Lasmiditan, an antimigraine drug with selective 5-HT1F receptor affinity, prejunctionally inhibits calcitonin gene-related peptide release in peripheral and central trigeminal nerve terminals of rodents.
Keywords: 5-HT1F receptors, CGRP, Lasmiditan, Migraine, Trigeminovascular system
Abstract
Migraine headache pathophysiology involves trigeminovascular system activation, calcitonin gene-related peptide (CGRP) release, and dysfunctional nociceptive transmission. Triptans are 5-HT1B/1D/(1F) receptor agonists that prejunctionally inhibit trigeminal CGRP release, but their vasoconstrictor properties limit their use in migraine patients with cardiovascular disease. By contrast, lasmiditan is a novel antimigraine and selective 5-HT1F receptor agonist devoid of vasoconstrictor properties. On this basis, this study has investigated the modulation of trigeminal CGRP release by lasmiditan. For this purpose, we have comparatively analysed the inhibition of several components of the trigeminovascular system induced by lasmiditan and sumatriptan through: ex vivo KCl-induced CGRP release from isolated dura mater, trigeminal ganglion, and trigeminal nucleus caudalis of mice; and in vivo dural vasodilation in the rat closed-cranial window model induced by endogenous (electrical stimulation and capsaicin) and exogenous CGRP. The ex vivo release of CGRP was similarly inhibited by sumatriptan and lasmiditan in all trigeminovascular system components. In vivo, intravenous (i.v.) lasmiditan or higher doses of sumatriptan significantly attenuated the vasodilatory responses to endogenous CGRP release, but not exogenous CGRP effects. These data suggest that lasmiditan prejunctionally inhibits CGRP release in peripheral and central trigeminal nerve terminals. Because lasmiditan is a lipophilic drug that crosses the blood–brain barrier, additional central sites of action remain to be determined.
1. Introduction
Migraine is a chronic neurovascular disorder characterized by recurrent severe attacks of throbbing headache, which can be accompanied by nausea, vomiting, photophobia, and phonophobia.30 Although the pathophysiology of migraine has not been elucidated completely, it has been proposed that the headache phase results from activation and sensitization of trigeminal afferents from meningeal nociceptors, neuropeptide release, and dysfunctional nociceptive transmission.31
Calcitonin gene-related peptide (CGRP) is a key neuropeptide, widely expressed in the peripheral and central components of the trigeminovascular system, involved in craniofacial nociception modulation.11,25,48 Infusion of CGRP triggers immediate headaches in healthy volunteers and immediate headaches as well as delayed migraine-like attacks in migraine patients.30 Moreover, during spontaneous migraine attacks, this peptide is released in the extracerebral circulation.15 Moreover, blocking CGRP or its receptor with monoclonal antibodies, which do not cross the blood–brain barrier, has revealed that migraine attacks can be prevented through peripheral blockade of CGRP.16,44
For almost 30 years, the triptans, 5-HT1B/1D receptor agonists, of which some also display affinity for the 5-HT1F receptor,41 have been the specific treatment of choice for terminating migraine attacks. It has previously been shown that during a migraine attack, triptans normalize the elevated CGRP plasma levels by inhibiting further release from trigeminal afferents and, consequently, decrease nociceptive transmission from the periphery to the central nervous system.14 However, due to their peripheral vasoconstrictor potential, including coronary vasoconstriction, they are contraindicated in migraine patients with cardiovascular diseases.35 This problem led to the search for antimigraine agents lacking vasoconstrictor activity, which culminated in the development of lasmiditan.
Lasmiditan is a novel high-affinity, lipophilic, highly selective 5-HT1F receptor agonist (“ditan,” Table 1) that is currently under development for the acute treatment of migraine.37 Phase III trials revealed clinical efficacy in migraine patients, and preclinical data showed that it is devoid of vasoconstrictor properties in several blood vessels including human isolated coronary arteries.40 However, few studies have investigated its exact (antimigraine) mechanism(s) of action. Lasmiditan may possess central as well as peripheral antinociceptive effects because it reduced c-Fos expression in the trigeminal nucleus caudalis and inhibited the dural plasma protein extravasation, both induced by electrical stimulation of the trigeminal ganglion.37 However, the latter effect has not been demonstrated to occur in migraine patients, and several plasma protein extravasation inhibitors failed to be effective in clinical trials.10 Therefore, based on the neurobiology of migraine headache, we set out to analyze the effects of lasmiditan on peripheral components as well as central projections of the trigeminovascular CGRPergic system, and compared our results with the responses to sumatriptan, as a positive control. For this purpose, we investigated the ex vivo CGRP release in the peripheral (dura mater and trigeminal ganglion) and central (trigeminal nucleus caudalis) trigeminal components of mice, as well as the dural vasodilation induced by endogenous (capsaicin and electrical stimulation) and exogenous CGRP in rats in vivo. Our results show that selective 5-HT1F receptor activation by lasmiditan inhibits CGRP release and, consequently, may attenuate nociceptive transmission in the trigeminovascular system.
Table 1.
Summary of functional (pEC50 values of cAMP assays) and binding (pIC50 values of radioligand binding assays) data of sumatriptan and lasmiditan at 5-HT receptors.
2. Material and methods
2.1. Experimental animals
Experiments were performed in 36 male C57BL/6J (22-24 g; 13-14 weeks of age) mice and 54 male Sprague-Dawley (300-350 g; 8-10 weeks of age) rats, purchased from Charles River. Animals were housed under a 12-hour dark–light cycle in a special room at constant temperature (22 ± 2°C) and humidity (50%), with free access to food and water. Only male animals were used to avoid the well-known interaction of CGRP with cycling estrogen,29 previously reported in the cranial-window model.22 All experimental protocols of the study were approved by the Erasmus University Medical Center's institutional animal ethics committee (permission protocol numbers EMC 2891/3393), in accordance with the European directive 2010/63/EU and ARRIVE (Animal Research: Reporting In Vivo Experiments) reporting guidelines for the care and use of laboratory animals. All animals were randomly assigned into the different experimental protocols. For the CGRP assays (see next section), the analyst was not blinded to the compounds but to the research hypothesis. For the in vivo experiments, values were calculated using the dose–response autoanalyse selection feature of LabChart.
