Abstract
Background
This is an update of a Cochrane Review first published in The Cochrane Library in Issue 4, 2006.
Otitis media with effusion (OME) is common and may cause hearing loss with associated developmental delay. Treatment remains controversial. The effectiveness of antihistamines, decongestants and antihistamine/decongestant combinations in promoting the resolution of effusions has been assessed by randomized controlled trials.
Objectives
The objective of this review was to determine whether antihistamine, decongestant or combination therapy is effective in treating children who present with OME.
Search methods
We searched the Cochrane Ear, Nose and Throat Disorders Group Trials Register; the Cochrane Central Register of Controlled Trials (CENTRAL); PubMed; EMBASE; CINAHL; Web of Science; BIOSIS Previews; Cambridge Scientific Abstracts; ISRCTN and additional sources for published and unpublished trials. The date of the most recent search was 1 February 2011, following a previous search in 2006.
Selection criteria
Randomized controlled trials (RCTs) using antihistamines, decongestants or antihistamine/decongestant combinations as treatment for OME in children. We excluded trials that randomized on the basis of acute otitis media (AOM) even though OME was also studied in follow up.
Data collection and analysis
Two authors independently extracted data from the published reports using standardized data extraction forms and methods. The two authors assessed the methodological quality of the included studies independently. We expressed dichotomous results as a risk ratio with 95% confidence intervals using a fixed‐effect model when homogeneous and a random‐effects model when heterogeneous. Nearly all outcomes analyzed were homogeneous. We discussed continuous results qualitatively. We conducted statistical analysis using RevMan 5.1 software.
Main results
Sixteen studies (1880 participants) were included in the review. No statistical or clinical benefit was found for any of the interventions or outcomes studied. However, treated study subjects experienced 11% more side effects than untreated subjects (number needed to treat to harm = 9).
Authors' conclusions
The pooled data demonstrate no benefit and some harm from the use of antihistamines or decongestants alone or in combination in the management of OME, therefore we recommend against their use.
Keywords: Child; Humans; Drug Therapy, Combination; Drug Therapy, Combination/methods; Histamine H1 Antagonists; Histamine H1 Antagonists/adverse effects; Histamine H1 Antagonists/therapeutic use; Nasal Decongestants; Nasal Decongestants/adverse effects; Nasal Decongestants/therapeutic use; Otitis Media with Effusion; Otitis Media with Effusion/drug therapy; Randomized Controlled Trials as Topic
Plain language summary
Antihistamines with or without decongestants for otitis media with effusion (OME) ('glue ear') in children
Otitis media with effusion (OME), also known as glue ear or serous otitis media, is a condition in which there is fluid persisting in the middle ear. Many treatments have been suggested. This review summarizes the studies using antihistamines, decongestants or a combination of antihistamines and decongestants and finds no benefit for any of the short or long‐term outcomes including resolution of the fluid, hearing problems or the necessity of additional referral to specialists. Further, using these medications causes significant side effects, such as gastrointestinal upset, irritability, drowsiness or dizziness, in approximately 10% of patients. Therefore antihistamines, decongestants or antihistamine/decongestant combinations are not recommended treatments for OME. Watchful waiting is the best approach with consideration of referral for evaluation by an ENT consultant if symptoms persist beyond 12 weeks.
Background
This is an update of a Cochrane Review first published in The Cochrane Library in Issue 4, 2006. It is one of a number of reviews prepared within the Cochrane Ear, Nose and Throat Disorders Group on management options for patients with otitis media with effusion.
Description of the condition
Symptoms, prevalence and aetiology
Otitis media with effusion (OME), or 'glue ear', is characterized by an accumulation of fluid in the middle ear, in the absence of acute inflammation. It is very common in children, especially between the ages of one and three years and in seasons where the prevalence of upper respiratory tract infections ('colds') is high, with an incidence of 10% to 30%. It occurs frequently even up to the age of seven, with a prevalence of 3% to 8% (Fiellau 1977; Fiellau 1983; Lous 1981; Teele 1989). Otitis media with effusion is the commonest cause of acquired hearing loss in childhood. The reason why it develops is uncertain, but low‐grade infection, poor Eustachian tube function and adenoidal infection or hypertrophy have all been implicated (Bluestone 1995). Otitis media with effusion usually resolves spontaneously within a few months (Fiellau 1979; Rosenfeld 1999).
Acutely, OME may be associated with earache (otalgia), hearing loss and/or balance difficulty. Long‐term complications are reported to include hearing loss and linguistic, developmental or social consequences, especially if the OME is bilateral and of long duration (Fiellau 1983; Golz 1998; Grace 1990; Lous 1995). However, a report for the Agency for Health Research and Quality did not find evidence to support an effect of early‐life OME on language development or cognitive verbal intelligence (Shekelle 2003). The same report did find evidence to support a link between early‐life OME and increased risk of conductive hearing loss. Some children have nearly normal hearing despite the presence of fluid within the middle ear.
Diagnosis
The best technique for diagnosing OME is pneumatic otoscopy, with a sensitivity of 94% and a specificity of 80% based on a meta‐analysis of eight different methods of diagnosis (Takata 2003). OME is may also be present when tympanometry results in a flat curve (relative gradient less than 0.1, type B), when mobility of the tympanic membrane is absent or reduced, or fluid or air bubbles are evident behind the eardrum. The presence of a significant (10 dB) air‐bone gap correlates well with the presence of fluid in the middle ear. Persistence of OME in this review will be determined by pneumatic otoscopy or a combination of the aforementioned techniques.
Management options
Many patients with OME require no specific treatment. Commonly proposed medical treatment options include the use of decongestants, mucolytics, steroids, antihistamines, antibiotics and autoinflation. Surgical treatment options include grommet insertion (ventilation tubes), myringotomy (tympanocentesis, i.e. surgical incision of the eardrum, with or without aspiration of fluid from the middle ear cavity) and adenoidectomy. Grommets, autoinflation, steroids and adenoidectomy for OME have already been addressed in Cochrane Reviews (Browning 2010; Perera 2006b; Simpson 2011; van den Aardweg 2010).
Decongestants alone or in combination with antihistamines were studied in a published meta‐analysis (Witmer 1998) but only two randomized controlled trials were found and the analysis had significant methodological flaws. Wide international variation in clinical practice exists and the optimal treatment strategy remains controversial although a recent guideline (AAFP 2004) recommended watchful waiting for three months and recommended against antihistamines and/or decongestants. Rosenfeld and Bluestone (Rosenfeld 1999) recommended modification of risk factors such as avoidance of tobacco smoke, use of breastfeeding and choice of small group (< 5 children) daycare when possible to assist in the management and prevention of OME.
Description of the intervention
Antihistamines and/or decongestants
Antihistamines and decongestants are relatively safe and inexpensive and are commonly used separately or in combination in the management of OME. Theoretically, antihistamines may reduce the congestion of mucous membranes and decrease obstruction of tubes lined by mucous membrane, such as the Eustachian tube. An open Eustachian tube would allow the middle ear pressure to equalize to ambient air pressure. It may also allow drainage of fluid from the middle ear. If mucous membrane congestion is caused by allergy then anti‐allergy medications such as antihistamines may work to reduce congestion and similarly improve Eustachian tube dysfunction. Some recent evidence suggests that viral or bacterial organisms contribute to middle ear inflammation and the release of histamines as well as other inflammatory mediators such as leukotrienes (Chonmaitree 2003). Decongestants are vasoconstrictors and should reduce mucous membrane swelling and enhance Eustachian tube function. However, the evidence to support the use of antihistamines or decongestants in the treatment of OME is underwhelming. This review will analyze the best evidence to date.
Objectives
The objective of this review was to determine whether antihistamine, decongestant or combination therapy is effective in treating children who present with OME.
Methods
Criteria for considering studies for this review
Types of studies
Studies eligible for inclusion were randomized controlled trials. Methods of randomization were not used to exclude studies but were considered in the quality assessment
Types of participants
We included studies evaluating children (age 18 or under) who had a diagnosis of OME. We chose specifically not to evaluate acute otitis media, patients with anatomical deformity, or patients with other chronic immunocompromised states. When studies included mixed populations, but contained extractable data for a population that met our criteria, we included the study.
Types of interventions
The intervention of interest was the use of oral or nasal decongestant and/or antihistamine as compared to no medication or placebo. Our study specifically did not address the use of oral or nasal steroids for OME; these interventions are addressed in a separate Cochrane Review (Simpson 2011).
Types of outcome measures
We anticipated a large number of possible outcomes to be reported in the trials, and thus adopted the following strategy of another Cochrane Review Group (Becker 2011). After the primary search strategy was completed, we reviewed abstracts for possible inclusion. One author then reviewed all articles considered for inclusion, extracting a list of measured outcomes. This list was given to the other investigators, blinded to the number of studies that reported any given outcome. From that list, the primary outcomes of interest were selected. These then became the outcome criteria for inclusion in the review. Our primary interest was in outcomes of importance to patients. The general categories are listed below under primary and secondary outcomes.
Upon review of all included studies, however, it became clear that the only outcome measured consistently was resolution of the effusion. This, therefore, became our primary (that is, main) outcome.
Primary outcomes
Resolution of symptoms or signs (however measured).
Secondary outcomes
Duration of effusion.
Duration of hearing loss as defined by pure‐tone audiometric loss of over 20 dB.
Reduction of complications of OME.
Decreased necessity for tympanostomy or tympanocentesis.
Medication side effects or complications.
Search methods for identification of studies
We conducted systematic searches for randomized controlled trials. There were no language, publication year or publication status restrictions. The date of the last search was 1 February 2011, following a previous search in 2006.
Electronic searches
We searched the following databases from their inception for published, unpublished and ongoing trials: the Cochrane Ear, Nose and Throat Disorders Group Trials Register; the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library 2011, Issue 1); PubMed; EMBASE; CINAHL; LILACS; KoreaMed; IndMed; PakMediNet; CAB Abstracts; Web of Science; BIOSIS Previews; ISRCTN; ClinicalTrials.gov; ICTRP and Google.
We modeled subject strategies for databases on the search strategy designed for CENTRAL. Where appropriate, we combined subject strategies with adaptations of the highly sensitive search strategy designed by the Cochrane Collaboration for identifying randomized controlled trials and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, Box 6.4.b. (Handbook 2011)). Search strategies for major databases including CENTRAL are provided in Appendix 1
Searching other resources
We scanned the reference lists of identified publications for additional trials and contacted trial authors where necessary. In addition, we searched PubMed, TRIPdatabase, NHS Evidence ‐ ENT & Audiology and Google to retrieve existing systematic reviews relevant to this systematic review, so that we could scan their reference lists for additional trials. We searched for conference abstracts using the Cochrane Ear, Nose and Throat Disorders Group Trials Register.
In our previous search in 2006, we sent a letter to all first authors of included papers and to pharmaceutical companies manufacturing decongestants or antihistamines requesting data and references for any additional published or unpublished trials.
Data collection and analysis
Selection of studies
Two authors independently reviewed all the titles and abstracts and determined which studies met the inclusion criteria. Articles chosen by either author were retrieved and we applied the full inclusion criteria. We chose not to blind authors to study authors and journals (Berlin 1997; Jadad 1996).
Data extraction and management
At least two authors independently extracted and recorded data from included studies into a standardized article abstraction form. Disagreements were settled by consensus between the authors.
Assessment of risk of bias in included studies
Two review authors then graded the quality of each included study using the method of Mohar (Mohar 1995). Quality score depended on the randomization process (one point for mentioning randomization and one added point for describing the method of randomization), follow up (one point for describing all losses to follow up) and blinding (one point for mentioning blinding and one added point for describing a reasonable blinding process) for a maximum of five points. We also assessed allocation concealment as acceptable or not acceptable. Disagreements were discussed and resolved through consensus. We used quality scores and method of allocation concealment for sensitivity analysis.
Data synthesis
Our primary analysis compared outcomes for four groups of patients: those who received antihistamine versus placebo, those who received decongestant versus placebo, those who received antihistamine/decongestant combination versus placebo, and those who received any medication (antihistamine, decongestant or antihistamine/decongestant combination) versus placebo.
Anticipating some heterogeneity, we planned specific sub‐analyses for the following factors for the primary outcome:
setting (primary versus tertiary care);
age of study participants;
patient's history of allergies;
oral versus nasal decongestant;
patient's history of recent acute otitis media;
type of decongestant (specific medications);
type of antihistamine;
method to diagnose resolution of OME;
timing of dichotomous outcomes (i.e. follow up at two weeks versus six weeks versus 12 or more weeks);
study validity score;
year of publication.
We found a limited number of studies and there was minimal heterogeneity, therefore the value of these sub‐analyses and those planned for diagnostic criteria were not meaningful and thus they were not conducted. Only sub‐analyses based on study quality were warranted. See Results and Discussion for related qualitative comments.
Continuous measures were not included in the meta‐analysis but were described narratively. For dichotomous data, we calculated risk ratio with confidence intervals using the fixed‐effect method, unless heterogeneity was found, in which case we discussed results in the text. We used approximate Chi² tests for homogeneity to assess the comparability of included data.
Results
Description of studies
Results of the search
In the 2011 update searches, we retrieved a total of 37 references: we removed 21 of these in first‐level screening (i.e. removal of duplicates and clearly irrelevant references), leaving 22 references for further consideration. Out of these we selected one study for inclusion in the review (Choung 2008).
From the original review searches in 2006, we identified 70 studies: we removed 28 of these in first‐level screening and selected 42 for further consideration. Of these, 16 met our final inclusion criteria and were included in the review.
There was full agreement on the final selection of included studies. For more information please see the Characteristics of included studies and Characteristics of excluded studies.
Included studies
A total of 1880 patients, nearly all of whom were under the age of 18, participated in the studies.
Ten studies took place in ENT clinics and five in community‐based clinics. Thirteen studies involving 1659 patients provided dichotomous outcomes and were included in the statistical meta‐analyses. One of the studies (Olson 1978) provided continuous outcomes and could not be included in the statistical meta‐analyses. The author of this study was contacted but no longer had the original data for re‐calculation to provide dichotomous results. Two other studies (Fraser 1977; Khan 1981) did not provide sufficient individual patient data to allow inclusion of their results in the meta‐analysis. All three studies gave a simple statement of their results.
Though five studies reported outcomes using ears as the unit of measure rather than persons, we were able to convert the data into a usable, consistent format using a design effect method (Perera 2006a). All but one study was in English; the single Swedish study was translated into English and the data are included here.
Two pairs of studies reported on the same populations; we did not include duplicated data, but where studies reported different outcomes in different manuscripts, we pooled data as appropriate.
All articles used tympanometry (the closest thing to a 'gold standard' test for OME) in their methods of diagnosis except for three (Edstrom 1977; Haugeto 1981a; Saunte 1978). Edstrom used clinical examination only (dullness of the tympanic membrane and immobility on pneumatic otoscopy) and Haugeto and Saunte used clinical (pneumatic otoscopy) and audiometric assessments.
Six studies provided data on the primary outcome (lack of cure at or before one month) and a further seven studied our secondary outcome (lack of cure at one to three months). Two articles reported on the late outcome of lack of cure after three months and seven studies reported on other outcomes including hearing loss and school performance.
Six articles provided data on side effects of interventions and controls and nine studies considered complications such as surgery, recurrent OME or acute otitis media.
Excluded studies
We excluded 24 studies: nine were not actually randomized, four turned out to be reviews, four did not assess otitis media with effusion, two evaluated prevention rather than treatment, two did not measure patient‐oriented outcomes, in one trial we were not able to separate the intervention of interest and in one we were not able to separate the population of interest from the study population. See Characteristics of excluded studies.
Risk of bias in included studies
Eight papers had a quality score of three or more and eight had a score of two or less. Adequacy of allocation concealment was determined separately and only two of 16 papers demonstrated appropriate concealment of allocation to intervention or control groups. Only two of the included studies used intention‐to‐treat analysis.
Effects of interventions
OME persistence
None of the included studies provided an assessment of symptoms other than hearing loss. We therefore chose OME persistence as our primary outcome. As OME is often asymptomatic and because most studies provided this as their primary outcome, we chose to do the same. Outcomes were measured at one month or less, at one to three months (secondary outcome) and after three months (late outcome).
Six of the 16 included studies (all of which had a quality score of three or more) reported dichotomous results for the primary outcome: cure or no cure at one month or less. Pooling data for any medication combination resulted in a risk ratio (RR) of 0.99 with a 95% confidence interval (CI) from 0.92 to 1.05 (Analysis 4.1). These studies were statistically homogenous. In fact, the meta‐analysis results for all interventions and outcomes except one were homogeneous (i.e. not heterogeneous) using a P value for heterogeneity of 0.10 (Barker 2005).
4.1. Analysis.

