Abstract
Objective
Non-alcoholic fatty liver disease (NAFLD) is the most common cause of abnormal results of liver function tests. Earlier research showed that polyenylphosphatidylcholine (PPC) has hepatoprotective effects and thus can be used for the treatment of NAFLD and the prevention of its progression. Accordingly, the aim of this observational study was to evaluate if PPC administered as adjunctive therapy in routine clinical practice can effectively improve liver function tests of NAFLD in Russian patients with associated metabolic comorbidities.
Design
A total of 2843 adult patients with newly diagnosed NAFLD, who had a least one of four comorbidities, namely, overweight/obesity, hypertension, type 2 diabetes mellitus, and hypercholesterolaemia, and who were prescribed 1.8 g/day of PPC as an adjunctive treatment to standard care, were enrolled during 2015–2016. Laboratory data were collected at baseline and 12 and 24 weeks of the study, and included liver function tests (aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyl transferase (GGT)), fasting plasma glucose, and lipid profile.
Results
Overall, 2263 patients (79.6%) had at least two metabolic comorbidities associated with NAFLD, and overweight/obesity was the most common comorbidity reported in 2298 (80.8%) patients. At 24 weeks, there was a significant decrease in liver enzyme levels (all p<0.001 compared with baseline). Across the four comorbidity subgroups, there was a mean drop of ALT levels ranging from 19.7 to 22.0 U/L, AST from 16.9 to 18.4 U/L, and GGT from 17.2 to 18.7 U/L. Similar findings were reported in subgroups with either one, two, three, or four comorbidities, with a significant decrease in liver enzyme levels ranging from 18.4 to 22.4 U/L for ALT, 14.8 to 18.7 U/L for AST, and 15.5 to 19.5 U/L for GGT.
Conclusions
Adjuvant treatment with PPC resulted in consistent improvements in liver enzymes in patients with newly diagnosed NAFLD and associated metabolic comorbidities.
Trial registration number
Keywords: liver function test, fatty liver, nutritional supplementation
Summary box.
What is already known about this subject?
Non-alcoholic fatty liver disease (NAFLD) is currently the most common cause of abnormal results of liver function tests.
Aspartate aminotransferase (AST), alanine aminotransferase (ALT), and gamma-glutamyl transferase (GGT), which are markers of liver injury, are still considered as useful surrogate measures of NAFLD for clinical practitioners; recently, however, their diagnostic value has been challenged.
Previous research has indicated that polyenylphosphatidylcholine (PPC) might have a positive influence on liver enzyme levels, as well as on serum lipid profile.
What are the new findings?
PPC administered as adjunctive therapy in patients with NAFLD with metabolic comorbidities consistently lowered AST, ALT and GGT levels, irrespective of the nature and number of associated comorbidities.
Although a low rate of achieving total cholesterol levels below 5.0 mmol/L was noted at the end of the study in the overall patient population, significant improvements in lipid parameters were observed in both patients receiving PPC only and in those receiving PPC in addition to statins and/or fibrates.
Summary box.
How might it impact on clinical practice in the foreseeable future?
PPC has a promising role in the management of both NAFLD and its extrahepatic manifestations, including abnormal liver enzyme and lipid levels.
Liver enzymes can be useful markers for monitoring the progression of NAFLD over time. There is a need for further investigation on the association of liver enzymes with NAFLD, which might help improve the diagnosis, management and risk prediction of NAFLD progression and provide a more accurate indicator for chronic metabolic liver injury.
Individualised intervention strategies should be adopted in Russia to improve the management of patients with NAFLD with associated dyslipidaemia.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is an increasingly common chronic liver disease characterised by significant lipid deposition in the hepatocytes of the liver parenchyma and persistent abnormalities in liver enzymes.1 The spectrum of NAFLD is a continuum ranging from simple steatosis to non-alcoholic steatohepatitis (NASH), and finally cirrhosis.2 3 NAFLD is recognised as the hepatic manifestation of the metabolic syndrome, which includes obesity, dyslipidaemia, hypertension and type 2 diabetes mellitus (T2DM).3–5 Obesity, in particular, is often associated with NAFLD, as the degree of steatosis was found to be correlated with body mass index (BMI).6 7
The World Gastroenterology Organisation recommends a hierarchical resource-sensitive approach to the diagnosis of NAFLD.8 Even though liver biopsy remains the gold standard, it is impractical as a diagnostic tool because it is invasive and expensive.9 Thus, non-invasive and more affordable tools, based on a biological approach (such as serum biomarkers and liver enzymes), have been proposed for the diagnosis and staging of NAFLD.8 9 Indeed, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and gamma-glutamyl transferase (GGT) have been shown to be useful surrogate measures of NAFLD.8 10
As a result of increasing rates of obesity worldwide, NAFLD is currently the most common cause of abnormal results of liver function tests.11–13 The usual observed biochemical pattern in hepatic steatosis due to NAFLD is of increased levels of transaminases, with ALT levels exceeding those of AST.14 However, with the progression of hepatic steatosis to NASH and associated hepatic fibrosis, AST levels increase with a resultant rise in the AST to ALT ratio.14–16 GGT levels may also be modestly increased.14 At the same time, however, it has been shown that liver enzyme levels do not correlate with the histological severity of NAFLD.17 Of note, up to 80% of patients with NAFLD have no liver enzyme abnormalities, implying that normal transaminase levels do not exclude steatosis.18 19 Nevertheless, ALT, AST, the AST/ALT ratio and GGT are included in different multibiomarker panels aimed to optimise the diagnostic accuracy of NAFLD.20 The AST to ALT ratio, in particular, can provide important diagnostic clues. The normal AST to ALT ratio is approximately 0.8, and it is usually <1.0 in NAFLD, although it may be >1.0 with the development of cirrhosis.21 22
There is a wide variety of pharmaceutical agents currently in use or in clinical development for NAFLD and NASH. However, there are insufficient data supporting their beneficial effects on liver enzyme levels. For example, ursodeoxycholic acid, metformin, and vitamin E failed to have a significant influence on liver histology and on liver function tests in several large placebo-controlled trials.23–25 In contrast, pioglitazone led to significant improvements in hepatic steatosis, inflammation, insulin resistance, and liver enzyme levels in the phase III, placebo-controlled Pioglitazone Versus Vitamin E Versus Placebo for the Treatment of Non-diabetic Patients with Non-alcoholic Steatohepatitis (PIVENS) trial.26 However, pioglitazone use is associated with several safety concerns, including postmenopausal bone loss and increased risk of bladder cancer.27 Similarly, although vitamin E was found to be superior to placebo in the PIVENS trial,26 studies have shown that vitamin E supplementation significantly increases the risk of haemorrhagic stroke and prostate cancer.28 29 Among emerging pharmacological options, both obeticholic acid and selonsertib failed to achieve the primary endpoint of NASH resolution and of fibrosis improvement in the phase III Randomized Global Phase 3 Study to Evaluate the Impact on NASH With Fibrosis of Obeticholic Acid Treatment (REGENERATE) and Safety and Efficacy of Selonsertib in Adults With Compensated Cirrhosis Due to Nonalcoholic Steatohepatitis (STELLAR)-4 trials, respectively.30 31 Hence, despite considerable research and multiple clinical trials, the management of NAFLD and of NASH is not satisfactory at the present time.32 Thus, any therapeutic intervention, which can target fat accumulation in the liver and improve liver enzyme levels, would be of great value.33 34 Essential phospholipids (EPLs) are highly purified phosphatidylcholine fractions extracted from the semen of soybeans, containing linoleic acid and other unsaturated fatty acids.33–35 Polyenylphosphatidylcholine (PPC) is the main active ingredient in EPL.35 Earlier research on animal studies showed that PPC has protective effects against lipid peroxidation, oxidative stress and hepatic fibrosis, and thus can be used for the treatment of NAFLD and the prevention of its further progression.36–38
In Russia, the estimated prevalence of NAFLD in the adult population is 37%.39 Considering that EPL was indicated as antioxidant therapy for NAFLD in the Russian Guidelines for the Diagnosis and Management of NAFLD40 and having several clinical trials in which PPC had a positive influence on liver enzyme levels,5 41 42 the purpose of this real-life observational study was to evaluate if PPC administered as adjunctive therapy can effectively improve liver function tests of newly diagnosed NAFLD in Russian patients with associated metabolic comorbidities.