2.2. Ex vivo: calcitonin gene-related peptide release in trigeminal nucleus caudalis, trigeminal ganglion, and dura mater
This technique has been previously reported8; in brief, mice were anesthetized using intraperitoneal (i.p.) sodium pentobarbital (80 mg/kg) and decapitated at the atlanto-occipital joint. The scalp was retracted from the cranium. First, the trigeminal caudal nuclei, which run 9 to 13 mm caudally from bregma, were isolated from the brainstem. The skull was divided into halves by a clear cut along the sagittal suture, and the cerebral hemispheres were carefully removed while the cranial dura was left attached to the skull. Second, the trigeminal ganglia were harvested by dissection 1 mm proximal and distal to the point where the mandibular nerve branches off. Third, the remaining brain tissues were extracted from the hemisected skulls without damaging the dura of the middle cranial fossa. Each trigeminal nucleus caudalis, trigeminal ganglion, and hemisected skull with the dura mater were immersed and washed in carbogenated synthetic interstitial fluid, containing (mM): NaCl (108), KCl (3.48), MgSO4 (3.5), NaHCO3 (26), NaH2PO4 (11.7), CaCl2 (1.5), sodium gluconate (9.6), glucose (5.55), and sucrose (7.6) for 30 minutes at 37°C. The isolated tissues were randomized and placed in a 24-well plate containing 500-μL synthetic interstitial fluid. The 24-well plate was fixed in a water bath that formed a closed humid chamber of 37°C. Basal CGRP values were measured in each tissue; then, CGRP release was induced by superfusion with 60-mM KCl. Vehicle (saline), sumatriptan, or lasmiditan (30 μM each, concentration based on our earlier experiments with sumatriptan)8 was applied 10 minutes before the challenge with 60-mM KCl. For every sample, including the baseline measurement, the solution was collected after 10-minute incubation and mixed with aprotinin (500 KIU/mL). For the assessment of CGRP content, samples were stored at −20°C until processed with a commercial CGRP RIA kit according to the manual (Phoenix Pharmaceuticals, Burlingame, CA). Calcitonin gene-related peptide (pg/mL) absorbance values were calculated through an interpolation method using an equation derived from the standard curve. The assay has a detection level of ∼0.1 pg/mL. Blank wells without CGRP were used as a control to exclude possible false-positive measurements, as previously described.21
2.3. In vivo: intravital microscopy and dural artery vasodilation
Rats were anaesthetized throughout the experiments using sodium pentobarbital (60 mg/kg, i.p. followed by 18 mg/kg intravenously [i.v.] per hour). The adequacy of anesthesia was judged by a negative tail flick test, mean arterial pressure levels, and the absence of ocular reflexes, among others. Tracheal intubation was performed, and a capnograph was used to monitor pCO2 levels. As previously described,50 the femoral vein and artery were cannulated for i.v. administration of drugs and for continuous monitoring of mean arterial pressure, respectively. During the experiment, the core temperature of each animal was maintained between 36.5°C and 37.5°C using a homeothermic blanket system for rodents (Harvard Instruments, Edenbridge, United Kingdom). Subsequently, the rat was placed in a stereotaxic frame, and the parietal bone was drilled thin until the dural middle meningeal artery was clearly visible. Because the rat skull is thin, care was taken to drill with constant application of ice-cold saline. The drilled area was covered with saline to prevent drying of the skull and to facilitate visualization of the artery. The dural artery diameter was captured with an intravital microscope (Leica MZ 16; Leica Microsystem Ltd, Heerbrugg, Switzerland), using a cyan filter on a cold light source. A zoom lens (80-400x magnification) and camera (DCx V3.52, Thorlabs LTD, Ely, United Kingdom) were used to capture the image of the dural artery, which was displayed and measured on a computer using a dedicated software package (IDA-Intravital Dimension Analyser; http://www.beneryx.co.uk) integrated with a ADC/DAC board (DI-158, DATAQ instruments, 's-Hertogenbosch, The Netherlands). Dural artery diameter was calculated from the area under the curve of the intensity measured and expressed in arbitrary units. For periarterial electrical stimulation, a bipolar stimulating electrode (NE 200X, Clark Electromedical, Edenbridge, Kent, United Kingdom) was placed on the surface of the bone approximately 200 µM from the artery. The surface of the cranial window was stimulated at 5 Hz, 1 ms for 10 seconds (Stimulator model S88, Grass Instruments, West Warwick, RI) with a current intensity of 100 µA (monitored on an oscilloscope, model 54601A; Hewlett Packard, Palo Alto, CA) and increasing with 50-µA steps until a maximal level of vasodilation was achieved, usually at 200 µA.23 Data of dural artery diameter and mean arterial blood pressure were recorded using a LabChart data acquisition system (AD Instruments Ltd, Oxford, United Kingdom). Dural artery vasodilator responses produced by periarterial electrical stimulation (100-200 µA), capsaicin (10 µg/kg, i.v.), or exogenous α-CGRP (1 µg/kg, i.v.) were elicited before and after consecutive i.v. bolus injections of vehicle (saline), sumatriptan, or lasmiditan (0.3, 1, 3, and 10 mg/kg each). These doses were based on clinically relevant doses previously calculated40 as well as, for sumatriptan, previous studies performed in the same model.50 In brief, for sumatriptan, assuming a 25 to 100 mg oral dose in a 70-kg adult with a 15% bioavailability would be equivalent to i.v. doses of about 0.05 to 0.21 mg/kg. For lasmiditan, a 50 to 200 mg oral dose in a 70-kg adult with a 40% bioavailability would be equivalent to i.v. doses of about 0.28 to 1.14 mg/kg. Each dose of the aforementioned compounds was administered 5 minutes before a subsequent treatment with electrical stimulation, capsaicin, or CGRP. Dural artery diameter and mean arterial pressure values, were both restored to preinjection levels by the time the next vasodilation was induced, and 30 minutes was allowed to elapse after each of the vasodilator treatments for the recovery of baseline diameter.
2.4. Statistical analysis
All data are expressed as mean ± SEM. Calcitonin gene-related peptide release in the different trigeminal components of the mice was expressed as relative stimulated CGRP release, which was calculated by the ratio of KCl-induced CGRP release and basal CGRP values.12 Statistical differences of the basal CGRP values, the relative stimulated CGRP release in the presence of vehicle, and the % inhibition of CGRP release between groups were calculated using a one-tailed Mann–Whitney test for unpaired observations, as previously described.8 For comparing the relative stimulated CGRP release in the presence of vehicle to its corresponding treatment (sumatriptan or lasmiditan), a one-tailed Wilcoxon matched-pairs test for nonparametric analysis of paired was used. The peak changes in dural artery diameter were expressed as percent change from baseline. Changes in mean arterial pressure were expressed in absolute values (mm Hg). The differences between the variables within one group of animals were compared by using a one-way repeated-measures analysis of variance followed by Dunnett test. Statistical significance was accepted at P < 0.05.
