Aromatic amino acids such as tryptophan are primarily used as additives in the animal feed industry and are typically produced using genetically engineered heterotrophic organisms such as Escherichia coli. This involves a two-step process, where the substrate such as molasses is first obtained from plants followed by fermentation by heterotrophic organisms. We have engineered photoautotrophic cyanobacterial strains by a combination of random mutagenesis and metabolic engineering. These strains grow on CO2 as the sole carbon source and utilize light as the sole energy source to produce tryptophan, thus converting the two-step process into a single step. Our results show that combining random mutagenesis and metabolic engineering was superior to either approach alone. This study also builds a foundation for further engineering of cyanobacteria for industrial tryptophan production.
KEYWORDS: Synechocystis, aromatic amino acids, l-tryptophan, metabolic engineering, gene overexpression, cyanobacteria
ABSTRACT
Tryptophan (Trp) is an essential aromatic amino acid that has value as an animal feed supplement, as the amount found in plant-based sources is insufficient. An alternative to production by engineered microbial fermentation is to have tryptophan biosynthesized by a photosynthetic microorganism that could replace or supplement both the plant and industrially used microbes. We selected Synechocystis sp. strain PCC 6803, a model cyanobacterium, as the host and studied metabolic engineering and random mutagenesis approaches. Previous work on engineering heterotrophic microbes for improved Trp titers has targeted allosteric feedback regulation in enzymes 3-deoxy-d-arabinoheptulosonate 7-phosphate synthase (DAHPS) and anthranilate synthase (AS) as major bottlenecks in the shikimate pathway. In this work, the genes encoding feedback-resistant enzymes from Escherichia coli, aroGfbr and trpEfbr, were overexpressed in the host wild-type (WT) strain. Separately, the WT strain was subjected to random mutagenesis and selection using an amino acid analog to isolate tryptophan-overproducing strains. The randomly mutagenized strains were sequenced in order to identify the mutations that resulted in the desirable phenotypes. Interestingly, the tryptophan overproducers had mutations in the gene encoding chorismate mutase (CM), which catalyzes the conversion of chorismate to prephenate. The best tryptophan overproducer from random mutagenesis was selected as a host for metabolic engineering where aroGfbr and trpEfbr were overexpressed. The best strain developed produced 212 ± 23 mg/liter of tryptophan after 10 days of photoautotrophic growth under 3% (vol/vol) CO2. We demonstrated that a combination of random mutagenesis and metabolic engineering was superior to either individual approach.
IMPORTANCE Aromatic amino acids such as tryptophan are primarily used as additives in the animal feed industry and are typically produced using genetically engineered heterotrophic organisms such as Escherichia coli. This involves a two-step process, where the substrate such as molasses is first obtained from plants followed by fermentation by heterotrophic organisms. We have engineered photoautotrophic cyanobacterial strains by a combination of random mutagenesis and metabolic engineering. These strains grow on CO2 as the sole carbon source and utilize light as the sole energy source to produce tryptophan, thus converting the two-step process into a single step. Our results show that combining random mutagenesis and metabolic engineering was superior to either approach alone. This study also builds a foundation for further engineering of cyanobacteria for industrial tryptophan production.
INTRODUCTION
Aromatic amino acids are high-value biochemicals with industrial and agricultural applications (1–3). Tryptophan is used in feed, food, and pharmaceutical industries and serves as the precursor for the synthesis of 5-hydroxytryptophan and for the treatment of pellagra, a disease caused by the deficiency of niacin (1, 2, 4). Tryptophan is produced in biotechnological processes using engineered heterotrophic microorganisms such as Escherichia coli and Corynebacterium glutamicum from carbon sources such as glucose (1, 5). As an alternative, photoautotrophic cyanobacteria can fix carbon dioxide to produce aromatic amino acids via the shikimate pathway. This approach converts the two-step process of obtaining a carbon source such as molasses/glucose from plants and its subsequent conversion to aromatic amino acids into a single step.
Hall et al. (6) surveyed the regulation of the shikimate pathway in their study of 48 cyanobacterial strains and found they differ greatly from those of E. coli and C. glutamicum. They tested allosteric feedback inhibition of the first enzyme, 3-deoxy-d-arabinoheptulosonate 7-phosphate synthase (DAHPS), across several species and showed that it is predominantly due to phenylalanine (6), whereas the three isoforms of DAHPS are each individually inhibited by phenylalanine, tyrosine, and tryptophan in E. coli (3). Further differences between E. coli and cyanobacteria include the regulation of the Phe/Tyr and Trp branches. Chorismate mutase (CM) was only found to be feedback inhibited by Phe, Tyr, or Trp in a few cyanobacterial strains (6), whereas CM-prephenate dehydrogenase (PDH) is inhibited by tyrosine and the CM-prephenate dehydratase (PD) complex is inhibited by phenylalanine in E. coli (1). A summary of the observed regulation of the pathways of aromatic amino acid biosynthesis in cyanobacteria is provided in Fig. 1, but does not necessarily represent regulation in any single cyanobacterial strain.
FIG 1.
Observed enzyme regulation of the shikimate and aromatic amino acid pathways in cyanobacteria. Not all regulation necessarily exists in a single cyanobacterial species. Adapted and modified from references 43 and 44. E4P, erythrose-4-phosphate; PEP, phosphoenolpyruvate; DAHP, 3-deoxy-d-arabinoheptulosonate 7-phosphate; DHQ, 3-dehydroquinate; DHS, 3-dehydroshikimate; SHK, shikimate; S3P, shikimate-3-phosphate; EPSP, 5-enolpyruvylshikimate 3-phosphate; CHA, chorismate; ANT, anthranilate; PRA, phosphoribosylanthranilate; CRP, 1-(2-carboxyphenylamino)-1-deoxy-d-ribulose 5-phosphate; IGP, indole glycerol phosphate; TRP, tryptophan; PPA, prephenate; PPY, phenylpyruvate; AGN, arogenate; PHE, phenylalanine; TYR, tyrosine; DAHPS, 3-deoxy-d-arabinoheptulosonate 7-phosphate synthase; DHQS, 3-dehydroquinate synthase; DHQD, 3-dehydroquinate dehydrogenase; SDH, shikimate dehydrogenase; SK, shikimate kinase; EPSPS, 5-enolpyruvylshikimate 3-phosphate synthase; CS, chorismate synthase; AS, anthranilate synthase; APRT, anthranilate phosphoribosyltransferase; PRAI, phosphoribosylanthranilate isomerase; TRPS, tryptophan synthase; CM, chorismate mutase; PD, prephenate dehydratase; PAT, prephenate aminotransferase.
