Abstract
Objective
Maturity-onset diabetes of the young caused by hepatocyte nuclear factor-1 alpha (HNF1A) variants (HNF1A-MODY) is a common form of monogenetic diabetes. Although patients with HNF1A-MODY might specifically benefit from sulfonylurea treatment, available methods for screening this specific type of diabetes are not cost-effective. This study was designed to establish an optimized clinical strategy based on multiple biomarkers to distinguish patients with HNF1A-MODY from clinically diagnosed early-onset type 2 diabetes (EOD) for genetic testing in a Chinese population.
Research design and methods
A case–control study including 125 non-related young patients with EOD and 15 probands with HNF1A-MODY (cohort 1) was conducted to evaluate reported biomarkers for HNF1A-MODY. A cut-off for the fasting insulin (Fins) level, the 97.5 percentile of 150 healthy subjects with normal components of metabolic syndrome (cohort 2), was used to filter out individuals with obvious insulin resistance (Fins <102 pmol/L). An optimized clinical screening strategy (HNF1A-CSS) was established, and its effectiveness was assessed in another group of 410 young patients with EOD (cohort 3).
Results
In cohort 1, body mass index (BMI), serum high-density lipoprotein cholesterol (HDL-c) and high-sensitivity C reactive protein (hs-CRP) levels were confirmed to be useful for the differential diagnosis of HNF1A-MODY. In cohort 3, eight probands with HNF1A-MODY were identified. In cohort 3 and young relatives with HNF1A-MODY, meeting three of four criteria (BMI <28 kg/m2, hs-CRP <0.75 mg/L, Fins <102 pmol/L and HDL-c >1.12 mmol/L), the sensitivity and specificity of HNF1A-CSS were 100% and 69.3%, respectively. In the pooled analysis of all young patients, HNF1A-CSS displayed 90.5% sensitivity and 73.6% specificity for identifying patients with HNF1A-MODY among those with clinically diagnosed EOD.
Conclusion
Our HNF1A-CSS is useful for distinguishing patients with HNF1A-MODY from patients with EOD in a young Chinese population.
Keywords: screening strategies, HNF1a, MODY, type 2 diabetes
Significance of this study.
What is already known about this subject?
The overlap of phenotypes makes patients with maturity-onset diabetes of the young (MODY) difficult to distinguish from patients with early-onset type 2 diabetes (EOD) in the clinical setting.
A potentially efficient clinical screening strategy (CSS) including readily available clinical and biochemical features confirmed to be associated with hepatocyte nuclear factor-1 alpha (HNF1A)-MODY is needed.
What are the new findings?
Our study is the first to establish an HNF1A-CSS with the largest sample in an East Asian population. In this study, we confirmed the effectiveness of several reported biomarkers (high-sensitivity C reactive protein (hs-CRP), body mass index (BMI) and high-density lipoprotein cholesterol (HDL-c)) for differentiating young patients with HNF1A-MODY from patients with EOD in a Chinese population.
A cut-off for the fasting insulin (Fins) level derived from upper limit of normal reference range further excluded patients with type 2 diabetes.
By including multiple biomarkers (hs-CRP, BMI, HDL-c and Fins), we established and evaluated a CSS with better performance than the reported models and a single biomarker for identifying HNF1A-MODY in a Chinese population with EOD.
How might these results change the focus of research or clinical practice?
The CSS composed of the four criteria established in our study is practical and easy to be widely used in the real world.
Introduction
Maturity-onset diabetes of the young (MODY) caused by hepatocyte nuclear factor-1 alpha (HNF1A) gene variants is one of the most common types of MODY (HNF1A-MODY),1 2 usually misdiagnosed with either type 1 (T1DM) or type 2 diabetes mellitus (T2DM), and often receive incorrect treatment.2–4 Patients with HNF1A-MODY are sensitive to sulfonylureas (SUs),5 and their therapy is often changed from insulin to SUs once the diagnosis is established.6
Early-onset T2DM (EOD, diagnosed before 40 years old) has become increasingly prevalent in the past decades worldwide. Because of the overlap of EOD phenotypes with the classical forms of MODY,7 clinicians experience challenges in distinguishing patients with MODY from EOD. Despite tremendous advances in DNA sequencing technology in recent years, the application of genetic testing for common MODY subtypes to all patients remains very costly. And the interpretation of genetic findings without a clinical context is challenging. Therefore, a cost-effective screening strategy to identify subjects for genetic testing is urgently needed. In fact, using basic clinical characteristics, a prediction model to determine the probability of MODY has been developed,8 and if other important clinical markers such as insulin secretion and insulin resistance were included, the model would be improved. Particularly, some MODY subtype-specific biomarkers have been discovered, and they make it possible to establish a strategy to clinically screen patients with MODY due to single gene.9 10
Some researchers recommended clinical screening strategies (CSS) for identifying individuals with diabetes who have a higher probability of carrying causal variants in the HNF1A gene before molecular testing.2 7 These strategies are very attractive since they have the potential to reduce both the labor and costs associated with diagnosing a disease condition. In the past, single index or compositional indices, including the absence of autoantibodies associated with T1DM, young age at diabetes diagnosis, a non-obese status, a strong family history of diabetes in multiple generations, high-sensitivity C reactive protein (hs-CRP) and high-density lipoprotein cholesterol (HDL-c) levels, have been used to screen for HNF1A-MODY.7 11–13 However, the performance of these approaches in clinical settings has been suboptimal. In the present study, we used multiple biomarkers to establish a new optimized screening strategy to identify HNF1A-MODY in patients with EOD.
Methods
Study subjects
First, 125 patients with EOD and 15 patients with HNF1A-MODY were included (cohort 1). The cohort was used to evaluate the ability of the reported clinical biomarkers (body mass index (BMI), HDL-c and hs-CRP) associated with HNF1A-MODY to differentially diagnose HNF1A-MODY.
The patients with EOD were consecutively recruited from the Department of Endocrinology and Metabolism of Peking University People’s Hospital (PUPH) in Beijing from June 2016 to January 2018. They were diagnosed at 14–40 years old. The 125 patients with EOD were confirmed not to carry any rare variants of HNF1A, HNF4A, HNF1B, GCK or mitochondrial DNA 3243A>G mutation by DNA sequencing.