2.5. Compounds
The compounds used in this study (obtained from the sources indicated) were: rat α-CGRP (NeoMPS S.A., Strasbourg, France); sumatriptan succinate and capsaicin (Sigma Chemical, Co, Steinheim, Germany); and lasmiditan hydrochloride (provided by Eli Lilly & Co, Indianapolis, IN). Calcitonin gene-related peptide, sumatriptan, and lasmiditan were dissolved in physiological saline. Capsaicin (1 mg/mL) was dissolved in a mixture of Tween-80, ethanol 70%, and water (1:1:8). The doses mentioned in the text refer to the free base of substances in all cases.
3. Results
3.1. Ex vivo: basal calcitonin gene-related peptide levels and relative stimulated calcitonin gene-related peptide release after KCl stimulation
A total of 108 tissues were analyzed. Tissues with basal CGRP measurement errors (CGRP below detection limit, n = 2) or that did not generate CGRP release in response to 60 mM KCl (n = 14) were excluded. In addition, in one experiment, the positive control (inhibition of CGRP by sumatriptan) failed (instead we measured an increase in CGRP release of >15 times); this measurement was a statistically significant outlier (P = 0.016, Dixon outlier test) and was therefore excluded. Because data were paired between the left side and right side, in these cases, both the left and right sides were excluded.
There were no significant differences between the basal CGRP levels from the left and right side components of the trigeminovascular system in each experimental group (data not shown). Moreover, basal CGRP levels (in absolute values; pg/mL) were not different between the sumatriptan (S) and lasmiditan (L) groups in the dura mater (S: 10.7 ± 1.8 vs L: 9.7 ± 3.0; n = 10 and 9 respectively; P = 0.302), trigeminal ganglion (S: 14.7 ± 3.3 vs L: 18.4 ± 10.2; n = 12 each; P = 0.130), and trigeminal nucleus caudalis (S: 25.9 ± 13.2 vs L: 48.5 ± 21.9; n = 10 and 9 respectively; P = 0.219). Moreover, the basal CGRP release values were not modified by the incubation per se of vehicle, sumatriptan, or lasmiditan in all the components of the trigeminovascular system studied (data not shown). The relative stimulated CGRP release (ie, the fold increase compared to baseline) induced by KCl in the presence of vehicle (control) was comparable between both groups in the dura mater (S: 6.0 ± 1.4 vs L: 6.4 ± 1.2; n = 10 and 9, respectively; P = 0.275), trigeminal ganglion (S: 5.4 ± 1.5 vs L: 7.4 ± 2.6; n = 12 each; P = 0.267), and trigeminal nucleus caudalis (S: 9.2 ± 2.8 vs L: 10.9 ± 3.0; n = 10 and 9 respectively; P = 0.330).
3.2. Ex vivo: relative stimulated calcitonin gene-related peptide release in the presence of sumatriptan and lasmiditan
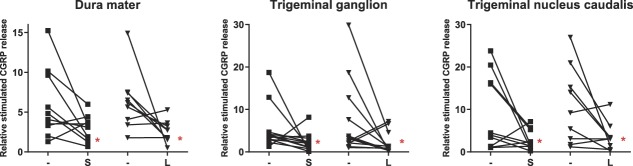
The effects of pretreatment with sumatriptan or lasmiditan (30 µM) on CGRP release in the trigeminovascular components are shown in Figure 1. In the presence of sumatriptan, relative stimulated CGRP release was significantly attenuated in the dura mater (6.0 ± 1.4 vs 3.0 ± 0.5; n = 10; P = 0.032), trigeminal ganglion (5.4 ± 1.5 vs 2.2 ± 0.6; n = 12; P = 0.013), and trigeminal nucleus caudalis (9.2 ± 2.8 vs 2.8 ± 0.7; n = 10; P = 0.032).
Figure 1.
Relative stimulated CGRP release after KCl in the absence (−) or presence of sumatriptan (S) and lasmiditan (L) in the dura mater (n = 9-10), trigeminal ganglion (n = 12), and trigeminal nucleus caudalis (n = 9-10). *P < 0.05 vs vehicle response. CGRP, calcitonin gene-related peptide.
Moreover, lasmiditan also significantly attenuated the relative stimulated CGRP release in the dura mater (6.4 ± 1.2 vs 2.6 ± 0.5; n = 9; P = 0.027), trigeminal ganglion (7.4 ± 2.6 vs 2.1 ± 0.7; n = 12 P = 0.032), and trigeminal nucleus caudalis (10.9 ± 3.0 vs 3.6 ± 1.1; n = 9; P = 0.037). Furthermore, both compounds were equieffective in inhibiting CGRP release in the dura mater (S: 50% vs L: 59%; n = 9-10; P = 0.257), trigeminal ganglion (S: 59% vs L: 71%; n = 12; P = 0.244), and trigeminal nucleus caudalis (S: 70% vs L: 67%; n = 9-10; P = 0.275).
3.3. In vivo: effects of ES, capsaicin, and calcitonin gene-related peptide on mean arterial pressure and dural diameter
At the beginning of the experiments, the average mean arterial pressure from all animals was 99 ± 2 mm Hg. Because there were no significant differences on the hemodynamic and dural artery changes in the control interventions (ES, capsaicin, and CGRP) between groups, data were pooled for further analysis. In none of the experiments, periarterial ES (100-200 μA) affected the mean arterial pressure. By contrast, there was a significant decrease in mean arterial pressure as compared to baseline after the i.v. administration of 10 µg/kg capsaicin (27 ± 5 mm Hg, n = 19) or 1 µg/kg CGRP (29 ± 4 mm Hg, n = 14), which was not different between capsaicin and CGRP (P = 0.744). At the end of the experiments, the value of mean arterial pressure (97 ± 2 mm Hg) was not significantly different from the initial baseline value (P = 0.140; n = 53).
