Abstract
Heterotrimeric G proteins are the core upstream elements that transduce and amplify the cellular signals from G protein–coupled receptors (GPCRs) to intracellular effectors. GPCRs are the largest family of membrane proteins encoded in the human genome and are the targets of about one-third of prescription medicines. However, to date, no single therapeutic agent exerts its effects via perturbing heterotrimeric G protein function, despite a plethora of evidence linking G protein malfunction to human disease. Several recent studies have brought to light that the Gq family–specific inhibitor FR900359 (FR) is unexpectedly efficacious in silencing the signaling of Gq oncoproteins, mutant Gq variants that mostly exist in the active state. These data not only raise the hope that researchers working in drug discovery may be able to potentially strike Gq oncoproteins from the list of undruggable targets, but also raise questions as to how FR achieves its therapeutic effect. Here, we place emphasis on these recent studies and explain why they expand our pharmacological armamentarium for targeting Gq protein oncogenes as well as broaden our mechanistic understanding of Gq protein oncogene function. We also highlight how this novel insight impacts the significance and utility of using G(q) proteins as targets in drug discovery efforts.
Keywords: G protein, G protein-coupled receptor (GPCR), signal transduction, signaling, cancer biology, cancer therapy, cancer, cell signaling, heterotrimeric G protein, oncogene, FR900359, Gq inhibitor, oncoprotein, uveal melanoma, YM254890, Gq, uveal melanoma
Introduction
GTP/GDP exchange and the intrinsic activity of GTP-binding proteins constitute widespread regulatory mechanisms in cells. These are utilized by heterotrimeric αβγ G proteins, downstream effectors of G protein–coupled receptors (GPCRs),2 to directly or indirectly regulate numerous physiological processes in mammals (1–6). Despite the discovery of G proteins about 40 years ago and their relevance for maintaining homeostasis in response to a myriad of extracellular cues, remarkably little effort has been devoted to development of selective and cell-permeable pharmacological agents for inhibition of members of this protein family (7–15). This is in stark contrast to the plethora of modulators currently available for pharmacological control of GPCRs (16, 17) and likely relates to the fact that perturbation of receptor function rather than their shared signaling cascades is a more specific approach to interfere with pathologies. However, such specific approaches may fail, if pathology is complex and involves dysregulation of more than one receptor and/or its associated signaling circuitry, as is the case in certain diseases of the lung (18–23) as well as various forms of pain (24–27) and cancer (28–35). Therefore, development of G protein–targeting pharmacological agents that are active in intact cells, on the level of an isolated organ and ideally also in the living organism, would offer unique opportunities to explore the biological consequences that arise from more broad inhibition of signaling components.
G proteins are grouped into four major families (Gq, Gi, Gs, and G12) based on α subunit homology and function (1–6). Missense mutations to codons within almost all of these (Gq, Gi, and Gs) result in diverse pathological conditions, yet all but Gq are lacking effective pharmacological inhibitors (i.e. remain untapped from a drug development perspective) (1–6). Note that members of the Gαi/o family except for Gαz are effectively hindered from signal transmission by pertussis toxin through ADP-ribosylation of a C-terminal cysteine residue (36–38). However, cell-permeable small-molecule inhibitors specifically targeting the Gαi/o branch have yet to be identified. Therefore, this review will focus primarily on the more recent discoveries obtained with the Gq family–specific inhibitors FR900359 (FR) and YM254890 (YM) (Fig. 1) and will highlight the conceptual advances originating therefrom for basic biological research and drug discovery. Specifically, we will single out a subset of Gq protein activities, namely aberrant signaling in cancer, to advance the ideas on drug–G protein interaction for therapeutic advantage. Because much of today's progress in this field traces back to a resurgence of interest in Gq protein inhibitors, a brief historical perspective will also be included.
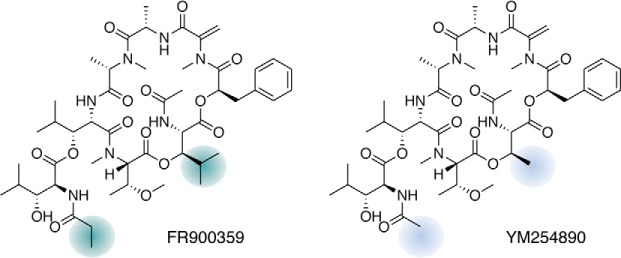
Figure 1.
Chemical structures of Gq inhibitors FR and YM. Colored areas highlight the components of the amino acid building blocks that differ between FR and YM, accounting for the higher hydrophobicity of FR as well as for the distinct pharmacological features of the two inhibitors (123, 124).
G protein signaling
The delicate balance between on and off states
To maintain organismal homeostasis, mammalian cells require an exquisite balance between G protein activation and deactivation. They achieve this by tight control over GDP/GTP exchange and GTP hydrolysis rates. Ligand-activated GPCRs act as guanine nucleotide exchange factors (GEFs) to stimulate GDP/GTP exchange on the G protein α subunit (Fig. 2). Upon GTP binding, Gα changes its conformation, and this is followed by separation of the heterotrimer (the extent of physical separation may vary however (39–45)) into GαGTP and a Gβγ dimer, each of which interacts with downstream effectors (Fig. 2) (1–6). GTP hydrolysis by the inherent GTPase activity, which is often supported by GTPase-activating proteins (GAPs), then terminates G signaling and allows GαGDP to associate with Gβγ to return the G protein to the inactive state (Fig. 2) (1, 46–48). This activation-inactivation cycle suffices to explain why guanine nucleotide dissociation inhibitors (GDIs), such as FR and YM, are efficient terminators of G protein signaling; they block the rate-limiting step of the cycle, which is GDP release (Fig. 2) (11, 49). It also rationalizes why G protein activity may be elevated in cancer cells because (i) GPCRs and/or their activating ligands are present in excess, (ii) cancer cells may harbor constitutively active receptor variants, (iii) cancer cells may have activating mutations within the Gα protein itself (29–31, 35), or (iv) may be deficient in expression of GAPs as well as carry mutated versions of these effective terminators of G protein–dependent signaling (50–53). Unlike the conventional GPCR-targeted therapies that intervene with categories (i) and (ii), the therapeutic concept discussed in this review is also, and perhaps especially, effective for category (iii). GAPs, category (iv), are not within the scope of this review and interested readers may refer to several excellent reviews on this topic elsewhere (46, 47, 54–56).
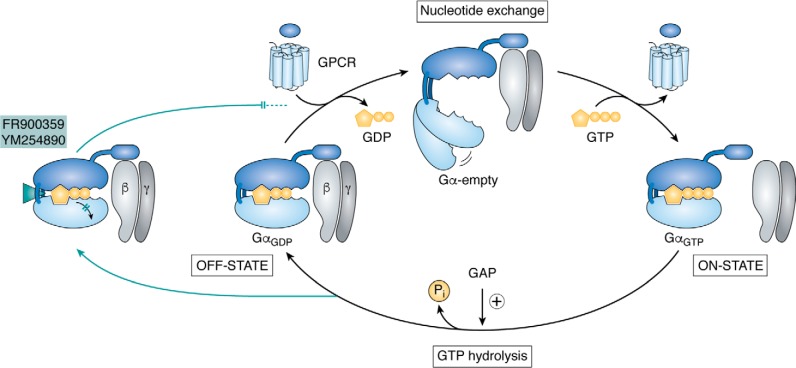
Figure 2.
Schematic of the guanine nucleotide cycle and Gα signaling states. Heterotrimeric G protein signaling commences when ligand-activated GPCRs act as GEFs, causing the release of bound GDP and its replacement by GTP via a short-lived intermediate “empty pocket” state. Exchange of the bound nucleotide results in ternary complex disassembly, separation of Gα from Gβγ, and initiation of downstream signaling. Intrinsic GTP hydrolysis, which is accelerated by GAPs, then resets GαGDP to form the inactive heterotrimer. FR and YM block G protein signaling by preventing GDP release. They freeze the heterotrimer in an inactive conformation by intercalating between the interdomain cleft at a site distinct from the nucleotide-binding pocket, thereby preventing domain separation (11, 49).