The 15 patients with a confirmed genetic diagnosis of HNF1A-MODY by DNA sequencing were from other clinical centers in Beijing. Each carried one of 13 rare variants of HNF1A (p.Thr10Met, p.Pro33Leu, p.Arg131Trp, p.Gln176Ter, p.Arg203His, p.Gly292Argfs*25, p.Ala311Asp, p.Pro379Thr, IVS2 +1 G>A, p.Pro519Leu, p.Gln541Ter, p.Val567Ile and p.Ile618Met) (online supplementary table S1), which were pathogenic or likely pathogenic according to the standards and guidelines recommended by the American College of Medical Genetics (ACMG) (online supplementary table S2).
bmjdrc-2019-000745supp001.pdf (1.3MB, pdf)
Second, 150 subjects (cohort 2) with normal glucose tolerance (NGT) and normal components of metabolic syndrome14 were used to determine the cut-off value for the fasting serum insulin (Fins) level to identify patients with HNF1A-MODY.
Increased insulin secretion from beta cells compensates for insulin resistance and does not result in hyperglycemia in normal individuals with NGT15; metabolic syndrome is strongly correlated with insulin resistance. Thus, in the subjects with NGT and no signs of insulin resistance (a condition with normal components of metabolic syndrome), an upper limit of the reference range of Fins levels would help to screen individuals with insulin resistance because an increase in Fins beyond the upper limit would indicate the occurrence of insulin resistance. In contrast to HNF1A-MODY, which is characterized by impaired beta cell function, insulin resistance is a major clinical feature of T2DM.
Cohort 2 were randomly selected from the 3345 subjects of the Pinggu cohort, which was described in our previous study.10 The subjects were aged ≤45 years at examination. Their fasting plasma glucose (FPG) levels were less than 6.1 mmol/L, 2-hour plasma glucose levels during the oral glucose tolerance test were less than 7.8 mmol/L and hemoglobin A1c (HbA1c) levels were less than 42 mmol/mol (6.0%). The clinical characteristics of the 150 subjects are presented in online supplementary table S3.
Third, a group of patients with clinically diagnosed as EOD were recruited in PUPH from January 2014 to May 2016 and used to validate the effectiveness of the CSS established in this study for HNF1A-MODY.
To avoid the interference from other subtypes of monogenic diabetes, four patients with other common subtype monogenic diabetes (two mitochondrial 3243A>G, one HNF4A-MODY and one GCK-MODY) (shown in online supplementary table S4) were excluded, and the remaining 410 patients (cohort 3) were used for analysis.
Clinical examinations and laboratory tests
All participants were examined in the morning after an overnight fast of 10–12 hours. Their clinical information and blood samples were collected.10 HbA1c, C-peptide, insulin, plasma glucose, total cholesterol (TC), HDL-c, low-density lipoprotein cholesterol (LDL-c), triglyceride (TG) and hs-CRP levels were measured. Fresh urine samples were collected to assess the urinary albumin/creatinine ratio (ACR).
The serum for quality control and serum samples from the patients were determined for glutamic acid decarboxylase antibody (GADA) (RSR Limited, UK), islet cell antibodies (ICA) (Biomerica, USA) and insulin antibody (IA) (Orgentec, Germany) using commercialized ELISA kit. The assays were performed according to the instructions from the manufacturer.
According to the Chinese-specific diagnostic criteria for obesity, a BMI ≥28 kg/m2 was defined as obesity.16 The diagnosis of obesity in subjects aged between 14 and 18 years was based on weight-for-age curves for Chinese individuals.17
Inclusion and exclusion criteria for the study subjects with EOD
All subjects were of Chinese Han ethnicity and aged ≤45 years. Diabetes was clinically diagnosed before 40 years according to WHO criteria.18 Patients were excluded from this study according to the following criteria: (1) the presence of typical clinical features of T1DM (absolute insulin deficiency and insulin-dependent treatment after diagnosis); (2) other specific forms of diabetes (eg, chronic pancreatitis and steroid-induced diabetes; (3) if any autoantibodies (GADAs, ICAs and IAs) were positive, but for the patients with exogenous insulin treatment, if only IA was positive and they had no other typical features of T1DM, they were also included; (4) a fasting serum C-peptide level <0.1 ng/mL; (5) the presence of an autoimmune disease; (6) the presence of an acute infectious disease or the acute stage of myocardial infarction and stroke and (7) an hs-CRP level >10 mg/L.
DNA sequencing and genetic diagnosis
Genomic DNA was extracted from whole blood. The patients in cohort 1 were sequenced to screen for the variants in exons of HNF1A, GCK, HNF4A, HNF1B or mitochondrial 3243A>G mutation by Sanger method. The patients in cohort 3 were screened for mitochondrial 3243A>G mutation by Sanger sequencing, and their DNA samples were also used for exon capture and next-generation sequencing. An Agilent SureselectXT2 customized gene panel was designed for HNF1A and other genes for common MODY subtypes (GCK, HNF4A and HNF1B). A HiSeq2000 Sequencing System (Illumina, San Diego, California, USA) was used for sequencing. Rare variants (≤0.005 in the Exome Aggregation Consortium) with a sequencing depth of at least 20 and variants located in coding regions with non-synonymous and frameshift variants or in splicing sites near the introns were included and validated by PCR and Sanger sequencing.
Three hundred controls with NGT and normal HbA1c were used to screen the rare variants identified in this study by Sanger method, which is helpful for determining whether the variants are damaging or not.
The pathogenicity of these variants was evaluated using the standards and guidelines recommended by ACMG19 (online supplementary table S2). If patients carried pathogenic or likely pathogenic variants, they were diagnosed with HNF1A-MODY.
