Abstract
Objective:
Cannabidiol (CBD) has been suggested as a potential antihypertensive drug. The aim of our study was to investigate its vasodilatory effect in isolated human pulmonary arteries (hPAs) and rat small mesenteric arteries (sMAs).
Methods:
Vascular effects of CBD were examined in hPAs obtained from patients during resection of lung carcinoma and sMAs isolated from spontaneously hypertensive (SHR); 11-deoxycorticosterone acetate (DOCA-salt) hypertensive rats or their appropriate normotensive controls using organ bath and wire myography, respectively.
Results:
CBD induced almost full concentration-dependent vasorelaxation in hPAs and rat sMAs. In hPAs, it was insensitive to antagonists of CB1 (AM251) and CB2 (AM630) receptors but it was reduced by endothelium denudation, cyclooxygenase inhibitors (indomethacin and nimesulide), antagonists of prostanoid EP4 (L161982), IP (Cay10441), vanilloid TRPV1 (capsazepine) receptors and was less potent under KCl-induced tone and calcium-activated potassium channel (KCa) inhibitors (iberiotoxin, UCL1684 and TRAM-34) and in hypertensive, overweight and hypercholesteremic patients. The time-dependent effect of CBD was sensitive to the PPARγ receptor antagonist GW9662. In rats, the CBD potency was enhanced in DOCA-salt and attenuated in SHR. The CBD-induced relaxation was inhibited in SHR and DOCA-salt by AM251 and only in DOCA-salt by AM630 and endothelium denudation.
Conclusion:
The CBD-induced relaxation in hPAs that was reduced in hypertensive, obese and hypercholesteremic patients was endothelium-dependent and mediated via KCa and IP, EP4, TRPV1 receptors. The CBD effect in rats was CB1-sensitive and dependent on the hypertension model. Thus, modification of CBD-mediated responses in disease should be considered when CBD is used for therapeutic purposes.
Keywords: cannabidiol, cannabinoid CB1 receptor, endothelium, hypertension, mesenteric artery, prostacyclin IP receptor, prostanoid EP4 receptor, pulmonary artery, transient receptor potential vanilloid 1, vasorelaxation
INTRODUCTION
Cannabidiol (CBD) is a nonpsychotropic constituent of Cannabis sativa. It possesses tremendous therapeutic potential because of its anti-inflammatory, antioxidative, anticancer, neuroprotective, antinociceptive and many other beneficial properties [1–3]. CBD has already been approved by the US Food and Drug Administration (FDA) for the treatment of epilepsy [4], and internationally approved for the therapy of spasticity in multiple sclerosis, and has also received orphan designation for the treatment of neonatal hypoxia--ischaemic encephalopathy [5]. Moreover, it has been postulated to play a beneficial role in cardiovascular disorders including hypertension [1,6–10].
Indeed, acute and chronic CBD administration reduced increases in blood pressure (BP) induced by stress but not under control conditions [9,11]. Its potential antihypertensive role has been suggested based on its vasodilatory effects under in-vitro conditions in systemic vasculature. Furthermore, near-maximal and almost half-maximal relaxation has been demonstrated in rat [12] and human [5] small mesenteric arteries (sMAs), respectively. The vasodilatory effects of CBD in the human mesenteric artery were dependent on endothelium and potassium channels, the transient receptor potential vanilloid 1 (TRPV1) and the cannabinoid CB1[5] receptors, and in rat aorta on peroxisome proliferator-activated receptors (PPARs), calcium channel inhibition and superoxide dismutase [13]. Moreover, CBD augmented the endothelium-dependent vasodilatation in response to acetylcholine in mesenteric arteries of Zucker diabetic fatty rats both ex vivo and in vivo[14,15] via cannabinoid CB2 and prostanoid EP4 receptors, elevated superoxide dismutase (SOD) and cyclooxygenase (COX) activity [16]. Importantly, CBD decreased DBP in pithed (but not in anaesthetized) SHR and their normotensive control Wistar–Kyoto (WKY) animals confirming the involvement of the CBD-induced peripheral vasodilatation in decreasing BP [17]. In contrast to the increasing evidence of the protective and/or hypotensive potential of CBD in the systemic vasculature, there is no data concerning the pulmonary arterial bed although pulmonary administration is the most common route of recreational and experimental cannabis consumption in humans [18]. We have demonstrated previously that exocannabinoids and endocannabinoids caused full relaxation of both human and rat pulmonary arteries (hPAs, rPAs [19–21]) and they were suggested to represent a possible future option in the co-therapy of pulmonary hypertension [22].
Taking the above into consideration, the first aim of our study was to determine the vasodilatory effect of CBD in hPAs, the chosen mechanisms underlying this action and post hoc analysis of trends in the effects of patient characteristics or medication on CBD responses in order to determine CBD effects in pulmonary vasculature. Our second aim was to check effect of CBD on vascular function in resistance sMAs isolated from rats with primary (spontaneously hypertensive rats, SHR) and secondary 11-deoxycorticosterone acetate-salt (DOCA-salt) hypertension in order to determine the influence of hypertension in response to CBD and its dependence on the model of hypertension.
MATERIALS AND METHODS
All of the protocols were approved by the Animal and Human Ethics Committee of the Medical University of Białystok, Poland (no. R-I-002/445/2015; 52/2015) and were performed in accordance with the European Directive (2010/63/EU). Animal studies were carried out in compliance with the replacement, refinement or reduction (the 3Rs). Male rats were obtained from the Centre for Experimental Medicine of the Medical University of Białystok (Poland). The tissue donors provided written informed consent for the use of their blood vessels.
The nomenclature of the molecular targets conforms to ‘THE CONCISE GUIDE TO PHARMACOLOGY 2017/18: G protein-coupled receptors’ [23].
Human tissue studies
Human lung tissue was obtained from 32 patients (21 men and 11 women, with a mean age of 65.2 ± 1.9 years) undergoing a lobectomy or pneumonectomy during the resection of lung carcinoma with a surgical margin of healthy tissue. Preoperative echocardiography revealed normal left and right ventricular function. A summary of control patient characteristics is shown in Table 1. Prior to the operation, all of the patients received treatment with cephalosporin and low-molecular-weight heparin. Seventy-two hours before surgery, patients stopped taking pain relief. This allowed time to metabolize and eliminate them from the organism. The tissue from the surgical margin was transported to the laboratory within 30 min in cold (4 °C), pregassed Tyrode's bicarbonate solution (concentration in mmol/l: NaCl, 139.2; KCl, 2.7; CaCl2, 1.8; MgCl2, 0.49; NaHCO3, 11.9; NaH2PO4, 0.4; glucose, 5.5) at pH 7.4.
TABLE 1.
Patient characteristics and post hoc analysis of its effects on the vasorelaxant responses to the cannabidiol-induced control concentration--response curves in human pulmonary artery with intact endothelium
| Vasodilatory effects of CBD | ||||
| Characteristic | n | Range (mean ± SEM) | pEC50 | Rmax (%) |
| Ethnicity | 21 | Polish white | 5.0 ± 0.1 | 90.3 ± 3.7 |
| Male | 15 | 4.9 ± 0.1 | 91.2 ± 4.6 | |
| Female | 6 | 4.8 ± 0.1 | 86.5 ± 7.9 | |
| Age (years) | 21 | 51–81 (66.5 ± 1.4) | ||
| Younger (≤ 60) | 6 | 4.9 ± 0.2 | 91.9 ± 8.9 | |
| Older (>60) | 15 | 4.8 ± 0.1 | 86.9 ± 8.9 | |
| BMI (kg/m2) | 21 | 18.8–30.6 (24.6 ± 0.7) | ||
| Lean (≤26) | 14 | 5.0 ± 0.1 | 93.2 ± 4.8 | |
| Overweight (>26) | 7 | 4.4 ± 0.1** | 76.6 ± 5.0* | |
| Smokers | 15 | 4.9 ± 0.1 | 90.9 ± 3.9 | |
| No smokers | 6 | 4.8 ± 0.2 | 91.5 ± 7.3 | |
| SBP (mmHg) | 21 | 102–205 (135 ± 4) | ||
| DBP (mmHg) | 21 | 132–57 (81 ± 3) | ||
| Normotensive | 13 | 4.8 ± 0.1 | 93.6 ± 4.8 | |
| Hypertensive | 8 | 4.2 ± 0.3* | 93.1 ± 5.5* | |
| No COPD | 17 | 4.7 ± 0.1 | 94.2 ± 4.2 | |
| COPD | 4 | 4.9 ± 0.2 | 91.6 ± 9.0 | |
| No CAD | 17 | 5.0 ± 0.1 | 83.5 ± 10.7 | |
| CAD | 4 | 4.7 ± 0.1 | 87.2 ± 5.3 | |
| Nondiabetic | 18 | 4.8 ± 0.1 | 91.2 ± 3.8 | |
| Diabetic | 3 | 4.9 ± 0.1 | 95.5 ± 7.6 | |
| Normocholesteraemia | 13 | 5.0 ± 0.1 | 91.2 ± 3.8 | |
| Hypercholesteraemia | 8 | 4.4 ± 0.2** | 87.5 ± 8.6 | |
| No α1 adrenoceptor antagonists | 18 | 4.8 ± 0.1 | 88.0 ± 3.7 | |
| α1 adrenoceptor antagonists | 3 | 4.7 ± 0.2 | 102.6 ± 9.7 | |
| No β blockers | 13 | 5.1 ± 0.1 | 87.1 ± 5.4 | |
| β blockers | 8 | 4.9 ± 0.1 | 95.1 ± 3.1 | |
| No ACE inhibitors | 17 | 4.9 ± 0.1 | 87.1 ± 4.0 | |
| ACE inhibitors | 4 | 4.5 ± 0.1 | 103.1 ± 3.9 | |
| No NSAIDs | 12 | 4.9 ± 0.1 | 85.3 ± 4.9 | |
| NSAIDs | 9 | 4.7 ± 0.1 | 96.6 ± 4.7 | |
| No statins | 16 | 4.9 ± 0.1 | 87.7 ± 4.2 | |
| Statins | 5 | 4.6 ± 0.1 | 98.0 ± 6.1 | |
| No PPIs | 14 | 5.0 ± 0.1 | 86.4 ± 4.3 | |
| PPIs | 7 | 4.9 ± 0.1 | 99.5 ± 5.2 | |
| AT1 antagonists | 2 | N.D. | N.D. | |
| Calcium channel blockers | 2 | N.D. | N.D. | |
| Hypoglycaemic medication | 2 | N.D. | N.D. | |
ACE, angiotensin-converting enzyme; AT1, angiotensin receptor type 1; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; NSAIDs, nonsteroidal anti-inflammatory drugs; PPIs, proton pump inhibitors.
Values of pEC50 and Rmax for vasodilatory effects of CBD are based on the concentration--response curves and represent mean percentage relaxations of the isometric contraction induced by U46619 and, with the standard error of the mean (SEM) fit to nonlinear Regression (Curve Fit) (Prism Version 5; GraphPad Software, California, USA). n represents the number of patients.
*P < 0.05.
**P < 0.01; compared with the respective control group as determined by Student's t-test for unpaired data.
Animals
Male rats housed in plastic cages had free access to water and food pellets and they were maintained under a 12 h/12 h light/dark cycle. We used age-matched rats with comparable primary (SHR; the most frequently studied genetic hypertensive model) and secondary DOCA-salt hypertension, in order to distinguish changes induced by hypertension from those related to any one particular hypertension model. The DOCA-salt model applied as a salt-rich diet is one of the main lifestyle factors leading to hypertension.
DOCA-salt hypertension was induced in Wistar male rats (6–7 weeks old; weighing 260–300 g) as described previously [24]. Animals were anaesthetized by intraperitoneal (i.p.) injection of pentobarbital sodium (70 mg/kg). The right kidney was removed via a right lateral abdominal incision. After 1 week of recovery, hypertension was induced by subcutaneous injections of DOCA (25 mg/kg, 0.4 ml/kg) twice weekly for 4 weeks and replacement of drinking water with 1% NaCl solution. Control sham-operated rats (SHAM) were uninephrectomized and received the vehicle for DOCA (N, N-dimethylformamide) twice weekly and drank tap water.
