Abstract
Objective:
The Rotterdam Healthy Aging Score (HAS) is a validated multidimensional index constructed from five health domains. We describe the HAS distribution in a cohort of HIV-positive adults and correlate it with health outcomes.
Design:
A cross-sectional pilot study of 101 adults aged at least 40 years, on suppressive antiretroviral therapy attending a tertiary HIV clinic in Toronto, Canada.
Methods:
Participants completed questionnaires to calculate their HAS (range 0–14). Demographics, HAS and sub-scores were compared by age and sex. The HAS was compared with results of the Fried Frailty Score, Short Performance Physical Battery score (SPPB) and measures of health utilization. Kruskal--Wallis Rank-Sum and Fisher's exact tests were used for all comparisons.
Results:
Median (IQR) age was 56 (50--62), 81 (80%) men and 50 (50%) born in Canada. Median (IQR) CD4+ cell count was 574 (417--794) cells/μl. Median (IQR) HAS was 12 (10--13) with 39 (39%) achieving a score more than 12 (considered healthy aging). Younger participants experienced more depression, whereas women had greater pain. The HAS score correlated with the Fried Frailty Score (P = 0.008) and trended with the SPPB Score (P = 0.077). Those with the poorest HAS scores were more likely to have been hospitalized in the preceding 6 months (P = 0.034).
Conclusion:
The HAS ranged from 5 to 14 in this cohort of older HIV adults with 39% attaining scores in the ‘healthy’ range. The HAS correlated with measures of physical performance and health utilization. Further validation of an objective outcome in HIV-positive patients will facilitate evaluation of interventional studies to improve healthy aging.
Keywords: aging, cohort study, frailty, health, HIV, pilot
Background
The aging of the HIV population is a remarkable success but comes with new challenges and questions for persons living with HIV (PLWH) and their caregivers [1–4]. Although long-term survival of effectively treated PLWH approaches that of the general population [5,6], age-related comorbidities are more prevalent and the overall quality of life may differ [3,7–9]. Critical knowledge gaps exist on how HIV and its therapies might impact and interact with normal aging processes.
As this cohort continues to grow, it is critical to develop strategies to support healthy aging in PLWH [10]. In order to do so, we need to define health and identify the modifiable factors that impact it. Researchers and advocates have called for a revision to the UN AIDS 90 : 90 : 90 goals for HIV [11]. Not only should 90% of those with HIV be aware of their disease, 90% be engaged in care, 90% be receiving ART and 90% have viral suppression but that 90% should have good health-related quality of life [12].
There are little data on how to measure healthy aging in the context of HIV and no HIV-specific indexes of health. Although older adults may present with more advanced HIV disease and have less immune recovery than younger adults [13,14], for PLWH on effective combination antiretroviral therapy (ART) neither absolute CD4+ cell counts, CD4+/CD8+ ratios, nor HIV viral load are useful surrogate markers of healthy aging [15–17]. The Veterans Aging Cohort Study Index [16] creates a score by summing preassigned points for age, CD4+ cell count and HIV-1 RNA, and general indicators of organ system injury including hemoglobin, platelets, aspartate and alanine transaminase, creatinine, and viral hepatitis C infection. The VACS Index predicts all-cause mortality, cause specific mortality, and other outcomes in those living with HIV infection. Despite these predictions, it is focused on physical health, does not capture other health domains or quality of life and has not been used to assess interventions to maintain or improve health.
In geriatric medicine, frailty is often used to assess health status [18]. A commonly used measure is the Fried frailty phenotype, which assesses five specific features: self-reported weight loss, self-reported exhaustion, low levels of physical activity, measured 15 feet walk time and measured grip strength. The Short Physical Performance Battery (SPPB) has also been used for over 20 years to measure physical performance or functional status [19]. It is a brief battery with a timed walk, repeated chair stands, and balance tests. Although useful, these two measures are largely focused on physical status.
There are many components to healthy aging beyond physical function or the simple presence or absence of comorbid disease [20]. The new framework of the WHO's Global Strategy and Action Plan on Aging and Health[21,22] defines healthy aging as ‘the process of developing and maintaining the functional ability that enables well being in older age’. The framework acknowledges that older people experience significant losses in their physical and cognitive capacities and that although some are inevitable, others may be avoided. The framework stresses that the study of healthy aging must consider a composite of an individual's physical and mental capacities (intrinsic capacity) in addition to factors in the extrinsic world that form the context of an individual's life (environments). In the Action Plan of the strategy, the WHO challenges researchers to improve measuring, monitoring, and understanding healthy aging in different populations [22].
The Rotterdam HAS was constructed using factor analysis from a prospective population-based study of 3527 Dutch participants at least 55 years old [23]. It considers five biopsychosocial domains of health: mental health, cognitive function, physical function (three sub-components of pain, comorbidity, and activities of daily living), social support, and quality of life. For each of the seven measures a score of 0 (low, corresponding to a worse status within the domain), 1 (moderate), or 2 (high, corresponding to an optimal status within the domain) is assigned and the total summed to determine the HAS score.
To respond to the WHO challenge for standardization, we selected The Rotterdam Healthy Aging Score- HAS [23] to examine the concept of healthy aging in an HIV cohort. Determination of a valid, standardized measure of healthy aging would be useful in the clinic to follow the health trajectory of patients and as an objective outcome of clinical trials of interventions designed to improve it.