Methods
Patients
A detailed description of the study methodology has already been published elsewhere.43 Briefly, patients aged 18–60 years, with newly diagnosed NAFLD and who had already been receiving PPC (Essentiale Forte N, which contains 300 mg of EPL) prescribed by a physician as an adjunctive treatment to standard care (within 30 days before enrolment), were prospectively and consecutively enrolled at 174 medical sites between 2015 and 2016, and were afterwards prospectively observed. NAFLD diagnosis was based on clinical examination, laboratory tests, and ultrasonography. In addition, eligible patients had at least one of the following concomitant diseases: (1) hypertension diagnosed by a cardiologist, (2) T2DM diagnosed by an endocrinologist, (3) high-serum cholesterol (defined as a total cholesterol (TC) level of ≥5.0 mmol/L), and/or (4) overweight/obesity (BMI≥27 kg/m2). Major exclusion criteria were the presence of other severe acute or chronic conditions (including other liver diseases and cancers)and treatment with other hepatoprotective drugs within 30 days prior to study enrolment. All study participants gave signed informed consent prior to study inclusion.
Data collection
Individual patient data were collected at three timepoints: baseline and 12 and 24 weeks. All patients had a complete history and physical examination performed at baseline. The history of prescription drug use and the prescribed dosage and duration of PPC therapy were assessed by the examining physicians.
Laboratory data collected from all patients at each study visit included liver function tests (AST, ALT, GGT, total bilirubin, alkaline phosphatase (ALP), albumin, prothrombin time, gamma-globulin, and serum iron); fasting plasma glucose (FPG); and lipid profile (TC, low-density lipoprotein (LDL) cholesterol, very-low-density lipoprotein (VLDL) cholesterol, high-density lipoprotein (HDL) cholesterol, and triglyceride (TG)). Furthermore, at baseline, additional test results, including hepatitis B serology, hepatitis C antibodies, serum creatinine, ferritin, blood urea nitrogen, immunoreactive insulin, and urine microalbumin, were analysed. In the study analysis, prothrombin time results were reported as percentage activity (also known as prothrombin activity).
The reference intervals of liver function tests used in this study were based on patient data from different laboratories across Russia, and included ALT, up to 41 U/L for men and 33 U/L for women; AST, up to 50 U/L for men and 35 U/L for women; GGT, up to 49 U/L for men and 32 U/L for women; ALP, 40–150 U/L; total bilirubin, 3–17 µmol/L; and albumin, 35–50 g/L.
Study endpoints
This study aimed to evaluate the effectiveness of PPC adjunctive therapy in improving liver function tests in the overall study population throughout the 24-week study period, and also according to the nature and number of metabolic comorbidities associated with NAFLD.
Since previous randomised controlled trials have reported that EPL intake was associated with an improved lipid profile,44 45 a post hoc analysis was performed to evaluate changes in lipid profile (ie, TC, LDL cholesterol, HDL cholesterol, VLDL cholesterol, and TG) from baseline to both weeks 12 and 24 of the study in two subgroups: patients receiving PPC only, and those receiving PPC in addition to lipid-lowering agents (mainly the most used in routine clinical practice in Russia, ie, statins and/or fibrates).
Statistical analysis
The population set used for statistical analysis comprised all eligible enrolled patients who provided adequate laboratory data. Continuous variables were represented as mean, SD, median, and IQR. Categorical variables were reported as numbers and percentages. At weeks 12 and 24 of the study, mean±SD changes in laboratory parameters from baseline were calculated.
To compare the means of normally distributed variables, Student’s t-test was performed. Non-parametric tests, such as the Wilcoxon-Mann-Whitney test, were used to compare continuous non-normally distributed variables. The χ2 test and Fisher's exact test were used for comparison of frequency data. As multiple comparisons were performed in the post hoc analysis, p values were adjusted using the Benjamini-Hochberg procedure.46
All statistical tests were two-sided and were performed at a 0.05 significance level. Statistical analyses were conducted using SAS V.9.3.
Results
Patient characteristics
A total of 2843 patients with newly diagnosed NAFLD were included, of which 2827 (99.4%) completed the study. Demographic and baseline characteristics of the study population are summarised in table 1. The mean±SD age of the female study participants was significantly higher than that of their male counterparts (49.7±8.2 vs 47.2±9.0 years, p<0.001). The great majority (2434/2843, 85.6%) of patients were non-smokers, and only 201 (7.1%) consumed alcohol at least once a week.
Table 1.
Demographic and baseline characteristics of the study population (n=2843)
| Baseline characteristic | Study population (n=2843) |
| Age (years), mean±SD (median, IQR) | 48.7±8.6 (50.7, 43.6–55.6) |
| Male/female, n (%) | 1076 (37.8)/1767 (62.2) |
| Weight (kg), mean±SD (median, IQR) | 91.0±14.1 (90.0, 82.0–99.5) |
| BMI (kg/m2), mean±SD (median, IQR) | 32.0±4.6 (31.8, 29.2–34.6) |
| Waist circumference (cm), mean±SD (median, IQR) | 98.4±12.4 (98.0, 90.0–105.0) |
| Comorbid conditions | |
| According to the nature of the disease, n (%)* | |
| Overweight/obesity | 2298 (80.8) |
| Elevated cholesterol | 2122 (74.6) |
| Hypertension | 1642 (57.8) |
| T2DM | 477 (16.8) |
| According to the number of diseases, n (%) | |
| 1 | 580 (20.4) |
| 2 | 1112 (39.1) |
| 3 | 869 (30.6) |
| 4 | 282 (9.9) |
Percentages are calculated as n/N.
*Patients may have more than one comorbid condition.
BMI, body mass index; T2DM, type 2 diabetes mellitus.
Overweight/obesity was the most common comorbid condition in the study population (80.8%). The majority of patients (2263/2843, 79.6%) had at least two metabolic comorbidities (table 1). At baseline, statins were the most frequently prescribed medications in patients with overweight/obesity (601/2298, 26.2%) and in those with hypercholesterolaemia (698/2122, 32.9%). Among hypertensive patients, ACE inhibitors were the most commonly prescribed medications (588/1642, 35.8%). Biguanides (specifically metformin) were prescribed to 241/477 (50.5%) patients with T2DM. Overall, 1092/2843 (38.4%) patients did not receive any comorbidity-related medications.
Staging of NAFLD showed that simple steatosis was the most frequently seen clinical form of NAFLD, diagnosed in 2128 patients (74.9%). NASH was detected in 712 patients (25.0%), and only three patients (0.1%) suffered from fibrosis. Cirrhosis was not detected in any of the patients.