3.4. In vivo: dural artery dilation induced by electrical stimulation, capsaicin, and calcitonin gene-related peptide
Periarterial ES (100-200 µA) increased dural artery diameter by 62 ± 3% (n = 20), whereas i.v. administration of 10 µg/kg capsaicin or 1 µg/kg CGRP increased the diameter by 59 ± 3% (n = 20) and 66 ± 3% (n = 14), respectively. In accordance with our previous controls, repeated treatment (up to 5 times) with electrical stimulation (P = 0.349; n = 4), capsaicin (P = 0.878; n = 4), or CGRP (P = 0.912; n = 4) produced reproducible increases in dural artery diameter.
3.5. In vivo: effects of sumatriptan and lasmiditan per se on mean arterial pressure and dural diameter
Because there were no significant differences on the hemodynamic and dural artery effects among sumatriptan (or lasmiditan) doses in the experimental interventions, data were pooled for further analysis. As shown in Figure 2 (upper panels), sumatriptan injection produced a significant (P < 0.0001, n = 21) short-lasting and dose-dependent vasodepressor response starting at clinically relevant doses. After each injection, mean arterial pressure returned to baseline values before the next systemic vasodilation was elicited. By contrast, lasmiditan was devoid of significant (P = 0.274, n = 20) effects on mean arterial blood pressure at all doses tested. When considering dural artery diameter (Fig. 2; lower panels), all doses of sumatriptan significantly (P < 0.0001, n = 21) and dose-dependently increased the artery diameter, whereas lasmiditan only increased it significantly (P = 0.006, n = 20) after injecting the 2 highest doses.
Figure 2.
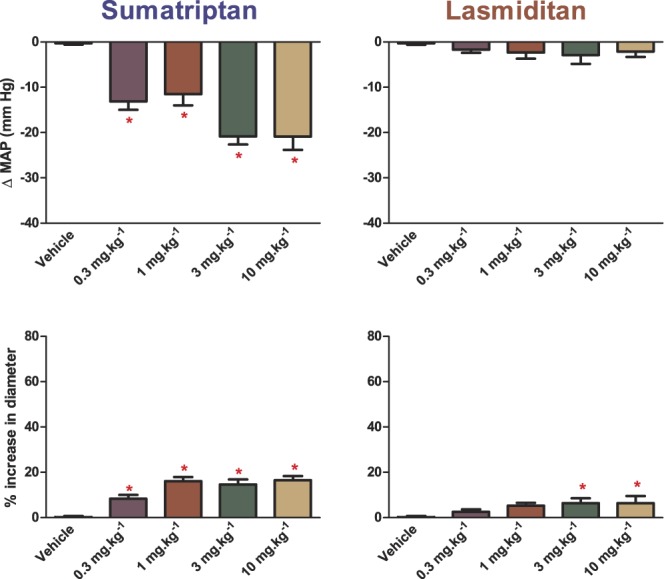
Effects per se of increasing doses of sumatriptan or lasmiditan on mean arterial pressure (MAP) and dural artery diameter. n = 20 to 21. *P < 0.05 vs vehicle response.
3.6. In vivo: effects of sumatriptan and lasmiditan on dural artery dilation induced by electrical stimulation, capsaicin, and calcitonin gene-related peptide
As shown in Figure 3, as compared to their corresponding control, the pretreatment with high doses (3 and 10 mg/kg) of sumatriptan significantly inhibited the vasodilation induced by electrical stimulation (control increase in dural artery diameter: 60 ± 3% vs 3 mg/kg: 30 ± 3%; 10 mg/kg: 21 ± 2%; P < 0.0001; n = 8) and capsaicin (control: 65 ± 6% vs 3 mg/kg: 44 ± 3%; 10 mg/kg: 34 ± 3%; P < 0.0001; n = 8). By contrast, i.v. dural artery diameter responses to exogenous (i.v.) CGRP were not affected by any dose of sumatriptan (P = 0.210; n = 5).
Figure 3.
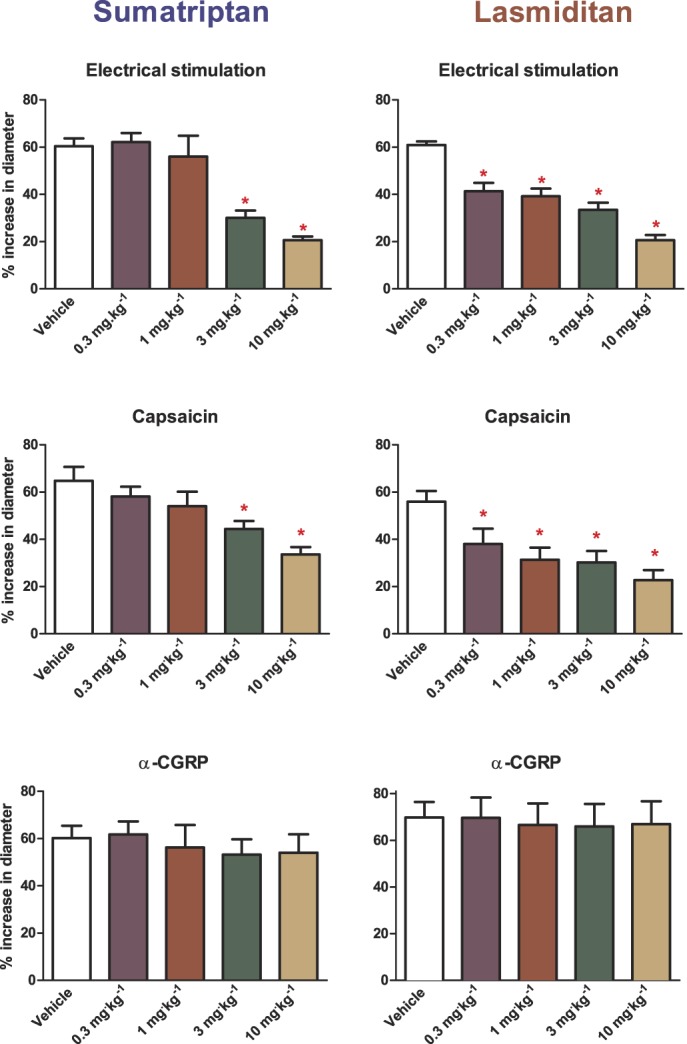
Effects of increasing doses of sumatriptan or lasmiditan on the vasodilation of the dural artery induced by electrical stimulation (upper panels, n = 8 each), capsaicin (middle panels, n = 8 each), and CGRP (lower panels, n = 5 each). *P < 0.05 vs vehicle response. CGRP, calcitonin gene-related peptide.
