Abstract
Background
Recent evidence suggests that an altered intestinal microbiota, specifically a reduction of bacterial diversity or a shift in microbial composition, is associated with the development of hypersensitivity disorders in humans, but this is unknown for horses.
Objectives
In this study we hypothesized that horses affected by either Culicoides hypersensitivity or severe equine asthma or both show a decreased diversity of their intestinal microbiota. We also investigated environmental effects.
Methods
Rectal swab samples of a total of 140 horses were collected and the owners completed a detailed questionnaire about their horse. For each allergic horse, a healthy peer from the same stable was equally sampled as an environmentally matched control. Microbiota in the swabs was determined by assessing the V4 region of the bacterial 16S rRNA gene. Structures of bacterial communities were investigated by means of alpha and beta diversity indices.
Results
Group wise comparisons between healthy and allergic horses showed no significant differences regarding alpha (p = 0.9) and beta diversity (p = 0.5). However, the microbial structure was associated with environmental factors such as the type of stable (p = 0.001), access to pasture (p = 0.001) or the type of feeding (p = 0.003). There was also a strong location effect meaning that the microbiota was more similar within the same as compared between farms within this study.
Conclusion
Our observations suggest that hypersensitivity disorders in adult horses are not associated with an alteration of the intestinal microbiota, but environmental and/or location factors strongly influence these bacteria.
Keywords: Horse, equine, intestinal microbiota, allergy, culicoides hypersensitivity, severe equine asthma, environment
1. Introduction
There is recent evidence for a role of the intestinal microbiome in the development and maintenance of hypersensitivity disorders. The microbiota is a potent immune modulator (Geuking et al. 2011; Hooper et al. 2012; Sommer and Bäckhed 2013) and the gastro-intestinal tract has been referred to as the largest immunological organ of the body (Kelly and Coutts 2000; Wershil and Furuta 2008). The microflora hypothesis as an expansion of the well-known hygiene hypothesis (Strachan 1989) suggests that a dysbiosis of the intestinal microbiota may result in the development of hypersensitivity disorders (Noverr and Huffnagle 2005; Stiemsma et al. 2015). Especially early infancy is a critical period of microbiological and immunological development (Fujimura and Lynch 2015) and an imbalance in the bacterial composition at this time may lead to an increased risk of allergic sensitization (Legatzki et al. 2014; Rutten et al. 2015). In human medicine this has been shown for several allergic disorders, like asthma, rhinitis or atopic dermatitis (Bottcher et al. 2000; Bisgaard et al. 2011; Abrahamsson et al. 2014; Marsland et al. 2015; Zheng et al. 2016). Furthermore, children with atopic dermatitis are also at an increased risk to subsequently develop other manifestations of hypersensitivities, a phenomenon, which is known as the allergic march (Linneberg 2008; Spergel 2010; Nissen et al. 2013). But there is also evidence for an association of allergic disorders and an altered intestinal microbiota in adults. In a large-scale study with a total of 1879 fecal samples provided by the publicly available American Gut Project, Hua et al. (2016) showed that adults with allergies, especially to nuts and seasonal pollen, also have a lower microbial diversity in their feces than healthy persons.
In horses, few studies using next-generation sequencing technologies for the investigation of the intestinal microbiome are available at this time and none have focused on the association of the intestinal microbiota with equine hypersensitivity disorders. The two best characterized and most common hypersensitivity disorders are Culicoides hypersensitivity (CH, also known as insect bite hypersensitivity, sweet itch or summer eczema) and severe equine asthma (SEA, formerly known as recurrent airway obstruction). CH is caused by type 1 allergic reactions against salivary gland proteins of biting midges of the genus Culicoides spp. and is frequently seen in certain horse breeds, such as the Icelandic Horse where the prevalence of CH can reach up to >50% in horses imported from Iceland to Europe (Schaffartzik et al. 2012). SEA is a chronic disorder of the airways, which is characterized by hypersensitivity-mediated airway inflammation and affects approximately 10-20% of adult horses living in the Northern Hemisphere (Hotchkiss et al. 2007; Ramseyer et al. 2007; Couëtil et al. 2016). Clinical signs of SEA are triggered or exacerbated by the inhalation of dust particles present in the stables, particularly those associated with hay feeding (Leclere et al. 2011). Since there is no causative treatment, the management of CH and SEA is frequently unsatisfactory and expensive. It is centered around the reduction or even complete avoidance of allergen exposure, which often needs to be complemented by medical treatment (Léguillette 2003; Cunningham and Dunkel 2008; Jackson et al. 2010; Schaffartzik et al. 2012). Interestingly, there is recent evidence for an association between the occurrence of CH and SEA. Horses suffering from SEA have an increased risk for developing CH (Kehrli et al. 2015) and, vice versa, horses with CH also have a higher risk for airway hyperreactivity (Lanz et al. 2017). We have therefore selected both diseases for our study and hypothesized that horses affected by either CH, SEA or both show a disordered intestinal microbiota in comparison to healthy stable mates.
Since environmental factors can exert strong effects on the intestinal bacterial composition, we also investigated this aspect. Such effects have been shown especially for humans (Candela et al. 2012; Song et al. 2013), for laboratory animals (Terán-Ventura et al. 2010; Campbell et al. 2012; Thoene-Reineke et al. 2014) and in several species for diet effects (Muegge et al. 2011; Albenberg and Wu 2014; David et al. 2014). Therefore, we controlled for some environmental factors, but also investigated which factors exert the most important effects on the equine intestinal bacterial diversity and composition.
2. Materials and methods
2.1. Study design
The project was designed as a case-control study in a field research environment, which means that all horses were sampled at their home stables. Three study groups were formed: horses suffering from Culicoides Hypersensitivity (group “CH”), horses suffering from severe equine asthma (group “SEA”) and horses suffering from both disorders (group “CH-SEA”). For each allergic horse of one of these three groups a healthy peer from the same stable was sampled as a control. Horses were thus matched primarily regarding housing and management. As further criteria breed, age and sex were also matched as far as possible.