The following four experimental groups were created: hypertensive DOCA-salt (4 weeks after the onset of DOCA-salt administration); 12–14-week-old male SHR (250–320 g) and their respective normotensive controls: SHAM (4 weeks after the onset of vehicle for DOCA-salt administration,) and Wistar–Kyoto rats (WKY; 290–370 g). Rats were assigned randomly to the different experimental groups. The SBP was recorded in conscious rats by the noninvasive tail-cuff method using the Rat Tail Blood Pressure Monitor from Hugo Sachs Elektronik-Harvard Apparatus (March–Hugstetten, Germany). SBPs (in mmHg) were higher in SHR (190 ± 6, n = 11, P < 0.001) and DOCA-salt animals (206 ± 8, n = 11, P < 0.001) than in their respective controls (WKY 94 ± 3, n = 12, SHAM 125 ± 7, n = 12).
The mesenteric arterial bed was removed from rats anaesthetized with pentobarbitone sodium (70 mg/kg; intraperitoneally) and placed into a cold Krebs--Henseleit solution with the following composition (in mmol/l): NaCl 118; KCl 4,8; CaCl2 2.5; MgSO4 1.2; NaHCO3 24; KH2PO4 1.2; glucose 11; EDTA 0.03 at pH 7.4.
Vessel preparation
The isolation of lobar and segmental hPA branches and sMAs have been described previously [19,25]. The lobar and segmental hPA branches were cleaned from the lung parenchyma and cut into rings (from the middle portion of each artery; 3–5 mm length and 2–4 mm outer diameter). They were suspended on stainless steel wires in 10 ml organ baths containing Tyrode's solution and muscle tensions were recorded using a force-displacement transducer (PIM 100RE; BIO-SYS-TECH, Białystok, Poland) and displayed on a computer.
From the rat mesenteric arterial bed, 2 mm long segments of the third-order of the superior artery were dissected from surrounding adipose tissue. Isolated sMAs (∼250 μm internal diameter) were mounted in a Mulvany--Halpern type wire myograph (Multi Wire Myograph System DMT 620M; Danish Myo Technology, Aarhus, Denmark) filled with 5 ml Krebs--Henseleit solution. Muscle tension was recorded by the software LabChart 7.3.7 Pro (ADInstruments, Hastings, UK).
The hPAs and sMAs were allowed to equilibrate for 90 and 45 min (resting tension ∼20–25 mN; ∼2.5 mN, respectively) and were gassed continuously with 95% O2 and 5% CO2 at 37 °C, pH 7.4. After the equilibration period, all rings were exposed to high KCl (60 mmol/l [19] and 120 mmol/l [25], respectively) to establish tissue viability. Then, the integrity of the vessel endothelium was checked for sub-maximal preconstriction of hPAs and sMAs with (−)-phenylephrine (10 μmol/l) followed by the induction of at least 80% relaxation in response to acetylcholine (1 and 10 μmol/l, respectively). Before certain experiments, the endothelium was removed by gently rubbing off the intima. A successful endothelial denudation was confirmed by the absence of acetylcholine-induced vasorelaxation.
Functional studies
In each individual preparation, only one concentration--response curve (CRC) was determined. All experiments were performed in paired vessels, that is, the effect of a drug or of endothelium denudation was studied in one vessel and another vessel from the same patient or animal, which served as a control.
Vasorelaxation study
Human pulmonary arteries and rat sMAs and were submaximally constricted with U46619 (0.01 μmol/l; a prostanoid TP receptor agonist) and (–)-phenylephrine (10 μmol/l), respectively; concentrations approximately equivalent to their EC60. After a stable level of tone was maintained, CRCs were generated by the cumulative application of CBD or its vehicle. To determine the mechanisms of the vasorelaxant effects of CBD, tissues were pretreated for 30 min with either the following antagonists or inhibitors (the respective concentrations are given in parentheses): AM251 (1 μmol/l; cannabinoid CB1 receptor antagonist); AM630 (1 μmol/l; cannabinoid CB2 receptor antagonist); O-1918 (10 μmol/l; antagonist of endothelium-dependent relaxation to cannabinoids; see Discussion); capsazepine (1 μmol/l; vanilloid TRPV1 receptor antagonist), Cay10441 (10 μmol/l; prostanoid IP receptor antagonist) [19]; RN1734 (20 μM; vanilloid TRPV4 receptor antagonist) [26]; L161982 (1 μmol/l; prostanoid EP4 receptor antagonist); nimesulide (10 μmol/l; cyclooxygenase COX-2 inhibitor); sodium diethyldithiocarbamate trihydrate (DETCA; 300 μmol/l; SOD inhibitor) [16]; indomethacin (INDO; 10 μmol/l; cyclooxygenase COX-1/COX-2 inhibitor); Nω-nitro- l-arginine methyl ester (l-NAME; 300 μmol/l, nitric oxide synthase inhibitor); and UCL1684 (0.1 μmol/l), TRAM-34 (10 μmol/l), and iberiotoxin (0.1 μmol/l), which are small (KCa2.3), intermediate (KCa3.1) and large (KCa1.1) conductance Ca2+- activated K+ channel blockers (KCa), respectively [27]. To assess the contribution of PPARγ activation, experiments were performed in the presence of the PPARγ antagonist GW9662 (1 and 10 μmol/l) [5,28] added 10 min prior to precontraction. Following this, only one concentration of CBD (10 μmol/l) was administered in order to examine its time-dependent effect.
In control tissues of both series, the effects of the appropriate volume of the respective vehicle of inhibitors or antagonists, ethanol (for CBD, O-1918, GW9662, nimesulide, Cay10441, RN1734), or dimethyl sulfoxide (DMSO; for TRAM-34), on the relaxation to CBD were also tested when the final bath concentration was equal to or exceeded 0.1% (v/v).
U46619 (0.01 μmol/l) and KCl (60 mmol/l) induced comparable increases in tone of the endothelium-intact and endothelium-denuded hPAs (11.5 ± 0.1 mN, n = 105, 9.5 ± 0.2 mN, n = 6 and 10.8 ± 0.2 mN, n = 15, respectively). A 30 min incubation with CBD 10 μmol/l, the antagonists, inhibitors or vehicles did not modify the U46619-induced contractions.
The mean tensions generated (in mN) by phenylephrine (10 μmol/l; a concentration approximately equivalent to its EC60) in sMAs were similar between all normotensive WKY, SHAM and hypertensive SHR and DOCA-salt groups (7.1 ± 1.0, n = 30; 6.9 ± 0.6, n = 29; 7.7 ± 0.7, n = 29; 7.1 ± 0.9, n = 27, respectively). Endothelium removal, the vehicle for CBD and receptor antagonists did not change the basal tone.
Vasoconstriction study
To examine the potential effect of CBD on vasoconstriction increasing concentrations of U46619 in isolated hPAs or phenylephrine in sMAs some rings were treated for 30 min with one concentration (hPA 10 μmol/l; sMAs 1 μmol/l, a concentration approximately equivalent to its EC60) of CBD or its vehicle and then a concentration–response curve for U46619 or phenylephrine were constructed.
RNA isolation and gene expression analysis
Rat sMAs were isolated from hypertensive SHR, DOCA-salt and normotensive WKY and SHAM animals (pooled from six rats from each group). The RNA isolation and Cnr1 and Cnr2 gene expression analysis were performed according to methodology previously described [27,29]. Tissue samples were immediately flash-frozen in liquid nitrogen and stored at − 80 °C. Next, up to 5 mg of tissue was finely ground with a chilled stainless-steel mortar and pestle. Total RNA was purified using NucleoSpin RNA XS (Macherey-Nagel GmbH and Co., Düren, Germany) with carrier RNA and rDNase treatment, according to the manufacturer's protocol. Spectrophotometric measurements (A260/A280) were made to assess the quantity and quality of the extracted RNA (NanoPhotometer, Implen, Germany). Synthesis of the cDNA was performed using the PrimeScript RT Reagent Kit (Takara) following the manufacturer's instructions. Briefly, 500 ng of purified total RNA was used in a 10 μl reaction mixture containing random octamers, oligo dT-16 primers, dNTPs and PrimeScript Reverse Transcriptase. cDNA (2 μl) served as a template for real-time qPCR reactions. Amplification of the product was performed using SsoAdvanced Universal SYBR Green Supermix (Bio-Rad, Hercules, California, USA). A set of predesigned primer pairs for Cnr1 and Cnr2 was purchased from Bio-Rad (PrimePCR PreAmp for SYBR Green Assay: Cnr1/and Cnr2, Rat, accession number for Cnr1 NM_012784, and for Cnr2 NM_001164143, NM_001164142, NM_020543). As an internal control, two reference genes GAPDH[30] and Cyclophili A[31] were tested, and Cyclophilin A was chosen for further analysis. The following reaction parameters were applied: initial denaturation at 95 °C for 3 min, followed by 40 cycles of 95 °C for 1 min, 57 °C for 30 s, and 72 °C for 45 s. Melt curve analysis was performed from 65 to 95 °C in 0.5 °C steps, 10 s for the first step and 5 s for each step thereafter. The CFX Connect Real-Time PCR System (Bio-Rad) was used to perform a real-time quantitative PCR (PCR) assay. Reactions were run in triplicates and expression was analyzed using the relative quantification method modified by Pfaffl [32].
Immunohistochemistry
In the immunohistochemical study, the EnVision method was used according to Baranowska-Kuczko et al.[25] using an antibody against the cannabinoid CB1 receptor [1: 1000; rabbit Anti-CB1 (no cat. ab23703); Abcam, Cambridge, UK], cannabinoid CB2 receptor [1: 200; rabbit Anti-CB2 (no cat. ab3561); Abcam, Cambridge, UK], TPRV1 [1: 1000; rabbit Anti-TRPV1 (no cat. bs-1931R); Bioss Antibodies, Woburn, Massachusetts, USA] and GPR18 [1: 1000; rabbit Anti-GPR18 (no cat. ab150618); Abcam, Cambridge, UK]. The specificity of all antibodies included a negative control, in which the antibodies were replaced by normal rabbit serum (Vector Laboratories, Burlingame, California, USA) at the respective dilution (no staining), and a positive control, where the tissue recommended by the antibody manufacturer was used for staining, which was prepared: for CB1 from human cerebellum, CB2 human tonsil and TPRV1 human endometrial cancer and for GPR18 from human testis. All control reactions performed gave positive results. The obtained results of immunohistochemical staining were evaluated on the Olympus BX41 microscope with the Olympus DP12 camera under a magnification of 200× (20× lens and 10× eyepiece; each field was 0.785 mm2) and submitted to morphometric evaluation by using software for image analysis (NIS-Elements Advanced Research software of Nikon). In each blood vessel, the intensity of the immunohistochemical reaction was measured in 10 randomly selected sites.
Statistical analysis
The results are given as the mean ± SEM (n = number of animals or patients). The exact group size (n) for each experimental group/condition is provided in Tables 2 and 3 and ‘n’ refers to independent values, not replicates.
TABLE 2.
