Abstract
Objective
Metformin, an oral medication used for type 2 diabetes mellitus, is the most commonly prescribed drug with less economic burden of patients. Although metformin’s efficacy and safety have long been recognized, approximately 5% of the patients treated with this drug develop severe diarrhea as an adverse effect and have to abandon treatment. Because there is no animal model to study metformin-induced diarrhea, it is hard to develop methods to maintain quality of life of patients prescribed with metformin.
Research design and methods
Using mouse models, we tried to develop an evaluation system for metformin-induced diarrhea to improve diarrheal symptoms in patients with diabetes. Healthy (C57BL/6J) and diabetic obese (db/db) mice were subjected to a stepwise dose escalation of metformin (250 mg/kg/day (125 mg/kg twice daily oral dose)—1000 mg/kg/day (500 mg/kg twice daily oral dose)), and fecal moisture contents and their score were monitored. To evaluate anti-diarrheal medications, wood creosote (a traditional medicine) was tested. Several groups of enterobacteria in fresh feces were examined by using PCR.
Results
1000 mg/kg/day (four times maximal effective dose) of metformin significantly increased fecal moisture content. Although no symptoms of diarrhea were observed in healthy C57BL/6J mice, the same dose of metformin induced severe diarrhea in diabetic obese db/db mice. A reduction in PCR signals for the Firmicutes group was associated with metformin-induced diarrhea. Wood creosote reduced diarrhea (high water-content) without affecting metformin’s efficacy or enterobacterial flora levels.
Conclusions
We have created the first animal model of metformin-induced diarrhea using db/db mice, which will provide better quality of life for patients suffering from diarrhea caused by metformin.
Keywords: metformin, diarrhea, mouse model(s), oral antidiabetics
Significance of this study.
What is already known about this subject?
Several risk factors of metformin-induced diarrhea have been proposed, such as obesity and liver disfunctions.
It is hard to provide a clue to reduce metformin-induced diarrhea.
What are the new findings?
A combination of a moderately high dose of metformin and diabetic obese mice with liver dysfunctions reproduced diarrheal symptoms observed in human patients.
The diarrheal symptoms in our mice models were ameliorated by the co-treatment with wood creosote, a traditional medicine commercially available for diarrhea.
How might these results change the focus of research or clinical practice?
Our extrapolation model will provide new insights in metformin-induced diarrhea and better quality of life for patients with not only severe symptoms (5% of patients), but also mild symptoms (30% of patients).
Introduction
Metformin, the first-line drug for the treatment of type 2 diabetes mellitus, is the most frequently prescribed medication worldwide.1 It attenuates mitochondrial electron transport, which increases the AMP/ATP ratio and lead to activation of AMP-activated kinase (AMPK).2 Active AMPK inhibits gluconeogenesis and promotes glycolysis, improving impaired glucose tolerance. Despite the well-established clinical benefits of treatment with metformin, digestive disorders, diarrhea and vomiting, are reported in approximately 30% of the patients who have been prescribed this drug.3–5 Additionally, 5% of patients suffered symptoms so severe they had to abandon the treatment.
Retrospective studies on metformin induced diarrhea have identified several risk factors, such as daily dose, age, sex, body mass index, and liver and biliary disorders.6 However, no digestive disorders have been observed in experimental animal models. Nevertheless, a few descriptions of diarrhea in experimental animals have been reported in interview forms for the approval of metformin.7 Animals administered with a semi-lethal dose of metformin had diarrhea with other adverse effects such as breathing difficulty, body weight loss, a decrease in a spontaneous activity, ataxia, and asitia (PubChem CID: 14219). These backgrounds show that there is a lack of extrapolating animal models of metformin-induced diarrhea, which makes it difficult to develop methods to treat patients suffering from metformin-induced diarrhea.
Here we report that administration of a high dose (500 mg/kg twice daily oral dose) of metformin to healthy mice (C57BL/6J) increases their fecal moisture content. Diabetic obese mice C57BL/6Jdb/db(db/db) with impaired liver function had severe diarrhea after the same treatment. The diarrheal symptom was ameliorated by the co-treatment with wood creosote, a traditional medicine commercially available for diarrhea. These results demonstrated that our study is the first experimental model for metformin-induced diarrhea, and the model may provide new mechanistic insights into metformin-induced digestive disorders and be used to design drugs to improve the quality of life of patients with diabetes.
Research design and methods
Mouse experiments
C57BL/6J and db/db mice (10W male) were purchased from SLC Japan (Shizuoka, Japan). The animals were maintained under a 12-hour light/dark cycle (08:00–20:00) and received a standard rodent diet CE-2 (CLEA Japan, Tokyo, Japan).
Metformin hydrochloride (Combi-Blocks, San Diego, California, USA) and wood creosote8 were suspended in 0.5% Tween 80 (Kanto Kagaku, Tokyo, Japan). The drugs were orally administrated to the mice twice daily at 18:00–20:00 and 8:00–10:00. Body weight was measured, and feces samples were collected in the evening following drug administration.
Measurement of fecal moisture content, scoring of feces, and blood biochemistry
Four to six mouse feces were collected into 1.5 mL sample tubes and weighed. The samples were dehydrated overnight and the weight was measured again. Scoring of feces are as follows; 0: normal, 1: stick to forceps, 2: easily collapsed, 3: unformed feces.
