study, the disability-adjusted life years (DALYs) of FBTs had been reported to be 1.8 million, and estimated to have increased by 8.5% within 2007–2017 [12]. FBTs, particularly liver flukes, have been implicated in biliary tract obstruction, bile flux block, and icterus [13]. Infections by intestinal flukes cause fatigue and mild gastrointestinal symptoms, such as epigastric pain, anorexia, and diarrhea, however, in severe infections they cause abdominal cramps, malabsorption, and weight loss [14].
Despite advances in the development of aquaculture in Bangladesh in recent years, small wild freshwater fishes are in high demand and they are used as a delicacy from villages to large cities, and even among people living abroad. These wild fishes generally live in natural water bodies such as rivers and low lying marshy lands, which are inhabited by many intermediate host snails. Reservoir hosts of FBTs, such as street dogs, cats, foxes, and jackals, also inhabit areas alongside these natural water bodies. About half a century ago, FBTs, such as C. sinensis and O. felineus, were detected in street dogs and wild carnivores in Bangladesh [15]. Moreover, Metorchis orientalis, a zoonotic liver fluke affecting humans, carnivores, and aquatic birds, was also found in the livers of ducks [16], indicating that wild fishes in Bangladesh are infected with the infective stage of FBTs. However, despite being of enormous importance, these parasites have not yet been investigated in fishes in Bangladesh. Therefore, we herein isolated and identified the MC of different FBTs in common wild freshwater fishes marketed in Bangladesh for human consumption.
2. Materials and methods
2.1. Study area
Two districts, Mymensingh (24°45ʹ22.90”N and 90°24ʹ23.26”E) and Kishoreganj (24°45ʹ83”N and 90°88ʹ33”E) (Figure 1), were selected because they are rich in natural water bodies and are regarded as the hub of indigenous fishes in Bangladesh.
Figure 1.
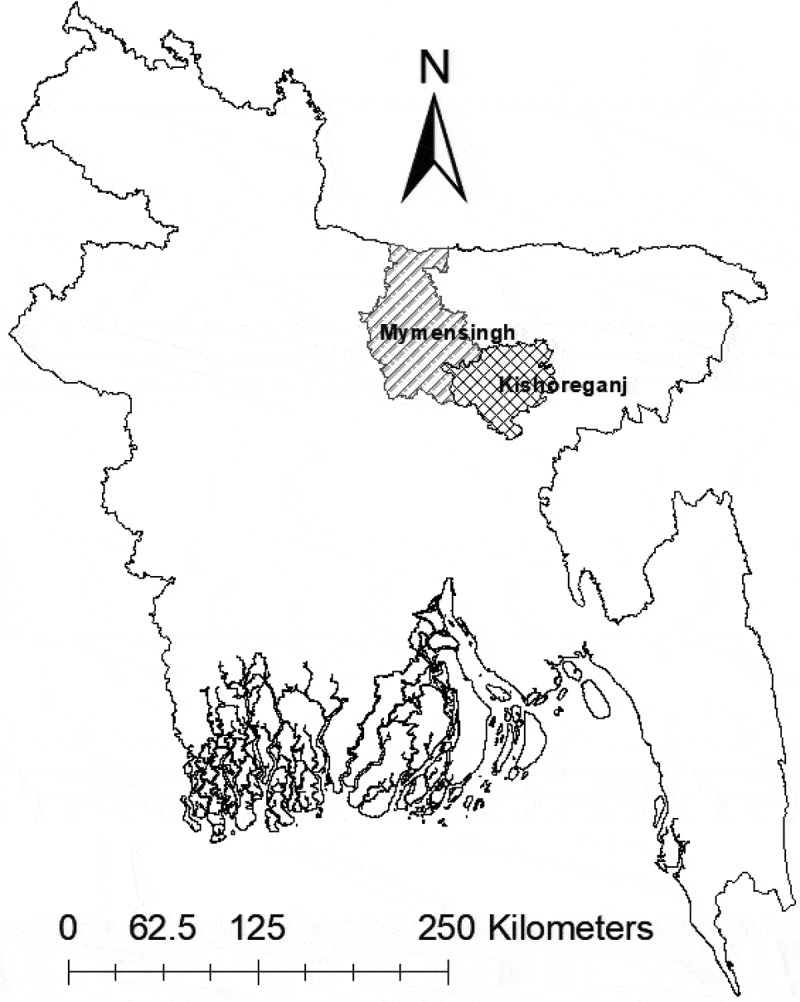
Map of Bangladesh showing study areas. The locations selected were two of the most important areas that produce wild fishes.