Methods
We evaluated the HAS in a cross-sectional cohort of HIV-positive older adults attending a tertiary care HIV clinic in Toronto, Canada between May and August 2018. To be eligible, patients were required to be on ART with HIV RNA less than 50 copies/ml for at least 6 months. A priori, we elected to include 30, 35, and 35 participants in the age categories 40–50, 51–60 and greater than 60 years, respectively, in order to assess the impact of age on the score. Exclusion criteria were: pregnancy, previous liver cirrhosis, and current treatment with systemic steroids, chemotherapy, immunosuppressive agents or radiation therapy. A convenience sample of 101 PLWH was chosen from consecutive patients attending the clinic who consented to participate and met the inclusion criteria. Participants completed a series of questionnaires to calculate the HAS (score range 0–14).
To assess construct validity of the score in our population, we also calculated the Fried Frailty Index [24] and determined the Short Performance Physical Battery Score (SPPB) [19] for each participant. In addition, we estimated a measure of health utilization by administering questionnaires on the number of emergency room visits and overnight hospital stays in the previous 6 months and the need for home assistance.
The study was approved by the University Health Network Ethics review board, and each participant provided written informed consent. The study was conducted in accordance with the Declaration of Helsinki.
Sample size considerations
This pilot study was not powered for statistical significance but rather to determine the patient acceptability of completing the tasks and determining some preliminary performance characteristics of the score. With 100 participants, we would be able to detect a difference in HAS of 1.30 between two groups of participants, one with and one without a characteristic of interest, assuming a standard deviation in the HAS score of 2.3, a significance level of 0.05, power of 80% and that 50% of the study population has the characteristic of interest.
Statistical analysis
The Kruskal--Wallis Rank-Sum and Fisher's Exact tests were used to make the following comparisons: demographics, HAS, and HAS domains by age group (40–50, 51–60, and ≥61 years) and sex; the Fried Frailty Score [24], its individual components, and the frailty stage by HAS category (healthy, intermediate, or poor); the overall and individual component scores of the SPPB by HAS category; and the health utilization parameters by HAS category.
Results
Of 107 persons approached, 104 agreed to participate and 3 were excluded for not meeting eligibility criteria. The demographics of the participants are shown in Table 1. The median [interquartile range (IQR)] age was 56 years (50--62), 81 (80%) were men, and 50 (50%) were born in Canada reflective of our clinic population. Participants aged at least 61 years were more often Caucasian (75%) compared with those 40–50 years (47%) and 51–60 years (60%, P = 0.051). More women were black (55%) than men (7%, P < 0.001). Participants at least 61 years of age had longer median durations of HIV infection (25 years, P = 0.001) and lower CD4+ nadirs (153 cells/μl, P = 0.047) compared with 40–50 years (16 years, 267 cells/μl) or 51–60-year categories (22 years, 180 cells/μl). Median (IQR) CD4+ cell count at enrollment was 574 (417--794) cells/μl.
Table 1.
Demographic and clinical characteristics of HIV cohort by age category.
| Missing (%) | Overall, N (%) | 40--50 years | 51--60 years | ≥61 years | P | |