Influence of various antagonists and inhibitors and endothelium denudation on the vasorelaxant effects of cannabidiol in the U46619- or KCl1-preconstricted human pulmonary arteries
| Group | Pharmacological activity | n | pEC25 | pEC50 | Rmax |
| CONTROL | 21 | 5.8 ± 0.1 | 5.0 ± 0.1 | 90.3 ± 3.7 | |
| -ENDOTHELIUM | 6 | 5.0 ± 0.1*** | N.D. | 37.4 ± 9.7*** | |
| l-NAME (300) | (−) endothelial nitric oxide synthase | 6 | 5.7 ± 0.2 | 5.0 ± 0.1 | 80.1 ± 12.0 |
| DETCA (300) | (−) superoxide dismutase | 6 | 5.8 ± 0.2 | 5.2 ± 0.1 | 78.5 ± 11.3 |
| INDOMETHACIN (10) | (−) cyclooxygenase | 5 | 5.2 ± 0.2** | N.D. | 45.0 ± 13.6*** |
| NIMESULIDE (10) | (−) cyclooxygenase 2 | 5 | 4.9 ± 0.1*** | N.D. | 26.8 ± 6.8*** |
| Cay10441 (10) | (−) prostacyclin IP receptor | 5 | 4.9 ± 0.1*** | N.D. | 38.6 ± 6.8*** |
| L161982 (1) | (−) prostanoid EP4 receptor | 7 | 5.1 ± 0.1*** | N.D. | 42.2 ± 11.0*** |
| IBERIOTOXIN (0.1) | (−) large conductance Ca2+-activated potassium channel (KCa1.1) | 5 | 5.0 ± 0.2** | N.D. | 39.0 ± 7.0*** |
| UCL1684 (0.1) + TRAM-34 (10) | (−) small (KCa2.3) and intermediate (KCa3.1) conductance Ca2+- activated potassium channel, respectively | 7 | 4.9 ± 0.2*** | N.D. | 40.7 ± 10.4*** |
| AM251 (1) | (−) cannabinoid CB1 receptor | 5 | 5.6 ± 0.2 | 5.3 ± 0.1 | 64.2 ± 9.4* |
| AM630 (1) | (−) cannabinoid CB2 receptor | 6 | 5.6 ± 0.2 | 5.1 ± 0.1 | 72.6 ± 14.2 |
| O1918 (10) | (−) endothelium-dependent relaxation to cannabinoids | 5 | 5.4 ± 0.1 | 4.9 ± 0.1 | 72.3 ± 9.4 |
| CAPSAZEPINE (1) | (−) vanilloid TRPV1 receptor | 5 | 5.5 ± 0.2 | N.D. | 46.1 ± 1.2*** |
| RN1734 (20) | (−) vanilloid TRPV4 receptor | 6 | 5.8 ± 0.2 | 5.0 ± 0.1 | 96.5 ± 9.6 |
| KCl (60 mmol/l)1 | 5 | N.D. | N.D. | 21.8 ± 5.8***,2 |
Values are based on the concentration--response curves shown in Figs. 1-6. Data are expressed as mean ± SEM. n represents the number of patients, DETCA, sodium diethyldithiocarbamate trihydrate; l-NAME, Nω-nitro-l-arginine methyl ester; ND, not determined. Molar concentrations (μmol/l) of chemicals are provided in parentheses.
*P < 0.05.
**P < 0.01.
***P < 0.001 compared wit the control group as determined by one-way ANOVA followed by Dunnett's post hoc test or Student's t-test2 for unpaired data.
TABLE 3.
Influence of cannabinoid CB1, CB2 and vanilloid TRPV1 receptor antagonists and endothelium denudation on the vasorelaxant effects of cannabidiol in the phenylephrine-preconstricted small mesenteric arteries of hypertensive spontaneously and deoxycorticosterone acetate rats and their respective normotensive controls, Wistar–Kyoto and SHAM rats
| Group | WKY | SHR | SHAM | DOCA-salt | ||||||||
| n | pEC50 | Rmax (%) | n | pEC50 | Rmax (%) | n | pEC50 | Rmax (%) | n | pEC50 | Rmax (%) | |
| CONTROL | 11 | 6.0 ± 0.1 | 92.5 ± 3.1 | 11 | 5.6 ± 0.1$$,1 | 82.2 ± 4.5 | 12 | 5.5 ± 0.1 | 91.9 ± 4.9 | 12 | 5.9 ± 0.1**1 | 90.5 ± 3.0 |
| +AM251 (1) | 7 | 5.9 ± 0.1 | 93.1 ± 3.6 | 6 | 5.1 ± 0.1### | 84.6 ± 5.9 | 5 | 5.4 ± 0.1 | 77.3 ± 7.0 | 5 | 5.3 ± 0.1& | 76.5 ± 10.5 |
| +AM630 (1) | 6 | 5.7 ± 0.1 | 91.6 ± 3.5 | 7 | 5.7 ± 0.1 | 84.1 ± 3.7 | 7 | 5.3 ± 0.1 | 80.5 ± 3.9 | 5 | 5.2 ± 0.2& | 76.8 ± 5.0 |
| + CAPSAZEPINE (1) | 6 | 6.0 ± 0.1 | 87.6 ± 4.8 | 5 | 5.5 ± 0.1 | 96.4 ± 1.9 | 5 | 5.5 ± 0.1 | 87.2 ± 4.6 | 5 | 6.2 ± 0.3 | 83.8 ± 3.8 |
| −ENDOTHELIUM | 5 | 5.6 ± 0.2 | 98.6 ± 3.4 | 5 | 5.5 ± 0.1 | 79.0 ± 8.1 | 7 | 5.5 ± 0.1 | 89.0 ± 7.4 | 6 | 4.6 ± 0.3&&& | 67.0 ± 6.4&&& |
| −ENDOTHELIUM + AM251 (1) | 5 | 5.5 ± 0.1 | 97.9 ± 1.7 | 5 | 5.7 ± 0.1 | 89.2 ± 4.5 | 6 | 5.0 ± 0.3 | 92.1 ± 6.6 | 6 | 4.7 ± 0.1 | 90.3 ± 1.0 |
Values are based on the concentration--response curves shown in Figs. 5 and 6. Data are expressed as mean ± SEM. n represents the number of animals. Molar concentrations (μmol/l) of chemicals are provided in parentheses. DOCA-salt, deoxycorticosterone acetate-salt; SHR, spontaneously hypertensive rats; WKY, Wistar–Kyoto;
&P < 0.05; **,$$,##P < 0.01; &&&,###P < 0.001 compared with the respective control group ($ WKY, *SHAM, #CONTROL SHR, &CONTROL DOCA-salt); as determined by one-way ANOVA followed by Dunnett's post hoc test or Student's t-test1 for unpaired data.
The relaxation elicited by CBD or the appropriate solvent was expressed as a percentage of the precontraction induced by U46619 and high KCl (hPAs) or phenylephrine (sMAs). Contractile responses to U46619 in hPAs and phenylephrine in sMAs were presented as a percentage of the reference contraction response to KCl (60 mmol/l in hPAs and 120 mmol/l in sMAs). GraphPad Prism 5.0 software (La Jolla, California, USA) was used to plot the mean data as sigmoidal CRCs. The curves were then used to determine the potency (pEC50, the negative logarithm of the concentration causing a half-maximum effect) and the maximal response (Rmax) values as the effect of the highest concentration of each particular agonist. In analogy to our previous study [19,25], the extent of relaxation at 30 μmol/l CBD was quantified as a rough measure of the maximum extent of relaxation obtainable (Rmax). When some experimental procedures attenuated the Rmax of CBD by greater than 50%, the concentrations causing a relaxation of 25% of the precontracted vessel, EC25 values, were used as the parameter for analysis (determined graphically from the individual CRCs). The rightward shifts of CRCs relative to the control curve were calculated on the basis of the EC25 values. EC25 values were transformed into pEC25 values (the negative logarithms of EC25 values, respectively). The antagonistic potency (pA2) of the latter was calculated from the equation
where [B] is the molar concentration of Cay10441 or L161982 and CR is the concentration ratio of the EC25 values of CBD in the presence and absence of an antagonist.
When two or more treatment groups were compared with the same control, a one-way analysis of variance (ANOVA) followed by Dunnett's test was used. A Student's t-test for unpaired data was used wherever appropriate. Data were analyzed using GraphPad Prism version 5.00 for Windows, GraphPad Software. Differences were considered to be statistically significant at P less than 0.05.
Drugs
Cremophor EL, DOCA, dimethyl sulfoxide (DMSO), N,N-dimethylformamide (DMF) and Tween-80; pentobarbitone sodium (Biowet, Puławy, Poland) and sodium chloride (NaCl) (Chempur, Piekary Śląskie, Poland).
Unless otherwise stated, all chemicals were purchased from Sigma-Aldrich (Munich, Germany). U46619, CBD, GW9662, L161982, O-1918, TRAM-34, AM251, and AM630 were bought from Tocris Bioscience (Bristol, UK). Cay10441 was purchased from Cayman Chemical Co. (Ann Arbor, Michigan, USA). Stock solutions of CBD, U46619, GW9662, AM251, O-1918, capsazepine, nimesulide, Cay10441 and RN1734 were made to 10 mmol/l in ethanol; AM630, L161982, UCL1684 and TRAM-34 were made to 10 mmol/l in dimethylsulfoxide. Their final concentrations were prepared by dilutions with deionized water, which adjusted the final concentrations of ethanol or DMSO 0.1% v/v or less with the exception of RN1734 (ethanol final concentration 0.2% v/v). Stock solutions of l-NAME (300 mmol/l), phenylephrine and acetylcholine (all 10 mmol/l) were made in distilled water. Indomethacin was dissolved in 0.5 mol/l NaHCO3 and DETCA directly into Krebs.
RESULTS
Influence of cannabidiol on the preconstricted human pulmonary arteries
U46619 induced a concentration-dependent contraction of hPA rings (pEC50 = 8.4 ± 0.1, Rmax = 101.5 ± 1.7, n = 6). CBD 10 μmol/l and its vehicle did not modify the CRC of U46619 (pEC50 = 8.4 ± 0.1, Rmax = 98.8 ± 1.8, n = 6, pEC50 = 8.4 ± 0.1, Rmax = 98.5 ± 1.9, n = 5, respectively, Fig. 1a).
FIGURE 1.
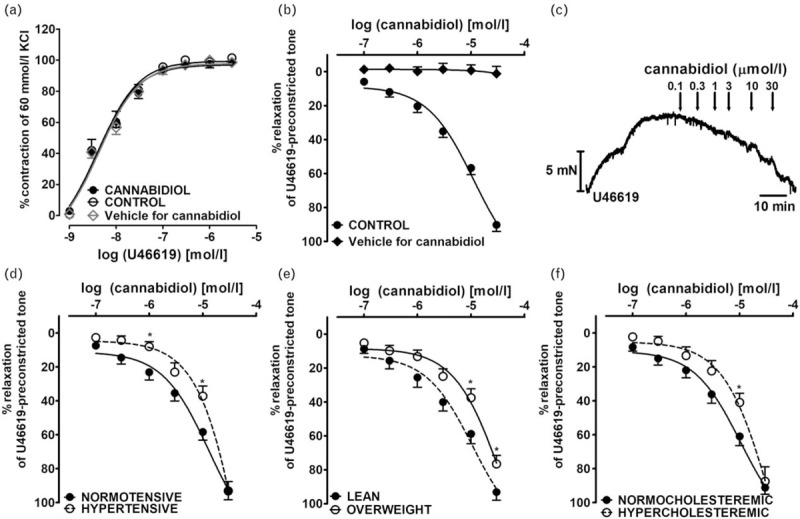
Influence of cannabidiol (10 μmol/l) on the concentration--response curve of U46619-induced contraction (a), its vasodilatory effect in endothelium-intact isolated human pulmonary arteries (b; c -- representative original trace) and post hoc analysis of the patients comorbidities on cannabidiol-mediated vasorelaxation (d–f). ∗P less than 0.05 according to Student's t-test. The results are presented as the mean ± SEM of n tissue samples for each curve. See Table 1 for n and the statistical analysis (b). The control curve for cannabidiol vehicle [ethanol, 0.1% v/v (a) and 0.001 – 0.3% v/v final concentration (b), respectively]. In a few cases, SEM is smaller than or equal to the size of symbols. Arrows show the moment of application of a particular concentration of CBD. CBD, cannabidiol. hPAs, human pulmonary arteries.
CBD (0.1–30 μmol/l) but not its vehicle caused almost full relaxation of the hPAs preconstricted with U46619 (Fig. 1b); the pEC25 and pEC50 values were 5.8 and 5.0, respectively (Table 2). The vehicle controls with CBD were comparable (ethanol, pEC50 = 5.0 ± 0.1, n = 5; DMSO, 5.0 ± 0.1, n = 6; water, pEC50 = 4.9 ± 0.1, n = 10). They are presented in the Figures as one merged control group (pEC50 = 5.0 ± 0.1, n = 21). The vasodilatation induced by CBD was gradual in onset. Thus, it took 80 min to construct the whole CRCs (for the original traces see Fig. 1c).