Almost all study participants (2837/2843, 99.8%) were prescribed by their physicians 1.8 g of PPC administered in three divided doses per day.
Laboratory findings in the overall study population
The baseline biochemical parameters of the study population are presented in table 2. As expected, mean transaminases levels at baseline were above the upper limit of normal. The mean serum levels of GGT and total bilirubin at baseline were also moderately increased. However, other liver function parameters, including albumin, ALP, and prothrombin activity were within the normal reference ranges defined in our study. Results of the baseline lipid and glycaemic profiles revealed that the study population had on average high TC and TG levels, impaired FPG, and increased haemoglobin A1c levels (table 2). Overall, 36.2%, 52.3%, and 39.8% of patients had normal serum levels of ALT, AST, and GGT, respectively, at baseline (table 3).
Table 2.
Baseline laboratory data
| Laboratory tests | N | Mean±SD (normal range) |
| Carbohydrate metabolism | ||
| Fasting plasma glucose (mmol/L) | 2827 | 5.6±1.4 (<5.5) |
| 2-hour postprandial plasma glucose (mmol/L) | 383 | 7.3±2.2 (<7.8) |
| Haemoglobin A1c (%) | 843 | 6.1±1.4 (4.0–5.6) |
| Blood urea nitrogen (mmol/L) | 1915 | 7.0±7.7 (2.5–7.1) |
| Serum creatinine (µmol/L) | 1095 | 78.9±23.3 (60–110) |
| Lipid profile | ||
| Total cholesterol (mmol/L) | 2786 | 6.3±1.2 (3.5–5.0) |
| High-density lipoprotein cholesterol (mmol/L) | 1780 | 1.4±0.7 (0.9–1.5) |
| Low-density lipoprotein cholesterol (mmol/L) | 1795 | 3.7±1.1 (<3.4) |
| Very-low-density lipoprotein cholesterol (mmol/L) | 499 | 1.2±0.9 (0.1–1.7) |
| Serum triglyceride (mmol/L) | 2080 | 2.1±0.9 (<1.7) |
| Liver function tests | ||
| Alanine aminotransferase (U/L) | 2843 | 50.2±33.2 (M<41, F<33) |
| Aspartate aminotransferase (U/L) | 2843 | 43.6±25.7 (M<50, F<35) |
| Gamma-glutamyl transferase (U/L) | 2076 | 51.7±37.6 (M<49, F<32) |
| Alkaline phosphatase (U/L) | 2076 | 137.4±80.7 (40–150) |
| Total bilirubin (µmol/L) | 2588 | 17.5±9.0 (3–17) |
| Albumin (g/L) | 990 | 47.3±12.8 (35–50) |
| Prothrombin activity (%) | 1001 | 87.9±25.9 (70–100) |
| Gamma-globulin (g/L) | 361 | 17.1±9.9 (7–16) |
| Serum iron (µmol/L) | 939 | 18.5±5.8 (10–30) |
| Urine albumin (mg/L) | 130 | 9.8±11.9 (<20) |
N refers to the number of patients with adequate laboratory data.
F, female; M, male.
Table 3.
Proportion of patients with normal and abnormal liver enzyme levels at each study visit
| Laboratory value | Baselinea | 12 weeksb | 24 weeksc |
| Alanine aminotransferase (U/L) | n=2843 | n=2697 | n=2764 |
| Normal (M<41, F<33) | 1028 (36.2) | 1427 (52.9) | 2095 (75.8) |
| Abnormal (1–2 times upper limit) | 1286 (45.2) | 1127 (41.8) | 622 (22.5) |
| >2 times upper limit | 529 (18.6) | 143 (5.3) | 47 (1.7) |
| Aspartate aminotransferase (U/L) | n=2843 | n=2696 | n=2761 |
| Normal (M<50, F<35) | 1487 (52.3) | 1907 (70.7) | 2464 (89.2) |
| Abnormal (1–2 times upper limit) | 1162 (40.9) | 754 (28.0) | 278 (10.1) |
| >2 times upper limit | 194 (6.8) | 35 (1.3) | 19 (0.7) |
| Gamma-glutamyl transferase (U/L) | n=2076 | n=1936 | n=1964 |
| Normal (M<49, F<32) | 827 (39.8) | 1017 (52.5) | 1228 (62.5) |
| Abnormal (1–2 times upper limit) | 928 (44.7) | 759 (39.2) | 666 (33.9) |
| >2 times upper limit | 321 (15.5) | 160 (8.3) | 70 (3.6) |
Data are expressed as n (%). Percentages are calculated as n/N.
P value (χ2 test with Benjamini-Hochberg correction for multiple comparisons): b vs a, p<0.001, c vs a, p<0.001, c vs b, p<0.001.
F, female; M, male.
There was a consistent and significant reduction of serum ALT, AST, and GGT levels throughout the 24-week study period (figure 1). Compared with baseline, mean ALT levels decreased by 20.0 U/L, mean AST by 16.5 U/L, and mean GGT by 15.9 U/L at 24 weeks of the study. These changes were already statistically significant at 12 weeks (all p<0.001 by paired t-test for both timepoints). At the end of the study, ALT, AST, and GGT levels were normal in 75.8%, 89.2%, and 62.5% of patients, respectively (all p<0.001 compared with baseline). Serum liver enzyme levels were higher than twice the upper limit of normal in up to 3.6% of patients (table 3).
Figure 1.
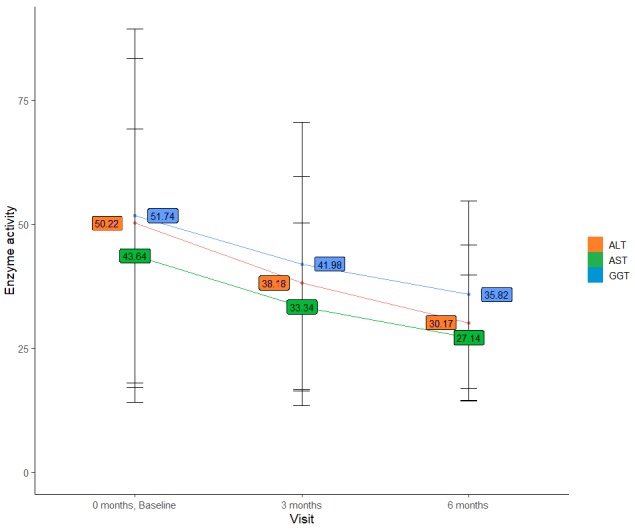
Mean±SD liver function tests (U/L) at baseline and weeks 12 and 24 in the overall study population. ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transferase.
Laboratory findings by nature and number of comorbidities
At baseline, high levels of liver enzymes, namely, ALT, AST and GGT, were reported in all four comorbidity subgroups: hypertension, overweight/obesity, T2DM, and hypercholesterolaemia. Baseline liver enzyme levels were similar across comorbidity subgroups, with the highest mean ALT, AST, and GGT values recorded in patients with NAFLD with T2DM (table 4). At 24 weeks of the study, there was a significant decrease in liver enzyme levels in all four comorbidity subgroups (all p<0.001 compared with baseline), with a mean drop of ALT levels ranging from 19.7 to 22.0 U/L, of AST from 16.9 to 18.4 U/L, and of GGT from 17.2 to 18.7 U/L (table 4).
Table 4.
