Abstract
Aims
We assessed the change from baseline in vitamin E, steroid hormones, adrenocorticotropic hormone (ACTH), and gonadotropins, overall and by lowest achieved low‐density lipoprotein‐cholesterol (LDL‐C) level, in patients with type 2 diabetes and dyslipidaemia after 12 weeks of treatment with evolocumab.
Materials and Methods
This was a prespecified analysis of vitamin E, cortisol, ACTH, gonadal hormones and gonadotropins in the 12‐week, placebo‐controlled BERSON trial of evolocumab in patients with type 2 diabetes and dyslipidaemia. In BERSON, 981 (451 in China) patients on daily atorvastatin 20 mg were randomized to placebo or one of two doses of evolocumab. We measured analyte levels at baseline and week 12 (vitamin E in all patients; steroid/gonadal hormones only in Chinese patients).
Results
In both the global and Chinese populations, absolute vitamin E levels decreased from baseline to week 12 by approximately 6 μmol/L (P < .0001) among evolocumab‐treated patients; however, when normalized for LDL‐C, apoB or non–HDL‐C, we observed no decrease in vitamin E levels. In Chinese patients, levels of cortisol and ACTH as well as the cortisol:ACTH ratio did not change significantly from baseline to week 12. No patient had a cortisol:ACTH ratio <3.0 (nmol/pmol), suggestive of adrenocortical deficiency. We did not observe clinically relevant changes for gonadal hormones and gonadotropins (oestradiol and testosterone in female and male patients, respectively, luteinizing and follicle‐stimulating hormones for both).
Conclusions
In the BERSON study, evolocumab did not adversely affect vitamin E, steroid hormone or gonadotropin levels in the Chinese or global type 2 diabetic populations.
ClinicalTrials.gov NCT02662569.
Keywords: diabetes, dyslipidaemia, hypercholesterolaemia, PCSK9 inhibitor
In this prespecified analysis of the BERSON study in Chinese and global patients with type 2 diabetes and dyslipidaemia, we assessed the change from baseline in vitamin E, steroid hormones, adrenocorticotropic hormone (ACTH), and gonadotropins, overall and by lowest achieved low‐density lipoprotein‐cholesterol (LDL‐C) level after 12 weeks of treatment with evolocumab. We found that evolocumab did not adversely affect vitamin E, steroid hormone or gonadotropin levels in the Chinese or global type 2 diabetic populations.

1. INTRODUCTION
Statins are the first‐line treatment for patients with established cardiovascular disease or high cardiovascular risk. In those unable to achieve low‐density lipoprotein‐cholesterol (LDL‐C) goals with maximally tolerated doses of statins, additional therapies may be prescribed including ezetimibe and proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors. Addition of the PCSK9 inhibitor evolocumab to maximally tolerated statin therapy markedly reduces LDL‐C among patients with primary hypercholesterolaemia and mixed dyslipidaemia,1, 2 and significantly reduces the risk of cardiovascular events (myocardial infarction, stroke and coronary revascularization) among patients with established atherosclerotic cardiovascular disease.1
A recently reported clinical trial (BERSON; NCT02662569) confirmed the efficacy of evolocumab in reducing LDL‐C over 12 weeks among patients with type 2 diabetes and hypercholesterolaemia or mixed dyslipidaemia.3, 4 The BERSON study is the largest diabetes trial with evolocumab performed to date and the first to include a large cohort of patients enrolled in China.5
A possible concern of achieving low or very low levels of LDL‐C is the effect on vitamin E, which is predominantly transported in apolipoprotein B (apoB)‐containing lipoproteins, and lowering these lipoproteins markedly could theoretically impact the delivery of vitamin E to tissues.6, 7, 8 While a prior analysis in mostly nondiabetic patients showed that the effects of evolocumab on vitamin E levels were not clinically significant,9 the unique context of diabetes requires additional evaluation. Oxidative stress is thought to play a key role in the pathogenesis of diabetes and diabetic vascular complications,10, 11 and vitamin E levels are decreased in patients experiencing oxidative stress. Further, vitamin E deficiency has been associated with neurological dysfunction, myopathies and retinopathy.6, 12 For these reasons, evaluation of vitamin E levels in diabetic patients treated with PCSK9 inhibitors is of interest.
Low LDL‐C levels achievable with PCSK9 inhibitors have also led to a concern about decreases in steroid hormone levels (cortisol, oestradiol and testosterone), since steroidogenesis is based on an enzymatic modification of cholesterol.13, 14 Since decreases in values of steroid hormone levels have been related to adverse outcomes in patients with diabetes,15, 16, 17, 18, 19 further evaluation of the effects of low LDL‐C on steroid hormone levels in this population is relevant.
In the present work, we analysed levels of vitamin E in the BERSON study with atorvastatin 20 mg plus evolocumab or placebo in patients with type 2 diabetes and hypercholesterolaemia or mixed dyslipidaemia. We also analysed levels of cortisol, adrenocorticotropic hormone (ACTH), gonadal hormones (oestradiol and testosterone) and gonadotropins (luteinizing hormone [LH] and follicle‐stimulating hormone [FSH]) in the Chinese cohort of this study.
2. METHODS
2.1. Study design
The design and results of the BERSON study (ClinicalTrials.gov, NCT02662569) have been previously described.3, 4, 5 Briefly, this was a double‐blind, randomized, placebo‐controlled clinical trial conducted in 10 countries (Argentina, Brazil, Canada, China, Colombia, France, Republic of Korea, Russian Federation, Turkey and the United States). This study was designed to recruit one‐half of its patients from China. The primary objective was to assess the effect of 12 weeks of evolocumab, versus placebo, in combination with statin therapy, on the per cent change from baseline in LDL‐C in patients with type 2 diabetes and hypercholesterolaemia or mixed dyslipidaemia.