The vasodilator responses to CBD varied between patients (the maximal response to CBD ranged from 63 to 120% relaxation), so post hoc analysis was performed to establish any relationships between CBD responses and patient characteristics (see Table 1). The potency of CBD was reduced by hypertension (vs. normotension; pEC50 = 4.1 ± 0.3, n = 8 vs. 4.9 ± 0.1, n = 13, P < 0.05; Fig. 1d), obesity (vs. lean; pEC50 = 4.4 ± 0.1, n = 7 vs. 5.0 ± 0.1, n = 14, P < 0.01; Fig. 1e) and hypercholesteraemia (vs. normocholesteraemia; pEC50 = 4.4 ± 0.2, n = 8 vs. 5.0 ± 0.1, n = 13, P < 0.01; Fig. 1f), whereas the maximal response was diminished only in obese patients (Rmax = 76.6 ± 5.0, n = 7 vs. 93.2 ± 4.8, n = 14, P < 0.05; Fig. 1e). Moreover, the vasorelaxation to lower concentrations of CBD was attenuated in those taking β-blockers (vs. no β-blockers; pEC25 = 5.9 ± 0.1, n = 8 vs. 5.5 ± 0.1, n = 13; P < 0.01; no differences in pEC50 values). The following parameters, concurrent diseases and cardiovascular therapy had no influence on the CBD-evoked relaxation: sex, age, smoking, prevalence of chronic obstructive pulmonary disease, type 2 diabetes and angiotensin-converting enzyme inhibitors, α1-adrenolytics statins, nonsteroidal anti-inflammatory drugs (NSAIDs and proton pump inhibitors (PPIs) (for pEC50 and Rmax see Table 1). In a few cases, coronary artery disease, angiotensin receptor blockers, calcium channel blockers and hypoglycaemics virtually did not change CBD-mediated effects, however patient numbers were too small for adequate statistical analysis.
Influence of endothelium denudation and inhibitors of cyclooxygenase, nitric oxide synthetase and superoxide dismutase pathways and antagonists of prostacyclin and prostanoid receptors on the cannabidiol-induced vasorelaxation in human pulmonary arteries
The endothelium removal reduced the potency and efficacy of CBD (Fig. 2a). Inhibition of COX-1 or COX-2 activity using indomethacin (10 μmol/l; nonselective) or nimesulide (10 μmol/l; COX-2), reduced the vasorelaxant effects of CBD at 30 μmol/l by about 50 and 70%, respectively, and shifted the CRC for CBD to the right by a factor of 4 and 8, respectively (Fig. 2b). Therefore, further experiments were performed to test prostanoids (or other metabolite) pathways. The IP receptor antagonist Cay10441 (10 μmol/l) as well as the EP4 receptor antagonist L161982 (1 μmol/l), shifted the CRC for CBD to the right by a factor of 8 and 5, respectively, and reduced the relaxant effect of the highest concentration of CBD (30 μmol/l) to about 40% (Fig. 3b). The estimated antagonistic potency (pA2) for Cay10441 and L161982 was approximately 5.8 and 6.6, respectively. Incubation with l-NAME and DETCA (NOS and SOD inhibitors, respectively) did not modify CRCs for CBD (Fig. 2a). For pEC50, pEC25, and Rmax values, see Table 2.
FIGURE 2.
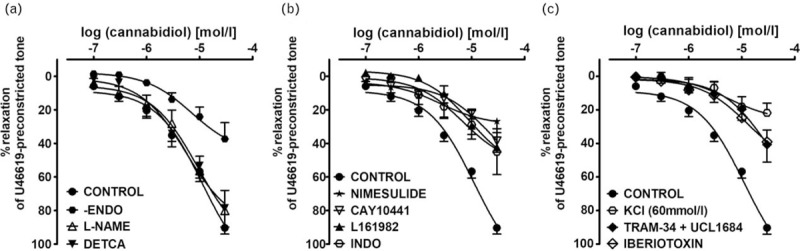
Concentration--response curve of the cannabidiol-induced vasorelaxation after endothelium removal (-ENDO) or 30 min incubation (before precontraction) with inhibitors/antagonists of various enzymes, receptors or ion channels in hPAs. Panel a: nitric oxide synthase -- Nω-nitro-l-arginine methyl ester (l-NAME) 300 μmol/l; superoxide dismutase -- DETCA 300 μmol/l. Panel b: COX-2 -- nimesulide 10 μmol/l; COX-1/COX-2 - indomethacin (INDO) 10 μmol/l; IP receptor -- Cay10411 10 μmol/l; EP4 receptor -- L161982 1 μmol/l. Panel c: large conductance Ca2+-activated potassium channels (KCa1.1) -- iberiotoxin 0.1 μmol/l; small (KCa2.3) -- UCL1684 0.1 μmol/l with intermediate conductance Ca2+-activated potassium channels -- (KCa3.1) TRAM-34 10 μmol/l. Results are expressed as the percentage relaxation of the isometric contraction induced by U46619 and, in the case of the open hexagons, of the contraction induced by KCl 60 mmol/l. Values are presented as the mean ± SEM of n tissue samples for each curve. See Table 2 for n and the statistical analysis. In a few cases, SEM is smaller than or equal to the size of symbols. SEM, standard error of the mean.
FIGURE 3.
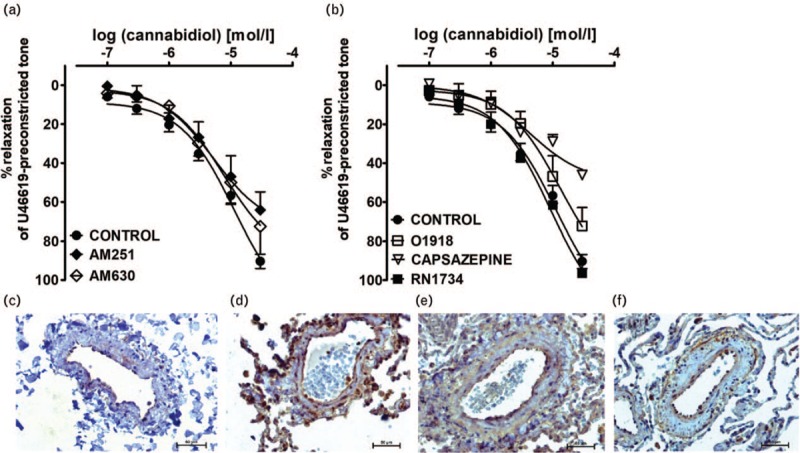
Mechanisms of the cannabidiol-induced vasorelaxation in isolated human pulmonary arteries. Concentration--response curve of the cannabidiol-induced vasorelaxation after 30 min incubation (before precontraction) with antagonists of CB1 AM251 (1 μmol/l), CB2 AM630 (1 μmol/l) (a) and endothelium-dependent relaxation to cannabinoids O-1918 (10 μmol/l), TRPV1 capsazepine (1 μmol/l) and TRPV4 RN1734 (20 μmol/l) (b) in endothelium-intact hPAs. The results are presented as the mean ± SEM of n tissue samples for each curve. In a few cases, SEM is smaller than or equal to the size of symbols. See Table 2 for n and the statistical analysis if not stated here. Representative CB1 (c), CB2 (d), TRPV1 (e) and GPR18 (f) immunoreactivity. Scale bars: 50 μm. hPAs, human pulmonary arteries; SEM, standard error of the mean.
Influence of KCa channel inhibitors on the cannabidiol-induced vasorelaxation in human pulmonary arteries
In order to determine if potassium channels contribute to the vasodilatory effects of CBD, some experiments were carried out in high KCl-preconstricted arteries. The vasorelaxation induced by CBD at a concentration of 30 μmol/l was reduced by about 75% in high KCl-preconstricted arteries compared with those preconstricted with U46619 (Fig. 2c). Pretreatment with the selective inhibitors of large (KCa 1.1, iberiotoxin, 0.1 μmol/l) and given together small (KCa 2.3, UCL1684, 0.1 μmol/l) and intermediate (KCa 3.1, TRAM-34, 10 μmol/l) conductance KCa shifted the CRC for CBD (determined for the U46619-preconstricted hPAs) to the right by a factor of 6, and 8, respectively. The effect elicited by the highest concentration of CBD was reduced in both cases by about 55% (for pEC25 and Rmax values, see Table 2 and Fig. 2c).
Influence of AM251, AM630, capsazepine, O-1918 and RN1734 on the cannabidiol-induced vasorelaxation in human pulmonary arteries
The antagonists of the CB1 receptor, AM251 (1 μmol/l) and CB2 receptor, AM630 (1 μmol/l) and endothelium-dependent relaxation to cannabinoids, O-1918 (10 μmol/l) and the inhibitor of TRPV4, RN1734 (20 μmol/l) did not change the CRCs for CBD (Fig. 3a and b). The only exception was inhibition by AM251 (by about 30%) of the vasodilatory response to the highest concentration of CBD (30 μmol/l; Fig. 3a). The TRPV1 antagonist capsazepine (10 μmol/l), shifted the CRC for CBD to the right by a factor of 2 and reduced the relaxant effect of the highest concentration of CBD (30 μmol/l) by about 50% (Fig. 3b). For pEC50, pEC25, and Rmax values, see Table 2.
Immunostaining revealed the presence of CB1, CB2 and TRPV1 receptors in endothelial and smooth muscle cells of the walls of the hPAs (Fig. 3c–e, respectively). Immunohistochemical staining of GPR18 was detected in the endothelial and adventitial layer of hPAs (Fig. 3f).
Influence of GW9662 on the time-dependent cannabidiol-induced vasorelaxation in human pulmonary arteries
A single concentration of CBD (10 μmol/l; approximately equivalent to its pEC60) but not its vehicle, ethanol caused an initial vasorelaxation of 22.4 ± 7.8% at 15 min, developing to 67.3 ± 7.4% at 120 min (n = 8). The PPARγ antagonist GW9662 at concentrations of 1 and 10 μmol/l tended towards and significantly reduced, the respective time-dependent relaxation induced by CBD 10 μmol/l. Thus, its vasodilatory effects at 120 min were 49.5 ± 7.2% (n = 3) and 40.7 ± 6.6% (n = 5; P < 0.05), respectively (Fig. 4a; for the original traces of control and GW9662 10 μmol/l-treated arteries, see Fig. 4b).
FIGURE 4.
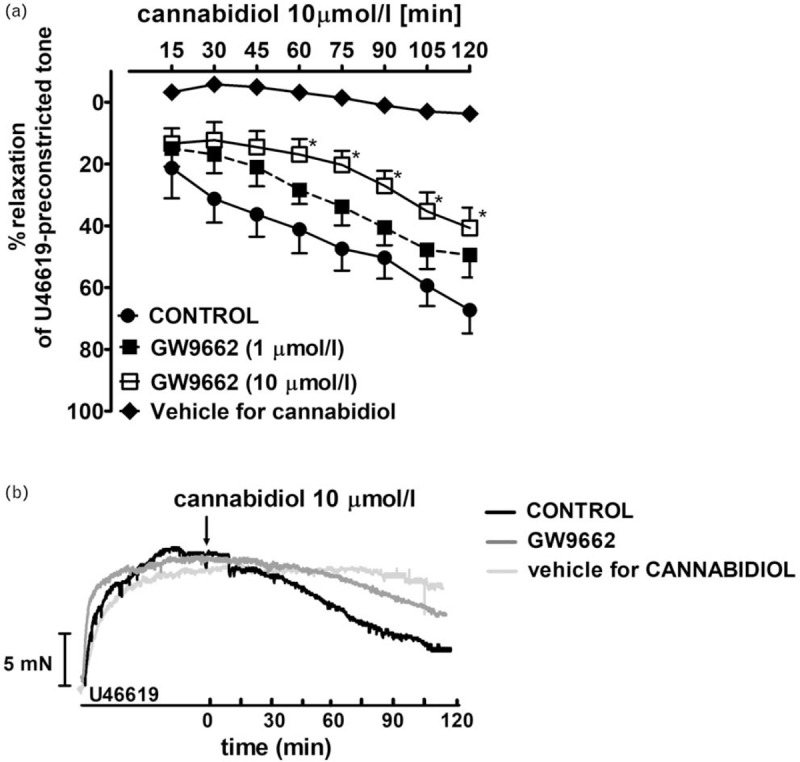
Influence of PPAR γ antagonist GW9662 (1 μmol/l; n = 3 and 10 μmol/l; n = 5) on the time-dependent cannabidiol-induced (n = 8) vasodilatory effect in endothelium-intact isolated human pulmonary arteries. The results are presented as the mean ± SEM of n tissue samples for each curve. P less than 0.05 according to one-way ANOVA followed by the Dunnett's post hoc test (a). Representative original traces of the cannabidiol-induced time-dependent (also in the presence of GW9662 10 μmol/l) vasodilatory effect in endothelium-intact isolated hPAs (b) The control--time curve for cannabidiol vehicle (ethanol, 0.1% v/v) was also shown. Arrow show the moment of application of CBD and/or vehicle. ANOVA, analysis of variance; CBD, cannabidiol; hPAs, human pulmonary arteries; SEM, standard error of the mean.