When the balance is tipped toward the on state
It has been known for many years that activating point mutations in Gα proteins are important causative factors in several human cancers (31, 57). Of the four families of heterotrimeric G proteins, gain-of-function mutations were found in GNAS (Gαs) (58–61), GNAI2 (Gαi2) (62), GNAO1 (Gαo1) (63, 64), and GNAQ/GNA11 (Gαq/Gα11) (65–68) gene loci. Whereas GNAS and GNAI mutations occur in subsets of human endocrinopathies (57, 62, 69, 70), the first activating somatic GNAO1 mutation was found in breast cancer (63). Within the GNAQ and GNA11 genes, two particular codons are frequently mutated: arginine 183 and glutamine 209. Mutations at these two positions cause diminished GTPase function and so are linked to gain-of-signaling phenotypes (7, 9, 11, 31, 35, 71). Interestingly, both are also considered oncogenic driver mutations in ocular (uveal) melanoma (UM), an aggressive malignancy of the adult eye (72–76). Aside from mutationally activated Gα subunits, an additional recurrent hotspot mutation in UM was recently identified in the CYSLTR2 gene, which codes for the G protein–coupled cysteinyl-leukotriene receptor type 2: CysLTR2L129Q (77). A hallmark feature of this mutant receptor is an overactive Gq signaling cascade coupled with impaired arrestin-mediated down-regulation, abolished responsiveness to its cognate endogenous ligands, and insensitivity to CysLTR2 antagonist/inverse agonist ligands (78). It follows that inhibitors of Gq function such as FR or YM should have therapeutic potential to suppress the aberrant activity of this signaling module originating on either the receptor or the G protein level. In other words, targeting a convergence point in signal transduction with a single agent might bring therapeutic benefit irrespective of the precise nature of the upstream activating oncoprotein.
Pharmacological inhibitors of G protein function: Focus on FR900359
Discovery of a cyclic depsipeptide with the code name FR900359 from a methanol extract of the evergreen plant Ardisia crenata dates back to 1988 (Fig. 3) (79). Along with the elucidation of its chemical structure, a preliminary description of biological effects was provided: FR inhibits platelet aggregation, decreases blood pressure, and is cytotoxic to cultured rat fibroblasts and myelocytic leukemia cells (data not shown in Ref. 79). Whereas all of the observed biological effects may be explained entirely by specific inhibition of Gq family proteins, it was not until 2010 that FR was rediscovered as “compound 362-63-08” in a plant extract library screen searching for inhibitors of the gut hormone cholecystokinin type 1 (CCK1, formerly CCK-A) receptor (Fig. 3) (10). The structural similarity of compound 362-63-08 with YM together with its in vitro selectivity profile led the authors to conclude that the screening hit 362-63-08 does not target the receptor itself but rather hinders CCK1 receptor signaling by specific inhibition of its signal transducing Gq/11 proteins (10). Selective inhibition by FR of Gq, G11, and G14 over all other mammalian G proteins, its molecular mechanism of GDI action, and the potential to probe the Gq contribution to complex biological processes in physiology and disease were not addressed until 2015, when a comprehensive study provided in vitro and ex vivo characterization at a level of detail sufficient to reinvigorate the field of Gq protein inhibitors (11) (Fig. 3). Indeed, this very study impacted G protein inhibitor research in manifold beneficial ways: it (i) created scientific community awareness for the existence of a most valuable signal transduction inhibitor, (ii) triggered independent confirmatory studies to re-examine FR's selectivity profile (80–82), (iii) helped fuel the competitive efforts to identify the best-suited synthetic methodology for preparing the complex molecule by chemical synthesis (83–86), (iv) sparked broad interest for the application of FR and YM to explore the biological consequences that arise from specific Gq inhibition (7, 87–107), and (v) provided experimental evidence that Gq inhibition may qualify as an effective postreceptor strategy to target oncogenic signaling in cancer cells with elevated Gq activity.
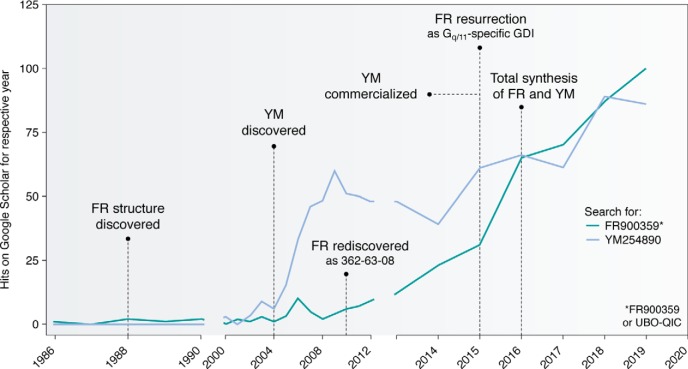
Figure 3.
Google scholar hits for Gq inhibitors FR and YM. 1988: Isolation and structure elucidation of FR; biology and mechanism of action unknown (79). 2004: Discovery of the structurally close analog YM (9) by Yamanouchi Pharmaceutical Co., later combined in a merger with Fujisawa to form Astellas Pharma, which chose to provide YM to the scientific community in a rather restrictive manner. Until commercialization (see below), YM was available for a small number of researchers only. 2010: Rediscovery of FR, code-named “362-63-08,” from a plant extract library as inhibitor of the Gq-coupled cholecystokinin CCK1 receptor (10). 2015: Resurrection of FR by in-depth characterization of its in vitro specificity and mechanism of action by a concerted effort of members of the signal transduction community (11). 2016: Commercialization of YM by Fujifilm Wako Chemicals, as well as total synthesis of YM and FR (83). Coincidentally, worldwide awareness of and interest in FR and YM has risen steeply. During a short period of time, FR was commercialized under the code name “UBO-QIC” (University of Bonn–Gq-inhibiting component), which indicated market potential and, in turn, encouraged commercialization of the competing molecule YM.
FR suppresses oncogenic signaling in melanoma cells with elevated Gq activity
The first signs for FR efficacy in cancer treatment were obtained when exposing a panel of skin melanoma cells to FR in cell culture (11). Interestingly, despite an intrinsically activated Gq cascade in a number of these lines, and despite potent suppression by FR of Gq-mediated inositol phosphate accumulation across all of these, proliferation, cell cycle progression, and mitogenic signaling were abolished in all but MZ7 cells. MZ7 cells harbor the constitutively active GαqR183C variant, considered susceptible to FR treatment (9, 83). These data provided the first hint that aberrant Gq activity per se does not suffice to instruct MZ7 cancer cells to proliferate. Apparently, an overactive Gq system is required but not sufficient to define the molecular subtype of melanoma that responds to FR treatment or else to forecast therapeutic efficacy of Gq-inhibiting agents. Given the rich mutational landscape of skin melanoma and the high frequency of mutations in the BRAF, NRAS, CDK4, PTK2B, and ERBB4 genes (108, 109), along with the notion that MZ7 cells also harbor the constitutively active BRAFV600E allele, the findings argue that BRAFV600E but not Gα11R183C must act as the dominant oncogenic driver and that the occurrence of R183C may merely be a consequence of the general mutational burden in this melanoma cell line. Indeed, mitogenic signaling in MZ7 cells is completely blunted by the BRAF inhibitors vemurafenib and trametinib (11). Regardless, Gq inhibition with FR provided the proof of principle for a novel route to reprogram a range of skin melanoma cells—those that are instructed by Gq to proliferate—to a less aggressive phenotype (11). Because mutant Gαq or Gα11 proteins are found in only 4% of skin melanoma but in 90% of uveal melanoma, it was not surprising to observe researchers turn to the study of FR in cell lines from uveal melanoma tumors: four independent studies on similar subject matter emerged within just a 6-month time frame (97, 100, 110, 111).
FR inhibition of uveal melanoma Gα oncoproteins: A mechanistic surprise?