2.2. Patients
Eligible patients were ≥18 to ≤80 years of age with type 2 diabetes, were receiving stable pharmacological therapy for diabetes for ≥6 months and had screening haemoglobin A1c (HbA1c) ≤10% and fasting triglycerides ≤4.5 mmol/L. Lipid‐lowering therapy status had to be unchanged for ≥4 weeks before LDL‐C screening. Patients on statin therapy at screening with an LDL‐C of ≥2.6 mmol/L and those not on statin therapy at screening with an LDL‐C of ≥3.4 mmol/L entered in a lipid stabilization period and started daily oral atorvastatin 20 mg, after which eligible patients were randomized to subcutaneous evolocumab 140 mg every 2 weeks (Q2W), evolocumab 420 mg monthly (QM), placebo Q2W or placebo QM (2:2:1:1) added to daily oral atorvastatin 20 mg. Dosing groups were pooled in the present analysis of vitamin E and steroidal and gonadal hormones because the doses were clinically equivalent in prior analyses.4, 5, 20 The co‐primary end‐points of the BERSON study were per cent change in LDL‐C from baseline to week 12 and to the mean of weeks 10 and 12.
2.3. Vitamin E, cortisol, ACTH, gonadal hormone and gonadotropin populations
The analysis of vitamin E, steroid hormones and gonadotropins in the BERSON study is similar in methodology to the previously reported analysis in the Durable Effect of PCSK9 Antibody Compared with Placebo Study (DESCARTES) study.9 We measured vitamin E, cortisol, ACTH, gonadal hormone and gonadotropin levels at baseline and week 12. All laboratory values were analysed at a central laboratory (Medpace Reference Laboratories: Cincinnati, Ohio, USA; Leuven, Belgium; Beijing, China; Singapore, Singapore).
Vitamin E was measured at baseline in all patients who received at least one dose of study drug (evolocumab or placebo). Patients taking vitamin E supplements at baseline were ineligible for study enrolment; no patient initiated vitamin E supplements after baseline. All other patients with at least one recorded postbaseline vitamin E level were included in the analysis population. Both the uncorrected vitamin E levels and an analysis in which the values were normalized for change in total cholesterol are presented. A secondary analysis assessed the vitamin E levels normalized for LDL‐C, total apoB (apoB‐100 + apoB‐48) and non–HDL‐C.
Cortisol, ACTH, gonadal hormone and gonadotropin levels were measured only in patients randomized in Chinese centres who received at least one dose of study drug (evolocumab or placebo). Patients receiving systemic steroids (eg intravenous, intramuscular or oral administration) at screening were excluded from the BERSON trial. In the main analysis, no Chinese patients were excluded from the analyses of gonadal hormones and gonadotropin levels. Gonadal steroid metabolism in female patients was evaluated by measuring oestradiol, LH and FSH. Gonadal steroid metabolism in male patients was evaluated by measuring total testosterone, LH and FSH.
As was done in the prior report of steroid hormones after evolocumab use,9 exclusion criteria were applied to the analyses of gonadal hormones and gonadotropin levels to minimize confounding. Male patients were excluded if they were receiving testosterone supplementation or had an LH level ≥15 IU/L at baseline; female patients were excluded if they were receiving hormone replacement therapy, had an FSH level ≥25 IU/L at baseline or were ≥50 years old. Because of the age of the study population enrolled in China (mean 60.0 years), the number of premenopausal female patients was very low, and it was thus not possible to meaningfully analyse gonadal hormones in female patients owing to the very low baseline levels (Table S1).
Therefore, this report presents male gonadal hormones and gonadotropin levels with exclusions for possible confounders as described above. The results of the analysis of male and female patients without exclusions as well as the details after applying the exclusion criteria can be found in the supplementary appendix (Table S5).
2.4. Laboratory methods
Vitamin E, LH, FSH, testosterone, oestradiol and cortisol were collected in a serum separation tube and shipped at an ambient temperature (vitamin E) or frozen to ≤ −25°C (all others). ACTH was collected in a K2 EDTA plasma tube and shipped at ≤−25°C. Samples were shipped daily to the central laboratory.
Vitamin E levels were defined as the sum of alpha and gamma tocopherol concentration in serum and were measured using ultraperformance liquid chromatography with photodiode array detection or ultraviolet detection. Gonadal steroids, ACTH, FSH and LH were measured with an electrochemiluminescence immunoassay (Immunoanalyser, Roche Diagnostics, Basel, Switzerland).
2.5. Statistical analyses
Data were presented as means and standard deviations where the data follow a normal distribution. Fasting serum glucose and HbA1C are presented as both mean (SD) and median (range) due to some skewing in the data.
The objective of the analysis was to assess the change from baseline in vitamin E, steroid hormones, ACTH and gonadotropins, overall and by lowest achieved postbaseline LDL‐C, in patients with type 2 diabetes and dyslipidaemia after 12 weeks of treatment within the evolocumab group. For comparison purposes, the change from baseline after 12 weeks was also analysed for the placebo group.
The analysed subgroups of evolocumab‐treated patients by their minimum postbaseline LDL‐C level were as follows: <0.4 mmol/L, <0.6 mmol/L, <1.0 mmol/L and ≥1.0 mmol/L. A patient could be included in more than one group (eg patients with <0.4 mmol/L were counted in all other groups). As few patients in the placebo group experienced a meaningful reduction in LDL‐C, the overall placebo group was used for comparison. We analysed the proportion of patients with gonadal hormones or gonadotropins greater than the upper limit of normal (ULN) or less than the lower limit of normal (LLN). This analysis used the same patient populations as the main gonadal hormone and gonadotropin analyses (male with exclusions applied, female without exclusions applied as explained in section 3.4). The reference ranges for male steroid hormone and gonadotropins were 1.7‐8.6 IU/L for LH; 1.6‐11.0 IU/L for FSH; and 9.37‐37.13 nmol/L for testosterone. The reference ranges for female steroid hormones and gonadotropins were 1.0‐95.6 IU/L for LH; 1.8‐20.4 IU/L for FSH; and 0‐1655 pmol/L for oestradiol.