Selection of clinical biomarkers and establishment of a CSS to differentiate HNF1A-MODY from EOD
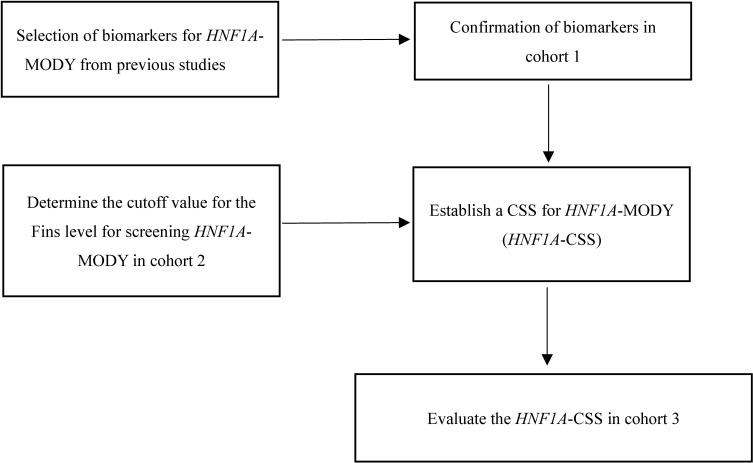
As reported in previous studies, most patients with HNF1A-MODY have no insulin resistance.8 20–22 However, a recent study pointed that the patients with both HNF1A-MODY and overweight/obesity should not be ignored.23 They also present low insulin secretion, low serum hs-CRP levels,9 24 25 low prevalence of dyslipidemia (such as a normal or higher HDL-c level than patients with T2DM) and a low rate of insulin resistance.4 7 All relevant biomarkers were used to establish the clinical screening model for identifying individuals with a higher probability of HNF1A-MODY. If different cut-off values were reported for the same variable, the cut-off value with higher sensitivity was used in this study. The flowchart showing the design of our study is presented in figure 1.
Figure 1.
Flowchart showing the design of our study. CSS, clinical screening strategy; HNF1A, hepatocyte nuclear factor-1 alpha; MODY, maturity-onset diabetes of the young.
Statistical analysis
Statistical analyses were performed using the Statistical Package for Social Sciences (SPSS for Windows, V.23.0; Chicago, Illinois, USA). Student’s t-test was used to analyze quantitative traits. The χ2 test or Fisher’s exact test was used to assess categorical traits. Normally distributed continuous variables are presented as means and SDs (means±SDs), and non-normally distributed variables are presented as medians (IQRs). Categorical variables are presented as numbers and percentages. The ability of each biomarker and HNF1A score to discriminate HNF1A-MODY from EOD was assessed by calculating the area under the receiver operating characteristic curve (ROC) curve (C-statistics). A p value <0.05 was considered significant.
Results
Evaluation of three reported clinical biomarkers for HNF1A-MODY in cohort 1
Compared with patients with EOD (table 1), a lower percentage of patients with HNF1A-MODY exhibited obesity and dyslipidemia; these patients were also diagnosed at a younger age and presented lower levels of BMI, HbA1c, TC, TGs, LDL-c, uric acid and hs-CRP, as well as higher levels of HDL-c.
Table 1.
Clinical characteristics of the patients with HNF1A-MODY and young patients with EOD in cohort 1
| Characteristic | EOD (N=125) | HNF1A-MODY (N=15) | P value |
| Sex, male/female | 91/34 | 8/7 | 0.138 |
| Age, mean (SD), years | 32.4 (6.6) | 29.4 (9.3) | 0.120 |
| Age at diagnosis, mean (SD), years | 29.7 (6.0) | 25.3 (7.5) | 0.010* |
| Duration of DM, median (IQR), years | 1.0 (0.1 to 5.0) | 3.0 (1.0 to 6.0) | 0.083 |
| Family history of DM, n (%) | 105 (84.0) | 14 (93.3) | 0.469 |
| BMI, mean (SD), kg/m2 | 28.1 (4.1) | 23.0 (4.0) | <0.0001*** |
| SBP, mean (SD), mm Hg | 125.2 (13.9) | 122.2 (14.3) | 0.433 |
| DBP, mean (SD), mm Hg | 78.8 (11.2) | 76.6 (10.2) | 0.474 |
| FPG, median (IQR), mmol/L | 7.32 (6.16 to 9.75) | 7.20 (5.10 to 7.83) | 0.222 |
| HbA1c, median (IQR), mmol/mol | 76.0 (58.0 to 95.5) | 56.0 (42.0 to 67.0) | 0.001** |
| HbA1c, median (IQR), % | 9.10 (7.50 to 10.85) | 7.30 (6.00 to 8.30) | 0.001** |
| TC, mean (SD), mmol/L | 4.87 (1.10) | 4.21 (0.69) | 0.024* |
| LDL-c, mean (SD), mmol/L | 3.14 (0.87) | 2.58 (0.75) | 0.019* |
| HDL-c, mean (SD), mmol/L | |||
| Male | 0.96 (0.19) | 1.15 (0.35) | 0.016* |
| Female | 1.02 (0.24) | 1.45 (0.46) | 0.001** |
| Triglycerides, median (IQR), mmol/L | 1.72 (1.22 to 2.70) | 1.10 (0.74 to 1.53) | 0.002** |
| hs-CRP, median (IQR), mg/L | 2.14 (1.22 to 4.02) | 0.41 (0.10 to 2.13) | 0.002* |
| CRE, mean (SD), µmol/L | 60.3 (14.8) | 68.7 (21.4) | 0.056 |
| UA, mean (SD), µmol/L | |||
| Male | 390.8 (92.7) | 313.7 (53.5) | 0.033* |
| Female | 353.2 (92.6) | 266.6 (61.0) | 0.024* |
| ACR, median (IQR), mg/g | 8.36 (3.90 to 20.25) | 5.00 (3.85 to 6.50) | 0.078 |
| eGFR, median (IQR), mL/min/1.73 m2 | 126.0 (113.3 to 139.0) | 134.5 (119.0 to 160.2) | 0.272 |
| Comorbidities and complications | |||
| Hypertension, n (%) | 19 (15.2) | 1 (6.7) | 0.696 |
| Dyslipidemia, n (%) | 69 (55.2) | 2 (13.3) | 0.002** |
| Diabetic nephropathy, n (%) | 9 (7.2) | 1 (6.7) | 1.000 |
| Diabetic retinopathy, n (%) | 11 (8.8) | 4 (26.7) | 0.058 |
| Coronary heart disease, n (%) | 1 (0.8) | 1 (6.7) | 0.203 |
| Cerebrovascular disease, n (%) | 2 (1.6) | 1 (6.7) | 0.290 |
| Obesity, n (%) | 62 (49.6) | 1 (6.7) | 0.002** |
| Treatment | |||
| OHA, n (%) | 65 (52.0) | 10 (66.7) | 0.258 |
| Insulin, n (%) | 40 (32.0) | 8 (53.3) | 0.077 |
Data are presented as means (SD) or medians (IQRs); categorical variables are presented as n (%).
eGFR (mL/min per 1.73m2) = 175×CRE(mg/dL)-1.234×age (years)-0.179×0.79 (if female).