Comparison 4 Any medication: antihistamine, decongestant or antihistamine/decongestant combination versus control, Outcome 1 Primary outcome: cure or no cure at or before 1 month.
Separating out the various interventions had little effect. For decongestant alone the RR for the primary outcome was 1.06 (95% CI 0.92 to 1.22) (Analysis 2.1) and for antihistamine/decongestant combinations the RR was 0.97 (95% CI 0.89 to 1.04) (Analysis 3.1), both non‐significant, statistically and clinically.
2.1. Analysis.

Comparison 2 Decongestant versus control, Outcome 1 Primary outcome: cure or no cure at or before 1 month.
3.1. Analysis.

Comparison 3 Antihistamine/decongestant combination versus control, Outcome 1 Primary outcome: cure or no cure at or before 1 month.
The results of studies that could not be combined in the meta‐analysis are consistent with the summary findings. Olson 1978 found that decongestants alone were not effective for the short‐term resolution of OME; in fact, some of the analyses in this study statistically favored the placebo group! Khan 1981 found that for the antihistamine/decongestant combination the primary outcome (cure or no cure of OME at one month or less), there was no statistical difference between intervention and control. Fraser 1977 studied the interventions of decongestant versus control and antihistamine/decongestant combination versus control and also found no difference in outcomes.
For the outcome of delayed persistence of OME (one to three months), pooling data for any medication combination found no statistical benefit (RR 1.06, 95% CI 0.92 to 1.22) (Analysis 4.2). For decongestant alone the RR was 1.05 with the 95% CI from 0.85 to 1.30 (Analysis 2.2). For the antihistamine/decongestant combination there was also no estimated benefit (RR 1.09, 95% CI 0.85 to 1.40) (Analysis 3.2).
4.2. Analysis.

Comparison 4 Any medication: antihistamine, decongestant or antihistamine/decongestant combination versus control, Outcome 2 Secondary outcome: cure or no cure at 1‐3 months.
2.2. Analysis.

Comparison 2 Decongestant versus control, Outcome 2 Secondary outcome: cure or no cure at 1‐3 months.
3.2. Analysis.

Comparison 3 Antihistamine/decongestant combination versus control, Outcome 2 Secondary outcome: cure or no cure at 1‐3 months.
Our main outcome for antihistamines alone (due to lack of other evidence) was the measure of delayed persistence of OME with RR 1.05 and 95% CI 0.8 to 1.38 or, more usefully, RD (actual risk difference) of 0.02 with 95% CI ‐0.11 to 0.16 (Analysis 1.1; Figure 1). This confidence interval is somewhat wider than the previous three examples because of a smaller sample size.
1.1. Analysis.

Comparison 1 Antihistamine versus control, Outcome 1 Secondary outcome: cure or no cure at 1‐3 months.
1.

Forest plot of comparison: 1 Antihistamine versus control, outcome: 1.1 Secondary outcome: cure or no cure at 1‐3 months.
In summary, for our two main outcome measures, no clinical benefit was found for any of the interventions studied and there was sufficient power in the meta‐analysis to have detected any clinically significant benefit.
Late outcome (cure or no cure after three months) was studied only for the antihistamine/decongestant combination and was found to favor control by a small but statistically non‐significant amount (RR 1.24, 95% CI 0.72 to 2.13) (Analysis 3.3). The wider confidence intervals indicate less power to show a statistically significant outcome but the trend is clearly toward harm.
3.3. Analysis.

Comparison 3 Antihistamine/decongestant combination versus control, Outcome 3 Late outcome: cure or no cure after 3 months.
Complications (recurrent OME or acute otitis media)
Recurrent OME was evaluated as a complication by two studies for antihistamine/decongestant versus control and the outcome was found to be statistically non‐significant (RR 1.30, 95% CI 0.80 to 2.11) (Analysis 3.9).
3.9. Analysis.

Comparison 3 Antihistamine/decongestant combination versus control, Outcome 9 Complication: recurrent OME.
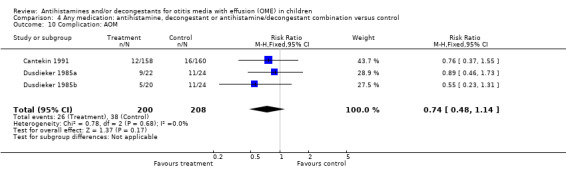
Acute otitis media (AOM) was studied as another complication of OME in three studies; one each for antihistamine alone, decongestant alone and antihistamine/decongestant combination and for each the results were not statistically significant (antihistamine alone (RR 0.89, 95% CI 0.46 to 1.73) (Analysis 1.2); decongestant alone (RR 0.55, 95% CI 0.23 to 1.31) (Analysis 2.6) and antihistamine/decongestant combination (RR 0.76, 95% CI 0.46 to 1.26) (Analysis 3.10)). Due to the small size of the studied population for this outcome, the confidence intervals are fairly wide indicating only fair power to detect a difference between treatment and control.
1.2. Analysis.

Comparison 1 Antihistamine versus control, Outcome 2 Complication: AOM.
2.6. Analysis.

Comparison 2 Decongestant versus control, Outcome 6 Complication: AOM.
3.10. Analysis.

Comparison 3 Antihistamine/decongestant combination versus control, Outcome 10 Complication: AOM.
Other outcomes
Other measured outcomes were hearing loss (early: four studies and late: one study), school performance (one study) and surgery (tympanostomy: three studies).
Hearing loss
No treatment lessened hearing loss at one‐month follow up (four studies) (Analysis 4.5) and the trend was toward harm (RR 1.08, 95% CI 0.93 to 1.27). At late follow up (one study) (Analysis 4.6) the trend was again toward harm (RR 1.50, 95% CI 0.63 to 3.56). Khan 1981 did not provide dichotomous data but also found no difference in hearing loss between groups of children treated with antihistamine/decongestant combination and placebo.
4.5. Analysis.