Summary of vitamin E, steroid hormone and gonadotropin levels was based on observed data, and no imputation was used for missing values. Mean analyte/ratio values at baseline and week 12 were compared for statistical significance (P < .05) using a paired t test. The paired t test was chosen because the sample sizes (n ≥ 30) are sufficient to apply the central limit theorem, and the quantile‐quantile plots indicated normal distribution of the data. The one exception was the cortisol:ACTH ratio in the minimum postbaseline LDL‐C ≥ 1.0 mmol/L subgroup of the evolocumab group. In this case, we also performed the Wilcoxon signed‐rank test. Placebo results are descriptive and presented only for comparison. All analyses were done using SAS software version 9.4 (SAS Institute).
3. RESULTS
3.1. Patients
As previously reported,4, 5 the BERSON study randomized 324 patients to placebo and 657 to evolocumab (149 and 302, respectively, in China) (Table 1). Overall, the mean age was 61 years, and the proportion of female patients was 57%. The Chinese population had a lower rate of statin use than the global population (38% vs 57% in the evolocumab group), and baseline lipid measurements were similar between the populations (mean baseline LDL‐C 2.2‐2.4 mmol/L). Within each population, demographics and baseline characteristics were balanced between treatment groups, except for HbA1c, fasting serum glucose, HbA1c ≥ 8% and insulin use (Table 1).
Table 1.
Demographics and baseline characteristics in the global and Chinese populations
| Characteristics | Global population (n = 981) | Chinese population (n = 451) | ||
|---|---|---|---|---|
| Placebo (n = 324) | Evolocumab (n = 657) | Placebo (n = 149) | Evolocumab (n = 302) | |
| Age, years, mean (SD) | 61.3 (8.8) | 61.3 (8.5) | 59.8 (8.6) | 60.2 (8.4) |
| Sex, female, n (%) | 195 (60.2) | 367 (55.9) | 81 (54.4) | 149 (49.3) |
| Race, n (%) | ||||
| Asian | 160 (49.4) | 330 (50.2) | 149 (100) | 302 (100) |
| White | 139 (42.9) | 278 (42.3) | 0 | 0 |
| Black | 13 (4.0) | 29 (4.4) | 0 | 0 |
| Other | 12 (3.7) | 20 (3.0) | 0 | 0 |
| Statin use, n (%) | 186 (57.4) | 374 (56.9) | 59 (39.6) | 116 (38.4) |
| Intensive a | 14 (4.3) | 36 (5.5) | 3 (2.0) | 8 (2.6) |
| Nonintensive b | 172 (53.1) | 338 (51.4) | 56 (37.6) | 108 (35.8) |
| Lipid values, mmol/L, mean (SD) | ||||
| LDL‐C | 2.4 (0.8) | 2.4 (0.9) | 2.2 (0.8) | 2.3 (0.9) |
| Non–HDL‐C | 3.1 (1.0) | 3.1 (1.0) | 2.9 (0.9) | 3.0 (0.9) |
| HDL‐C | 1.3 (0.4) | 1.2 (0.3) | 1.2 (0.4) | 1.2 (0.3) |
| Triglycerides | 1.6 (1.4) | 1.6 (0.7) | 1.6 (1.8) | 1.5 (0.7) |
| Total cholesterol | 4.3 (1.0) | 4.4 (1.0) | 4.2 (0.9) | 4.2 (1.0) |
| Insulin use, n (%) | 91 (28.1) | 211 (32.1) | 50 (33.6) | 138 (45.7) |
| Glucose metabolism parameters | ||||
| Fasting serum glucose, mmol/L | ||||
| Mean (SD) | 7.1 (1.2) | 7.3 (1.2) | 7.6 (2.0) | 8.1 (2.2) |
| Median (range) | 7.0 (5.9,8.6) | 7.4 (6.1,9.2) | 7.1 (6.1,8.8) | 7.8 (6.5,9.3) |
| HbA1c, % | ||||
| Mean (SD) | 7.1 (1.2) | 7.3 (1.2) | 7.3 (1.2) | 7.5 (1.1) |
| Median (range) | 6.9 (6.2,7.9) | 7.1 (6.4,8.2) | 7.0 (6.5,7.9) | 7.4 (6.7,8.3) |
| HbA1c category, n (%) | ||||
| <7% | 176 (54.3) | 275 (41.9) | 74 (49.7) | 99 (32.8) |
| 7% to <8% | 72 (22.2) | 187 (28.5) | 38 (25.5) | 99 (32.8) |
| 8% to <9% | 39 (12.0) | 119 (18.1) | 19 (12.8) | 60 (19.9) |
| ≥9% | 37 (11.4) | 76 (11.6) | 18 (12.1) | 44 (14.6) |
Abbreviations: HbA1c, haemoglobin A1c; HDL‐C, high‐density lipoprotein‐cholesterol; LDL‐C, low‐density lipoprotein‐cholesterol; Q1, Q3, quartile 1, quartile 3; SD, standard deviation.
Adapted with permission from Lorenzatti et al4 and Chen et al5 © 2019 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Patient had at least one of the following recorded for the last 4 weeks before screening: atorvastatin ≥ 40 mg daily; rosuvastatin ≥20 mg daily; simvastatin ≥80 mg daily (simvastatin 80 mg daily is not approved in some countries, [eg the United States]); and any statin (atorvastatin, fluvastatin, lovastatin, pitavastatin, pravastatin, rosuvastatin and simvastatin) daily plus ezetimibe.
Patient had been taking any dose of a statin at least weekly for the last 4 weeks before screening and was not included in the intensive statin usage.
3.2. LDL‐C reduction
As reported previously,4, 5 compared with placebo, evolocumab reduced LDL‐C from baseline by the least squares mean (standard error [SE]) of 70.3% (2.6%) with the Q2W dose and 70.0% (2.4%) with the QM dose for the mean of weeks 10 and 12, and by 71.8% (3.0%) with the Q2W dose and 64.9% (2.6%) with the QM dose at week 12 (adjusted P < .0001) (Table S2). In the Chinese population, placebo‐corrected reductions with evolocumab were greater by approximately 10%: 80.4% (3.3%) and 81.0% (2.7%) (Q2W and QM doses, respectively) for the mean of weeks 10 and 12, and 85.0% (3.9%) and 74.8% (3.3%) (Q2W and QM doses, respectively) at week 12 (P < .0001) (Table S3).