*p<0.05, **p<0.01 and ***p<0.001.
ACR, urinary albumin/creatinine ratio; BMI, body mass index; CRE, serum creatinine; DBP, diastolic blood pressure; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; EOD, young early-onset type 2 diabetes; FPG, fasting plasma glucose; HbA1c, hemoglobin A1c; HDL-c, high-density lipoprotein cholesterol; HNF1A, hepatocyte nuclear factor-1 alpha; hs-CRP, high-sensitivity C reactive protein; LDL-c, low-density lipoprotein cholesterol; MODY, maturity-onset diabetes of the young; OHA, oral hypoglycemic agent; SBP, systolic blood pressure; TC, total cholesterol; UA, uric acid.
As shown in online supplementary figure S1, the C-statistic for hs-CRP was 0.902 (95% CI 0.840 to 0.946), and the cut-off value was 0.72 mg/L, with 76.9% sensitivity and 92.0% specificity. The C-statistic for BMI was 0.811 (95% CI 0.736 to 0.872), and the cut-off value was 22.7 kg/m2, with 60.0% sensitivity and 92.0% specificity. The C-statistic for HDL-c was 0.763 (95% CI 0.684 to 0.831), and the cut-off value was 1.20 mmol/L, with 66.7% sensitivity and 88.0% specificity.
When the reported cut-off values for hs-CRP (<0.75 mg/L),9 HDL-c (>1.12 mmol/L)26 and BMI (the cut-off for obesity in Chinese individuals is 28 kg/m2)16 that have been used to identify HNF1A-MODY were applied to cohort 1, the sensitivity and specificity were 76.9% and 92.0% for hs-CRP, 73.3% and 78.4% for HDL-c, and 93.3% and 62.4% for BMI, respectively. These cut-off values displayed higher sensitivity than those determined in cohort 1.
Establishment of a cut-off value for the Fins level for diagnosing HNF1A-MODY
In cohort 2, the 97.5 percentile of the Fins level was 102 pmol/L. In cohort 1, all of 15 patients with HNF1A-MODY presented Fins levels less than 102 pmol/L or received insulin treatment, 88 individuals of 125 patients with EOD had Fins levels of <102 pmol/L. Among the subjects in the Pinggu cohort with Fins levels greater than 102 pmol/L (n=390, unpublished data), 95.1% were diagnosed with metabolic syndrome. Thus, using the Fins cut-off of 102 pmol/L, we were able to exclude most patients with T2DM presenting obvious insulin resistance from patients with EOD.
Establishment of the CSS for HNF1A-MODY (HNF1A-CSS)
Due to our limited sample size, the cut-off values defined in the current study might not be the most precise values for the clinical screening of HNF1A-MODY. Among the reported cut-off values and our values for screening patients with HNF1A-MODY, we selected those with the highest sensitivity and established an HNF1A-CSS. To maximally avoid missing patients with HNF1A-MODY, this HNF1A-CSS comprises the following four criteria: (1) non-obesity, BMI <28 kg/m2,16 (2) hs-CRP level <0.75 mg/L,9 (3) insulin deficiency or a lack of insulin resistance (insulin treatment or Fins <102 pmol/L in patients without insulin treatment) and (4) an HDL-c level >1.12 mmol/L.26
Screening of patients with HNF1A-MODY in cohort 3
In cohort 3, nine rare heterozygous HNF1A variants (p.Arg131Trp, p.Arg200Trp, p.Arg263Cys, p.Gln324Ter, IVS8−1G>A, p.Pro112Leu, p.Glu240Val, p.Glu548Gly and p.Arg159fs (c.474_475insA)) (online supplementary table S1) were identified in nine study subjects. The mothers of the probands carrying p.Arg200Trp, p.Arg131Trp and IVS8−1G>A variants also carried the same variants and were diagnosed with EOD (online supplementary figure S2). The parents of the probands carrying the p.Arg263Cys variant did not have diabetes and did not carry the p.Arg263Cys variant. Unfortunately, we failed to recruit the family members of the other probands. According to the guidelines of the ACMG, eight rare variants (p.Arg131Trp, p.Arg200Trp, p.Arg263Cys, p.Gln324Ter, IVS8−1G>A, p.Glu240Val, p.Pro112Leu and p.Arg159fs (c.474_475insA)) are pathogenic or likely pathogenic (online supplementary table S2). Thus, eight patients with HNF1A-MODY were identified in cohort 3. Nine patients with HNF1A-MODY, including eight probands and one younger family member with the p.Arg200Trp variant and diabetes (clinical features are summarized in online supplementary table S5), were compared with patients with EOD. Similar to cohort 1, patients with HNF1A-MODY from cohort 3 were also characterized by a lower percentage of obesity and dyslipidemia, and displayed a lower age at diagnosis and lower levels of BMI, TGs and hs-CRP, as well as higher levels of HDL-c (table 2).
Table 2.