Comparison 4 Any medication: antihistamine, decongestant or antihistamine/decongestant combination versus control, Outcome 5 Outcome: hearing on or about 1 month.
4.6. Analysis.

Comparison 4 Any medication: antihistamine, decongestant or antihistamine/decongestant combination versus control, Outcome 6 Late outcome: hearing at 1 year.
School performance
For school performance (studied only for the intervention of antihistamine/decongestant) the RR was 0.81 (95% CI 0.35 to 1.86) (Analysis 3.7).
3.7. Analysis.

Comparison 3 Antihistamine/decongestant combination versus control, Outcome 7 Late outcome: school performance at 1 year.
Surgery
For surgery after any medication, antihistamine alone, decongestant alone or antihistamine/decongestant combination, results were not statistically significant. The risk ratio and confidence intervals for any medication were RR 1.07, 95% CI 0.81 to 1.41 (Analysis 4.8). For antihistamine alone the result was RR 2.08, 95% CI 0.77 to 5.58 (Analysis 1.3; Figure 2). For decongestant alone the result was RR 1.07, 95% CI 0.71 to 1.62 (Analysis 2.5). The antihistamine/decongestant analysis for this outcome (RR 0.54, 95% CI 0.09 to 3.41) (Analysis 3.8) was the only outcome to have been found to be heterogeneous (P = 0.06 for heterogeneity) therefore we shall describe the studies rather than trying to interpret an inappropriate pooling of results. This analysis looked at the two included studies that measured the outcome of surgical complications for the intervention of antihistamine/decongestant combination. One of these studies was clearly an outlier (Saunte 1978) (see Analysis 4.1). It was a very small study (n = 21) with few outcomes and was the only study to show any significant benefit for several of our measured outcomes. The positive results from this study were always overwhelmed by the other studies in its comparison group. The other study in Analysis 3.8 was slightly larger and showed no hint of benefit from the intervention. In summary, this intervention (antihistamine/decongestant combination) shows no clinically significant benefit for the outcome of surgical complications but the power to detect such a benefit is only fair because of the small study sizes.
4.8. Analysis.

Comparison 4 Any medication: antihistamine, decongestant or antihistamine/decongestant combination versus control, Outcome 8 Outcome: surgery (tympanostomy (myringotomy)).
1.3. Analysis.

Comparison 1 Antihistamine versus control, Outcome 3 Outcome: surgery (tympanostomy (myringotomy)).
2.

Forest plot of comparison: 1 Antihistamine versus control, outcome: 1.3 Outcome: surgery (tympanostomy (myringotomy)).
2.5. Analysis.

Comparison 2 Decongestant versus control, Outcome 5 Outcome: surgery (tympanostomy (myringotomy)).
3.8. Analysis.

Comparison 3 Antihistamine/decongestant combination versus control, Outcome 8 Outcome: surgery (tympanostomy (myringotomy)).
Medication side effects
Pooling the data from the six studies that evaluated medication side effects found a rate of 17% in the treated group versus 6% in the placebo group. This was both statistically significant (RR 2.70, 95% CI 1.87 to 3.88) (Analysis 4.4) and clinically meaningful. The number needed to treat to harm was nine, based on an actual risk reduction of 11%.
4.4. Analysis.