In general, the first step for microbial overproduction of phenylalanine or tryptophan is the removal of allosteric feedback inhibition of DAHPS to increase flux into the shikimate pathway (1). Further deregulation of enzymes downstream of DAHPS is also required to improve the productivity of the microbes. In E. coli, the branch point enzyme CM is deregulated to produce phenylalanine, and anthranilate synthase (AS) is deregulated to produce tryptophan (7). The removal of feedback inhibition of DAHPS by phenylalanine alone in a mutant of Synechocystis sp. strain 29108 was sufficient to confer resistance to phenylalanine analogs and produce measurable amounts of extracellular Phe and Tyr compared to nondetectable amounts in the wild type (8).
Random mutagenesis followed by selection on amino acid analogs is a common strain development technique for the production of valuable amino acids. With the reduced cost of whole-genome sequencing using next-generation sequencing (NGS), random mutagenesis has become a feasible option to discover favorable mutations and has been used in Corynebacterium for enhancing the production of lysine, methionine, and serine (9). Moreover, random mutagenesis, screening, and resequencing may identify gene regulation of key features of the shikimate pathway in less-characterized species. Previous work demonstrated that DAHPS and other shikimate pathway enzymes lost feedback inhibition in analog-resistant cyanobacterial mutants through enzyme assays (8, 10, 11) but did not determine the biochemical nature, either of alterations in the gene coding region or amino acids in the sequence of the resistant enzyme. In contrast, specific amino acid substitutions are known to occur in feedback-resistant DAHPS (12), CM-PD (13), and AS in E. coli, allowing for direct expression of genes encoding these allosteric feedback-resistant enzymes.
In this study, we heterologously overexpressed aroGL175D (12) and trpES40F (14) from E. coli, which code for feedback-resistant DAHPS and AS, respectively, individually as well as in combination to study their effect on aromatic amino acid production. In parallel, methyl methanesulfonate (MMS), a potent DNA alkylating agent that induces base substitutions (15), was used for random mutagenesis, followed by selection on a tryptophan analog. Three overproducing mutants were sequenced using the Illumina MiSeq platform to identify novel mutations that could lead to overproduction of aromatic amino acids. Finally, the randomly mutagenized strain with the highest tryptophan titer was selected to be further engineered by the individual and combined overexpression of feedback-resistant forms of DAHPS and AS to develop strains with higher tryptophan titers.
We show that the combination of metabolic engineering and random mutagenesis approaches was a strategy superior to either approach alone. This method could be used in the future to guide the development of cyanobacterial strains or other microbes for production of valuable chemicals on larger scales.
RESULTS
Metabolic engineering of wild-type Synechocystis sp. strain PCC 6803 (SYNY3).
The enzymes DAHPS and AS are, in general, subject to feedback inhibition by phenylalanine and tryptophan, respectively, in cyanobacteria (Fig. 1). To overcome the feedback regulation of the shikimate pathway by these end products, we expressed the feedback-resistant (fbr) genes aroGfbr(L175D) and trpEfbr(S40F) from E. coli individually and in combination. The gene(s) was expressed after integration by homologous recombination of a plasmid with a light-driven promoter as listed in Table 1. Both the intracellular and extracellular Trp concentrations were measured, and the final titers were calculated for different strains (Fig. 2). The major fraction of aromatic amino acids was obtained in the extracellular component, and the contribution of the intracellular accumulation to the total was minimal (data not shown). Similar to that for Trp, the total titer of Phe was significantly increased in strains containing feedback-resistant DAHPS (SYNY3-AD2 and SYNY3-AD4), while Tyr was unaffected (see Fig. S3 in the supplemental material).
TABLE 1.
Plasmids used in this study
| Plasmid | Integration site | Promoter | Antibiotic resistance | Gene(s) expressed (E. coli) | Reference |
|---|---|---|---|---|---|
| pEERM1 | psbA2 | PpsbA2 | Km | 36 | |
| pEERM1a | psbA2 | PpsbA2 | Km | aroGL175D | This work |
| pEERM1b | psbA2 | PpsbA2 | Km | trpES40F | This work |
| pEERM1c | psbA2 | PpsbA2 | Km | aroGL175D trpES40F | This work |
FIG 2.

Trp production by metabolically engineered Synechocystis strains after 168 h under atmospheric CO2, 240 μmol photons m−2 s−1 light at 30°C, and 200 rpm. Error bars indicate standard errors (n = 3, biological replicates). Multiple comparison using Tukey’s test (α = 0.05) showed two distinct groups for Trp production, A and B.
The overexpression of the feedback-resistant DAHPS alone and in combination with feedback-resistant AS resulted in elevated tryptophan titers; however, the individual overexpression of trpEfbr (AS) showed no significant difference with respect to the empty vector control (Fig. 2). The strain SYNY3-AD4, expressing both feedback-resistant genes, showed a 190-fold increase in the mean tryptophan production, with a mean titer of 12.6 mg/liter, compared to that with the empty vector control. A multiple-comparison test was performed using the Tukey’s test (α = 0.05), which grouped the tryptophan production by these engineered strains into two distinct groups, A and B; the higher titer value group B contained a feedback-resistant DAHPS. The growth rates of the engineered SYNY3 strains (SYNY3-AD2 to SYNY3-AD4) were statistically not different from either the wild type (WT) or the empty vector control (SYNY3-AD1) according to Tukey’s test (α = 0.05) (see Table S2). This result shows that relieving the allosteric inhibition at DAHPS is a crucial first step in aromatic amino acid overproduction and results in greater flux to the shikimate pathway.