| n | 101 | 30 | 35 | 36 | ||
| Age [median (IQR)] | 56 [50--62] | 46 [44--49] | 56 [53--58] | 66 [62--69] | <0.001 | |
| Male | 81 (80.2) | 21 (70.0) | 29 (82.9) | 31 (86.1) | 0.257 | |
| BMI [median (IQR)] | 3 | 26 [23--29] | 27 [25--30] | 25 [21--29] | 25 [21--28] | 0.040 |
| Born in Canada | 50 (49.5) | 12 (40.0) | 19 (54.3) | 19 (52.8) | 0.475 | |
| Race | 0.051 | |||||
| Asian | 10 (9.9) | 4 (13.3) | 5 (14.3) | 1 (2.8) | ||
| Black | 17 (16.8) | 8 (26.7) | 4 (11.4) | 5 (13.9) | ||
| Caucasian | 62 (61.4) | 14 (46.7) | 21 (60.0) | 27 (75.0) | ||
| Hispanic | 5 (5.0) | 3 (10.0) | 0 (0.0) | 2 (5.6) | ||
| Other | 7 (6.9) | 1 (3.3) | 5 (14.3) | 1 (2.8) | ||
| Risk factor | 0.473 | |||||
| MSM only | 74 (73.3) | 18 (60.0) | 27 (77.1) | 29 (80.6) | ||
| IDU only | 1 (1.0) | 1 (3.3) | 0 (0.0) | 0 (0.0) | ||
| MSM and IDU | 2 (2.0) | 1 (3.3) | 1 (2.9) | 0 (0.0) | ||
| Heterosexual contact | 15 (14.9) | 5 (16.7) | 5 (14.3) | 5 (13.9) | ||
| Other/unknown | 9 (8.9) | 5 (16.7) | 2 (5.7) | 2 (5.6) | ||
| Duration of HIV (Y) | 21 [12--28] | 16 [11--19] | 22 [8--29] | 25 [18--29] | 0.001 | |
| CD4+ Nadir [median (IQR)] | 208 [83--306] | 267 [170--368] | 180 [80--320] | 153 [48--266] | 0.047 | |
| Current CD4+ [median (IQR)] | 574 [417--794] | 690 [550--869] | 535 [362--822] | 544 [404--678] | 0.063 | |
| Education | 0.335 | |||||
| Elementary | 6 (5.9) | 1 (3.3) | 2 (5.7) | 3 (8.3) | ||
| Secondary | 35 (34.7) | 7 (23.3) | 17 (48.6) | 11 (30.6) | ||
| Post-secondary | 45 (44.6) | 17 (56.7) | 13 (37.1) | 15 (41.7) | ||
| Masters or PhD | 15 (14.9) | 5 (16.7) | 3 (8.6) | 7 (19.4) | ||
| Income | 1 | 0.142 | ||||
| <$20 000 | 35 (35.0) | 8 (26.7) | 17 (48.6) | 10 (28.6) | ||
| $20 000--$49 999 | 32 (32.0) | 7 (23.3) | 9 (25.7) | 16 (45.7) | ||
| $50 000--$99 999 | 24 (24.0) | 10 (33.3) | 7 (20.0) | 7 (20.0) | ||
| >$100 000 | 9 (9.0) | 5 (16.7) | 2 (5.7) | 2 (5.7) | ||
| Smoking | 0.066 | |||||
| Current | 22 (21.8) | 7 (23.3) | 9 (25.7) | 6 (16.7) | ||
| Past | 40 (39.6) | 6 (20.0) | 16 (45.7) | 18 (50.0) | ||
| Never | 39 (38.6) | 17 (56.7) | 10 (28.6) | 12 (33.3) | ||
| Alcohol use | 0.306 | |||||
| None | 32 (31.7) | 11 (36.7) | 12 (34.3) | 9 (25.0) | ||
| Occasional | 38 (37.6) | 12 (40.0) | 13 (37.1) | 13 (36.1) | ||
| 2–3 per week | 14 (13.9) | 3 (10.0) | 7 (20.0) | 4 (11.1) | ||
| Daily | 11 (10.9) | 1 (3.3) | 2 (5.7) | 8 (22.2) | ||
| Weekends | 6 (5.9) | 3 (10.0) | 1 (2.9) | 2 (5.6) | ||
| Recreational drug use | 0.626 | |||||
| Current | 29 (28.7) | 8 (26.7) | 13 (37.1) | 8 (22.2) | ||
| Past | 30 (29.7) | 10 (33.3) | 10 (28.6) | 10 (27.8) | ||
| Never | 42 (41.6) | 12 (40.0) | 12 (34.3) | 18 (50.0) | ||
| Average daily fiber (g) (IQR) | 18 [11--25] | 17 [7--28] | 16 [11--19] | 20 [15--26] | 0.082 | |
| Average daily fiber ≥20 g (IQR) | 40 (39.6) | 13 (43.3) | 8 (22.9) | 19 (52.8) | 0.032 |
IDU, injection drug use; IQR, interquartile range.
The results of the HAS summary score are shown in Fig. 1 and Table 2. The median (IQR) HAS was 12 (10--13) with 39 (39%) achieving a score greater than 12 (considered by the Rotterdam study as healthy aging). Overall 32% participants scored in the intermediate and 30% in the range considered to be poor health. There was not an overall 1.3-point difference in the HAS score by age category (P = 0.79) or sex (P = 0.76).
Fig. 1.
Distribution of the Healthy Aging Score in the HIV cohort.
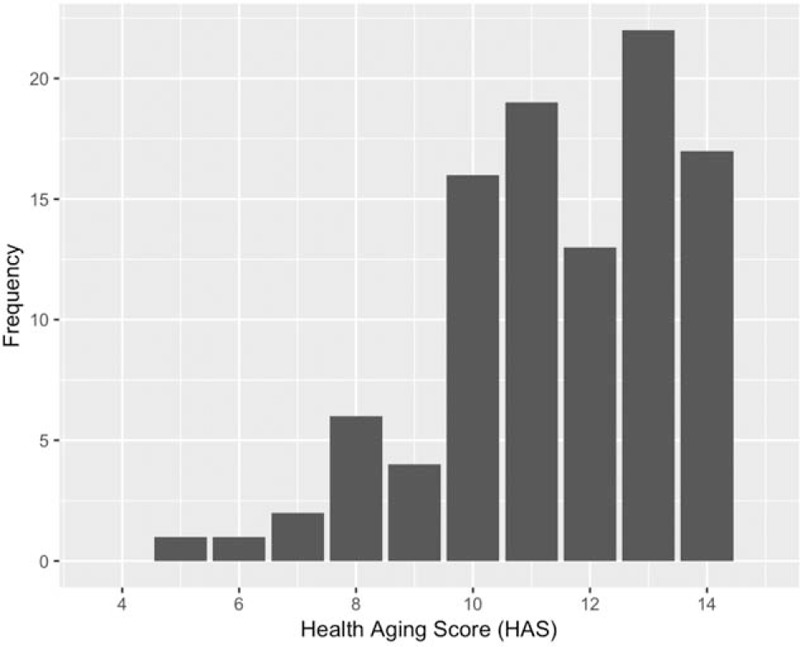
HAS, healthy aging score.
Table 2.
Health aging score and sub-categories by age category.