3.3. Vitamin E levels
There were 977 patients in the global population analysis with both baseline and postbaseline vitamin E measurements (321 receiving placebo, 656 evolocumab) (Figure 1) and 448 patients in the Chinese population (147 placebo, 301 evolocumab) (Figure 2). Among evolocumab‐treated patients in the global population, absolute vitamin E levels decreased from baseline to week 12 by approximately 6 µmol/L (P < .0001) (Figure 1A). The observed change was consistent regardless of minimum postbaseline LDL‐C level (Figure 1B). There was no decrease in vitamin E from baseline to week 12 when normalized for LDL‐C, apoB and non–HDL‐C in evolocumab‐treated patients (Figure 1C,E,G). This result was maintained regardless of LDL‐C level (Figure 1D,F,H). No statistically significant differences in absolute or normalized vitamin E were observed from baseline to week 12 among patients in the placebo group (Figure 1A,C,E,G). Results for vitamin E were consistent between the global and Chinese populations (Figure 2).
Figure 1.
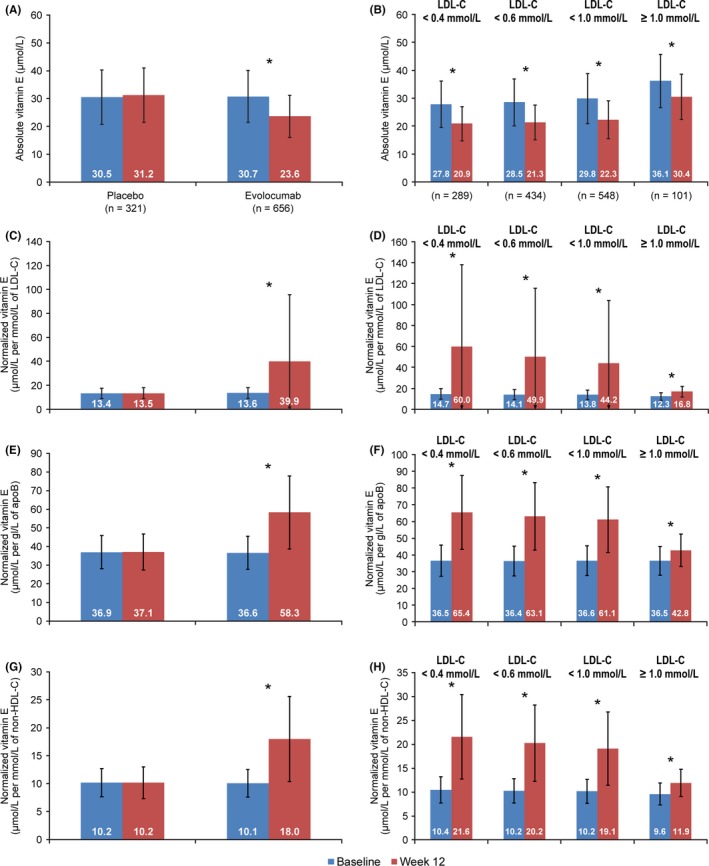
Mean (SD) Vitamin E Levels in the Global Population at Baseline and Week 12 and by Minimum Postbaseline LDL‐C. A, Absolute vitamin E levels; B, absolute vitamin E levels in the evolocumab group; C, vitamin E levels normalized for LDL‐C; D, vitamin E levels in the evolocumab group normalized for LDL‐C; E, vitamin E levels normalized for apoB; F, vitamin E levels in the evolocumab group normalized for apoB; G, vitamin E levels normalized for non–HDL‐C; H, vitamin E levels in the evolocumab group normalized for non–HDL‐C. ApoB, apolipoprotein B; LDL‐C, low‐density lipoprotein‐cholesterol; non–HDL‐C, non–high‐density lipoprotein‐cholesterol; SD, standard deviation. Patients with vitamin E supplementation at baseline were excluded (3 in the placebo group, 1 in the evolocumab group). *P < .0001; P‐values (paired t test) are nonsignificant unless given above the comparison
Figure 2.
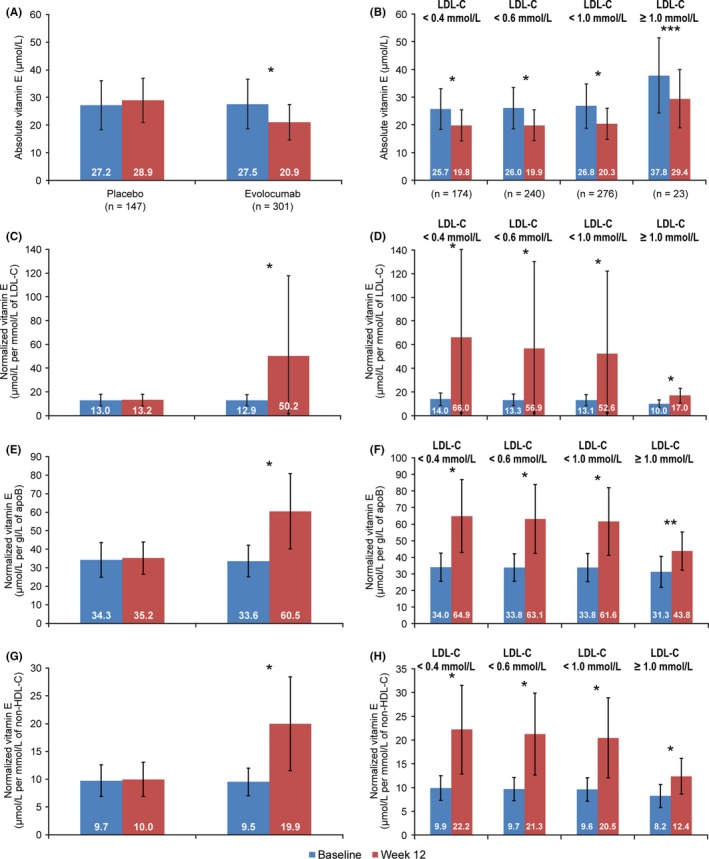
Mean (SD) Vitamin E Levels in the Chinese Population at Baseline and Week 12 and by Minimum Postbaseline LDL‐C. A, Absolute vitamin E levels; B, absolute vitamin E levels in the evolocumab group; C, vitamin E levels normalized for LDL‐C; D, vitamin E levels in the evolocumab group normalized for LDL‐C; E, vitamin E levels normalized for apoB; F, vitamin E levels in the evolocumab group normalized for apoB; G, vitamin E levels normalized for non–HDL‐C; H, vitamin E levels in the evolocumab group normalized for non–HDL‐C. ApoB, apolipoprotein B; LDL‐C, low‐density lipoprotein‐cholesterol; non–HDL‐C, non–high‐density lipoprotein‐cholesterol; SD, standard deviation. Patients with vitamin E supplementation at baseline were excluded (3 in the placebo group, 1 in the evolocumab group). *P < .0001; **P < .0005; ***P = .0007. P‐values (paired t test) are nonsignificant unless given above the comparison