Clinical characteristics of the patients with HNF1A-MODY and young patients with EOD in cohort 3
| Characteristic | EOD (N=402) | HNF1A-MODY (N=9) | P value |
| Sex, male/female | 273/129 | 3/6 | 0.064 |
| Age, median (IQR), years | 33.0 (29.0 to 36.0) | 31.0 (21.0 to 33.5) | 0.146 |
| Age at diagnosis, median (IQR), years | 30.0 (26.0 to 33.0) | 18.0 (13.0 to 30.5) | 0.006** |
| Duration of DM, median (IQR), years | 2.0 (1.0 to 6.0) | 4.0 (1.0 to 17.0) | 0.077 |
| Family history of DM, n (%) | 307 (76.4) | 8 (88.9) | 0.692 |
| Waist circumference†, mean (SD), cm | 92.5 (12.5) | 78.9 (9.0) | 0.002** |
| BMI, mean (SD), kg/m2 | 26.8 (4.6) | 20.9 (1.9) | <0.0001*** |
| SBP, mean (SD), mm Hg | 122.6 (15.7) | 113.0 (22.3) | 0.073 |
| DBP, mean (SD), mm Hg | 78.8 (12.1) | 69.9 (15.2) | 0.058 |
| FPG, median (IQR), mmol/L | 8.37 (6.53 to 11.05) | 6.86 (5.58 to 8.63) | 0.068 |
| HbA1c, median (IQR), mmol/mol | 77.0 (57.3 to 98.0) | 60.5 (47.0 to 75.0) | 0.095 |
| HbA1c, median (IQR), % | 9.20 (7.43 to 11.10) | 7.65 (6.45 to 8.98) | 0.095 |
| TC, mean (SD), mmol/L | 4.82 (1.18) | 4.73 (1.41) | 0.709 |
| LDL-c, mean (SD), mmol/L | 2.82 (0.85) | 2.84 (0.90) | 0.940 |
| HDL-c†, mean (SD), mmol/L | 1.04 (0.27) | 1.41 (0.20) | <0.0001*** |
| Triglycerides, median (IQR), mmol/L | 1.74 (1.16 to 2.74) | 1.05 (0.87 to 1.53) | 0.033* |
| hs-CRP, median (IQR), mg/L | 1.47 (0.71 to 3.21) | 0.17 (0.11 to 0.32) | <0.0001*** |
| CRE, median (IQR), µmol/L | 62.0 (50.0 to 72.0) | 69.0 (46.0 to 89.0) | 0.520 |
| UA†, median (IQR), µmol/L | 345.0 (283.0 to 411.0) | 350.0 (264.5 to 363.5) | 0.796 |
| ACR, median (IQR), mg/g | 8.61 (4.19 to 30.00) | 10.87 (6.67 to 1004.42) | 0.368 |
| eGFR, median (IQR), mL/min/1.73 m2 | 139.4 (120.6 to 167.9) | 126.4 (73.4 to 180.4) | 0.518 |
| Comorbidities and complications | |||
| Hypertension, n (%) | 56 (13.9) | 1 (11.1) | 1.000 |
| Dyslipidemia, n (%) | 375 (93.3) | 2 (22.2) | <0.0001*** |
| Diabetic nephropathy, n (%) | 50 (12.4) | 3 (33.3) | 0.097 |
| Diabetic retinopathy, n (%) | 36 (9.0) | 3 (33.3) | 0.044* |
| Coronary heart disease, n (%) | 9 (2.2) | 0 (0) | 1.000 |
| Cerebrovascular disease, n (%) | 5 (1.2) | 0 (0) | 1.000 |
| Obesity, n (%) | 156 (38.8) | 0 (0) | 0.015* |
| Treatment | |||
| OHA, n (%) | 171 (42.5) | 5 (55.6) | 0.506 |
| Insulin, n (%) | 174 (43.3) | 4 (44.4) | 1.000 |
Data are presented as the means (SD) or medians (IQR); categorical variables are presented as n (%).
eGFR (mL/min per 1.73m2)=175×CRE(mg/dL)-1.234×age (years)-0.179×0.79 (if female).
*p<0.05, **p<0.01 and ***p<0.001.
†Three male patients were diagnosed with HNF1A-MODY, and therefore, the waist circumference, HDL-c level and uric acid level of all patients with HNF1A-MODY (males and females) were calculated in this analysis.
ACR, urinary albumin/creatinine ratio; BMI, body mass index; CRE, serum creatinine; DBP, diastolic blood pressure; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; EOD, early-onset type 2 diabetes; FPG, fasting plasma glucose; HbA1c, hemoglobin A1c; HDL-c, high-density lipoprotein cholesterol; HNF1A, hepatocyte nuclear factor-1 alpha; hs-CRP, high-sensitivity C reactive protein; LDL-c, low-density lipoprotein cholesterol; MODY, maturity-onset diabetes of the young; OHA, oral hypoglycemic agent; SBP, systolic blood pressure; TC, total cholesterol; UA, uric acid.
Evaluation of the effectiveness of the HNF1A-CSS in cohort 3
As shown in figure 2, we tested the effectiveness of HNF1A-CSS on all probands with HNF1A-MODY in cohort 3 and the young relatives with HNF1A-MODY (n=9). Nine individuals met three criteria, eight met four criteria and none met one criterion or two criteria. There were 113 patients including 9 with HNF1A-MODY meeting at least three of four criteria (HNF1A-CSS1), according to HNF1A-CSS1. This indicates that out of sequencing 12.6 patients only one patient could be screened with HNF1A-MODY. The sensitivity and specificity of HNF1A-CSS1 were 100% and 69.7%, respectively, and the positive and negative prediction values were 6.9% and 100%, respectively. On the other hand, there were 51 patients including eight with HNF1A-MODY meeting all of four criteria (HNF1A-CSS2). Through sequencing only 6.4 patients, we could identify one patient with HNF1A-MODY according to HNF1A-CSS2. The sensitivity and specificity of CSS2 were 88.9% and 89.6%, respectively. The positive and negative prediction values were 15.7% and 99.7%, respectively. Notably, HNF1A-CSS1 exhibited the highest sensitivity and HNF1A-CSS2 exhibited the highest specificity for clinically diagnosing HNF1A-MODY.
Figure 2.
Flowchart for the approach used to distinguish between patients with HNF1A-MODY and young patients with early-onset type 2 diabetes in cohort 3. *A family member with HNF1A-MODY is included in this analysis. HNF1A-CSS0 indicates that patients meet two criteria, one criterion or zero criteria, and HNF1A-CSS1 indicates that patients meet three or four criteria, and HNF1A-CSS2 indicates that patients meet four criteria. BMI, body mass index; CSS, clinical screening strategy; EOD, early-onset type 2 diabetes; Fins, fasting insulin; HDL-c, high-density lipoprotein cholesterol; HNF1A, hepatocyte nuclear factor-1 alpha; hs-CRP, high-sensitivity C reactive protein; MODY, maturity-onset diabetes of the young.
We conducted an analysis by pooling all patients with EOD and HNF1A-MODY in this study (24 with HNF1A-MODY and 527 with EOD). As shown in figure 3 and online supplementary figures S3 and S4, after excluding three patients with missing data for the four criteria from cohort 1, 21 patients with HNF1A-MODY were included in the analysis. Nineteen of these patients (90.5%) met the HNF1A-CSS1 criteria and 15 patients (71.4%) met the HNF1A-CSS2 criteria, with specificities of 73.6% and 91.3%, respectively.