Blood biochemistry
One to 2 µL of blood was collected via the tail vein of the mice, and glucose levels were measured by using G-sensor (Arkray, Kyoto, Japan). Blood samples with glucose levels over 600 mg/dL were diluted with phosphate buffered saline (PBS) and the levels were measured by using the glucose assay kit-WST (Dojin, Tokyo, Japan). Serum bile acid and alanine aminotransferase (ALT) were measured with Total Bile Acids Assay Kit (Diazyme, Poway, California, USA) and ALT activity colorimetric kit (BioVison, Milpitas, California, USA), respectively.
Western blotting
For western blotting, a piece of liver was homogenized in PBS buffer with the protease inhibitor cocktail (Nacalai, Kyoto, Japan), and 50 µg of total protein was suspended in Laemmli SDS sample buffer. Antibodies are as follows: Anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (1E6D9, Proteintech, Rosemont, Illinois, USA), anti-AMPK (ABV10739, ABGENT, San Diego, California, USA), anti-phospho AMPK (pT172) (40H9, Cell Signaling Technology, Danvers, Massachusetts, USA), anti-total Protein Kinase B (AKT) (200–401 N98, Rockland, Limerick, Pennsylvania, USA), and anti-phosho AKT (pS473) (D9E, Cell Signaling Technology).
Analysis of enterobacteria
To analyze the enterobacteria in mouse feces, one drop of fresh feces was directly suspended in 600 µL denaturation buffer associated with a DNA purification kit (GP1 buffer in Fast Gene Gel/PCR Extraction kit, Nippon Genetics Co., Tokyo, Japan). After the removal of insoluble reside by centrifugation at 12 000 G for 20 min, 500 µL of the supernatant was applied to a silica gel columns in accordance with the manufacturer’s recommendation. To amplify the 16S rRNA of enterobacteria, Expand High Fidelity PCR System (Merck, Burlington, Massachusetts, USA) was used with 1 µL of purified DNA solution and primer sets that had been previously validated9 : Universal (926F), AAACTCAAAKGAATTGACGG and (1062R), CTCACRRCACGAGCTGAC; Betaproteobacteria (Beta979F), AACGCGAAAAACCTTACCTACC and (Beta1130R), TGCCCTTTCGTAGCAACTAGTG; Firmicutes (Firm934F), GGAGYATGTGGTTTAATTCGAAGCA and (Firm1060R), AGCTGACGACAACCATGCAC; Bacteroidetes (Bac960F), GTTTAATTCGATGATACGCGAG and (Bac1100R) TTAASCCGACACCTCACGG. To obtain amplicon images, the following PCR conditions were applied: 94°C, 20 s; 55°C, 20 s; 72°C, 20 s (19 cycles for Universal, 20 cycles for Firmicutes and Bacteroidetes, and 25 cycles for Betaproteobacteria). To quantify the amplicons, quantitative PCR (qPCR) was used with Thunderbird Sybr qPCR Mix (Toyobo, Osaka, Japan) and MyiQ (BioRad, Hercules, California, USA). The signals for Betaproteobacteria, Firmicutes, and Bacteroidetes were normalized to that of Universal.
Antibiotics, penicillin (1000 U/mL), and streptomycin (1000 µg/mL) (Nacalai Tesque, Kyoto, Japan), were dissolved in drinking water and provided for 5 days. For the final 2 days, metformin (2×500 mg/kg/day) was also administered to the mice.
Statistical analysis
For all experiments, data are expressed as mean±SEM. One-way analysis of variance (ANOVA) was used to analyzed data of control, metformin, and wood creosote groups, and Student’s t-test was used to compare data from the metformin and wood creosote group.
Data and resource availability
The data sets generated during the current study are available from websites (see Data Availability Statement). We use only commercially available materials except for wood creosote. To request the wood creosote, contact Dr. Masafumi Ito (masafumi.itoh@seirogan.co.jp, Department of Quality Assurance, Taiko Pharmaceutical Co., Osaka, 550–0005, Japan). For other requests, contact to corresponding author or The Organization for Research and Community Development, Gifu University (orchid@gifu-u.ac.jp).
Results
Metformin induces severe diarrhea in obese diabetic mice
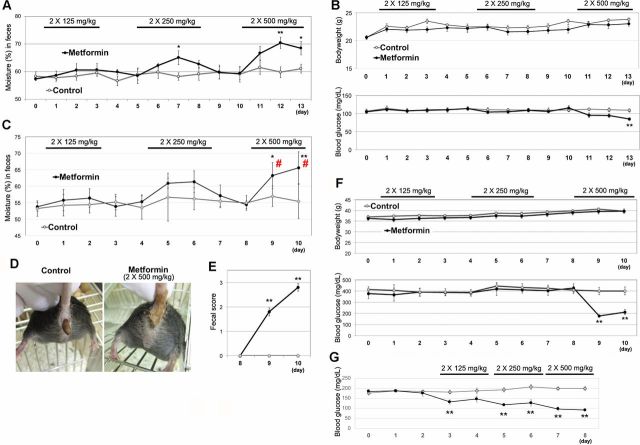
Hypoglycemic effects of metformin are observed at the dose of 250 mg/kg in mice.10 However, the median lethal dose, LD50, of metformin in mice had been reported to be approximately 1500 mg/kg.11 We first carried out a stepwise increase in administration of metformin to healthy mice (C57BL/6J), from 250 mg/kg to 1000 mg/kg. For clinical relevancy, metformin was orally administered twice per day, for 3 days with 2-day intervals. Although metformin at even 500 mg/kg twice daily (daily dose of 1000 mg/kg) did not cause diarrhea in mice, we observed an increase in fecal moisture levels when metformin was administrated at 250 mg/kg twice daily and 500 mg/kg twice daily (figure 1A). There was no significant change in the body weights of the mice (figure 1B). Unexpectedly, blood glucose levels were lowered only when a high dose (500 mg/kg twice daily) of metformin was administered under fed conditions.