3.4. Cortisol, ACTH, gonadal hormone and gonadotropin levels in the Chinese population
No statistically significant differences were observed from baseline to week 12 among patients in the placebo group in cortisol or ACTH—overall or when analysed by minimum postbaseline LDL‐C (Figure 3). Levels of cortisol and the cortisol:ACTH ratio did not change significantly from baseline to week 12 among evolocumab‐treated patients (Figure 3A,E). There was a marginally statistically significant but not clinically relevant change in ACTH (0.4 pmol/L increase, P = .047) with a nonstatistically significant change in cortisol (Figure 3C). No patient had a cortisol:ACTH ratio <3.0 (nmol/pmol). Differences from baseline to week 12 were not statistically significant within the evolocumab group across LDL‐C levels for cortisol, ACTH and cortisol:ACTH ratio except for ACTH in the LDL‐C <1 mmol/L group (mean 0.47 pmol/L increase, P = .02) (Figure 3B,D,F).
Figure 3.
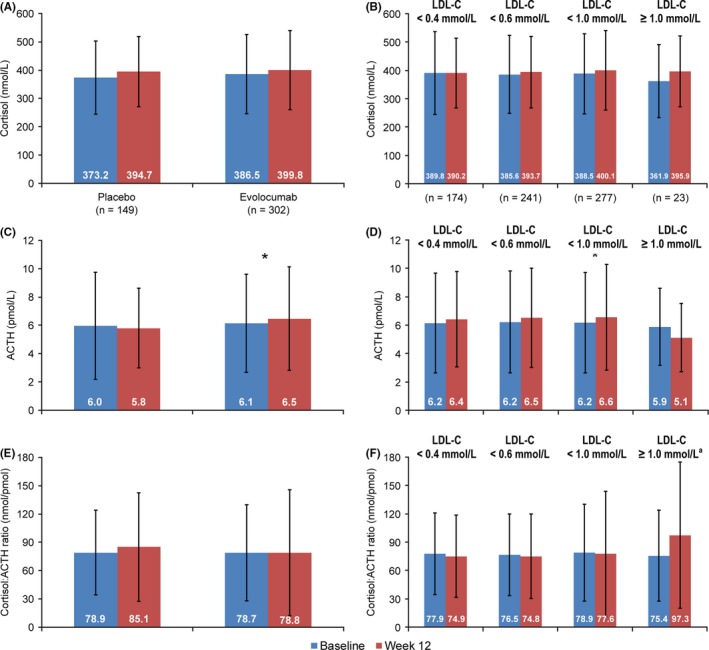
Mean (SD) Cortisol Levels, ACTH Levels and Cortisol:ACTH Ratio in the Chinese Population at Baseline and Week 12 and by Minimum Postbaseline LDL‐C. A, Cortisol levels; B, cortisol levels in the evolocumab group. C, ACTH levels; D, ACTH levels in the evolocumab group; E, cortisol:ACTH ratio; F, cortisol:ACTH ratio in the evolocumab group. ACTH, adrenocorticotropic hormone; LDL‐C, low‐density lipoprotein‐cholesterol; SD, standard deviation. *P = .02; P‐values (paired t test) are nonsignificant unless given above the comparison. aDue to abnormal distribution of the data in this group, the Wilcoxon signed‐rank test was also used in addition to the paired t test. The result (P = .06) confirmed the lack of a statistically significant difference
Changes from baseline to week 12 in male gonadal hormones (testosterone, LH) excluding those with possibly confounding characteristics are presented in Figure 4A,B,E,F. There were no statistically significant changes within the evolocumab and placebo groups when analysed overall and by LDL‐C level. The decrease in FSH levels in the evolocumab group was not clinically relevant (Figure 4C,D). The analysis without excluding male patients receiving testosterone supplementation or with an LH level ≥15 IU/L at baseline is presented in Table S5. For analysis of female gonadal hormone and gonadotropins, when patients with possibly confounding characteristics were excluded—hormone replacement therapy, age ≥50 years or FSH ≥25 IU/L at baseline—there were only four female evolocumab‐treated patients available for analysis (Table S1). No meaningful results could be derived from this sample size. When the population was analysed without excluding these patients, there was no clinically relevant difference from baseline to week 12 overall or by LDL‐C level (Table S6).
Figure 4.