Random mutagenesis of wild-type Synechocystis sp. PCC 6803 (SYNY3).
Random mutagenesis was performed using MMS as the chemical mutagen, which alters the DNA by causing mainly GC-to-TA or TA-to-GC transversions (16). Six colonies resistant to the highest level (0.9 mg/ml) of analog 5-fluoro-dl-tryptophan were selected; however, only 3 survived inoculation into liquid medium (SYNY3-JV1 to SYNY3-JV3). Amino acid analogs are effectors that can bind allosteric inhibition sites of enzymes in the shikimate pathway (17). They also may inhibit growth by incorporating into protein, resulting in lesser or nonfunctional proteins, or by competitive inhibition of protein synthesis (18). The strain SYNY3-JV1, the highest Trp producer among the randomly mutagenized strains, produced 17.2 ± 1.5 mg/liter of tryptophan, which is significantly higher than the wild type (0.041 ± 0.003 mg/liter). However, the other aromatic amino acids, Phe and Tyr, were unchanged (Fig. S4). Similar to that in the engineered strains, a majority of the Trp was extracellular. Interestingly, the mutant SYNY3-JV1 grew at a slightly higher rate than the parent SYNY3 strain (P < 0.05) (Table S2). An additional electron sink could result in a boost in the photosynthetic activity and growth as seen in Synechocystis sp. PCC 6803 (19). Similar observations were also made with 2,3-butanediol- and sucrose-overproducing Synechococcus elongatus strains (20, 21).
To identify the mutations that led to the improved phenotype, we sequenced the genomic DNA from multiple isolated strains (SYNY3-JV1, SYNY3-JV2, and SYNY3-JV3). Mutations were identified in these overproducing strains with respect to the resequenced SYNY3 as a reference. In each case, the number of single nucleotide polymorphisms (SNPs) was less than 0.1% of the genome (3.6 Mb). SYNY3-JV1, SYNY3-JV2, and SYNY3-JV3 showed 3,189, 1,718, and 3,000 SNPs, respectively, which included missense, silent, and nonsense mutations. No indels were identified in the variant calling pipeline. Because Synechocystis is polyploid, not all mutations observed were identified on all copies of the genome if they did not completely segregate (22). Several SNPs were identified in the shikimate pathway in the overproducing strains developed; however, not all were fully segregated. Table 2 shows the fully segregated mutations in the shikimate pathway, while all the mutations observed in this pathway are listed in Table S3.
TABLE 2.
Summary of fully segregated mutations in shikimate pathway in Trp-overproducing strains
| Trp-overproducing strain | Mutation | Enzyme |
|---|---|---|
| SYNY3-JV1 | V52F | CM |
| SYNY3-JV2 | F50V | CM |
| SYNY3-JV3 | I64N | CM |
All the Trp-overproducing randomly mutagenized strains, SYNY3-JV1, SYNY3-JV2, and SYNY3-JV3, showed fully segregated mutations in the gene aroH, which encodes CM (Table 2). All aroH mutations identified were in the same region. Thus, the mutations observed likely result in a mutated CM that results in redirection of flux to the Trp branch. Surprisingly, no fully segregated mutations were observed in either DAHPS or AS in our randomly mutagenized tryptophan-overproducing strains. Thus, our random mutagenesis strategy led to the identification of novel mutations in CM that lead to improved Trp accumulation.
Table S4 shows the other fully segregated mutations identified. Two of the three strains sequenced, interestingly, showed mutations in the gene encoding ATP synthase subunit c (atpH).
Combining random mutagenesis and metabolic engineering approaches for tryptophan overproduction.
The randomly mutagenized strain with highest tryptophan titer, SYNY3-JV1, was chosen for further metabolic engineering. The feedback-resistant enzyme encoding genes aroGfbr (DAHPS) and trpEfbr (AS) were overexpressed both individually and in combination to determine the effect on tryptophan production with the pEERM-derived plasmids used earlier in the study (Table 1). All the newly engineered strains (SYNY3-AD5 to SYNY3-AD8) grew equally as well as SYNY3-JV1 (see Table S2).
These strains (SYNY3-AD6 to SYNY3-AD8) performed better than the empty vector control in the best Trp producer obtained from random mutagenesis (SYNY3-AD5) as well as the wild-type strain overexpressing both aroGfbr and trpEfbr (SYNY3-AD4), as can be seen from the two separate groups A and B deduced using Tukey’s test (Fig. 3). The best strain, SYNY3-AD8, containing both feedback-resistant DAHPS and AS, produced 46.1 mg/liter of tryptophan, which is ∼2.5-fold that by SYNY3-AD5 (Fig. 3) and ∼3.5-fold that by the wild type overexpressing aroGfbr and trpEfbr (SYNY3-AD4). This result shows that a combination of random mutagenesis and metabolic engineering is superior to either approach alone. We analyzed the other aromatic amino acids, Phe and Tyr, produced by SYNY3-AD4 to SYNY3-AD8 and found that Phe and Tyr were not changed (see Fig. S5).
FIG 3.
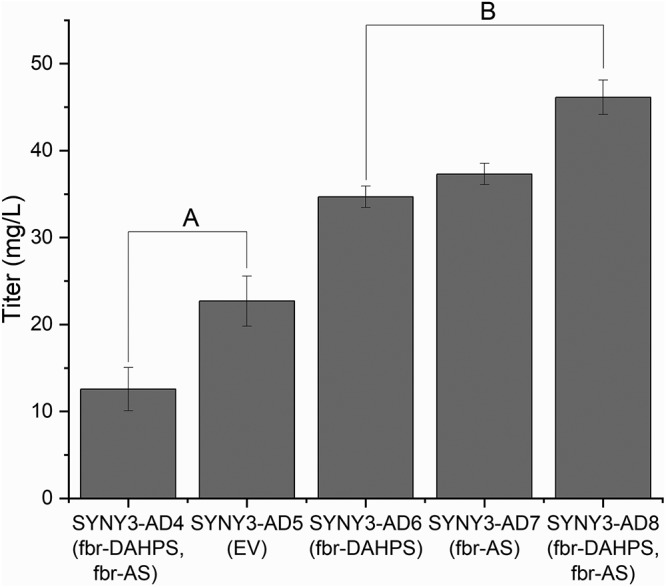
Trp production by a combination of randomly mutagenized and metabolically engineered Synechocystis strains after 168 h under atmospheric CO2, 240 μmol photons m−2 s−1 light at 30°C, and 200 rpm. Error bars indicate standard errors (n = 3, biological replicates). Multiple comparison using Tukey’s test (α = 0.05) showed two distinct groups for Trp production, A and B.