Figure 1.
Metformin induces severe diarrhea in diabetic obese mice. (A) Ten-week-old male C57BL/6J mice (n=5) were orally administered metformin with a stepwise increase in dose from 125 mg/kg twice daily to 500 mg/kg twice daily, for 3 days with 2-days interval (without administration). Wet weight of feces, collected into sample tubes, was measured. The feces were dehydrated (90°C for overnight), and the dry weight was measured to determine the feces moisture content. Data are shown as means±SEM. *P<0.05, **p<0.01 between the control group (saline alone) and the metformin group. (B) Bodyweight (upper) and blood glucose (lower) changes of 10-week-old male C57BL/6J mice (in figure 1A) were monitored. P<0.05, **p<0.01 between the metformin group and the co-treatment group. (C) Ten-week-old male db/db mice (n=6) also received metformin with a stepwise increase in dose from 125 mg/kg twice daily to 500 mg/kg twice daily, for 2 days with 2-days interval. # indicates that the values (feces moisture content) are not accurate, due to severe diarrhea. (D) Representative image of the severe diarrhea. (E) Fecal score (at day 8–10) was calculated as follows. 0: normal, 1: stick to forceps, 2: collapsed by forceps, 3: unformed feces. (F) Bodyweight (upper) and blood glucose (lower) changes of 10-week-old male db/db mice (in figure 1B) were monitored. (G) Blood glucose levels after a 3-hour fast were monitored in a different group of db/db mice (10-week-old male mice, n=5).
In patients with diabetes, the incidence of metformin-induced diarrhea increases in the presence of risk factors such as body mass index. Therefore, we carried out the same dose-escalation study in diabetic obese mice. Feces moisture content did not change when db/db mice were administrated low (125 mg/kg twice daily) to medium (250 mg/kg twice daily) doses of metformin (figure 1C). However, severe diarrhea was observed when a high dose (500 mg/kg twice daily) of metformin was administrated (figure 1D, E). We observed that basal levels (in the absence of metformin treatment) of the moisture in the feces of db/db mice were lower than those in healthy C57BL/6J mice, suggesting the presence of unknown differences in the physiological environment of the db/db mice gut.
A loss in body weight was not observed during the experiment (figure 1F). Only db/db mice treated with the high dose (500 mg/kg twice daily) of metformin showed a decrease in the blood glucose levels. We surmised that the apparent lack of effect of the low and medium doses of metformin on blood glucose levels may have been a result of the fed condition of mice. Therefore, we examined blood glucose levels in mice after a 3-hour fast (figure 1G) and confirmed that the low dose (125 mg/kg twice daily) of metformin decreased the blood glucose level in db/db mice, indicating that 500 mg/kg twice daily metformin was four times higher than the effective dose for db/db mice.
Administration of wood creosote reduced metformin-induced diarrhea in mice
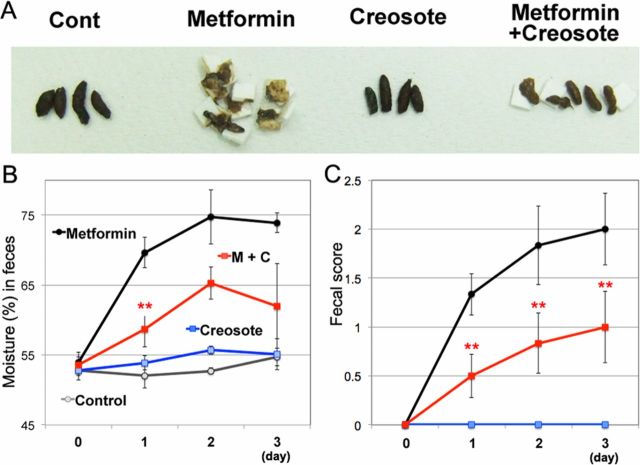
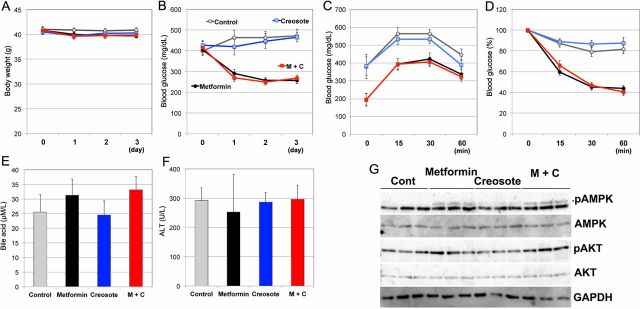
Next, we examined whether our mouse model was able to evaluate anti-diarrheal medications. We assessed the effects of wood creosote, a mixture that is used as an anti-diarrheal medication. First, we examined effective dose of wood creosote (figure 2A): 5 mg/kg twice daily of wood creosote decreased excess fecal moisture content in C57BL/6J mice administered 500 mg/kg twice daily of metformin. Additionally, wood creosote (5 mg/kg twice daily) had no effects on fecal moisture content (figure 2B) or fecal score (figure 2C) in animals, which did not receive metformin. We did not observe adverse effects such as weight loss (figure 2D), interferences with hypoglycemic effects (glucose tolerance (figure 2E)), changes in serum bile acid levels (figure 2F), and liver function (ALT (figure 2G)).