Comparison 4 Any medication: antihistamine, decongestant or antihistamine/decongestant combination versus control, Outcome 4 Side effects: any significant side effects at or before 1 month.
None of the studies included in this review evaluated side effects for antihistamines so we used an alternative resource, the Lexi‐Drugs program online (Lexi‐Drugs 2006) (accessed 27 June 2006). We looked at the side effect profile of three commonly used antihistamines with a low side effect frequency: cetirizine, chlorpheniramine and loratadine. All three had two or more side effects with a frequency of more than 10% with many other side effects in the frequency range from 1% to 10%. We accessed an online medication monitoring service (Mediguard.org) on 29 May 2011 to determine the frequency of side effects of phenylephrine and pseudoephedrine, two of the decongestants used in some of the studies included in this review. The overall rate of side effects was 24% for the former and 6% for the latter. This is in agreement with our review which showed a general frequency of side effects of approximately 10% for antihistamine/decongestant combinations or decongestants alone. The commonest side effects found in the studies evaluated in our review were irritability, sedation and gastrointestinal upset
Sub‐analyses
Validity
We performed sub‐analyses on the basis of quality of study. Those studies with a quality score of three or more and those with a quality score of less than three had similar statistically non‐significant results, except when the study by Saunte 1978 was the lone comparator. Allocation concealment also did not influence the findings as studies with adequate concealment had the same statistically non‐significant results, except when the study by Saunte 1978 stood alone in the comparison. This study was small, used a different method to diagnose OME and found benefit where other studies did not (see Discussion below). We did not include quality sub‐analyses in the meta‐analysis because they added no important information.
Discussion
One study (Saunte 1978) is an outlier (Analysis 4.1). This, at first glance, seems not to make sense as this study had a high quality score for methodology and had adequate allocation concealment. Reading the study, however, shows that this study was one of only three that did not include tympanometry as one of the methods of diagnosis of OME, and the number of study subjects was very small (n = 21) (see Characteristics of included studies). The small size of this trial might lead some authors to discard it on this basis alone (Bandolier 2000).
This meta‐analysis and systematic review found no benefit from any treatment combination ‐ antihistamine, decongestant or antihistamine/decongestant combination ‐ for any of the measured patient oriented outcomes for OME. It did find, however, an increased rate of medication side effects (number needed to treat to harm = 9). There was a paucity of included studies comparing antihistamines to controls but we did review three studies (Bhambhani 1983; Chonmaitree 2003; Klein 1980) that were excluded because the researchers randomized by acute otitis media (rather than by OME) and then followed the groups for prevention and treatment of OME. All of these studies found no benefit for antihistamines in the prevention or treatment of OME.
One limitation of our analysis is the relatively small number of studies found. However, we were unlikely to miss studies given our comprehensive search, and we found many more than the previous systematic review on this topic. Furthermore, the studies were so consistent in their findings that even if we missed a study, the summary results are unlikely to be overturned.
Another limitation of our review is that the included articles had some methodological flaws. They varied in duration and thoroughness of follow up, had poor allocation concealment, unclear randomization and blinding processes and lack of intention‐to‐treat analysis in most studies. In general, all of these design flaws tend to overestimate the benefit of treatment (Bandolier 2000). Since we did not find benefit from any of the interventions, the faults of the included studies strengthen our conclusion.
Strengths of this review are the comprehensive search strategy, the rigorous protocol that was used, the general homogeneity of studies with regard to diagnosis, quality and interventions and the homogeneity and consistency of the outcomes. Sub‐analysis by quality showed that both good quality and poor quality studies resulted in no benefit for any of the interventions. This also strengthens the conclusions of the review.
It is possible that these medications may provide some benefit in specific situations such as for allergic‐related OME and for OME related to upper respiratory infection. This cannot be determined from our meta‐analysis that lumps them together but the consistency of the lack of benefit makes this possibility unlikely.
Authors' conclusions
Implications for practice.
We found no benefit for any of the studied interventions for any of the outcomes measured and we found harm from the side effects of the interventions, therefore we recommend that practitioners not use antihistamines, decongestants or antihistamine/decongestant combinations to treat OME in children. This recommendation is consistent with the American Academy of Family Physicians, American Academy of Otolaryngology ‐ Head and Neck Surgery and American Academy of Pediatrics joint guideline on the management of OME (AAFP 2004).
Implications for research.
This review of the use of antihistamines, decongestants or antihistamine/decongestant combination does not show any significant benefit but does show harm from the interventions assessed. The results are surprisingly consistent. It appears highly unlikely that further research studies of these interventions for the treatment of OME would change the outcomes, and harm may be caused by the interventions, therefore we feel that further research on this question is not justified. It is possible, though there was no suggestion of it in any of the studied articles, that antihistamines might be useful specifically for allergic OME or decongestants might be helpful for OME related to upper respiratory infection or postacute otitis media. These are possible, though not likely fruitful, areas for future research.
What's new
| Date | Event | Description |
|---|---|---|
| 1 February 2011 | New citation required but conclusions have not changed | Change of authors. |
| 1 February 2011 | New search has been performed | New searches run in February 2011. One new study was included (Choung 2008) but this resulted in no changes to the conclusions of the review. |
History
Protocol first published: Issue 1, 2002 Review first published: Issue 4, 2006
| Date | Event | Description |
|---|---|---|
| 20 October 2008 | Amended | Converted to new review format. |
Acknowledgements
We acknowledge the invaluable assistance of Bette Jean Ingui in providing thorough search procedures, Ulla Hedstrom for translation services and John Smucny for providing useful advice. We also acknowledge R Eugene Bailey and Jennifer K Schultz who co‐authored the original version of this review.
Appendices
Appendix 1. Search strategies
| CENTRAL | Cochrane Ear, Nose and Throat Disorders Group Trials Register (ProCite database) | PubMed | EMBASE (Ovid) |
| #1 MeSH descriptor Histamine Antagonists explode all trees #2 antihistam* OR anti NEXT histam* #3 histamine* NEAR antagonist* #4 acrivastne OR antazoline OR azelastine OR azatadine OR astemizole OR brompheniramine OR buclizine OR carbinoxamine OR cetirizine OR clemastine OR chlorpheniramine OR chlorphenamine OR cyproheptadine OR cinnarizine OR cyclizine OR doxylamine OR desloratadine OR dexbrompheniramine OR dexchlorpheniramine OR dimetapp OR dimenhydrinate #5 dimethindene OR diphenhydramine OR drixoral OR ebastine OR fexofenadine OR flunarizine OR hydrazine OR ketotifen OR levocetirizine OR levocabastine OR loratadine OR meclizine or meclozine OR methapyrilene OR methdilazine OR mequitazine OR mizolastine #6 MeSH descriptor Vasoconstrictor Agents explode all trees #7 vasoconstrictor NEXT agent* OR decongest* #8 pseudoephedrine OR propylhexedrine OR methoxamine OR midrodrine OR mephentermine OR phenylephrine OR phenylpropanolamine OR oxymetazoline OR xylometazoline OR naphazoline OR metizoline OR fenoxazoline OR tramazoline OR tetrahydrozoline #9 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #7 OR #8) #10 MeSH descriptor Otitis Media with Effusion explode all trees #11 MeSH descriptor Ear, Middle explode all trees with qualifier: SE #12 glue NEXT ear OR otitis NEXT media NEAR effusion* OR middle NEXT ear NEAR effusion* OR nonsuppurative NEXT otitis OR non NEXT suppurative NEXT otitis #13 tympanitis OR serous NEXT otitis OR secretory NEXT otitis OR otitis NEXT serosa #14 mucoid NEAR otitis OR mucous NEAR otitis OR seromuco* NEAR otitis OR sero NEXT muco* NEAR otitis #15 mucoid NEAR middle NEXT ear OR mucous NEAR middle NEXT ear OR seromuc* NEAR middle NEXT ear #16 adhesive NEAR otitis OR exudative NEAR otitis #17 (#10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16) #18 (#9 AND #17) | ((ear OR otitis) AND (effusion OR glue OR serous OR secretory OR mucoid)) OR OME | #1 "Histamine Antagonists"[mh] #2 antihistam* OR (anti histam*) #3 histamine* AND antagonist* #4 acrivastne OR antazoline OR azelastine OR azatadine OR astemizole OR brompheniramine OR buclizine OR carbinoxamine OR cetirizine OR clemastine OR chlorpheniramine OR chlorphenamine OR cyproheptadine OR cinnarizine OR cyclizine OR doxylamine OR desloratadine OR dexbrompheniramine OR dexchlorpheniramine OR dimetapp OR dimenhydrinate #5 dimethindene OR diphenhydramine OR drixoral OR ebastine OR fexofenadine OR flunarizine OR hydrazine OR ketotifen OR levocetirizine OR levocabastine OR loratadine OR meclizine or meclozine OR methapyrilene OR methdilazine OR mequitazine OR mizolastine #6 "Vasoconstrictor Agents"[mh] #7 vasoconstrictor AND (agent* OR decongest*) #8 pseudoephedrine OR propylhexedrine OR methoxamine OR midrodrine OR mephentermine OR phenylephrine OR phenylpropanolamine OR oxymetazoline OR xylometazoline OR naphazoline OR metizoline OR fenoxazoline OR tramazoline OR tetrahydrozoline #9 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #7 OR #8) #10 "Otitis Media with Effusion"[mh] #11 "Ear, Middle/secretion"[Mesh] #12 (glue ear) OR (otitis AND media AND effusion*) OR (middle AND ear AND effusion*) OR (nonsuppurative AND otitis) OR (non suppurative otitis) #13 tympanitis OR (serous otitis) OR (secretory otitis) OR (otitis serosa) #14 (mucoid AND otitis) OR (mucous AND otitis) OR (seromuco* AND otitis) OR (sero muco* AND otitis) #15 (mucoid AND middle ear) OR (mucous AND middle ear) OR (seromuc* AND middle ear) #16 (adhesive AND otitis) OR (exudative AND otitis) #17 #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 #18 #9 AND #17 | 1 exp *Antihistaminic Agent/ 2 (ANTIHISTAM* or (ANTI adj HISTAM*)).tw. 3 (HISTAMINE* and ANTAGONIST*).tw. 4 (ACRIVASTINE or 87848‐99‐5 or ANTAZOLINE or 91‐75‐8 or ASTEMIZOLE or 68844‐77‐9 or AZATADINE or 3964‐81‐6 or AZELASTINE or 58581‐89‐8 or BROMPHENIRAMINE or 86‐22‐6 or CARBINOXAMINE or 486‐16‐8 or CETIRIZINE or 83881‐51‐0 or CHLORPHENIRAMINE or CHLORPHENAMINE or 132‐22‐9 or CINNARIZINE or 298‐57‐7 or CLEMASTINE or 15686‐51‐8 or CYCLIZINE or 82‐92‐8 or CYPROHEPTADINE or 129‐03‐3 or DEXCHLORPHENIRAMINE or 25523‐97‐1 or DEXBROMPHENIRAMINE or 132‐21‐8 or DESLORATADINE or 100643‐71‐8 or DIMETHINDENE or 5636‐83‐9 or DIPHENHYDRAMINE or 58‐73‐1 or DIMENHYDRINATE or 523‐87‐5 or DOXYLAMINE or 469‐21‐6 or DIMETAPP or 58660‐10‐9 or DRIXORAL or 76404‐09‐6 or EBASTINE or 90729‐43‐4 or FEXOFENADINE or 83799‐24‐0 or FLUNARIZINE or 52468‐60‐7).rn. 5 (HYDROXYZINE or 68‐88‐2 or KETOTIFEN or 34580‐13‐7 or LEVOCETIRIZINE or 130018‐77‐8 or LEVOCABASTINE or 79516‐68‐0 or LORATADINE or 79794‐75‐5 or MECLIZINE or 569‐65‐3 or MEPYRAMINE or METHAPYRILENE or 91‐80‐5 or MEQUITAZINE or 29216‐28‐2 or MIZOLASTINE or 108612‐45‐9 or METHDILAZINE or 1982‐37‐2 or OXATOMIDE or 60607‐34‐3 or PHENIRAMINE or 86‐21‐5 or PROMETHAZINE or 60‐87‐7 or PYRILAMINE or 91‐84‐9 or PHENYLTOLOXAMINE or 92‐12‐6 or TRIPROLIDINE or 486‐12‐4 or TRIMEPRAZINE or ALIMEMAZIN or TRIPELENNAMINE or 91‐81‐6 or TRITOQUALINE or 14504‐73‐5 or TERFENADINE or 50679‐08‐8).rn. 6 exp *Decongestive Agent/ 7 exp *Vasoconstrictor Agent/ 8 (VASOCONSTRICTOR adj (AGENT* or DECONGEST*)).tw. 9 (PSEUDOEPHEDRINE or 345‐78‐8 or 90‐82‐4 or PROPYLHEXEDRINE or 101‐40‐6 or METHOXAMINE or 390‐28‐3 or MEPHENTERMINE or 100‐92‐5 or PHENYLEPHRINE or 59‐42‐7 or PHENYLPROPANOLAMINE or 14838‐15‐4 or OXYMETAZOLINE or 1491‐59‐4 or XYLOMETAZOLINE or 526‐36‐3 or NAPHAZOLINE or 835‐31‐4 or METIZOLINE or 17692‐22‐7 or FENOXAZOLINE or 4846‐91‐7 or TRAMAZOLINE or 1082‐57‐1 or TETRAHYDROZOLINE or 84‐22‐0).rn. 10 6 or 3 or 7 or 9 or 2 or 8 or 1 or 4 or 5 11 exp Mucoid Otitis Media/ 12 exp Secretory Otitis Media/ 13 exp Serous Otitis Media/ 14 (GLUE adj EAR).tw. 15 ((OTITIS adj MEDIA) and EFFUSION*).tw. 16 (TYMPANITIS or (SEROUS adj OTITIS) or (SECRETORY adj OTITIS) or (OTITIS adj SEROSA)).tw. 17 ((MIDDLE adj EAR) and EFFUSION).tw. 18 ((NONSUPPURATIVE adj OTITIS) or (NON adj SUPPURATIVE adj OTITIS)).tw. 19 (((MUCOID and MIDDLE) adj EAR) or (((SERO adj MUC*) and MIDDLE) adj EAR) or ((SEROMUC* and MIDDLE) adj EAR)).tw. 20 ((ADHESIVE and OTITIS) or (EXUDATIVE and OTITIS)).tw. 21 ((OME or SOM) and (OTITIS or EAR*)).tw. 22 11 or 21 or 17 or 12 or 20 or 15 or 14 or 18 or 13 or 16 or 19 23 22 and 10 |
| Web of Science | BIOSIS (Web of Knowledge) | CAB Abstracts (Ovid) | ISRCTN (mRCT) |
| #1 TS=((ear OR otitis) AND (effusion OR serous OR glue)) #2 TS=(OME) #3 #1 OR #2 #4 TS=(antihist* OR (histamin* and (antagonist* or anti))) #5 TS=(acrivastne OR antazoline OR azelastine OR azatadine OR astemizole OR brompheniramine OR buclizine OR carbinoxamine OR cetirizine OR clemastine OR chlorpheniramine OR chlorphenamine OR cyproheptadine OR cinnarizine OR cyclizine OR doxylamine desloratadine OR dexbrompheniramine OR dexchlorpheniramine OR dimetapp OR dimenhydrinate) #6 TS=(dimethindene OR diphenhydramine OR drixoral OR ebastine OR fexofenadine OR flunarizine OR hydrazine OR ketotifen OR levocetirizine OR levocabastine OR loratadine OR meclizine or meclozine OR methapyrilene OR methdilazine OR mequitazine OR mizolastine) #7 TS=(oxatomide OR pheniramine OR promethazine OR pyrilamine OR mepyramine OR phenyltoloxamine OR trimeprazine OR alimemazine OR triprolidine OR tritoqualine OR tripelennamine OR terfenadine) #8 TS=(vasoconstrictor AND (agent* OR decongest*)) #9 TS=(pseudoephedrine OR propylhexedrine OR methoxamine OR midrodrine OR mephentermine OR phenylephrine OR phenylpropanolamine OR oxymetazoline OR xylometazoline OR naphazoline OR metizoline OR fenoxazoline OR tramazoline OR tetrahydrozoline) #10 #4 OR #5 OR #6 OR #7 OR #8 OR #9 #11 #3 AND #10 | #1 TS=((ear OR otitis) AND (effusion OR serous OR glue)) #2 TS=(OME) #3 #1 OR #2 #4 TS=(antihist* OR (histamin* and (antagonist* or anti))) #5 TS=(acrivastne OR antazoline OR azelastine OR azatadine OR astemizole OR brompheniramine OR buclizine OR carbinoxamine OR cetirizine OR clemastine OR chlorpheniramine OR chlorphenamine OR cyproheptadine OR cinnarizine OR cyclizine OR doxylamine desloratadine OR dexbrompheniramine OR dexchlorpheniramine OR dimetapp OR dimenhydrinate) #6 TS=(dimethindene OR diphenhydramine OR drixoral OR ebastine OR fexofenadine OR flunarizine OR hydrazine OR ketotifen OR levocetirizine OR levocabastine OR loratadine OR meclizine or meclozine OR methapyrilene OR methdilazine OR mequitazine OR mizolastine) #7 TS=(oxatomide OR pheniramine OR promethazine OR pyrilamine OR mepyramine OR phenyltoloxamine OR trimeprazine OR alimemazine OR triprolidine OR tritoqualine OR tripelennamine OR terfenadine) #8 TS=(vasoconstrictor AND (agent* OR decongest*)) #9 TS=(pseudoephedrine OR propylhexedrine OR methoxamine OR midrodrine OR mephentermine OR phenylephrine OR phenylpropanolamine OR oxymetazoline OR xylometazoline OR naphazoline OR metizoline OR fenoxazoline OR tramazoline OR tetrahydrozoline) #10 #4 OR #5 OR #6 OR #7 OR #8 OR #9 #11 #3 AND #10 | 1 (ANTIHISTAM* or (ANTI adj HISTAM*)).tw. 2 (HISTAMINE* and ANTAGONIST*).tw. 3 (ACRIVASTINE or 87848‐99‐5 or ANTAZOLINE or 91‐75‐8 or ASTEMIZOLE or 68844‐77‐9 or AZATADINE or 3964‐81‐6 or AZELASTINE or 58581‐89‐8 or BROMPHENIRAMINE or 86‐22‐6 or CARBINOXAMINE or 486‐16‐8 or CETIRIZINE or 83881‐51‐0 or CHLORPHENIRAMINE or CHLORPHENAMINE or 132‐22‐9 or CINNARIZINE or 298‐57‐7 or CLEMASTINE or 15686‐51‐8 or CYCLIZINE or 82‐92‐8 or CYPROHEPTADINE or 129‐03‐3 or DEXCHLORPHENIRAMINE or 25523‐97‐1 or DEXBROMPHENIRAMINE or 132‐21‐8 or DESLORATADINE or 100643‐71‐8 or DIMETHINDENE or 5636‐83‐9 or DIPHENHYDRAMINE or 58‐73‐1 or DIMENHYDRINATE or 523‐87‐5 or DOXYLAMINE or 469‐21‐6 or DIMETAPP or 58660‐10‐9 or DRIXORAL or 76404‐09‐6 or EBASTINE or 90729‐43‐4 or FEXOFENADINE or 83799‐24‐0 or FLUNARIZINE or 52468‐60‐7).rn. 4 (HYDROXYZINE or 68‐88‐2 or KETOTIFEN or 34580‐13‐7 or LEVOCETIRIZINE or 130018‐77‐8 or LEVOCABASTINE or 79516‐68‐0 or LORATADINE or 79794‐75‐5 or MECLIZINE or 569‐65‐3 or MEPYRAMINE or METHAPYRILENE or 91‐80‐5 or MEQUITAZINE or 29216‐28‐2 or MIZOLASTINE or 108612‐45‐9 or METHDILAZINE or 1982‐37‐2 or OXATOMIDE or 60607‐34‐3 or PHENIRAMINE or 86‐21‐5 or PROMETHAZINE or 60‐87‐7 or PYRILAMINE or 91‐84‐9 or PHENYLTOLOXAMINE or 92‐12‐6 or TRIPROLIDINE or 486‐12‐4 or TRIMEPRAZINE or ALIMEMAZIN or TRIPELENNAMINE or 91‐81‐6 or TRITOQUALINE or 14504‐73‐5 or TERFENADINE or 50679‐08‐8).rn. 5 (VASOCONSTRICTOR adj (AGENT* or DECONGEST*)).tw. 6 (PSEUDOEPHEDRINE or 345‐78‐8 or 90‐82‐4 or PROPYLHEXEDRINE or 101‐40‐6 or METHOXAMINE or 390‐28‐3 or MEPHENTERMINE or 100‐92‐5 or PHENYLEPHRINE or 59‐42‐7 or PHENYLPROPANOLAMINE or 14838‐15‐4 or OXYMETAZOLINE or 1491‐59‐4 or XYLOMETAZOLINE or 526‐36‐3 or NAPHAZOLINE or 835‐31‐4 or METIZOLINE or 17692‐22‐7 or FENOXAZOLINE or 4846‐91‐7 or TRAMAZOLINE or 1082‐57‐1 or TETRAHYDROZOLINE or 84‐22‐0).rn. 7 (GLUE adj EAR).tw. 8 ((OTITIS adj MEDIA) and EFFUSION*).tw. 9 (TYMPANITIS or (SEROUS adj OTITIS) or (SECRETORY adj OTITIS) or (OTITIS adj SEROSA)).tw. 10 ((MIDDLE adj EAR) and EFFUSION).tw. 11 ((NONSUPPURATIVE adj OTITIS) or (NON adj SUPPURATIVE adj OTITIS)).tw. 12 (((MUCOID and MIDDLE) adj EAR) or (((SERO adj MUC*) and MIDDLE) adj EAR) or ((SEROMUC* and MIDDLE) adj EAR)).tw. 13 ((ADHESIVE and OTITIS) or (EXUDATIVE and OTITIS)).tw. 14 ((OME or SOM) and (OTITIS or EAR*)).tw. 15 6 or 4 or 1 or 3 or 5 or 2 16 8 or 11 or 7 or 13 or 10 or 9 or 12 or 14 17 15 and 16 | ((ear OR otitis) AND (effusion OR glue OR serous OR secretory OR mucoid)) |
Data and analyses
Comparison 1. Antihistamine versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Secondary outcome: cure or no cure at 1‐3 months | 3 | 206 | Risk Difference (M‐H, Fixed, 95% CI) | 0.02 [‐0.11, 0.16] |
| 2 Complication: AOM | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.46, 1.73] |
| 3 Outcome: surgery (tympanostomy (myringotomy)) | 1 | 66 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.08 [0.77, 5.58] |
Comparison 2. Decongestant versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary outcome: cure or no cure at or before 1 month | 3 | 276 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.92, 1.22] |
| 2 Secondary outcome: cure or no cure at 1‐3 months | 2 | 216 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.85, 1.30] |
| 3 Side effect: any significant side effects at or before 1 month | 1 | 172 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.05 [0.66, 185.38] |
| 4 Outcome: hearing on or about 1 month | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.16, 4.68] |
| 5 Outcome: surgery (tympanostomy (myringotomy)) | 1 | 172 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.71, 1.62] |
| 6 Complication: AOM | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.23, 1.31] |
2.3. Analysis.