All horses underwent a general examination before the sample collection that included respiratory tract and cardiac auscultation, rectal temperature, mucous membrane colour, capillary refill time and submandibular lymph node palpation. Examination and sampling were performed by the same veterinarian (SK) in all horses. Owners were asked to complete a detailed and standardized questionnaire consisting of four parts. The first part comprised general information about the horse (e.g., breed, age). The second part comprised questions about the housing, feeding and management of the horse. Part three was used to collect information about the worst clinical signs of CH that owners had ever observed in their horse. To complement the historical information, current clinical signs were recorded by the examining veterinarian (SK) and horses were then scored as previously described by Lanz et al. (2017). In brief, this score comprises five severity grades, each with an additional characteristic to the preceding one with 0) no signs of CH; 1) increased skin flaking with visible epithelial debris; 2) areas of broken hairs or alopecia from scratching; 3) indurated skin folds; 4) obvious crusts from serous exudate, but without bleeding; 5) bleeding from self-inflicted abrasions. As diagnosis of CH is based on clinical signs and a characteristic medical history (Oliveira-Filho et al. 2012), horses were classified as CH affected if their current CH-score was >0 and their history was compatible with CH. Finally, in part four the historical lung health status of the horse was assessed using the questions from the Horse Owner Assessed Respiratory Signs Index (HOARSI) questionnaire developed by Ramseyer et al. (2007). This index also refers to the worst clinical signs in the horse’s medical history and defines four categories according to the severity of respiratory signs (HOARSI 1-4: healthy, mild, moderate and severe). It has been shown that SEA affected horses can be reliably identified by their HOARSI category (Laumen et al. 2010). Accordingly, all SEA affected horses had been graded HOARSI 3 (moderate) or 4 (severe) and, in addition, all these horses had formerly been presented to the equine clinics in Bern or Zurich, where they were diagnosed with SEA based on standard examination of the lower respiratory tract.
Horses could only serve as controls if both their CH score was 0 and their HOARSI score was 1. Furthermore, horses were excluded from the study if they showed acute systemic illness on the day of sampling, if they had received antibiotics within the last 3 months, or corticosteroids within the last 4 weeks, or if a matching healthy peer was not available.
2.2. Sample collection
Samples were collected between June 2017 and November 2017. We focused on the summer and fall season as clinical signs of CH are exacerbated when specific biting midges (genus Culicoides) are most active (Sanders et al. 2011).
Rectal swabs were obtained using sterile collection swabs (Sarstedt, PS/Viscose). Swabs were inserted 12 cm into the rectum for 15 sec while rotating and gently rubbing them against the rectal mucosa. Afterwards swabs were put into their transport tubes and placed into a cold box (at 4 °C). Upon arrival at the equine clinic, swabs were immediately put at −80 °C until further processing.
2.3. DNA extraction, 16S rRNA amplification, purification and sequencing
DNA was extracted from the swabs using the commercial QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s recommendations. Forward (5′-GTGCCAGCMGCCGCGGTAA-3′) and reverse (5′-GGACTACHVGGGTWTCTAAT-3′) primers (Caporaso et al. 2011) were used to amplify the V4 region of the bacterial 16S rRNA gene and an Illumina adaptor sequence was attached at the 5′ end. PCR was then run in the same way as described by Kraemer et al. (2018), including negative controls. PCR products were purified by QIAquick PCR Purification Kit (Qiagen, Hilden, Germany) and the purified products were evaluated by gel electrophoresis.
The samples were then submitted to the Next Generation Sequencing Platform at the University of Bern for indexing and paired-end 2 × 250 bp sequencing (Reagent Kit v2) on the Illumina MiSeq platform (Illumina Inc., San Diego, USA).
2.4. Sequence analysis
The open-source software R version 3.3.2 (R Core Team 2018) with the DADA2 package version 1.6.0 was used to process the sequences as described by Callahan et al. (2016). Specifically, forward reads were trimmed at 180 base pairs (bp) and reverse reads at 150 bp. We decided for these rather conservative borders to make sure that the Phred quality scores for all reads at any point up to the trimming borders were well over 30, which corresponds to a base call accuracy of 99.9%. Although the DADA2 algorithm is relatively robust to lower quality sequences, deliberate trimming improves the algorithm’s sensitivity to detect rare sequence variants. Reads containing ambiguous base calls or sequences with inappropriate length (<245 bp and >257 bp) were filtered out and chimeras were also identified and removed. Taxonomy was then assigned to the remaining amplicon sequence variants (ASVs) by making use of the Silva 16S rRNA reference database version 128 (Quast et al. 2013). Finally, sequences corresponding to chloroplasts, mitochondria, Archaea and Eukaryotes were removed as well (207 sequences removed = 1,7% of sequences).
2.5. Statistical analysis
Data were analyzed in R using the base, vegan, microbiome and pairwiseAdonis packages (Lahti and Shetty 2018; Martinez Arbizu 2018; Oksanen et al. 2018; R Core Team 2018).**** To ensure that sequencing depth was sufficient for all samples rarefaction curves were visually assessed at first. Based on the authors of DADA2 and others, rarefying microbiome data for statistical analyses is discouraged and was therefore waived (McMurdie and Holmes 2014; Callahan et al. 2016; Kraemer et al. 2018). For the evaluation of alpha diversity (within-sample diversity) we investigated the number of ASVs in each sample, Pielou’s evenness index and Shannon diversity. We compared the aforementioned alpha diversity indices by groups (e.g., allergic vs. healthy) using an unpaired Wilcoxon rank-sum test or a Kruskal-Wallis test as data were not normally distributed. We also performed pairwise comparisons of all three alpha diversity indices between affected horses and their matched control horses using a paired Wilcoxon test as well as a Jonckheere’s trend test for environmental comparisons since the respective subcategories can be brought into a logical order (e.g., ordered by the amount of roughage and concentrate).
