The genus Arcobacter was unexpectedly abundant in the effluent from 14 Danish wastewater treatment plants treating municipal wastewater, and the species included the human-pathogenic A. cryaerophilus and A. butzleri. Recent studies have shown that Arcobacter is common in wastewater worldwide, so the study indicates that discharge of members of the genus Arcobacter may be a global problem, and further studies are needed to quantify the risk and potentially minimize the discharge. The study also shows that culture-based analyses are insufficient for proper effluent quality control, and new standardized culture-independent measurements of effluent quality encompassing most pathogens should be considered.
KEYWORDS: Arcobacter, wastewater, activated sludge, full-scale wastewater treatment plants, removal efficiency
ABSTRACT
Pathogenic bacteria in wastewater are generally considered to be efficiently removed in biological wastewater treatment plants. This understanding is almost solely based on culture-based control measures, and here we show, by applying culture-independent methods, that the removal of species in the genus Arcobacter was less effective than for many other abundant genera in the influent wastewater. Arcobacter was one of the most abundant genera in influent wastewater at 14 municipal wastewater treatment plants and was also abundant in the “clean” effluent from all the plants, reaching up to 30% of all bacteria as analyzed by 16S rRNA gene amplicon sequencing. Metagenomic analyses, culturing, genome sequencing of Arcobacter isolates, and visualization by fluorescent in situ hybridization (FISH) confirmed the presence of the human-pathogenic Arcobacter cryaerophilus and A. butzleri in both influent and effluent. The main reason for the high relative abundance in the effluent was probably that Arcobacter cells, compared to those of other abundant genera in the influent, did not flocculate and attach well to the activated sludge flocs, leaving a relatively large fraction dispersed in the water phase. The study shows there is an urgent need for new standardized culture-independent measurements of pathogens in effluent wastewaters, e.g., amplicon sequencing, and an investigation of the problem on a global scale to quantify the risk for humans and livestock.
IMPORTANCE The genus Arcobacter was unexpectedly abundant in the effluent from 14 Danish wastewater treatment plants treating municipal wastewater, and the species included the human-pathogenic A. cryaerophilus and A. butzleri. Recent studies have shown that Arcobacter is common in wastewater worldwide, so the study indicates that discharge of members of the genus Arcobacter may be a global problem, and further studies are needed to quantify the risk and potentially minimize the discharge. The study also shows that culture-based analyses are insufficient for proper effluent quality control, and new standardized culture-independent measurements of effluent quality encompassing most pathogens should be considered.
INTRODUCTION
The development of sanitary systems, including urban wastewater treatment, has been one of the most important factors in preventing the spread of fecal contaminants and pathogens. Advanced wastewater treatment plants (WWTPs) remove pathogens, carbon, nitrogen, phosphorus, and micropollutants until the load is no longer harmful to recipients (1). A range of pathogenic bacteria and fecal contaminants, such as Escherichia coli, Campylobacter, Salmonella, and Shigella, are removed from the wastewater at efficiencies of 99% or more (2). However, the standard methods for determining removal efficiencies of bacterial pathogens during the treatment process are based on culture and on the detection of only a few indicator species, such as E. coli or coliforms (3–5). As some pathogens are difficult to culture under the standard conditions (6) or are not looked for at all (7), these high removal efficiencies of more than 99% (8) may not apply to all important pathogens in full-scale facilities, thus underestimating the transfer of certain pathogens into the receiving waters. In some countries, the effluent is treated with UV light, ozone, or chlorine or by other means to make sure the pathogens are removed (9). However, in many countries, this is not the case, especially in temperate climate areas with advanced treatment, where pathogens are considered to be efficiently removed by the activated-sludge process in combination with simple tertiary treatment, such as sand filtration (6).
Recent DNA-based molecular studies indicate that many previously unknown potential fecal contaminants are entering wastewater treatment plants in high numbers, including the genera Ruminococcus and Clostridium, which contain potential pathogens (5). Recently, it was shown that emergent pathogens belonging to the genus Arcobacter can be very abundant in the influent wastewater in several countries (5, 6, 10–12). The genus contains several pathogenic species, including Arcobacter butzleri and A. cryaerophilus (13). A. butzleri has been detected in both effluent from WWTPs and receiving waters (14) by culture-based methods and quantitative PCR (qPCR), but no studies have addressed the removal efficacy. Furthermore, the bacteria are not detected by standard culture-based methods because the methods of culturing Arcobacter rely heavily on enrichment before culturing (15). A. butzleri and other Arcobacter species, such as A. cryaerophillus, have previously been isolated from humans and animals with diarrhea (13) but are also found in other environments, and some species are capable of surviving in open waters, such as sewer systems (5, 12).
Removal of influent microbes, including pathogens, in biological wastewater treatment plants is usually considered to take place by adsorption to activated-sludge flocs and subsequent removal by the excess sludge (2). Many may also die off or are consumed by protozoa (2, 16), while some species act as seeding for the process-critical microorganisms in the plant and ensure the presence of nitrification, denitrification, and other key processes (17, 18). However, it is not known how the highly abundant Arcobacter organisms in the influent are removed in WWTPs, so the aim of this study was to investigate whether Arcobacter spp. in the influent wastewater are efficiently removed in well-working, advanced WWTPs or whether they pose an unexpected threat to the environment.
RESULTS
Overall microbial composition of influent wastewater, activated sludge, and effluent.
The microbial community composition was analyzed in 14 full-scale Danish WWTPs with nutrient removal from cities distributed across the country during a 3-month period at three different process stages—influent wastewater, process tanks with activated sludge, and effluent water—using 16S rRNA gene amplicon sequencing, resulting in a total of 252 samples.
Principal-component analysis (Fig. 1) showed that microbial community compositions for individual process stages were similar across different WWTPs, as the samples clustered by process stage rather than plant location, and groupings from different locations were significantly different from each other (Adonis test; P = 0.001). There were large differences in the microbial community composition in the influent and process tanks, while the effluent samples showed a different pattern, with some samples indicating a microbial community closer to that of the process tank, some closer to that of the influent, and some in between.
FIG 1.

Microbial community structures of influent, process tank, and effluent of 14 full-scale WWTPs over a 3-month period as determined by 16S rRNA amplicon sequencing and principal-component analysis. All the samples are shown.