Figure 2.
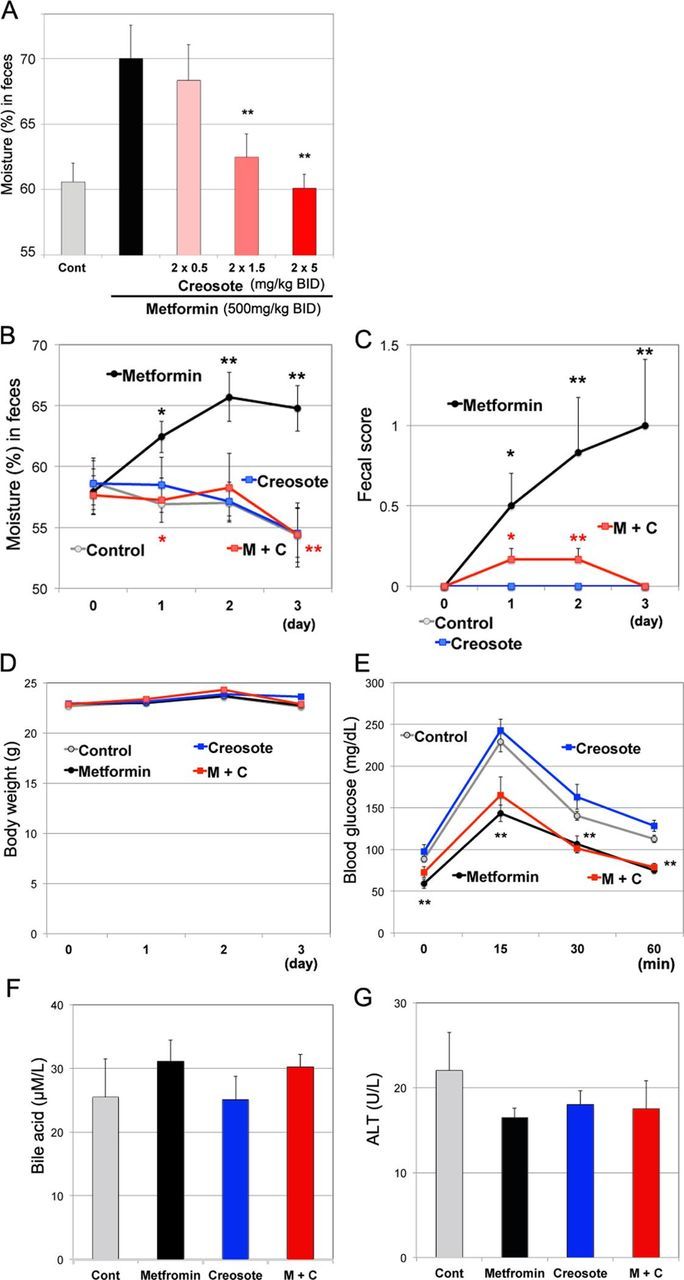
Effects of wood creosote on healthy mice treated with metformin. (A) Ten-week-old male C57BL/6J mice (n=4) were orally administered metformin (500 mg/kg twice daily) and different dose of wood creosote (<5 mg/kg twice daily). At 8 hours after the second treatment, feces were collected into sample tubes. Bars indicate mean fecal moisture (%)±SEM. *P<0.05, **p<0.01 between the metformin group and the co-treatment group. Ten-week-old male C57BL/6J mice (n=6) received metformin (500 mg/kg twice daily) with or without wood creosote (5 mg/kg twice daily) for 3 days. The changes of fecal moisture (B), fecal score (C), and body weight (D) were monitored. (E) Mice were fasted for 3 hours after the final treatment. One g/kg glucose was administrated via orally. One to 2 µL of blood was collected via tail vein at indicated time points, and blood glucose was measured using GlucoSensor. At day 4, blood was collected from the ventricle from anesthetized animals. The levels of serum bile acids (F) and alanine aminotransferase (ALT) (G) were measured. M+C, metformin+creosote.
We also assessed the effect of wood creosote administration on metformin-induced diarrhea in diabetic obese mice. Co-administration of wood creosote (5 mg/kg twice daily) and metformin (500 mg/kg twice daily) significantly suppressed diarrhea (figure 3A, B). Mice co-administered wood creosote and metformin excreted feces that could be picked up with forceps (figure 3C). No adverse effects or interactions between metformin and wood creosote were observed (figure 4A–F). Moreover, wood creosote did not inhibit the effects of metformin on AMPK and AKT phosphorylation in the liver of db/db mice (figure 4G). These results suggest that our mouse model can be used to evaluate the efficacy of commercially available medications for metformin-induced diarrhea.