The best Trp overproducer developed using a combination of random mutagenesis and metabolic engineering approaches, SYNY3-AD8, was then chosen to be cultivated under enhanced conditions. Cultures were inoculated with a starting optical density (OD) of ∼0.2 and grown photoautotrophically for 10 days in an incubator at 30°C with 3% (vol/vol) CO2 and under 240 microeinsteins (μmol photons m−2 s−1) light. The culture pH after 10 days was 7.67 ± 0.01. The elevated CO2 levels coupled with higher starting density and growth time led to a Trp titer of 212 ± 23 mg/liter. The Phe and Tyr titers were 18.6 ± 1.5 mg/liter and 6.7 ± 2.2 mg/liter, respectively. The growth under enhanced conditions is shown in Fig. S6.
DISCUSSION
This study shows that the combination of random mutagenesis and metabolic engineering is superior to either of the approaches alone for the biosynthesis of tryptophan. Our results show that alleviating feedback inhibition by the end products is a crucial first step in the overproduction of aromatic amino acids, consistent with previous work (1). In this work, we found that overexpressing fbr-DAHPS can significantly increase the overall flux to the shikimate pathway by alleviating feedback inhibition at DAHPS (Fig. 2). This is consistent with the work done in other organisms, such as E. coli, where genes encoding feedback-resistant DAHPS and AS are overexpressed to engineer Trp-overproducing strains (1, 4). Recent studies have also shown that successfully increasing the flux to the shikimate pathway improves Phe accumulation by transgenic expression of aroGfbr and pheAfbr or tyrAfbr from E. coli in Synechococcus elongatus (23) and Synechocystis sp. PCC 6803 (24).
Random mutagenesis coupled with amino acid analog selection was previously shown to be successful in selecting amino-acid-overproducing mutants (6, 8, 10). Synechocystis sp. PCC 6803 typically contains multiple copies of its genome depending on the phase of growth (22); thus, the selection of mutants requires multiple rounds of restreaking under selection. The independently obtained strains from random mutagenesis, SYNY3-JV1, SYNY3-JV2, and SYNY3-JV3, all had fully segregated mutations in aroH, which codes for CM, and all had increased production of Trp. The mutations observed (Table 2) may have lowered the activity of CM, thereby redirecting flux to the tryptophan biosynthesis branch. However, although the flux split was altered, the flux toward Phe and Tyr was not altered significantly. All three strains showed mutations in the region of CM between amino acids 50 and 64. Synechocystis sp. PCC 6803 has a single CM enzyme that belongs to the type I or AroH class (25). This is a monofunctional CM protein similar to Bacillus subtilis CM, which is a nonallosteric homotrimeric enzyme (25) and has a published crystal structure (26). We mapped the mutations listed in Table 2 onto the crystal structure of the CM protein in Bacillus subtilis (https://doi.org/10.2210/pdb1DBF/pdb) and found that all the mutations lie in the chorismate binding region. This potentially means that the mutant CM has its activity reduced due to reduced binding of chorismate. Thus, it is possible that larger chorismate pools are available to be used by the competing enzyme, AS. Moreover, this could be the reason why overexpression of trpEfbr only increases Trp accumulation in the background of our CM mutation. Future work will test CM in vitro to see if the mutant CM shows reduced activity compared to that of the WT.
Our work also shows the importance of sequencing the genome of randomly mutagenized strains to avoid redundant targeting of the same genes. Since fully segregated mutations to genes encoding feedback-sensitive DAHPS and AS were not observed in the genome sequences of SYNY3-JV1, SYNY3-JV2, and SYNY3-JV3, we chose to overexpress feedback-resistant DAHPS and AS from E. coli. Overexpression of feedback-resistant DAHPS improved tryptophan titers, which shows that the total flux into the pathway is a limiting factor in SYNY3-JV1. Overexpression of feedback-resistant AS also improved the tryptophan titers, indicating that relieving the feedback inhibition at AS is also crucial. However, when both the feedback-resistant genes were overexpressed, we saw only a modest increase and not an additive effect. The diminishing returns may be due to negative epistasis between the beneficial genotypes or successful removal of Trp overproduction bottleneck by overexpression of either aroGfbr or trpEfbr in the background of our CM mutation. Our best strain, SYNY3-AD8, combines overexpression of feedback-resistant forms of DAHPS and AS which are complemented by the mutated CM that likely redirects flux to the Trp branch by possibly lowering its affinity for chorismate.
Trp titer and productivity of 212 ± 23 mg/liter and 0.88 ± 0.09 mg/liter · h, respectively, are the highest reported in a photoautotrophic system to the best of our knowledge. The titers and productivities of heterotrophic systems are significantly higher. For example, in a metabolically engineered E. coli strain (27), a titer of 1.85 g/liter and a productivity of 38.5 mg/liter · h were observed in shake flasks. In another study, C. glutamicum (28) had a titer of 50 g/liter with a productivity of 0.625 g/liter · h in fed-batch operation. However, despite a lower productivity and titer, an entire technical economic evaluation of a bioprocess would involve a comparison of the different substrate costs (CO2 versus sugars) and reactor types (photobioreactor versus fermenter).