Microbial composition of the influent wastewater.
The microbial community composition of the influent wastewater in each WWTP was very diverse, although the abundant genera in the wastewater influent were highly similar across all the WWTPs. Among the 10 most abundant genera were Trichococcus, Streptococcus, Arcobacter, and Blautia, with relative read abundances of up to 45% in some influent samples (Fig. 2A). Together, the 25 most abundant genera accounted for 60% of the reads, while 128 genera accounted for 80% of the reads.
FIG 2.
Average abundances (percent read abundance) of the 10 most abundant genera in the influent, process tank, and effluent in 14 WWTPs (n = 6) over 3 months. The data are sorted according to abundance in influent (A), process tank (B), and effluent (C). The shade of red indicates the percentage value. Light red and dark red represent low and high values, respectively (refer to scale bar in Fig. 3).
Each genus contained a number of abundant species, which were constant among treatment plants and stable over time, with only small variations of the most abundant species (see Fig. S1 in the supplemental material). The genus Arcobacter had 30 different amplicon sequence variants (ASVs) above 0.1% read abundance, and they clustered into 15 species using MiDAS 3 taxonomy, which enabled species level identification (98.7% identity) (19). Other potentially pathogenic genera, such as Legionella, were present in relative read abundances lower than 0.1% in influent wastewater and were not further considered in this study.
Arcobacter was highly abundant in the effluent.
Several studies have found a high removal rate of influent bacteria in WWTPs, so it was expected that the most abundant bacteria in the effluent would be the process-critical genera growing in the process tanks, such as nitrifiers and denitrifiers (20). Surprisingly, this was not the case in most WWTPs. Instead, several of the highly abundant genera in the influent were also present at high relative abundance in the effluent without having a similar high relative abundance in the process tanks (Fig. 2A to C). Figure 2B shows that most of the abundant genera in the process tanks (except for Trichococcus) did not have a high relative abundance in the influent and that the majority of the most abundant influent bacteria were not abundant in the process tanks.
The three most abundant genera in the effluent (Fig. 2C), Arcobacter, Trichococcus, and Streptococcus, were also very abundant in the influent, and Arcobacter was detected as the most abundant genus in effluent to 8 of 14 WWTPs and in relative abundances close to that observed in the influent (1.6 to 13.3%). Some process-critical genera in the process tank, such as Tetrasphaera and Dechloromonas (both involved in biological nitrogen and phosphorus removal), were also present in the effluent, but in general, unexpected high abundances of influent bacteria were observed.
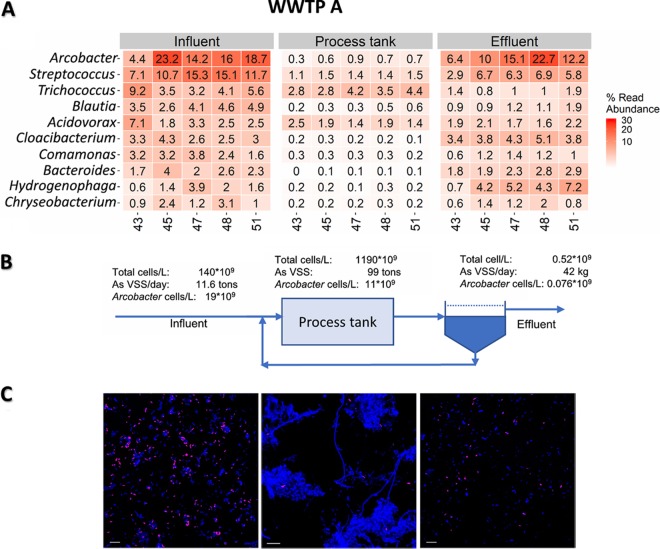
These observations were consistent among all WWTPs included in the 3 months of investigation. An example of the community composition over time of the 10 most abundant genera is shown for WWTP A in Fig. 3A. In this plant, Arcobacter was the most abundant genus in the influent and effluent at all time points (4.4 to 23.1%), while in the remaining 13 WWTPs, it was always present among the most abundant genera in the effluent (0.4 to 16.9%) (see Fig. S2 in the supplemental material).
FIG 3.
(A) Dominant genera of the influent and their abundance (percent read abundance) in process tank and effluent measured every second week (weeks 43 to 51) for 3 months for wastewater treatment plant A, treating wastewater from 300,000 PE. (B) Examples of numbers of cells per liter, total kilograms of VSS, and numbers of Arcobacter cells per liter at different sampling locations for week 47 in 2014. (C) FISH images from influent, process tank, and effluent with Arcobacter probe (purple) and EUBmix (blue). Bars, 20 μm.
The fate of Arcobacter in the process tank.
In order to quantify the removal efficiency of Arcobacter, we established mass balances in all 14 WWTPs by considering the difference in suspended organic material (volatile suspended solids [VSS]) and estimated total bacterial cell numbers in the influent and effluent. During the sampling period, the total cell numbers were reduced by 96.5 to 100% (average, 98.9%) in the different WWTPs. An example for WWTP A is shown in Fig. 3B for week 47 (detailed calculations can be found in the supplemental RMarkdown [RMD] file at https://github.com/janniemunk/Publications/tree/master/2020_Arcobacter and in Table S1 in the supplemental material). The flow to WWTP A was approximately 41,500 m3/day, providing a load of VSS of 11.6 tons/day with an average bacterial cell content, based on staining and fluorescence microscopy, of 1.4 × 1011 cells/liter ± 25% (standard deviation) (10). The total number of cells in the influent was reduced by 99.6%, and hence, only 42 kg VSS/day was present in the effluent, showing a general high removal of incoming bacteria in the process tanks.
When the same mass balance was made for the specific genus Arcobacter, the removal efficiency varied among the wastewater treatment plants. For example, in a specific sample, Arcobacter bacteria had an abundance of 19 × 109 cells/liter in the influent to plant A, and they were reduced to 0.079 × 109 cells/liter in the effluent. For all 14 plants, the abundance-range of Arcobacter in the influents was 4.2 × 108 to 3.2 × 1010 cells/liter in the different WWTPs, and the effluent range was 3.5 × 107 to 5.1 × 109 cells/liter. The corresponding removal was in the range of 79.6 to 100%, with an average removal efficiency of 97.3%. The removal of Arcobacter cells, however, was not statistically different from the removal of total cells (P = 0.2) (see Fig. S2). In order to verify that the most abundant incoming bacteria were removed in the WWTPs, their net growth rates were calculated based on mass balances and relative read abundances (10). The results (see the supplemental RMD file [https://github.com/janniemunk/Publications/tree/master/2020_Arcobacter]) showed that all Arcobacter cells and most others of the incoming highly abundant genera did not grow in the process tanks. This shows that their abundance in the process tanks (less than 1%) primarily depended on their continuous immigration with the influent due to the die off in the process tank and removal with the surplus activated sludge.