2.2. Sampling of fishes
Varieties of small wild freshwater fishes were collected from local markets in the study areas. A total of 14 kg of fishes, belonging to five species, were collected and examined. Fishes were identified as described previously [17,18], and the identified species were ticto barb (Puntius ticto), flying barb (Esomus danricus), banded gourami (Colisa fasciata), freshwater garfish (Xenentodon cancila), and reba carp (Cirrhinus reba). Detail is in the Table 1. During collection, the sources of fishes were confirmed by fishermen. After collection, samples were preserved in ice and transported to the laboratory.
Table 1.
Size, weight and amount of different species of fishes used.
| Common name/species of fishes | Length (cm) |
Weight (g) |
Amount used (g) | ||
|---|---|---|---|---|---|
| Ranges | Mean±SD | Ranges | Mean±SD | ||
| Ticto barb (Puntius ticto) |
2.20–9.4 | 6.63 ± 1.41 | 1.54–11.66 | 4.61 ± 2.99 | 3,415 |
| Banded gourami (Colisa fasciata) |
2.10–9.3 | 7.95 ± 0.68 | 5.22–15.15 | 9.71 ± 2.34 | 2,037 |
| Freshwater garfish (Xenentodon cancila) |
6.55–22.0 | 17.59 ± 2.64 | 5.18–17.75 | 10.51 ± 3.91 | 2,576 |
| Flying barb (Esomus danricus) |
1.53–5.6 | 3.88 ± 0.61 | 0.24–1.5 | 0.47 ± 0.28 | 3,078 |
| Reba carp (Cirrhinus reba) |
2.4–14.3 | 9.72 ± 4.21 | 3.25–20.98 | 12.79 ± 5.95 | 2,894 |
| Total weight | 14,000 | ||||
2.3. Fish processing and preparation
Different species of fishes were weighed and used immediately for the detection of MC or kept separately in polythene bags at −20 ºC until examined within one week. Pooled samples were used, with each sample consisting of 150–200 g of fishes of the same species. The visceral organs of each fish were carefully removed. The head and body of fishes were separated, cut into small pieces, and blended in a blender to which artificial gastric juice containing 0.3% Pepsin (LOBA Chemie Pvt. Ltd., Mumbai, India) and 1% conc. HCl (Merck, Germany) were added.
2.4. Artificial digestion and recovery of MC
Processed samples were digested according to the procedures described by Sohn [19] with slight modifications. Briefly, processed samples were incubated at 37 ºC overnight in artificial gastric juice under vigorous stirring conditions. The next morning, larger particles (scales, fins, bones, and undigested materials) were removed by filtering through a sieve (1 × 1-mm mesh). The filtrate was washed extensively by adding normal saline (2 L), stirring vigorously, and leaving to stand for 30 minutes for sedimentation. The supernatant was then discarded and washing with normal saline was repeated until the filtrate became clear. The sediment was centrifuged at 3000 rpm for 3 min, the supernatant was discarded, and the pellet was examined.
2.5. Detection of MC
The pellet was re-suspended with PBS to a total volume of 15 ml and mixed thoroughly. A 0.15-ml aliquot of the suspension was added to a clean glass slide and examined under a microscope (Labomed, USA) using the 10X objective. MC were identified to the genus level following previously reported keys and descriptions [19–24]. Each sample was examined at least in triplicate and averaged. The average number of MC was multiplied by 100 to assess the total number of MC present in 15 ml of the suspension, and MC per 100 g of fishes was calculated. Microphotographs of MC were taken using an inverted microscope to which a digital camera (Labomed, USA) had been fit.
2.6. MC size measurements
The length and width or diameter of MC were estimated using a pre-calibrated eye piece.