Influence of hypertension and receptor antagonists on vasodilatory effects of cannabidiol in rat endothelium-intact and denuded small mesenteric arteries
CBD (1 μmol/l) did not modify the CRC of phenylephrine in all groups (WKY pEC50 = 5.5 ± 0.1, Rmax = 93.5 ± 8.0, n = 6 vs. CBD pEC50 = 5.6 ± 0.1, Rmax = 107.2 ± 10.7, n = 6; SHR pEC50 = 5.6 ± 0.1, Rmax = 102.7 ± 7.8, n = 7 vs. CBD pEC50 = 5.3 ± 0.2, Rmax = 107.7 ± 4.8, n = 7; SHAM pEC50 = 5.9 ± 0.1, Rmax = 109.6 ± 9.4, n = 5 vs. CBD pEC50 = 5.7 ± 0.2, Rmax = 110.2 ± 10.7, n = 6; DOCA-salt pEC50 = 5.8 ± 0.1, Rmax = 119.5 ± 8.5, n = 6 vs. CBD pEC50 = 5.6 ± 0.2, Rmax = 122.0 ± 9.4, n = 6; Fig. 5a and b).
FIGURE 5.
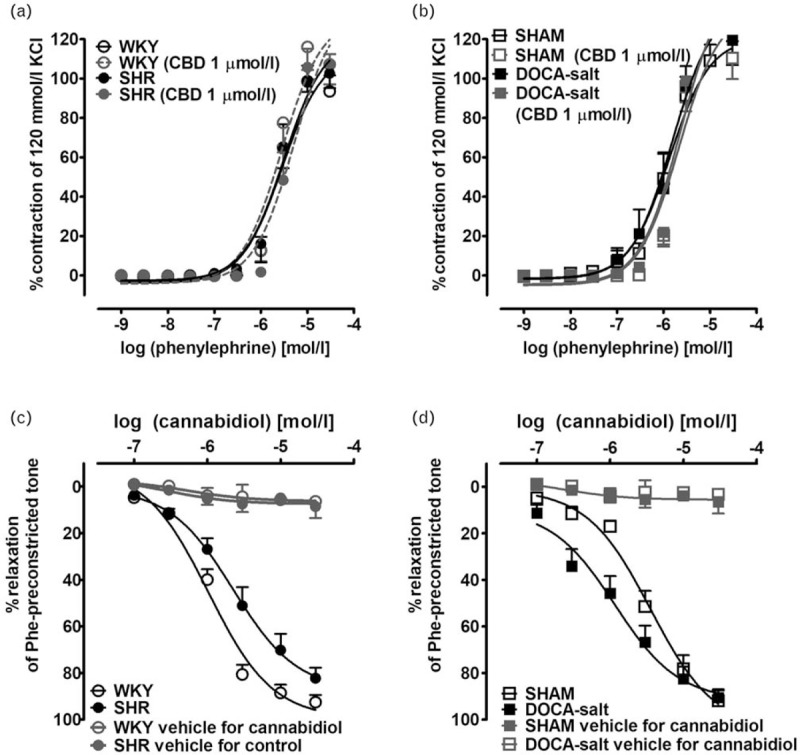
Influence of cannabidiol (1 μmol/l) on the concentration--response curve of phenyleprine-induced contraction (a and b), and its vasodilatory effects (c and d) in endothelium-intact isolated small mesenteric arteries from normotensive control (a and c, WKY; b and d, SHAM) and hypertensive (a and c, SHR; b and d, DOCA-salt) rats. The results are presented as the mean ± SEM of n tissue samples for each curve. See Table 3 for n and the statistical analysis (c and d). The control--time curve for cannabidiol vehicle (ethanol, 0.001–0.3% v/v final concentration, n = 5 in each group). In a few cases, SEM is smaller than or equal to the size of symbols. SHR, spontaneously hypertensive rats; SEM, standard error of the mean; SHAM, control sham-operated rats; WKY, Wistar–Kyoto.
CBD (0.1–30 μmol/l) but not its vehicle produced a concentration-dependent acute, almost full relaxation of endothelium-intact phenylephrine-preconstricted sMAs isolated from hypertensive (SHR and DOCA-salt) and normotensive animals (WKY and SHAM) (Fig. 5c and d). The maximal relaxation (Rmax ∼90%) was comparable in all experimental groups. The vasorelaxant potency of CBD was stronger in DOCA-salt than in respective normotensive controls, and in SHR. In contrast, in SHR, CBD was less potent as a vasodilator than in WKY (for pEC50 values, see Table 3).
Interestingly, in endothelium-intact sMAs of SHR and DOCA-salt, the relaxation response to CBD was CB1-dependent, as the blockade of CB1 receptors by AM251 (1 μmol/l) resulted in a significant ∼3-fold and ∼4-fold rightward displacement of the relaxation response, respectively, and reduction of its potency but not its maximal effects. In DOCA-salt animals, it was additionally sensitive to the CB2-receptor antagonist, AM630 (1 μmol/l) with attenuation in relaxant potency, and to endothelium removal that caused both reduced affinity and efficacy. The TRPV1 receptor antagonist capsazepine (1 μmol/l) and AM251 (1 μmol/l) did not change the CRC for CBD in endothelium intact and denuded sMAs of hypertensive rats, respectively. None of the antagonists or endothelium denudation changed the potency and efficacy of CBD in normotensive controls (for respective pEC50 and Rmax values, see Table 3 and Fig. 6).
FIGURE 6.
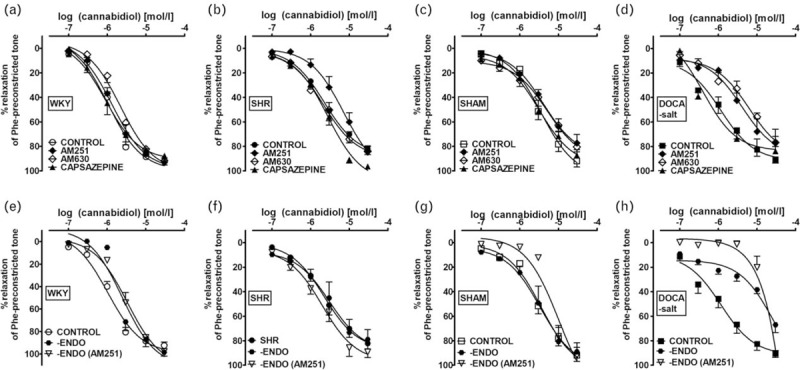
Mechanisms of the cannabidiol-induced vasorelaxation in isolated rat small mesenteric arteries. Concentration--response curve of the cannabidiol-induced vasorelaxation after 30 min incubation (before precontraction) with antagonists of CB1 AM251 (1 μmol/l; a–h), CB2 AM630 (1 μmol/l; a–d), TRPV1 capsazepine (1 μmol/l; a–d) in endothelium-intact (a–d) and endothelium-denuded (-ENDO; e–h) rat sMAs. The results are presented as the mean ± SEM of n tissue samples for each curve. See Table 3 for n and the statistical analysis. In a few cases, SEM is smaller than or equal to the size of symbols. SEM, standard error of the mean; sMAs, small mesenteric arteries.
Influence of hypertension on Cnr1 and Cnr2 gene expression in rat small mesenteric arteries
Expression of Cnr1 and Cnr2 genes were analyzed by real-time qPCR (Fig. 7a and b). It has been shown that Cnr1 expression was up-regulated in the case of both SHR and DOCA-salt rats in comparison to controls, with over 2-fold and 3-fold increases, respectively (Fig. 7a). The corresponding effect was observed regarding the Cnr2 gene in sMAs of DOCA-salt animals (Fig. 7b).
FIGURE 7.
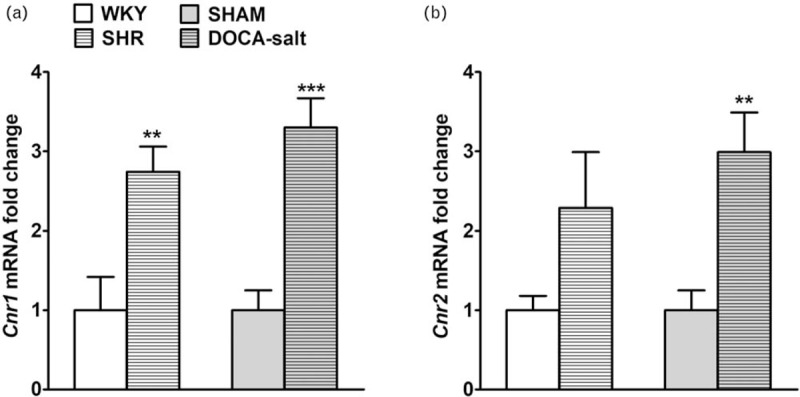
The relative Cnr1 (a) and Cnr2 (b) gene expression evaluated by real-time quantitative PCR in small mesenteric arteries of hypertensive SHR and DOCA-salt vs. normotensive controls WKY and SHAM, respectively. Results are shown mean ± SEM (n = 6 per group) as relative fold change in mRNA expression in comparison to respective control, where expression level was set as 1. ∗∗P less than 0.01 or ∗∗∗P less than 0.001 according to the Student's t-test. DOCA-salt, 11-deoxycorticosterone acetate-salt; SEM, standard error of the mean; SHR, spontaneously hypertensive rats; SHAM, control sham-operated rats; WKY, Wistar–Kyoto.
Influence of hypertension on immunostaining of CB1, CB2, transient receptor potential vanilloid 1 and GPR18 receptors in rat small mesenteric arteries
CB1, CB2, TRPV1 and GPR18 receptor immunostaining revealed their presence in the endothelial and smooth muscle cells of the walls of the sMAs in all groups with the exception of GPR18 immunostaining in WKY and SHAM. A more intense staining of CB1 and GPR18 receptors was observed in endothelium and smooth muscle cells of sMAs of SHR and DOCA-salt hypertensive animals (Fig. 8).
FIGURE 8.
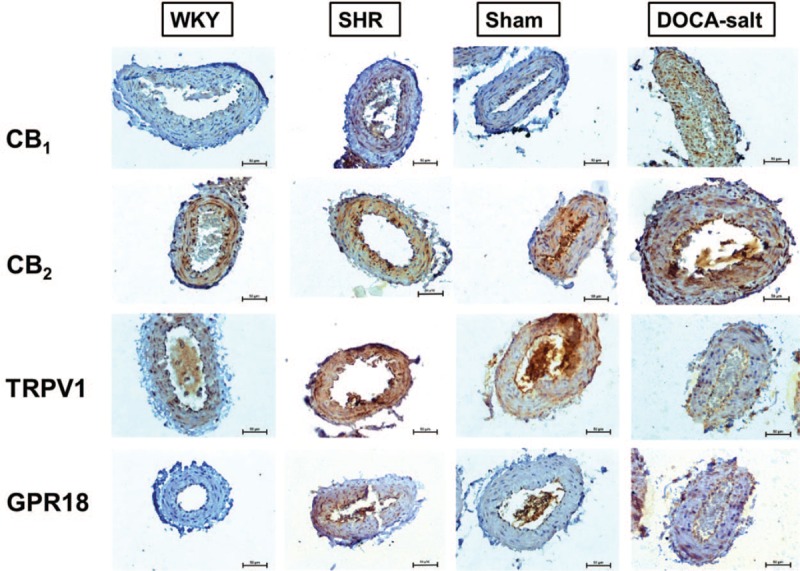
Representative micrographs of immunohistochemical staining of CB1, CB2, TRPV1 and GPR18 receptors in cross-sections of rat small mesenteric arteries from normotensive control (WKY; SHAM) and hypertensive (SHR; DOCA-salt) rats. Scale bars: 50 μm. DOCA-salt, 11-deoxycorticosterone acetate-salt; SHR, spontaneously hypertensive rats; SHAM, control sham-operated rats; WKY, Wistar–Kyoto.