Beta diversity measurements (between-sample diversity) were assessed by means of weighted (abundance-based) and unweighted (presence/absence-based) Bray-Curtis indices. Nonmetric multidimensional scaling was then performed of the generated distance matrices in order to plot dissimilarities. Differences between groups of samples were statistically evaluated by a permutational multivariate analysis of variance using the distance matrices. Post-hoc testing for multilevel pairwise comparisons was performed with the pairwise.adonis function. Mean dissimilarity distances within specific groups (e.g., all horses from the same stable) were investigated by compiling all appropriate pairwise distance values from the distance matrix based on weighted and unweighted Bray-Curtis dissimilarity calculations. As these data were not normally distributed, groups were compared by an unpaired Wilcoxon rank-sum test. A p-value of <0.05 was considered for statistical significance for all comparisons.
3. Results
3.1. Study population
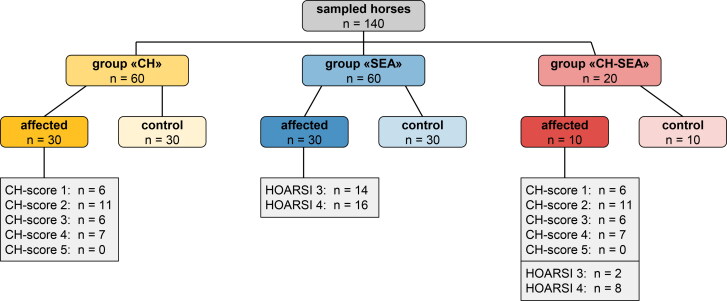
A total of 140 horses met the inclusion criteria, 70 were affected by a hypersensitivity disorder and the other 70 were the respective healthy and stable-matched controls. Of these, three study groups (“CH”, “SEA” and “CH-SEA”) were formed (Figure 1). The horses originated from altogether 48 farms from all over Switzerland and further details about each horse can be found in the supplementary material (Additional Table A1).
Figure 1.
Flow chart of the study population including the distribution of CH- and HOARSI scores. CH = Culicoides hypersensitivity, SEA = severe equine asthma, HOARSI = horse owner assessed respiratory signs index.
Table 1.
Comparisons of the three alpha diversity indices richness, evenness and Shannon index within several subcategories.
| n | Richness |
Evenness |
Shannon Index |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mean and SD | p-value unpaired1 | p-value combined2 | mean and SD | p-value unpaired1 | p-value combined2 | mean and SD | p-value unpaired1 | p-value combined2 | ||||
| Disease | allergic vs. healthy | allergic horses healthy controls |
70 70 |
848 ± 249 820 ± 221 |
0.5 | 0.6 | 0.79 ± 0.06 0.80 ± 0.07 |
0.4 | 0.6 | 5.35 ± 0.56 5.34 ± 0.57 |
0.9 | 1.0 |
| CH vs. healthy | CH-affected horses healthy controls |
30 30 |
887 ± 218 881 ± 252 |
0.8 | 0.8 | 0.81 ± 0.05 0.79 ± 0.08 |
0.9 | 0.7 | 5.46 ± 0.44 5.35 ± 0.68 |
0.7 | 0.6 | |
| SEA vs. healthy | SEA-affected horses healthy controls |
30 30 |
802 ± 284 775 ± 218 |
0.5 | 0.7 | 0.79 ± 0.07 0.80 ± 0.06 |
0.6 | 0.5 | 5.25 ± 0.70 5.31 ± 0.51 |
0.8 | 0.8 | |
| CH-SEA vs. healthy | CH-SEA-affected horses healthy controls |
10 10 |
873 ± 192 860 ± 237 |
1.0 | 0.9 | 0.79 ± 0.03 0.81 ± 0.02 |
0.3 | 0.2 | 5.35 ± 0.23 5.45 ± 0.32 |
0.5 | 0.6 | |
| Environment/ Management | type of stable | interior box exterior box open stable pasture only |
25 24 82 9 |
909 ± 274 730 ± 209 875 ± 231 630 ± 155 |
0.003 *** |
0.2 (decr.) |
0.77 ± 0.10 0.79 ± 0.07 0.81 ± 0.04 0.79 ± 0.03 |
0.1 | 0.3 (decr.) |
5.22 ± 0.82 5.17 ± 0.69 5.47 ± 0.39 5.08 ± 0.34 |
0.02 * |
0.4 (decr.) |
| access to pasture | 0-4 hours per day 4-12 hours per day >12 hours per day |
28 50 62 |
863 ± 272 880 ± 242 799 ± 228 |
0.2 | 0.05 * (decr.) |
0.80 ± 0.07 0.79 ± 0.08 0.81 ± 0.04 |
0.8 | 0.3 (incr.) |
5.35 ± 0.62 5.33 ± 0.65 5.36 ± 0.44 |
0.7 | 0.2 (decr.) |
|
| type of feeding | roughage only roughage dominating roughage and concentrate |
17 62 61 |
867 ± 263 872 ± 193 802 ± 280 |
0.1 | 0.04 * (decr.) |
0.80 ± 0.02 0.82 ± 0.04 0.78 ± 0.08 |
0.001 *** |
0.03 * (decr.) |
5.37 ± 0.32 5.53 ± 0.38 5.16 ± 0.69 |
0.003 *** |
0.02 * (decr.) |
|
| Signalment | type of breed | horse breed pony breed |
64 76 |
836 ± 273 844 ± 218 |
0.5 | – | 0.79 ± 0.07 0.81 ± 0.05 |
0.2 | – | 5.27 ± 0.64 5.42 ± 0.48 |
0.3 | – |
| gender | male female |
89 51 |
850 ± 261 823 ± 213 |
0.4 | – | 0.80 ± 0.07 0.80 ± 0.06 |
0.6 | – | 5.34 ± 0.61 5.37 ± 0.47 |
0.5 | – | |
The unpaired comparisons (1) were done by means of unpaired Wilcoxon rank-sum tests or Kruskal-Wallis tests. The combined comparisons (2) were done by means of paired Wilcoxon rank-sum tests (within the category “disease”) or Jonckheere’s trend tests (within the category “environment/management”). CH = Culicoides hypersensitivity, SEA = severe equine asthma. Levels of significance: * p ≤ 0.05 | ** p ≤ 0.01 | *** p ≤ 0.001.