| Overall (n, %) | 40–50 years (n, %) | 51–60 years (n, %) | At least 61 years (n, %) | P | |
| N | 101 | 30 | 35 | 36 | |
| HAS [median (IQR)] | 12 [10--13] | 12 [11--13] | 11 [10--13] | 12 [10--13] | 0.791 |
| HAS [mean (SD)] | 11 (2) | 12 (2) | 11 (2) | 11 (2) | 0.611 |
| Overall HAS category | 0.907 | ||||
| Poor | 30 (29.7) | 7 (23.3) | 11 (31.4) | 12 (33.3) | |
| Intermediate | 32 (31.7) | 11 (36.7) | 11 (31.4) | 10 (27.8) | |
| Healthy | 39 (38.6) | 12 (40.0) | 13 (37.1) | 14 (38.9) | |
| Chronic disease | 0.107 | ||||
| Poor | 11 (10.9) | 1 (3.3) | 4 (11.4) | 6 (16.7) | |
| Intermediate | 21 (20.8) | 4 (13.3) | 6 (17.1) | 11 (30.6) | |
| Healthy | 69 (68.3) | 25 (83.3) | 25 (71.4) | 19 (52.8) | |
| Mental health | 0.040 | ||||
| Poor | 19 (18.8) | 8 (26.7) | 9 (25.7) | 2 (5.6) | |
| Intermediate | 22 (21.8) | 3 (10.0) | 9 (25.7) | 10 (27.8) | |
| Healthy | 60 (59.4) | 19 (63.3) | 17 (48.6) | 24 (66.7) | |
| Cognitive function | 0.371 | ||||
| Poor | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Intermediate | 5 (5.0) | 0 (0.0) | 2 (5.7) | 3 (8.3) | |
| Healthy | 96 (95.0) | 30 (100.0) | 33 (94.3) | 33 (91.7) | |
| Physical function | 0.703 | ||||
| Poor | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Intermediate | 10 (9.9) | 2 (6.7) | 3 (8.6) | 5 (13.9) | |
| Healthy | 91 (90.1) | 28 (93.3) | 32 (91.4) | 31 (86.1) | |
| Pain | 0.968 | ||||
| Poor | 4 (4.0) | 1 (3.3) | 2 (5.7) | 1 (2.8) | |
| Intermediate | 26 (25.7) | 8 (26.7) | 8 (22.9) | 10 (27.8) | |
| Healthy | 71 (70.3) | 21 (70.0) | 25 (71.4) | 25 (69.4) | |
| Social well being | 0.702 | ||||
| Poor | 18 (17.8) | 6 (20.0) | 6 (17.1) | 6 (16.7) | |
| Intermediate | 19 (18.8) | 3 (10.0) | 8 (22.9) | 8 (22.2) | |
| Healthy | 64 (63.4) | 21 (70.0) | 21 (60.0) | 22 (61.1) | |
| Quality of life | 0.803 | ||||
| Poor | 6 (5.9) | 2 (6.7) | 3 (8.6) | 1 (2.8) | |
| Intermediate | 37 (36.6) | 12 (40.0) | 13 (37.1) | 12 (33.3) | |
| Healthy | 58 (57.4) | 16 (53.3) | 19 (54.3) | 23 (63.9) |
HAS, healthy aging score.
The distribution of the HAS domain sub-scores by age category is demonstrated in Table 2. Younger participants were more likely to have low mental health scores (27% for age 40–50 years and 26% for age 51–60 years) compared with those at least 61 years (6%, P = 0.04). Women were more likely to have low-to-moderate scores on the pain sub-scale (corresponding to greater amounts of pain) (45%) compared with men (26%, P = 0.02), data not shown. Other HAS domains did not differ by age or sex (data not shown).
Table 3 shows the outcome of the Fried Frailty Score, stages, and individual components by HAS category. Overall, 10.4% are classified as frail, 79% as prefrail and 10.4% as not frail. A lower HAS score was associated with increased frailty (P = 0.008). This was most strongly correlated with the sub-component of the frailty index that related to exhaustion. There was also a positive correlation between the HAS and grip strength (P = 0.03).
Table 3.
Comparison of the Fried Frailty Score and Components by healthy aging score status.
| Missing (%) | Overall (n, %) | Healthy (n, %) | Intermediate (n, %) | Poor (n, %) | P | |
| N | 101 | 39 | 32 | 30 | ||
| Fried Frailty Score | 6.9 | 0.008 | ||||
| 0 | 10 (10.6) | 6 (16.2) | 1 (3.7) | 3 (10.0) | ||
| 1 | 48 (51.1) | 24 (64.9) | 16 (59.3) | 8 (26.7) | ||
| 2 | 26 (27.7) | 6 (16.2) | 8 (29.6) | 12 (40.0) | ||
| 3 | 9 (9.6) | 1 (2.7) | 2 (7.4) | 6 (20.0) | ||
| 4 | 1 (1.1) | 0 (0.0) | 0 (0.0) | 1 (3.3) | ||
| Frailty stage | 5 | 0.036 | ||||
| Not frail | 10 (10.4) | 6 (16.2) | 1 (3.4) | 3 (10.0) | ||
| Prefrail | 76 (79.2) | 30 (81.1) | 26 (89.7) | 20 (66.7) | ||
| Frail | 10 (10.4) | 1 (2.7) | 2 (6.9) | 7 (23.3) | ||
| ≥10 lbs of unintentional weight loss in the previous year | 6.9 | 7 (7.4) | 2 (5.4) | 3 (11.1) | 2 (6.7) | 0.708 |
| Everything was an effort or could not get going most or a moderate amount of time in the previous year | 0 | 40 (39.6) | 6 (15.4) | 15 (46.9) | 19 (63.3) | <0.001 |
| 30 min of activity one to three times in the past 2 weeks (lowest option) | 0 | 15 (14.9) | 3 (7.7) | 5 (15.6) | 7 (23.3) | 0.187 |
| Less than 0.8 m/s speed on faster of two 4 m walks | 0 | 60 (59.4) | 25 (64.1) | 20 (62.5) | 15 (50.0) | 0.492 |
| Less than 20 kg for women or less than 30 kg for men on best of three grip strength tests | 0 | 21 (20.8) | 7 (17.9) | 3 (9.4) | 11 (36.7) | 0.033 |
Table 4 shows the relationship between the overall SPPB score and the SPPB component sub-scores with the HAS categories. In this case, there was a trend towards poorer performance and with lower HAS scores (P = 0.077).
Table 4.
