Abstract
Convulsive seizures are known to cause severe cardiopulmonary changes and increased autonomic activity. Limited reports describe peri-ictal cardiac arrhythmias such as atrial fibrillation (AF) with generalized tonic–clonic seizures (GTCS). We present a unique case of a healthy 23-year-old male patient with new onset prolonged AF in the setting of new onset seizures, occurring on three independent occasions. Over two years, our patient had multiple hospitalizations for seizures with an electrocardiogram (ECG) diagnosis of AF made on three different occasions, occurring during his post-ictal state (all within 30 min of seizure onset). These seizures were never captured by electroencephalography (EEG) or witnessed by the medical staff, but were reported by family and/or reviewed on video provided by them. After his first GTCS, his AF persisted and was medically cardioverted. Two additional instances of AF after witnessed GTCS have been captured. After his second unprovoked seizure, an anti-seizure drug (ASD) was prescribed. A multi-disciplinary approach may be adopted to address comorbidities associated with seizures. Aggressive evaluation and treatment should be employed for newly diagnosed and refractory seizure patients associated with arrhythmias, in our case AF. Peri-ictal arrhythmias may be considered a potential marker for increased sudden unexpected death in epilepsy (SUDEP) risk.
Keywords: Atrial fibrillation, Seizures, Epilepsy, Arrhythmias, EEG, ECG, SUDEP
Highlights
-
•
Association of new onset post-ictal atrial fibrillation (AF) with new onset generalized tonic-clonic seizure (GTCS) is rare
-
•
Over a two year period, an overall healthy 23-year-old male was found to have three independent AF occurrences after GTCS
-
•
A multi-disciplinary approach and aggressive treatment with anti-seizure drugs may be adopted to address such events
1. Introduction
Convulsive seizures are associated with severe changes in cardiopulmonary activity that can result in central apnea, severe bradycardia and transient asystole [1]. Cardiac rhythm abnormalities such as sinus tachycardia, sinus bradycardia, ictal asystole, ventricular tachycardia (VT), ventricular fibrillation (VF), atrioventricular (AV) nodal block, atrial flutter and atrial fibrillation (AF) can occur during the ictal- and post-ictal phases. Among these, sinus tachycardia was identified as the most common arrhythmia associated with the generalized tonic–clonic seizures (GTCS) [2]. AF, an independent risk factor for stroke and a leading cause of death, is the most prevalent arrhythmia in general and epilepsy populations, with its prevalence relatively higher in men [3] and in patients with temporal lobe epilepsy [4]. However, there are only a few cases in the literature that report AF during the ictal- or post-ictal state [5,6]. We present a unique case of an epilepsy patient having new onset transient AF after his generalized convulsions.
2. Case
A 23-year-old African-American male with new onset seizure was also found to have new onset transient AF in his postictal period. His past medical history was significant for asthma, obesity (BMI 33.1 kg/m2), and smoking (cigarettes, marijuana). His neurological examination, initial brain imaging, electroencephalography (EEG) and trans-thoracic echocardiogram (ECG) were unremarkable during his first admission for seizure. His first GTCS was at work, described by family as shaking all over, without reported tongue biting, or urinary/bowel incontinence. With unremarkable initial work-up, along with new onset of AF at that time, an initial diagnosis of convulsive syncope was made, with no anti-seizure drug (ASD) being initiated. Subsequent seizures consisted of psychomotor arrest, progressing to GTCS as reported by family/co-workers (patient denied any auras). Hospitalization during the post-ictal phase recorded AF with a rapid ventricular response (RVR) as recorded in the ECG (Fig. 1). Within two days, he spontaneously converted to normal sinus rhythm and was started on apixaban, diltiazem and metoprolol. Follow-up portable cardiac monitoring showed no evidence of recurrent arrhythmia. Beta-blocker and apixaban were discontinued due to symptomatic bradycardia, and respectively low CHA2DS2-VASc score. Multiple prior hospitalizations over a 5-year period for knee-related injuries included an ECG/cardiac monitoring, showing normal sinus rhythm.