Changes in liver function tests at 24 weeks of the study, according to to the nature and number of comorbidities
| Hypertension | Overweight/obesity | T2DM | Elevated cholesterol | ||
| ALT (U/L) | Baseline values | 50.3±32.8 | 52.3±34.7 | 53.9±31.0 | 51.4±33.4 |
| Change from baseline* | −19.7±26.5 | −21.6±29.4 | −22.0±22.7 | −20.8±27.3 | |
| AST (U/L) | Baseline values | 44.3±27.3 | 45.1±26.6 | 46.4±24.9 | 44.8±26.8 |
| Change from baseline* | −16.9±21.8 | −17.7±21.6 | −18.4±19.9 | −17.1±21.6 | |
| GGT (U/L) | Baseline values | 53.7±39.3 | 52.6±38.0 | 57.7±38.2 | 52.3±37.4 |
| Change from baseline* | −17.6±31.6 | −17.9±31.6 | −18.7±31.2 | −17.2±30.6 | |
| One comorbidity | Two comorbidities | Three comorbidities | Four comorbidities | ||
| ALT (U/L) | Baseline values | 44.5±30.9 | 49.7±33.0 | 52.8±34.2 | 56.3±33.5 |
| Change from baseline* | −18.4±28.1 | −20.4±28.2 | −21.2±28.3 | −22.4±23.9 | |
| AST (U/L) | Baseline values | 38.2±20.5 | 42.8±23.3 | 47.1±30.5 | 47.5±25.6 |
| Change from baseline* | −14.8±18.2 | −16.2±19.3 | −18.7±24.3 | −17.9±20.3 | |
| GGT (U/L) | Baseline values | 47.8±39.0 | 48.8±33.6 | 56.5±40.7 | 57.2±39.7 |
| Change from baseline* | −15.7±33.5 | −15.5±28.4 | −19.5±32.4 | −18.6±32.9 | |
Data are expressed as mean±SD.
*There was a significant decrease in the mean values of the liver function tests (p<0.001 compared with baseline, paired t-test).
ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transferase; T2DM, type 2 diabetes mellitus.
In the subgroups of patients with either one, two, three, or four comorbidities, elevated baseline levels of ALT, AST and GGT were also noted (table 4). Moreover, it appears that the increasing number of metabolic comorbidities at baseline was associated with higher liver enzyme levels (table 4). At 24 weeks of the study, there was a significant decrease in liver enzyme levels (p<0.001 compared with baseline) in the subgroups with either one, two, three, or four comorbidities, ranging from 18.4 to 22.4 U/L for ALT, from 14.8 to 18.7 U/L for AST, and from 15.5 to 19.5 U/L for GGT.
Post hoc analysis results: changes in serum lipid profile
Of the 2843 enrolled patients, 2077 (73.1%) were not undergoing any form of lipid-lowering therapy, and 766 (26.9%) reported the use of statins (in 764 patients) or fenofibrate (in 2 patients). At baseline, patients treated with lipid-lowering therapy (compared with untreated patients) had higher levels of LDL cholesterol (4.0±1.1 vs 3.5±1.1 mmol/L, respectively), VLDL cholesterol (1.2±0.8 vs 1.1±0.9 mmol/L), TC (6.9±1.1 vs 6.1±1.2 mmol/L), and TG (2.3±0.9 vs 2.0±0.9 mmol/L), and almost the same level of HDL cholesterol (1.4±0.7 vs 1.4±0.6 mmol/L, respectively).
Compared with baseline, all lipid parameters were significantly improved at 12 and 24 weeks of the study in both subgroups (all p<0.05). On post hoc comparison of the mean differences in lipid profiles between the two subgroups (figure 2), patients undergoing lipid-lowering therapy had significantly greater improvements in all lipid parameters at both 12 and 24 weeks (all p<0.05).
Figure 2.
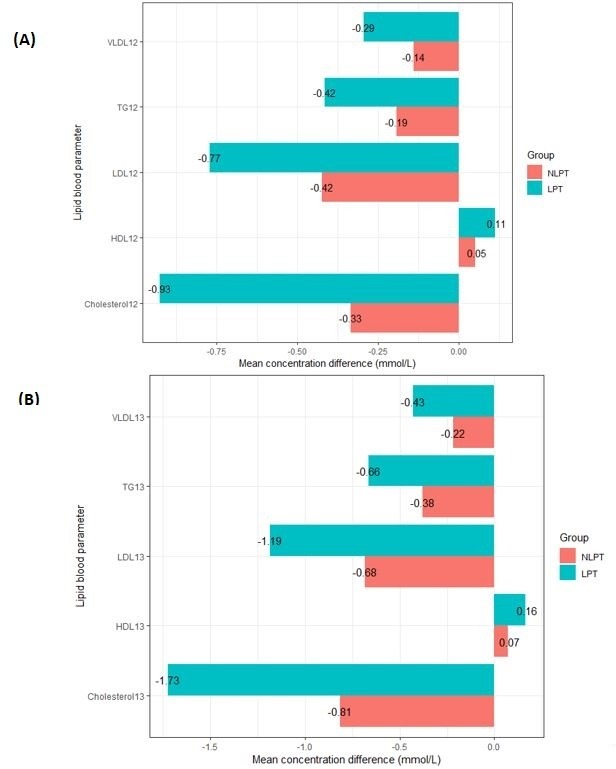
Mean changes in serum lipid profile from baseline to 12 weeks (A) and 24 weeks (B) of the study in patients who were NLPT (n=2077) and in patients who were LPT (n=766): post hoc analysis. All within-subgroup and between-subgroup differences were statistically significant (p<0.05, by Wilcoxon-Mann-Whitney test with Benjamini-Hochberg correction for multiple comparisons). HDL, high-density lipoprotein; LDL, low-density lipoprotein; LPT, undergoing lipid-lowering therapy; NLPT, not undergoing lipid-lowering therapy; TG, triglyceride; VLDL, very-low-density lipoprotein.
Among patients who underwent any form of lipid-lowering therapy in addition to PPC, 96.6% had higher than normal TC levels at baseline vs 62.7% at the end of the study. Similarly, among those who did not undergo lipid-lowering therapy, 79.6% and 59.7% had elevated TC levels at baseline and at study end, respectively. The proportions of patients with normal TC levels at 24 weeks of the study were comparable in both subgroups (table 5).
Table 5.
Proportion of patients with controlled (<5.0) and uncontrolled (≥5.0) levels of TC (mmol/L) throughout the study in both subgroups: post hoc analysis
| Lipid-lowering drug treatment (n=766) | Baseline | 12 weeks | 24 weeks |
| TC<5.0 mmol/L | 23 (3.0) | 86 (11.2) | 279 (36.4) |
| TC≥5.0 mmol/L | 740 (96.6) | 660 (86.2) | 480 (62.7) |
| Unavailable data | 3 (0.4) | 20 (2.6) | 7 (0.9) |
| No lipid-lowering drug treatment (n=2077) | Baseline | 12 weeks | 24 weeks |
| TC<5.0 mmol/L | 370 (17.8) | 482 (23.2) | 691 (33.3) |
| TC≥5.0 mmol/L | 1653 (79.6) | 1387 (66.8) | 1240 (59.7) |
| Unavailable data | 54 (2.6) | 208 (10.0) | 146 (7.0) |
Data are expressed as n (%). Percentages are calculated as n/N.
TC, total cholesterol.