In contrast to sumatriptan, already lower doses of lasmiditan induced a significant dose-dependent attenuation of the vasodilator responses to electrical stimulation (control increase in dural artery diameter: 61 ± 2% vs 0.3 mg/kg: 41 ± 4%; 1 mg/kg: 39 ± 3%; 3 mg/kg: 34 ± 3%; 10 mg/kg: 21 ± 2%; P < 0.0001; n = 8) and capsaicin (control: 56 ± 5% vs 0.3 mg/kg: 38 ± 7%; 1 mg/kg: 31 ± 5%; 3 mg/kg: 30 ± 5%; 10 mg/kg: 23 ± 4%; P < 0.0001; n = 8) as compared to their corresponding control. Similar as with sumatriptan, the exogenous CGRP responses were not affected by any dose of lasmiditan (P = 0.911; n = 5).
4. Discussion
In this study, we investigated the potential antimigraine site of action of the selective 5-HT1F receptor agonist lasmiditan in relation to the trigeminovascular CGRPergic system, through inhibition of chemically induced (KCl and capsaicin, through voltage-gated calcium and TRPV1 channel activation, respectively) and electrically induced CGRP release from trigeminal sensory fibers.
We compared our results with data obtained with sumatriptan because this was the first triptan developed, and this class of drugs is the current gold standard for the specific acute treatment of migraine attacks. In accordance with our previous work,8 pretreatment with 30-µM sumatriptan attenuated KCl-relative stimulated CGRP release in the peripheral and central components of the trigeminovascular system of mice ex vivo (Fig. 1). This inhibition has previously been shown to be mediated through activation of 5-HT1D and 5-HT1F receptors in rats, whereas a lower concentration (3 µM) of sumatriptan was ineffective.3 Moreover, 30-µM lasmiditan was equieffective in attenuating CGRP release in the dura mater, trigeminal ganglion, as well as trigeminal nucleus caudalis; however, because theoretically multiple 5-HT1 (ie, 5-HT1A and 5-HT1F) inhibitory-Gi protein receptor subtypes expressed in the mice trigeminovascular system7 could be activated at the nonselective concentration used,40 we performed more in-depth experiments with a range of clinically relevant doses in the in vivo rat closed cranial window, an experimental neurovascular model of migraine.50
In accordance with the ex vivo experiments, high doses (3 and 10 mg/kg) of sumatriptan were required to inhibit the neurogenic dural vasodilation in response to i.v. capsaicin and periarterial electrical stimulation. These high-dose inhibitory effects have been previously shown with sumatriptan, and also with rizatriptan, whereas low doses of both compounds (1 mg/kg) were ineffective in this model.50,51 Based on data available on receptor affinity profiles and pharmacological antagonism,5,41 the inhibition of CGRP release by triptans seems to be mediated through activation of 5-HT1D and possibly also 5-HT1F receptors; unfortunately, highly selective antagonists for the 5-HT1F receptors are not yet commercially available to further determine the receptor(s) involved. Remarkably, lasmiditan inhibited the neurogenic dural vasodilation in response to i.v. capsaicin and periarterial electrical stimulation starting at lower, clinically relevant doses; this effect is most likely mediated by selective activation of the 5-HT1F receptor. By contrast, like sumatriptan, lasmiditan was not capable of attenuating the nonneurogenic dural vasodilation in response to exogenous CGRP. Thus, we demonstrate that lasmiditan prejunctionally inhibits the release of CGRP from trigeminal sensory fibers innervating the dural vasculature.
The activation of either 5-HT1B, 5-HT1D, or 5-HT1F receptors on trigeminal fibers results in a direct inhibition of adenylyl cyclase and a subsequent decrease in the cAMP-signaling pathway,1 which in turn alters the phosphorylation of TRPV1 channels6 and decreases the release of CGRP, as evidenced by the inhibition of CGRP- and capsaicin-induced dural vasodilation by sumatriptan and lasmiditan. In addition, activation of 5-HT1 receptors also inhibits neurotransmitter release occurring after stimulation with KCl or electrical stimulation.8,18,20 However, if both compounds act through the same second messenger cascade, why does agonism of the 5-HT1F receptor seem to be more effective?
Although the mRNA expression of the 5-HT1D and 5-HT1F receptors has been detected in the peripheral and central components of the rat trigeminovascular system,3 and both receptors have been found to be colocalized with CGRP,2,34 a small study (n = 3) found that the 5-HT1D receptor is more abundantly expressed in the trigeminal ganglion, whereas the highest concentration of 5-HT1F receptors was found in the trigeminal nucleus caudalis, and this expression pattern could result in diverse inhibition profiles.3 However, more in-depth studies have found that the 5-HT1B, 5-HT1D, and 5-HT1F receptors are equally expressed in the trigeminal ganglion.9,39 Therefore, the difference between sumatriptan and lasmiditan inhibition results does not seem to be explained by the expression/density profile of the 5-HT1B/1D/1F receptors, and may involve (although this study does not prove it) drug-dependent factors such as the affinity and intrinsic activity of these compounds for each of these receptors at the specific components of the trigeminovascular system.
It is noteworthy that species differences have been described for the 5-HT1 receptor subtypes mediating the inhibition of trigeminal CGRP release by 5-HT1B and 5-HT1D receptor agonists (including sumatriptan). This inhibition has previously been attributed to activation of 5-HT1B receptors in rats and mice, and 5-HT1D receptors in guinea pigs, cats, and humans.49 Moreover, the rat and mouse 5-HT1B receptor has been considered to represent the counterpart to the 5-HT1D receptor in other species.24 Thus, sumatriptan's receptor affinity for the 5-HT1 receptors in rats (5-HT1D >5-HT1B) correlates with the high doses required to inhibit CGRP release through 5-HT1B receptors and, possibly also, 5-HT1F receptors. In addition, as described above, the CGRPergic system is influenced by cycling estrogen, which we have previously described in both the human26 and rodent22 trigeminovascular system, whereas a link between testosterone and CGRP release remains to be demonstrated. Thus, in future studies, it would be interesting to investigate the effects of lasmiditan on trigeminovascular CGRP release in female animals with different hormonal status, but also in male animals that are intact or orchiectomized.