Figure 3.
Distribution of patients with HNF1A-MODY among the four groups according to the four criteria. In this figure, patients with HNF1A-MODY were divided into four groups according to the following four criteria: BMI <28 kg/m2, hs-CRP level <0.75 mg/L, insulin treatment or Fins level <102 pmol/L without insulin treatment and an HDL-c level >1.12 mmol/L. Twenty-one patients with HNF1A-MODY were included in our study. The hs-CRP level was not available for two patients with HNF1A-MODY, and the Fins level was not available for one patient; thus, these patients were excluded from this analysis. Fifteen patients met four criteria, two patients met the BMI, Fins and HDL-c criteria, one patient met the BMI, Fins and hs-CRP criteria, and another patient met the Fins, hs-CRP and HDL-c criteria. Only two patients met two criteria (BMI and Fins). BMI, body mass index; CSS, clinical screening strategy; Fins, fasting insulin; HDL-c, high-density lipoprotein cholesterol; HNF1A, hepatocyte nuclear factor-1 alpha; hs-CRP, high-sensitivity C reactive protein; MODY, maturity-onset diabetes of the young.
Discussion
In the present study, we confirmed the utility of several reported biomarkers (hs-CRP, BMI and HDL-c) for differentiating young patients with HNF1A-MODY from EOD in a Chinese population. A new HNF1A-CSS composed of all the aforementioned biomarkers and the Fins level was established for the clinical identification of individuals with a high probability of having HNF1A-MODY among patients with EOD. This study is the first to establish a CSS for HNF1A-MODY in East Asian population using the largest sample to date.
The major obstacle for the diagnosis of patients with HNF1A-MODY is that the diagnosis rate based on existing clinical screening criteria is relatively low (10%–14%).7 11 A recent study confirmed that the addition of hs-CRP levels to other clinical parameters used for identifying HNF1A-MODY slightly improved the performance of the predictive model.27 Different cut-off values for hs-CRP levels (0.25–0.75 mg/L) have been reported in different study populations, with a sensitivity ranging from 71% to 83% and a specificity ranging from 68% to 86%.9 24 25 Our study confirmed that the hs-CRP level is a useful biomarker for the differential diagnosis of HNF1A-MODY in Chinese population with EOD using a similar cut-off value (0.72 mg/L).
A higher HDL-c level has also been observed in patients with HNF1A-MODY compared with those with T2DM or MODY-like phenotypes.7 28 However, only one study reported that the cut-off value for HDL-c (1.12 mmol/L) was able to distinguish patients with HNF1A-MODY from patients with T2DM.26 These findings were similar to our results, and with greater effectiveness in diagnosing females (online supplementary figure S3) in our study. In fact, in HNF1A-/- mice, increased plasma cholesterol levels are predominantly located in large, buoyant HDL particles, likely due to the reduced activity of the HDL-catabolic enzyme hepatic lipase and increased expression of the HDL-esterifying enzyme lecithin: cholesterol acyl transferase.29 Not surprisingly, HDL-c is a relatively specific biomarker for HNF1A-MODY.
HNF1A encodes a transcription factor that increases insulin secretion. Impaired insulin secretion, but not insulin resistance, is the major pathogenic factor associated with HNF1A-MODY. Although the clinical parameters associated with impaired insulin secretion or insulin resistance differ between patients with HNF1A-MODY and T2DM, their application in the differential diagnosis of HNF1A-MODY had not been well assessed. In our study, we adopted the upper limit of the normal reference range for the Fins level as a cut-off and expected to exclude most patients with obvious insulin resistance. Unfortunately, 88/125 patients with EOD in cohort 1 and 336/402 with EOD in cohort 3 had the Fins levels of <102 pmol/L or insulin treatment, which indicates the Fins cut-off was not a good indicator for distinguishing HNF1A-MODY and EOD when it was used as a single marker, but it remained helpful to filter out some patients with both T2DM and insulin resistance, and in turn reduce the number of individuals for genetic screening.
The absence of obesity is one criterion for clinically screening HNF1A-MODY in Caucasians,30 and we confirmed that the obesity-specific cut-off for BMI in the Chinese population is also sufficiently sensitive to identify patients with HNF1A-MODY in a Chinese population. In fact, it was reported that a few of patients with monogenic diabetes had overweight or even obesity,23 and some patients with HNF1A-MODY would become overweight or obesity when they were treated with insulin. As observed in our study, one patient with HNF1A-MODY (carrier of p.Pro33Leu in cohort 1) was obese, which might attribute to long-term insulin injection because her maximum body weight occurred after insulin treatment. Thus, we recommended 28 kg/m2 as the cut-off for clinically identifying HNF1A-MODY instead of the values identified in our ROC analysis (online supplementary figures S1 and S3) to avoid as much as possible missing patients with HNF1A-MODY.
Similar to other studies, 13 of the 23 patients with HNF1A-MODY (15 patients from cohort 1 and 8 from cohort 3) identified in our study (56.5%) were diagnosed before the age of 25 years old, but the family history of diabetes did not differ significantly between the patients with HNF1A-MODY and those with EOD. Thus, many patients with HNF1A-MODY would be missed if a stringent criterion2 7 11 (eg, onset before 25 years of age or a family history of diabetes) is adopted to clinically screen patients for genetic testing.
Multiple biomarkers have been used in previous studies to improve the sensitivity and specificity of predictive models for HNF1A-MODY. One study, which included the largest sample of patients with HNF1A-MODY,7 reported that a clinical predictive model for HNF1A-MODY based on age, BMI, number of generations diagnosed with diabetes, presence of diabetes symptoms and geographic origin only exhibited 49% specificity when the sensitivity was set to 90%. However, its performance has not been confirmed in other populations, and this model must be improved by introducing new biomarkers. Our CSS for HNF1A-MODY, which consists of multiple criteria, would be effective and partly make up the shortcoming of single criterion. For example, the individuals with obesity might have opportunity to be picked up through other criteria, such as Fins, HDL-c or hs-CRP. Although insulin treatment could cause weight gain, insulin treatment itself is one of four criteria.