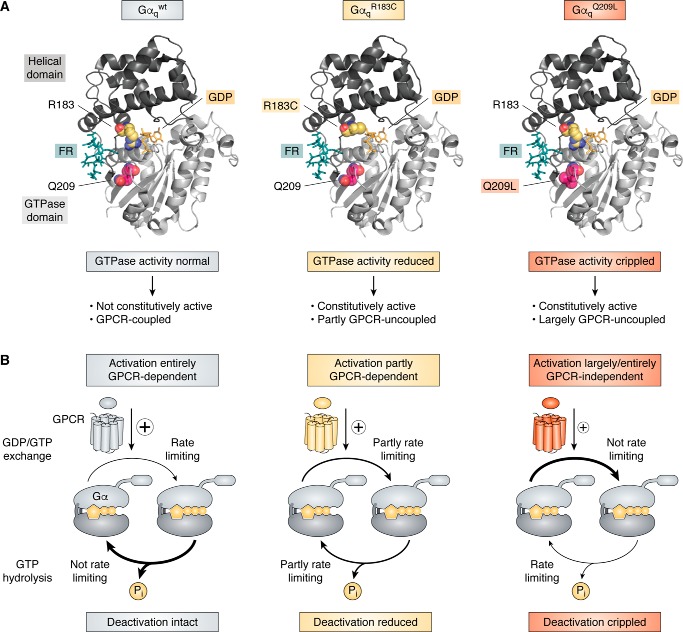
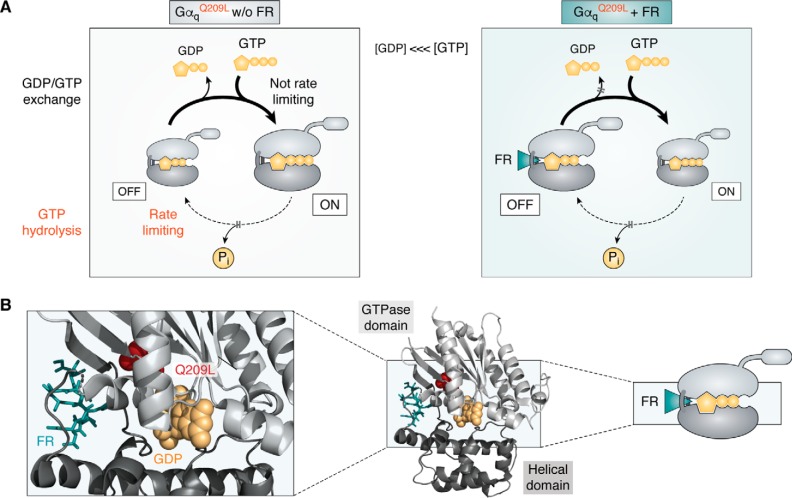
Uveal melanoma is the most common cancer of the adult eye, originating from melanocytes in the choroid, iris, or ciliary body (72–76). The genetic signature and evolution of this particularly lethal form of melanoma is distinct from skin melanoma in that mutations within a Gq signaling module comprising the gene loci for GNAQ, GNA11, their downstream effector PLCB4, or the upstream activating CYSLTR2 occur in a mutually exclusive fashion (65–67, 77, 112). Particularly predominant are gain-of-function mutations within the two highly homologous G protein α subunits, Gαq and Gα11, at the recurrent hotspots Gln-209 and Arg-183 (65–67), with mutations at Gln-209 being 13 times more frequent than those at Arg-183 (67). Both mutation hotspots are located in the GTPase domain (Fig. 4A) and are catalytically important for the GTPase turn-off reaction by stabilizing the transition state for GTP hydrolysis. Gln-209 of Gαq and Gα11 is analogous to Gln-204 within Gαi, Gln-227 within Gαs, and Gln-61 within the small GTPase Ras, the latter mutated in multiple human cancers (61, 113). If altered by mutation, Gαq/11 deactivation is disturbed, driving inappropriate proliferative signaling, yet different in extent for each of the two hotspots: the Gln-209 mutations (GαqQ209L/P or Gα11Q209L/P) cripple the GTPase activity to create persistently active Gα subunits (as inferred from pioneering X-ray crystallographic studies with Gαi (114, 115) and recent biochemical investigations (71), whereas Arg-183 mutants (GαqR183C or Gα11R183C) retain the capacity to hydrolyze GTP, albeit at a reduced catalytic rate (Fig. 4A) (116). Thus, both mutants differ in their oncogenic properties because R183C prefers GTP over GDP yet still responds to receptor stimulation, whereas Q209L/P is largely, if not entirely, uncoupled from activation by upstream acting GPCRs (9, 110, 116–118) (Fig. 4A). This mechanistic difference explains why Gαq/11Q209L/P but not Gαq/11R183C mutants were long considered unresponsive to inhibitors of receptor-mediated nucleotide exchange (so-called GDIs). FR and YM are precisely such GDIs, viewed as unsuited for manipulating the oncogenic signaling driven by GTPase-deficient Gαq proteins for experimental or therapeutic purposes. However, FR in particular has shown convincing efficacy against UM cancer cells as brought to focus by four independent studies (97, 100, 110, 111). How come?
Figure 4.
Tertiary structure and signaling phenotypes of WT Gαq and GTPase-inactivating mutations R183C and Q209L. A, ribbon drawings of WT and mutant Gαq subunits bound to GDP and FR and based on the atomic coordinates of the Gαi/q-YM-inhibitor complex crystal structure (Protein Data Bank entry 3AH8 (49)). The GTPase-inactivating mutational hotspots R183C and Q209L are shown as space-filling models, and Gq inhibitor FR is illustrated as a stick model located in the interdomain cleft between the GTPase and the helical domain. B, schematic showing intrinsic properties of WT and mutationally activated Gαq oncoproteins. Curved arrows indicate rates of nucleotide exchange (top) or GTP hydrolysis (bottom), with thin arrows depicting rate-limiting reactions and thick arrows representing non-rate-limiting reactions: for Gαqwt, for example, nucleotide exchange is rate-limiting, but GTP hydrolysis is not, placing Gαqwt under upstream control of a GEF, the GPCR.
Experimental efficacy of FR in UM cancer cells: Solving an apparent paradox
G protein signaling requires both activation and deactivation. In normal cells, deactivation is an intrinsic property of the Gα subunit and is not rate-limiting (Fig. 4B). Mammalian Gα proteins typically deactivate by hydrolyzing GTP to GDP at catalytic rates kcat between 0.01 and 3.5 min−1 (116). Because GTP hydrolysis is faster than GDP release, the steady-state pool of activated Gα subunits is tightly linked to the amount of agonist-occupied GPCRs (Fig. 4B). In this way, G protein signaling is largely controlled by and dependent on catalytic input from the upstream acting receptors. However, in Gαq/11Q209L/P mutant cells, the inherent hydrolysis rate is far too slow to reset GDP-Gα (Fig. 4B). It follows that the nucleotide state of Gαq/11Q209L/P becomes more dependent on nucleotide affinity and concentration. Because GTP is in molar excess over GDP in living cells (119) and because GTP dissociates an order of magnitude slower than GDP (120), GTPase-deficient mutants predominantly exist in the GTP-bound state (Fig. 5). However, inhibitors of nucleotide dissociation may shift the nucleotide preference to enrich the fraction of inactive GαqGDP-βγ heterotrimers over time (Fig. 5). Their onset of action will depend on the rate of nucleotide exchange and/or the rate of GTP hydrolysis in a given cellular environment. Let us pause for a moment to reiterate this point: For a GTPase-deficient Gq to become GDP-bound at a relatively fast pace, it must either exchange nucleotides in cells at rates much faster than those believed to occur in in vitro experiments and/or hydrolyze GTP better than predicted from in vitro studies. It may therefore be advisable to revisit the molecular details underlying these quintessential processes of nucleotide exchange and GTP hydrolysis in the living cell context. This does not only appear timely but may also be technically feasible, given the availability of CRISPR-Cas9 genome-edited cells depleted of multiple G protein α subunits (103). So far, only FR (and not YM) has shown efficacy in the UM context. It is conceivable that this efficacy is in keeping with the kinetic parameters recently determined for direct interaction between tritiated FR and Gq; unlike YM, FR dissociates from Gq with a remarkably slow off rate (t½diss(FR) ∼92 min versus t½diss(YM) ∼4 min (124)), suggesting interaction in a pseudo-irreversible manner. Long Gq residence times may therefore be decisively advantageous for duration of action as well as experimental and therapeutic efficacy of Gq inhibitors in UM. Regardless of the kinetic differences, inhibitors of guanine nucleotide dissociation diminish the signaling of GTP-bound Gα in an indirect manner, clearly illustrating their dual value to blunt signaling not only of WT GTPases but also of mutationally activated GTPase-deficient oncogenes.
Figure 5.
Schematic for FR inhibition of oncogenic Gαq/11 GTPases. A, oncogenic Gln-209 mutations result in functional activation of Gαq/11 family proteins by impairing GTP hydrolysis. With diminished regulation by GTPase activity (GTP hydrolysis is rate-limiting), the nucleotide state of mutant Gq becomes more dependent on nucleotide affinity and concentration. Because GTP is in higher abundance than GDP in cells, GαGTP freed from its Gβγ binding partner is the major nucleotide-bound form of GTPase-deficient Gln-209 mutants. Inhibitors of nucleotide dissociation, such as FR, shift the equilibrium toward GαGDP-βγ heterotrimers over time, thereby enriching the fraction of G proteins in a signaling-incompetent state. Subversion of the nucleotide preference of Gln-209 mutants to favor GDP over GTP is an allosteric mechanism whereby FR gains control over aberrant signaling of oncogenic GTPase-deficient Gαq/11 proteins. B, schematic, overall structural fold and detailed view of the heterotrimeric G protein Gαq subunit (Protein Data Bank entry 3AH8) in its inactive, GDP-bound form; Q209L is visualized with a space-filling model. FR does not directly interact with Gln-209 but allosterically stabilizes the GDP-bound fraction of the oncoprotein, a conformation that cannot be maintained when Gαq is GTP-bound (49).
Heterotrimeric Gα subunits as drug targets?