DISCUSSION
The present study demonstrated a potent influence of CBD in the regulation of vascular tone in pulmonary circulation of human and that it could be modified by disease, including hypertension. The vasorelaxant effects of CBD in hPAs preconstricted with thromboxane analogue, U46619 is endothelium-dependent and include the following mechanisms: IP, EP4, TRPV1, PPARγ receptors and KCa channels. We have also shown species-specific, vascular-bed-specific and hypertension model differences in response to CBD, as in phenylephrine-preconstricted rat sMAs, CBD-evoked vasorelaxation was enhanced in DOCA-salt but diminished in SHR compared with respective normotensive controls. Moreover, in rats but not in humans, CB1 receptors could play potential protective role in both models of hypertension. Our findings provide evidence that CBD could be a potential relevant vasorelaxant; however, modification of its responses in disease should be considered whenever CBD is used for therapeutic purposes.
Cannabidiol causes strong relaxation of human pulmonary arteries via complex mechanisms
We are the first to demonstrate the vasodilatory effect of CBD on the pulmonary circulation. Thus, CBD induced a concentration-dependent, slowly developed full relaxation (at a concentration of 30 μmol/l) in isolated endothelium-intact hPAs precontracted with U46619. It neither affected basal tone nor the U46619-induced vasoconstriction by itself. The pEC50 value for its vasodilatory response was in the mid-micromolar range (∼5.0). Importantly, it could be extrapolated to in-vivo concentrations that decreased BP in stress [9,11] and are achieved in serum after oral administration of 800 mg [33] in humans. Moreover, a high oral dose reaching 1280 mg/day of CBD was used in psychiatric patients without any side effects [34].
Although mechanisms are responsible for the vasodilatory effect of CBD in hPAs? Firstly, it is to a large extent endothelium-dependent as its removal reduced the maximal effect of the highest concentration used (30 μmol/l) by about 60%. Thus, all further experiments were carried out in rings with an intact endothelium. We found that the CBD-induced relaxation was reduced by about 50–70% by the nonselective COX inhibitor indomethacin, the selective COX-2 inhibitor nimesulide, the IP receptor antagonist Cay10441 and the EP4 receptor antagonist L161982, demonstrating that CBD caused vasorelaxation via an arachidonic acid COX/COX-2-derived metabolite acting at IP and/or EP4 receptors. On the other hand, we excluded the possibility that it is related to a direct antagonistic effect of CBD at prostanoid TP receptors as CBD at a concentration that evoked ∼60% relaxation (10 μmol/l) did not influence the CRC of U46619. Similarly, the vasodilatory effects of anandamide in hPAs and rPAs were mediated via IP receptors [19,20] and EP4 receptors participated in the enhancement by CBD and acetylcholine-evoked [16] and 2-AG-evoked relaxation in rat and hMA [35]. On the other hand, the effects of CBD in hPAs were not related to activation of NO and/or SOD as incubation with l-NAME or DETCA did not modify vascular responses to CBD.
Secondly, two sets of experiments suggest the involvement of potassium channels in the effect of CBD. The relaxation to CBD was significantly attenuated in hPAs preconstricted with a high concentration of K+ that not only blocks outward potassium channels but also in addition, also leads to membrane depolarization. The latter phenomenon may interfere with the quantitative contribution of ion channels to the vasodilator effect of CBD. Accordingly, CBD-induced responses were strongly, by a comparable degree, inhibited by iberiotoxin (KCa1.1 blocker) and TRAM-34 (KCa3.1 blocker) given in the presence of UCL1684 (KCa2.3 blocker), indicating a major role for KCa in the vascular action of CBD in endothelium-intact hPAs. It is generally accepted [36] that KCa3.1 and KCa2.3 channels are located in the endothelium and KCa1.1 in the smooth muscle. However, as we used iberiotoxin in endothelium-intact hPAs, we cannot exclude a minor role for endothelial KCa1.1 that has been recently suggested in CB1/CB2 receptor-independent cannabinoid-induced vasodilation [37]. Similarly to the present results, CBD and its synthetic analogue abn-CBD relaxed endothelium-intact hMAs and hPAs via potassium [5] and KCa channels [21], respectively.
Thirdly, we have determined the possible involvement of cannabinoid-sensitive receptors in the CBD-induced relaxation of hPAs. CBD might activate them directly or indirectly leading to enhancement of endocannabinoid tone, as it is known to inhibit fatty acid amide hydrolase (FAAH; an enzyme responsible for the degradation of endocannabinoid anandamide activity and transport [38]) and to activate phospholipase A2 with subsequent elevation in arachidonic acid-derived products.
The expression of CB1-receptors has been determined in hPAs (present study, 39]). CBD has a low affinity at CB1 receptors, including human receptors [38]. Accordingly, in our study, AM251 failed to modify the vasorelaxation induced by CBD 0.1–10 μmol/l pointing to a CB1 receptor-independent mechanism. The inhibitory effect of AM251 against the response to the highest CBD concentration 30 μmol/l, results probably from nonspecific and also CB1-independent actions of CBD, such as increasing membrane fluidity demonstrated for CBD 30 μmol/l [38,40], a negative allosteric modulation [41] and/or enhancement of endocannabinoid tone [38].
CBD has also been demonstrated to activate TRPV1 [42]. Indeed, we demonstrated that in hPAs TRPV1 receptors are involved in the vasodilatory response to CBD, as their antagonist capsazepine reduced the vasodilatory effect of CBD and they are expressed in the endothelium and smooth muscle cells of hPAs.
The PPARγ receptors mediate a slowly developed relaxation of hPAs (e.g. rosiglitazone maximum relaxation 75%, pEC50 5.5 [28]). CBD is considered to be a PPARγ ligand capable of eliciting time-dependent relaxation in rat-isolated aorta, maximally by about 50% [13]. In our hands, CBD (10 μmol/l) evoked a nearly 70% time-dependent relaxation in hPAs that was not significantly (by 25%) and significantly (by 40%) reduced by the potent PPARγ antagonist, GW9662 1 and 10 μmol/l, respectively.
We excluded the potential involvement of CB2 and TRPV4 receptors in the CBD-induced relaxation of hPAs as they were not altered by the respective antagonists AM630, and RN1734. In addition, we have examined the vasodilatory effect of CBD in the presence of O-1918 known to antagonize endothelial-dependent vasodilatory effects of various cannabinoids including anandamide and abn-CBD (see below). However, in our hands, O-1918 failed to modify the response to CBD in hPAs. Thus, we can exclude the involvement of the following mechanisms connected with this antagonist: still questionable endothelial atypical cannabinoid receptors, the Na+-Ca2+ exchanger or GPR18 receptors (according to [37,43]). The latter despite the fact that CBD is a weak GPR18 partial agonist with pEC50 51.1 μmol/l [44] and we determined the expression of GPR18 in the endothelium of hPAs. O-1918 has also been demonstrated to inhibit KCa1.1 channels [45,46] but in cell cultures only.
The CBD-induced relaxation of hPAs in the current study was comparable with that demonstrated by us previously in endothelial-intact hPAs relaxed by endocannabinoids, that is, anandamide [19], 2-AG [39] and virodhamine [47], the CBD analogue abn-CBD [21] and LPI [27]. In all cases, we noticed almost full relaxation, with similar respective pEC50 values: 5.0, 5.4, 5.1, 4.8 and 6.4, the involvement of endothelium, potassium channels and sensitivity to O-1918 (not examined in the case of 2-AG). The COX metabolites mediated the relaxation induced by unstable anandamide and virodhamine but not by stable abn-CBD. CB1 receptors were involved in responses to 2-AG only (see above) and CB2 receptors were excluded in the actions of anandamide and virodhamine.
Our results demonstrated that the vasodilatory effect of CBD in human arteries is dependent on the vascular bed, as it relaxed hMAs with similar potency (pEC50 was 5.1) but only in about 40% [5]. The difference in efficacy results probably comes from the enhanced physiological role of the endothelium in the regulation of smooth muscle tone in the pulmonary circulation compared with mesenteric arteries [19,39,27]. However, both responses involved similar mechanisms, that is, endothelium-dependency, involvement of TRPV1 receptors and via inhibition of potassium efflux. In addition, they were not modified by AM630, O-1918 and l-NAME. There are following differences between the CBD-induced relaxation in hPAs and in hMAs. Thus, in hMAs, CBD-induced vasorelaxation was reduced by two CB1 receptor antagonists (including AM251). It also gradually increased with time, but it was not inhibited by PPARγ antagonism [5], confirming that these receptors are involved in the relaxation of conduit vessels, such as rat aorta [13], but not in small arteries. Moreover, indomethacin was ineffective in hMAs excluding the involvement of COX metabolites in the vasodilatory effect of CBD.
The post hoc analysis revealed that the potency of the CBD-induced relaxation of hPAs was reduced by hypertension, obesity and hypercholesterolemia and in patients taking β-blockers compared with respective controls. The efficacy was diminished by obesity only. The patient age, sex, smoking habits and comorbidities, such as chronic obstructive pulmonary disease and type 2 diabetes did not influence the potency of CBD in hPAs. Similarly, hypercholesterolemia and β-blockers reduced the CBD responses in hMAs [5]. However, these data of comorbidities and the medication taken showed only rough approximations. It is not yet known whether this might result from drug interactions or the disorder for which the medication is being taken. Hypertension, obesity and hypercholesterolemia are well known important risk factors for endothelial dysfunction [25] that in this study could impair the CBD-mediated vasorelaxation. Interestingly, patients taking and not taking NSAIDs exhibited the same degree of CBD-induced vasodilation in spite of evident attenuation of the CBD-induced vasodilation by selective and nonselective COX inhibitors. However, one should keep in mind, that 72 h before surgery patients stopped taking pain relief, that is, the time was sufficient for drug metabolization and elimination and in our hands, COX inhibitors were given in vitro 30 min before CBD.
Influence of hypertension on the cannabidiol-mediated vasorelaxation in rat small mesenteric arteries
We decided to examine the vasodilatory response to CBD in experimental hypertension as CBD is suggested to have antyhypertensive potency. We applied the same models of primary (SHR) and secondary (DOCA-salt) hypertension in which we noticed previously different effects of chronic FAAH inhibition on the vasodilator effect of methanandamide (the stable anandamide analogue; see below).
In normotensive rats, acute CBD did not affect basal tone and the phenylephrine-induced vasoconstriction but caused the concentration-dependent full relaxation of sMAs preconstricted with phenylephrine with similar mid-micromolar potency as demonstrated previously in rat sMAs [12] and hMAs [5]. Importantly, CBD caused full relaxation of rat sMAs (present study [12]) but only almost half-maximal relaxation of hMAs [5], confirming the suggestion of Stanley et al.[5] that CBD efficacy might be reduced in human mesenteric vasculature.
We are the first to demonstrate the influence of hypertension on the vascular response to CBD. Interestingly, it was enhanced in DOCA-salt hypertension but reduced in SHR. The reactivity of the CBD-mediated relaxation by endothelium removal in DOCA-salt but not in SHR argues for its possible crucial role in compensatory mechanisms in secondary hypertension, like DOCA-salt [48]. Similarly, despite comparable values of enhanced blood pressure in both models of hypertension in comparison with respective controls secondary [25] and primary [49,50] hypertension enhanced and reduced the vasodilator responses to methanadamide and/or anandamide in rat sMAs and/or the mesenteric vascular bed, respectively; endocannabinoids levels were lower in SHR and higher in DOCA-salt ([51]; one should keep in mind that CBD is antagonist of anandamide degradation), pressor responses of MAs to periarterial nerve stimulation and to exogenous noradrenaline infusion were higher in SHRs and lower or without change in DOCA-salt, respectively [52]. Thus, various genetic and environmental triggers or key pathophysiological mechanisms characterized both models of hypertension [53] might determine opposite responses to CBD determined by us in SHR and DOCA-salt.