Comparison 2 Decongestant versus control, Outcome 3 Side effect: any significant side effects at or before 1 month.
2.4. Analysis.

Comparison 2 Decongestant versus control, Outcome 4 Outcome: hearing on or about 1 month.
Comparison 3. Antihistamine/decongestant combination versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary outcome: cure or no cure at or before 1 month | 4 | 901 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.89, 1.04] |
| 2 Secondary outcome: cure or no cure at 1‐3 months | 3 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.85, 1.40] |
| 3 Late outcome: cure or no cure after 3 months | 2 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.72, 2.13] |
| 4 Side effect: any significant side effects at or before 1 month | 5 | 972 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.54 [1.76, 3.67] |
| 5 Outcome: hearing at or less than 3 months | 3 | 343 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.93, 1.27] |
| 6 Late outcome: hearing at 1 year | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.63, 3.56] |
| 7 Late outcome: school performance at 1 year | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.35, 1.86] |
| 8 Outcome: surgery (tympanostomy (myringotomy)) | 2 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.09, 3.41] |
| 9 Complication: recurrent OME | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Quality score 3 or greater | 1 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.92, 3.01] |
| 9.2 Quality score less than 3 | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.31, 1.83] |
| 9.3 Allocation concealment adequate | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.4 Allocation concealment not adequate | 2 | 142 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.3 [0.80, 2.11] |
| 10 Complication: AOM | 1 | 636 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.46, 1.26] |
| 10.1 Quality score 3 or greater | 1 | 318 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.37, 1.55] |
| 10.2 Quality score less than 3 | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.3 Allocation concealment adequate | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.4 Allocation concealment not adequate | 1 | 318 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.37, 1.55] |
3.4. Analysis.

Comparison 3 Antihistamine/decongestant combination versus control, Outcome 4 Side effect: any significant side effects at or before 1 month.
3.5. Analysis.

Comparison 3 Antihistamine/decongestant combination versus control, Outcome 5 Outcome: hearing at or less than 3 months.
3.6. Analysis.

Comparison 3 Antihistamine/decongestant combination versus control, Outcome 6 Late outcome: hearing at 1 year.
Comparison 4. Any medication: antihistamine, decongestant or antihistamine/decongestant combination versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary outcome: cure or no cure at or before 1 month | 7 | 1177 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.92, 1.05] |
| 2 Secondary outcome: cure or no cure at 1‐3 months | 8 | 580 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.92, 1.22] |
| 3 Late outcome: cure or no cure after 3 months | 2 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.72, 2.13] |
| 4 Side effects: any significant side effects at or before 1 month | 6 | 1144 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.70 [1.87, 3.88] |
| 5 Outcome: hearing on or about 1 month | 4 | 358 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.93, 1.27] |
| 6 Late outcome: hearing at 1 year | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.63, 3.56] |
| 7 Late outcome: school performance at 1 year | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.35, 1.86] |
| 8 Outcome: surgery (tympanostomy (myringotomy)) | 4 | 295 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.81, 1.41] |
| 9 Complication: recurrent OME | 2 | 142 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.3 [0.80, 2.11] |
| 10 Complication: AOM | 3 | 408 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.48, 1.14] |
4.3. Analysis.

Comparison 4 Any medication: antihistamine, decongestant or antihistamine/decongestant combination versus control, Outcome 3 Late outcome: cure or no cure after 3 months.
4.7. Analysis.

Comparison 4 Any medication: antihistamine, decongestant or antihistamine/decongestant combination versus control, Outcome 7 Late outcome: school performance at 1 year.
4.9. Analysis.