To verify that Arcobacter bacteria were likely viable in both influent and effluent, fluorescent in situ hybridization (FISH) was applied for detection of Arcobacter, as only cells with many ribosomes are detected by FISH, indicating viability (21) (Fig. 3C). In the influent, Arcobacter cells made up 16.3% of all FISH-positive cells, while the relative read abundance was 16.1% by amplicon sequencing. In the effluent, the numbers were 5.4% and 8.2%, respectively.
One hypothesis as to why Arcobacter bacteria were relatively abundant in the effluent is that Arcobacter cells do not flocculate and attach well to the activated-sludge flocs, leaving a relatively large fraction dispersed in the water phase. Those that stay in the bulk water may not die off, as the residence time of the water was only 18 to 30 h in the plants investigated in contrast to Arcobacter bound in the flocs, where the sludge had a retention time for solids of 25 to 35 days. In order to investigate possible differences in the bacterial compositions of activated-sludge flocs and bulk water, we sampled fresh activated sludge from 12 of the WWTPs and analyzed the relative abundances of bacterial genera in the supernatant and total sludge (Fig. 4A). In all the supernatant samples, Arcobacter and several genera normally abundant in the influent and process tanks (e.g., Trichococcus, Tetrasphaera, and Acidovorax) were present in high relative abundance, but only Arcobacter was found significantly more abundantly in the supernatant than in activated sludge (P = 0.002) (see Table S2 in the supplemental material). This supported our hypothesis that a fraction of incoming Arcobacter cells did not attach well to the activated-sludge flocs.
FIG 4.
(A) Box plot with the top 10 abundant genera in the total activated sludge and the sludge supernatant showing that Arcobacter was significantly more abundant (P = 0.002) in the supernatant than in activated sludge. The box plot is based on median values of 16S rRNA amplicon sequencing relative read abundance, where the box displays the first to third quartiles of data; lines show the spread of data; and points show outliers. (B) FISH image from WWTP A of Arcobacter cells loosely attached as single cells (red) and EUBmix showing all bacteria (green). The bacteria are shown as a maximum-intensity projection image.
FISH combined with confocal microscopy additionally showed that Arcobacter cells attached to the activated-sludge flocs were distributed as single cells on the surfaces, supporting the hypothesis that they did not multiply as an incorporated part of the activated sludge (Fig. 4B).
For most WWTPs, the microbial composition in the effluent was significantly different from the community in the process tank (P < 0.001). Only in plants D and F was this not the case (P = 0.18 and 0.21), and in those effluent samples, Arcobacter and other abundant influent genera had low relative abundance (Fig. 1 and 2; see Fig. S2). This could be due to poor settling properties of the activated sludge causing the process tank bacteria to make up a larger fraction of the effluent bacteria, but we were not able to find any strong correlation between effluent biomass (range, 0.1 to 20 mg VSS/liter) and the effluent content of Arcobacter and other abundant bacteria from the influent.
Presence of pathogenic Arcobacter species.
To investigate if potential pathogenic Arcobacter species were present, we carried out a more detailed analysis of the species. In all 14 plants, amplicon sequencing detected 15 Arcobacter species, with the pathogenic A. cryaerophilus and A. butzleri present in both influent and effluent and with A. cryaerophilus being the most abundant (Fig. 5). This was confirmed by sequencing metagenomes from the influent and effluent at WWTP A. Three full-length 16S rRNA genes from Arcobacter species were assembled, and they were most similar to those of A. cryaerophilus and A. suis (Fig. 6), and A. cryaerophilus 16S rRNA gene sequences were among the most abundant in the metagenomes (see Fig. S4 in the supplemental material). An overview of the different Arcobacter species found in the metagenomes, isolates, and amplicons is shown in Fig. 6, with the most closely related strains of the most abundant Arcobacter species highlighted.
FIG 5.
Abundance (percent read abundance) of species within the genus Arcobacter in influent, process tank, and effluent. Fifteen species were found, including the two pathogenic species A. cryaerophilus (most abundant) and A. butzleri (less abundant).
FIG 6.
Maximum-likelihood phylogenetic tree of the 16S rRNA genes from metagenomes, isolates, and reference species within the genus Arcobacter. Bootstrap values (%) based on 1,000 replications are shown at the nodes of the tree. Sequence accession numbers are shown in parentheses. The Arcobacter species found in metagenomes and isolates are in boldface, and the closest related strains of the most abundant Arcobacter ASVs are in red.
In order to verify the potential virulence of Arcobacter species found in influent and effluent, 10 isolates were obtained, and the genomes of four of them were sequenced, one originating from influent and three from effluent. All 4 isolates were verified as Arcobacter with 97% average nucleotide identity (ANIb) to the human pathogen A. butzleri RM4018, suggesting they belonged to the same species. They all contained virulence genes responsible for the pathogenicity of A. butzleri (cadF, ciaB, cj1349, mviN, pldA, and tlyA) (12, 22, 23). Due to the complexity and diversity of species in the original samples, metagenome-assembled genomes could not be recovered for the in situ Arcobacter populations, but some of the virulence genes could be found in the metagenome (pldA and tlyA) (see Fig. S4).
DISCUSSION
The genus Arcobacter includes several emerging pathogens, such as A. butzleri and A. cryaerophilus, that can be a threat to human and animal health (24). Arcobacter species have been shown to be present in domestic wastewater, sometimes in high abundance (5, 6, 25). However, until now, it has been assumed that they are removed in well-working WWTPs. In this study, we show that pathogenic Arcobacter bacteria pass through the WWTPs and, despite good removal in the process tank, are present in high relative abundance in the effluent. This is very concerning, since all the treatment plants investigated were working well, with advanced biological and chemical treatment, obeying Danish and European effluent standards. Furthermore, all the plants have a long solid residence time (SRT) (25 to 35 days) that should ensure efficient removal of most incoming bacteria, including the pathogens, so only process-critical bacteria growing in the process tanks, such as nitrifiers, would be in the effluent. It is therefore likely that other WWTPs with shorter SRTs or generally poor performance would have even worse removal of Arcobacter and other potential pathogens.