3.2. Sequences
One sample of every participating horse (n = 140) was included in the study. A total of 14’909’138 reads were recorded and these reads were clustered into 11’658 amplicon sequence variants (ASVs). The mean number of reads per sample was 106’494 (with a standard deviation of 53’494), ranging from 28’295 to 508’739 reads. A rarefaction curve was used to investigate the sequencing depth and due to the low slope of the curve, the sequencing depth was found to be sufficient (Supplementary material, Additional Figure A1).
3.3. Alpha diversity comparisons
We defined three root categories (“disease”, “environment/management” and “signalment”) and compared the three indices richness, evenness and Shannon index within several subcategories (Table 1). Within the category “disease” we could not detect significant differences, neither for unpaired comparisons between groups, nor for paired comparisons between the affected horses and their respective controls. The same applies for the type of breed and the sex (category “signalment”). In contrast, environment/management factors had significant influences on the alpha diversity values. Specifically, richness, evenness and Shannon indices were decreasing from irregular to daily (pasture access) and from a roughage-based diet to larger amounts of concentrate (feeding type).
We also investigated age as a factor. The horses included in this study ranged in age from 2 to 30 years (mean age: 15.6 years, ±6.5 years). Scatterplots with regression lines and 95% confidence intervals were created, but the regression lines remained almost parallel to the x axis (Supplementary material, Additional Figure A2), indicating that there was no significant change within our range of ages.
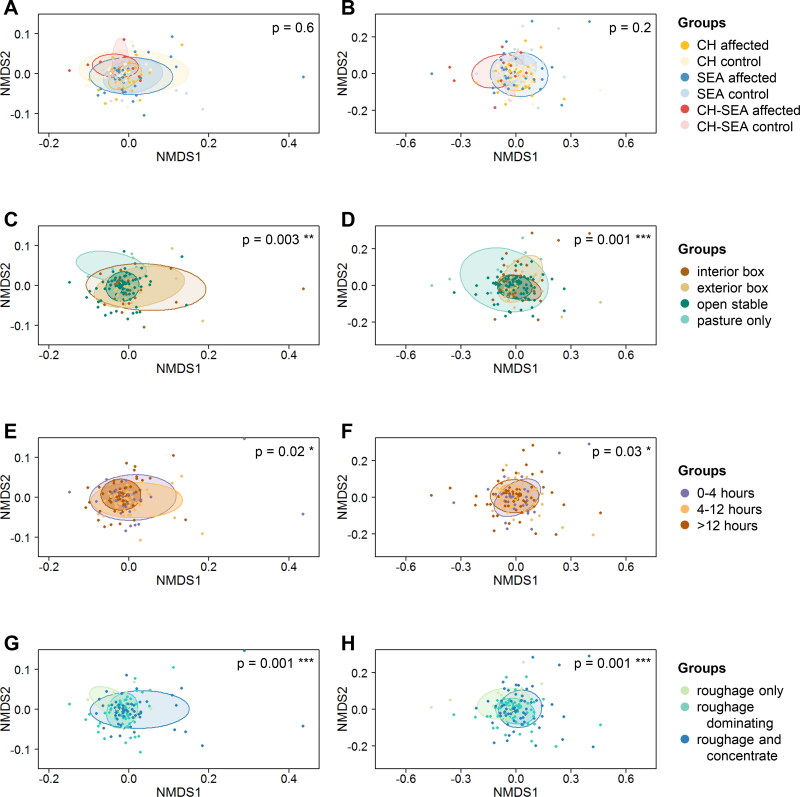
Figure 2.
Nonmetric multidimensional scaling (NMDS) plots. The plots are based on the weighted (left side) and unweighted (right side) Bray-Curtis dissimilarity distance matrices. A and B: Comparisons of study groups (CH = Culicoides hypersensitivity, SEA = severe equine asthma). C and D: Comparisons of stable types. E and F: Comparisons of pasture access rates. G and H: Comparisons of feeding types.
3.4. Beta diversity comparisons
Differences in weighted and unweighted Bray-Curtis dissimilarities were tested among the same categories as described above. Again, significant differences could only be found for comparisons of the category “environment/management”. Nonmetric multidimensional scaling (NMDS) plots were created for these comparisons (Figure 2(C–D)) as well as for the comparisons of study groups (Figure 2(A–B)).
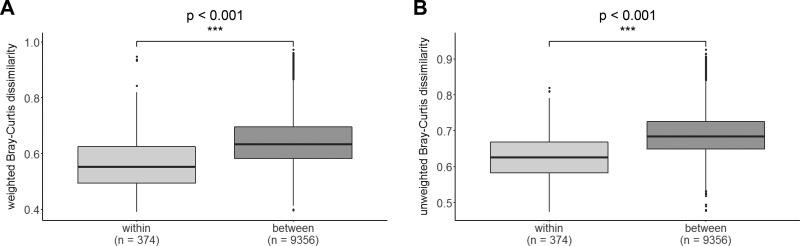
In order to determine the impact of the location (stable ID) on the equine intestinal microbiota, we then compared the weighted and unweighted Bray-Curtis dissimilarity distances of pairwise combinations of horses from the same stable (“within stable”) to all pairwise distances of horses that originated from different stables (“between stables”). There was a highly significant difference (Wilcoxon rank-sum test, p < 0.001) between these two groups with “within” distances being smaller than “between” distances (Figure 3(A–B)).
Figure 3.
Boxplots for the investigation of a “location effect”. Calculations were made based on weighted (A) and unweighted (B) pairwise dissimilarity distances. Pairwise distances between horses originating from the same stable (“within stable”) are significantly lower than pairwise distances between horses originating from different stables (“between stables”).
3.5. Diversity of bacterial taxa
The majority of ASVs belonged to the phylum Verrucomicrobia (36.3% ± 9.7%), followed by Bacteroidetes (28.0% ± 10.0%), Firmicutes (21.8% ± 5.1%), Cyanobacteria (5.1% ± 3.4%) and Proteobacteria (2.8% ± 3.2%). The remaining 6% of the bacterial population belonged to one of 24 phyla, all of which made up less than 1.5% of the relative abundance each. The most frequent ASV within each of those top 5 phyla was determined (Additional Table A2), as well as the top 20 most abundant ASVs overall (Additional Table A3). Twelve of these 20 belonged to unassigned genera from Subdivision 5 of the phylum Verrucomicrobia. Even by manually checking with the online RDP sequence match tool they could not be assigned further on a species level (Cole et al. 2005).