Inhibition of GαGTP and, thereby, its downstream signaling repertoire, may be relevant to treat pathologies that are driven by overactive G proteins as is the case in various types of human cancers (31, 35, 57). Provided that targeting of heterotrimeric G proteins in a subfamily- or even isoform-specific manner will be expanded beyond the Gq/11 branch, the issue of ubiquitous Gα expression will still remain a perceived safety concern for potential medications. One possibility to overcome systemic toxicity is local drug application. For FR treatment of ocular melanoma, this may be achieved by local delivery directly into the eye just as established for a number of clinically used intravitreal therapeutics. Topical application, for the avoidance of systemic adverse effects, has already proven successful for FR inhibition of Gq-GPCR signaling in the airways using various in vivo models for acute and chronic lung diseases (94). Whereas the pulmonary administration route of an FR aerosol effectively suppressed Gq signaling, as evidenced by remarkable bronchodilation, systemic side effects that would directly result from Gq inhibition, such as blood pressure or heart rate alterations, were not detected (94). Long-term toxicity studies will be required to assess whether FR accumulates in certain cells, tissues, or organs to judge its potential to be administered to humans.
In the current absence of precision pharmacological targeting for mutationally activated Gα proteins, one can only speculate about possible advantages of targeted GαGTP therapeutics. Such a strategy does spring to mind as an attempt to preferentially diminish the aberrant Gα activity in cancer cells only, akin to therapies targeting mutationally activated BRAFV600E in metastatic melanoma. Yet, mutation-specific inhibitors for active Gα have not been reported to date, and, moreover, GαGTP antagonizing agents will likely also block the signaling of WT GTPases in that only a low dosage might afford a therapeutic window for targeted (preferential) inhibition of the oncogenic over the WT GαGTP pool. In light of these considerations and the current absence of X-ray structural information on GTPase-deficient Gαq, the recent successes to target mutationally activated Gq with FR in uveal melanoma must be viewed as a considerable breakthrough (97, 110, 111). Guanine nucleotide dissociation inhibitors of heterotrimeric G proteins such as FR may therefore evolve to be cornerstones of “anti-GαGTP therapies,” given their proven capacity to shift the nucleotide preference of Gα proteins toward the GDP-bound inactive state (Fig. 5). If combined with tissue- or cell-specific targeting, such as antibody-drug conjugates, systemic side effects may be kept at a minimum or even be spared. In such a scenario, concomitant inhibition of both mutationally activated and WT Gα may even be of advantage to harm the aberrant cells.
Conclusions and outlook
It has been known for decades that GTPase-inactivating point mutations in Gα proteins are important causative factors in many human cancers. However, there have been few attempts to establish approaches for inhibition of Gα oncoproteins (97, 100, 110, 111). One possible daunting challenge may have been that G protein–targeted pharmacological agents must enter the cell to exert their desired biological effect. However, molecules like FR or YM are beginning to bring this goal within reach. As far as pharmacological strategies are concerned, direct competition with GTP binding, in analogy to kinase inhibitors that compete with ATP binding, has not been seriously considered. This is because of the extremely high affinities of GTP and GDP for their nucleotide-binding pockets along with their micromolar abundance in cells, meaning that nucleotide binding to the catalytic site is very hard to overcome by any competitive inhibitor (121). What other strategies do come to mind to hinder constitutively active Gα proteins from aberrant signaling? Pharmacological reactivation of deficient Gα-GTPase activity may be a way to go (122), but conceivably very hard to implement. Thus, in the current absence of pharmacological agents to directly antagonize persistently active Gα, targeting nucleotide exchange, for long viewed ineffective for this purpose, appears particularly straightforward. In this respect, the re-emergence of FR, a highly specific Gαq-directed inhibitor of GDP/GTP exchange and cellular signaling, has not only revitalized the idea of targeting G protein oncogenes but also provided proof of principle in vitro (97, 100, 110, 111) and in vivo (110) that this is indeed experimentally feasible.
The authors declare that they have no conflicts of interest with the contents of this article.
- GPCR
- G protein–coupled receptor
- FR
- YM
- YM254890
- GEF
- guanine nucleotide exchange factor
- GAP
- GTPase-activating protein
- UM
- uveal melanoma
- GDI
- guanine nucleotide dissociation inhibitor.
References
- 1. Gilman A. G. (1987) G proteins: transducers of receptor-generated signals. Annu. Rev. Biochem. 56, 615–649 10.1146/annurev.bi.56.070187.003151 [DOI] [PubMed] [Google Scholar]
- 2. Neves S. R., Ram P. T., and Iyengar R. (2002) G protein pathways. Science 296, 1636–1639 10.1126/science.1071550 [DOI] [PubMed] [Google Scholar]
- 3. Johnston C. A., and Siderovski D. P. (2007) Receptor-mediated activation of heterotrimeric G-proteins: current structural insights. Mol. Pharmacol. 72, 219–230 10.1124/mol.107.034348 [DOI] [PubMed] [Google Scholar]
- 4. Oldham W. M., and Hamm H. E. (2008) Heterotrimeric G protein activation by G-protein-coupled receptors. Nat. Rev. Mol. Cell Biol. 9, 60–71 10.1038/nrm2299 [DOI] [PubMed] [Google Scholar]
- 5. Preininger A. M., Meiler J., and Hamm H. E. (2013) Conformational flexibility and structural dynamics in GPCR-mediated G protein activation: a perspective. J. Mol. Biol. 425, 2288–2298 10.1016/j.jmb.2013.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Milligan G., and Kostenis E. (2006) Heterotrimeric G-proteins: a short history. Br. J. Pharmacol. 147, S46–S55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Campbell A. P., and Smrcka A. V. (2018) Targeting G protein-coupled receptor signalling by blocking G proteins. Nat. Rev. Drug Discov. 17, 789–803 10.1038/nrd.2018.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Smrcka A. V. (2013) Molecular targeting of Gα and Gβγ subunits: a potential approach for cancer therapeutics. Trends Pharmacol. Sci. 34, 290–298 10.1016/j.tips.2013.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Takasaki J., Saito T., Taniguchi M., Kawasaki T., Moritani Y., Hayashi K., and Kobori M. (2004) A novel Gαq/11-selective inhibitor. J. Biol. Chem. 279, 47438–47445 10.1074/jbc.M408846200 [DOI] [PubMed] [Google Scholar]
- 10. Nesterov A., Hong M., Hertel C., Jiao P., Brownell L., and Cannon E. (2010) Screening a plant extract library for inhibitors of cholecystokinin receptor CCK1 pathways. J. Biomol. Screen. 15, 518–527 10.1177/1087057110369702 [DOI] [PubMed] [Google Scholar]
- 11. Schrage R., Schmitz A.-L., Gaffal E., Annala S., Kehraus S., Wenzel D., Büllesbach K. M., Bald T., Inoue A., Shinjo Y., Galandrin S., Shridhar N., Hesse M., Grundmann M., Merten N., et al. (2015) The experimental power of FR900359 to study Gq-regulated biological processes. Nat. Commun. 6, 10156 10.1038/ncomms10156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bonacci T. M., Mathews J. L., Yuan C., Lehmann D. M., Malik S., Wu D., Font J. L., Bidlack J. M., and Smrcka A. V. (2006) Differential targeting of Gβγ-subunit signaling with small molecules. Science 312, 443–446 10.1126/science.1120378 [DOI] [PubMed] [Google Scholar]