Discussion
This study showed that adjuvant treatment with EPL resulted in consistent improvements in liver enzymes compared with baseline in patients with newly diagnosed NAFLD and metabolic comorbidities. Although the beneficial effect of PPC adjunctive therapy might have been enhanced by the use of concomitant oral antidiabetic, antihypertensive, and/or lipid-lowering agents for the treatment of the associated metabolic comorbidities, the improvement of liver function tests seems to be genuinely induced by EPL regardless of the types of concomitant medications prescribed. Patients in our study had already been receiving medications for their comorbidities at least 6 months prior to their enrolment, yet at baseline, most of them presented with abnormal liver enzyme levels. In addition, liver enzyme levels were significantly reduced after PPC therapy in both patients receiving comorbidity-related medications (n=1751) and in those not receiving any comorbidity-related medications (n=1092).
In our study population, mildly raised transaminases (with ALT>AST) and GGT were detected at baseline. This is in line with previous studies describing the biochemical alterations of NAFLD in patients with associated metabolic comorbidities.10 47 For instance, in a cross-sectional study from Eastern India evaluating the profile of liver enzymes in 310 patients with impaired glucose tolerance and newly diagnosed untreated T2DM, subjects with NAFLD had significantly higher ALT, AST, GGT and AST/ALT ratio compared with subjects without NAFLD (p≤0.02 by unpaired t-test).10 However, no significant difference in ALP levels was detected between patients with NAFLD and subjects without NAFLD (p=0.09).10 Similarly, in our study, ALP levels were within the normal reference range. Angulo and colleagues have reported that an AST to ALT ratio of >1.0 significantly correlated (p=0.03) with the presence of advanced liver fibrosis in 144 patients with well-defined NASH.15 Thus, the AST to ALT ratio can be of help in detecting progression to more advanced liver disease in patients with NAFLD who initially present with no or trivial fibrosis.15 In our study, most patients presented with an AST to ALT ratio of <1.0. That is expected, since liver fibrosis was reported in only 3/2843 patients (0.1%).
The pathogenesis of liver damage in NAFLD is not thoroughly understood. Hepatic insulin resistance can play an important role in liver dysfunction, and inflammatory cytokines, including tumour necrosis factor and interleukin-6, have also been proposed in the pathogenesis of hepatocellular injury, leading to mild to moderate increase of liver enzymes.47 48 Thus, ALT, AST and GGT are markers of liver injury.10 In a prospective study of 5237 healthy Korean men, serum ALT concentrations were found to be more closely associated with the development and progression of NAFLD than either AST or GGT concentrations.49 This finding could be partially explained by the higher specificity of ALT for liver injury, as well as by the contribution of ALT as a glucogenic enzyme.49 50 It has also been suggested that ALT might be a preclinical marker of NAFLD, since slightly increased serum ALT levels might reflect subclinical (or ultrasonography-undetectable) early fatty changes in the liver (hepatic steatosis), which predate the overtly detectable NAFLD.49
Although the exact mechanism through which EPLs exert their beneficial hepatoprotectant effect is still unknown,51 EPL administration has been shown to increase the percentage of PPC in the membranes of hepatocytes, blood corpuscles and pancreatic tissue, among other tissues.52 Due to the intriguing ability of PPC to incorporate into damaged sections of hepatic cell membranes,53 increasing the amount of PPC in membranes results in increased membrane fluidity and maintains membrane-dependent functions.52 53 PPC administration has also been found to ameliorate an ethanol-induced decrease in phosphatidylethanolamine N-methyltransferase activity and to correct phospholipid and phosphatidylcholine depletions.54 This could be one of the mechanisms through which PPC exerts its antifibrotic effect.53
The hepatoprotective effect of EPL has been widely investigated in several animal studies.55–61 In alcohol-fed baboons with fatty livers, long-term administration of PPC prevented the development of septal fibrosis.55 56 Moreover, PPC stimulated collagenase activity in cultured hepatic stellate cells,56 an effect that may contribute to the prevention of fibrosis by promoting the breakdown of collagen in alcoholic as well as in non-alcoholic forms of liver injury.56 57 In a 2009 study conducted in ethanol-fed peroxisome proliferator-activated receptor alpha-null mice, PPC markedly ameliorated ethanol-induced hepatocyte damage and hepatitis.58 These effects were likely a consequence of decreased oxidative stress, as shown by a significant improvement in serum AST and ALT levels (p<0.01 for both enzymes) and the histology of hepatitis (p<0.01), and by the downregulation of reactive oxygen species-generating enzymes.58 More recently, Chen and colleagues61 demonstrated an improved liver function in rats with liver injury treated orally with phosphatidylcholine. These phosphatidylcholine-treated rats also exhibited a significantly lower portal pressure compared with untreated rats with liver injury. Additionally, liver histopathological changes were improved after phosphatidylcholine therapy, as collagen fibres gradually decreased and damaged hepatic lobules were partly repaired.61
In the present study, there was a mean drop of ALT levels ranging from 19.7 to 22.0 U/L, of AST from 16.9 to 18.4 U/L, and of GGT from 17.2 to 18.7 U/L across the four comorbidity subgroups. This is line with the findings of previous clinical studies evaluating PPC in patients with NAFLD with metabolic comorbidities.5 33 35 In one study conducted in 28 patients with NAFLD with T2DM who received 2.1 g/day of PPC as an adjuvant treatment, a significant reduction in liver function tests was observed after EPL treatment.33 ALT, AST and GGT levels, respectively, decreased by a mean of 17.4, 10.4 and 9.1 U/L after 6 months of treatment with PPC adjunctive therapy, and this decrease was observed from the beginning of PPC therapy (p<0.05 compared with baseline for all three liver enzymes at months 2, 4, and 6 of treatment).33 Similarly, in a non-randomised, open-label trial by Padma and colleagues in 293 patients with NAFLD treated with EPL administered three times per day daily for 90 days, there was a decrease in liver enzyme levels, which was significant at days 60 and 90 of the study (p<0.05 compared with baseline for both timepoints).35 More recently, Dajani et al conducted a prospective, multicentre, open-label study evaluating EPL administered as an adjunctive treatment at 1.8 g/day for 24 weeks, followed by 0.9 g for 48 weeks in a cohort of 324 patients with either lone NAFLD (n=113), NAFLD with T2DM (n=107), or NAFLD with hyperlipidaemia (n=104).5 EPL led to notable symptomatic improvement and a mean reduction of ALT of 50.8 U/L and AST of 46.1 U/L per patient (p<0.01 compared with baseline for both liver enzymes); liver transaminases levels were reduced after the first 6 months of EPL treatment in 80.5% of patients with lone NAFLD, 84.1% of patients with NAFLD with T2DM, and 87.5% of patients with NAFLD with hyperlipidaemia.5 In a double-blind trial also from Russia conducted in 215 diabetic patients with NASH who were randomly allocated to either metformin taken at 1000 mg per day or metformin+1368 mg of PPC per day, a mean reduction of ALT of 21.3 U/L (p=0.02), of AST of 12.5 U/L (p=0.04) and of GGT of 10.7 U/L (p=0.03) was observed after 6 months of PPC therapy.42 Thus, the findings of these studies, along with our study results, point at a marked support to the liver function by EPL.