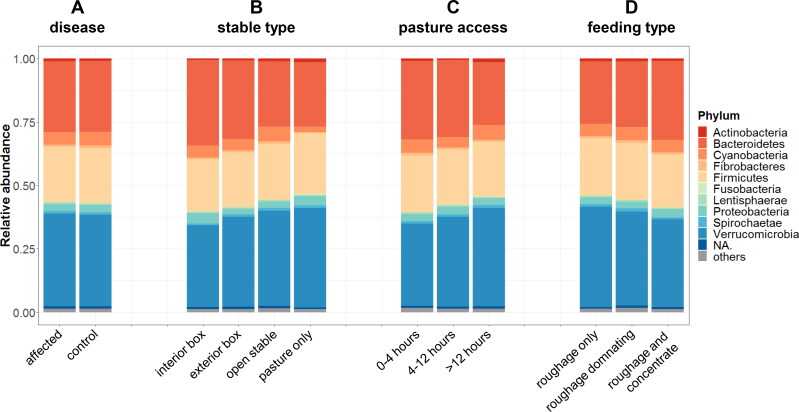
Barplots were created in order to visualize the percentage distributions of bacterial phyla for the comparison of affected vs. healthy (Figure 4(A)) and for the three environmental categories “type of stable” (Figure 4(B)), “pasture access” (Figure 4(C)) and “type of feeding” (Figure 4(D)).
Figure 4.
Mean percentage distribution of bacterial phyla. Relative bacterial abundances as a function of hypersensitivity disease (A), stable type (B), pasture access per day (C) and feeding type (D).
4. Discussion
This study offers new data regarding the equine intestinal microbiota and important influencing factors. Derived from findings in human medicine, we hypothesized that the intestinal microbiota of adult horses suffering from either CH or SEA or both differs from that of healthy horses, specifically in terms of microbial diversity and composition. However, when comparing these in affected and healthy horses, we found that neither alpha nor beta diversity indices were significantly different. This may be due to the chosen age groups, since all horses were adults. Although Hua et al. (2016) could demonstrate alterations in the fecal microbiota of adult humans suffering from allergies, many other studies focus on the neonatal time period (Kalliomäki et al. 2001; Penders et al. 2007; Craig 2016), suggesting that perturbations are more likely associated with hypersensitivities when occurring in early life (Arrieta et al. 2014; Fujimura and Lynch 2015; Tamburini et al. 2016). Abrahamsson et al. (2014) found that a reduced bacterial diversity during the first month of life is associated with the development of asthma at school age and Zheng et al. (2016) showed in a case-control study that taxa abundances in fecal samples differed between healthy infants and infants with eczema. Apparently, the resulting alterations of the microbiota in early infancy may diminish over time and may not be necessarily detectable in adulthood any longer (Bisgaard et al. 2011).
The distribution of bacterial phyla in our results agrees with some previous findings, but not others. Verrucomicrobia, for example, have not been described as a key component in the equine intestinal microbiome by some reports (Dougal et al. 2013; Venable et al. 2016; Salem et al. 2018), but the larger proportion that we found is in accordance with others (Steelman et al. 2012; Costa et al. 2015; Venable et al. 2016). There might be several reasons for this finding. First of all, different primers may have been used during PCR in the respective studies (Tremblay et al. 2015). Also, the pipeline used during sequence analysis could have an influence. In our study we used the DADA2 pipeline which infers exact amplicon sequence variants (ASVs) instead of the more traditionally employed operational taxonomic units (OTUs) with the advantage that biological differences of even 1 or 2 nucleotides can be resolved (Callahan et al. 2016). Discussion is ongoing about the respective advantages and disadvantages of ASVs and OTUs (Callahan et al. 2017). However, applying the DADA2 algorithm on three mock communities and comparing results to four other common clustering algorithms (UPARSE, MED, mothur and QIIME), Callahan et al. (2016) showed that the overall performance of DADA2 is accurate. Also, it should be kept in mind that the reference reports on the equine intestinal microbiota vary in topicality and that taxa assignment always depends on the existing databases. Regardless of this, the assignment of taxa has no influence on the calculation of alpha and beta diversities, which do not show any significant differences here between affected horses and healthy controls.
As for environmental factors affecting the microbiota we could confirm a significant influence of especially feeding as well as husbandry conditions in terms of stable type and grazing period. Clearly, the three mentioned aspects are not independent of one another. For example, horses that are kept solely on pastures also eat more or even exclusively grass (roughage), because they have unlimited access to it, whereas sport horses are frequently kept in boxes and are also fed higher amounts of concentrate to meet their energy requirements. Figure 4(B–D) illustrate this correlation. A roughly linear change especially in the percentages of Verrucomicrobia (increasing) and Bacteroidetes (decreasing) is apparent in correlation with how much a stable type limits the horse’s access to the outdoors (with “interior box” being at one end of the range to “pasture only” being at the other end). A similar interdependence can be found with regard to the feeding: with increasing amounts of concentrate fed the percentage of Verrucomicrobia decreases and the percentage of Bacteroidetes increases roughly linearly. These results underline the strong impact diet has on the gut microbiota (Albenberg and Wu 2014; David et al. 2014; Harlow et al. 2016; Julliand and Grimm 2017).
Another important observation, which we took into account, is that humans co-habiting the same household frequently share a significant proportion of their microbiota with one another (Song et al. 2013). To investigate this effect in our study population we chose to compare pairwise dissimilarity distances between two samples (Figure 3(A–B)). We found that these pairwise distances are the smallest for horses originated from the same stable, indicating that the microbiota of horses within the same stable is the most similar, i.e., a higher proportion is shared among horses co-inhabiting the same location. This is also in accordance with the findings by Kraemer et al. (2018) that found a similar “location effect” in pigs.
In the face of environmental effects as possible pitfalls, a strength of our study is the close matching of the sampled horse pairs. For every allergic horse we chose a healthy peer from the same stable for comparison and these horse pairs were therefore matched with the main focus on housing and management as well as breed, age and sex, since it is known that the latter factors can also have an effect on the intestinal microbiota (Yatsunenko et al. 2012; Hollister et al. 2014; Gomez et al. 2015).