Comparison 4 Any medication: antihistamine, decongestant or antihistamine/decongestant combination versus control, Outcome 9 Complication: recurrent OME.
4.10. Analysis.
Comparison 4 Any medication: antihistamine, decongestant or antihistamine/decongestant combination versus control, Outcome 10 Complication: AOM.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cantekin 1983.
| Methods | Randomized controlled trial Quality score: 3/5 based on Jadad scoring (randomization: 1 + 0; blinding: 1 + 1; withdrawals: 0) Follow up was 87% and the RCT was conducted over 3 years, from 1978 to 1981 Intention‐to‐treat analysis: yes |
|
| Participants | 553 pediatric ENT patients were referred from outpatient clinics Diagnosis was based on an algorithm involving otoscopy, tympanometry and middle ear muscle reflex testing Exclusion criteria included: congenital craniofacial malformation, Down's syndrome, history of tonsillectomy, adenoidectomy or tympanostomy (myringotomy) tubes, structural middle ear abnormality, underlying hearing loss, severe upper airway obstruction, acute otitis media, purulent rhinitis, any sinusitis, or history of antihistamine or decongestant use in the preceding 30 days |
|
| Interventions | Antihistamine (chlorpheniramine) and decongestant (pseudoephedrine) combination versus placebo | |
| Outcomes | The primary outcome was effusion or no effusion at 4 weeks. Other outcomes measured were hearing, medication side effects and the complication of recurrent OME | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Cantekin 1991.
| Methods | Randomized controlled trial Quality score: 3/5 (randomization: 1 + 0; blinding: 1 + 1; withdrawals: 0) Intention‐to‐treat analysis: no Follow up was 89%. RCT was conducted over 3 years from 1981 to 1984 |
|
| Participants | 318 children aged 7 months to 12 years were recruited from community practices and a hospital ambulatory care center Diagnosis was based on an algorithm using otoscopy and tympanometry Exclusion criteria included: congenital craniofacial malformation, systemic illness, history of tonsillectomy, adenoidectomy, insertion of tubes (tympanostomy), structural middle ear abnormality, hearing loss ... |
|
| Interventions | Antihistamine (chlorpheniramine) and decongestant (pseudoephedrine) combination versus placebo | |
| Outcomes | The primary outcome was effusion or no effusion at 4 weeks with a secondary outcome measured at 12 weeks. Hearing improvement or no improvement was measured at 4 weeks and the complication of acute otitis media was assessed. Side effects of medications were counted. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Choung 2008.
| Methods | Randomized controlled trial Quality score: 1/5 (randomization: 1 + 0; blinding: 0 + 0; withdrawals: 0) Intention‐to‐treat analysis: no Follow up was 84%. The trial took place between June 2003 and March 2005. |
|
| Participants | Children aged 5 months to 12 years in a tertiary care ENT clinic in Korea with otitis media with effusion defined as Type B or C tympanometry and 25 dB hearing loss on audiometry. Children with acute otitis media or fever or otalgia were excluded as were children with cleft palate, developmental difficulties, contraindications to medications and those children lost to follow up. | |
| Interventions | Ebastine, an antihistamine | |
| Outcomes | The primary outcome for this study was cure or no cure at a mean of 7 weeks after initiation of treatment. A secondary outcome was surgery (ventilation tube insertion) or no surgery after 3 months of observation. | |
| Notes | There were 2 differently treated control groups combined into one for the review. One control group used an antibiotic with and without the antihistamine and the other used an antibiotic and a steroid with and without the antihistamine. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Dusdieker 1985a.
| Methods | Randomized controlled trial Quality score: 4/5 (randomization: 1 + 0; blinding: 1 + 1; withdrawals: 1) Intention‐to‐treat analysis: no Follow up was 89% |
|
| Participants | 66 children aged 6 months to 10 years were recruited from a pediatric outpatient clinic. All had completed antibiotic treatment before enrolment. Diagnosis was based on pneumatic otoscopy and tympanometry Exclusion criteria included: history of cleft lip or palate, chronic disease, immunodeficiency disease, recent use of corticosteroids or known hearing loss > 25 dB bilaterally or > 35 dB unilaterally |
|
| Interventions | Antihistamine (chlorpheniramine), decongestant (pseudoephedrine) and placebo | |
| Outcomes | Our primary outcome was not measured (cure at or before 4 weeks), but a secondary outcome of effusion or no effusion at 12 weeks was measured as was the complication of acute otitis media (AOM) | |
| Notes | Antihistamine arm of the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Dusdieker 1985b.
| Methods | See Notes | |
| Participants | — | |
| Interventions | — | |
| Outcomes | — | |
| Notes | Same study as Dusdieker 1985a but different arm ‐ decongestant arm | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
Edstrom 1977.
| Methods | Randomized controlled trial Quality score: 1/5 (randomization: 0; blinding: 1 + 0; withdrawals: 0) Intention‐to‐treat analysis: no Follow up was 78%. The trial took place in 1974 |
|
| Participants | 94 children mainly less than 10 years were seen in an ENT clinic Diagnosis was based on pneumatic otoscopy Exclusion criteria: none reported |
|
| Interventions | Antihistamine (cinnarizine) and placebo | |
| Outcomes | The only outcome measured was the secondary outcome of effusion or no effusion at less than 12 weeks (7 weeks here) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Fabian 1986.
| Methods | Randomized controlled trial Quality score: 3/5 (randomization: 1; binding: 1 + 0; withdrawals: 1) Intention‐to‐treat analysis: yes Follow up 100% |
|
| Participants | 172 children aged 6 months to 15 years were recruited from ENT clinics in Sweden Diagnosis was based on pneumatic otoscopy and tympanometry Exclusion criteria included: need for acute tympanocentesis, chronic illness, refusal to participate, difficult child to examine, previous side effects to one drug or the other |
|
| Interventions | Decongestant (oxymetazoline nasal drops or phenylpropanolamine orally) versus no treatment | |
| Outcomes | The primary outcome of effusion or no effusion at 4 weeks was measured as was the secondary outcome of cure or no cure at 4 to 8 weeks, all significant side effects and the complication of surgery (tympanostomy) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Fraser 1977.
| Methods | Randomized controlled trial Quality score: 1/5 (randomization: 1 + 0; blinding: 0; withdrawals: 0) Intention‐to‐treat analysis: unable to determine Follow up was 96% |
|
| Participants | 85 children aged 3 to 12 years with bilateral OME were recruited Diagnosis was based on an algorithm using otoscopy and tympanometry Exclusion criteria: none given |
|
| Interventions | Decongestant (ephedrine nasal drops) or antihistamine/decongestant combination (brompheniramine/phenylpropanolamine) versus autoinflation (control) | |
| Outcomes | No individual patient data were given. The authors gave a simple statement that outcomes from all 3 interventions were the same. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
Haugeto 1981a.
| Methods | Randomized controlled trial Quality score: 3/5 (randomization: 1; blinding: 1 + 1; withdrawals: 0) Intention‐to‐treat analysis: no Follow up was 94% |
|
| Participants | 61 children aged 1 to 14 years were seen in an ENT Department in Norway Diagnosis was based on pneumatic otoscopy, otomicroscopy, tympanometry and audiometry Exclusion criterion: age less than 1 year |
|
| Interventions | Decongestant (phenylpropanolamine) or decongestant/antihistamine combination (phenylpropanolamine/brompheniramine) versus placebo | |
| Outcomes | The primary outcome of effusion or not at 4 weeks was measured as was improvement or no improvement in hearing at 4 weeks | |
| Notes | For 'any medication' comparison, this will be decongestant arm only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Haugeto 1981b.
| Methods | See Notes | |
| Participants | — | |
| Interventions | — | |
| Outcomes | — | |
| Notes | Same study as Haugeto 1981a but different arm, antihistamine/decongestant arm | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
Hayden 1984.
| Methods | Randomized controlled trial Quality score: 4/5 (randomization: 1 + 0; blinding: 1 + 1; withdrawals: 1) Intention‐to‐treat analysis: no Follow up was 50% and the duration of the study was 4 years from 1978 to 1982 |
|
| Participants | 67 children aged 9 months to 10 years were recruited from private pediatrics offices approximately 2 weeks after treatment for AOM Diagnosis was based on either clinical criteria (pneumatic otoscopy) or tympanometry. We used only tympanometry data. Exclusion criteria: none given |
|
| Interventions | Decongestant (phenylephrine) given intranasally versus intranasal placebo | |
| Outcomes | The primary outcome of effusion or not at 4 weeks was measured | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Hughes 1984.
| Methods | Randomized controlled trial Quality score: 3/5 (randomization: 1 + 0; blinding: 1 + 1; withdrawals: 0) Intention‐to‐treat analysis: no Follow up was 88% |
|
| Participants | 42 children (no age range given) from GP practices were referred to a single ENT specialist Diagnosis was based on clinical examination and tympanometry Exclusion criteria: previous surgery to ears, nose or throat and abnormal palatal function |
|
| Interventions | Antihistamine/decongestant combination (triprolidine/pseudoephedrine) versus placebo | |
| Outcomes | Our primary outcome (effusion or not at 4 weeks or less) was not measured. Secondary (1 to 3 months) and late (greater than 3 months) were measured as was the outcome of surgery (tympanostomy). | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Khan 1981.
| Methods | Randomized controlled trial Quality score: 2/5 (randomization: 1; blinding: 1; withdrawals: 0) Intention‐to‐treat analysis: unable to determine Follow up was 97% |
|
| Participants | 58 children aged 5 to 14 years were recruited from an ENT clinic Diagnosis was based on a combination of history, otoscopy and audiology Exclusion criterion: presence of AOM |
|
| Interventions | Antihistamine/decongestant combination (brompheniramine/phenylephrine and phenylpropanolamine) versus placebo | |
| Outcomes | No individual patient data are available. The outcome measured was hearing loss at 4 weeks. The authors stated that no significant difference in hearing was found between the antihistamine/decongestant combination and the placebo group. All children got tympanostomies. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Lesser 1986.
| Methods | Randomized controlled trial Quality score: 2/5 (randomization: 1; blinding: 0; withdrawals: 1) Intention‐to‐treat analysis: no Follow up 95%. Study duration from September 1984 to January 1985 |
|
| Participants | 39 children aged 3 to 12 years with OME after tympanostomy and (adenoidectomy or tonsillectomy) were recruited from an ENT practice Diagnosis was based on a thick effusion at tympanostomy at entry to the study and on, at outcome, an algorithm that included tympanometry, audiography and otoscopy Exclusion criteria included: previous surgery for OME and use of mucolytics, antihistamines or decongestants in the preceding 72 days |
|
| Interventions | Antihistamine/decongestant combination (brompheniramine/phenylephrine and phenylpropanolamine) versus no treatment | |
| Outcomes | Our primary outcome of effusion or not at 4 weeks was not measured. A secondary outcome of effusion or not at 6 weeks (by counting ears) was measured as was the side effect of nosebleed. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