To further develop cyanobacteria for aromatic amino acid production, significant enhancements of yield and productivity are needed. After removing the allosteric feedback inhibitions, improving the flux requires identifying rate limitations. One approach to identify the rate-limiting enzymes employed combinatorial overexpression of all the shikimate pathway enzymes, which successfully led to greater titers of aromatic amino acids in E. coli and Saccharomyces cerevisiae (29, 30). Targets that have shown success in other organisms are primary metabolism enzymes that provide the precursors of the shikimate pathway (31). Transketolase, which catalyzes the formation of erythrose-4-phosphate (E4P), was shown to be a limiting factor for synthesis of DAHP in E. coli with a feedback-resistant DAHP synthase (31). Transketolase may additionally improve carbon fixation, further increasing aromatic amino acid production, because of its central position in the Calvin cycle (32, 33). Interestingly, we observed one partially segregated mutation in transketolase (tkt) in SYNY3-JV1, but the effect due to this mutation has not been examined in separate experiments.
Strain improvement could continue by using other means of mutagenesis, such as UV irradiation or chemical mutagens, that explore other types of nucleotide conversions (34). Taking advantage of reduced costs for genome sequencing allows one to learn from strains selected for improved production of metabolite after random mutagenesis. As shown in this work, combining traits from randomly mutated strains with metabolic engineering targets has the potential to significantly outperform results from either approach alone.
MATERIALS AND METHODS
Bacterial strains, media, and growth conditions.
In this study, the cyanobacterial wild-type strain is Synechocystis sp. PCC 6803 substrain GT-I. All the strains (Table 3) were grown in triplicates in 250-ml Erlenmeyer flasks at 30°C and 200 rpm under 240 microeinsteins (μmol photons m−2 s−1) fluorescent light and atmospheric CO2 conditions in 50 ml of BG-11 medium (pH 8) (35) with a starting optical density (OD) of 0.05 or on BG-11 agar plates with 1.5% (wt/vol) Difco Bacto agar (Becton, Dickinson) with 3 g/liter sodium thiosulfate unless stated otherwise. Randomly mutagenized strains were regularly cultured with 0.3 mg/ml of analog 5-fluoro-dl-tryptophan apart from when used in an experiment to maintain their observed phenotype, whereas rationally engineered strains were grown with 50 μg/ml kanamycin. The growth of the cultures was determined by measuring the optical density at 730 nm (OD730) using a Beckman DU Series 500 spectrophotometer. The relationship between the cell dry weight and the OD730 was determined to be 0.286 g (dry weight) cells · liter−1 · OD730−1.
TABLE 3.
Strains used in this study
| Strain | Parent strain | Characteristic or integrative plasmid | Source |
|---|---|---|---|
| SYNY3 | Synechocystis sp. PCC 6803 substrain GT-I | ATCC | |
| SYNY3-JV1 | Tryptophan overproducer | This work | |
| SYNY3-JV2 | |||
| SYNY3-JV3 | |||
| SYNY3-AD1 | SYNY3 | pEERM1 | This work |
| SYNY3-AD2 | SYNY3 | pEERM1a | This work |
| SYNY3-AD3 | SYNY3 | pEERM1b | This work |
| SYNY3-AD4 | SYNY3 | pEERM1c | This work |
| SYNY3-AD5 | SYNY3-JV1 | pEERM1 | This work |
| SYNY3-AD6 | SYNY3-JV1 | pEERM1a | This work |
| SYNY3-AD7 | SYNY3-JV1 | pEERM1b | This work |
| SYNY3-AD8 | SYNY3-JV1 | pEERM1c | This work |
Construction of plasmids for gene expression.
Plasmid pEERM1 was a gift from Pia Lindberg and acquired from Addgene (Addgene plasmid 64024; http://n2t.net/addgene:64024) (36). Genes encoding feedback-resistant DAHPS and AS were codon optimized for expression in cyanobacteria and synthesized by GenScript (GenScript, Piscataway, NJ). To develop plasmids pEERM1a and pEERM1b, aroGL175D and trpES40F were inserted in the open reading frame between cloning sites XbaI and SpeI, respectively, in plasmid pEERM1. Plasmid pEERM1c contains both aroGL175D and trpES40F and was constructed by insertion of gene trpES40F between cloning sites SpeI and PstI in plasmid pEERM1a. Table 1 lists all the plasmids used in this study. Successful cloning was verified by gel electrophoresis.
Construction of metabolically engineered strains.
Transgenic SYNY3 strains were constructed by overexpression of feedback-resistant DAHPS and AS from E. coli. Briefly, the integrative plasmid pEERM1 integrates into the Synechocystis genome at the psbA2 site, and expression of the cloned genes is driven by the strong light-inducible promoter PpsbA2 (36). The plasmid has a kanamycin antibiotic resistance cassette, which was used for selecting transformed strains. The plasmids pEERM1a, pEERM1b, and pEERM1c were transformed into the SYNY3 (wild type) or randomly mutagenized strain SYNY3-JV1 by a previously described protocol (37). The transformed cells were selected by restreaking individual colonies on BG-11 plates containing 50 μg/ml kanamycin a minimum of three times. Individual colonies were then selected and grown in liquid medium containing 50 μg/ml kanamycin, and successful transformation was verified by PCR amplification followed by gel electrophoresis.
Verification of cloning and bacterial transformation using PCR and gel electrophoresis.
Plasmids in this study were separated on 1% agarose gels in 1× Tris-acetate-EDTA (TAE) buffer with 0.5 μg/liter ethidium bromide (EtBr) on a Bio-Rad Mini-Sub Cell GT gel electrophoresis system prior to UV light imaging using a transilluminator. A supercoiled DNA ladder was used as a marker to verify that the inserts were as expected. Figure S1 in the supplemental material shows the DNA bands obtained after the plasmids were run on the gel. The plasmid pEERM1 is 3,728 bp, whereas the genes aroGfbr and trpEfbr are 1,053 bp and 1,563 bp long, respectively. Figure S1 thus confirms that the plasmids pEERM1a to pEERM1c have the expected sizes (∼4.7 kb, 5.3 kb, and 6.3 kb, respectively).