2.7. Statistical analyses
Data were analyzed using SPSS software. The infection rate of fishes was analyzed using the Z-test and the loads of MC in different species were compared using the chi-squared (χ2) test. P < 0.05 was considered to be significant.
3. Results
3.1. The rate of FBT infections is pretty high in small wild freshwater fishes
A total of five species of fishes marketed for human consumption were purchased from local markets. After collection, fishes were transported to the laboratory, identified, and processed for the isolation of the MC of FBTs. All five species of fishes: ticto barb, flying barb, banded gourami, freshwater garfish, and reba carp, were infected with MC (Figure 2). We examined 65 pooled samples and of them 43 (66.2%) were positive for the MC of FBTs, suggesting that the selected small wild fishes play important roles as the second intermediate hosts. The highest infection rate was in ticto barb (87.5%) and the lowest in flying barb (41.7%), and this difference was significant (p < 0.05). Furthermore, the mean load of MC per 100 g of fishes was the highest in ticto barb (1978.8 ± 5053.7) and the lowest in flying barb (117.6 ± 102.3) (Table 2).
Figure 2.
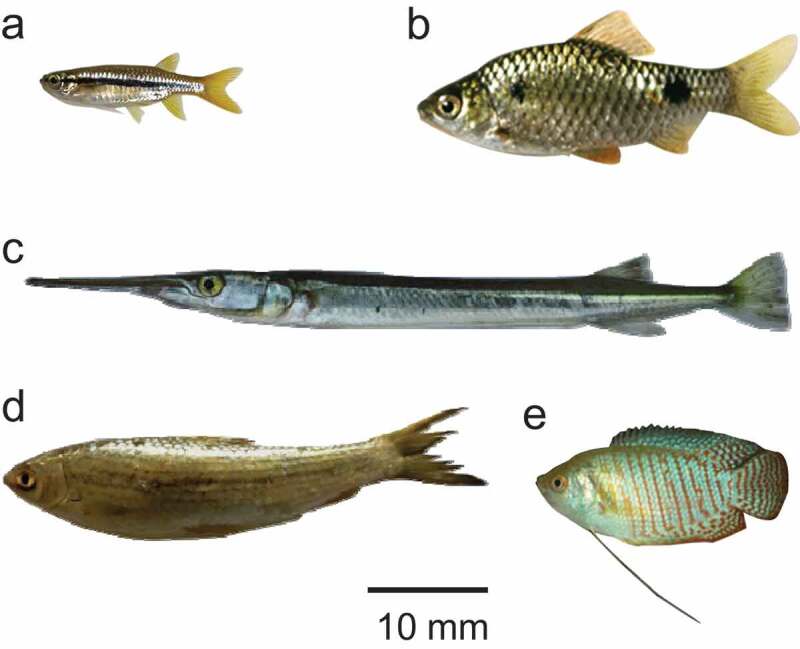
Small wild fishes infected with MC of FBTs. Five species of fishes, (a) flying barb (Esomus danricus), (b) ticto barb (Puntius ticto), (c) freshwater garfish (Xenentodon cancila), (d) reba carp (Cirrhinus reba), and (e) banded gourami (Colisa fasciata) were collected and identified. The selected fishes are commonly used for human consumption in Bangladesh.
Table 2.
Metacercariae detected in small wild fishes.
| Fishes |
Sample infected (%) | Load of MC |
Parasites | |
|---|---|---|---|---|
| Family | species | Range (Mean ± SD) | ||
| Cyprinidae | Ticto barb n = 16 |
14/16 (87.5a) | 13–19,200 (1978.8a ± 5053.7) |
Clonorchis/Opisthorchis Metagonimus, Metorchis |
| Flying barb n = 12 |
5/12 (41.7b) |
14–200 (117.6b ± 102.3) |
Clonorchis/Opisthorchis | |
| Banded gourami n = 12 |
8/12 (66.7a) |
27–363 (134.3b ± 109.2) |
Clonorchis/Opisthorchis Metagonimus, Metorchis |
|
| Reba carp n = 11 |
7/11 (63.7a) |
15–1,250 (268.3b ± 440.7) |
Metorchis, Metagonimus Clonorchis/Opisthorchis | |
| Belonidae | Freshwater garfish n = 14 |
9/14 (64.3a) |
23–223 (140b ± 105.4) |
Clonorchis/Opisthorchis Metorchis, Metagonimus Echinostoma |
| Total n = 65 |
43/65 (66.2a) | 13–19,200 (748.3b ± 2,947.5) |
||
n, Sample number; MC, metacercariae
Values with different superscripts in the same column are significant (P < 0.05).