It should be kept in mind that our rodent model is suitable for studying inhibition of CGRP release, but not hemodynamic changes in response to 5-HT1 receptor stimulation. In support of this is the fact that lower doses of i.v. sumatriptan immediately activated systemic vascular 5-HT receptors before reaching the 5-HT1D (and later 5-HT1B) receptors in the trigeminal sensory fibers. Consequently, the injection of already clinically relevant doses of sumatriptan resulted in a significant drop in mean arterial pressure and dural artery vasodilation (rather than vasoconstriction; Fig. 2), which have been shown to be mediated through activation of 5-HT7 receptors in rodents.46 However, as previously pointed out, rodents are not suitable for studying hemodynamic changes associated with 5-HT1 receptor activation because receptor expression and vascular responses are not similar to nonrodent species, including humans. Hence, we have recently addressed the vasoconstrictor effects of sumatriptan in appropriate and translational (human isolated arteries and canine in vivo) models.40 Similar to our previous findings, i.v. infusions of sumatriptan, through 5-HT1B receptor activation,32 increased systemic and pulmonary arterial pressure in humans,36 which represents a major limitation for triptans use in cardiovascular compromised patients. By contrast, lasmiditan was devoid of hemodynamic effects at all doses tested. Thus, as demonstrated by the above-mentioned studies, clinically relevant doses of lasmiditan are not associated with changes in mean arterial pressure or dural artery diameter. These results suggest that the antimigraine efficacy of lasmiditan is unrelated to direct vasoconstrictive mechanisms, although the inhibition of CGRP release may indirectly affect dural artery diameter.
The 5-HT1F receptor is also widely expressed throughout the central nervous system, and because lasmiditan is a lipophilic drug that crosses the blood–brain barrier (in contrast with sumatriptan), it has been associated with mild central side effects. The most common side effects described in clinical trials are dizziness, paresthesia, somnolence, fatigue, nausea, and lethargy.17,28 Given that lasmiditan inhibits the peripheral components of the trigeminovascular system, it is tempting to speculate what would happen to the side-effect profile or antimigraine efficacy of a selective 5-HT1F receptor agonist that does not enter the brain. Whether inhibiting the trigeminovascular system centrally as well as peripherally is more effective than just peripherally or centrally, or whether a mixed mechanism is associated with increased side effects remains to be determined. Unfortunately, there are no hydrophilic (selective) 5-HT1F receptor agonists available yet.
Additional (antimigraine) mechanisms of action described with previous 5-HT1F receptor agonists include modulation of glutamate release from trigeminal sensory fibers and mitochondrial biogenesis.38,42 Clearly, future experiments, beyond the scope of our study, are needed to determine whether lasmiditan can inhibit glutamatergic neurons in the central nervous system and/or enhance mitochondrial biogenesis. Moreover, it would be interesting to evaluate whether lasmiditan is able to attenuate cortical spreading depression, a key pathogenic event in migraine with aura,47 and whether these mechanisms are also associated with its clinical antimigraine efficacy.
In conclusion, our results indicate that lasmiditan prejunctionally inhibits CGRP release in peripheral and central trigeminal nerve terminals. This effect may, at least partly, explain the clinical efficacy of lasmiditan. Because activation of 5-HT1F receptors is not associated with vasoconstriction, lasmiditan may represent a cardiovascular safety advantage over the vasoactive triptans.
Conflict of interest statement
A. Labastida-Ramírez and E. Rubio-Beltrán received travel support from Eli Lilly. C.M. Villalón received consultation fees from Eli Lilly. K.W. Johnson is employee of Eli Lilly. A. MaassenVanDenBrink received research grants and/or consultation fees from Amgen/Novartis, Eli Lilly, Teva, and ATI. The remaining authors declare no conflicts of interests.
Acknowledgments
This work was supported by grants of the Netherlands Organization for Scientific Research (AMVD; Vidi grant nr. 917.11.349); International Headache Society fellowship (KAH); the Consejo Nacional de Ciencia y Tecnología (CONACyT; ALR, ERB, and CMV grant nr. 410778, 409865, and 219707, respectively); and a research grant from Eli Lilly (AMvdB). None of the above funding sources were involved in the study design, collection, analysis or interpretation of data, or in the writing of the manuscript.
This work has been previously presented as oral and poster presentations and has been published only as abstracts.
Author contributions: A. Labastida-Ramírez: performed the experiments, analyzed the data, and drafted the manuscript; E. Rubio-Beltrán: drafted, revised, and approved the final manuscript; K.A. Haanes: revised and approved the final manuscript; K.Y. Chan: revised and approved the final manuscript; I.M. Garrelds: CGRP quantification, and revised and approved the manuscript; K.W. Johnson: revised and approved the final manuscript; A.H. Danser: revised and approved the final manuscript; C.M. Villalón: revised and approved the final manuscript; A. MaassenVanDenBrink: supervised the experiments and data analysis, and revised and approved the final manuscript.