Figure 3.
Wood creosote reduces diarrhea induced by metformin in obese diabetic mice. (A) Collected feces from the different experimental groups. (B) Effects of metformin (500 mg/kg twice daily) and wood creosote (5 mg/kg twice daily) co-administration on feces moisture content was examined (n=6). Red ** indicates p<0.01 between the metformin group and the co-administered group (M+C). (C) Fecal score. Data are shown as mean±SEM. *P<0.05, **p<0.01 between the control group (saline alone) and the metformin group. M+C, metformin+creosote.
Figure 4.
Effects of wood creosote on diabetic obese mice treated with metformin. Wood creosote does not affect efficacy of metformin. Weight change (A) and blood glucose levels (fed condition) (B) were examined during the experiment in figure 3. Data are shown as means±SEM. No significant differences between the metformin group and the metformin with wood creosote (M+C) group were observed. Mice were fasted for 3 hours after the final administration of drugs. (C) One g/kg glucose was administrated via oral gavage. One to 2 µL blood was collected via tail vein at indicated time points, and blood glucose was measured by using GlucoSensor. (D) At day 4 of figure 3, mice were fasted for 2 hours and then injected intraperitoneally with 36 µg/kg insulin. Blood glucose levels are shown as % initial. At day 5 in figure 3, blood was collected and measured serum bile acid levels (E) and alanine aminotransferase (F) were measured. (G) Western blots were carried out using liver protein (50 µg) to detect levels of phospho-AMPK and phoshopho-AKT. AKT, Protein Kinase B; AMPK, AMP-activated kinase.
Reduced PCR signals for the Firmicutes phylum was partially associated with metformin-induced diarrhea
Diarrhea symptoms are often associated with changes in the enterobacterial flora, which alter the metabolic environment of the gut.12 13 Because the gastrointestinal microbiome modulator improved gastrointestinal intolerance in human patients treated with metformin, the modification of enterobacterial flora has been proposed to be a cause of the gastrointestinal intolerance.14 In addition, in mice, the genetic background, including obesity and diabetic phenotypes (such as db/db mice), and metformin treatment are known to induce the modification of enterobacterial flora.15–18 Therefore, we examined the differences in enterobacterial flora in metformin-induced diarrhea.
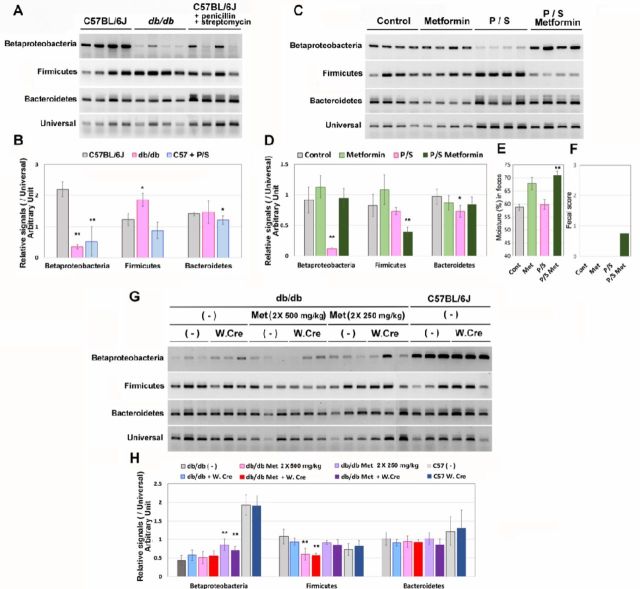
As the flora of Betaproteobacteria and Firmicutes were found to change in db/db or metformin-treated mice,18 we measured the ratio of these groups. Bacteroidetes, the major enterobacterial group, is often used for the normalization of the target enterobacterial flora group. To forcibly modify the flora, mice (healthy, C57BL/6J) were treated with penicillin and streptomycin for 3 days. Fresh feces were directly suspended in denaturation buffer, and fecal DNA was purified and amplified by using 16S rRNA from each primer set. To quantify the amplicons, qPCR analysis followed by normalization to signals from the Universal primer set was used.
As shown in figure 5A, B, the signals for Bacteroidetes were low in db/db mice feces, and those for Firmicutes were slightly high. The penicillin and streptomycin treatment also reduced the signals for Bacteroidetes. To determine if these differences were associated with metformin-induced diarrhea, C57BL/6J mice were treated with metformin (500 mg/kg twice daily) for 2 days after the 3 days of penicillin and streptomycin treatment (figure 5C, D). Interestingly, the level of the signals for Bacteroidetes was restored by the metformin treatment; in contrast, the level of the signals for Firmicutes was significantly lowered. Fecal moisture (figure 5E) and score (figure 5F) were also increased by the metformin treatment in mice pretreated with penicillin and streptomycin, suggesting that the reduction in Firmicutes group levels may be associated with the elevation of fecal moisture in the metformin-treated mice.
Figure 5.