3.2. Morphological characteristics of different MC in freshwater wild fishes
Based on morphology and morphometry, the MC of Clonorchis spp. were elliptical and measured 0.17 ± 0.01 × 0.14 ± 0.01 mm in size. A detailed morphological study revealed oral and ventral suckers of similar sizes and an O-shaped excretory bladder. Morphologically, the internal structures of MC of Opisthorchis spp. were similar to those of Clonorchis spp., except the size of the cyst was a bit bigger (0.19 ± 0.01 × 0.14 ± 0.01 mm). The size of the MC of Metorchis spp. was 0.17 ± 0.06 × 0.17 ± 0.05 mm and was characterized by the presence of a clearly visible double-layered cyst wall. The MC of Metagonimus spp. were distinct both in size and shape. The MC of Metagonimus spp. were subglobular or disc-shaped, measuring 0.15–0.14 mm in diameter, and were distinguished by the presence of yellow-brownish pigments and a V-shaped excretory bladder. On the other hand, echinostome-MC were elliptical in shape with an average size of 0.15–0.16 × 0.11–0.13 mm, and were typically characterized by the presence of collar spines (Figure 3).
Figure 3.
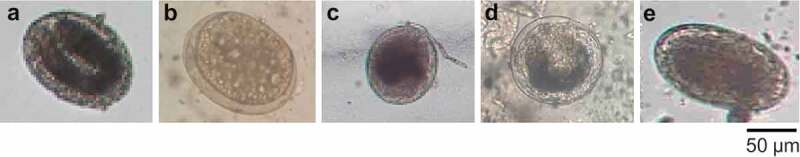
MC isolated and identified from wild fishes. Five species of fishes were collected, processed, and digested using artificial gastric juice as described in the Materials and Methods. Digested fishes was sieved and washed extensively with PBS. The sediment was examined under a microscope and microphotographs were taken. MC of (a) Clonorchis spp., (b) Echinostoma spp., (c) Metagonimus spp., (d) Metorchis spp., and (e) Opisthorchis spp.
3.3. MC of FBTs in different fishes
The MC of FBTs, such as Clonorchis spp., Opisthorchis spp., Metorchis spp., Metagonimus spp., and Echinostoma spp., were identified. In most samples, infections with more than one type of FBT were detected. Clonorchis/Opisthorchis were isolated from all species of fishes examined. Metorchis-MC were found in four species of fishes, namely, ticto barb, banded gourami, freshwater garfish, and reba carp. The MC of Metagonimus spp. were also detected in ticto barb, banded gourami, freshwater garfish, and reba carp fishes, whereas Echinostoma-MC were only detected in freshwater garfish (Table 2).
3.4. Distribution of FBTs in the head and body of fishes
During the pr o head and body segments and digested separately using artificial gastric juice, as described in the Materials and Methods section. The infection rate was significantly higher (P < 0.05) in the body (55.4%) than in the head (40%) of fishes (data not shown). The infection rate and load both differed in the head and body of the same species of fishes. The infection rate with MC was the highest in the head (81.3%) of ticto barb, but was higher in the body than in the head of the other fishes examined. The lowest infection rates with MC were detected in the head (16.7%) and body (25%) of flying barb. There was no tissue preference in freshwater garfish; MC were equally detected in both the head and body (36.4%) (Table 3). On the other hand, the mean load of MC was significantly higher in the heads of ticto barb (2082.7 ± 5,245.7), reba carp (215 ± 78.8), and freshwater garfish (450.4 ± 533.8). In contrast, the load of MC was significantly higher in the bodies of flying barb (176.7 ± 87.4) and banded gourami (131.8 ± 111.7) (Table 3).