O'Shea 1980.
| Methods | Randomized controlled trial Quality score: 2/5 (randomization: 0; blinding: 1 + 1; withdrawals: 0) Intention‐to‐treat analysis: no Follow up was 91%. The study took place between March and December 1977. |
|
| Participants | 83 children aged 3 to 9 years with their first episode of OME were recruited from Pediatrics and ENT practices in Rhode Island Diagnosis was based on a combination of otoscopy, audiometry and tympanometry Exclusion criteria included: oral temperature greater than 37.8 °C and ear or nose deformity |
|
| Interventions | Antihistamine/decongestant combination (diphenhydrinate/pseudoephedrine) versus placebo | |
| Outcomes | Our primary outcome of effusion or not at 4 weeks was not measured. A secondary outcome was measured by patient‐ear‐visit at 3 months. Hearing at 3 months was measured for improvement (by at least 20 dB) or no improvement. Adverse effects of sedation, hyperactivity or any of the above were measured after 1 month. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
O'Shea 1982.
| Methods | This study was a report of late follow up of the previous study so the methods were the same | |
| Participants | Participants were the same as above | |
| Interventions | Interventions were the same as above | |
| Outcomes | A late outcome of effusion or no effusion was measured by ears at 1 year. Improvement in hearing and school performance were also measured at 1 year. The complication of recurrence of OME at 1 year was measured. | |
| Notes | Late follow up of O'Shea 1980 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Olson 1978.
| Methods | Randomized controlled trial Quality score: 2/5 (randomization: 1; blinding: 1; withdrawals: 0) Intention‐to‐treat analysis: unable to determine Follow up was 67% |
|
| Participants | 78 children over 6 months of age were recruited from a community‐based pediatric practice in upstate New York after a recent diagnosis of AOM treated with antibiotics and decongestant Diagnosis was based on tympanometry Exclusion criterion was presence of ear grommets (ventilation tubes). A history of previous OME and of allergies was recorded and used to generate outcomes. |
|
| Interventions | Decongestant (pseudoephedrine) versus placebo | |
| Outcomes | No individual patient data were given for our primary outcome. The authors stated that in all comparisons measured, patients who received oral decongestant consistently did worse than those on placebo although the difference did not always reach statistical significance. | |
| Notes | See outcomes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Saunte 1978.
| Methods | Randomized controlled trial Quality score: 3/5 (randomization: 1; blinding 1 + 1; withdrawals: 0) Intention‐to‐treat analysis: no Follow up was 68% |
|
| Participants | 21 children aged 1 to 12 were recruited from an ENT clinic. A history of allergies was noted. Diagnosis was based on audiometry and pneumatic otoscopy Exclusion criteria included: no AOM within 2 weeks of OME and normal hearing prior to OME |
|
| Interventions | Antihistamine/decongestant (brompheniramine/phenylpropanolamine) versus placebo | |
| Outcomes | The primary outcome of effusion or not at 4 weeks was measured as was hearing. The surgical complication of tympanostomy was measured and side effects were assessed. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
AOM: acute otitis media; OME: otitis media with effusion; RCT: randomized controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Altman 1998 | ALLOCATION:
Randomized, double‐blind PARTICIPANTS: Patients undergoing myringotomy ‐ not confined to OME |
| Bhambhani 1983 | ALLOCATION: Randomized by AOM, not OME |
| Brown 1985 | ALLOCATION: Review, not a trial |
| Brownoff 1998 | ALLOCATION:
Randomized, double‐blind PARTICIPANTS: Did not have OME, this was a prevention study |
| Cantekin 1980 | ALLOCATION: Not randomized |
| Chonmaitree 2003 | ALLOCATION: Randomization by AOM, not OME |
| Collins 1983 | ALLOCATION:
Randomized, not blinded PARTICIPANTS: Had OME, were awaiting adenoidectomy INTERVENTION: Antihistamine/decongestant, sodium cromoglycate, control OUTCOME: Mean free histamine content in middle ear fluid. Excluded because no patient‐oriented outcome was measured and none of our primary or secondary outcomes were measured. |
| Gates 1986 | ALLOCATION: Treatment group only ‐ no control |
| Grundfast 1981 | ALLOCATION: Review, not a trial |
| Kjellman 1978 | ALLOCATION: Did not study OME; this was a prevention study |
| Klein 1980 | ALLOCATION: Randomized, double‐blind but randomized by AOM, not OME |
| Kraemer 1984 | ALLOCATION: Not a randomized controlled trial |
| Lildholdt 1982 | ALLOCATION:
Randomized, double‐blind PARTICIPANTS: Did not have OME, had only a history of treated OME |
| Malm 1985 | ALLOCATION: Review, not a trial |
| Marshall 1984 | ALLOCATION: Review, not a trial |
| McCormick 2003 | ALLOCATION: Randomized by AOM, not OME |
| Moller 1980 | ALLOCATION:
No randomization PARTICIPANTS: All children had cleft palate |
| Moran 1982 | ALLOCATION:
Randomized and multiply blinded PARTICIPANTS: Patients had AOM with effusion, not OME |
| Ogino 1992 | ALLOCATION: No mention of randomization |
| Otten 1990 | ALLOCATION:
Randomized but not blinded PARTICIPANTS: Children with URTI and OME INTERVENTION: Combination treatment of antibiotic with decongestant. Excluded because we could not separate out the decongestant effect. |
| Sorri 1982 | ALLOCATION: Not randomized |
| Suzuki 1999 | ALLOCATION:
Randomized PARTICIPANTS: Included adults, could not separate out children |
| Theoharides 1994 | ALLOCATION:
Randomized PARTICIPANTS: Children with OME INTERVENTION: Non‐feasible intervention‐direct instillation of antihistamine into the middle ear through a grommet |
| van Heerbeek 2002 | ALLOCATION:
Randomized and double‐blinded PARTICIPANTS: Children with OME INTERVENTION: Nasal decongestant or placebo OUTCOME: Eustachian tube function at 15 minutes after Tx. Excluded because no patient‐oriented outcomes were measured and none of our primary or secondary outcomes was measured. |
AOM: acute otitis media; OME: otitis media with effusion; Tx: treatment; URTI: upper respiratory tract infection
Differences between protocol and review
Change of authorship.
Contributions of authors
GH Griffin: primary review manager and author. CA Flynn: consultant, editor and co‐author.
Sources of support
Internal sources
None, Not specified.
External sources
None, Not specified.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Cantekin 1983 {published data only}
- Cantekin EI, Mandel EM, Bluestone CD, Rockette HE, Paradise JL, Stool SE, et al. Lack of efficacy of a decongestant‐antihistamine combination for otitis media with effusion ("secretory" otitis media) in children. Results of a double‐blind, randomized trial. New England Journal of Medicine 1983;308(6):297‐301. [DOI] [PubMed] [Google Scholar]
Cantekin 1991 {published data only}
- Cantekin EI, McGuire TW, Griffith TL. Antimicrobial therapy for otitis media with effusion ('secretory' otitis media) [see comment]. JAMA 1991;266(23):3309‐17. [PubMed] [Google Scholar]
- Mandel EM, Rockette HE, Bluestone CD, Paradise JL, Nozza RJ. Efficacy of amoxicillin with and without decongestant‐antihistamine for otitis media with effusion in children. Results of a double‐blind, randomized trial. New England Journal of Medicine 1987;316(8):432‐7. [DOI] [PubMed] [Google Scholar]
Choung 2008 {published data only}
- Choung Y‐H, Shin YR, Choi SJ, Park K, Park HY, Lee JB, et al. Management for the children with otitis media with effusion in the tertiary hospital. Clinical and Experimental Otorhinolaryngology 2008;1(4):201‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dusdieker 1985a {published data only}
- Dusdieker LB, Smith G, Booth BM, Woodhead JC, Milavetz G. The long‐term outcome of nonsuppurative otitis media with effusion. Clinical Pediatrics 1985;24(4):181‐6. [DOI] [PubMed] [Google Scholar]
Dusdieker 1985b {published data only}
- Dusdieker LB, Smith G, Booth BM, Woodhead JC, Milavetz G. The long‐term outcome of nonsuppurative otitis media with effusion. Clinical Pediatrics 1985;24(4):181‐6. [DOI] [PubMed] [Google Scholar]
Edstrom 1977 {published data only}
- Edstrom S, Lundin K, Jeppsson PH. Secretory otitis media. Aspects on treatment and control. ORL: Journal of Oto‐Rhino‐Laryngology and its Related Specialties 1977;39(2):68‐73. [DOI] [PubMed] [Google Scholar]
Fabian 1986 {published data only}
- Fabian P, Lonnerholm G, Jerrmark‐Tera IB, Kleberg A, Melander H. Effects and adverse effects of decongestants in otosalpingitis. Lakartidningen 1986;83(42):3508‐13. [PubMed] [Google Scholar]
Fraser 1977 {published data only}
- Fraser JG, Mehta M, Fraser PA. The medical treatment of secretory otitis media. A clinical trial of three commonly used regimes. Journal of Laryngology and Otology 1977;91(9):757‐65. [DOI] [PubMed] [Google Scholar]
Haugeto 1981a {published data only}
- Haugeto OK, Schroder KE, Mair IW. Secretory otitis media, oral decongestant and antihistamine. Journal of Otolaryngology 1981;10(5):359‐62. [PubMed] [Google Scholar]
Haugeto 1981b {published data only}
- Haugeto OK, Schroder KE, Mair IW. Secretory otitis media, oral decongestant and antihistamine. Journal of Otolaryngology 1981;10(5):359‐62. [PubMed] [Google Scholar]
Hayden 1984 {published data only}
- Hayden GF, Randall JE, Randall JC, Hendley JO. Topical phenylephrine for the treatment of middle ear effusion. Archives of Otolaryngology 1984;110(8):512‐4. [DOI] [PubMed] [Google Scholar]
Hughes 1984 {published data only}
- Hughes KB. Management of middle‐ear effusions in children. Journal of Laryngology and Otology 1984;98(7):677‐84. [DOI] [PubMed] [Google Scholar]
Khan 1981 {published data only}
- Khan JA, Marcus P, Cummings SW. S‐carboxymethylcysteine in otitis media with effusion (a double‐blind study). Journal of Laryngology and Otology 1981;95(10):995‐1001. [DOI] [PubMed] [Google Scholar]
Lesser 1986 {published data only}
- Lesser TH, Clayton MI, Skinner D. Efficacy of medical treatment as an adjunct to surgery in the treatment of secretory otitis media. Journal of Laryngology and Otology 1986;100(12):1347‐50. [DOI] [PubMed] [Google Scholar]
O'Shea 1980 {published data only}
- O'Shea JS, Langenbrunner DJ, McCloskey DE, Pezzullo JC, Regan JB. Diagnostic and therapeutic studies in childhood serous otitis media. Results of treatment with an antihistamine‐adrenergic combination. Annals of Otology, Rhinology, and Laryngology. Supplement 1980;89(3 Pt 2):285‐9. [DOI] [PubMed] [Google Scholar]
O'Shea 1982 {published data only}
- O'Shea JS, Langenbrunner DJ, McCloskey DE, Pezzullo JC, Regan JB. Childhood serous otitis media: fifteen months' observations of children untreated compared with those receiving an antihistamine‐adrenergic combination. Clinical Pediatrics 1982;21(3):150‐3. [DOI] [PubMed] [Google Scholar]
Olson 1978 {published data only}
- Olson AL, Klein SW, Charney E, MacWhinney JB Jr, McInerny TK, Miller RL, et al. Prevention and therapy of serous otitis media by oral decongestant: a double‐blind study in pediatric practice. Pediatrics 1978;61(5):679‐84. [PubMed] [Google Scholar]
Saunte 1978 {published data only}
- Saunte C. Clinical trial with Lunerin mixture and Lunerin mite in children with secretory otitis media. Journal of International Medical Research 1978;6(1):50‐5. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Altman 1998 {published data only}
- Altman JS, Haupert MS, Hamaker RA, Belenky WM. Phenylephrine and the prevention of postoperative tympanostomy tube obstruction. Archives of Otolaryngology ‐ Head and Neck Surgery 1998;124(11):1233‐6. [DOI] [PubMed] [Google Scholar]
Bhambhani 1983 {published data only}