Dyslipidaemia is a well-established risk factor for NAFLD.62 In fact, patients with NAFLD usually have an atherogenic dyslipidaemia characterised by high levels of TG, LDL cholesterol and VLDL cholesterol, as well as a higher concentration of remnant lipoprotein cholesterol coupled with low HDL cholesterol levels.63 64 In this context, due to an increased cardiovascular risk, the treatment of dyslipidaemia should be considered in the management framework of NAFLD.65 However, a recent retrospective study in 2566 patients with NAFLD found that the use of statins and other lipid-lowering agents did not have a positive effect on overall or cardiovascular mortality in NAFLD.66 In our study, even though treatment with PPC in addition to statins and/or fibrates resulted in greater reductions in TG, TC, LDL and VLDL cholesterol levels and greater increases in HDL cholesterol compared with PPC only, the goal of TC<5.0 mmol/L was not achieved in one-third of patients on lipid-lowering therapy at 24 weeks of the study. This might be related to inadequate intensity and/or duration of statin therapy, and/or patient compliance with prescribed treatment. Nevertheless, this finding should be considered, as high blood cholesterol and impaired cholesterol metabolism may play a part in the development of cardiovascular disease in NAFLD.66 It is also worth mentioning that in the present study, PPC-treated patients who did not undergo any form of lipid-lowering therapy had statistically significant improvements in lipid parameters throughout the 24-week study period, although to a lesser extent than patients treated with statins and/or fibrates. Although the observational nature of our study does not allow drawing conclusions on the efficacy of EPL in improving lipid parameters, we think that the effect of EPL supplementation on lipid profile is worthy of further investigation, considering that EPLs were found in non-clinical studies to have lipid-regulating effects.34 This was also highlighted in several clinical studies. In 74 diabetic patients with NAFLD treated with either metformin alone or in combination with PPC for 3 months, Sun et al reported a significant decline in TC and TG levels in PPC-treated patients (p<0.01 compared with metformin monotherapy).45 Similarly, in 185 diabetic patients with fatty liver who received basic drug therapy with or without PPC for 84 days, TG, TC and LDL cholesterol levels significantly decreased in the PPC group, and HDL cholesterol significantly increased (all p<0.05 compared with control).44
Our study had certain limitations. First, this study, like all observational studies, was susceptible to information bias, and the lack of a comparator arm made it difficult to assign a causal relationship to any possible treatment effect. Second, we used liver enzymes as a surrogate to assess the effectiveness of PPC administered as adjunctive therapy in patients with NAFLD. However, normal levels of liver enzymes have been detected in subjects with the entire histological spectrum of NAFLD.67 Sanyal et al have suggested that novel cut-offs for liver enzymes are needed in order to prevent unnecessary diagnostic work-ups and to improve early detection of NAFLD.10 Third, liver biopsy was rarely performed in the present study to assess NAFLD. Although ultrasonography is a practical method with reasonable sensitivity and specificity, it may underestimate the actual rate of NAFLD, because ultrasonography changes appear at a hepatocyte fat content of 15% to 30%.49 68 69 Finally, although one of the most effective ways to reduce hepatic steatosis is weight loss, its effect could not be evaluated in our study population because most patients remained in the same weight range at the end of the study. Nevertheless, our study had several strengths, including its prospective and multicentric design; the large number of participants, which allowed us to identify the effect of PPC among stratified subgroup analyses; and the relatively long study duration. The inclusion of a large number of patients with comorbidities and from different cities reflects actual patient populations in daily clinical practice in Russia.
In conclusion, the results of our study suggest that PPC adjunctive therapy is effective in improving liver function markers of NAFLD in patients with associated metabolic comorbidities and that PPC might have a positive impact on serum lipid profile. The present study also indicates that liver transaminases can be useful markers for monitoring the progression of NAFLD over time. Importantly, a low rate of achieving lipid level targets was noted among patients treated with statins and/or fibrates, which might reflect potential patient non-compliance and inadequate dosing. Further data on the relation between liver transaminases and NAFLD might help to improve the diagnosis, management and risk prediction of NAFLD progression and to provide a more accurate indicator for chronic metabolic liver injury.
Acknowledgments
The authors thank Thomas Rohban, MD (Partner 4 Health, France) for providing medical writing support (sponsored by Sanofi) in accordance with Good Publication Practice or GPP3 guidelines.
Footnotes
Contributors: IVM was the study’s national coordinator who contributed to the design and supervision of the study. All authors participated in the acquisition, analysis, and interpretation of the data. CSP, ENS, LKP, AAS, EIV, and KMS contributed to the drafting of the initial manuscript and its finalisation. All authors have read and approved the final version of the manuscript.
Funding: This study was funded by Sanofi, France.
Competing interests: KMS is a Sanofi employee. The other authors declare that they have no conflict of interest.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request. Qualified researchers may request access to patient level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications. Patient-level data will be anonymised and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at: https://www.clinicalstudydatarequest.com.
References
- 1.Abd El-Kader SM, El-Den Ashmawy EMS. Non-Alcoholic fatty liver disease: the diagnosis and management. World J Hepatol 2015;7:846–58. 10.4254/wjh.v7.i6.846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hassan K, Bhalla V, El Regal ME, et al. Nonalcoholic fatty liver disease: a comprehensive review of a growing epidemic. World J Gastroenterol 2014;20:12082–101. 10.3748/wjg.v20.i34.12082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Demir M, Lang S, Steffen H-M. Nonalcoholic fatty liver disease - current status and future directions. J Dig Dis 2015;16:541–57. 10.1111/1751-2980.12291 [DOI] [PubMed] [Google Scholar]
- 4.Alexander M, Loomis AK, Fairburn-Beech J, et al. Real-World data reveal a diagnostic gap in non-alcoholic fatty liver disease. BMC Med 2018;16:130 10.1186/s12916-018-1103-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dajani AIM, Abu Hammour AM, Zakaria MA, et al. Essential phospholipids as a supportive adjunct in the management of patients with NAFLD. Arab J Gastroenterol 2015;16:99–104. 10.1016/j.ajg.2015.09.001 [DOI] [PubMed] [Google Scholar]