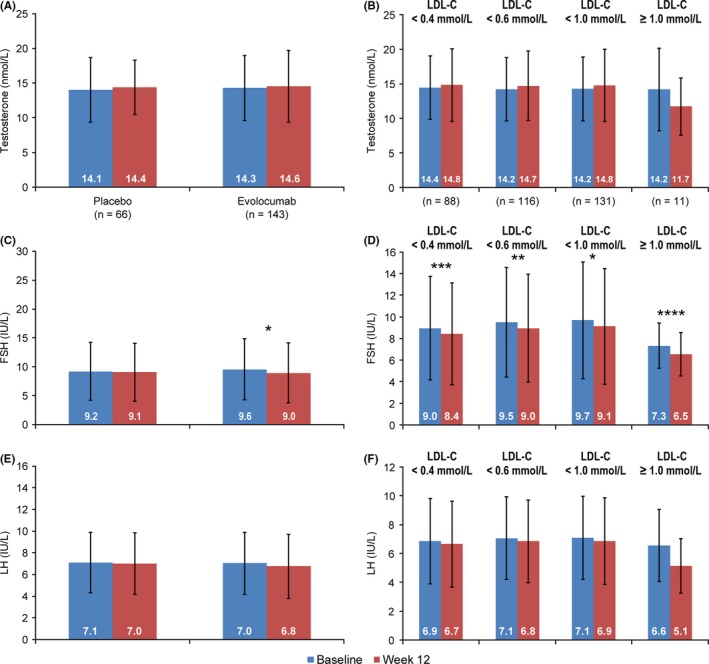
Mean (SD)Testosterone, FSH and LH Levels among Male Patients With Exclusion for Possibly Confounding Baseline Factors in the Chinese Population at Baseline and Week 12 and by Minimum Postbaseline LDL‐C. A, Testosterone; B, testosterone in the evolocumab group; C, FSH; D, FSH in the evolocumab group; E, LH; F, LH in the evolocumab group. Possibly confounding factors included the use of testosterone supplementation or LH levels ≥15 IU/L at baseline. Abbreviations: FSH, follicle‐stimulating hormone; LDL‐C, low‐density lipoprotein‐cholesterol; LH, luteinizing hormone; SD, standard deviation. Patients with testosterone supplementation or LH ≥15 IU/L at baseline were excluded (two in the placebo group, 10 in the evolocumab group). *P < .0001; **P = .0003;***P = .0032; ****P = .047; P‐values (paired t test) are nonsignificant unless given above the comparison
At baseline, the percentage of male and female patients with gonadal hormones or gonadotropins less than the LLN or greater than the ULN was similar in the placebo and evolocumab groups. At week 12, there was no increase in the proportion of evolocumab‐treated patients with gonadal hormones or gonadotropins <LLN or >ULN (Table S4). Results were consistent regardless of LDL‐C level (data not shown).
4. DISCUSSION
In this study of 977 patients (448 from China) with type 2 diabetes, treatment with evolocumab markedly lowered LDL‐C levels without adversely affecting adrenal and gonadal steroid hormone production even in the trial participants with the lowest levels of LDL‐C (<0.4 mmol/L). Although absolute vitamin E levels were lower in the patients receiving evolocumab, vitamin E concentrations normalized for plasma lipid concentrations (LDL‐C, apoB and non–HDL‐C) did not decrease. Instead, we observed an increase in the concentration of vitamin E normalized for LDL‐C, apoB and non–HDL‐C.
The results of this analysis suggest that patients receiving evolocumab incorporate vitamin E normally into their lipoproteins which are then cleared more rapidly, delivering their vitamin E content to various tissues as they are cleared. This situation is fundamentally different from that seen in abetalipoproteinaemia where the underlying defect is a failure to lipidate nascent chylomicrons and VLDL particles in the intestine and liver, respectively.12, 14 These unlipidated nascent lipoproteins are not secreted, and vitamin E never enters the circulation, causing vitamin E deficiency at the tissue level.12, 14 Red cell membrane (RCM) vitamin E concentrations are a good surrogate marker of tissue vitamin E levels.21, 22, 23 We did not measure RCM vitamin E levels in this study, but in a previous study with evolocumab, RCM vitamin E remained unchanged after 52 weeks of treatment.9
For statins, multiple studies have shown that the adrenal gland continues to synthesize cortisol normally, despite marked LDL lowering.24, 25, 26, 27, 28, 29, 30, 31 However, even in patients with markedly impaired LDL receptor function (ie, homozygous familial hypercholesterolaemia), adrenal function is normal and remains normal on lipid‐lowering therapy.28 Further studies have shown that adrenal cells receive cholesterol from multiple sources, including endogenous synthesis and HDL‐mediated delivery.13, 32 In keeping with this, cortisol and ACTH levels remained unchanged during the study. The cortisol:ACTH ratio also remained unchanged and did not decrease as one would expect with decreased adrenal cortisol output followed by a compensatory increase in the level of ACTH. We, therefore, did not see any evidence of impaired adrenal function during this study.
Gonadal hormones are also synthesized from cholesterol. Assessment of gonadal hormone production in women receiving lipid‐lowering therapy is complicated by the menstrual cycle and menopause and has thus not been studied extensively. In men, most statin studies have not found any impairment in testosterone production.24, 25, 27, 29, 30, 33, 34 Similarly, we did not find any change in serum testosterone or gonadotropins during this study. Because the mean age of female participants was 61.5 years, most patients were postmenopausal, as indicated by mean FSH of more than 60 IU/L, and it is thus not possible to detect impaired gonadal steroidogenesis in this cohort.
Our results are consistent with those previously published from a 1‐year study of evolocumab in mostly nondiabetic patients.9 In the DESCARTES study, serum vitamin E also decreased, but this decrease was attributable to a decrease in the number of lipoproteins rather than inadequate incorporation of vitamin E into lipoproteins. Cortisol, ACTH and gonadal hormones remained unchanged.9 In an analysis of pooled safety data for alirocumab, no meaningful decreases in the levels of the fat‐soluble vitamins A, D and K were observed.35 As seen in our study, serum vitamin E levels decreased, while vitamin E normalized for LDL‐C increased. Cortisol was not measured systematically across the alirocumab development programme and was mostly only measured in patients with LDL‐C lower than 25 mg/dL on two consecutive occasions or when sham alerts for low LDL‐C were generated to maintain the blind. However, in these patients, there was no difference in the number of patients treated with alirocumab or placebo who had cortisol or ACTH levels outside the laboratory reference ranges. ACTH stimulation testing was performed in a small number of patients and did not reveal any differences between the two treatment arms.35
This is the first study, to our knowledge, reporting the effects of a PCSK9 inhibitor on vitamin E, steroid hormone or gonadotropin levels in the Chinese population or in a global type 2 diabetic population.