- Bhambhani K, Foulds DM, Swamy KN, Eldis FE, Fischel JE. Acute otitis media in children: are decongestants or antihistamines necessary?. Annals of Emergency Medicine 1983;12(1):13‐6. [DOI] [PubMed] [Google Scholar]
Brown 1985 {published data only}
- Brown DT, Potsic WP, Marsh RR, Litt M. Drugs affecting clearance of middle ear secretions: a perspective for the management of otitis media with effusion. Annals of Otology, Rhinology, and Laryngology. Supplement 1985;117:3‐15. [DOI] [PubMed] [Google Scholar]
Brownoff 1998 {published data only}
- Brownoff R, Hutchison LJ. An antihistamine decongestant in the prevention of acute otitis media in children with colds. Canadian Family Physician 1998;34:2389‐93. [PMC free article] [PubMed] [Google Scholar]
Cantekin 1980 {published data only}
- Cantekin EI, Bluestone CD, Rockette HE, Beery QC. Effect of decongestant with or without antihistamine on Eustachian tube function. Annals of Otology, Rhinology, and Laryngology. Supplement 1980;89(3 Pt 2):290‐5. [DOI] [PubMed] [Google Scholar]
Chonmaitree 2003 {published data only}
- Chonmaitree T, Saeed K, Uchida T, Heikkinen T, Baldwin CD, Freeman DH Jr, et al. A randomized, placebo‐controlled trial of the effect of antihistamine or corticosteroid treatment in acute otitis media. Journal of Pediatrics 2003;143(3):377‐85. [DOI] [PubMed] [Google Scholar]
Collins 1983 {published data only}
- Collins MP, Church MK. The effect of an anti‐allergic, nasal decongestant combination ('Dimotapp') and sodium cromoglycate nose drops on the histamine content of adenoids, middle ear fluid and nasopharyngeal secretions of children with secretory otitis media. Current Medical Research and Opinion 1983;8(4):392‐4. [DOI] [PubMed] [Google Scholar]
Gates 1986 {published data only}
- Gates GA, Wachtendorf C, Holt GR, Hearne EM. Medical treatment of chronic otitis media with effusion (secretory otitis media). Otolaryngology ‐ Head and Neck Surgery 1986;94(3):350‐4. [DOI] [PubMed] [Google Scholar]
Grundfast 1981 {published data only}
- Grundfast KM. A review of the efficacy of systemically administered decongestants in the prevention and treatment of otitis media. Otolaryngology ‐ Head and Neck Surgery 1981;89(3 Pt 1):432‐9. [DOI] [PubMed] [Google Scholar]
Kjellman 1978 {published data only}
- Kjellman NI, Harder H, Lindwall L, Synnerstad B. Longterm treatment with brompheniramine and phenylpropanolamine in recurrent otitis media ‐ a double‐blind study. Journal of Otolaryngology 1978;7(3):257‐61. [PubMed] [Google Scholar]
Klein 1980 {published data only}
- Klein SW, Olson AL, Perrin J, Cunningham D, Hengerer A, Hoekelman RA, et al. Prevention and treatment of serous otitis media with an oral antihistamine. A double‐blind study in pediatric practice. Clinical Pediatrics 1980;19(5):342‐7. [DOI] [PubMed] [Google Scholar]
Kraemer 1984 {published data only}
- Kraemer MJ, Marshall SG, Richardson MA. Etiologic factors in the development of chronic middle ear effusions. Clinical Reviews in Allergy 1984;2(4):319‐28. [PubMed] [Google Scholar]
Lildholdt 1982 {published data only}
- Lildholdt T, Cantekin EI, Bluestone CD, Rockette HE. Effect of a topical nasal decongestant on Eustachian tube function in children with tympanostomy tubes. Acta Oto‐Laryngologica 1982;94(1‐2):93‐7. [DOI] [PubMed] [Google Scholar]
Malm 1985 {published data only}
- Malm L. Oral decongestants in acute rhinitis, acute sinusitis, acute otitis media and secretory otitis media: prognostic implications. Workshop: Treatment of Ear, Nose and Throat Infections. 1985.
Marshall 1984 {published data only}
- Marshall SG, Bierman CW, Shapiro GG. Otitis media with effusion in childhood. Annals of Allergy 1984;53(5):370‐8, 394. [PubMed] [Google Scholar]
McCormick 2003 {published data only}
- McCormick DP, Saeed K, Uchida T, Baldwin CD, Deskin R, Lett‐Brown MA, et al. Middle ear fluid histamine and leukotriene B4 in acute otitis media: effect of antihistamine or corticosteroid treatment. International Journal of Pediatric Otorhinolaryngology 2003;67(3):221‐30. [DOI] [PubMed] [Google Scholar]
Moller 1980 {published data only}
- Moller P. Negative middle ear pressure and hearing thresholds in secretory otitis media. A double‐blind crossover study with Lunerin. Scandinavian Audiology 1980;9(3):171‐6. [DOI] [PubMed] [Google Scholar]
Moran 1982 {published data only}
- Moran DM, Mutchie KD, Higbee MD, Paul LD. The use of an antihistamine‐decongestant in conjunction with an anti‐infective drug in the treatment of acute otitis media. Journal of Pediatrics 1982;101(1):132‐6. [DOI] [PubMed] [Google Scholar]
Ogino 1992 {published data only}
- Ogino S, Harada T, Matsunaga T, Tominaga Y. Use of Tranilast [N‐(3,4‐dimethoxycinnamoyl) anthranilic acid] in secretory otitis media. Annals of Allergy 1992;68(5):407‐12. [PubMed] [Google Scholar]
Otten 1990 {published data only}
- Otten FW, Grote JJ. Otitis media with effusion and chronic upper respiratory tract infection in children: a randomized, placebo‐controlled clinical study. Laryngoscope 1990;100(6):627‐33. [DOI] [PubMed] [Google Scholar]
Sorri 1982 {published data only}
- Sorri M, Sipila P. Can secretory otitis media be prevented by oral decongestants?. Acta Otolaryngologica Supplement 1982;386:115‐6. [Google Scholar]
Suzuki 1999 {published data only}
- Suzuki M, Kawauchi H, Mogi G. Clinical efficacy of an antiallergic drug on otitis media with effusion in association with allergic rhinitis. Auris Nasus Larynx 1999;26(2):123‐9. [DOI] [PubMed] [Google Scholar]
Theoharides 1994 {published data only}
- Theoharides TC, Manolidis SS, Vliagoftis H, Manolidis LS. Treatment of secretory otitis media with local instillation of hydroxyzine. International Archives of Allergy and Immunology 1994;103(1):95‐101. [DOI] [PubMed] [Google Scholar]
van Heerbeek 2002 {published data only}
- Heerbeek N, Ingels KJ, Zielhuis GA. No effect of a nasal decongestant on Eustachian tube function in children with ventilation tubes. Laryngoscope 2002;112(6):1115‐8. [DOI] [PubMed] [Google Scholar]
Additional references
AAFP 2004
- American Academy of Family Physicians, American Academy of Otolaryngology ‐ Head, Neck Surgery. American Academy of Pediatrics Subcommittee on Otitis Media with Effusion. Otitis media with effusion. Pediatrics 2004;113(5):1412‐29. [DOI] [PubMed] [Google Scholar]
Bandolier 2000
- Bandolier Bias Guide. http://www.medicine.ox.ac.uk/bandolier/Extraforbando/Bias.pdf 2000 (accessed 11 October 2010).
Barker 2005
- Barker FG, Carter BS. Synthesizing medical evidence: systematic reviews and meta‐analyses. Neurosurgical Focus 2005;19(E5):1‐21. [DOI] [PubMed] [Google Scholar]
Becker 2011
- Becker LA, Hom J, Villasis‐Keever M, Wouden JC. Beta2‐agonists for acute bronchitis. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD001726.pub4] [DOI] [PubMed] [Google Scholar]
Berlin 1997
- Berlin J. Does blinding of readers affect the results of meta‐analyses?. Lancet 1997;350:185‐6. [DOI] [PubMed] [Google Scholar]
Bluestone 1995
- Bluestone CD, Klein JO. Otitis Media in Infants and Children. Second Edition. Philadelphia: W.B. Saunders, 1995. [Google Scholar]
Browning 2010
- Browning GG, Rovers MM, Williamson I, Lous J, Burton MJ. Grommets (ventilation tubes) for hearing loss associated with otitis media with effusion in children. Cochrane Database of Systematic Reviews 2010, Issue 10. [DOI: 10.1002/14651858.CD001801.pub3] [DOI] [PubMed] [Google Scholar]
Fiellau 1977
- Fiellau‐Nikolajsen M, Lous J, Pedersen SV, Schousboe HH. Tympanometry in three‐year‐old children. 1. A regional prevalence study on the distribution of tympanometric results in a non‐selected population of three‐year‐old children. Scandinavian Audiology 1977;6:199‐204. [DOI] [PubMed] [Google Scholar]
Fiellau 1979
- Fiellau‐Nikolajsen M, Lous J. Prospective tympanometry in three‐year‐old children: a study of spontaneous course of tympanometry types in a non‐selected population. Archives of Otolaryngology 1979;105:461‐6. [DOI] [PubMed] [Google Scholar]
Fiellau 1983
- Fiellau‐Nikolajsen M. Tympanometry and secretory otitis media. Observations on diagnosis, epidemiology, treatment, and prevention in prospective cohort studies of three‐year‐old children [thesis]. Acta Otolaryngologica Stockholm Supplement 1983;394:1‐73. [PubMed] [Google Scholar]
Golz 1998
- Golz A Netzer A, Angel‐Yeger B, Westerman ST, Gilbert LM, Joachims HZ. Effects of middle ear effusions on the vestibular system in children. Otolaryngology ‐ Head and Neck Surgery 1998;119:695‐9. [DOI] [PubMed] [Google Scholar]
Grace 1990
- Grace AR, Pfleiderer AG. Dysequilibrium and otitis media with effusion: what is the association?. Journal of Laryngology and Otology 1990;104:682‐4. [DOI] [PubMed] [Google Scholar]
Handbook 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jadad 1996
- Jadad A, Moore R, Carroll D. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
Lexi‐Drugs 2006
- Lexi‐Drugs. http://www.cma.ca/index.cfm?la_id=1&ci_id=370&gmAction=/lexidrug/displayMonograph.do&drugId=959 (accessed 27 June 2006).
Lous 1981
- Lous J, Fiellau‐Nikolajsen M. Epidemiology of middle ear effusion and tubal dysfunction: a one‐year prospective study comprising monthly tympanometry in 387 non‐selected 7‐year‐old children. International Journal of Pediatric Otorhinolaryngology 1981;3:303‐17. [DOI] [PubMed] [Google Scholar]
Lous 1995
- Lous J. Secretory otitis media in schoolchildren. Is screening for secretory otitis media advisable?. Danish Medical Bulletin 1995;42:71‐99. [PubMed] [Google Scholar]
Mohar 1995
- Mohar D, Jadad AR, Nichol G. Assessing the quality of randomized controlled trials: an annotated bibliography of scales and checklists. Controlled Clinical Trials 1995;16:62‐73. [DOI] [PubMed] [Google Scholar]
Perera 2006a
- Perera R, Glasziou P. Design effect adjustment for paired data. (submitted to Statistics in Medicine) 2006.
Perera 2006b
- Perera R, Haynes J, Glasziou PP, Heneghan CJ. Autoinflation for hearing loss associated with otitis media with effusion. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD006285] [DOI] [PubMed] [Google Scholar]
Rosenfeld 1999
- Rosenfeld R, Bluestone C. Evidence Based Otitis Media. Hamilton, Ontario: BC Decker, 1999. [Google Scholar]
Shekelle 2003
- Shekelle P, Takata G, Chan LS, Mangione‐Smith R, Corley PM, Morphew T, et al. Diagnosis, natural history, and late effects of otitis media with effusion. Evidence Report/Technology Assessment No. 55 (Prepared by Southern California Evidence‐based Practice Center under Contract No 290‐97‐0001, Task Order No. 4). AHRQ Publication No. 03‐E023. Rockville, MD: Agency for Healthcare Research and Quality May 2003.
Simpson 2011
- Simpson SA, Lewis R, Voort J, Butler CC. Oral or topical nasal steroids for hearing loss associated with otitis media with effusion in children. Cochrane Database of Systematic Reviews 2011, Issue 5. [DOI: 10.1002/14651858.CD001935.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Takata 2003
- Takata GS, Chan LS, Morphew T, Mangione‐Smith R, Morton SC, Shekelle P. Evidence assessment of the accuracy of methods of diagnosing middle ear effusion in children with otitis media with effusion. Pediatrics 2003;112(6 Pt 1):1379‐87. [DOI] [PubMed] [Google Scholar]
Teele 1989
- Teele DW, Klein JO, Rosner B. Epidemiology of otitis media during the first seven years of life in children in greater Boston: a prospective, cohort study. Journal of Infectious Diseases 1989;160:83‐94. [DOI] [PubMed] [Google Scholar]
van den Aardweg 2010
- Aardweg MTA, Schilder AGM, Herkert E, Boonacker CWB, Rovers MM. Adenoidectomy for otitis media in children. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD007810.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Witmer 1998
- Witmer A, Wells AM, Seymour RJ. A comparison of the effectiveness of pharmacologic treatment of otitis media with effusion in children: integrative and meta‐analysis. Online Journal of Knowledge Synthesis for Nursing 1998; Vol. 5, issue 4. [PubMed]