Availability of data and materials: The data set supporting the conclusion of this article is available upon reasonable request to the corresponding author.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
References
- [1].Adham N, Kao HT, Schecter LE, Bard J, Olsen M, Urquhart D, Durkin M, Hartig PR, Weinshank RL, Branchek TA. Cloning of another human serotonin receptor (5-HT1F): a fifth 5-HT1 receptor subtype coupled to the inhibition of adenylate cyclase. Proc Natl Acad Sci U S A 1993;90:408–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ahn SK, Khalmuratova R, Jeon SY, Kim JP, Park JJ, Hur DG, Balaban CD. Colocalization of 5-HT1F receptor and calcitonin gene-related peptide in rat vestibular nuclei. Neurosci Lett 2009;465:151–6. [DOI] [PubMed] [Google Scholar]
- [3].Amrutkar DV, Ploug KB, Hay-Schmidt A, Porreca F, Olesen J, Jansen-Olesen I. mRNA expression of 5-hydroxytryptamine 1B, 1D, and 1F receptors and their role in controlling the release of calcitonin gene-related peptide in the rat trigeminovascular system. PAIN 2012;153:830–8. [DOI] [PubMed] [Google Scholar]
- [4].Beer MS, Heald MA, McAllister G, Stanton JA. Pharmacological characterisation of a cloned dog 5-HT1B receptor cell line. Eur J Pharmacol 1998;360:117–21. [DOI] [PubMed] [Google Scholar]
- [5].Bhatt DK, Gupta S, Jansen-Olesen I, Andrews JS, Olesen J. NXN-188, a selective nNOS inhibitor and a 5-HT1B/1D receptor agonist, inhibits CGRP release in preclinical migraine models. Cephalalgia 2013;33:87–100. [DOI] [PubMed] [Google Scholar]
- [6].Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RWt. cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron 2002;35:721–31. [DOI] [PubMed] [Google Scholar]
- [7].Bonnavion P, Bernard JF, Hamon M, Adrien J, Fabre V. Heterogeneous distribution of the serotonin 5-HT(1A) receptor mRNA in chemically identified neurons of the mouse rostral brainstem: implications for the role of serotonin in the regulation of wakefulness and REM sleep. J Comp Neurol 2010;518:2744–70. [DOI] [PubMed] [Google Scholar]
- [8].Chan KY, Labastida-Ramirez A, Ramirez-Rosas MB, Labruijere S, Garrelds IM, Danser AH, van den Maagdenberg AM, MaassenVanDenBrink A. Trigeminovascular calcitonin gene-related peptide function in Cacna1a R192Q-mutated knock-in mice. J Cereb Blood Flow Metab 2019;39:718–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Classey JD, Bartsch T, Goadsby PJ. Distribution of 5-HT1B, 5-HT1D and 5-HT1F receptor expression in rat trigeminal and dorsal root ganglia neurons: relevance to the selective anti-migraine effect of triptans. Brain Res 2010;1361:76–85. [DOI] [PubMed] [Google Scholar]
- [10].Diener HC. Rpr100893, A substance-P antagonist, is not effective in the treatment of migraine attacks. Cephalalgia 2003;23:183–5. [DOI] [PubMed] [Google Scholar]
- [11].Eftekhari S, Warfvinge K, Blixt FW, Edvinsson L. Differentiation of nerve fibers storing CGRP and CGRP receptors in the peripheral trigeminovascular system. J Pain;14:1289–303. [DOI] [PubMed] [Google Scholar]
- [12].Fioretti B, Catacuzzeno L, Sforna L, Gerke-Duncan MB, van den Maagdenberg AM, Franciolini F, Connor M, Pietrobon D. Trigeminal ganglion neuron subtype-specific alterations of Ca(V)2.1 calcium current and excitability in a Cacna1a mouse model of migraine. J Physiol 2011;589:5879–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Glennon RA, Hong SS, Dukat M, Teitler M, Davis K. 5-(Nonyloxy) tryptamine: a novel high-affinity 5-HT1D. Beta. Serotonin receptor agonist. J Med Chem 1994;37:2828–30. [DOI] [PubMed] [Google Scholar]
- [14].Goadsby PJ, Edvinsson L. The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurol 1993;33:48–56. [DOI] [PubMed] [Google Scholar]
- [15].Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol 1990;28:183–7. [DOI] [PubMed] [Google Scholar]
- [16].Goadsby PJ, Reuter U, Hallstrom Y, Broessner G, Bonner JH, Zhang F, Sapra S, Picard H, Mikol DD, Lenz RA. A controlled trial of erenumab for episodic migraine. N Engl J Med 2017;377:2123–32. [DOI] [PubMed] [Google Scholar]
- [17].Goadsby PJ, Wietecha LA, Dennehy EB, Kuca B, Case MG, Aurora SK, Gaul C. Phase 3 randomized, placebo-controlled, double-blind study of lasmiditan for acute treatment of migraine. Brain 2019;142:1894–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Göthert M. Presynaptic serotonin receptors in the central nervous system. Ann N Y Acad Sci 1990;604:102–12. [DOI] [PubMed] [Google Scholar]
- [19].Grånäs C, Larhammar D. Identification of an amino acid residue important for binding of methiothepin and sumatriptan to the human 5-HT1B receptor. Eur J Pharmacol 1999;380:171–81. [DOI] [PubMed] [Google Scholar]
- [20].Gupta S, Akerman S, van den Maagdenberg A, Saxena P, Goadsby P, MaassenVanDenBrink A. Intravital microscopy on a closed cranial window in mice: a model to study trigeminovascular mechanisms involved in migraine. Cephalalgia 2006;26:1294–303. [DOI] [PubMed] [Google Scholar]
- [21].Gupta S, Amrutkar DV, Mataji A, Salmasi H, Hay-Schmidt A, Sheykhzade M, Messlinger K, Olesen J, Jansen-Olesen I. Evidence for CGRP re-uptake in rat dura mater encephali. Br J Pharmacol 2010;161:1885–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gupta S, Villalon CM, Mehrotra S, de Vries R, Garrelds IM, Saxena PR, MaassenVanDenbrink A. Female sex hormones and rat dural vasodilatation to CGRP, periarterial electrical stimulation and capsaicin. Headache 2007;47:225–35. [DOI] [PubMed] [Google Scholar]
- [23].Haanes KA, Labastida-Ramírez A, Chan KY, de Vries R, Shook B, Jackson P, Zhang J, Flores CM, Danser AHJ, Villalón CM, MaassenVanDenBrink A. Characterization of the trigeminovascular actions of several adenosine A2A receptor antagonists in an in vivo rat model of migraine. J Headache Pain 2018;19:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hamblin MW, Metcalf MA, McGuffin RW, Karpells S. Molecular cloning and functional characterization of a human 5-HT1B serotonin receptor: a homologue of the rat 5-HT1B receptor with 5-HT1D-like pharmacological specificity. Biochem Biophysical Res Commun 1992;184:752–9. [DOI] [PubMed] [Google Scholar]
- [25].Hendrikse ER, Bower RL, Hay DL, Walker CS. Molecular studies of CGRP and the CGRP family of peptides in the central nervous system. Cephalalgia 2019;39:403–19. [DOI] [PubMed] [Google Scholar]