Difference in enterobacterial flora in db/db mice and metformin-treated mice. Enterobacterial DNA was prepared from the fresh feces of healthy (C57BL/6J), db/db, and healthy mice treated with antibiotics (penicillin and streptomycin) treatment for 3 days (10-week-old male mice, n=4, each). The DNA was analyzed by PCR, gel electrophoresis (A), and qPCR (B). The bars indicate the SD; * and ** indicate p<0.05 and p<0.01, respectively. C57BL/6J mice (n=4) that had been treated with the antibiotics (P/S: penicillin/streptomycin) for 3 days were treated with or without metformin (500 mg/kg twice daily) for 2 days with the antibiotics. The enterobacterial flora were analyzed by PCR (C and D). Fecal moisture (E) and score (F) were also analyzed on the final day. db/db mice (10-week-old male mice, n=6) were treated with metformin (Met: 250 mg/kg twice daily for 2 days followed by 500 mg/kg twice daily for 2 days) with or without wood creosote (W. CRE: 5 mg/kg twice daily). (-) indicates without metformin or without wood creosote, as appropriate. Diarrhea was observed in db/db mice treated only with metformin (500 mg/kg twice daily). (G) and (H) show the results of PCR analyses of the enterobacterial flora.
Finally, enterobacterial flora was examined in db/db mice treated with both metformin and wood creosote (figure 5G, H). Although the levels of Betaproteobacteria were partially restored after treatment with the medium dose of metformin (250 mg/kg twice daily), the levels were restored to those of the untreated db/db mice (control) by treatment with the high dose of metformin (500 mg/kg twice daily), suggesting that different sub-species in the Betaproteobacteria group may contribute to the discrepancies between the modulate and high doses of metformin.
Similar to the antibiotic treated mice, the Firmicutes levels were suppressed in mice treated with the high dose of metformin, and the mice showed diarrhea symptoms. However, the treatment of mice (both db/db and C57BL/6J) with wood creosote did not alter the levels for these enterobacterial groups. These results suggested that the reduced levels of Firmicutes may be associated with diarrhea symptom, but that this may not be the only factor.
Discussion
Descriptions about metformin-induced diarrhea in model animals have only been documented as preclinical information (eg, National Library of Medicine, Toxicology Data Network). Diarrhea has been described (PubChem CID: 14219), along with other adverse effects such as reduced spontaneous activity and ataxia, in healthy mice that received an extremely high dose of metformin (≧ 1500 mg/kg, (lethal dose is ≧ 1920 mg/kg)).19 Administration of metformin over the lethal dose (≧ 2280 mg/kg) to rats also caused diarrhea. In rabbits and dogs, only 100 mg/kg and 250 mg/kg of metformin, respectively, induces diarrhea.20 However, this dose has been shown to lead to a number of adverse effects such as breathing difficulty, ataxia, asitia, and, in some case, death, suggesting that these diarrheal symptoms may not reflect those in human patients with diabetes.
A moderately high dose of metformin administrated to db/db mice may be a clinically representative model. Studies suggest that in patients with diabetes, age, sex, body mass index, and liver and biliary disorders can increase the incidence of metformin-induced diarrhea.6 Patients with more than five risk factors definitely developed diarrhea when they were initially treated with metformin at a high dose (750 mg/kg). The model we have developed using db/db mice can be used to study metformin-induced diarrhea in patients with diabetes and obesity as well as impaired liver function.
The fluctuations in enterobacterial flora are even found to be associated with diarrhea that is not caused by pathogenic bacteria, such as in patients with irritable bowel syndrome (IBS).12 The db/db mice used in this study were subjected to analysis of their enterobacterial flora and the effect of metformin treatment. The population of the three major groups of enterobacteria (Betaproteobacteria, Firmicutes, and Bacteroidetes) were analyzed approximately by qPCR and it was found that the ratio of PCR signals for Firmicutes/Universal was lower in diarrhea conditions (db/db mice treated with a high dose of metformin).
Interestingly, although penicillin and streptomycin treatment did not decrease enterobacterial levels (as detected by the primer set for Universal), the mice excreted soft feces after metformin treatment, which was associated with the reduced Firmicutes levels. In addition, metformin-induced diarrhea in db/db mice was also associated with reduced Firmicutes levels. The Firmicutes group is composed of a variety of enterobacterial species; some are decreased in patients with IBS, and others are increased.12
In experiments with mice, the ratio of Firmicutes group has been reported to be increased by anti-diarrheal reagents or natural materials13. These reports agree with our observation of metformin-induced diarrhea. However, wood creosote, which we choose as the anti-diarrheal material, did not modulate enterobacterial flora, including Firmicutes, suggesting that the reduced ratio of the Firmicutes group may be a risk factor for metformin-induced diarrhea, but not the only factor.