Successful transformation was checked using a combination of PCR and gel electrophoresis. Transformed strains listed in Table 3 were grown until they reached exponential growth phase, when samples were taken. Genomic DNA was extracted using the Applied Biosystems DNA All Reagents kit to serve as the template for PCR. Primers were designed to amplify the psbA2 integration region. The forward primer was 5′-CCAATCTGAACATCGACAAATACAT-3′, while 5′-CCCATTGAAGGAGAGTGCAA-3′ served as the reverse primer. Thermo Scientific DreamTaq green PCR mastermix was used for the PCR, and the conditions were set as follows: denaturation at 95°C for 5 min on initial cycle followed by 30 cycles of 94°C for 30 s, 60°C for 30 s and 68°C for 210 s. This was followed by a final extension at 68°C for 5 min before holding at 4°C. The amplified PCR products obtained were then run on 1% agarose gels as previously described with a 1-kb DNA ladder as a marker lane. Figure S2 shows the DNA bands obtained with the amplified PCR products. The size integration region amplified by the primers is roughly 1.2 kb. Figure S2 shows successful transformation and segregation for all our developed strains, where incorporation of the empty vector (EV) corresponds to ∼1.2 kb, while aroGfbr, trpEfbr, and a combination of both genes correspond to DNA bands of ∼2.3 kb, 2.8 kb, and 3.8 kb, respectively.
Random mutagenesis and selection.
Random mutagenesis was carried out using a modified procedure previously described using methyl methanesulfonate (MMS) as the chemical mutagen (16). Briefly, 1% (vol/vol) MMS was added to cyanobacterial cultures in exponential growth phase for 60 s and quenched with sodium thiosulfate for reaction volumes greater than 1.5 ml. The cells were then pelleted by centrifugation at 2,760 × g and washed a total of three times in fresh growth medium to remove the mutagen.
Cells were then incubated at room temperature in the dark for 24 h before spreading on agar plates containing increasing concentrations of amino acid analog 5-fluoro-dl-tryptophan (Sigma-Aldrich, St. Louis, MO) ranging from 0.3 mg/ml to 0.9 mg/ml. Individual colonies resistant to the highest analog concentration were restreaked on analog-containing plates a minimum of three times before being shifted to liquid medium to test for amino acid production.
Metabolite extraction.
Synechocystis cells were pelleted by centrifugation at 2,760 × g. The supernatant was collected and stored at −20°C until further analysis for extracellular metabolites. The cell pellet was extracted with 500 μl of methanol followed by incubation at −20°C for 30 min. Two more extractions using 50% methanol with p-fluoro-dl-phenylalanine as the internal standard were performed, and all the extracts were pooled. Both the supernatant and the extracts were dried to completeness either using a stream of pure N2 or with a Labconco CentriVap concentrator. The dried samples were reconstituted in 50% methanol before injection into the liquid chromatography tandem mass spectrometer (LC-MS/MS).
Quantification of amino acid production.
Intracellular and extracellular aromatic amino acid concentrations were separately calculated using reverse-phase chromatography on a Shimadzu HPLC-20 AD system (Shimadzu, Columbia, MD) and quantified by external standards using an AB Sciex 5500 triple quadrupole mass spectrometer (AB Sciex, Redwood City, CA) in negative ion mode. The cell extracts were spiked with the internal standard p-fluoro-dl-phenylalanine to account for volume changes. Chromatographic separations were performed on a 150-mm by 4.6-mm Zorbax Eclipse C8 column, 5 μm (Agilent Technologies, Santa Clara, CA), at a column temperature of 30°C and a flow rate of 1 ml/min. The injection volume was set to 10 μl. A linear gradient of aqueous solvent A (2.5 mM ammonium acetate in ultrapure water, adjusted to pH 5.3 using glacial acetic acid) and organic solvent B (97.8% acetonitrile, 2% ultrapure water, and 0.2% formic acid) was used as follows: 10% B (vol/vol) for 1 min, 10% to 20% B over 3 min, 20% to 70% B over 3 min, hold at 70% B for 3 min, return to 10% B for 1 min followed by equilibration at 10% B for 3 min, resulting in a 14-min runtime for each sample.
For metabolite profiling, the mass spectrometer was equipped with electrospray ionization (ESI), and all the analyses were performed using the Analyst 1.5.1 software. The ESI parameters, such as declustering potential (DP), entrance potential (EP), collision energy (CE), and cell exit potential (CXP), were manually tuned for all the metabolites and are listed in Table S1. All the results report the total production of aromatic amino acids in milligrams of total aromatic amino acid per liter medium.
DNA sequencing and SNP and indel analysis.
Genomic DNA was extracted using a modified procedure (38). While cultures were in exponential phase, cells were pelleted and resuspended in 0.034 volumes of lysis buffer (25% sucrose in 50 mM Tris-HCl [pH 8], 10 mM disodium EDTA), and lysozyme was added to a final concentration of 2 mg/ml. Cells were then incubated at 37°C for 45 min. Proteinase K (0.4% [vol/vol]) and Sarkosyl (1% [vol/vol]) were added to the cell lysis solution before incubation at 55°C for 30 min. The lysis solution was centrifuged, and DNA was extracted by chloroform-isoamyl alcohol extraction followed by extraction with equilibrated phenol and again by chloroform-isoamyl alcohol.
DNA sequencing was performed on the Illumina MiSeq platform (2 × 150 bp) at the Purdue University Genomics Core Facility. The raw paired-end data were trimmed using Trimmomatic (39) to remove low-quality reads and known adaptor sequences. Filtered paired-end reads were then mapped to reference genomes for Synechocystis sp. PCC 6803 substrain GT-I as well as SYNY3 using Bowtie2 (40). SNPs and indels were called using Genome Analysis Toolkit (GATK) UnifiedGenotyper (41) after indel realignment. Ploidy for SNP and indel calls was set to 15 for Synechocystis (22). SNPs and indels with read depths of less than 5 or greater than 200 (two times the average read depth) or quality scores lower than 20 were filtered out of the call set. Functional effects of SNPs were annotated using SnpEff (42).
Statistical analysis.
Data are presented as means ± standard errors (SEs; biological replicates). Pairwise comparison and Tukey’s test for multiple comparisons were performed using either SAS (SAS Institute) or Origin 2019b (OriginLab). A P value of <0.05 was considered statistically significant.