- 13. Ayoub M. A., Damian M., Gespach C., Ferrandis E., Lavergne O., De Wever O., Banères J.-L., Pin J.-P., and Prévost G. P. (2009) Inhibition of heterotrimeric G protein signaling by a small molecule acting on Gα subunit. J. Biol. Chem. 284, 29136–29145 10.1074/jbc.M109.042333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schmitz A.-L., Schrage R., Gaffal E., Charpentier T. H., Wiest J., Hiltensperger G., Morschel J., Hennen S., Häussler D., Horn V., Wenzel D., Grundmann M., Büllesbach K. M., Schröder R., Brewitz H. H., et al. (2014) A cell-permeable inhibitor to trap Gαq proteins in the empty pocket conformation. Chem. Biol. 21, 890–902 10.1016/j.chembiol.2014.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Prévost G. P., Lonchampt M. O., Holbeck S., Attoub S., Zaharevitz D., Alley M., Wright J., Brezak M. C., Coulomb H., Savola A., Huchet M., Chaumeron S., Nguyen Q.-D., Forgez P., Bruyneel E., et al. (2006) Anticancer activity of BIM-46174, a new inhibitor of the heterotrimeric Gα/Gβγ protein complex. Cancer Res. 66, 9227–9234 10.1158/0008-5472.CAN-05-4205 [DOI] [PubMed] [Google Scholar]
- 16. Hauser A. S., Attwood M. M., Rask-Andersen M., Schiöth H. B., and Gloriam D. E. (2017) Trends in GPCR drug discovery: new agents, targets and indications. Nat. Rev. Drug Discov. 16, 829–842 10.1038/nrd.2017.178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rask-Andersen M., Almén M. S., and Schiöth H. B. (2011) Trends in the exploitation of novel drug targets. Nat. Rev. Drug Discov. 10, 579–590 10.1038/nrd3478 [DOI] [PubMed] [Google Scholar]
- 18. Panettieri R. A., Pera T., Liggett S. B., Benovic J. L., and Penn R. B. (2018) Pepducins as a potential treatment strategy for asthma and COPD. Curr. Opin. Pharmacol. 40, 120–125 10.1016/j.coph.2018.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bel E. H. (2013) Clinical practice: mild asthma. N. Engl. J. Med. 369, 549–557 10.1056/NEJMcp1214826 [DOI] [PubMed] [Google Scholar]
- 20. Scadding G. W., and Scadding G. K. (2010) Recent advances in antileukotriene therapy. Curr. Opin. Allergy Clin. Immunol. 10, 370–376 10.1097/ACI.0b013e32833bfa20 [DOI] [PubMed] [Google Scholar]
- 21. Barnes P. J. (2017) Cellular and molecular mechanisms of asthma and COPD. Clin. Sci. 131, 1541–1558 10.1042/CS20160487 [DOI] [PubMed] [Google Scholar]
- 22. Moulton B. C., and Fryer A. D. (2011) Muscarinic receptor antagonists, from folklore to pharmacology; finding drugs that actually work in asthma and COPD. Br. J. Pharmacol. 163, 44–52 10.1111/j.1476-5381.2010.01190.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Erle D. J., and Sheppard D. (2014) The cell biology of asthma. J. Cell Biol. 205, 621–631 10.1083/jcb.201401050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Geppetti P., Veldhuis N. A., Lieu T., and Bunnett N. W. (2015) G protein-coupled receptors: dynamic machines for signaling pain and itch. Neuron 88, 635–649 10.1016/j.neuron.2015.11.001 [DOI] [PubMed] [Google Scholar]
- 25. Yim Y. Y., Zurawski Z., and Hamm H. (2018) GPCR regulation of secretion. Pharmacol. Ther. 192, 124–140 10.1016/j.pharmthera.2018.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Han S.-K., and Simon M. I. (2011) Intracellular signaling and the origins of the sensations of itch and pain. Sci. Signal. 4, pe38 10.1126/scisignal.2002353 [DOI] [PubMed] [Google Scholar]
- 27. Wirotanseng L. N., Kuner R., and Tappe-Theodor A. (2013) Gq rather than G11 preferentially mediates nociceptor sensitization. Mol. Pain 9, 54 10.1186/1744-8069-9-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nieto Gutierrez A., and McDonald P. H. (2018) GPCRs: emerging anti-cancer drug targets. Cell. Signal. 41, 65–74 10.1016/j.cellsig.2017.09.005 [DOI] [PubMed] [Google Scholar]
- 29. Wu V., Yeerna H., Nohata N., Chiou J., Harismendy O., Raimondi F., Inoue A., Russell R. B., Tamayo P., and Gutkind J. S. (2019) Illuminating the Onco-GPCRome: novel G protein-coupled receptor-driven oncocrine networks and targets for cancer immunotherapy. J. Biol. Chem. 294, 11062–11086 10.1074/jbc.REV119.005601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nohata N., Goto Y., and Gutkind J. S. (2017) Onco-GPCR signaling and dysregulated expression of microRNAs in human cancer. J. Hum. Genet. 62, 87–96 10.1038/jhg.2016.124 [DOI] [PubMed] [Google Scholar]
- 31. O'Hayre M., Degese M. S., and Gutkind J. S. (2014) Novel insights into G protein and G protein-coupled receptor signaling in cancer. Curr. Opin. Cell Biol. 27, 126–135 10.1016/j.ceb.2014.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Adlere I., Caspar B., Arimont M., Dekkers S., Visser K., Stuijt J., de Graaf C., Stocks M., Kellam B., Briddon S., Wijtmans M., de Esch I., Hill S., and Leurs R. (2019) Modulators of CXCR4 and CXCR7/ACKR3 function. Mol. Pharmacol. 96, 737–752 10.1124/mol.119.117663 [DOI] [PubMed] [Google Scholar]
- 33. Lappano R., Jacquot Y., and Maggiolini M. (2018) GPCR modulation in breast cancer. Int. J. Mol. Sci. 19, E3840 10.3390/ijms19123840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Arakaki A. K. S., Pan W.-A., and Trejo J. (2018) GPCRs in cancer: protease-activated receptors, endocytic adaptors and signaling. Int. J. Mol. Sci. 19, E1886 10.3390/ijms19071886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. O'Hayre M., Vázquez-Prado J., Kufareva I., Stawiski E. W., Handel T. M., Seshagiri S., and Gutkind J. S. (2013) The emerging mutational landscape of G-proteins and G-protein coupled receptors in cancer. Nat. Rev. Cancer 13, 412–424 10.1038/nrc3521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Codina J., Hildebrandt J., Iyengar R., Birnbaumer L., Sekura R. D., and Manclark C. R. (1983) Pertussis toxin substrate, the putative Ni component of adenylyl cyclases, is an αβ heterodimer regulated by guanine nucleotide and magnesium. Proc. Natl. Acad. Sci. U.S.A. 80, 4276–4280 10.1073/pnas.80.14.4276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Casey P. J., Fong H. K., Simon M. I., and Gilman A. G. (1990) Gz, a guanine nucleotide-binding protein with unique biochemical properties. J. Biol. Chem. 265, 2383–2390 [PubMed] [Google Scholar]
- 38. Murayama T., and Ui M. (1983) Loss of the inhibitory function of the guanine nucleotide regulatory component of adenylate cyclase due to its ADP ribosylation by islet-activating protein, pertussis toxin, in adipocyte membranes. J. Biol. Chem. 258, 3319–3326 [PubMed] [Google Scholar]
- 39. Hommers L. G., Klenk C., Dees C., and Bünemann M. (2010) G proteins in reverse mode: receptor-mediated GTP release inhibits G protein and effector function. J. Biol. Chem. 285, 8227–8233 10.1074/jbc.M109.015388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bünemann M., Frank M., and Lohse M. J. (2003) Gi protein activation in intact cells involves subunit rearrangement rather than dissociation. Proc. Natl. Acad. Sci. U.S.A. 100, 16077–16082 10.1073/pnas.2536719100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bondar A., and Lazar J. (2014) Dissociated GαGTP and Gβγ protein subunits are the major activated form of heterotrimeric Gi/o proteins. J. Biol. Chem. 289, 1271–1281 10.1074/jbc.M113.493643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Katada T., Northup J. K., Bokoch G. M., Ui M., and Gilman A. G. (1984) The inhibitory guanine nucleotide-binding regulatory component of adenylate cyclase: subunit dissociation and guanine nucleotide-dependent hormonal inhibition. J. Biol. Chem. 259, 3578–3585 [PubMed] [Google Scholar]
- 43. Higashijima T., Ferguson K. M., Smigel M. D., and Gilman A. G. (1987) The effect of GTP and Mg2+ on the GTPase activity and the fluorescent properties of Go. J. Biol. Chem. 262, 757–761 [PubMed] [Google Scholar]
- 44. Frank M., Thümer L., Lohse M. J., and Bünemann M. (2005) G protein activation without subunit dissociation depends on a Gαi-specific region. J. Biol. Chem. 280, 24584–24590 10.1074/jbc.M414630200 [DOI] [PubMed] [Google Scholar]