Excess bile acid has been shown to be correlated with the incidence of diarrhea. Approximately 25%–33% of patients with functional diarrhea may show dysregulation of bile acid homeostasis.21 Studies have shown that AMPK, the major target of metformin, upregulates bile acid synthesis in the liver by disrupting a negative feedback for CYP7a (bile acid synthase) gene expression.22 Although the db/db mice had liver dysfunction, they did not have biliary disorders. In addition, the population of Firmicutes flora has been reported to be increased by excess bile acids in the rat gut23; however, it was decreased in our model, suggesting that the excess bile acids did not cause metformin-induced diarrhea in our mouse model. In case of patients with diabetes, chronic biliary disorders may be associated with liver dysfunctions.4
A conflicting observation in rats has been reported that AMPK inhibits Cl- channel that possibly leads to water uptake from the colon lumens.24 Cholera toxin-mediated and bile acid-mediated cAMP signal results in fluid accumulation in isolated jejunal loops, which is countered by treatment with AMPK agonists,24 25 suggesting that metformin-induced diarrhea may not be caused by AMPK activation.
A low concentration of metformin (subthreshold-activating AMPK) has been found to stimulate the release of 5-hydroxytryptamine (5-HT) in tissue biopsy specimens of human duodenal mucosa,26 which can explain diarrhea and vomiting via central nervous system. However, 5-HT3 receptor antagonists show no effect on the metformin-stimulated 5-HT release in the specimens or on diarrheal symptoms in patients prescribed metformin.27 In contrast, wood creosote inhibits not only 5-HT3 receptors, but also 5-HT4 receptors,28 suggesting the relevance of the parasympathetic nerve system. Moreover, it has been reported that wood creosote does not modulate enterobacterial flora,29 which we also found in this study. In contrast, a gastrointestinal microbiome modulator (a mixture of purified ingredients of foods) improves diarrhea symptoms induced by metformin and lowered fasting blood glucose levels.14 These evidences suggest that wood creosote does not act directly in the gut or alter the metformin effects or glucose tolerance.
Using our mouse model, we evaluated the anti-diarrheal effects of wood creosote on metformin-induced diarrhea. Understanding the mechanism by which wood creosote improves the symptoms of metformin-induced diarrhea may provide new insights into how metformin causes diarrhea. In addition, correlations between metabolites that are produced by enterobacteria and the severity of diarrheal symptom induced by metformin should be examined. Especially, human patients consume a variety of diets, which may contribute to the difference in the severity of diarrheal symptom. Therefore, other risk factors for metformin-induced diarrhea in human patients, age, sex, as well as dietary patterns (for example, a high-fat diet), should also be examined in mouse models.
Footnotes
Contributors: AH, KI, MI, and MT performed experiments. HM, and TM analyzed data. HT wrote this manuscript. HM, TM, KO, and TS designed the overall study and analyzed the data. HT is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding: We declare that the present study is not supported by any funders. All expenses (about US$ 20 000) in the present study were borne by Taiko Pharmaceutical Co., Ltd.
Competing interests: We declare that MI, MT, HM, TM, KO, and TS are employed by Taiko Pharmaceutical Co., Ltd. AH and KI have nothing to declare.
Patient consent for publication: Not required.
Ethics approval: All animal experimental procedures were approved by the Animal Committee in Gifu University (H30-040 and 2019–192).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available in a public, open access repository. Data Sharing: Original data is available on the web (DOI: 10.13140/RG.2.2.28403.68645: https://www.researchgate.net/publication/338655326_Data_original. and DOI: 10.13140/RG.2.2.35953.43365: https://www.researchgate.net/publication/338649294_Entero_PCR).
References
- 1.Bailey CJ. Metformin: historical overview. Diabetologia 2017;60:1566–76. 10.1007/s00125-017-4318-z [DOI] [PubMed] [Google Scholar]
- 2.Zhou G, Myers R, Li Y, et al. . Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 2001;108:1167–74. 10.1172/JCI13505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouchoucha M, Uzzan B, Cohen R. Metformin and digestive disorders. Diabetes Metab 2011;37:90–6. 10.1016/j.diabet.2010.11.002 [DOI] [PubMed] [Google Scholar]
- 4.Appleby RN, Moghul I, Khan S, et al. . Non-Alcoholic fatty liver disease is associated with dysregulated bile acid synthesis and diarrhea: a prospective observational study. PLoS One 2019;14:e0211348 10.1371/journal.pone.0211348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ji L, Liu J, Yang J, et al. . Comparative effectiveness of metformin monotherapy in extended release and immediate release formulations for the treatment of type 2 diabetes in treatment-naïve Chinese patients: analysis of results from the consent trial. Diabetes Obes Metab 2018;20:1006–13. 10.1111/dom.13190 [DOI] [PubMed] [Google Scholar]