- 6.Moretto M, Kupski C, Mottin CC, et al. Hepatic steatosis in patients undergoing bariatric surgery and its relationship to body mass index and co-morbidities. Obes Surg 2003;13:622–4. 10.1381/096089203322190853 [DOI] [PubMed] [Google Scholar]
- 7.Hsiao P-J, Kuo K-K, Shin S-J, et al. Significant correlations between severe fatty liver and risk factors for metabolic syndrome. J Gastroenterol Hepatol 2007;22:2118–23. 10.1111/j.1440-1746.2006.04698.x [DOI] [PubMed] [Google Scholar]
- 8., LaBrecque DR, Abbas Z, et al. , Review Team . World gastroenterology organisation global guidelines: nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. J Clin Gastroenterol 2014;48:467–73. 10.1097/MCG.0000000000000116 [DOI] [PubMed] [Google Scholar]
- 9.Sebastiani G, Ghali P, Wong P, et al. Physicians' practices for diagnosing liver fibrosis in chronic liver diseases: a nationwide, Canadian survey. Can J Gastroenterol Hepatol 2014;28:23–30. 10.1155/2014/675409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanyal D, Mukherjee P, Raychaudhuri M, et al. Profile of liver enzymes in non-alcoholic fatty liver disease in patients with impaired glucose tolerance and newly detected untreated type 2 diabetes. Indian J Endocrinol Metab 2015;19:597–601. 10.4103/2230-8210.163172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark J, Brancati F, Diehl A. Nonalcoholic fatty liver disease: the most common cause of abnormal liver enzymes in the U.S. population. Gastroenterology 2001;120:A65 10.1016/S0016-5085(01)80321-8 [DOI] [Google Scholar]
- 12.Dyson JK, Anstee QM, McPherson S. Non-Alcoholic fatty liver disease: a practical approach to diagnosis and staging. Frontline Gastroenterol 2014;5:211–8. 10.1136/flgastro-2013-100403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armstrong MJ, Houlihan DD, Bentham L, et al. Presence and severity of non-alcoholic fatty liver disease in a large prospective primary care cohort. J Hepatol 2012;56:234–40. 10.1016/j.jhep.2011.03.020 [DOI] [PubMed] [Google Scholar]
- 14.Sattar N, Forrest E, Preiss D. Non-Alcoholic fatty liver disease. BMJ 2014;349:g4596 10.1136/bmj.g4596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Angulo P, Keach JC, Batts KP, et al. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology 1999;30:1356–62. 10.1002/hep.510300604 [DOI] [PubMed] [Google Scholar]
- 16.Malakouti M, Kataria A, Ali SK, et al. Elevated liver enzymes in asymptomatic patients – what should I do? J Clin Transl Hepatol 2017;5:1–10. 10.14218/JCTH.2017.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McPherson S, Stewart SF, Henderson E, et al. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut 2010;59:1265–9. 10.1136/gut.2010.216077 [DOI] [PubMed] [Google Scholar]
- 18.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 2004;40:1387–95. 10.1002/hep.20466 [DOI] [PubMed] [Google Scholar]
- 19.Kotronen A, Yki-Järvinen H. Fatty liver: a novel component of the metabolic syndrome. Arterioscler Thromb Vasc Biol 2008;28:27–38. 10.1161/ATVBAHA.107.147538 [DOI] [PubMed] [Google Scholar]
- 20.Noureddin M, Loomba R. Nonalcoholic fatty liver disease: indications for liver biopsy and noninvasive biomarkers. Clin Liver Dis 2012;1:104–7. 10.1002/cld.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Obika M, Noguchi H. Diagnosis and evaluation of nonalcoholic fatty liver disease. Exp Diabetes Res 2012;2012:1–12. 10.1155/2012/145754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nallagangula KS, Nagaraj SK, Venkataswamy L, et al. Liver fibrosis: a compilation on the biomarkers status and their significance during disease progression. Future Sci OA 2018;4:FSO250 10.4155/fsoa-2017-0083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindor KD, Kowdley KV, Heathcote EJ, et al. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology 2004;39:770–8. 10.1002/hep.20092 [DOI] [PubMed] [Google Scholar]
- 24.Leuschner UFH, Lindenthal B, Herrmann G, et al. High-Dose ursodeoxycholic acid therapy for nonalcoholic steatohepatitis: a double-blind, randomized, placebo-controlled trial. Hepatology 2010;52:472–9. 10.1002/hep.23727 [DOI] [PubMed] [Google Scholar]
- 25.Lavine JE, Schwimmer JB, Van Natta ML, et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the tonic randomized controlled trial. JAMA 2011;305:1659–68. 10.1001/jama.2011.520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med 2010;362:1675–85. 10.1056/NEJMoa0907929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA 2015;313:2263–73. 10.1001/jama.2015.5370 [DOI] [PubMed] [Google Scholar]
- 28.Schürks M, Glynn RJ, Rist PM, et al. Effects of vitamin E on stroke subtypes: meta-analysis of randomised controlled trials. BMJ 2010;341:c5702 10.1136/bmj.c5702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klein EA, Thompson IM, Tangen CM, et al. Vitamin E and the risk of prostate cancer: the selenium and vitamin E cancer prevention trial (select). JAMA 2011;306:1549–56. 10.1001/jama.2011.1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pharmaceuticals I. Intercept Reports Additional Positive Data from REGENERATE, the First Successful Phase 3 Study in NASH [Press release]. April 11, 2019. Available: http://ir.interceptpharma.com/news-releases/news-release-details/intercept-reports-additional-positive-data-regenerate-first [Accessed 4 Dec 2019].
- 31.Sciences G. Gilead Announces Topline Data From Phase 3 STELLAR-4 Study of Selonsertib in Compensated Cirrhosis (F4) Due to Nonalcoholic Steatohepatitis (NASH) [Press release]. February 11, 2019. Available: https://www.gilead.com/news-and-press/press-room/press-releases/2019/2/gilead-announces-topline-data-from-phase-3-stellar4-study-of-selonsertib-in-compensated-cirrhosis-f4-due-to-nonalcoholic-steatohepatitis-nash [Accessed 4 Dec 2019].
- 32.Filozof C, Goldstein BJ, Williams RN, et al. Non-Alcoholic steatohepatitis: limited available treatment options but promising drugs in development and recent progress towards a regulatory approval pathway. Drugs 2015;75:1373–92. 10.1007/s40265-015-0437-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poongothai S, Karkuzhali K, Prakash GSiva, et al. Effect of essentiale in diabetic subjects with Non - Alcoholic fatty liver. Int J Diabetes Dev Ctries 2005;25:12–19. 10.4103/0973-3930.26859 [DOI] [Google Scholar]
- 34.Gundermann K-J, Gundermann S, Drozdzik M, et al. Essential phospholipids in fatty liver: a scientific update. Clin Exp Gastroenterol 2016;9:105–17. 10.2147/CEG.S96362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Padma L, Mukaddam Q, Trailokya A. An observational study of Essentiale-L in the treatment of patients with fatty liver disease. Indian Journal of Clinical Practice 2013;23:735–9. [Google Scholar]
- 36.Lieber CS, Robins SJ, Li J, Jianjun L, et al. Phosphatidylcholine protects against fibrosis and cirrhosis in the baboon. Gastroenterology 1994;106:152–9. 10.1016/S0016-5085(94)95023-7 [DOI] [PubMed] [Google Scholar]
- 37.Lieber CS, Leo MA, Aleynik SI, et al. Polyenylphosphatidylcholine decreases alcohol-induced oxidative stress in the baboon. Alcohol Clin Exp Res 1997;21:375–9. 10.1111/j.1530-0277.1997.tb03776.x [DOI] [PubMed] [Google Scholar]
- 38.Lieber CS. New concepts of the pathogenesis of alcoholic liver disease lead to novel treatments. Curr Gastroenterol Rep 2004;6:60–5. 10.1007/s11894-004-0027-0 [DOI] [PubMed] [Google Scholar]