- 45. Galés C., Van Durm J. J. J., Schaak S., Pontier S., Percherancier Y., Audet M., Paris H., and Bouvier M. (2006) Probing the activation-promoted structural rearrangements in preassembled receptor-G protein complexes. Nat. Struct. Mol. Biol. 13, 778–786 10.1038/nsmb1134 [DOI] [PubMed] [Google Scholar]
- 46. Kimple A. J., Bosch D. E., Giguère P. M., and Siderovski D. P. (2011) Regulators of G-protein signaling and their Gα substrates: promises and challenges in their use as drug discovery targets. Pharmacol. Rev. 63, 728–749 10.1124/pr.110.003038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. O'Brien J. B., Wilkinson J. C., and Roman D. L. (2019) Regulator of G protein signaling (RGS) proteins as drug targets: progress and future potentials. J. Biol. Chem. 294, 18571–18585 10.1074/jbc.REV119.007060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kaziro Y., Itoh H., Kozasa T., Nakafuku M., and Satoh T. (1991) Structure and function of signal-transducing GTP-binding proteins. Annu. Rev. Biochem. 60, 349–400 10.1146/annurev.bi.60.070191.002025 [DOI] [PubMed] [Google Scholar]
- 49. Nishimura A., Kitano K., Takasaki J., Taniguchi M., Mizuno N., Tago K., Hakoshima T., and Itoh H. (2010) Structural basis for the specific inhibition of heterotrimeric Gq protein by a small molecule. Proc. Natl. Acad. Sci. U.S.A. 107, 13666–13671 10.1073/pnas.1003553107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hurst J. H., and Hooks S. B. (2009) Regulator of G-protein signaling (RGS) proteins in cancer biology. Biochem. Pharmacol. 78, 1289–1297 10.1016/j.bcp.2009.06.028 [DOI] [PubMed] [Google Scholar]
- 51. Hayes M. P., and Roman D. L. (2016) Regulator of G protein signaling 17 as a negative modulator of GPCR signaling in multiple human cancers. AAPS J. 18, 550–559 10.1208/s12248-016-9894-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ahlers K. E., Chakravarti B., and Fisher R. A. (2016) RGS6 as a novel therapeutic target in CNS diseases and cancer. AAPS J. 18, 560–572 10.1208/s12248-016-9899-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. DiGiacomo V., Maziarz M., Luebbers A., Norris J. M., Laksono P., and Garcia-Marcos M. (2020) Probing the mutational landscape of regulators of G protein signaling proteins in cancer. Sci. Signal. 13, eaax8620 10.1126/scisignal.aax8620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hepler J. R. (1999) Emerging roles for RGS proteins in cell signalling. Trends Pharmacol. Sci. 20, 376–382 10.1016/S0165-6147(99)01369-3 [DOI] [PubMed] [Google Scholar]
- 55. Dohlman H. G., Song J., Apanovitch D. M., DiBello P. R., and Gillen K. M. (1998) Regulation of G protein signalling in yeast. Semin. Cell Dev. Biol. 9, 135–141 10.1006/scdb.1998.0218 [DOI] [PubMed] [Google Scholar]
- 56. Dohlman H. G., and Thorner J. (1997) RGS proteins and signaling by heterotrimeric G proteins. J. Biol. Chem. 272, 3871–3874 10.1074/jbc.272.7.3871 [DOI] [PubMed] [Google Scholar]
- 57. Spiegel A. M., and Weinstein L. S. (2004) Inherited diseases involving G proteins and G protein-coupled receptors. Annu. Rev. Med. 55, 27–39 10.1146/annurev.med.55.091902.103843 [DOI] [PubMed] [Google Scholar]
- 58. Weinstein L. S., Liu J., Sakamoto A., Xie T., and Chen M. (2004) Minireview: GNAS: normal and abnormal functions. Endocrinology 145, 5459–5464 10.1210/en.2004-0865 [DOI] [PubMed] [Google Scholar]
- 59. Innamorati G., Wilkie T. M., Kantheti H. S., Valenti M. T., Dalle Carbonare L., Giacomello L., Parenti M., Melisi D., and Bassi C. (2018) The curious case of Gαs gain-of-function in neoplasia. BMC Cancer 18, 293 10.1186/s12885-018-4133-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Landis C. A., Masters S. B., Spada A., Pace A. M., Bourne H. R., and Vallar L. (1989) GTPase inhibiting mutations activate the α chain of Gs and stimulate adenylyl cyclase in human pituitary tumours. Nature 340, 692–696 10.1038/340692a0 [DOI] [PubMed] [Google Scholar]
- 61. Forbes S. A., Bhamra G., Bamford S., Dawson E., Kok C., Clements J., Menzies A., Teague J. W., Futreal P. A., and Stratton M. R. (2008) The Catalogue of Somatic Mutations in Cancer (COSMIC). Curr. Protoc. Hum. Genet. Chapter 10, Unit 10.11 10.1002/0471142905.hg1011s57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lyons J., Landis C. A., Harsh G., Vallar L., Grünewald K., Feichtinger H., Duh Q. Y., Clark O. H., Kawasaki E., and Bourne H. R. (1990) Two G protein oncogenes in human endocrine tumors. Science 249, 655–659 10.1126/science.2116665 [DOI] [PubMed] [Google Scholar]
- 63. Kan Z., Jaiswal B. S., Stinson J., Janakiraman V., Bhatt D., Stern H. M., Yue P., Haverty P. M., Bourgon R., Zheng J., Moorhead M., Chaudhuri S., Tomsho L. P., Peters B. A., Pujara K., et al. (2010) Diverse somatic mutation patterns and pathway alterations in human cancers. Nature 466, 869–873 10.1038/nature09208 [DOI] [PubMed] [Google Scholar]
- 64. Leyme A., Marivin A., Casler J., Nguyen L. T., and Garcia-Marcos M. (2014) Different biochemical properties explain why two equivalent Gα subunit mutants cause unrelated diseases. J. Biol. Chem. 289, 21818–21827 10.1074/jbc.M114.549790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lamba S., Felicioni L., Buttitta F., Bleeker F. E., Malatesta S., Corbo V., Scarpa A., Rodolfo M., Knowles M., Frattini M., Marchetti A., and Bardelli A. (2009) Mutational profile of GNAQQ209 in human tumors. PLoS ONE 4, e6833 10.1371/journal.pone.0006833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Van Raamsdonk C. D., Bezrookove V., Green G., Bauer J., Gaugler L., O'Brien J. M., Simpson E. M., Barsh G. S., and Bastian B. C. (2009) Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature 457, 599–602 10.1038/nature07586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Van Raamsdonk C. D., Griewank K. G., Crosby M. B., Garrido M. C., Vemula S., Wiesner T., Obenauf A. C., Wackernagel W., Green G., Bouvier N., Sozen M. M., Baimukanova G., Roy R., Heguy A., Dolgalev I., et al. (2010) Mutations in GNA11 in uveal melanoma. The New England journal of medicine 363, 2191–2199 10.1056/NEJMoa1000584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Möller I., Murali R., Müller H., Wiesner T., Jackett L. A., Scholz S. L., Cosgarea I., van de Nes J. A., Sucker A., Hillen U., Schilling B., Paschen A., Kutzner H., Rütten A., Böckers M., Scolyer R. A., Schadendorf D., and Griewank K. G. (2017) Activating cysteinyl leukotriene receptor 2 (CYSLTR2) mutations in blue nevi. Mod. Pathol. 30, 350–356 10.1038/modpathol.2016.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Weinstein L. S., Shenker A., Gejman P. V., Merino M. J., Friedman E., and Spiegel A. M. (1991) Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. N. Engl. J. Med. 325, 1688–1695 10.1056/NEJM199112123252403 [DOI] [PubMed] [Google Scholar]
- 70. Shenker A., Weinstein L. S., Sweet D. E., and Spiegel A. M. (1994) An activating Gsα mutation is present in fibrous dysplasia of bone in the McCune-Albright syndrome. J. Clin. Endocrinol. Metab. 79, 750–755 10.1210/jcem.79.3.8077356 [DOI] [PubMed] [Google Scholar]
- 71. Maziarz M., Leyme A., Marivin A., Luebbers A., Patel P. P., Chen Z., Sprang S. R., and Garcia-Marcos M. (2018) Atypical activation of the G protein Gαq by the oncogenic mutation Q209P. J. Biol. Chem. 293, 19586–19599 10.1074/jbc.RA118.005291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sisley K., Doherty R., and Cross N. A. (2011) What hope for the future? GNAQ and uveal melanoma. Br. J. Ophthalmol. 95, 620–623 10.1136/bjo.2010.182097 [DOI] [PubMed] [Google Scholar]