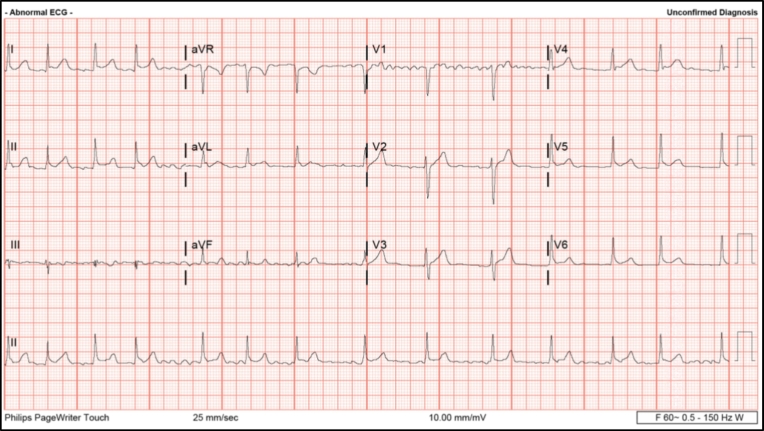
Fig. 1.
AF after the initial GTCS episode. The above EKG demonstrates the AF observed within 30 min, after the initial GTCS episode (August 2017). The heart rate during this period was 116 BPM.
Six months later, levetiracetam was started after the second unprovoked GTCS and changed to valproic acid due to behavioral side effects. Poor compliance led to several admissions after sustaining seizures. During these encounters, multiple 24-hour EEG recording showed mild background slowing and no seizure activity after the anti-seizure medication load. Subsequent brain MRI showed mild asymmetry of hippocampi with no abnormality in the temporal lobes.
Fourteen months after the first seizure, the patient was brought to the emergency department with a prolonged seizure, lasting over 5 min, when the second prolonged AF with RVR was identified. A third seizure associated with AF and RVR prompting hospitalization was captured by family on video and consisted of confusion, right arm tonic posturing, forced head deviation to the right, and progression to a GTCS. A follow-up epilepsy monitoring unit (EMU) recording to determine seizure localization prompting anti-seizure medication discontinuation, captured one focal seizure with left temporal onset and psychomotor arrest but no changes in the cardiac rate or rhythm (Fig. 2). As per EMU protocol, he was immediately treated with lorazepam to avoid secondary generalization and to prevent sudden unexpected death in epilepsy (SUDEP). At this time the patient realized the importance of medication compliance and his epilepsy is currently well controlled with two ASDs.
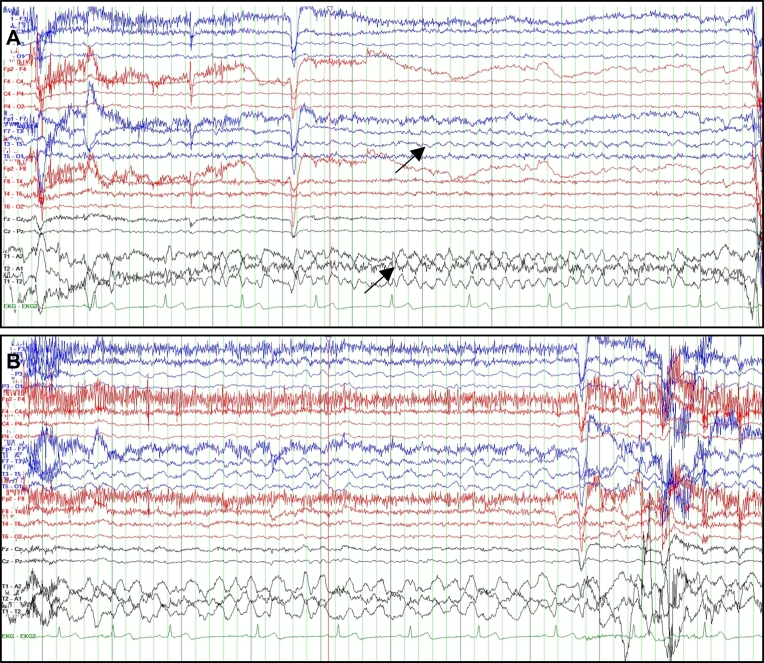
Fig. 2.
EEG during EMU admission (Feb 2019). The EEG of the patient, (A) at the beginning of the seizure episode (the top arrow shows left temporal moderate rhythmic theta activity), and (B) at the end of the seizure (one-minute duration). The heart rate (green), was consistent during episode. The patient did not progress to a GTCS. The two images are at sensitivity 10.
3. Discussion
In convulsive seizures, there is an increased sympathetic activity that is reflected as a peak in catecholamine and electrodermal activity [7]. During the post-ictal state following GTCS, there is an autonomic dysregulation with increased sympathetic and decreased parasympathetic outflow. This may generate fatal arrhythmias and potential SUDEP [7,8]. In addition, the association between arrhythmias and seizures could be due to the direct stimulation of the central autonomic centers [2,9,10]. Seizures originating from the amygdala, hippocampus, insular cortex, and the frontotemporal regions can produce a wide range of cardiac abnormalities [11,12] with studies suggesting that the insular cortex has a direct influence on the cardiac rate and rhythm [4,11]. We observed that our patient had a past history of hypertension and obesity. Cardiovascular comorbidities and their associated risk factors are more common in adults with chronic epilepsy [2,13], particularly those who have a higher incidence of hypertension and obesity. Several of these factors pose a risk towards the development of stroke.