The high relative proportion of Arcobacter and some other influent genera in the effluent was unexpected. Culture-based studies have reported general removal percentages of pathogens in activated sludge systems of 99% or more (2). We found similar overall removal efficiencies (98.5 to 99.9%) in the WWTPs investigated in this study, based on biomass estimates as VSS or total bacteria. The relatively high proportion of Arcobacter in the effluent (up to 22%) was detected by culture-independent molecular methods, as there is no routine standard culturing method that would detect Arcobacter. Only a few other studies have detected Arcobacter bacteria in the effluent from WWTPs or in the receiving water by molecular methods (5, 6, 14, 25, 26), and to our knowledge, no comprehensive studies have described this potential problem. Amplicon sequencing and metagenomics may potentially also analyze dead cells (“naked” DNA), but our quantitative-FISH (qFISH) analyses of Arcobacter cells in both influent and effluent confirmed the high abundance of likely viable Arcobacter.
The high relative abundance of Arcobacter in the effluent may be due to poor attachment to the flocs in the well-mixed process tanks. We found that a substantial fraction of Arcobacter cells stayed in the water phase in the process tank and the effluent, not attached to the flocs and thus not removed by the surplus sludge as usually expected (27). Incoming microorganisms are anticipated to be adsorbed to the activated-sludge flocs and to slowly die off and/or be ingested by protozoans (27), and if so, we should expect to find a community composition in the effluent very similar to that in the process tank. Little is known about the surface properties of Arcobacter strains, but it is known that negatively charged hydrophilic cells do not flocculate well (28), so this could be investigated further in relevant strains.
The sources of Arcobacter in wastewater are not well described, but it is possible that the bacteria grow in biofilms in the sewer systems, as they attach to pipe surfaces (29), or that they grow in the wastewater (30). Some studies suggest that sewers may hold a unique habitat, where Arcobacter thrives, which indicates that sewers potentially hold a large reservoir of pathogens (5, 12, 25, 26). They are likely also common in some types of wastewater collected in the sewers, as they are found in a wide range of animal and environmental sources, such as raw meat products (31, 32) and surface waters (14), but only a few of them are known opportunistic pathogens (13).
Fifteen different Arcobacter species were detected in the influent wastewater and the “clean” effluent, with A. cryaerophilus the most abundant. This species and A. butzleri are both known pathogens and have been associated with persistent diarrhea and bacteremia in humans and animals (13, 23, 33). Historically, Arcobacter infections have often been mistaken for Campylobacter infections because the infection symptoms are very similar, and application of molecular methods is needed for reliable identification (34). Analyses of A. butzleri isolates and metagenomes confirmed the presence of virulence genes (13) in both the influent and effluent. Other studies found similar results and suggest that Arcobacter species should be considered bacterial weed species (35, 36), well suited to survive harsh conditions (37), such as those within wastewater treatment plants. In addition to its pathogenicity, Arcobacter has also been considered a keystone genus involved in plasmid diffusion, including the transfer of antibiotic resistance genes (32) from sewage to the environment (35, 38). Some Arcobacter species have been found in stools from nondiarrheic people, stressing that not all are pathogenic or that the pathogenicity could be regulated by the status of the host (39).
A high number of Arcobacter cells enter the WWTPs, and while a large proportion of these cells are removed during treatment, they still form a substantial fraction of the bacteria discharged with the effluent on a daily basis. For example, in WWTP A, this corresponded to a discharge of more than 10 kg/day as Arcobacter biomass. As Arcobacter may pose a threat of broad redistribution to freshwater environments even at low abundance (12, 35), it is a concern if the treated wastewater is reused or supplied to recipients for, e.g., bathing water or livestock drinking water. There are, however, no reports about problems related to the discharge of the 14 Danish WWTPs investigated.
Discharge of Arcobacter is likely a global problem, with Arcobacter cells common in most municipal wastewater (30), so further studies are needed to quantify the risk and potentially minimize the discharge. The study also shows that culture-based analyses are insufficient for proper control of Arcobacter in effluent. However, this is a more general challenge for monitoring and evaluation of wastewater effluent quality in relation to potential pathogens in wastewater and their fate through treatment facilities. Monitoring and evaluation of wastewater effluent quality could be carried out with amplicon sequencing, as demonstrated in this study, or Arcobacter and its virulence genes could be analyzed by combining metagenomic and quantitative-PCR studies (40). Our study stresses the need for developing and testing such standardized culture-independent measurements of all pathogens in wastewater, treatment facilities, effluent, and the receiving environment.
MATERIALS AND METHODS
Sampling.
Samples (10 liters) were taken from the influent wastewater at 14 Danish wastewater treatment plants as 24-h flow-proportional samples at a location that represented the average influent to the process tanks at each WWTP. The same was done for effluents. A subsample of 50 ml was frozen for further analysis. From activated sludge, grab samples of 50 ml were frozen until analysis. Samples were taken every 14 days for 3 months. All the plants were large (37,000 to 330,000 person equivalents [PE]) full-scale plants in cities treating mainly domestic wastewater. All the plants had biological N removal and either chemical or biological removal of phosphorus. They all obeyed the Danish rules for effluent quality (total chemical oxygen demand [COD] < 70 mg/liter, total N < 8 mg/liter, and total P < 1 mg/liter). Supplemental information about wastewater treatment plants can be found in Table S1. Samples for studying the microbial community distribution in sludge flocs and supernatant were collected from the aeration tanks of 12 of the WWTPs described above. The samples were transported overnight and analyzed immediately upon arrival. Samples of sludge supernatant (activated-sludge water phase) were prepared by gentle centrifugation of sludge samples (2 min; 3,000 rpm; 854 × g) in a laboratory centrifuge (model 1e6; Sigma Laboratory Centrifuges, Germany).
DNA extraction, amplification, and sequencing.