Table 3.
Relative distribution of metacercariae in different body parts of fishes.
| Fish species | No. of infected body parts (%) |
Load of MC Range (Mean ± SD) |
||
|---|---|---|---|---|
| Head | Body | Head | Body | |
| Ticto barb | 13/16 (81.3a) | 12/16 (75a) |
13–19200 (2082.7a ± 5245.7) |
6–3376 (625.5b ± 1016.2) |
| Flying barb | 2/12 (16.7a) |
3/12 (25a) |
14–44 (29a ± 21.2) |
80–250 (176.7b ± 87.4) |
| Banded gourami | 3/12 (25a) |
8/12 (66.7b) |
47–83 (62.3a ± 18.6) |
27–363 (131.8a ± 111.7) |
| Reba carp | 5/14 (35.7a) |
9/14 (64.3a) |
127–303 (215a ± 78.8) |
23–106 (49.6b ± 25.1) |
| Freshwater garfish | 4/11 (36.4a) |
4/11 (36.4a) |
150–1250 (450.5a ± 533.8) |
15–50 (31.5b ± 17.1) |
MC, metacercariae
Values with different superscripts in the same row and parameter are significant (P < 0.05).
4. Discussion
FBT infections, particularly diseases caused by human liver flukes (C. sinensis and O. viverrini), are neglected tropical diseases (NTDs). FBTs have a negative impact on public health and are of great concern due to the associated complications [25]. We herein investigated the current status of FBT infections in wild, small, freshwater fishes in Bangladesh. The results obtained revealed that FBT infections in wild freshwater fishes were very high in Bangladesh, with 66% of the pool samples examined being infected. Although the MC of FBTs had not previously been identified in freshwater fishes in Bangladesh, adult flukes were detected in reservoir animals and birds [15,16], indicating that these parasites had existed for a long time. Since wild fishes are available throughout the country and are in high demand both in rural and urban areas in Bangladesh, the entire population is at high risk of FBT infections.
The present study revealed that four species of wild freshwater fishes belonging to the family Cyprinidae, namely, ticto barb, flying barb, banded gourami, and reba carp, and one species of the family Belonidae (e.g. freshwater garfish) play roles as the second intermediate hosts to complete the lifecycle of different FBTs prevalent in the study areas. In Bangladesh, the types of freshwater fishes that act as the second intermediate hosts of FBTs have not yet been identified. However, previous studies conducted in other countries suggested that cyprinid fishes typically play central roles in the transmission cycle of FBTs [20,26–28]. In addition to cyprinid fishes, MC have been identified in freshwater garfish (Belonidae) in Thailand [29] and Tilapia spp. (family: Cichlidae) in northern Vietnam [30], suggesting that fishes other than cyprinids can act as the second intermediate hosts for FBTs.
In the present study, the MC of Clonorchis/Opisthorchis were detected in all types of fish species examined, suggesting that these fishes play roles as the second intermediate hosts of opisthorchid flukes. C. sinensis is the most significant species of FBTs in Southeast Asia [31–33]. Until now, endemic areas of clonorchiasis included China, South Korea, North Vietnam, Taiwan, and Far East Russia [34]. A nationwide survey in China revealed that 102 species of fishes act as the second intermediate hosts of Clonorchis [35]. In Korea, Pungtungia herzi, Ladislabia taczanowskii, and Acheilognathus rhombeus fishes were identified as the second intermediate hosts of C. sinensis [36]. On the other hand, only two fish species have been shown to act as the second intermediate hosts for O. viverrini in Phu Yen province, Vietnam, which is considered to be the ‘hot spot’ of the parasite. The prevalence of O. viverrini was 10–29% in the crucian carp, Carassius carassius, in Phu Yen province [37] and 1.9% in the snakehead fish, Channa striata, in An Giang province in southern Vietnam [38]. The MC of O. viverrini have been detected in fishes of the genera Carassius, Channa, Cyclocheilicthys, Hampala, Esomus, Osteochilus, Puntioplites, and Puntius [39].