Importantly, none of the mechanisms involved in the CBD-induced vasorelaxation in hypertension was detected in normotension despite the fact that all the receptors described below were expressed in sMAs of normotensive and hypertensive rats (present study, [25,49]). Thus, CB1 receptors were involved in the CBD-induced vasorelaxation as it was reduced by their antagonist AM251 and increased Cnr1 expression was observed in sMAs in both models of hypertension. Their up-regulation, shown by the higher expression level and their involvement in methanandamide-induced relaxation and potential protective role, has been previously demonstrated in sMAs isolated from primary [49] and secondary [25] hypertensive rats. Moreover, the enhancement and a potential protective effect of CB1 receptor-mediated presynaptic inhibition of the sympathetic nerve endings innervating resistance vessels of pithed rats was noticed in DOCA-salt hypertension [54] but not in SHR [55]. In contrast to hPAs, in rat sMAs, AM251 reduced CBD's potency, suggesting that CBD might rather act directly at CB1 receptors in this vascular bed. Interestingly, in both models of hypertension, they are expressed in the endothelium and the smooth muscle, but AM251 reduced the CBD effect in endothelium-intact arteries only. In contrast, in hMAs CBD acted at CB1 receptors that are present on both the endothelium and smooth muscle [5].
The CBD-induced relaxation was diminished by the CB2 receptor antagonist AM630 in DOCA-salt, but not in SHR. This is in line with the increased Cnr2 expression and CB2 immunostaining observed in DOCA-salt only. Similarly, AM630 diminished the CBD-stimulated improvement of the acetylcholine-evoked vasorelaxation in sMAs of diabetic rats, resulting probably from the off-target effect of AM630 or the allosteric effect of CBD on CB2 receptors [16]. Thus, we could not exclude that these phenomena appeared also in hypertension.
In contrast to responses to CBD in hPAs (see above) and in hMAs [5], the TRPV1 receptor antagonist capsazepine failed to produce the vasodilator effect of CBD in normotensive and hypertensive rats. This is in agreement with the present and former studies, which have demonstrated unchanged TRPV1 receptor expression and/or no alternation in the TRPV1-sensitive component of cannabinoid-mediated vasorelaxant effects in DOCA-salt rats (methanandamide [25]) or in SHR (anandamide [50]).
Limitations and dichotomous cannabidiol pharmacology
It is important to mention the following limitations of our study. Polypharmacological profiles of CBD [38,56] makes it impossible to precisely determine its vasodilatory mechanism of action. The above was probably the reason that CBD was originally introduced to cardiovascular system pharmacology as the antagonist of the currently questionable endothelial atypical cannabinoid receptor [12,20,21,47] that given in nonpreconstricted arteries did not produce any or only slight vasodilatation. However, currently it is known as a potent vasodilator [5,12,13], but this effect is observed in preconstricted arteries. In the literature, there still exists two opposite pharmacological descriptions that complicated the final conclusions. The efforts to re-evaluate discrepancies between the studies on the pharmacological profile of CBD were made and described in detail by Bondarenko [43,37]. The lipophilic nature of CBD and other cannabinoid ligands could allow them (especially in μmol/l concentrations) to incorporate into membranes or associate with lipid rafts [37]. We have done our best to use the selective ligands of particular antagonists (e.g. according to [23]). However, AM251 has also been suggested to directly inhibit KCa1.1 channel activity independently of cannabinoid receptors [37] and/or act as a mixed CB1/GPR18 antagonist [57]. The complex effects of O-1918 were described above. Thus, the confirmation of the role of CB1, PPARγ and/or GPR18 receptors in the CBD-mediated vasorelaxation warrants further investigation and application of more selective antagonists combined with genetic tools.
Conclusion and perspectives
In conclusion, our results demonstrate that CBD caused a full concentration-dependent and endothelium-dependent relaxation of hPAs. This was mediated through IP, EP4 and TRPV1 receptors and KCa channels. Moreover, CBD produced a time-dependent slowly developing decrease in the tone of endothelium-intact hPAs sensitive to the PPARγ antagonist, GW9662. Pulmonary hypertension is a life-threatening condition that lacks effective therapy. The following facts underline the possibility that CBD might represent a future goal/option in the therapy of this disease that had been previously suggested to abn-CBD [22]: the strong vasorelaxant efficacy of CBD in hPAs is dependent among others on IP receptors, prostacyclin analogues belong to the frontline therapy against pulmonary hypertension [58] and inhalation is the main route of cannabinoid administration. The vasorelaxant effect of CBD was reduced by comorbidities, including hypertension, obesity and hypercholesterolemia and in patients taking β-blockers. In addition, response to CBD is species-specific, vascular-bed-specific and hypertension model differences, as in phenylephrine-preconstricted rat sMAs CBD-evoked vasorelaxation was enhanced in DOCA-salt but diminished in SHR compared with respective normotensive controls and the protective role of CB1 receptors has been suggested in animal models of hypertension but not in human. In light of the evidence that CBD could reduce BP in stressed patients, our data supplies a further rationale that the cardiovascular system could be indeed a valid therapeutic target for CBD and that preclinical responses to CBD will translate into the human cardiovascular system. Thus, CBD-derived therapies after years might have potential for the evaluation and development of CBD-based drugs not only for neuronal disorders but also in cardiovascular diseases. Importantly, CBD is a nonpsychotropic drug and it has been demonstrated to offer well tolerated therapy. Overall, our results suggest that although the beneficial effects of CBD in cardiovascular disorders are known, additional studies are required to fully understand its potential contributions in the clinical setting [1].
ACKNOWLEDGEMENTS
This study was supported by the National Science Centre (Poland) (grant no. NCN 2015/19/B/NZ7/02270); and by the Medical University of Białystok (Poland) (grant no. N/ST/ZB/17/003/2213). We wish to thank Mrs. I. Malinowska and Mrs. A. Toczydłowska for their excellent technical assistance.
Previous presentations of part of this work: Baranowska-Kuczko M, Kozłowska H, Kloza M, Karpińska O, Toczek M, Kozłowski M, et al. Cannabidiol-induced vasorelaxation in isolated human pulmonary and small mesenteric arteries of hypertensive rat - preliminary study. In: 26th Annual Symposium on Cannabinoids, International Cannabinoid Research Society. Book of abstracts, 2016; pp. 1--19.
Karpińska O, Baranowska-Kuczko M, Kloza M, Kozłowski M, Kozłowska H, Malinowska B. Hypertension modifies the cannabidiol-mediated vascular response in isolated human pulmanory and rat small mesentric arteries. In: 28th Annual Symposium on Cannabinoids, International Cannabinoid Research Society. Book of abstracts, 2018; pp. 1--49.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Abbreviations: 2-AG, 2-arachidonoyl glycerol; abn-CBD, abnormal cannabidiol; ACE, angiotensin-converting enzyme; ARBs, angiotensin receptor blockers; AT1, angiotensin II receptor type 1; BMI, Body Mass Index; BP, blood pressure; CAD, coronary artery disease; CBD, cannabidiol; COPD, chronic obstructive pulmonary disease; COX-1, cyclooxygenase 1; COX-2, cyclooxygenase 2; CRCs, concentration-response curves; DETCA, sodium diethyldithiocarbamate trihydrate; DMF, N,N-dimethylformamide; DMSO, dimethyl sulfoxide; DOCA, 11-deoxycorticosterone acetate; DOCA-salt, 11-deoxycorticosterone acetate, high salt-diet treated and uninephrectomized; EP4, prostanoid EP4 receptor; FAAH, fatty acid amide hydrolase; hMAs, human mesenteric arteries, hPAs, human pulmonary arteries, Indo, indomethacin; IP, prostacyclin receptor; KCa, Ca2+-activated K+ channels; l-NAME, Nω-nitro-l-arginine methyl ester; LPI, L-alpha-lysophosphatidylinositol; NO, nitric oxide; NOS, nitric oxide synthetase; NSAIDs, nonsteroidal anti-inflammatory drugs; PGI2, prostacyclin; PPARγ, peroxisome proliferator-activated receptors γ; PPIs, proton pump inhibitors; rPA, rat pulmonary artery; SHAM, control sham-operated rats; SHR, spontaneously hypertensive rats; sMAs, small mesenteric arteries; SOD, superoxide dismutase; TP, prostanoid thromboxane receptor; TRPV1, transient receptor potential vanilloid 1; WKY, Wistar–Kyoto rats
REFERENCES
- 1.Pisanti S, Malfitano AM, Ciaglia E, Lamberti A, Ranieri R, Cuomo G, et al. Cannabidiol: State of the art and new challenges for therapeutic applications. Pharmacol Ther 2017; 175:133–150. [DOI] [PubMed] [Google Scholar]
- 2.Couch DG, Tasker C, Theophilidou E, Lund JN, O'Sullivan SE. Cannabidiol and palmitoylethanolamide are anti-inflammatory in the acutely inflamed human colon. Clin Sci (Lond) 2017; 131:2611–2626. [DOI] [PubMed] [Google Scholar]
- 3.Scharf EL. Translating endocannabinoid biology into clinical practice: cannabidiol for stroke prevention. Cannabis Cannabinoid Res 2017; 2:259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubin R. The path to the first FDA-approved cannabis-derived treatment and what comes next. JAMA 2018; 320:1227–1229. [DOI] [PubMed] [Google Scholar]
- 5.Stanley CP, Hind WH, Tufarelli C, O'Sullivan SE. Cannabidiol causes endothelium-dependent vasorelaxation of human mesentric arteries via CB1 activation. Cardiovasc Res 2015; 107:568–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Booz GW. Cannabidiol as an emergent therapeutic strategy for lessening the impact of inflammation on oxidative stress. Free Radic Biol Med 2011; 51:1054–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stanley CP, Hind WH, O'Sullivan SE. Is the cardiovascular system a therapeutic target for cannabidiol? Br J Clin Pharmacol 2013; 75:313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sultan SR, Millar SA, England TJ, O'Sullivan SE. A systematic review and meta-analysis of the haemodynamic effects of cannabidiol. Front Pharmacol 2017; 8:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sultan SR, England TJ, O‘Sullivan SE. Acute and chronic effects of cannabidiol on haemodynamics in healthy males [Abstract]. In: 29th Annual Symposium on the Cannabinoids, International Cannabinoid Research Society, Research Triangle Park, NC, USA, 2019. p. 6. [Google Scholar]
- 10.Malinowska B, Toczek M, Pędzińska-Betiuk A, Schlicker E. Cannabinoids in arterial, pulmonary and portal hypertension - mechanisms of action and potential therapeutic significance. Br J Pharmacol 2019; 176:1395–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jadoon KA, Tan GD, O'Sullivan SE. A single dose of cannabidiol reduces blood pressure in healthy volunteers in a randomized crossover study. JCI Insight 2017; 2: pii: 93760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Offertáler L, Mo FM, Bátkai S, Liu J, Begg M, Razdan RK, et al. Selective ligands and cellular effectors of a G protein-coupled endothelial cannabinoid receptor. Mol Pharmacol 2003; 63:699–705. [DOI] [PubMed] [Google Scholar]
- 13.O'Sullivan SE, Sun Y, Bennett AJ, Randall MD, Kendall DA. Time-dependent vascular actions of cannabidiol in the rat aorta. Eur J Pharmacol 2009; 612:61–68. [DOI] [PubMed] [Google Scholar]