Fatal arrhythmias and ECG abnormalities are more likely to be observed during or after a generalized seizure and with prolonged convulsive activity [9,10]. Studies indicate that ictal asystole, VT and VF are associated with SUDEP [7]. Concerns have been expressed over AF compromising ventricular output, and thus could be a precipitating factor for SUDEP [6]. There are very few reports showing post-ictal AF diagnosed during video-EEG monitoring [14]. A study reported a past medical history of AF in two patients with SUDEP but ictal or immediate post-ictal ECG recording in these patients were not available [15]. Clinical evidence showing association between AF and SUDEP is scant and it has been suggested that peri-ictal arrhythmias should be considered as a marker for increased SUDEP risk [7].
We report three instances of new onset and transient postictal AF after GTCS in our patient, occurring over a period of 24 months. Between his first and second AF associated with seizures events described above, the patient had multiple other seizures prompting five additional ER evaluations or ICU hospitalizations during which AF was not demonstrated on ECG. None of his GTCS occurred while in the hospital, therefore the exact timing of onset of AF in relation to the ictal onset cannot be quantified. It has been hypothesized that following GTCS, there is a period of severe autonomic dysregulation strongly characterized by excessive sympathetic activation in tandem with parasympathetic suppression in the early post-ictal phase; the later phase seems to be dominated by impaired vagal recovery. AF can induce cardiac failure with severe hemodynamic repercussions, and it is a well-known cause of stroke. To our knowledge, a single prior report was published showing post-ictal AF/flutter lasting just minutes as a marker for SUDEP [14]. We considered our patient to have a much higher risk of stroke and/or SUDEP, due to prolonged post-ictal AF, lasting over two days. The post-ictal AF connected with the GTCS presented as an RVR, hence it was important to maintain the sinus rhythm and prevent peripheral embolization [14]. It is worthwhile considering whether or not post-ictal AF needs to be treated, since seizure control in itself is likely to prevent this arrhythmia. An extensive cardiac work-up is vital in these patients to differentiate between peri-ictal AF related to GTCS and those unrelated to seizures. If at all, the risk of SUDEP is unclear, because AF is considered a low-mortality arrhythmia, an implantable cardioverter-defibrillator is not generally indicated, although each case must be individually assessed. A 24-hour Holter ECG is essential to exclude intrinsic cardiac abnormalities [4,11]. Simultaneous video EEG and ECG recordings can establish the relationship between the seizure and the arrhythmia [15].
Patients with AF are at the risk of stroke and systemic embolism without anticoagulant therapy. Seizure control is paramount in all patients, especially if associated with peri-ictal cardiac rhythm abnormalities. ASD selection plays an important role in the patients with intrinsic cardiac conductive tissue dysfunction. Lamotrigine, valproate and gabapentin have a minimal effect on the cardiac function whereas carbamazepine, phenytoin, barbiturates and benzodiazepines are linked with conduction abnormalities [4]. It is vital to closely monitor patients having post-ictal AF after seizures, with primary focus being given to the seizure control. Our patient had a low CHA2DS2-VASc score, therefore the anticoagulation therapy was discontinued. In general, a patient with a high CHA2DS2-VASc score requires anticoagulation therapy. Patients with history of ASD non-compliance or with refractory seizures, that are prescribed anticoagulation therapy, are at risk for prolonged or severe bleeding, especially if they sustain injuries during seizures. Hence, it is important to choose an ASD medication with good efficacy and minimal drug-to-drug interaction and an anticoagulant that can be quickly reversed.
4. Conclusions
Post-ictal AF, though rarely reported, is a highly dangerous seizure complication. GTCS associated with prolonged arrhythmias such as AF can complicate the healthcare management in patients. AF, when linked with GTCS, has been associated with post-ictal generalized EEG suppression and autonomic dysregulation, and can be potentially connected to SUDEP [14]. A multi-disciplinary approach should be an integral part of AF management if related to seizures, in order to prevent stroke. Aggressive control of the seizures can prevent post-ictal AF occurrence.
The following is the supplementary data related to this article.
The seizure episode of patient captured by family when the third AF was identified.