Comparison of Short Performance Physical Battery Score and subcomponents by healthy aging score status.
| Missing (%) | Overall (n, %) | Healthy (n, %) | Intermediate (n,%) | Poor (n, %) | P | |
| N | 101 | 39 | 32 | 30 | ||
| SPPB score [median, IQR] | 2 | 10 [9--11] | 10.5 [9.25--11.75] | 10 [10--11] | 10 [9-- 10] | 0.077 |
| Balance score | 2 | 0.521 | ||||
| 1 | 2 (2.0) | 0 (0.0) | 0 (0.0) | 2 (6.9) | ||
| 2 | 4 (4.0) | 1 (2.6) | 2 (6.2) | 1 (3.4) | ||
| 3 | 4 (4.0) | 1 (2.6) | 1 (3.1) | 2 (6.9) | ||
| 4 | 89 (89.9) | 36 (94.7) | 29 (90.6) | 24 (82.8) | ||
| Seconds to walk 4 m [median, IQR] | 0 | 3.00 [2.62--3.73] | 2.96 [2.56--3.56] | 2.90 [2.70--3.60] | 3.23 [2.62--4.07] | 0.397 |
| Gait score | 0 | 0.234 | ||||
| 1 | 1 (1.0) | 0 (0.0) | 0 (0.0) | 1 (3.3) | ||
| 2 | 1 (1.0) | 0 (0.0) | 0 (0.0) | 1 (3.3) | ||
| 3 | 5 (5.0) | 1 (2.6) | 1 (3.1) | 3 (10.0) | ||
| 4 | 94 (93.1) | 38 (97.4) | 31 (96.9) | 25 (83.3) | ||
| Seconds for five chair stands [median, IQR] | 2 | 14.13 [12.00--16.06] | 13.46 [11.20--16.50] | 14.02 [12.17--15.20] | 15.15 [12.58--16.91] | 0.264 |
| Chair stand score | 0 | 0.113 | ||||
| 0 | 2 (2.0) | 0 (0.0) | 0 (0.0) | 2 (6.7) | ||
| 1 | 22 (21.8) | 10 (25.6) | 4 (12.5) | 8 (26.7) | ||
| 2 | 32 (31.7) | 8 (20.5) | 14 (43.8) | 10 (33.3) | ||
| 3 | 28 (27.7) | 11 (28.2) | 11 (34.4) | 6 (20.0) | ||
| 4 | 17 (16.8) | 10 (25.6) | 3 (9.4) | 4 (13.3) |
IQR, interquartile range.
We also explored the relationship between measures of health utilization and the HAS. Of the 101 participants, seven had been hospitalized in the preceding 6 months. This included two of 39 (5.1%) participants who scored in the healthy range of the HAS, and five of 30 (16.7%) who scored in the poor range of the HAS, P = 0.034. The length of hospitals stay was a median of 2 days (IQR 2--4) and did not vary by HAS score, P = 0.32. Overall 24 (23.8%) participants had an emergency room visit in the preceding 6 months, the number did not vary by HAS score, P = 0.915 (data not shown), nor did the number of emergency room visits during this period vary by HAS score. Overall only three (3%) of participants had received help for activities of daily living at home during the previous 6 months (data not shown).
Discussion
This is the first study to evaluate the HAS in a cohort of HIV-infected individuals. In our pilot study of aging PLWH, the outcome of the score ranged from 5 to 14 with a median score of 12 [IQR (interquartile range) 10--13). The median score did not vary by age category or sex. According to the criteria developed by the Rotterdam group, only 39% of our participants scored in the healthy range of the score.
The WHO Global Strategy and Action Plan on Aging and Health has emphasized the need for standardizing measurements of healthy aging to use in care and research to enable comparisons across populations and disciplines [21]. Although there is currently no consensus or gold standard, researchers are attempting to develop and validate indexes to operationalize healthy aging in different disease settings [20,25–29]. The indexes are broadly similar, capturing multiple dimensions of health status within the domains of the WHO framework. It remains unclear, which health dimensions should best be captured in a global score [30–32]. It is likely that different domains of health have more impact in different disease cohorts [18].
After the review of existing scores, we chose to study the HAS score in our HIV population for the following reasons. The HAS was developed in a cohort of 3527 older Dutch participants [23]. We felt it ideal to study as the domains included in the score enabled an evaluation of health as a multidimensional state consistent with the framework established by the WHO. When developing the Rotterdam model, a number of socioeconomic and health behavioral factors that are relevant to those living with HIV were considered as covariates. Other strengths of this score included the demonstration of a mean decrease in score with age; sex differences in domain subscores, and correlations between scores of certain domains with each other. The score can be assessed as a mean score, or scores can be compared in the separate domains. Limitations of this score included: the three-part ordinal outcome, equal weighting of each dimension in the total score when some may have greater impact on healthy aging, and the inclusion of participant comorbidities without considering their severity.
In the Rotterdam study [23], the mean (SD) of the HAS was 11.2 (2.2) in men and 10.7 (2.30) in women and the score outcomes ranged across the entire score continuum. In the Rotterdam cohort, men had poorer scores in the chronic diseases domain than women. Women had poorer mental health, worse physical function, more pain, and lower quality of life compared with men. The Rotterdam study was also able to validate the score to mortality data. The age-adjusted hazard ratio per unit increase in the HAS with mortality was 0.86 (0.83–0.89) in men and 0.89 (0.87–0.91) in women.
The population studied in the Rotterdam cohort [23] has significant differences to that of our HIV cohort with 39.8% men, and a mean (SD) age of 75.3 (6.0) years. The majority were of Caucasian decent (>97%). Potential limitations of applying this score to an HIV population include the chronic diseases included in the domains. Many chronic diseases in the HAS are similar to those that aging PLWH are experiencing (cardiovascular disease, stroke, diabetes, chronic obstructive lung disease, chronic renal failure, and cancer) [33]; however, Parkinson's disease was included but not seen in our clinic population and liver disease commonly seen in HIV was not included [34]. There is some evidence that aging is ‘accelerated’ or ‘accentuated’ in HIV because of the residual immune inflammation and activation that persist despite control of HIV replication, which may affect the development of and age of presentation of comorbidities [35–37]. Whether or not this would be reflected in the score is uncertain. Further, other factors that can affect health in PLWH were not captured in the score, such as stigma, trauma, and discrimination [38] as only a depression scale was included in the mental health domain. Additionally, the underlying demographics of PLWH vary [39] and social determinants of health, such as income, food, and housing could affect quality of life and healthy aging [40–42]. Finally, the HIV cohort consists of many individuals who assume nontraditional sex roles and may have different caretaking and advocacy roles than aging heterosexual men and women, which could impact the domain of the score-related to activities of daily living. Despite these limitations, the mean and range of scores was similar in our cohort to the original Rotterdam study suggesting that it will be useful in this population.