- 73. Bastian B. C. (2014) The molecular pathology of melanoma: an integrated taxonomy of melanocytic neoplasia. Annu. Rev. Pathol. 9, 239–271 10.1146/annurev-pathol-012513-104658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Luke J. J., Triozzi P. L., McKenna K. C., Van Meir E. G., Gershenwald J. E., Bastian B. C., Gutkind J. S., Bowcock A. M., Streicher H. Z., Patel P. M., Sato T., Sossman J. A., Sznol M., Welch J., Thurin M., et al. (2015) Biology of advanced uveal melanoma and next steps for clinical therapeutics. Pigment Cell Melanoma Res. 28, 135–147 10.1111/pcmr.12304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Singh A. D., Turell M. E., and Topham A. K. (2011) Uveal melanoma: trends in incidence, treatment, and survival. Ophthalmology 118, 1881–1885 10.1016/j.ophtha.2011.01.040 [DOI] [PubMed] [Google Scholar]
- 76. Carvajal R. D., Schwartz G. K., Tezel T., Marr B., Francis J. H., and Nathan P. D. (2017) Metastatic disease from uveal melanoma: treatment options and future prospects. Br. J. Ophthalmol. 101, 38–44 10.1136/bjophthalmol-2016-309034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Moore A. R., Ceraudo E., Sher J. J., Guan Y., Shoushtari A. N., Chang M. T., Zhang J. Q., Walczak E. G., Kazmi M. A., Taylor B. S., Huber T., Chi P., Sakmar T. P., and Chen Y. (2016) Recurrent activating mutations of G-protein-coupled receptor CYSLTR2 in uveal melanoma. Nat. Genet. 48, 675–680 10.1038/ng.3549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ceraudo E., Horioka M., Mattheisen J. M., Hitchman T. D., Moore A. R., Kazmi M. A., Chi P., Chen Y., Sakmar T. P., and Huber T. (2019) Uveal melanoma oncogene CYSLTR2 encodes a constitutively active GPCR highly biased toward Gq signaling. bioRxiv 10.1101/663153 [DOI] [PMC free article] [PubMed]
- 79. Fujioka M., Koda S., Morimoto Y., and Biemann K. (1988) Structure of FR900359, a cyclic depsipeptide from Ardisia crenata sims. J. Org. Chem. 53, 2820–2825 10.1021/jo00247a030 [DOI] [Google Scholar]
- 80. Gao Z.-G., and Jacobson K. A. (2016) On the selectivity of the Gαq inhibitor UBO-QIC: a comparison with the Gαi inhibitor pertussis toxin. Biochem. Pharmacol. 107, 59–66 10.1016/j.bcp.2016.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Inamdar V., Patel A., Manne B. K., Dangelmaier C., and Kunapuli S. P. (2015) Characterization of UBO-QIC as a Gαq inhibitor in platelets. Platelets 26, 771–778 10.3109/09537104.2014.998993 [DOI] [PubMed] [Google Scholar]
- 82. Kukkonen J. P. (2016) G-protein inhibition profile of the reported Gq/11 inhibitor UBO-QIC. Biochem. Biophys. Res. Commun. 469, 101–107 10.1016/j.bbrc.2015.11.078 [DOI] [PubMed] [Google Scholar]
- 83. Xiong X.-F., Zhang H., Underwood C. R., Harpsøe K., Gardella T. J., Wöldike M. F., Mannstadt M., Gloriam D. E., Bräuner-Osborne H., and Strømgaard K. (2016) Total synthesis and structure-activity relationship studies of a series of selective G protein inhibitors. Nat. Chem. 8, 1035–1041 10.1038/nchem.2577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Rensing D. T., Uppal S., Blumer K. J., and Moeller K. D. (2015) Toward the selective inhibition of G proteins: total synthesis of a simplified YM-254890 analog. Org. Lett. 17, 2270–2273 10.1021/acs.orglett.5b00944 [DOI] [PubMed] [Google Scholar]
- 85. Kaur H., Harris P. W. R., Little P. J., and Brimble M. A. (2015) Total synthesis of the cyclic depsipeptide YM-280193, a platelet aggregation inhibitor. Org. Lett. 17, 492–495 10.1021/ol503507g [DOI] [PubMed] [Google Scholar]
- 86. Reher R., Kühl T., Annala S., Benkel T., Kaufmann D., Nubbemeyer B., Odhiambo J. P., Heimer P., Bäuml C. A., Kehraus S., Crüsemann M., Kostenis E., Tietze D., König G. M., and Imhof D. (2018) Deciphering specificity determinants for FR900359-derived Gqα inhibitors based on computational and structure-activity studies. ChemMedChem 13, 1634–1643 10.1002/cmdc.201800304 [DOI] [PubMed] [Google Scholar]
- 87. Karpinsky-Semper D., Volmar C.-H., Brothers S. P., and Slepak V. Z. (2014) Differential effects of the Gβ5-RGS7 complex on muscarinic M3 receptor-induced Ca2+ influx and release. Mol. Pharmacol. 85, 758–768 10.1124/mol.114.091843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Wauson E. M., Guerra M. L., Dyachok J., McGlynn K., Giles J., Ross E. M., and Cobb M. H. (2015) Differential regulation of ERK1/2 and mTORC1 through T1R1/T1R3 in MIN6 cells. Mol. Endocrinol. 29, 1114–1122 10.1210/ME.2014-1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Carr R. 3rd, Koziol-White C., Zhang J., Lam H., An S. S., Tall G. G., Panettieri R. A. Jr., and Benovic J. L. (2016) Interdicting Gq activation in airway disease by receptor-dependent and receptor-independent mechanisms. Mol. Pharmacol. 89, 94–104 10.1124/mol.115.100339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kim S. H., MacIntyre D. A., Hanyaloglu A. C., Blanks A. M., Thornton S., Bennett P. R., and Terzidou V. (2016) The oxytocin receptor antagonist, Atosiban, activates pro-inflammatory pathways in human amnion via Gαi signalling. Mol. Cell. Endocrinol. 420, 11–23 10.1016/j.mce.2015.11.012 [DOI] [PubMed] [Google Scholar]
- 91. Liao Y., Lu B., Ma Q., Wu G., Lai X., Zang J., Shi Y., Liu D., Han F., and Zhou N. (2016) Human neuropeptide S receptor is activated via a Gαq protein-biased signaling cascade by a human neuropeptide S analog lacking the C-terminal 10 residues. J. Biol. Chem. 291, 7505–7516 10.1074/jbc.M115.704122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Bolognini D., Moss C. E., Nilsson K., Petersson A. U., Donnelly I., Sergeev E., König G. M., Kostenis E., Kurowska-Stolarska M., Miller A., Dekker N., Tobin A. B., and Milligan G. (2016) A novel allosteric activator of free fatty acid 2 receptor displays unique Gi-functional bias. J. Biol. Chem. 291, 18915–18931 10.1074/jbc.M116.736157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Badolia R., Inamdar V., Manne B. K., Dangelmaier C., Eble J. A., and Kunapuli S. P. (2017) Gq pathway regulates proximal C-type lectin-like receptor-2 (CLEC-2) signaling in platelets. J. Biol. Chem. 292, 14516–14531 10.1074/jbc.M117.791012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Matthey M., Roberts R., Seidinger A., Simon A., Schröder R., Kuschak M., Annala S., König G. M., Müller C. E., Hall I. P., Kostenis E., Fleischmann B. K., and Wenzel D. (2017) Targeted inhibition of Gq signaling induces airway relaxation in mouse models of asthma. Sci. Transl. Med. 9, eaag2288 10.1126/scitranslmed.aag2288 [DOI] [PubMed] [Google Scholar]
- 95. Crüsemann M., Reher R., Schamari I., Brachmann A. O., Ohbayashi T., Kuschak M., Malfacini D., Seidinger A., Pinto-Carbó M., Richarz R., Reuter T., Kehraus S., Hallab A., Attwood M., Schiöth H. B., et al. (2018) Heterologous expression, biosynthetic studies, and ecological function of the selective Gq-signaling inhibitor FR900359. Angew. Chem. Int. Ed. Engl. 57, 836–840 10.1002/anie.201707996 [DOI] [PubMed] [Google Scholar]
- 96. Gao Z.-G., Inoue A., and Jacobson K. A. (2018) On the G protein-coupling selectivity of the native A2B adenosine receptor. Biochem. Pharmacol. 151, 201–213 10.1016/j.bcp.2017.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Onken M. D., Makepeace C. M., Kaltenbronn K. M., Kanai S. M., Todd T. D., Wang S., Broekelmann T. J., Rao P. K., Cooper J. A., and Blumer K. J. (2018) Targeting nucleotide exchange to inhibit constitutively active G protein α subunits in cancer cells. Sci. Signal. 11, eaao6852 10.1126/scisignal.aao6852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Lian X., Beer-Hammer S., König G. M., Kostenis E., Nürnberg B., and Gollasch M. (2018) RXFP1 receptor activation by relaxin-2 induces vascular relaxation in mice via a Gαi2-protein/PI3Kβ/γ/nitric oxide-coupled pathway. Front. Physiol. 9, 1234 10.3389/fphys.2018.01234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Cervantes-Villagrana R. D., Adame-García S. R., García-Jiménez I., Color-Aparicio V. M., Beltrán-Navarro Y. M., König G. M., Kostenis E., Reyes-Cruz G., Gutkind J. S., and Vázquez-Prado J. (2019) Gβγ signaling to the chemotactic effector P-REX1 and mammalian cell migration is directly regulated by Gαq and Gα13 proteins. J. Biol. Chem. 294, 531–546 10.1074/jbc.RA118.006254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lapadula D., Farias E., Randolph C. E., Purwin T. J., McGrath D., Charpentier T. H., Zhang L., Wu S., Terai M., Sato T., Tall G. G., Zhou N., Wedegaertner P. B., Aplin A. E., Aguirre-Ghiso J., and Benovic J. L. (2019) Effects of oncogenic Gαq and Gα11 inhibition by FR900359 in uveal melanoma. Mol. Cancer Res. 17, 963–973 10.1158/1541-7786.MCR-18-0574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Kienitz M.-C., Niemeyer A., König G. M., Kostenis E., Pott L., and Rinne A. (2019) Biased signaling of Ca2+-sensing receptors in cardiac myocytes regulates GIRK channel activity. J. Mol. Cell. Cardiol. 130, 107–121 10.1016/j.yjmcc.2019.03.022 [DOI] [PubMed] [Google Scholar]