Our study has important limitations. The most important limitation is the relatively short 12‐week duration of this trial. This may not have been sufficient time for cholesterol deficiency to affect steroid hormone synthesis. In this study, we did not measure vitamin E in RCM to assess tissue levels. We also did not perform any dynamic testing of endocrine function; in particular, we did not perform ACTH stimulation testing, which may have been able to detect subtle changes in adrenal function. Our study may not be fully representative of all diabetic patients. This is particularly relevant in the study participants from China since less than half of the patients were using statins at baseline and only approximately 2.0%‐2.5% of patients were taking high‐intensity statins prior to enrolment.
In conclusion, adding evolocumab to atorvastatin 20 mg daily in patients with type 2 diabetes lowered LDL‐C by approximately 70% in the global population and by 80% in patients from China compared to placebo. Despite marked lowering of LDL‐C, we did not identify any clinically concerning changes in vitamin E, steroid hormones and gonadotropins, even in patients with the lowest LDL‐C levels.
CONFLICT OF INTEREST
DJB reports personal fees from Amgen South Africa, during the conduct of the study; personal fees from Sanofi‐Aventis, outside the submitted work. JC, ZY, JLB and JG report no potential conflicts of interest. AWH and MLM are employees of and stockholders in Amgen Inc NW was an employee of Amgen when the work was conducted.
AUTHOR CONTRIBUTIONS
DJB interpreted the data. JC, ZY, JLB and JG. acquired the data. AWH, MLM and NW acquired and interpreted the data. NW analysed the data. All authors are responsible for the work described in this paper. All authors either drafted the manuscript or critically reviewed the manuscript for important intellectual content. All authors gave final approval of the version to be published and agree to be accountable for all aspects of the work ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
ETHICS STATEMENT
The study was conducted in accordance with the guidelines on Good Clinical Practice and with ethical standards for human experimentation established by the Declaration of Helsinki. An independent review board or independent ethics committee at each study site reviewed the study and approved the protocol and the subsequent amendments to the study protocol. All patients provided written informed consent before participation.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
This study was sponsored by Amgen Inc Editorial assistance was provided by Tim Peoples MA, ELS, CMPP and Maya Shehayeb, both of Amgen, and Wanda Krall PhD, on behalf of Amgen. Dr Borges' current affiliation is Centro de Pesquisa Clínica do Brasil, Brasilia, Brazil.
Blom DJ, Chen J, Yuan Z, et al. Effects of evolocumab therapy and low LDL‐C levels on vitamin E and steroid hormones in Chinese and global patients with type 2 diabetes. Endocrinol Diab Metab. 2020;3:e00123 10.1002/edm2.123
DATA AVAILABILITY STATEMENT
Qualified researchers may request data from Amgen clinical studies. Complete details are available at http://www.amgen.com/datasharing.
REFERENCES
- 1. Sharma K, Baliga RR. Genetics of dyslipidemia and ischemic heart disease. Curr Cardiol Rep. 2017;19:46. [DOI] [PubMed] [Google Scholar]
- 2. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713‐1722. [DOI] [PubMed] [Google Scholar]
- 3. Lorenzatti AJ, Eliaschewitz FG, Chen Y, et al. Rationale and design of a randomized study to assess the efficacy and safety of evolocumab in patients with diabetes and dyslipidemia: the BERSON clinical trial. Clin Cardiol. 2018;41:1117‐1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lorenzatti AJ, Eliaschewitz FG, Chen Y, et al. Randomised study of evolocumab in patients with type 2 diabetes and dyslipidaemia on background statin: primary results of the BERSON clinical trial. Diabetes Obes Metab. 2019;21(6):1455‐1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen Y, Yuan Z, Lu J, et al. Randomised study of evolocumab in patients with type 2 diabetes and dyslipidaemia on background statin: pre‐specified analysis of the China population from the BERSON clinical trial. Diabetes Obes Metab. 2019;21(6):1464‐1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hacquebard M, Carpentier YA. Vitamin E: absorption, plasma transport and cell uptake. Curr Opin Clin Nutr Metab Care. 2005;8:133‐138. [DOI] [PubMed] [Google Scholar]
- 7. Thurnham DI, Davies JA, Crump BJ, Situnayake RD, Davis M. The use of different lipids to express serum tocopherol: lipid ratios for the measurement of vitamin E status. Ann Clin Biochem. 1986;23(Pt 5):514‐520. [DOI] [PubMed] [Google Scholar]
- 8. Traber MG, Jialal I. Measurement of lipid‐soluble vitamins–further adjustment needed? Lancet. 2000;355:2013‐2014. [DOI] [PubMed] [Google Scholar]
- 9. Blom DJ, Djedjos CS, Monsalvo ML, et al. Effects of evolocumab on vitamin E and steroid hormone levels: results from the 52‐week, phase 3, double‐blind, randomized, placebo‐controlled DESCARTES study. Circ Res. 2015;117:731‐741. [DOI] [PubMed] [Google Scholar]
- 10. Balbi ME, Tonin FS, Mendes AM, et al. Antioxidant effects of vitamins in type 2 diabetes: a meta‐analysis of randomized controlled trials. Diabetol Metab Syndr. 2018;10:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ceriello A, Testa R, Genovese S. Clinical implications of oxidative stress and potential role of natural antioxidants in diabetic vascular complications. Nutr Metab Cardiovasc Dis. 2016;26:285‐292. [DOI] [PubMed] [Google Scholar]