- 6.Okayasu S, Kitaichi K, Hori A, et al. . The evaluation of risk factors associated with adverse drug reactions by metformin in type 2 diabetes mellitus. Biol Pharm Bull 2012;35:933–7. 10.1248/bpb.35.933 [DOI] [PubMed] [Google Scholar]
- 7.Quaile MP, Melich DH, Jordan HL, et al. . Toxicity and toxicokinetics of metformin in rats. Toxicol Appl Pharmacol 2010;243:340–7. 10.1016/j.taap.2009.11.026 [DOI] [PubMed] [Google Scholar]
- 8.Ogata N, Baba T. Analysis of beechwood creosote by gas chromatography-mass spectrometry and high-performance liquid chromatography. Res Commun Chem Pathol Pharmacol 1989;66:411–23. [PubMed] [Google Scholar]
- 9.Yang Y-W, Chen M-K, Yang B-Y, et al. . Use of 16S rRNA gene-targeted group-specific primers for real-time PCR analysis of predominant bacteria in mouse feces. Appl Environ Microbiol 2015;81:6749–56. 10.1128/AEM.01906-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bailey CJ, Flatt PR, Ewan C. Anorectic effect of metformin in lean and genetically obese hyperglycaemic (ob/ob) mice. Arch Int Pharmacodyn Ther 1986;282:233–9. [PubMed] [Google Scholar]
- 11.Lewis RJ. Sax's dangerous properties of industrial materials. 1405 11th Edition Wiley-Interscience, Wiley & Sons, Inc, 2004. [Google Scholar]
- 12.Rodiño-Janeiro BK, Vicario M, Alonso-Cotoner C, et al. . A review of microbiota and irritable bowel syndrome: future in therapies. Adv Ther 2018;35:289–310. 10.1007/s12325-018-0673-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu B, Yan Y, Huang J, et al. . Cortex Phellodendri extract's anti-diarrhea effect in mice related to its modification of gut microbiota. Biomed Pharmacother 2020;123:109720 10.1016/j.biopha.2019.109720 [DOI] [PubMed] [Google Scholar]
- 14.Burton JH, Johnson M, Johnson J, et al. . Addition of a gastrointestinal microbiome modulator to metformin improves metformin tolerance and fasting glucose levels. J Diabetes Sci Technol 2015;9:808–14. 10.1177/1932296815577425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang X, Zhao Y, Xu J, et al. . Modulation of gut microbiota by berberine and metformin during the treatment of high-fat diet-induced obesity in rats. Sci Rep 2015;5:14405 10.1038/srep14405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bauer PV, Duca FA, Waise TMZ, et al. . Metformin alters upper small intestinal microbiota that impact a Glucose-SGLT1-Sensing glucoregulatory pathway. Cell Metab 2018;27:101–17. 10.1016/j.cmet.2017.09.019 [DOI] [PubMed] [Google Scholar]
- 17.Beli E, Prabakaran S, Krishnan P, et al. . Loss of diurnal oscillatory rhythms in gut microbiota correlates with changes in circulating metabolites in type 2 diabetic db/db mice. Nutrients 2019;11 10.3390/nu11102310. [Epub ahead of print: 29 Sep 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang W, Xu J-H, Yu T, et al. . Effects of berberine and metformin on intestinal inflammation and gut microbiome composition in db/db mice. Biomed Pharmacother 2019;118:109131 10.1016/j.biopha.2019.109131 [DOI] [PubMed] [Google Scholar]
- 19.Zhou T, Xu X, Du M, et al. . A preclinical overview of metformin for the treatment of type 2 diabetes. Biomed Pharmacother 2018;106:1227–35. 10.1016/j.biopha.2018.07.085 [DOI] [PubMed] [Google Scholar]
- 20.Heller J. Metformin overdose in dogs and cats. Vet Med 2007;231:231–3. [Google Scholar]
- 21.Camilleri M. Advances in understanding of bile acid diarrhea. Expert Rev Gastroenterol Hepatol 2014;8:49–61. 10.1586/17474124.2014.851599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Hagedorn CH, Wang L. Role of nuclear receptor SHP in metabolism and cancer. Biochim Biophys Acta 2011;1812:893–908. 10.1016/j.bbadis.2010.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Islam KBMS, Fukiya S, Hagio M, et al. . Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology 2011;141:1773–81. 10.1053/j.gastro.2011.07.046 [DOI] [PubMed] [Google Scholar]
- 24.Rogers AC, Huetter L, Hoekstra N, et al. . Activation of AMPK inhibits cholera toxin stimulated chloride secretion in human and murine intestine. PLoS One 2013;8:e69050 10.1371/journal.pone.0069050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yibcharoenporn C, Chusuth P, Jakakul C, et al. . Discovery of a novel chalcone derivative inhibiting CFTR chloride channel via AMPK activation and its anti-diarrheal application. J Pharmacol Sci 2019;140:273–83. 10.1016/j.jphs.2019.07.012 [DOI] [PubMed] [Google Scholar]
- 26.Cubeddu LX, Bönisch H, Göthert M, et al. . Effects of metformin on intestinal 5-hydroxytryptamine (5-HT) release and on 5-HT3 receptors. Naunyn Schmiedebergs Arch Pharmacol 2000;361:85–91. 10.1007/s002109900152 [DOI] [PubMed] [Google Scholar]
- 27.Hoffmann IS, Roa M, Torrico F, et al. . Ondansetron and metformin-induced gastrointestinal side effects. Am J Ther 2003;10:447–51. 10.1097/00045391-200311000-00012 [DOI] [PubMed] [Google Scholar]
- 28.Ataka K, Kuge T, Fujino K, et al. . Wood creosote prevents CRF-induced motility via 5-HT3 receptors in proximal and 5-HT4 receptors in distal colon in rats. Auton Neurosci 2007;133:136–45. 10.1016/j.autneu.2006.11.002 [DOI] [PubMed] [Google Scholar]
- 29.Ogata N, Miura T. Absence of bactericidal activity of orally administered wood creosote on human intestinal bacterial flora. Integr Mol Med 2018;5:1–4. 10.15761/IMM.1000321 [DOI] [Google Scholar]