- 39.Ivashkin VT, Drapkina OM, Mayev IV, et al. The prevalence of non-alcoholic fatty liver disease in patients of outpatient practice in the Russian Federation: the results of the study DIREG 2. Russian Journal of Gastroenterology, Hepatology, Coloproctology 2015;25:31–8. [Google Scholar]
- 40.Lazebnik LB, Radchenko VG, Golovanova EV, et al. [NONALCOHOLIC FATTY LIVER DISEASE: DIAGNOSTIC, SYMPTOMS, TREATMENT. GUIDELINES WERE APPROVED BY THE XV GASTROENTEROLOGICAL SCIENTIFIC SOCIETY OF RUSSIA IN 2015]. Eksp Klin Gastroenterol 2015;7:85–96. [PubMed] [Google Scholar]
- 41.JH L, Chen XY, Zhong CF, et al. A randomized controlled study of essential phospholipids (Essentiale capsules) in the treatment of fatty liver. Infect Dis Info 2000;13:180–1. [Google Scholar]
- 42.Sas E, Grinevich V, Efimov O, et al. 1366 beneficial influence of polyunsaturated phosphatidylcholine enhances functional liver condition and liver structure in patients with nonalcoholic steatohepatitis. Results of prolonged randomized blinded prospective clinical study. J Hepatol 2013;58:S549 10.1016/S0168-8278(13)61365-3 [DOI] [Google Scholar]
- 43.Maev IV, Samsonov AA, Palgova LK, et al. Real-World comorbidities and treatment patterns among patients with non-alcoholic fatty liver disease receiving phosphatidylcholine as adjunctive therapy in Russia. BMJ Open Gastroenterol 2019;6:e000307 10.1136/bmjgast-2019-000307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yin D, Kong L. Observation for curative effect of Essentiale in treatment of fatty liver caused by diabetes mellitus. Med J Q Ilu 2000;15:277–8. [Google Scholar]
- 45.Sun C, Zheng X, Tan Z, et al. Clinical observation on polyene phosphatidyl choline and metformin in the treatment of type 2 diabetes and non-alcoholic fatty liver disease. Clin Focus 2008;23:1272–3. [Google Scholar]
- 46.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B 1995;57:289–300. 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- 47.Pardhe BD, Shakya S, Bhetwal A, et al. Metabolic syndrome and biochemical changes among non-alcoholic fatty liver disease patients attending a tertiary care hospital of Nepal. BMC Gastroenterol 2018;18:109 10.1186/s12876-018-0843-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Esteghamati A, Jamali A, Khalilzadeh O, et al. Metabolic syndrome is linked to a mild elevation in liver aminotransferases in diabetic patients with undetectable non-alcoholic fatty liver disease by ultrasound. Diabetol Metab Syndr 2010;2:65 10.1186/1758-5996-2-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chang Y, Ryu S, Sung E, et al. Higher concentrations of alanine aminotransferase within the reference interval predict nonalcoholic fatty liver disease. Clin Chem 2007;53:686–92. 10.1373/clinchem.2006.081257 [DOI] [PubMed] [Google Scholar]
- 50.Tiikkainen M, Bergholm R, Vehkavaara S, et al. Effects of identical weight loss on body composition and features of insulin resistance in obese women with high and low liver fat content. Diabetes 2003;52:701–7. 10.2337/diabetes.52.3.701 [DOI] [PubMed] [Google Scholar]
- 51.Varganova DL, Pavlov CS, Casazza G, et al. Essential phospholipids for people with non-alcoholic fatty liver disease. Cochrane Database Syst Rev 2019;67 10.1002/14651858.CD013301 [DOI] [Google Scholar]
- 52.Gundermann K-J, Kuenker A, Kuntz E, et al. Activity of essential phospholipids (EPL) from soybean in liver diseases. Pharmacol Rep 2011;63:643–59. 10.1016/S1734-1140(11)70576-X [DOI] [PubMed] [Google Scholar]
- 53.Valentino G, Zivko C, Weber F, et al. Synergy of Phospholipid-Drug formulations significantly deactivates profibrogenic human hepatic stellate cells. Pharmaceutics 2019;11:676 10.3390/pharmaceutics11120676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lieber CS, Robins SJ, Leo MA. Hepatic phosphatidylethanolamine methyltransferase activity is decreased by ethanol and increased by phosphatidylcholine. Alcohol Clin Exp Res 1994;18:592–5. 10.1111/j.1530-0277.1994.tb00915.x [DOI] [PubMed] [Google Scholar]
- 55.Lieber CS, DeCarli LM, Mak KM, et al. Attenuation of alcohol-induced hepatic fibrosis by polyunsaturated lecithin. Hepatology 1990;12:1390–8. 10.1002/hep.1840120621 [DOI] [PubMed] [Google Scholar]
- 56.Lieber CS, Robins SJ, Li J, et al. Phosphatidylcholine protects against fibrosis and cirrhosis in the baboon. Gastroenterology 1994;106:152–9. 10.1016/S0016-5085(94)95023-7 [DOI] [PubMed] [Google Scholar]
- 57.Ma X, Zhao J, Lieber CS. Polyenylphosphatidylcholine attenuates non-alcoholic hepatic fibrosis and accelerates its regression. J Hepatol 1996;24:604–13. 10.1016/S0168-8278(96)80147-4 [DOI] [PubMed] [Google Scholar]
- 58.Okiyama W, Tanaka N, Nakajima T, et al. Polyenephosphatidylcholine prevents alcoholic liver disease in PPARalpha-null mice through attenuation of increases in oxidative stress. J Hepatol 2009;50:1236–46. 10.1016/j.jhep.2009.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Buko V, Lukivskaya O, Nikitin V, et al. Hepatic and pancreatic effects of polyenoylphosphatidylcholine in rats with alloxan-induced diabetes. Cell Biochem Funct 1996;14:131–7. 10.1002/cbf.657 [DOI] [PubMed] [Google Scholar]
- 60.Navder KP, Baraona E, Leo MA, et al. Oxidation of LDL in baboons is increased by alcohol and attenuated by polyenylphosphatidylcholine. J Lipid Res 1999;40:983–7. [PubMed] [Google Scholar]
- 61.Chen M, Huang H, Zhou P, et al. Oral phosphatidylcholine improves intestinal barrier function in drug-induced liver injury in rats. Gastroenterol Res Pract 2019;2019:1–6. 10.1155/2019/8723460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Petrović G, Bjelaković G, Benedeto-Stojanov D, et al. Obesity and metabolic syndrome as risk factors for the development of non-alcoholic fatty liver disease as diagnosed by ultrasound. Vojnosanit Pregl 2016;73:910–20. 10.2298/VSP150514093P [DOI] [PubMed] [Google Scholar]
- 63.Bhusal KR, Simkhada R, Nepal P. Lipid profile in different grades of ultrasonic non-alcoholic fatty liver disease. J Coll Med Sci-Nepal 2017;13:258–61. 10.3126/jcmsn.v13i2.17773 [DOI] [Google Scholar]
- 64.Pastori D, Baratta F, Novo M, et al. Remnant lipoprotein cholesterol and cardiovascular and cerebrovascular events in patients with non-alcoholic fatty liver disease. J Clin Med 2018;7:378 10.3390/jcm7110378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American association for the study of liver diseases, American College of gastroenterology, and the American gastroenterological association. Hepatology 2012;55:2005–23. 10.1002/hep.25762 [DOI] [PubMed] [Google Scholar]
- 66.Shahab O, Biswas R, Paik J, et al. Among patients with NAFLD, treatment of dyslipidemia does not reduce cardiovascular mortality. Hepatol Commun 2018;2:1227–34. 10.1002/hep4.1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mofrad P, Contos MJ, Haque M, et al. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology 2003;37:1286–92. 10.1053/jhep.2003.50229 [DOI] [PubMed] [Google Scholar]
- 68.Joseph AE, Saverymuttu SH, al-Sam S, et al. Comparison of liver histology with ultrasonography in assessing diffuse parenchymal liver disease. Clin Radiol 1991;43:26–31. 10.1016/S0009-9260(05)80350-2 [DOI] [PubMed] [Google Scholar]
- 69.Siegelman ES, Rosen MA. Imaging of hepatic steatosis. Semin Liver Dis 2001;21:071–80. 10.1055/s-2001-12930 [DOI] [PubMed] [Google Scholar]