A possible limitation of our study is the use of rectal swabs, since several other reports have shown that the microbial composition is very site specific (Suchodolski et al. 2005; Zoetendal et al. 2008; Costa and Weese 2012). Furthermore, as Thomas and Fernández-Peñas pointed out (2017), fecal samples best reflect luminal organisms of the large intestine rather than mucosal organisms that may have a greater ability to influence the immune system. We tried to account for this by not only inserting the swab into the rectum, but also gently rubbing it against the rectal mucosa. Rectal swabs were chosen despite this limitation, since they offer the most practicable and noninvasive way of sampling. More invasive methods (e.g., rectal biopsies or cecal fistulation) would not have been an option for this field study in privately owned horses.
5. Conclusion
Our observations suggest that hypersensitivity diseases in adult horses are not associated with an alteration of the intestinal microbiota, but the environment of a horse strongly affects the microbial composition. Especially the stable type and feeding exert a strong influence on the bacteria and there is also evidence for a “location effect”, as cohabiting horses share more of their microbiota with each other. These observations contribute to a better understanding of the interrelation between the intestinal microbiota of horses and their environments. The importance of considering the environmental impact on the microbiota is thus highlighted, which is informative for future studies.
Acknowledgments
A special thank you goes to all owners and their horses that participated in this study and to Susanne Aebi for her help with the laboratory work.
Glossary
ABBREVIATIONS
- ASV
amplicon sequence variant
- bp
base pair
- CH
Culicoides hypersensitivity
- HOARSI
horse owner assessed respiratory signs index
- NMDS
nonmetric multidimensional scaling
- OTU
operational taxonomic unit
- SEA
severe equine asthma
- V4
variable region 4 (of the bacterial 16Sr RNA gene)
Funding Statement
This project was funded by the Swiss National Science Foundation (grant number 31003 A-162548/1) and the ISMEquine Research Fund (Swiss Institute of Equine Medicine).
Disclosure statement
The authors declare no competing interests.
Ethics approval and consent to participate
The study protocol was approved by the Veterinary Ethical Committees of all 26 cantons in Switzerland (BE110/16+). Written informed consent was obtained from all horse owners.
Availability of data and materials
DNA reads have been deposited in NCBI’s Short Read Archive (SRA) repository under accession number PRJNA548852. Additional data files and R codes are available on figshare (DOI: 10.6084/m9.figshare.11973093).
References
- Abrahamsson TR, Jakobsson HE, Andersson AF, Björkstén B, Engstrand L, Jenmalm MC.. 2014. Low gut microbiota diversity in early infancy precedes asthma at school age. Clin Exp Allergy. 44(6):842–850. [DOI] [PubMed] [Google Scholar]
- Albenberg LG, Wu GD.. 2014. Diet and the intestinal microbiome: associations, functions, and implications for health and disease. Gastroenterology. 146(6):1564–1572. 10.1053/j.gastro.2014.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrieta M-C, Stiemsma LT, Amenyogbe N, Brown EM, Finlay B.. 2014. The intestinal microbiome in early life: health and disease. Front Immunol. 5:427–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisgaard H, Li N, Bonnelykke K, et al. . 2011. Reduced diversity of the intestinal microbiota during infancy is associated with increased risk of allergic disease at school age. J Allergy Clin Immunol. 128:646–652. [DOI] [PubMed] [Google Scholar]
- Bottcher MF, Nordin EK, Sandin A, Midtvedt T, Bjorksten B.. 2000. Microflora-associated characteristics in faeces from allergic and nonallergic infants. Clin Exp Allergy. 30(11):1591–1596. [DOI] [PubMed] [Google Scholar]
- Callahan BJ, McMurdie PJ, Holmes SP.. 2017. Exact sequence variants should replace operational taxonomic units in marker-gene data analysis. ISME J. 11(12):2639–2643. 10.1038/ismej.2017.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP.. 2016. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods. 13(7):581–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan BJ, Sankaran K, Fukuyama JA, McMurdie PJ, Holmes SP.. 2016. Bioconductor workflow for microbiome data analysis: From raw reads to community analyses. F1000Res. 5:1492–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JH, Foster CM, Vishnivetskaya T, Campbell AG, Yang ZK, Wymore A, Palumbo AV, Chesler EJ, Podar M.. 2012. Host genetic and environmental effects on mouse intestinal microbiota. ISME J. 6(11):2033–2044. 10.1038/ismej.2012.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candela M, Biagi E, Maccaferri S, Turroni S, Brigidi P.. 2012. Intestinal microbiota is a plastic factor responding to environmental changes. Trends Microbiol. 20(8):385–391. 10.1016/j.tim.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Lozupone CA, Turnbaugh PJ, Fierer N, Knight R.. 2011. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci USA. 108(Suppl. 1):4516–4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole JR, Chai B, Farris RJ, et al. . 2005. The Ribosomal Database Project (RDP-II): sequences and tools for high-throughput rRNA analysis. Nucleic Acids Res. 33:294–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa MC, Stämpfli HR, Arroyo LG, et al. . 2015. Changes in the equine fecal microbiota associated with the use of systemic antimicrobial drugs. BMC Vet Res. 11:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa MC, Weese JS.. 2012. The equine intestinal microbiome. Anim Health Res Rev. 13(1):121–128. [DOI] [PubMed] [Google Scholar]