Multiple approaches have been used to operationalize health in the general aging population but the best tools to use in the clinical and research setting remain controversial [26,27,29,42–44]. It will be difficult to come up with a simple tool that can dichotomize aging as successful or not [20]. Despite the challenges, a global healthy aging score would be useful. An ideal score would be valid for the population under study, be relatively easy and inexpensive to use, and be responsive to detecting changes in health over time. A standardized score could support clinical decision-making around a person's strengths and quality of life, the need for investigation or intensity of follow-up, and monitoring health trajectories over time or with specific therapies. A score could help researchers identify areas for directed interventions to maintain or improve health and provide an outcome to monitor response. Population level assessments could provide policy makers with comprehensive data to inform the allocation of funds and services to keep persons healthy.
Other groups have evaluated the Fried Frailty phenotype and the SPPB in the context of HIV [45–50]. The limitations of using these as indicators of health is that they fail to take into consideration other domains of health, such as cognitive function, mental health, and social well being. We were able to demonstrate correlation of the HAS scores with these indices especially with respect to the subscales addressing physical function adding to the validity of the HAS.
In attempts to improve on the frailty phenotype in the context of HIV, Brothers et al.[51] used data from 963 participants in the Modena HIV Metabolic Clinic Cohort study to develop a frailty index. In this cohort, the baseline frailty index was an independent predictor of mortality [odds ratio [OR] 1.19, 95% confidence interval (CI) 1.02–1.38]. Despite validity demonstrated by this correlation, this index included 31 clinical, laboratory, and imaging variables making it impractical and expensive for use as an end point in care and research.
In order to be a useful endpoint in interventional studies, a measure of healthy aging needs to be predictive of clinically relevant endpoints, such as disease progression, hospitalization, and mortality. The original Rotterdam cohort was able to demonstrate an association between the HAS score and mortality. In our cohort, we were able to demonstrate a significant correlation between poor scores on the HAS and hospitalization in the preceding 6 months. However, as the numbers were small, this observation needs to be confirmed with a larger population.
This is the first step to assess a validated healthy aging score in an HIV-infected population. The strengths of our study are that we were able to demonstrate within a small cohort that individuals scored across the range of the score values without a significant floor or ceiling effect. We were able to correlate the score with other indicators of primarily physical health, such as the Fried Frailty phenotype and the SPPB score. We were also able to demonstrate a correlation between health and hospitalization, although this conclusion is limited by small numbers. Limitations include our small sample size and the small percentage of women. Further study is warranted in a larger HIV aging population and to validate it against clinical outcomes, such as disease progression, hospitalization, and mortality. Furthermore, as our study was cross-sectional, more work is required to determine if the score is responsive to change.
In conclusion, in this pilot study of 101 older PLWH, we demonstrated a range of healthy aging scores using an index that has been validated in a population of older adults in the Netherlands [23]. The score correlated with other indexes that measure health and with recent hospitalization. Only 39% of participants scored in the ‘healthy’ range despite viral suppression and good immunologic response to ART suggesting that other domains are important in evaluating health in this population. As the HIV population continues to age, we will need measures to assess outcomes and test interventions to maintain or optimize health [52]. This will enable us to determine whether or not we can achieve the proposed fourth 90 of the UN AIDS goals for HIV that 90% of those with HIV have good health and quality of life [12]. The data from our pilot study suggest we have work to do to reach this goal.
Acknowledgements
S.W. conceived the study, enrolled participants, drafted and finalized the manuscript; M.R., C.S. and R.C. completed participant assessments and reviewed the initial and final manuscripts; L.S. performed the statistical analysis and reviewed the initial and final manuscripts.
We acknowledge the support of the research nurses Adrianna D’Aquila, Bryan Boyachuk and Warmond Chan for completion of participant assessments and Chantale Sheehan for administrative support.
Disclaimers: S.W. receives support from the Ontario HIV Treatment Network. S.W. conducts clinical trials, serves on advisory boards and speaks at CME events for Viiv, Merck and Gilead. For the remaining authors, no conflict of interest is declared.
This work was supported through an investigator-sponsored research grant from Gilead, Sciences, Inc.
Conflicts of interest
There are no conflicts of interest.
This work was presented in part at the 21st International workshop on comorbidities and adverse drug reactions in HIV (abstract P03) and at the European AIDS Conference (abstract PE 9/5) in Basel, Switzerland in November 2019.