Data availability.
The raw data from the genome sequencing were deposited to GenBank and are available under accession numbers SAMN12364542 for the WT (SYNY3) and SAMN12364543 to SAMN12364545 for SYNY3-JV1 to SYNY3-JV3, respectively. All the data are under BioProject accession no. PRJNA556701.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the U.S. Department of Energy (DE-SC0008628). We declare no conflicts of interest.
We thank the Morgan group members for critical reading and feedback on the manuscript.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Ikeda M. 2006. Towards bacterial strains overproducing l-tryptophan and other aromatics by metabolic engineering. Appl Microbiol Biotechnol 69:615–626. doi: 10.1007/s00253-005-0252-y. [DOI] [PubMed] [Google Scholar]
- 2.Leuchtenberger W, Huthmacher K, Drauz K. 2005. Biotechnological production of amino acids and derivatives: current status and prospects. Appl Microbiol Biotechnol 69:1–8. doi: 10.1007/s00253-005-0155-y. [DOI] [PubMed] [Google Scholar]
- 3.Sprenger GA. 2007. From scratch to value: engineering Escherichia coli wild type cells to the production of l-phenylalanine and other fine chemicals derived from chorismate. Appl Microbiol Biotechnol 75:739–749. doi: 10.1007/s00253-007-0931-y. [DOI] [PubMed] [Google Scholar]
- 4.Bongaerts J, Kramer M, Muller U, Raeven L, Wubbolts M. 2001. Metabolic engineering for microbial production of aromatic amino acids and derived compounds. Metab Eng 3:289–300. doi: 10.1006/mben.2001.0196. [DOI] [PubMed] [Google Scholar]
- 5.Ikeda M, Takeno S. 2013. Amino acid production by Corynebacterium glutamicum, p 107–136 In Yukawa H, Inui M. (ed), Corynebacterium glutamicum: biology and biotechnology, vol 23, Springer, Berlin, Germany. [Google Scholar]
- 6.Hall GC, Flick MB, Gherna RL, Jensen RA. 1982. Biochemical diversity for biosynthesis of aromatic-amino-acids among the cyanobacteria. J Bacteriol 149:65–78. doi: 10.1128/JB.149.1.65-78.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodriguez A, Martinez JA, Flores N, Escalante A, Gosset G, Bolivar F. 2014. Engineering Escherichia coli to overproduce aromatic amino acids and derived compounds. Microb Cell Fact 13:126. doi: 10.1186/s12934-014-0126-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall GC, Jensen RA. 1980. Enzymological basis for growth-inhibition by l-phenylalanine in the cyanobacterium Synechocystis sp. 29108. J Bacteriol 144:1034–1042. doi: 10.1128/JB.144.3.1034-1042.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bose JL. 2016. Chemical and UV mutagenesis. Methods Mol Biol 1373:111–115. doi: 10.1007/7651_2014_190. [DOI] [PubMed] [Google Scholar]
- 10.Hall GC, Flick MB, Jensen RA. 1983. Regulation of the aromatic pathway in the cyanobacterium Synechococcus sp strain PCC 6301 (Anacystis nidulans). J Bacteriol 153:423–428. doi: 10.1128/JB.153.1.423-428.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rao NS, Shakila TM, Bagchi SN. 1995. De-regulated assimilation and over-production of amino-acids in analog-resistant mutants of a cyanobacterium, Phormidium uncinatum. World J Microbiol Biotechnol 11:665–668. doi: 10.1007/BF00361013. [DOI] [PubMed] [Google Scholar]
- 12.Hu CY, Jiang PH, Xu JF, Wu YQ, Huang WD. 2003. Mutation analysis of the feedback inhibition site of phenylalanine-sensitive 3-deoxy-d-arabino-heptulosonate 7-phosphate synthase of Escherichia coli. J Basic Microbiol 43:399–406. doi: 10.1002/jobm.200310244. [DOI] [PubMed] [Google Scholar]
- 13.Nelms J, Edwards RM, Warwick J, Fotheringham I. 1992. Novel mutations in the pheA gene of Escherichia coli K-12 which result in highly feedback inhibition-resistant variants of chorismate mutase prephenate dehydratase. Appl Environ Microbiol 58:2592–2598. doi: 10.1128/AEM.58.8.2592-2598.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramos I, Downs D. 2003. Anthranilate synthase can generate sufficient phosphoribosyl amine for thiamine synthesis in Salmonella enterica. J Bacteriol 185:5125–5132. doi: 10.1128/jb.185.17.5125-5132.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foster PL. 1991. In vivo mutagenesis. Methods Enzymol 204:114–125. doi: 10.1016/0076-6879(91)04007-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tillich UM, Lehmann S, Schulze K, Duhring U, Frohme M. 2012. The optimal mutagen dosage to induce point-mutations in Synechocystis sp. PCC6803 and its application to promote temperature tolerance. PLoS One 7:e49467. doi: 10.1371/journal.pone.0049467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Denenu EO, Demain AL. 1981. Enzymatic basis for overproduction of tryptophan and its metabolites in Hansenula polymorpha mutants. Appl Environ Microbiol 42:497–501. doi: 10.1128/AEM.42.3.497-501.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richmond MH. 1962. The effect of amino acid analogs on growth and protein synthesis in microorganisms. Microbiol Mol Biol Rev 26:398–420. doi: 10.1128/MMBR.26.4.398-420.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grund M, Jakob T, Wilhelm C, Bühler B, Schmid A. 2019. Electron balancing under different sink conditions reveals positive effects on photon efficiency and metabolic activity of Synechocystis sp. PCC 6803. Biotechnol Biofuels 12:43. doi: 10.1186/s13068-019-1378-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oliver J, Machado I, Yoneda H, Atsumi S. 2013. Cyanobacterial conversion of carbon dioxide to 2,3-butanediol. Proc Natl Acad Sci U S A 110:1249–1254. doi: 10.1073/pnas.1213024110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ducat D, Avelar-Rivas J, Way J, Silver P. 2012. Rerouting carbon flux to enhance photosynthetic productivity. Appl Environ Microbiol 78:2660–2668. doi: 10.1128/AEM.07901-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zerulla K, Ludt K, Soppa J. 2016. The ploidy level of Synechocystis sp PCC 6803 is highly variable and is influenced by growth phase and by chemical and physical external parameters. Microbiology 162:730–739. doi: 10.1099/mic.0.000264. [DOI] [PubMed] [Google Scholar]