In the present study, the MC of Metagonimus spp. were found in ticto barb, banded gourami, freshwater garfish, and reba carp. The MC of Metagonimus have been reported in several species of fishes, such as Abramis brama, A. ballerus, Aspius aspius, Blicca bjoerkna, C. carassius, Chondrostoma nasus, Hemibarbus labeo, Leuciscus idus, Pseudobagrus fulvidraco, Plecoglossus altivelis, Tribolodon taczanowskii (T. hakonensis), and Lateolabrax japonicus in China, Taiwan, and Korea [19,35]. In the present study, ticto barb, banded gourami, freshwater garfish, and reba carp were positive for the MC of Metorchis spp. Metorchis spp. have been detected in humans in Eurasia, North America, and East Asia [40]. Anisuzzaman et al. [16] reported that 48% of ducks in Bangladesh were infected with M. orientalis. Reservoir animals (dogs and cats) act as the source of M. orientalis in Japan and China. The MC of M. orientalis were detected in Pseudorasbora parva, Hemiculter leucisculus, Saurogobio dabryi, Rhynchocypris lagowskii, C. auratus, Rhodeus ocellatus, Perccottus glehnii, P. herzi, Misgurnus anguillicaudatus, Microphysogobio koreensis, and Gnathopogon strigatus in China and Korea [41,42], indicating its very wide host preference.
Overall, we found very high loads of MC in all five fish species examined. Previous studies reported that the average load of MC ranged between 1 and 485 [43,44]. Variations among previous findings on the load of MC obtained from different studies may be attributed to the fish species examined, the methods employed, the geoclimatic conditions of the countries, the availability of reservoir hosts, and even cropping patterns. In Bangladesh, measures to control snails have not yet been implemented, and all types of natural water bodies harbor varieties of snail species. The entire country is crisscrossed with rivers and other natural water sources, and most parts of the country are flooded during the long rainy season. The temperature is generally >30 °C throughout the year and rarely decreases to ~15 °C in the very short winter (from the second week of December to the first week of the February) [45]. Moreover, countrywide faulty drainage systems used for irrigation, particularly in the dry seasons, also favor the survival and propagation of snails, intermediate hosts, throughout the year. Thus, wild fishes are continuously exposed to the cercariae released from snails. Furthermore, in the present study, we digested fish samples for a long time, which possibly caused the release of all MC from the muscles and head tissues of the fishes, leading to as the detection of high infection rate and recovery of MC.
5. Conclusions
FBT infection rates are high in small wild fishes in Bangladesh, which is a public health concern. The present study confirmed that Bangladesh is an endemic area of fish-borne liver and intestinal parasites of humans, which is an emerging threat particularly to those who consume improperly cooked fish and fish products. The present results will be the basis for advanced studies on FBTs in Bangladesh. A country-wide extensive survey and further studies on more fish species are needed to reveal the extent of this infection.
Funding Statement
The present study was supported by grants (to Anisuzzaman) by the Ministry of Education, Government of Bangladesh, Bangladesh.
Acknowledgments
We thank the fishermen of the study area for providing information on fishes.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Andrews RH, Sithithawarn P, Petney TN.. Opisthorchis viverrini: an underestimated parasite in world health. Trends Parasitol. 2008;24:497–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chai JY, Murrell KD, Lymbery AJ.. Fish-borne parasitic zoonoses: status and issues. Int J Parasitol. 2005;35:1233–1254. [DOI] [PubMed] [Google Scholar]
- [3].WHO . The World Health Report, Changing History. 2004; 170.