- 12. Hentati F, El‐Euch G, Bouhlal Y, Amouri R. Ataxia with vitamin E deficiency and abetalipoproteinemia. Handb Clin Neurol. 2012;103:295‐305. [DOI] [PubMed] [Google Scholar]
- 13. Hu J, Zhang Z, Shen WJ, Azhar S. Cellular cholesterol delivery, intracellular processing and utilization for biosynthesis of steroid hormones. Nutr Metab (Lond). 2010;7:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee J, Hegele RA. Abetalipoproteinemia and homozygous hypobetalipoproteinemia: a framework for diagnosis and management. J Inherit Metab Dis. 2014;37:333‐339. [DOI] [PubMed] [Google Scholar]
- 15. Asih PR, Tegg ML, Sohrabi H, et al. Multiple mechanisms linking type 2 diabetes and Alzheimer's disease: testosterone as a modifier. J Alzheimers Dis. 2017;59:445‐466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Carcaillon L, Brailly‐Tabard S, Ancelin M‐L, et al. High plasma estradiol interacts with diabetes on risk of dementia in older postmenopausal women. Neurology. 2014;82:504‐511. [DOI] [PubMed] [Google Scholar]
- 17. Cheung KK, Luk AO, So WY, et al. Testosterone level in men with type 2 diabetes mellitus and related metabolic effects: a review of current evidence. J Diabetes Investig. 2015;6:112‐123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hamilton EJ, Davis WA, Makepeace A, et al. Prevalence and prognosis of a low serum testosterone in men with type 2 diabetes: the Fremantle Diabetes Study Phase II. Clin Endocrinol (Oxf). 2016;85:444‐452. [DOI] [PubMed] [Google Scholar]
- 19. Zhang YI, Zhang S‐W, Khandekar N, et al. Reduced serum levels of oestradiol and brain derived neurotrophic factor in both diabetic women and HFD‐feeding female mice. Endocrine. 2017;56:65‐72. [DOI] [PubMed] [Google Scholar]
- 20. Stroes E, Robinson JG, Raal FJ, et al. Consistent LDL‐C response with evolocumab among patient subgroups in PROFICIO: a pooled analysis of 3146 patients from phase 3 studies. Clinical Cardiology. 2018;41:1328‐1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Franco RS. The measurement and importance of red cell survival. Am J Hematol. 2009;84:109‐114. [DOI] [PubMed] [Google Scholar]
- 22. Lehmann J, Rao DD, Canary JJ, Judd JT. Vitamin E and relationships among tocopherols in human plasma, platelets, lymphocytes, and red blood cells. Am J Clin Nutr. 1988;47:470‐474. [DOI] [PubMed] [Google Scholar]
- 23. Saito M, Nakatsugawa K, Oh‐Hashi A, Nishimuta M, Kodama N. Comparison of vitamin E levels in human plasma, red blood cells, and platelets following varying intakes of vitamin E. J Clin Biochem Nutr. 1992;12:59‐68. [Google Scholar]
- 24. Arem R, Ghusn H, Ellerhorst J, Comstock JP. Effect of decreased plasma low‐density lipoprotein levels on adrenal and testicular function in man. Clin Biochem. 1997;30:419‐424. [DOI] [PubMed] [Google Scholar]
- 25. Azzarito C, Boiardi L, Zini M, et al. Long‐term therapy with high‐dose simvastatin does not affect adrenocortical and gonadal hormones in hypercholesterolemic patients. Metabolism. 1992;41:148‐153. [DOI] [PubMed] [Google Scholar]
- 26. Illingworth DR, Corbin D. The influence of mevinolin on the adrenal cortical response to corticotropin in heterozygous familial hypercholesterolemia. Proc Natl Acad Sci USA. 1985;82:6291‐6294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Krysiak R, Okopien B. The effect of aggressive rosuvastatin treatment on steroid hormone production in men with coronary artery disease. Basic Clin Pharmacol Toxicol. 2014;114:330‐335. [DOI] [PubMed] [Google Scholar]
- 28. Laue L, Hoeg JM, Barnes K, Loriaux DL, Chrousos GP. The effect of mevinolin on steroidogenesis in patients with defects in the low density lipoprotein receptor pathway. J Clin Endocrinol Metab. 1987;64:531‐535. [DOI] [PubMed] [Google Scholar]
- 29. Takeda Y, Miyamori I, Karayalcin U, Takeda R. Influence of 3‐hydroxy‐3‐methylglutaryl coenzyme A reductase inhibitor, pravastatin, on corticosteroid metabolism in patients with heterozygous familial hypercholesterolemia. Horm Res. 1991;36:75‐77. [DOI] [PubMed] [Google Scholar]
- 30. Travia D, Tosi F, Negri C, Faccini G, Moghetti P, Muggeo M. Sustained therapy with 3‐hydroxy‐3‐methylglutaryl‐coenzyme‐A reductase inhibitors does not impair steroidogenesis by adrenals and gonads. J Clin Endocrinol Metab. 1995;80:836‐840. [DOI] [PubMed] [Google Scholar]
- 31. Wani TA, Samad A, Tandon M, Saini GS, Sharma PL, Pillai KK. The effects of rosuvastatin on the serum cortisol, serum lipid, and serum mevalonic acid levels in the healthy Indian male population. AAPS PharmSciTech. 2010;11:425‐432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bochem AE, Holleboom AG, Romijn JA, et al. High density lipoprotein as a source of cholesterol for adrenal steroidogenesis: a study in individuals with low plasma HDL‐C. J Lipid Res. 2013;54:1698‐1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Farnsworth WH, Hoeg JM, Maher M, Brittain EH, Sherins RJ, Brewer HB Jr. Testicular function in type II hyperlipoproteinemic patients treated with lovastatin (mevinolin) or neomycin. J Clin Endocrinol Metab. 1987;65:546‐550. [DOI] [PubMed] [Google Scholar]
- 34. Santini SA, Carrozza C, Lulli P, Zuppi C, CarloTonolo G, Musumeci S. Atorvastatin treatment does not affect gonadal and adrenal hormones in type 2 diabetes patients with mild to moderate hypercholesterolemia. J Atheroscler Thromb. 2003;10:160‐164. [DOI] [PubMed] [Google Scholar]
- 35. Robinson JG, Rosenson RS, Farnier M, et al. Safety of very low low‐density lipoprotein cholesterol levels with alirocumab: pooled data from randomized trials. J Am Coll Cardiol. 2017;69:471‐482. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
Qualified researchers may request data from Amgen clinical studies. Complete details are available at http://www.amgen.com/datasharing.