- Couëtil LL, Cardwell JM, Gerber V, Lavoie J-P, Léguillette R, Richard EA.. 2016. Inflammatory airway disease of horses-revised consensus statement. J Vet Intern Med. 30(2):503–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig JM. 2016. Atopic dermatitis and the intestinal microbiota in humans and dogs. Vet Med Sci. 2(2):95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham FM, Dunkel B.. 2008. Equine recurrent airway obstruction and insect bite hypersensitivity: understanding the diseases and uncovering possible new therapeutic approaches. Vet J. 177(3):334–344. [DOI] [PubMed] [Google Scholar]
- David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, et al. . 2014. Diet rapidly and reproducibly alters the human gut microbiome. Nature [Internet]. 505(7484):559–563. 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougal K, de la Fuente G, Harris PA, Girdwood SE, Pinloche E, Newbold CJ.. 2013. Identification of a core bacterial community within the large intestine of the horse. PLoS One. 8(10):e77660–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura KE, Lynch SV.. 2015. Microbiota in allergy and asthma and the emerging relationship with the gut microbiome. Cell Host Microbe. 17(5):592–602. 10.1016/j.chom.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuking MB, Cahenzli J, Lawson MAE, Ng DCK, Slack E, Hapfelmeier S, McCoy KD, Macpherson AJ.. 2011. Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity. 34(5):794–806. 10.1016/j.immuni.2011.03.021. [DOI] [PubMed] [Google Scholar]
- Gomez A, Luckey D, Taneja V.. 2015. The gut microbiome in autoimmunity: sex matters. Clin Immunol. 159(2):154–162. 10.1016/j.clim.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow BE, Lawrence LM, Hayes SH, Crum A, Flythe MD.. 2016. Effect of dietary starch source and concentration on equine fecal microbiota. PLoS One. 11(4):e0154037–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollister EB, Gao C, Versalovic J.. 2014. Compositional and functional features of the gastrointestinal microbiome and their effects on human health. Gastroenterology. 146(6):1449–1458. 10.1053/j.gastro.2014.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper LV, Littman DR, Macpherson AJ.. 2012. Interactions between the microbiota and the immune system. Science. 336(6086):1268–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss JW, Reid SWJ, Christley RM.. 2007. A survey of horse owners in Great Britain regarding horses in their care. Part 2: risk factors for recurrent airway obstruction. Equine Vet J. 39(4):301–308. [DOI] [PubMed] [Google Scholar]
- Hua X, Goedert JJ, Pu A, Yu G, Shi J.. 2016. Allergy associations with the adult fecal microbiota: analysis of the American gut project. EBioMedicine. 3:172–179. 10.1016/j.ebiom.2015.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson CA, Berney C, Jefcoat AM, Robinson NE.. 2010. Environment and prednisone interactions in the treatment of recurrent airway obstruction (heaves). Equine Vet J. 32(5):432–438. [DOI] [PubMed] [Google Scholar]
- Julliand V, Grimm P.. 2017. The impact of diet on the hindgut microbiome. J. Equine Vet Sci. 52:23–28. 10.1016/j.jevs.2017.03.002. [DOI] [Google Scholar]
- Kalliomäki M, Kirjavainen P, Eerola E, Kero P, Salminen S, Isolauri E.. 2001. Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. J Allergy Clin Immunol. 107(1):129–134. [DOI] [PubMed] [Google Scholar]
- Kehrli D, Jandova V, Fey K, Jahn P, Gerber V.. 2015. Multiple hypersensitivities including recurrent airway obstruction, insect bite hypersensitivity, and urticaria in 2 warmblood horse populations. J Vet Intern Med. 29(1):320–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly D, Coutts A.. 2000. Early nutrition and the development of immune function in the neonate. Proc Nutr Soc. 59(2):177–185. [DOI] [PubMed] [Google Scholar]
- Kraemer JG, Ramette A, Aebi S, et al. . 2018. Influence of pig farming on the human nasal microbiota: key role of airborne microbial communities. Appl Environ Microbiol. 84: e02470–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahti L, Shetty S.. 2018. Microbiome R package.
- Lanz S, Brunner A, Graubner C, Marti E, Gerber V.. 2017. Insect bite hypersensitivity in horses is associated with airway hyperreactivity. J Vet Intern Med. 31(6):1877–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laumen E, Doherr MG, Gerber V.. 2010. Relationship of horse owner assessed respiratory signs index to characteristics of recurrent airway obstruction in two warmblood families. Equine Vet J. 42(2):142–148. [DOI] [PubMed] [Google Scholar]
- Leclere M, Lavoie-Lamoureux A, Lavoie JP.. 2011. Heaves, an asthma-like disease of horses. Respirology. 16(7):1027–1046. [DOI] [PubMed] [Google Scholar]
- Legatzki A, Rösler B, von Mutius E.. 2014. Microbiome diversity and asthma and allergy risk. Curr Allergy Asthma Rep. 14:1–9. [DOI] [PubMed] [Google Scholar]
- Léguillette R. 2003. Recurrent airway obstruction - heaves. Vet Clin North Am Equine Pract. 19(1):63–86. [DOI] [PubMed] [Google Scholar]
- Linneberg A. 2008. The allergic march in early childhood and beyond. Clin Exp Allergy. 38(9):1419–1421. [DOI] [PubMed] [Google Scholar]
- Marsland BJ, Trompette A, Gollwitzer ES.. 2015. The gut-lung axis in respiratory disease. Ann Am Thorac Soc. 12:150–156. [DOI] [PubMed] [Google Scholar]
- Martinez Arbizu P. PairwiseAdonis: pairwise multilevel comparison using adonis. 2018.
- McMurdie PJ, Holmes S.. 2014. Waste not, want not: why rarefying microbiome data is inadmissible. PLoS Comput. Biol. 10: e1003531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muegge BD, Kuczynski J, Knights D, Clemente JC, Gonzalez A, Fontana L, Henrissat B, Knight R, Gordon JI.. 2011. Diet drives convergence in gut microbiome functions across mammalian phylogeny and within humans. Science (80-.). 332(6032):970–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen SP, Kjaer HF, Høst A, Nielsen J, Halken S.. 2013. The natural course of sensitization and allergic diseases from childhood to adulthood. Pediatr Allergy Immunol. 24(6):549–555. [DOI] [PubMed] [Google Scholar]
- Noverr MC, Huffnagle GB.. 2005. The ‘microflora hypothesis’ of allergic diseases. Clin Exp Allergy. 35(12):1511–1520. [DOI] [PubMed] [Google Scholar]
- Oksanen J, Blanchet FG, Friendly M, et al. . 2018. Vegan: community ecology package.