According to the available clinical screening criteria and after considering the cut-off values for several new biomarkers and pathophysiological characteristics of HNF1A-MODY,2 7 22 we established the HNF1A-CSS to identify patients with HNF1A-MODY in the clinic. Because a higher sensitivity is necessary to increase the efficiency of screening for HNF1A-MODY, when balancing specificity and sensitivity, HNF1A-CSS1 exhibited better performance (90.5% sensitivity and 73.6% specificity) in analyzing pooled data than previously reported models (90% sensitivity and 49% specificity)7 9 24 25 and single biomarker (figure 3 and online supplementary figure S4). Certainly, HNF1A-CSS2 exhibited higher specificity, but it might miss more patients with HNF1A-MODY.
In this study, we established an approach to identify candidates for genetic testing of HNF1A-MODY rather than the full range of MODYs. In fact, each subtype MODY has its specific clinical features and proper therapeutic regimen. For example, hs-CRP is a biomarker for HNF1A-MODY9 but not for HNF4A-MODY; and GCK-MODY was characterized by stable mild hyperglycemia. American Diabetes Association recommended that SUs were first-line therapy for HNF1A/4A-MODY and the patients with GCK-MODY did not require antihyperglycemic therapy except sometimes during pregnancy, so they are important to establish MODY subtype-specific CSS and correctly determine the subtype of MODY. Certainly, the CSS for the full range of MODY was also useful if only basic clinical characteristics were available. A clinical prediction model to determine the probability of common MODY subtypes (GCK-MODY, HNF1A/4A-MODY) had been developed.8 Using that model, candidate patients could be picked up, and then separate gene for subtype of MODY need to be sequenced if they meet CSS for single subtype of MODY or have additional clinical characteristics.
Diabetes due to variants of mitochondrial DNA, GCK, HNF4A or HNF1B was also common among monogenic diabetes. To avoid the potential interference from other subtype monogenic diabetes, we excluded four patients (two mitochondrial 3243A>G, one GCK-MODY and one HNF4A-MODY) in evaluating the effectiveness of the HNF1A-CSS. Of them, only the patient with GCK-MODY met our HNF1A-CSS, but because he had stable mild hyperglycemia, it was easy to pick him up as GCK-MODY.10
Our study has some limitations. (1) This HNF1A-CSS was established for a selected Chinese population of young patients with EOD and must be tested in other populations. (2) Although the sample size of the patients with HNF1A-MODY in this study is the largest sample of Asians reported to date, it is not large enough to obtain the best predictive model for HNF1A-MODY. Cut-off values with the highest sensitivity reported in previous studies and in our analysis were selected, which was verified feasible in the study, to improve the performance of HNF1A-CSS. (3) Indeed, a fasting C peptide level would be helpful to define the insulin resistance. Unfortunately, we did not detect the serum C peptide in cohort 2 and some patients in cohort 1 and cohort 3. (4) Because only the patients carrying pathogenic or likely pathogenic variants of HNF1A were diagnosed with HNF1A-MODY in this study, it is possible that those patients with uncertain significance variants of HNF1A (eg, p.Gly47Arg and p.Glu548Gly in online supplementary table S2) were misdiagnosed as EOD, future functional studies and other information help to further evaluate the pathogenicity of these variants. (5) Due to the limited sensitivity of these autoantibody kits, we are not able to exclude all the patients with T1DM, although GADA, ICA and IA were analyzed in this study. However, basing on the fact of low prevalence of T1DM in China, we thought the effect was ignorable.
In summary, we found a HNF1A-CSS which combining hs-CRP, BMI, HDL-c and Fins levels is very useful for distinguishing patients with HNF1A-MODY from young patients with EOD in a Chinese population. This screening strategy is practical and easy to use in a wide variety of settings in the real world.
Acknowledgments
We thank all the research staff for collecting data and blood samples. We also thank all study participants for their participation and contributions.
Footnotes
YM, SG and XW contributed equally.
XHa and LJ contributed equally.
Contributors: YM performed a literature review, collected data, analyzed the data, and wrote the manuscript. LJ and XH designed the study, contributed to the discussion, and reviewed and edited the manuscript. SG, XW, XC, XX, WG, JY, LZhon, JX, ML, WL, SZ, XZho, YLi, LZhou, YZ, YLu, QR, XHu, XG, XZha, RZ, LC, FW, QW and MH help collect the data and reviewed the manuscript. YM, XHa and LJ are the guarantors of this work and, as such, had full access to all the data reported in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors approved the final draft.
Funding: This research was supported by grants from the National Key Research and Development Program (2016YFC1304901) and the Beijing Municipal Commission of Science and Technology funds (Z141100007414002 and D131100005313008).