- [26].Ibrahimi K, Vermeersch S, Frederiks P, Geldhof V, Draulans C, Buntinx L, Lesaffre E, MaassenVanDenBrink A, de Hoon J. The influence of migraine and female hormones on capsaicin-induced dermal blood flow. Cephalalgia 2017;37:1164–72. [DOI] [PubMed] [Google Scholar]
- [27].Kovalchin J, Ghiglieri A, Zanelli E, Ings R, Mathers T. Lasmiditan acts specifically on the 5-HT1F receptor in the central nervous system. Cephalalgia 2016;36:103.25944815 [Google Scholar]
- [28].Kuca B, Silberstein SD, Wietecha L, Berg PH, Dozier G, Lipton RB; Group CM-S. Lasmiditan is an effective acute treatment for migraine: a phase 3 randomized study. Neurology 2018;91:e2222–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Labastida-Ramírez A, Rubio-Beltrán E, Villalón CM, MaassenVanDenBrink A. Gender aspects of CGRP in migraine. Cephalalgia 2019;39:435–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lassen LH, Haderslev PA, Jacobsen VB, Iversen HK, Sperling B, Olesen J. Cgrp may play A causative role in migraine. Cephalalgia 2002;22:54–61. [DOI] [PubMed] [Google Scholar]
- [31].Levy D, Labastida-Ramirez A, MaassenVanDenBrink A. Current understanding of meningeal and cerebral vascular function underlying migraine headache. Cephalalgia 2019;39:1606–22. [DOI] [PubMed] [Google Scholar]
- [32].Longmore J, Razzaque Z, Shaw D, Davenport AP, Maguire J, Pickard JD, Schofield WN, Hill RG. Comparison of the vasoconstrictor effects of rizatriptan and sumatriptan in human isolated cranial arteries: immunohistological demonstration of the involvement of 5-HT1B-receptors. Br J Clin Pharmacol 1998;46:577–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lovenberg TW, Erlander MG, Baron BM, Racke M, Slone AL, Siegel BW, Craft CM, Burns JE, Danielson PE, Sutcliffe JG. Molecular cloning and functional expression of 5-HT1E-like rat and human 5-hydroxytryptamine receptor genes. Proc Natl Acad Sci U S A 1993;90:2184–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ma QP, Hill R, Sirinathsinghji D. Colocalization of CGRP with 5-HT1B/1D receptors and substance P in trigeminal ganglion neurons in rats. Eur J Neurosci 2001;13:2099–104. [DOI] [PubMed] [Google Scholar]
- [35].MaassenVanDenBrink A, Reekers M, Bax WA, Ferrari MD, Saxena PR. Coronary side-effect potential of current and prospective antimigraine drugs. Circulation 1998;98:25–30. [DOI] [PubMed] [Google Scholar]
- [36].MacIntyre PD, Bhargava B, Hogg KJ, Gemmill JD, Hillis WS. Effect of subcutaneous sumatriptan, a selective 5HT1 agonist, on the systemic, pulmonary, and coronary circulation. Circulation 1993;87:401–5. [DOI] [PubMed] [Google Scholar]
- [37].Nelson DL, Phebus LA, Johnson KW, Wainscott DB, Cohen ML, Calligaro DO, Xu YC. Preclinical pharmacological profile of the selective 5-HT1F receptor agonist lasmiditan. Cephalalgia 2010;30:1159–69. [DOI] [PubMed] [Google Scholar]
- [38].Ramadan NM, Skljarevski V, Phebus LA, Johnson KW. 5-HT1F receptor agonists in acute migraine treatment: a hypothesis. Cephalalgia 2003;23:776–85. [DOI] [PubMed] [Google Scholar]
- [39].Reuter U, Salomone S, Ickenstein GW, Waeber C. Effects of chronic sumatriptan and zolmitriptan treatment on 5-HT1 receptor expression and function in rats. Cephalalgia 2004;24:398–407. [DOI] [PubMed] [Google Scholar]
- [40].Rubio-Beltrán E, Labastida-Ramírez A, Haanes KA, van den Bogaerdt A, Bogers AJJC, Zanelli E, Meeus L, Danser AHJ, Gralinski MR, Senese PB, Johnson KW, Kovalchin J, Villalón CM, MaassenVanDenBrink A. Characterization of binding, functional activity and contractile responses of the selective 5-HT1F receptor agonist lasmiditan. Br J Pharmacol 2019;176:4681–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Rubio-Beltran E, Labastida-Ramirez A, Villalon CM, MaassenVanDenBrink A. Is selective 5-HT1F receptor agonism an entity apart from that of the triptans in antimigraine therapy? Pharmacol Ther 2018;186:88–97. [DOI] [PubMed] [Google Scholar]
- [42].Scholpa NE, Lynn MK, Corum D, Boger HA, Schnellmann RG. 5-HT1F receptor-mediated mitochondrial biogenesis for the treatment of Parkinson's disease. Br J Pharmacol 2018;175:348–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Shepherd SL, Williamson DJ, Beer MS, Hill RG, Hargreaves RJ. Differential effects of 5-HT1B/1D receptor agonists on neurogenic dural plasma extravasation and vasodilation in anaesthetized rats. Neuropharmacology 1997;36:525–33. [DOI] [PubMed] [Google Scholar]
- [44].Silberstein SD, Dodick DW, Bigal ME, Yeung PP, Goadsby PJ, Blankenbiller T, Grozinski-Wolff M, Yang R, Ma Y, Aycardi E. Fremanezumab for the preventive treatment of chronic migraine. New Engl J Med 2017;377:2113–22. [DOI] [PubMed] [Google Scholar]
- [45].Terron JA. Role of 5-ht7 receptors in the long-lasting hypotensive response induced by 5-hydroxytryptamine in the rat. Br J Pharmacol 1997;121:563–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Terron JA, Martinez-Garcia E. 5-HT7 receptor-mediated dilatation in the middle meningeal artery of anesthetized rats. Eur J Pharmacol 2007;560:56–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Tozzi A, de Iure A, Di Filippo M, Costa C, Caproni S, Pisani A, Bonsi P, Picconi B, Cupini LM, Materazzi S, Geppetti P, Sarchielli P, Calabresi P. Critical role of calcitonin gene-related peptide receptors in cortical spreading depression. Proc Natl Acad Sci U S A 2012;109:18985–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Uddman R, Tajti J, Hou M, Sundler F, Edvinsson L. Neuropeptide expression in the human trigeminal nucleus caudalis and in the cervical spinal cord C1 and C2. Cephalalgia 2002;22:112–16. [DOI] [PubMed] [Google Scholar]
- [49].Williamson DJ, Hargreaves RJ. Neurogenic inflammation in the context of migraine. Microsc Res Tech 2001;53:167–78. [DOI] [PubMed] [Google Scholar]
- [50].Williamson DJ, Hargreaves RJ, Hill RG, Shepheard SL. Sumatriptan inhibits neurogenic vasodilation of dural blood vessels in the anaesthetized rat--intravital microscope studies. Cephalalgia 1997;17:525–31. [DOI] [PubMed] [Google Scholar]
- [51].Williamson DJ, Shepheard SL, Hill RG, Hargreaves RJ. The novel anti-migraine agent rizatriptan inhibits neurogenic dural vasodilation and extravasation. Eur J Pharmacol 1997;328:61–4. [DOI] [PubMed] [Google Scholar]