- 102. Ebner J. K., König G. M., Kostenis E., Siegert P., Aktories K., and Orth J. H. C. (2019) Activation of Gq signaling by Pasteurella multocida toxin inhibits the osteoblastogenic-like actions of Activin A in C2C12 myoblasts, a cell model of fibrodysplasia ossificans progressiva. Bone 127, 592–601 10.1016/j.bone.2019.07.031 [DOI] [PubMed] [Google Scholar]
- 103. Grundmann M., Merten N., Malfacini D., Inoue A., Preis P., Simon K., Rüttiger N., Ziegler N., Benkel T., Schmitt N. K., Ishida S., Müller I., Reher R., Kawakami K., Inoue A., et al. (2018) Lack of β-arrestin signaling in the absence of active G proteins. Nat. Commun. 9, 341 10.1038/s41467-017-02661-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Coombs C., Georgantzoglou A., Walker H. A., Patt J., Merten N., Poplimont H., Busch-Nentwich E. M., Williams S., Kotsi C., Kostenis E., and Sarris M. (2019) Chemokine receptor trafficking coordinates neutrophil clustering and dispersal at wounds in zebrafish. Nat. Commun. 10, 5166 10.1038/s41467-019-13107-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Olianas M. C., Dedoni S., and Onali P. (2016) Protection from interferon-β-induced neuronal apoptosis through stimulation of muscarinic acetylcholine receptors coupled to ERK1/2 activation. Br. J. Pharmacol. 173, 2910–2928 10.1111/bph.13570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Roszko K. L., Bi R., Gorvin C. M., Bräuner-Osborne H., Xiong X.-F., Inoue A., Thakker R. V., Strømgaard K., Gardella T., and Mannstadt M. (2017) Knockin mouse with mutant Gα11 mimics human inherited hypocalcemia and is rescued by pharmacologic inhibitors. JCI Insight 2, e91079 10.1172/jci.insight.91079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Lorenzen E., Ceraudo E., Berchiche Y. A., Rico C. A., Fürstenberg A., Sakmar T. P., and Huber T. (2018) G protein subtype-specific signaling bias in a series of CCR5 chemokine analogs. Sci. Signal. 11, eaao6152 10.1126/scisignal.aao6152 [DOI] [PubMed] [Google Scholar]
- 108. Dutton-Regester K., Irwin D., Hunt P., Aoude L. G., Tembe V., Pupo G. M., Lanagan C., Carter C. D., O'Connor L., O'Rourke M., Scolyer R. A., Mann G. J., Schmidt C. W., Herington A., and Hayward N. K. (2012) A high-throughput panel for identifying clinically relevant mutation profiles in melanoma. Mol. Cancer Ther. 11, 888–897 10.1158/1535-7163.MCT-11-0676 [DOI] [PubMed] [Google Scholar]
- 109. Davies H., Bignell G. R., Cox C., Stephens P., Edkins S., Clegg S., Teague J., Woffendin H., Garnett M. J., Bottomley W., Davis N., Dicks E., Ewing R., Floyd Y., Gray K., et al. (2002) Mutations of the BRAF gene in human cancer. Nature 417, 949–954 10.1038/nature00766 [DOI] [PubMed] [Google Scholar]
- 110. Annala S., Feng X., Shridhar N., Eryilmaz F., Patt J., Yang J., Pfeil E. M., Cervantes-Villagrana R. D., Inoue A., Häberlein F., Slodczyk T., Reher R., Kehraus S., Monteleone S., Schrage R., et al. (2019) Direct targeting of Gαq and Gα11 oncoproteins in cancer cells. Sci. Signal. 12, eaau5948 10.1126/scisignal.aau5948 [DOI] [PubMed] [Google Scholar]
- 111. Feng X., Arang N., Rigiracciolo D. C., Lee J. S., Yeerna H., Wang Z., Lubrano S., Kishore A., Pachter J. A., König G. M., Maggiolini M., Kostenis E., Schlaepfer D. D., Tamayo P., Chen Q., et al. (2019) A platform of synthetic lethal gene interaction networks reveals that the GNAQ uveal melanoma oncogene controls the Hippo pathway through FAK. Cancer Cell 35, 457–472.e5 10.1016/j.ccell.2019.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Robertson A. G., Shih J., Yau C., Gibb E. A., Oba J., Mungall K. L., Hess J. M., Uzunangelov V., Walter V., Danilova L., Lichtenberg T. M., Kucherlapati M., Kimes P. K., Tang M., Penson A., et al. (2017) Integrative analysis identifies four molecular and clinical subsets in uveal melanoma. Cancer Cell 32, 204–220.e15 10.1016/j.ccell.2017.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Slebos R. J., Kibbelaar R. E., Dalesio O., Kooistra A., Stam J., Meijer C. J., Wagenaar S. S., Vanderschueren R. G., van Zandwijk N., and Mooi W. J. (1990) K-ras oncogene activation as a prognostic marker in adenocarcinoma of the lung. N. Engl. J. Med. 323, 561–565 10.1056/NEJM199008303230902 [DOI] [PubMed] [Google Scholar]
- 114. Kleuss C., Raw A. S., Lee E., Sprang S. R., and Gilman A. G. (1994) Mechanism of GTP hydrolysis by G-protein alpha subunits. Proc. Natl. Acad. Sci. U.S.A. 91, 9828–9831 10.1073/pnas.91.21.9828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Coleman D. E., Berghuis A. M., Lee E., Linder M. E., Gilman A. G., and Sprang S. R. (1994) Structures of active conformations of Giα1 and the mechanism of GTP hydrolysis. Science 265, 1405–1412 10.1126/science.8073283 [DOI] [PubMed] [Google Scholar]
- 116. Sprang S. R. (2016) Invited review: activation of G proteins by GTP and the mechanism of Gα-catalyzed GTP hydrolysis. Biopolymers 105, 449–462 10.1002/bip.22836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Wu D., Katz A., Lee C. H., and Simon M. I. (1992) Activation of phospholipase C by α1-adrenergic receptors is mediated by the α subunits of Gq family. J. Biol. Chem. 267, 25798–25802 [PubMed] [Google Scholar]
- 118. Kalinec G., Nazarali A. J., Hermouet S., Xu N., and Gutkind J. S. (1992) Mutated α subunit of the Gq protein induces malignant transformation in NIH 3T3 cells. Mol. Cell. Biol. 12, 4687–4693 10.1128/MCB.12.10.4687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Traut T. W. (1994) Physiological concentrations of purines and pyrimidines. Mol. Cell. Biochem. 140, 1–22 10.1007/BF00928361 [DOI] [PubMed] [Google Scholar]
- 120. Chidiac P., Markin V. S., and Ross E. M. (1999) Kinetic control of guanine nucleotide binding to soluble Gαq. Biochem. Pharmacol. 58, 39–48 10.1016/S0006-2952(99)00080-5 [DOI] [PubMed] [Google Scholar]
- 121. John J., Sohmen R., Feuerstein J., Linke R., Wittinghofer A., and Goody R. S. (1990) Kinetics of interaction of nucleotides with nucleotide-free H-ras p21. Biochemistry 29, 6058–6065 10.1021/bi00477a025 [DOI] [PubMed] [Google Scholar]
- 122. Ja W. W., Wiser O., Austin R. J., Jan L. Y., and Roberts R. W. (2006) Turning G proteins on and off using peptide ligands. ACS Chem. Biol. 1, 570–574 10.1021/cb600345k [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Malfacini D., Patt J., Annala S., Harpsøe K., Eryilmaz F., Reher R., Crüsemann M., Hanke W., Zhang H., Tietze D., Gloriam D. E., Bräuner-Osborne H., Strømgaard K., König G. M., Inoue A., et al. (2019) Rational design of a heterotrimeric G protein α subunit with artificial inhibitor sensitivity. J. Biol. Chem. 294, 5747–5758 10.1074/jbc.RA118.007250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Kuschak M., Namasivayam V., Rafehi M., Voss J. H., Garg J., Schlegel J. G., Abdelrahman A., Kehraus K., Reher R., Küppers J., Sylvester K., Hinz S., Matthey M., Wenzel D., Fleischmann B. K., et al. (2020) Cell-permeable high-affinity tracers for Gq proteins provide structural insights, reveal distinct binding kinetics and identify small molecule inhibitors. Br. J. Pharmacolo. 177, 1898–1916 10.1111/bph.14960 [DOI] [PMC free article] [PubMed] [Google Scholar]