- Oliveira-Filho JP, Fabris VE, Goncalves RC, Amorim RM, Chiacchio SB, Borges AS.. 2012. Clinical and histopathological aspects of the insect bite hypersensitivity in horses. Sem Ci Agr. 33(3):1113–1122. [Google Scholar]
- Penders J, Thijs C, van den Brandt PA, Kummeling I, Snijders B, Stelma F, Adams H, van Ree R, Stobberingh EE.. 2007. Gut microbiota composition and development of atopic manifestations in infancy: the KOALA birth cohort study. Gut. 56(5):661–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quast C, Pruesse E, Yilmaz P, et al. . 2013. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41:590–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . 2018. R: a language and environment for statistical computing. Vienna, Austria. https://www.R-project.org [Google Scholar]
- Ramseyer A, Gaillard C, Burger D, Straub R, Jost U, Boog C, Marti E, Gerber V.. 2007. Effects of genetic and environmental factors on chronic lower airway disease in horses. J Vet Intern Med. 21(1):149–156. [DOI] [PubMed] [Google Scholar]
- Rutten NBMM, Gorissen DMW, Eck A, Niers LEM, Vlieger AM, Besseling-van der Vaart I, Budding AE, Savelkoul PHM, van der Ent CK, Rijkers GT, et al. . 2015. Long term development of gut microbiota composition in atopic children: impact of probiotics. PLoS One. 10(9):e0137681–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem SE, Maddox TW, Berg A, et al. . 2018. Variation in faecal microbiota in a group of horses managed at pasture over a 12-month period. Sci. Rep. 8:1–10. 10.1038/s41598-018-26930-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders CJ, Shortall CR, Gubbins S, Burgin L, Gloster J, Harrington R, Reynolds DR, Mellor PS, Carpenter S.. 2011. Influence of season and meteorological parameters on flight activity of culicoides biting midges. J. Appl. Ecol. 48(6):1355–1364. [Google Scholar]
- Schaffartzik A, Hamza E, Janda J, Crameri R, Marti E, Rhyner C.. 2012. Equine insect bite hypersensitivity: what do we know? Vet Immunol Immunopathol. 147(3-4):113–126. 10.1016/j.vetimm.2012.03.017. [DOI] [PubMed] [Google Scholar]
- Sommer F, Bäckhed F.. 2013. The gut microbiota-masters of host development and physiology. Nat Rev Microbiol. 11(4):227–238. 10.1038/nrmicro2974. [DOI] [PubMed] [Google Scholar]
- Song SJ, Lauber C, Costello EK, Lozupone CA, Humphrey G, Berg-Lyons D, Caporaso JG, Knights D, Clemente JC, Nakielny S, et al. . 2013. Cohabiting family members share microbiota with one another and with their dogs. Elife. 2:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spergel JM. 2010. From atopic dermatitis to asthma: the atopic march. Ann Allergy Asthma Immunol. 105(2):99–106. 10.1016/j.anai.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Steelman SM, Chowdhary BP, Dowd S, et al. . 2012. Pyrosequencing of 16S rRNA genes in fecal samples reveals high diversity of hindgut microflora in horses and potential links to chronic laminitis. BMC Vet Res. 8:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiemsma LT, Reynolds LA, Turvey SE, Finlay BB.. 2015. The hygiene hypothesis: current perspectives and future therapies. Immunotargets Ther. 4:143–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strachan DP. 1989. Hay fever, hygiene, and household size.pdf. BMJ. 299(6710):1259–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchodolski JS, Ruaux CG, Steiner JM, Fetz K, Williams DA.. 2005. Assessment of the qualitative variation in bacterial microflora among compartments of the intestinal tract of dogs by use of a molecular fingerprinting technique. Am J Vet Res. 66(9):1556–1562. [DOI] [PubMed] [Google Scholar]
- Tamburini S, Shen N, Wu HC, Clemente JC.. 2016. The microbiome in early life: implications for health outcomes. Nat Med. 22(7):713–722. 10.1038/nm.4142. [DOI] [PubMed] [Google Scholar]
- Terán-Ventura E, Roca M, Martin MT, Abarca ML, Martinez V, Vergara P.. 2010. Characterization of housing-related spontaneous variations of gut microbiota and expression of toll-like receptors 2 and 4 in rats. Microb Ecol. 60(3):691–702. [DOI] [PubMed] [Google Scholar]
- Thoene-Reineke C, Fischer A, Friese C, Briesemeister D, Göbel UB, Kammertoens T, Bereswill S, Heimesaat MM.. 2014. Composition of intestinal microbiota in immune- deficient mice kept in three different housing conditions. PLoS One. 9(11):e113406–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CL, Fernández-Peñas P.. 2017. The microbiome and atopic eczema: more than skin deep. Australas J Dermatol. 58(1):18–24. [DOI] [PubMed] [Google Scholar]
- Tremblay J, Singh K, Fern A, Kirton ES, He S, Woyke T, Lee J, Chen F, Dangl JL, Tringe SG, et al. . 2015. Primer and platform effects on 16S rRNA tag sequencing. Front Microbiol. 6:771–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venable EB, Bland SD, McPherson JL, Francis J.. 2016. Role of the gut microbiota in equine health and disease. Anim Front. 6(3):43–49. [Google Scholar]
- Wershil BK, Furuta GT.. 2008. Gastrointestinal mucosal immunity. J Allergy Clin Immunol. 121:380–383. [DOI] [PubMed] [Google Scholar]
- Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, et al. . 2012. Human gut microbiome viewed across age and geography. Nature. 486(7402):222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Liang H, Wang Y, Miao M, Shi T, Yang F, Liu E, Yuan W, Ji Z-S, Li D-K, et al. . 2016. Altered gut microbiota composition associated with eczema in infants. PLoS One. 11(11):e0166026–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoetendal EG, Rajilić-Stojanović M, De Vos WM.. 2008. High-throughput diversity and functionality analysis of the gastrointestinal tract microbiota. Gut. 57(11):1605–1615. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
DNA reads have been deposited in NCBI’s Short Read Archive (SRA) repository under accession number PRJNA548852. Additional data files and R codes are available on figshare (DOI: 10.6084/m9.figshare.11973093).