References
- 1.Negredo E, Back D, Blanco JR, Blanco J, Erlandson KM, Garolera M, et al. Aging in HIV-infected subjects: a new scenario and a new view. Biomed Res Int 2017; 2017:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guaraldi G. The transition from co-morbidities to geriatric syndromes in HIV. Germs 2016; 6:79–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guaraldi G, Palella FJ., Jr Clinical implications of aging with HIV infection: perspectives and the future medical care agenda. AIDS 2017; 31: Suppl 2: S129–S135. [DOI] [PubMed] [Google Scholar]
- 4.Vance DE, Cody SL. Predictions of geriatric HIV in 2030. Lancet Infect Dis 2015; 15:753–754. [DOI] [PubMed] [Google Scholar]
- 5.Marcus JL, Chao CR, Leyden WA, Xu L, Quesenberry CP, Jr, Klein DB, et al. Narrowing the gap in life expectancy between HIV-infected and HIV-uninfected individuals with access to care. J Acquir Immune Defic Syndr 2016; 73:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antiretroviral Therapy Cohort C Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. Lancet HIV 2017; 4:e349–e356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deeks SG, Phillips AN. HIV infection, antiretroviral treatment, ageing, and non-AIDS related morbidity. BMJ 2009; 338:a3172. [DOI] [PubMed] [Google Scholar]
- 8.Guaraldi G, Orlando G, Zona S, Menozzi M, Carli F, Garlassi E, et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis 2011; 53:1120–1126. [DOI] [PubMed] [Google Scholar]
- 9.Solomon P, O’Brien K, Wilkins S, Gervais N. Aging with HIV and disability: the role of uncertainty. AIDS Care 2014; 26:240–245. [DOI] [PubMed] [Google Scholar]
- 10.Solomon P, O’Brien KK, Nixon S, Letts L, Baxter L, Gervais N. Trajectories of episodic disability in people aging with HIV: a longitudinal qualitative study. J Int Assoc Provid AIDS Care 2018; 17:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. UNAIDS. 90-90-90: an ambitious treatment target to help end the AIDS epidemic. 2017. pp. 1--40. Available at: www.unaids.org. [Google Scholar]
- 12.Lazarus JV, Safreed-Harmon K, Barton SE, Costagliola D, Dedes N, Del Amo Valero J, et al. Beyond viral suppression of HIV - the new quality of life frontier. BMC Med 2016; 14:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szadkowski L, Tseng A, Walmsley SL, Salit I, Raboud JM. Short communication: effects of age on virologic suppression and CD4 cell response in HIV-positive patients initiating combination antiretroviral therapy. AIDS Res Hum Retroviruses 2012; 28:1579–1583. [DOI] [PubMed] [Google Scholar]
- 14.Gazzola L, Tincati C, Bellistri GM, Monforte A, Marchetti G. The absence of CD4+ T cell count recovery despite receipt of virologically suppressive highly active antiretroviral therapy: clinical risk, immunological gaps, and therapeutic options. Clin Infect Dis 2009; 48:328–337. [DOI] [PubMed] [Google Scholar]
- 15.Guaraldi G, Brothers TD, Zona S, Stentarelli C, Carli F, Malagoli A, et al. A frailty index predicts survival and incident multimorbidity independent of markers of HIV disease severity. AIDS 2015; 29:1633–1641. [DOI] [PubMed] [Google Scholar]
- 16.Justice AC, Freiberg MS, Tracy R, Kuller L, Tate JP, Goetz MB, et al. VACS Project Team Does an index composed of clinical data reflect effects of inflammation, coagulation, and monocyte activation on mortality among those aging with HIV?. Clin Infect Dis 2012; 54:984–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calcagno A, Piconi S, Foca E, Nozza S, Carli F, Montrucchio C, et al. GEPPO (GEriatric Patients living with HIV/AIDS: a Prospective Multidimensional cOhort) Study Group Role of normalized T-cell subsets in predicting comorbidities in a large cohort of geriatric HIV-infected patients. J Acquir Immune Defic Syndr 2017; 76:338–342. [DOI] [PubMed] [Google Scholar]
- 18.Guaraldi G, Milic J. The interplay between frailty and intrinsic capacity in aging and HIV infection. AIDS Res Hum Retroviruses 2019; 35:1013–1022. [DOI] [PubMed] [Google Scholar]
- 19.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994; 49:M85–M94. [DOI] [PubMed] [Google Scholar]
- 20.Depp CA, Jeste DV. Definitions and predictors of successful aging: a comprehensive review of larger quantitative studies. Am J Geriatr Psychiatry 2006; 14:6–20. [DOI] [PubMed] [Google Scholar]
- 21. Chatterji S, L Ustün B, Sadana R, Salomon J, D Mathers C, Jl Murray C. The conceptual basis for measuring and reporting health. In: Global programme on evidence for health policy discussion paper No 45. Geneva, Switzerland: World Health Organization; 2002. pp. 1--20. [Google Scholar]
- 22.Beard JR, Officer A, de Carvalho IA, Sadana R, Pot AM, Michel JP, et al. The World report on ageing and health: a policy framework for healthy ageing. Lancet 2016; 387:2145–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaspers L, Schoufour JD, Erler NS, Darweesh SK, Portegies ML, Sedaghat S, et al. Development of a healthy aging score in the population-based Rotterdam study: evaluating age and sex differences. J Am Med Dir Assoc 2017; 18:276.e1–276.e7. [DOI] [PubMed] [Google Scholar]
- 24.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–156. [DOI] [PubMed] [Google Scholar]
- 25.Fuchs J, Scheidt-Nave C, Hinrichs T, Mergenthaler A, Stein J, Riedel-Heller SG, Grill E. Indicators for healthy ageing--a debate. Int J Environ Res Public Health 2013; 10:6630–6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lara J, Godfrey A, Evans E, Heaven B, Brown LJ, Barron E, et al. Towards measurement of the Healthy Ageing Phenotype in lifestyle-based intervention studies. Maturitas 2013; 76:189–199. [DOI] [PubMed] [Google Scholar]