Subsamples of frozen influent and effluent were thawed and homogenized with an overhead stirrer (Heidolph, Schwabach, Germany), and samples were filtered on 0.2-μm polycarbonate filters. To validate that filtration of wastewater and effluent samples did not bias the community composition, a sample of activated sludge from WWTP A was diluted in triplicate to the representative average cell concentration of influent and effluent and filtered on 0.2-μm polycarbonate filters (see Fig. S3 in the supplemental material). The samples were processed through the pipeline described below, and no negative effect of dilution and filtration was found. For extraction of DNA, the polycarbonate filters or 0.5 ml of homogenized sludge sample was used for direct extraction of DNA with the FastDNA spin kit for soil (MP Biomedicals, Irvine, CA, USA), with the modification that the bead beating was extended to 4 times for 40 s each time at 6 m/s instead of 40 s at 6 m/s (41). Nucleic acids were quantified using a double-stranded DNA (dsDNA) BR assay kit on a Qubit 2.0 fluorometer (Invitrogen, Carlsbad, CA, USA). The 16S V1 to V3 library preparation was performed as described by Albertsen et al. (41) and McIlroy et al. (42), modified from Caporaso et al. (43), with the use of bacterial primers that amplify a DNA fragment of 500 bp of the V1 to V3 region of the 16S rRNA gene (27F [AGAGTTTGATCCTGGCTCAG; Lane {44}] and 534R [ATTACCGCGGCTGCTGG; Muyzer et al. {45}]). The library DNA concentration was measured with a dsDNA HS assay kit on a Qubit 2.0 fluorometer (Invitrogen, Carlsbad, CA, USA), and the quality was validated with a TapeStation 2200 using D1K screen tapes (Agilent Technologies, Santa Clara, CA, USA). Libraries were pooled in equimolar concentrations. The library pool was paired-end (2 × 300 bp) sequenced on a MiSeq (Illumina, San Diego, CA, USA).
All the sequenced libraries were screened for PhiX contamination. The reads were dereplicated and formatted for use in the USEARCH UNOISE workflow (46). The dereplicated reads were used to generate amplicon sequence variants (ASVs) (also called zero-radius operational taxonomic units [zOTUs]), using USEARCH v. 10 unoise3 with default settings. Taxonomy was assigned using the SINTAX classifier (47) as implemented in USEARCH v. 11, based on the concept of AutoTax (48), where a comprehensive full-length 16S rRNA gene database provides species level resolution using MiDAS 3 taxonomy (19), which is based on the SILVA taxonomy (49). The results were analyzed in R (R Development Core Team, 2018) using Rstudio IDE (RStudio) and the ampvis2 R package v. 2.4.2.1 (GitHub). Principal-component analysis (PCA) was produced using Vegan (50) with square-root-transformed ASV counts. P values were calculated using an Adonis test (Vegan) of a Bray-Curtis dissimilarity matrix across the sampling locations.
Culturing.
For verification of viability and species identification, Arcobacter was isolated from influent and effluent wastewater by the following procedure. Fifteen milliliters was filtered on 0.45-μm polycarbonate filters, and the filters were incubated under microaerobic conditions in 10 ml Arcobacter broth with cefoperazone, amphotericin B, and teicoplanin (CAT) supplement (Oxoid) for 24 h at 30°C. One hundred microliters of broth was suspended on a sterile 0.45-μm polyacetate filter placed on medium containing Arcobacter broth with 12 g/liter agar and CAT supplement. The filters were removed after 0.5 h, and the filtrates were plated out and incubated under microaerobic conditions for 7 days at 37°C. Pinpoint-shaped and slightly translucent colonies were restreaked and incubated under microaerobic conditions for 7 days at 37°C. Isolated colonies were verified as Arcobacter using FISH and genome sequencing.
Fluorescent in situ hybridization.
Fresh 24-h flow-proportional samples of influent wastewater and effluent were collected from WWTP A and processed immediately upon arrival at the laboratory. Ten microliters of the influent and 1 ml of the effluent were filtered on 0.22-μm polycarbonate filters. Samples were fixed with 4% paraformaldehyde (final concentration) for 2 h at 4°C and washed 3 times with 1 ml of sterile filtered tap water. FISH with the ARC1430 probe (labeled with Alexa 555) targeting Arcobacter spp. (51) and EUB-338-I, EUB338-II, and EUB338-III [EUBmix labeled with 5(6)-carboxyfluorescein-N-hydroxysuccinimide ester (FLUOS)] (52, 53) covering all bacteria was performed essentially as described previously (54) at a hybridization buffer formamide concentration of 20% (vol/vol). Cells were counted on the filter in a minimum of 10 different fields of view, using an Axioskop epifluorescence microscope (Carl Zeiss, Oberkochen, Germany; ×1,000 magnification), and the mean number of cells per liter was calculated for each sample. A white-light laser confocal microscope (TCS SP8 X; Leica, Wetzlar, Germany) was used to capture FISH images.
Genome/metagenome sequencing.
DNA from 4 isolated cultures and metagenomes from 1 influent and 1 effluent sample were prepared and sequenced as Illumina Nextera libraries, prepared according to the manufacturer’s protocol, and paired-end sequenced on the Illumina MiSeq platform. For metagenomes, a total of 63,868,036 reads were assembled using default settings in CLC genomic workbench (CLC Bio v.7.5.1; Qiagen). Reads were mapped to assemblies separately for each sample using minimap2 (v. 2-2.5) (55), coverage was determined from the bam files using SAMtools v. 1.3.1 (56). The read mappings and assemblies were exported as .CSV and .fasta files and processed in R with the mmgenome tool (57) for binning. Isolated genome assemblies were identified as A. butzleri by analyzing the 16S rRNA gene using NCBI BLAST in CLC workbench using default settings, and they were annotated using Prokka (v. 1.11) (58). The 4 genomes were assembled with CheckM (59) completeness scores of 99.6, 97.8, 99.6, and 96.8% and contamination scores of 0, 5.66, 0, and 3.46%, respectively. Similarity was analyzed with JSpeciesWS (60; http://jspecies.ribohost.com/jspeciesws/). Phylogenetic analyses were conducted in MEGA7 (61) using the maximum-likelihood method based on the Tamura-Nei model (62).
Calculation of biomass fractions and cell numbers.