- 14.Stanley CP, Wheal AJ, Randall MD, O'Sullivan SE. Cannabinoids alter endothelial function in the Zucker rat model of type 2 diabetes. Eur J Pharmacol 2013; 720:376–382. [DOI] [PubMed] [Google Scholar]
- 15.Wheal AJ, Jadoon KA, Randall MD, O'Sullivan SE. In vivo cannabidiol treatment improves endothelium-dependent vasorelaxation in mesentric arteries of zucker diabetic fatty rats. Front Pharmacol 2017; 8:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wheal AJ, Cipriano M, Fowler CJ, O'Sullivan SE. Cannabidiol improves vasorelaxation in zucker diabetic fatty rats through cyclooxygenase activation. J Pharmacol Exp Ther 2014; 351:457–466. [DOI] [PubMed] [Google Scholar]
- 17.Kossakowski R, Schlicker E, Toczek M, Weresa J, Malinowska B. Cannabidiol affects the Bezold-Jarisch reflex via TRPV1 and 5-HT3 receptors and has peripheral sympathomimetic effects in spontaneously hypertensive and normotensive rats. Front Pharmacol 2019; 10:500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hložek T, Uttl L, Kadeřábek L, Balíková M, Lhotková E, Horsley RR, et al. Pharmacokinetic and behavioural profile of THC, CBD, and THC+CBD combination after pulmonary, oral, and subcutaneous administration in rats and confirmation of conversion in vivo of CBD to THC. Eur Neuropsychopharmacol 2017; 27:1223–1237. [DOI] [PubMed] [Google Scholar]
- 19.Baranowska–Kuczko M, Kozłowska H, Kozłowski M, Schlicker E, Kloza M, Surażyński A, et al. Mechanism of endothelium – dependent relaxation evoked by anandamide in isolated human pulmonary arteries. Naunyn Schmiedebergs Arch Pharmacol 2014; 387:477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baranowska-Kuczko M, MacLean MR, Kozłowska H, Malinowska B. Endothelium-dependent mechanisms of the vasodilatory effect of the endocannabinoid, anandamide, in the rat pulmonary artery. Pharmacol Res 2012; 66:251–259. [DOI] [PubMed] [Google Scholar]
- 21.Kozłowska H, Baranowska M, Schlicker E, Kozłowski M, Laudański J, Malinowska B. Identification of the vasodilatory endothelial cannabinoid receptor in the human pulmonary artery. J Hypertens 2007; 25:2240–2248. [DOI] [PubMed] [Google Scholar]
- 22.Hornig B. Endothelial vasodilatory cannabinoid receptor in the human pulmonary artery: a future option in the therapy of pulmonary hypertension? J Hypertens 2007; 25:2202–2203. [DOI] [PubMed] [Google Scholar]
- 23.Alexander SPH, Christopoulos A, Davenport AP, Kelly E, Marrion NV, Peters JA, et al. THE CONCISE GUIDE TO PHARMACOLOGY 2017/18: G protein-coupled receptors. Br J Pharmacol 2017; 174: Suppl 1: S17–S129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pędzińska-Betiuk A, Weresa J, Toczek M, Baranowska-Kuczko M, Kasacka I, Harasim-Symbor E, et al. Chronic inhibition of fatty acid amide hydrolase by URB597 produces differential effects on cardiac performance in normotensive and hypertensive rats. Br J Pharmacol 2017; 174:2114–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baranowska-Kuczko M, Kozłowska H, Kloza M, Karpińska O, Toczek M, Harasim E, et al. Protective role of cannabinoid CB1 receptors and vascular effects of chronic administration of FAAH inhibitor URB597 in DOCA-salt hypertensive rats. Life Sci 2016; 151:288–299. [DOI] [PubMed] [Google Scholar]
- 26.Ho WS, Zheng X, Zhang DX. Role of endothelial TRPV4 channels in vascular actions of the endocannabinoid, 2-arachidonoylglycerol. Br J Pharmacol 2015; 172:5251–5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karpińska O, Baranowska-Kuczko M, Malinowska B, Kloza M, Kusaczuk M, Gęgotek A, et al. Mechanisms of l-alpha-lysophosphatidylinositol-induced relaxation in human pulmonary arteries. Life Sci 2018; 192:38–45. [DOI] [PubMed] [Google Scholar]
- 28.Kozłowska H, Baranowska-Kuczko M, Schlicker E, Kozłowski M, Kloza M, Malinowska B. Relaxation of human pulmonary arteries by PPARγ agonists. Naunyn Schmiedebergs Arch Pharmacol 2013; 386:445–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kusaczuk M, Krętowski R, Stypułkowska A, Cechowska-Pasko M. Molecular and cellular effects of a novel hydroxamate-based HDAC inhibitor – belinostat – in glioblastoma cell lines: a preliminary report. Invest New Drugs 2016; 34:552–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.AlSuleimani YM, Hiley CR. The GPR55 agonist lysophosphatidylinositol relaxes rat mesenteric resistance artery and induces Ca2+ release in rat mesenteric artery endothelial cells. Br J Pharmacol 2015; 172:3043–3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mikłosz A, Łukaszuk B, Żendzian-Piotrowska M, Brańska-Januszewska J, Ostrowska H, Chabowski A. Challenging of AS160/TBC1D4 alters intracellular lipid milieu in L6 myotubes incubated with palmitate. J Cell Physiol 2017; 232:2373–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pfaffl MW. A new mathematical model for relative quantification in real-time RT– PCR. Nucleic Acids Res 2001; 29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manini AF, Yiannoulos G, Bergamaschi MM, Hernandez S, Olmedo R, Barnes AJ, et al. Safety and pharmacokinetics of oral cannabidiol when administered concomitantly with intravenous fentanyl in humans. J Addict Med 2015; 9:204–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zuardi AW, Hallak JE, Dursun SM, Morais SL, Sanches RF, Musty RE, et al. Cannabidiol monotherapy for treatment-resistant schizophrenia. J Psychopharmacol 2006; 20:683–686. [DOI] [PubMed] [Google Scholar]
- 35.Stanley CP, O'Sullivan SE. Cyclooxygenase metabolism mediates vasorelaxation to 2-arachidonoylglycerol (2-AG) in human mesenteric arteries. Pharmacol Res 2014; 81:74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Köhler R, Kaistha BP, Wulff H. Vascular KCa-channels as therapeutic targets in hypertension and restenosis disease. Expert Opin Ther Targets 2010; 14:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bondarenko AI, Panasiuk O, Drachuk K, Montecucco F, Brandt KJ, Mach F. The quest for endothelial atypical cannabinoid receptor: BKCa channels act as cellular sensors for cannabinoids in in vitro and in situ endothelial cells. Vascul Pharmacol 2018; 102:44–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McPartland JM, Duncan M, Di Marzo V, Pertwee RG. Are cannabidiol and Δ9-tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review. Br J Pharmacol 2015; 172:737–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karpińska O, Baranowska-Kuczko M, Kloza M, Ambrożewicz E, Kozłowski T, Kasacka I, et al. Activation of CB1 receptors by 2-arachidonoylglycerol attenuates vasoconstriction induced by U46619 and angiotensin II in human and rat pulmonary arteries. Am J Physiol Regul Integr Comp Physiol 2017; 312:R883–R893. [DOI] [PubMed] [Google Scholar]
- 40.Howlett AC, Scott DK, Wilken GH. Regulation of adenylate cyclase by cannabinoid drugs. Insights based n thermodynamic studies. Biochem Pharmacol 1989; 38:3297–3304. [DOI] [PubMed] [Google Scholar]
- 41.Tham M, Yilmaz O, Alaverdashvili M, Kelly MEM, Denovan-Wright EM, Laprairie RB. Allosteric and orthosteric pharmacology of cannabidiol and cannabidiol-dimethylheptyl at the type 1 and type 2 cannabinoid receptors. Br J Pharmacol 2019; 176:1455–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iannotti FA, Hill CL, Leo A, Alhusaini A, Soubrane C, Mazzarella E, et al. Nonpsychotropic plant cannabinoids, cannabidivarin (CBDV) and cannabidiol (CBD), activate and desensitize transient receptor potential vanilloid 1 (TRPV1) channels in vitro: potential for the treatment of neuronal hyperexcitability. ACS Chem Neurosci 2014; 5:1131–1141. [DOI] [PubMed] [Google Scholar]
- 43.Bondarenko AI. Endothelial atypical cannabinoid receptor: do we have enough evidence? Br J Pharmacol 2014; 171:5573–5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McHugh D. GPR18 in microglia: implications for the CNS and endocannabinoid system signalling. Br J Pharmacol 2012; 167:1575–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bondarenko AI, Panasiuk O, Okhai I, Montecucco F, Brandt KJ, Mach F. Direct activation of Ca2+ and voltage-gated potassium channels of large conductance by anandamide in endothelial cells does not support the presence of endothelial atypical cannabinoid receptor. Eur J Pharmacol 2017; 805:14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Godlewski G, Offertáler L, Osei-Hyiaman D, Mo FM, Harvey-White J, Liu J, et al. The endogenous brain constituent N-arachidonoyl L-serine is an activator of large conductance Ca2+-activated K+ channels. J Pharmacol Exp Ther 2009; 328:351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kozłowska H, Baranowska M, Schlicker E, Kozłowski M, Laudañski J, Malinowska B. Virodhamine relaxes the human pulmonary artery through the endothelial cannabinoid receptor and indirectly through a COX product. Br J Pharmacol 2008; 155:1034–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kloza M, Baranowska-Kuczko M, Malinowska B, Karpińska O, Harasim-Symbor E, Kasacka I, et al. The influence of DOCA-salt hypertension and chronic administration of the FAAH inhibitor URB597 on KCa2.3/KCa3.1-EDH-type relaxation in rat small mesenteric arteries. Vascul Pharmacol 2017; 99:65–73. [DOI] [PubMed] [Google Scholar]
- 49.Kloza M, Baranowska-Kuczko M, Kozłowska H, Karpińska O, Harasim-Symbor E, Kasacka I, Malinowska B. Influence of the chronić administration of fatty acid amide hydrolase inhibitor, URB597, on the vascular reactivity in small mesenteric arteries of spontaneously hypertensive rats [abstract]. J Physiol Pharmacol 2017; 68: Suppl. 1: 61. [Google Scholar]
- 50.Wheal AJ, Randall MD. Effects of hypertension on vasorelaxation to endocannabinoids in vitro. Eur J Pharmacol 2009; 603:79–85. [DOI] [PubMed] [Google Scholar]
- 51.Biernacki M, Malinowska B, Timoszuk M, Toczek M, Jastrząb A, Remiszewski P, Skrzydlewska E. Hypertension and chronic inhibition of endocannabinoid degradation modify the endocannabinoid system and redox balance in rat heart and plasma. Prostaglandins Other Lipid Mediat 2018; 138:54–63. [DOI] [PubMed] [Google Scholar]
- 52.Gamoh S, Shiba T, DiPette DJ, Yamamoto R. Differences in the response to periarterial nerve stimulation or exogenous noradrenaline infusion in the mesenteric vascular bed with the intestinal tract harvested from commonly used rat models of hypertension. Clin Exp Pharmacol Physiol 2019; 46:427–434. [DOI] [PubMed] [Google Scholar]
- 53.Lerman LO, Kurtz TW, Touyz RM, Ellison DH, Chade AR, Crowley SD, et al. Animal models of hypertension: a scientific statement from the American Heart Association. Hypertension 2019; 73:e87–e120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Toczek M, Schlicker E, Grzęda E, Malinowska B. Enhanced function of inhibitory presynaptic cannabinoid CB1 receptors on sympathetic nerves of DOCA-salt hypertensive rats. Life Sci 2015; 138:78–85. [DOI] [PubMed] [Google Scholar]
- 55.Malinowska B, Toczek M, Kossakowski R. Function of presynaptic inhibitory cannabinoid CB1 receptors on sympathetic nerves of spontaneously hypertensive rats [abstract]. Naunyn Schmiedebergs Arch Pharmacol 2017; 390: Suppl 1: 36. [Google Scholar]
- 56.Di Marzo V. New approaches and challenges to targeting the endocannabinoid system. Nat Rev Drug Discov 2018; 17:623–639. [DOI] [PubMed] [Google Scholar]
- 57.Su EN, Kelly ME, Cringle SJ, Yu DY. Role of endothelium in abnormal cannabidiol-induced vasoactivity in retinal arterioles. Invest Ophthalmol Vis Sci 2015; 56:4029–4037. [DOI] [PubMed] [Google Scholar]
- 58.Baliga RS, MacAllister RJ, Hobbs AJ. New perspectives for the treatment of pulmonary hypertension. Br J Pharmacol 2011; 163:125–140. [DOI] [PMC free article] [PubMed] [Google Scholar]