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: This study was performed in accordance with the tenets of the Declaration of Helsinki and approved by the ethics committee of Peking University People’s Hospital. Written informed consent was obtained from all participants.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1.Pihoker C, Gilliam LK, Ellard S, et al. Prevalence, characteristics and clinical diagnosis of maturity onset diabetes of the young due to mutations in HNF1A, HNF4A, and glucokinase: results from the search for diabetes in youth. J Clin Endocrinol Metab 2013;98:4055–62. 10.1210/jc.2013-1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ellard SB-C C, Hattersley AT. European molecular genetics quality network, MODY group: best practice guidelines for the molecular genetic diagnosis of maturity-onset diabetes of the young. Diabetologia 2008;51:546–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gardner DS, Tai ES. Clinical features and treatment of maturity onset diabetes of the young (MODY). Diabetes Metab Syndr Obes 2012;5:101–8. 10.2147/DMSO.S23353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Isomaa B, Henricsson M, Lehto M, et al. Chronic diabetic complications in patients with MODY3 diabetes. Diabetologia 1998;41:467–73. 10.1007/s001250050931 [DOI] [PubMed] [Google Scholar]
- 5.Pearson ER, Liddell WG, Shepherd M, et al. Sensitivity to sulphonylureas in patients with hepatocyte nuclear factor-1alpha gene mutations: evidence for pharmacogenetics in diabetes. Diabet Med 2000;17:543–5. 10.1046/j.1464-5491.2000.00305.x [DOI] [PubMed] [Google Scholar]
- 6.Shepherd M, Shields B, Ellard S, et al. A genetic diagnosis of HNF1A diabetes alters treatment and improves glycaemic control in the majority of insulin-treated patients. Diabet Med 2009;26:437–41. 10.1111/j.1464-5491.2009.02690.x [DOI] [PubMed] [Google Scholar]
- 7.Bellanné-Chantelot C, Lévy DJ, Carette C, et al. Clinical characteristics and diagnostic criteria of maturity-onset diabetes of the young (MODY) due to molecular anomalies of the HNF1A gene. J Clin Endocrinol Metab 2011;96:E1346–51. 10.1210/jc.2011-0268 [DOI] [PubMed] [Google Scholar]
- 8.Shields BM, McDonald TJ, Ellard S, et al. The development and validation of a clinical prediction model to determine the probability of MODY in patients with young-onset diabetes. Diabetologia 2012;55:1265–72. 10.1007/s00125-011-2418-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDonald TJ, Shields BM, Lawry J, et al. High-sensitivity CRP discriminates HNF1A-MODY from other subtypes of diabetes. Diabetes Care 2011;34:1860–2. 10.2337/dc11-0323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma Y, Han X, Zhou X, et al. A new clinical screening strategy and prevalence estimation for glucokinase variant-induced diabetes in an adult Chinese population. Genet Med 2019;21:939–47. 10.1038/s41436-018-0282-3 [DOI] [PubMed] [Google Scholar]
- 11.Shields BM, Hicks S, Shepherd MH, et al. Maturity-Onset diabetes of the young (MODY): how many cases are we missing? Diabetologia 2010;53:2504–8. 10.1007/s00125-010-1799-4 [DOI] [PubMed] [Google Scholar]
- 12.Chambers C, Fouts A, Dong F, et al. Characteristics of maturity onset diabetes of the young in a large diabetes center. Pediatr Diabetes 2016;17:360–7. 10.1111/pedi.12289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grzanka M, Matejko B, Szopa M, et al. Assessment of Newly Proposed Clinical Criteria to Identify HNF1A MODY in Patients with an Initial Diagnosis of Type 1 or Type 2 Diabetes Mellitus. Adv Med 2016;2016:1–3. 10.1155/2016/4243784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.IDF IDF: IDF consensus worldwide definition of the metabolic syndrome, 2017. [Google Scholar]
- 15.Bergman RN, Ader M, Huecking K, et al. Accurate assessment of beta-cell function: the hyperbolic correction. Diabetes 2002;51 Suppl 1:S212–20. 10.2337/diabetes.51.2007.s212 [DOI] [PubMed] [Google Scholar]
- 16.Zhou B-F, Cooperative Meta-Analysis Group of the Working Group on Obesity in China . Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults--study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci 2002;15:83–96. [PubMed] [Google Scholar]
- 17.Li H, Ji C-Y, Zong X-N, et al. [Height and weight standardized growth charts for Chinese children and adolescents aged 0 to 18 years]. Zhonghua Er Ke Za Zhi 2009;47:487–92. [PubMed] [Google Scholar]
- 18.Alberti KG, Zimmet PZ, Definition Zimmet PZ:. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a who consultation. Diabet Med 1998;15:539–53. [DOI] [PubMed] [Google Scholar]
- 19.Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and genomics and the association for molecular pathology. Genet Med 2015;17:405–23. 10.1038/gim.2015.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ellard S, Lango Allen H, De Franco E, et al. Improved genetic testing for monogenic diabetes using targeted next-generation sequencing. Diabetologia 2013;56:1958–63. 10.1007/s00125-013-2962-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Owen KR. Rd Lawrence lecture 2012: assessing aetiology in diabetes: how C-peptide, CRP and fucosylation came to the Party! Diabet Med 2013;30:260–6. 10.1111/dme.12038 [DOI] [PubMed] [Google Scholar]
- 22.Carroll RW, Murphy R. Monogenic diabetes: a diagnostic algorithm for clinicians. Genes 2013;4:522–35. 10.3390/genes4040522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kleinberger JW, Copeland KC, Gandica RG, et al. Monogenic diabetes in overweight and obese youth diagnosed with type 2 diabetes: the today clinical trial. Genet Med 2018;20:583–90. 10.1038/gim.2017.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Owen KR, Thanabalasingham G, James TJ, et al. Assessment of high-sensitivity C-reactive protein levels as diagnostic discriminator of maturity-onset diabetes of the young due to HNF1A mutations. Diabetes Care 2010;33:1919–24. 10.2337/dc10-0288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thanabalasingham G, Shah N, Vaxillaire M, et al. A large multi-centre European study validates high-sensitivity C-reactive protein (hsCRP) as a clinical biomarker for the diagnosis of diabetes subtypes. Diabetologia 2011;54:2801–10. 10.1007/s00125-011-2261-y [DOI] [PubMed] [Google Scholar]
- 26.McDonald TJ, McEneny J, Pearson ER, et al. Lipoprotein composition in HNF1A-MODY: differentiating between HNF1A-MODY and type 2 diabetes. Clin Chim Acta 2012;413:927–32. 10.1016/j.cca.2012.02.005 [DOI] [PubMed] [Google Scholar]
- 27.Bellanne-Chantelot C, Coste J, Ciangura C, et al. Monogenic diabetes Study group of the Societe Francophone Du D: high-sensitivity C-reactive protein does not improve the differential diagnosis of HNF1A-MODY and familial young-onset type 2 diabetes: a grey zone analysis. Diabetes & metabolism 2016;42:33–7. [DOI] [PubMed] [Google Scholar]
- 28.Xu JY, Dan QH, Chan V, et al. Genetic and clinical characteristics of maturity-onset diabetes of the young in Chinese patients. Eur J Hum Genet 2005;13:422–7. 10.1038/sj.ejhg.5201347 [DOI] [PubMed] [Google Scholar]
- 29.Shih DQ, Bussen M, Sehayek E, et al. Hepatocyte nuclear factor-1alpha is an essential regulator of bile acid and plasma cholesterol metabolism. Nat Genet 2001;27:375–82. 10.1038/86871 [DOI] [PubMed] [Google Scholar]
- 30.Fajans SS, Bell GI, Polonsky KS. Molecular mechanisms and clinical pathophysiology of maturity-onset diabetes of the young. N Engl J Med 2001;345:971–80. 10.1056/NEJMra002168 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjdrc-2019-000745supp001.pdf (1.3MB, pdf)