- [4].Nguyen MH, Madsen H, Fried B. Global status of fish-borne zoonotic trematodiasis in humans. Acta Parasitol. 2013;58:231–258. [DOI] [PubMed] [Google Scholar]
- [5].Skov J, Kania PW, Dalsgaard A, et al. Life cycle stages of heterophyid trematodes in Vietnamese freshwater fishes traced by molecular and morphometric methods. Vet Parasitol. 2009;160:66–75. [DOI] [PubMed] [Google Scholar]
- [6].De NV, Le TH, Murrell KD. Prevalence and intensity of fish-borne zoonotic trematodes in cultured freshwater fish from rural and urban areas of Northern Vietnam. J Parasitol. 2012;98:1023–1025. [DOI] [PubMed] [Google Scholar]
- [7].Keiser J, Utzinger J. Food-borne trematodiases. Clin Microbiol Rev. 2009;22:466–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Qian M, Utzinger J, Keiser J, et al. Clonorchiasis. Lancet. 2016;387:800–810. [DOI] [PubMed] [Google Scholar]
- [9].Qian M, Zhou X. Human liver flukes in China and ASEAN: time to flight together. PLoS Negl Dis. 2019;13:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Yossepowitch O, Gotesman T, Assous M, et al. Opisthorchiasis from imported raw fish. Emerg Infect Dis. 2004;10:2122–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hung NM, Dung DT, Anh NTL, et al. Current status of fish-borne zoonotic trematode infections in Gia Vien district, Ninh Binh province, Vietnam. Parasit Vectors. 2015;8:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].GBD 2017 DALYs and HALE Collaborators . Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: a systematic analysis for the GLOBAL Burden of Disease Study 2017. Lancet. 2018;392:1859–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Scholte LLS, Pascoal-Xavier MA, Nahum LA. Helminths and cancers from the evolutionary perspective. Front Med. 2018;5:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mac Lean JD, Cross JH, Mahanty S. Liver, lung and intestinal fluke infections. In: Guerran RL, Walker DH, Weller PF, editors. Tropical Infectious Diseases. Vol. II. Philadelphia: Churchill, Livingston; 2006. p. 1039–1057. [Google Scholar]
- [15].Shaikh H, Huq MM. A survey on the parasites of zoonotic importance in Bangladesh. Annual report of Bangladesh Agricultural Research Council. 1984: 9–10.
- [16].Anisuzzaman, Alim MA, Islam MK, et al. Avian liver fluke infection in indigenous ducks in Bangladesh: prevalence and pathology. J Bangladesh Agrl Univ. 2005;3:87–94. [Google Scholar]
- [17].Rahman AKA. Freshwater fishes of Bangladesh. 2nd ed. Dhaka, Bangladesh: Zoological Society of Bangladesh; 2005. p. 394. [Google Scholar]
- [18].Talwar PK, Jhingran AG. Inland fishes of India and adjacent countries. Vol. 1&2. New Delhi, India: Oxford & IBH Publishing Co. Pvt. Ltd; 1991. p. 541–1158. [Google Scholar]
- [19].Sohn W. Fish-borne Zoonotic trematode metacercariae in the Republic of Korea. Korean J Parasitol. 2009;47:103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Scholtz T, Ditrich O, Giboda M. Differential diagnosis of opisthorchid and heterophyid metacercariae (Trematoda) infecting flesh of cyprinid fish from Nam Ngum Dam Lake in Lao PDR. Southeast Asian J Trop Med Public Health. 1991;22:171–173. [PubMed] [Google Scholar]
- [21].Kaewkes S. Taxonomy and biology of liver flukes. Acta Trop. 2003;88:177–186. [DOI] [PubMed] [Google Scholar]
- [22].Clausen JH, Madsen H, Murrell D, et al. Relationship between snail population density and infection status of snails and fish with zoonotic trematodes in Vietnamese carp nurseries. PLoS Negl Dis. 2012;6(12):e1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Yamaguti S. Systema Helminthum. Vol. I. The Digenetic Trematodes of Vertebrates-Part I&II. New York, London: Interscience Publishers; 1958. [Google Scholar]