- 23.Ni J, Liu H-Y, Tao F, Wu Y-T, Xu P. 2018. Remodeling of the photosynthetic chain promotes direct CO2 conversion into valuable aromatic compounds. Angew Chem Int Ed 57:15990–15994. doi: 10.1002/anie.201808402. [DOI] [PubMed] [Google Scholar]
- 24.Brey L, Włodarczyk A, Bang Thøfner J, Burow M, Crocoll C, Nielsen I, Zygadlo Nielsen A, Jensen P. 2020. Metabolic engineering of Synechocystis sp. PCC 6803 for the production of aromatic amino acids and derived phenylpropanoids. Metab Eng 57:129–139. doi: 10.1016/j.ymben.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Chook Y, Ke H, Lipscomb W. 1993. Crystal structures of the monofunctional chorismate mutase from Bacillus subtilis and its complex with a transition state analog. Proc Natl Acad Sci U S A 90:8600–8603. doi: 10.1073/pnas.90.18.8600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ladner JE, Reddy P, Davis A, Tordova M, Howard AJ, Gilliland GL. 2000. The 1.30 A resolution structure of the Bacillus subtilis chorismate mutase catalytic homotrimer. Acta Crystallogr D Biol Crystallogr 56:673–683. doi: 10.1107/s0907444900004625. [DOI] [PubMed] [Google Scholar]
- 27.Liu L, Duan X, Wu J. 2016. Modulating the direction of carbon flow in Escherichia coli to improve l-tryptophan production by inactivating the global regulator FruR. J Biotechnol 231:141–148. doi: 10.1016/j.jbiotec.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 28.Ikeda M, Katsumata R. 1999. Hyperproduction of tryptophan by Corynebacterium glutamicum with the modified pentose phosphate pathway. Appl Environ Microbiol 65:2497–2502. doi: 10.1128/AEM.65.6.2497-2502.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lütke-Eversloh T, Stephanopoulos G. 2008. Combinatorial pathway analysis for improved l-tyrosine production in Escherichia coli: identification of enzymatic bottlenecks by systematic gene overexpression. Metab Eng 10:69–77. doi: 10.1016/j.ymben.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez A, Kildegaard K, Li M, Borodina I, Nielsen J. 2015. Establishment of a yeast platform strain for production of p-coumaric acid through metabolic engineering of aromatic amino acid biosynthesis. Metab Eng 31:181–188. doi: 10.1016/j.ymben.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 31.Draths KM, Pompliano DL, Conley DL, Frost JW, Berry A, Disbrow GL, Staversky RJ, Lievense JC. 1992. Biocatalytic synthesis of aromatics from d-glucose: the role of transketolase. J Am Chem Soc 114:3956–3962. doi: 10.1021/ja00036a050. [DOI] [Google Scholar]
- 32.Liang F, Lindblad P. 2016. Effects of overexpressing photosynthetic carbon flux control enzymes in the cyanobacterium Synechocystis PCC 6803. Metab Eng 38:56–64. doi: 10.1016/j.ymben.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Yu King Hing N, Liang F, Lindblad P, Morgan J. 2019. Combining isotopically non-stationary metabolic flux analysis with proteomics to unravel the regulation of the Calvin-Benson-Bassham cycle in Synechocystis sp. PCC 6803. Metab Eng 56:77–84. doi: 10.1016/j.ymben.2019.08.014. [DOI] [PubMed] [Google Scholar]
- 34.Packer M, Liu D. 2015. Methods for the directed evolution of proteins. Nat Rev Genet 16:379–394. doi: 10.1038/nrg3927. [DOI] [PubMed] [Google Scholar]
- 35.Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY. 1979. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 111:1–61. doi: 10.1099/00221287-111-1-1. [DOI] [Google Scholar]
- 36.Englund E, Andersen-Ranberg J, Miao R, Hamberger B, Lindberg P. 2015. Metabolic engineering of Synechocystis sp. PCC 6803 for production of the plant diterpenoid manoyl oxide. ACS Synth Biol 4:1270–1278. doi: 10.1021/acssynbio.5b00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eaton-Rye JJ. 2011. Construction of gene interruptions and gene deletions in the cyanobacterium Synechocystis sp. strain PCC 6803 In Carpentier R. (ed), Photosynthesis research protocols. Methods in molecular biology (methods and protocols), vol 684 Humana Press, Totowa, NJ. [DOI] [PubMed] [Google Scholar]
- 38.Rosato E. 2007. Circadian rhythms: methods and protocols. Humana Press, Totowa, NJ. [Google Scholar]
- 39.Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Langmead B, Salzberg SL. 2012. Fast gapped-read alignment with Bowtie 2. Nat Methods 9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M, DePristo MA. 2010. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res 20:1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cingolani P, Platts A, Wang LL, Coon M, Nguyen T, Wang L, Land SJ, Lu XY, Ruden DM. 2012. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w(1118); iso-2; iso-3. Fly (Austin) 6:80–92. doi: 10.4161/fly.19695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Song J, Bonner CA, Wolinsky M, Jensen RA. 2005. The TyrA family of aromatic-pathway dehydrogenases in phylogenetic context. BMC Biol 3:13. doi: 10.1186/1741-7007-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Riccardi G, de Rossi E, Milano A. 1989. Amino-acid biosynthesis and its regulation in cyanobacteria. Plant Sci 64:135–151. doi: 10.1016/0168-9452(89)90018-6. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data from the genome sequencing were deposited to GenBank and are available under accession numbers SAMN12364542 for the WT (SYNY3) and SAMN12364543 to SAMN12364545 for SYNY3-JV1 to SYNY3-JV3, respectively. All the data are under BioProject accession no. PRJNA556701.