The total number of bacterial cells in the influent was calculated by assuming that the total number of microorganisms in the wastewater was equal to that in previous reports for wastewater from the same region (63): average dry weather wastewater has 1.4 × 1011 cells/liter ± 25% (standard deviation) (10) (as counted by fluorescence microscopy and DAPI [4′,6-diamidino-2-phenylindole] staining). This number is multiplied by the volume of wastewater received on the sampling day to get the total number received in the WWTP per day (see Table S1 and detailed calculations and supplemental RMarkdown [RMD] file at https://github.com/janniemunk/Publications/tree/master/2020_Arcobacter).
The total number of bacterial cells per liter in the process tank was found from the amount of VSS using a conversion factor of 5 × 1011 cells per gram VSS ± 15% (64). The total mass of cells in the process tanks was found from the amount of VSS as gram VSS per liter multiplied by the volume of water coming per day into the wastewater treatment plant, assuming a typical hydraulic residence time (HRT) of 1 day in the plants.
The total number of bacterial cells in the effluent was determined from analyses of VSS in the effluent (ranging from 0.1 to 20 mg VSS/liter) and the effluent water flow, assuming it to be 97% of the influent flow, as approximately 3% of the flow goes with the surplus sludge (10).
The percentage of mass from a given genus (such as Arcobacter) is found by multiplying the total number of cells by the relative read abundance percentage.
Data availability.
Data are available at the European Nucleotide Archive (ENA) under study accession number PRJEB28796. The RMarkdown files to generate the figures are available at GitHub (https://github.com/janniemunk/Publications/tree/master/2020_Arcobacter).
Supplementary Material
ACKNOWLEDGMENTS
The study was supported by the Villum Foundation (Dark Matter) and wastewater treatment plants in the MIDAS project.
We thank the staff at the WWTPs for help in providing samples.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Lee SH, Kang HJ, Park HD. 2015. Influence of influent wastewater communities on temporal variation of activated sludge communities. Water Res 73:132–144. doi: 10.1016/j.watres.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 2.Mølgaard K, Nickelsen C, Jansen J. 2002. Hygienic quality of wastewater from municipal treatment plant. Report no. 684. Danish Environmental Protection Agency, Odense, Denmark: https://www2.mst.dk/udgiv/publikationer/2002/87-7972-078-1/pdf/87-7972-080-3.pdf. [Google Scholar]
- 3.Frigon D, Biswal BK, Mazza A, Masson L, Gehr R. 2013. Biological and physicochemical wastewater treatment processes reduce the prevalence of virulent Escherichia coli. Appl Environ Microbiol 79:835–844. doi: 10.1128/AEM.02789-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koivunen J, Siitonen A, Heinonen-Tanski H. 2003. Elimination of enteric bacteria in biological-chemical wastewater treatment and tertiary filtration units. Water Res 37:690–698. doi: 10.1016/s0043-1354(02)00305-6. [DOI] [PubMed] [Google Scholar]
- 5.McLellan SL, Huse SM, Mueller-Spitz SR, Andreishcheva EN, Sogin ML. 2010. Diversity and population structure of sewage-derived microorganisms in wastewater treatment plant influent. Environ Microbiol 12:378–392. doi: 10.1111/j.1462-2920.2009.02075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu X, Zhang XX, Wang Z, Huang K, Wang Y, Liang W, Tan Y, Liu B, Tang J. 2015. Bacterial pathogens and community composition in advanced sewage treatment systems revealed by metagenomics analysis based on high-throughput sequencing. PLoS One 10:1–15. doi: 10.1371/journal.pone.0125549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.George I, Crop P, Servais P. 2002. Fecal coliform removal in wastewater treatment plants studied by plate counts and enzymatic methods. Water Res 36:2607–2617. doi: 10.1016/s0043-1354(01)00475-4. [DOI] [PubMed] [Google Scholar]
- 8.Kabler P. 1959. Removal of pathogenic microorganisms by sewage treatment processes. Sewage Ind Waste 31:1373–1382. [Google Scholar]
- 9.Naidoo S, Olaniran AO. 2013. Treated wastewater effluent as a source of microbial pollution of surface water resources. Int J Environ Res Public Health 11:249–270. doi: 10.3390/ijerph110100249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saunders AM, Albertsen M, Vollertsen J, Nielsen PH. 2016. The activated sludge ecosystem contains a core community of abundant organisms. ISME J 10:11–20. doi: 10.1038/ismej.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McIlroy SJ, Kirkegaard RH, McIlroy B, Nierychlo M, Kristensen JM, Karst SM, Albertsen M, Nielsen PH. 2017. MiDAS 2.0: an ecosystem-specific taxonomy and online database for the organisms of wastewater treatment systems expanded for anaerobic digester groups. Database 2017:1–9. doi: 10.1093/database/bax016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher JC, Levican A, Figueras MJ, McLellan SL. 2014. Population dynamics and ecology of Arcobacter in sewage. Front Microbiol 5:525. doi: 10.3389/fmicb.2014.00525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collado L, Figueras MJ. 2011. Taxonomy, epidemiology, and clinical relevance of the genus Arcobacter. Clin Microbiol Rev 24:174–192. doi: 10.1128/CMR.00034-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fera MT, Gugliandolo C, Lentini V, Favaloro A, Bonanno D, La Camera E, Maugeri TL. 2010. Specific detection of Arcobacter spp. in estuarine waters of Southern Italy by PCR and fluorescent in situ hybridization. Lett Appl Microbiol 50:65–70. doi: 10.1111/j.1472-765X.2009.02767.x. [DOI] [PubMed] [Google Scholar]
- 15.Webb AL, Taboada EN, Selinger LB, Boras VF, Inglis GD. 2016. Efficacy of wastewater treatment on Arcobacter butzleri density and strain diversity. Water Res 105:291–296. doi: 10.1016/j.watres.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Amaral AL, Da Motta M, Pons MN, Vivier H, Roche N, Mota M, Ferreira EC. 2004. Survey of Protozoa and Metazoa populations in wastewater treatment plants by image analysis and discriminant analysis. Environmetrics 15:381–390. doi: 10.1002/env.652. [DOI] [Google Scholar]