- [24].Kiyan VS, Bulashev AK, Katokhin AV. Opisthorchis felineus and Metorchis bilis metacercariae in cyprinid fish Leuciscus idus in Nura-Sarysu River, Kazakhstan. Korean J Parasitol. 2018;56(3):267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Keiser J, Utzinger J. Food-borne trematodiasis: current chemotherapy and advances with artemisinins and synthetic trioxolanes. Trends Parasitol. 2007;23:555–562. [DOI] [PubMed] [Google Scholar]
- [26].Petney TN, Andrews RH, Saijuntha W, et al. The zoonotic, fish-borne liver flukes Clonorchis sinensis, Opisthorchis felineus and Opisthorchis viverrini. Int J Parasitol. 2013;43:1031–1046. [DOI] [PubMed] [Google Scholar]
- [27].Lun ZR, Gasser RB, Lai DH, et al. Clonorchiasis: a key foodborne zoonosis in China. Lancet Infect Dis. 2005;5:31–41. [DOI] [PubMed] [Google Scholar]
- [28].Conlana JV, Sripa B, Attwood S, et al. A review of parasitic zoonoses in a changing Southeast Asia. Vet Parasitol. 2011;182:22–40. [DOI] [PubMed] [Google Scholar]
- [29].Wongsawad C, Rojanapaibul A, Mhad-arehin N, et al. Metacercaria from freshwater fishes of Mae Sa stream, Chiang Mai, Thailand. Southeast Asian J Trop Med Public Health. 2000;31(Suppl 1):54–57. [PubMed] [Google Scholar]
- [30].De NV. The fishborne trematodes in Vietnam. Southeast Asian J Trop Med Public Health. 2004;35:299–301. [Google Scholar]
- [31].Rim HJ. Clonorchiasis in Korea. Korean J Parasitol. 1990;28:63–78. [DOI] [PubMed] [Google Scholar]
- [32].Chen MG, Lu Y, Hua X, et al. Progress in assessment of morbidity due to Clonorchis sinensis infection: a review of recent literature. Trop Dis Bull. 1994;91:R7–R56. [Google Scholar]
- [33].Hong ST. Clonorchis sinensis. In: Miliotis MD, Bier JW, editors. International handbook of foodborne pathogens. New York, Basel: Marcel Dekker, Inc.; 2003. p. 581–592. [Google Scholar]
- [34].Mas-Coma S, Bargues MD. Human liver flukes: a review. Res Rev Parasitol. 1997;57:145–218. [Google Scholar]
- [35].Yuan R, Huang J, Zhang X, et al. Modeling the transmission dynamics of clonorchiasis in Foshan, China. Sci Rep. 2018;8:15176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Shin HC, Lee W, Kim T, et al. Prevalence of zoonotic Trematode metacercariae in freshwater fish from Gangwon-do, Korea. Korean J Parasitol. 2014;52:399–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Chuong NV, Tuan BV, Chau LV, et al. Several epidemiological characteristics of Opisthorchis viverrini. Malar Parasit Dis Prevent Bull. 1997;2:85–90. [Google Scholar]
- [38].Thu ND, Dalsgaard A, Loan LT, et al. Survey for zoonotic liver and intestinal trematode metacercariae in cultured and wild fish in An Giang Province, Vietnam. Korean J Parasitol. 2007;45:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].WHO . Control of food borne trematode infections. World Health Organ Tech Rep Ser. 1995;849:1–157. [PubMed] [Google Scholar]
- [40].Lv S, Tian LG, Liu Q, et al. Water-related parasitic diseases in China. Int J Environ Res Public Health. 2013;10:1977–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Qiu J, Zhang Y, Zhang X, et al. Metacercaria infection status of fishborne zoonotic trematodes, except for clonorchis sinensis in fish from the Heilongjiang Province, China. Foodborne Pathog Dis. 2017;14(8):440–446. [DOI] [PubMed] [Google Scholar]
- [42].Sohn W, Na B, Cho S, et al. Prevalence of Clonorchis sinensis metacercariae in fish from water systems of Seomjin-gang (river). Korean J Parasitol. 2017;55(3):305–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Rim HJ, Sohn WM, Yong TS, et al. Fishborne trematode metacercariae in Luang Prabang, Khammouane, and Saravane Province, Lao PDR. Korean J Parasitol. 2013;51(1):107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Sarun T, Chalit K, Prayong R, et al. Discovery of Opisthorchis viverrini metacercariae in freshwater fish in southern Cambodia. Acta Trop. 2009;111:108–113. [DOI] [PubMed] [Google Scholar]
- [45].Bammi YM. India Bangladesh relations: the way ahead. New Delhi, India: Vij Books India Pvt Ltd; 2010. p. 3. [Google Scholar]