- 27.Michel JP, Sadana R. Healthy aging’ concepts and measures. J Am Med Dir Assoc 2017; 18:460–464. [DOI] [PubMed] [Google Scholar]
- 28.Moore RC, Moore DJ, Thompson WK, Vahia IV, Grant I, Jeste DV. A case-controlled study of successful aging in older HIV-infected adults. J Clin Psychiatry 2013; 74:e417–e423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peel N, Bartlett H, McClure R. Healthy ageing: how is it defined and measured?. Australasian J Ageing 2004; 23:115–119. [Google Scholar]
- 30.Bousquet J, Bewick M, Cano A, Eklund P, Fico G, Goswami N, et al. Building bridges for innovation in ageing: synergies between action groups of the EIP on AHA. J Nutr Health Aging 2017; 21:92–104. [DOI] [PubMed] [Google Scholar]
- 31.Bousquet J, Kuh D, Bewick M, Standberg T, Farrell J, Pengelly R, et al. Operational definition of Active and Healthy Ageing (AHA): a conceptual framework. J Nutr Health Aging 2015; 19:955–960. [DOI] [PubMed] [Google Scholar]
- 32.Kim SH, Park S. A meta-analysis of the correlates of successful aging in older adults. Res Aging 2017; 39:657–677. [DOI] [PubMed] [Google Scholar]
- 33.Rasmussen LD, May MT, Kronborg G, Larsen CS, Pedersen C, Gerstoft J, Obel N. Time trends for risk of severe age-related diseases in individuals with and without HIV infection in Denmark: a nationwide population-based cohort study. Lancet HIV 2015; 2:e288–e298. [DOI] [PubMed] [Google Scholar]
- 34.Onen NF, Overton ET, Seyfried W, Stumm ER, Snell M, Mondy K, Tebas P. Aging and HIV infection: a comparison between older HIV-infected persons and the general population. HIV Clin Trials 2010; 11:100–109. [DOI] [PubMed] [Google Scholar]
- 35.Appay V, Almeida JR, Sauce D, Autran B, Papagno L. Accelerated immune senescence and HIV-1 infection. Exp Gerontol 2007; 42:432–437. [DOI] [PubMed] [Google Scholar]
- 36.Capeau J. Premature aging and premature age-related comorbidities in HIV-infected patients: facts and hypotheses. Clin Infect Dis 2011; 53:1127–1129. [DOI] [PubMed] [Google Scholar]
- 37.Deeks SG, Tracy R, Douek DC. Systemic effects of inflammation on health during chronic HIV infection. Immunity 2013; 39:633–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Emlet CA, Brennan DJ, Brennenstuhl S, Rueda S, Hart TA, Rourke SB. The impact of HIV-related stigma on older and younger adults living with HIV disease: does age matter?. AIDS Care 2015; 27:520–528. [DOI] [PubMed] [Google Scholar]
- 39.Brennan DJ, Emlet CA, Brennenstuhl S, Rueda S. Socio-demographic profile of older adults with HIV/AIDS: gender and sexual orientation differences. Can J Aging 2013; 32:31–43. [DOI] [PubMed] [Google Scholar]
- 40.Brown TH, Hargrove TW. Multidimensional approaches to examining gender and racial/ethnic stratification in health. Women, Gender, and Families of Color 2013; 1:180–206. [Google Scholar]
- 41.Hankivsky O. Women's health, men's health, and gender and health: implications of intersectionality. Soc Sci Med 2012; 74:1712–1720. [DOI] [PubMed] [Google Scholar]
- 42.Swindell WR, Ensrud KE, Cawthon PM, Cauley JA, Cummings SR, Miller RA, et al. Indicators of ‘healthy aging’ in older women (65-69 years of age). A data-mining approach based on prediction of long-term survival. BMC Geriatr 2010; 10:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rowe JW, Kahn RL. Successful aging. Gerontologist 1997; 37:433–440. [DOI] [PubMed] [Google Scholar]
- 44.Cosco TD, Prina AM, Perales J, Stephan BC, Brayne C. Operational definitions of successful aging: a systematic review. Int Psychogeriatr 2014; 26:373–381. [DOI] [PubMed] [Google Scholar]
- 45.Crane HM, Miller ME, Pierce J, Willig AL, Case ML, Wilkin AM, et al. Physical functioning among patients aging with human immunodeficiency virus (HIV) versus HIV uninfected: feasibility of using the short physical performance battery in clinical care of people living with HIV aged 50 or older. Open Forum Infect Dis 2019; 6:ofz038.(031-038). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Branas F, Jimenez Z, Sanchez-Conde M, Dronda F, Lopez-Bernaldo De Quiros JC, Perez-Elias MJ, et al. Frailty and physical function in older HIV-infected adults. Age Ageing 2017; 46:522–526. [DOI] [PubMed] [Google Scholar]
- 47.Wallace LM, Ferrara M, Brothers TD, Garlassi S, Kirkland SA, Theou O, et al. Lower frailty is associated with successful cognitive aging among older adults with HIV. AIDS Res Hum Retroviruses 2017; 33:157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tassiopoulos K, Abdo M, Wu K, Koletar SL, Palella FJ, Jr, Kalayjian R, et al. Frailty is strongly associated with increased risk of recurrent falls among older HIV-infected adults. Aids 2017; 31:2287–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Margolick JB, Bream JH, Martinez-Maza O, Lopez J, Li X, Phair JP, et al. Frailty and circulating markers of inflammation in HIV+ and HIV- men in the Multicenter AIDS Cohort Study. J Acquir Immune Defic Syndr 2017; 74:407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brothers TD, Kirkland S, Guaraldi G, Falutz J, Theou O, Johnston BL, et al. Frailty in people aging with human immunodeficiency virus (HIV) infection. J Infect Dis 2014; 210:1170–1179. [DOI] [PubMed] [Google Scholar]
- 51.Brothers TD, Kirkland S, Theou O, Zona S, Malagoli A, Wallace LMK, et al. Predictors of transitions in frailty severity and mortality among people aging with HIV. PLoS One 2017; 12:e0185352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Althoff KN, Smit M, Reiss P, Justice AC. HIV and ageing: improving quantity and quality of life. Curr Opin HIV AIDS 2016; 11:527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]