- 17.Jauffur S, Isazadeh S, Frigon D. 2014. Should activated sludge models consider influent seeding of nitrifiers? Field characterization of nitrifying bacteria. Water Sci Technol 70:1526–1532. doi: 10.2166/wst.2014.407. [DOI] [PubMed] [Google Scholar]
- 18.Ofiteru ID, Lunn M, Curtis TP, Wells GF, Criddle CS, Francis CA, Sloan WT. 2010. Combined niche and neutral effects in a microbial wastewater treatment community. Proc Natl Acad Sci U S A 107:15345–15350. doi: 10.1073/pnas.1000604107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nierychlo M, Andersen KS, Xu Y, Green N, Albertsen M, Dueholm MS, Nielsen PH. 2019. Species-level microbiome composition of activated sludge—introducing the MiDAS 3 ecosystem-specific reference database and taxonomy. bioRxiv https://www.biorxiv.org/content/10.1101/842393v1. [DOI] [PubMed]
- 20.Morgan-Sagastume F, Larsen P, Nielsen JL, Nielsen PH. 2008. Characterization of the loosely attached fraction of activated sludge bacteria. Water Res 42:843–854. doi: 10.1016/j.watres.2007.08.026. [DOI] [PubMed] [Google Scholar]
- 21.Nielsen JL, Juretschko S, Wagner M, Nielsen PH. 2002. Abundance and phylogenetic affiliation of iron reducers in activated sludge as assessed by fluorescence in situ hybridization and microautoradiography. Appl Environ Microbiol 68:4629–4636. doi: 10.1128/aem.68.9.4629-4636.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Douidah L, de Zutter L, Bare J, De Vos P, Vandamme P, Vandenberg O, Van den Abeele A-M, Houf K. 2012. Occurrence of putative virulence genes in Arcobacter species isolated from humans and animals. J Clin Microbiol 50:735–741. doi: 10.1128/JCM.05872-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Figueras MJ, Levican A, Pujol I, Ballester F, Quilez MJR, Gomez-Bertomeu F. 2014. A severe case of persistent diarrhoea associated with Arcobacter cryaerophilus but attributed to Campylobacter sp. and a review of the clinical incidence of Arcobacter spp. New Microbes New Infect 2:31–37. doi: 10.1002/2052-2975.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brightwell G, Mowat E, Clemens R, Boerema J, Pulford DJ, On SL. 2007. Development of a multiplex and real time PCR assay for the specific detection of Arcobacter butzleri and Arcobacter cryaerophilus. J Microbiol Methods 68:318–325. doi: 10.1016/j.mimet.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 25.Vandewalle JL, Goetz GW, Huse SM, Morrison HG, Sogin ML, Hoffmann RG, Yan K, McLellan SL. 2012. Acinetobacter, Aeromonas and Trichococcus populations dominate the microbial community within urban sewer infrastructure. Environ Microbiol 14:2538–2552. doi: 10.1111/j.1462-2920.2012.02757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levican A, Collado L, Figueras MJ. 2016. The use of two culturing methods in parallel reveals a high prevalence and diversity of Arcobacter spp. in a wastewater treatment plant. Biomed Res Int 2016:8132058. doi: 10.1155/2016/8132058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nielsen PH, Seviour RJ. 2010. Microbial ecology of activated sludge. IWA Publishing, London, United Kingdom. [Google Scholar]
- 28.Zita A, Hermansson M. 1997. Effects of bacterial cell surface structures and hydrophobicity on attachment to activated sludge flocs. Appl Environ Microbiol 63:1168–1170. doi: 10.1128/AEM.63.3.1168-1170.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Assanta MA, Roy D, Lemay M-J, Montpetit D. 2002. Attachment of Arcobacter butzleri, a new waterborne pathogen, to water distribution pipe surfaces. J Food Prot 65:1240–1247. doi: 10.4315/0362-028x-65.8.1240. [DOI] [PubMed] [Google Scholar]
- 30.McLellan SL, Roguet A. 2019. The unexpected habitat in sewer pipes for the propagation of microbial communities and their imprint on urban waters. Curr Opin Biotechnol 57:34–41. doi: 10.1016/j.copbio.2018.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Atabay HI, Corry JEL, On S. 1998. Diversity and prevalence of Arcobacter spp. in broiler chickens. J Appl Microbiol 84:1007–1016. doi: 10.1046/j.1365-2672.1998.00437.x. [DOI] [PubMed] [Google Scholar]
- 32.Zacharow I, Bystroń J, Wałecka-Zacharska E, Podkowik M, Bania J. 2015. Genetic diversity and incidence of virulence-associated genes of Arcobacter butzleri and Arcobacter cryaerophilus isolates from pork, beef, and chicken meat in Poland. Biomed Res Int 2015:956507. doi: 10.1155/2015/956507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang ZD, Dupont HL, Brown EL, Nandy RK, Ramamurthy T, Sinha A, Ghosh S, Guin S, Gurleen K, Rodrigues S, Chen JJ, McKenzie R, Steffen R. 2010. Microbial etiology of travelers’ diarrhea in Mexico, Guatemala, and India: importance of enterotoxigenic Bacteroides fragilis and Arcobacter species. J Clin Microbiol 48:1417–1419. doi: 10.1128/JCM.01709-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abdelbaqi K, Buissonnière A, Prouzet-Mauleon V, Gresser J, Wesley I, Mégraud F, Ménard A. 2007. Development of a real-time fluorescence resonance energy transfer PCR to detect Arcobacter species. J Clin Microbiol 45:3015–3021. doi: 10.1128/JCM.00256-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Narbenovich VM, Vladimirovich CS. 2017. Deciphering conjugative plasmid permissiveness in wastewater microbiomes. J Technol 32:139–146. [DOI] [PubMed] [Google Scholar]
- 36.Cray JA, Bell ANW, Bhaganna P, Mswaka AY, Timson DJ, Hallsworth JE. 2013. The biology of habitat dominance; can microbes behave as weeds? Microb Biotechnol 6:453–492. doi: 10.1111/1751-7915.12027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Driessche E, Houf K. 2008. Survival capacity in water of Arcobacter species under different temperature conditions. J Appl Microbiol 105:443–451. doi: 10.1111/j.1365-2672.2008.03762.x. [DOI] [PubMed] [Google Scholar]