Authorship of the paper
P.B., M.E., S.N., and A.P. collected and analyzed the literature, and wrote the manuscript. P.B., M.E., A.H., S.N., and R.B. analyzed the literature. C.J., V.H., M.E., and A.P. reviewed and edited the manuscript. All authors read and agreed to the final version of the manuscript.
Ethical statement
As referenced by Epilepsy and Behavior Case Reports, all authors report that they have conformed to the principles of ethics in publishing and ethical guidelines for journal publication. Patient consent is provided.
Declaration of competing interest
The authors have no conflicts of interest to disclose related to this manuscript.
References
- 1.Ryvlin P., Nashef L., Lhatoo S.D., Bateman L.M., Bird J., Bleasel A. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet Neurol. 2013;12(10):966–977. doi: 10.1016/S1474-4422(13)70214-X. [DOI] [PubMed] [Google Scholar]
- 2.Shmuely S., van der Lende M., Lamberts R.J., Sander J.W., Thijs R.D. The heart of epilepsy: current views and future concepts. Seizure. 2017;44:176–183. doi: 10.1016/j.seizure.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Desai R., Rupareliya C., Patel U., Naqvi S., Patel S., Lunagariya A. Burden of arrhythmias in epilepsy patients: a nationwide inpatient analysis of 1.4 million hospitalizations in the United States. Cureus. 2017;9(8):e1550. doi: 10.7759/cureus.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lim E.C., Lim S.H., Wilder-Smith E. Brain seizes, heart ceases: a case of ictal asystole. J Neurol Neurosurg Psychiatry. 2000;69(4):557–559. doi: 10.1136/jnnp.69.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basili L.M., Morano A., Fattouch J., Fanella M., Albini M., Avorio F. Ictal atrial fibrillation during focal seizures: a case report and literature review. Epileptic Disord. 2019;21(3):295–301. doi: 10.1684/epd.2019.1070. [DOI] [PubMed] [Google Scholar]
- 6.Surges R., Moskau S., Viebahn B., Schoene-Bake J., Schwab J., Elger C. Prolonged atrial fibrillation following generalized tonic–clonic seizures. Seizure - European Journal of Epilepsy. 2012;21(8):643–645. doi: 10.1016/j.seizure.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Lende M., Surges R., Sander J.W., Thijs R.D. Cardiac arrhythmias during or after epileptic seizures. J Neurol Neurosurg Psychiatry. 2016;87(1):69. doi: 10.1136/jnnp-2015-310559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poh M.Z., Loddenkemper T., Reinsberger C., Swenson N.C., Goyal S., Madsen J.R. Autonomic changes with seizures correlate with postictal EEG suppression. Neurology. 2012;78(23):1868–1876. doi: 10.1212/WNL.0b013e318258f7f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nei M., Ho R.T., Sperling M.R. EKG abnormalities during partial seizures in refractory epilepsy. Epilepsia. 2000;41(5):542–548. doi: 10.1111/j.1528-1157.2000.tb00207.x. [DOI] [PubMed] [Google Scholar]
- 10.Devinsky O. Effects of seizures on autonomic and cardiovascular function. Epilepsy Curr. 2004;4(2):43–46. doi: 10.1111/j.1535-7597.2004.42001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kishk N., Nawito A., El-Damaty A., Ragab A. Ictal asystole: a case presentation. BMC Neurol. 2018;18(1):100. doi: 10.1186/s12883-018-1105-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schernthaner C., Lindinger G., Potzelberger K., Zeiler K., Baumgartner C. Autonomic epilepsy—the influence of epileptic discharges on heart rate and rhythm. Wien Klin Wochenschr. 1999;111(10):392–401. [PubMed] [Google Scholar]
- 13.Kadima N., K. R., Z. M., Helmers S. Comorbidity in adults with epilepsy—United States, 2010. Center for Disease Control and Prevention MMWR. 2013;62:849–853. [PMC free article] [PubMed] [Google Scholar]
- 14.Sanchez-Larsen A., Aznar-Lain G., Benito B., Principe A., Ley M., Tauste Campo A. Post-ictal atrial fibrillation detected during video-EEG monitoring: case report, proposed physiopathologic mechanism and therapeutic considerations. Epilepsy Behav Case Rep. 2017;8:40–43. doi: 10.1016/j.ebcr.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nei M., Ho R.T., Abou-Khalil B.W., Drislane F.W., Liporace J., Romeo A. EEG and ECG in sudden unexplained death in epilepsy. Epilepsia. 2004;45(4):338–345. doi: 10.1111/j.0013-9580.2004.05503.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The seizure episode of patient captured by family when the third AF was identified.