- 38.Douidah L, De Zutter L, Van Nieuwerburgh F, Deforce D, Ingmer H, Vandenberg O, Van Den Abeele AM, Houf K. 2014. Presence and analysis of plasmids in human and animal associated Arcobacter species. PLoS One 9:e85487-8. doi: 10.1371/journal.pone.0085487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Webb AL, Boras VF, Kruczkiewicz P, Selinger LB, Taboada EN, Douglas G. 2016. Comparative detection and quantification of Arcobacter butzleri in stools from diarrheic and nondiarrheic people in southwestern Alberta, Canada. J Clin Microbiol 54:1082–1088. doi: 10.1128/JCM.03202-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shrestha RG, Sherchan SP, Kitajima M, Tanaka Y, Gerba CP, Haramoto E. 2019. Reduction of Arcobacter at two conventional wastewater treatment plants in southern Arizona, USA. Pathogens 8:1–11. doi: 10.3390/pathogens8040175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Albertsen M, Karst SM, Ziegler AS, Kirkegaard RH, Nielsen PH. 2015. Back to basics—the influence of DNA extraction and primer choice on phylogenetic analysis of activated sludge communities. PLoS One 10:e0132783-15. doi: 10.1371/journal.pone.0132783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McIlroy SJ, Saunders AM, Albertsen M, Nierychlo M, McIlroy B, Hansen AA, Karst SM, Nielsen JL, Nielsen PH. 2015. MiDAS: the field guide to the microbes of activated sludge. Database (Oxford) 2015:bav062. doi: 10.1093/database/bav062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Lozupone CA, Turnbaugh PJ, Fierer N, Knight R. 2011. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci U S A 108 Suppl:4516–4522. doi: 10.1073/pnas.1000080107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lane DJ. 1991. 16S/23S rRNA sequencing. In Stackebrandt E, Goodfellow M (ed), Nucleic acid techniques in bacterial systematics, p 115–175. John Wiley & Sons, Ltd., Chichester, England. [Google Scholar]
- 45.Muyzer G, De Waal EC, Uitterlinden AG. 1993. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Inst Appl Microbiol 59:695–700. doi: 10.1128/AEM.59.3.695-700.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Edgar RC. 2016. UNOISE2: improved error-correction for Illumina 16S and ITS amplicon sequencing. bioRxiv https://www.biorxiv.org/content/10.1101/081257v1.
- 47.Edgar R. 2016. SINTAX: a simple non-Bayesian taxonomy classifier for 16S and ITS sequences. bioRxiv https://www.biorxiv.org/content/10.1101/074161v1.
- 48.Dueholm MS, Andersen KS, Petriglieri F, McIlroy SJ, Nierychlo M, Petersen JF, Kristensen JM, Yashiro E, Karst SM, Albertsen M, Nielsen PH. 2019. Comprehensive ecosystem-specific 16S rRNA gene databases with automated taxonomy assignment (AutoTax) provide species-level resolution in microbial ecology. bioRxiv https://www.biorxiv.org/content/10.1101/672873v2. [DOI] [PMC free article] [PubMed]
- 49.Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO. 2013. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41:D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oksanen AJ, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, Hara RBO, Simpson GL, Solymos P, Stevens MHH, Szoecs E. 2016. vegan: community ecology package. R Project version 2.4-0. https://CRAN.R-project.org/package=vegan.
- 51.Snaidr J, Amann R, Huber I, Ludwig W, Schleifer KH. 1997. Phylogenetic analysis and in situ identification of bacteria in activated sludge. Appl Environ Microbiol 63:2884–2896. doi: 10.1128/AEM.63.7.2884-2896.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Amann RI, Binder BJ, Olson RJ, Chisholm SW, Devereux R, Stahl DA. 1990. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl Environ Microbiol 56:1919–1925. doi: 10.1128/AEM.56.6.1919-1925.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Daims H, Brühl A, Amann R, Schleifer KH, Wagner M. 1999. The domain-specific probe EUB338 is insufficient for the detection of all bacteria: development and evaluation of a more comprehensive probe set. Syst Appl Microbiol 22:434–444. doi: 10.1016/S0723-2020(99)80053-8. [DOI] [PubMed] [Google Scholar]
- 54.Lam P, Cowen JP. 2004. Processing deep-sea particle-rich water samples for fluorescence in situ hybridization: consideration of storage effects, preservation, and sonication. Appl Environ Microbiol 70:25–33. doi: 10.1128/aem.70.1.25-33.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li H. 2018. Minimap2: pairwise alignment for nucleotide sequences. Bioinformatics 34:3094–3100. doi: 10.1093/bioinformatics/bty191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li H, 1000 Genome Project Data Processing Subgroup, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R. 2009. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Karst SM, Kirkegaard RH, Albertsen M. 2016. mmgenome: a toolbox for reproducible genome extraction from metagenomes. bioRxiv https://www.biorxiv.org/content/10.1101/059121v1.full.
- 58.Seemann T. 2014. Prokka: rapid prokaryotic genome annotation. Bioinformatics 30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 59.Parks DH, Imelfort M, Skennerton CT, Hugenholtz P, Tyson GW. 2015. CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res 25:1043–1055. doi: 10.1101/gr.186072.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Richter M, Rosselló-Móra R, Oliver Glöckner F, Peplies J. 2016. JSpeciesWS: a web server for prokaryotic species circumscription based on pairwise genome comparison. Bioinformatics 32:929–931. doi: 10.1093/bioinformatics/btv681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kumar S, Stecher G, Tamura K. 2016. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tamura K, Nei M. 1993. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol 10:512–526. doi: 10.1093/oxfordjournals.molbev.a040023. [DOI] [PubMed] [Google Scholar]
- 63.Vollertsen J, Jahn A, Nielsen JL, Hvitved-Jacobsen T, Nielsen PH. 2001. Comparison of methods for determination of microbial biomass in wastewater. Water Res 35:1649–1658. doi: 10.1016/s0043-1354(00)00450-4. [DOI] [PubMed] [Google Scholar]
- 64.Frølund B, Palmgren R, Keiding K, Nielsen PH. 1996. Extraction of extracellular polymers from activated sludge using a cation exchange resin. Water Res 30:1749–1758. doi: 10.1016/0043-1354(95)00323-1. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available at the European Nucleotide Archive (ENA) under study accession number PRJEB28796. The RMarkdown files to generate the figures are available at GitHub (https://github.com/janniemunk/Publications/tree/master/2020_Arcobacter).