Abstract
Objectives
Regorafenib improved overall survival in patients with metastatic colorectal cancer (mCRC) refractory to standard therapies in two randomised, phase III trials, but has not been evaluated in Turkey. REGARD evaluated the safety and efficacy of regorafenib in Turkish patients with treatment-refractory mCRC.
Design
Open-label, single-arm, phase IIIb study conducted between July 2013 and April 2015.
Setting
11 tertiary centres in Turkey.
Participants
Eligible patients were adults with mCRC who had disease progression within 3 months after receiving their last dose of approved standard therapies and who had an Eastern Cooperative Oncology Group performance status ≤1. Patients were excluded if they had previously received regorafenib. Of 139 patients screened, 100 were treated and completed the study, and all 100 were analysed. Fifty-eight per cent were male.
Interventions
Patients received oral regorafenib, 160 mg once daily, for the first 3 weeks of each 4-week cycle until disease progression, death or unacceptable toxicity.
Primary and secondary outcome measures
The primary endpoint was safety, assessed by incidence of treatment-emergent adverse events (TEAEs). Progression-free survival (PFS) per investigator was the primary efficacy endpoint. There were no secondary endpoints.
Results
The median treatment duration was 2.5 months (range 0.1 to 20.6). Ninety-six per cent of patients had at least one TEAE and 77% had a grade ≥3 TEAE. The most common grade ≥3 regorafenib-related TEAEs were hypophosphataemia (11%), fatigue (8%), hyperbilirubinaemia (6%), hand–foot skin reaction (5%), hypertension (5%), anorexia (5%) and increased alanine aminotransferase (5%). TEAEs led to dose reduction in 30% of patients. Regorafenib-related TEAEs led to treatment discontinuation in 17% of patients. Median PFS was 3.1 months (95% CI 2.9 to 3.8).
Conclusion
The regorafenib safety profile and PFS in REGARD were consistent with the results of previous trials of regorafenib in mCRC. Regorafenib is an option for patients in Turkey with treatment-refractory mCRC.
Trial registration number
NCT01853319, ClinicalTrials.gov.
Keywords: multikinase inhibitor, clinical trial, Turkey, progression-free survival, toxicities
Strengths and limitations of this study.
Open-label, single-arm, multicentre phase IIIb study.
Inclusion and exclusion criteria were consistent with the objectives of the study, that is, to determine the safety and efficacy of regorafenib in patients with metastatic colorectal cancer who had disease progression on prior treatment, which had to include bevacizumab.
Safety was measured by treatment-emergent adverse events and laboratory abnormalities, graded by National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) V.4.0.
Progression-free survival was assessed per investigator at intervals and with methods consistent with the best standard of care of each institution.
Statistics were descriptive.
Introduction
Few treatment options are available for patients with metastatic colorectal cancer (mCRC) who have disease progression on standard therapies. Standard regimens for intensive therapy for mCRC include chemotherapy based on a fluoropyrimidine used in combination or sequence with oxaliplatin or irinotecan, and biological agents targeting vascular endothelial growth factor and epidermal growth factor receptor in patients with RAS wild-type tumours.1 2 Patients who have disease progression during these standard therapies may benefit from additional treatment options.
Regorafenib is an oral multikinase inhibitor that blocks the activity of multiple protein kinases involved in the regulation of tumour angiogenesis, oncogenesis, tumour immunity and the tumour microenvironment.3 4 Regorafenib is approved for the treatment of patients with mCRC who have been previously treated with standard therapies, based on results of the phase III, randomised, double-blind, international CORRECT study. In CORRECT, regorafenib significantly improved overall survival versus placebo in patients with mCRC refractory to available standard therapies (HR 0.77, 95% CI 0.64 to 0.94; one-sided p=0.0052),5 and the benefit of regorafenib in Asian patients was confirmed in the phase III CONCUR study (HR 0.55; 95% CI 0.40 to 0.77; one-sided p=0.00016).6 Frequently reported regorafenib-related treatment-emergent adverse events (TEAEs) in the CORRECT and CONCUR studies included hand–foot skin reaction (HFSR), fatigue, diarrhoea and hypertension.5 6 The results of CORRECT and CONCUR were supported by the findings of the phase IIIb international CONSIGN study that further characterised the safety profile of regorafenib in a large cohort of patients.7 However, no patients from Turkey were randomised or treated in the CORRECT, CONCUR or CONSIGN trials. We present results of the REGARD study, which was designed to provide additional information about the safety and efficacy of regorafenib in patients with mCRC in Turkey who had disease progression after receiving all approved standard therapies.
Patients and methods
Study design
REGARD was an open-label, single-arm, phase IIIb trial of regorafenib conducted in 11 centres in Turkey. Eligible patients were adults with mCRC, with a life expectancy of at least 3 months and an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1, who had disease progression within 3 months after their last dose of approved standard therapies. Approved prior standard therapies must have included a fluoropyrimidine, oxaliplatin, irinotecan, bevacizumab and, for patients with KRAS wild-type tumours, cetuximab/panitumumab. Patients were also required to have adequate liver, renal and bone marrow function, defined by the following laboratory requirements: total bilirubin ≤1.5 × upper limit of normal (ULN), alanine aminotransferase (ALT) and aspartate aminotransferase (AST) ≤2.5 × ULN (≤5 × ULN for patients with hepatic involvement of their cancer), alkaline phosphatase ≤2.5 × ULN (≤5 × ULN for patients with hepatic involvement), lipase ≤1.5 × ULN, serum creatinine ≤1.5 × ULN, international normalised ratio/partial thromboplastin time ≤1.5 × ULN, platelets ≥100,000/mm3, haemoglobin ≥9 g/dL and absolute neutrophil count ≥1500/mm3. Patients were excluded if they had clinically significant comorbidities or had previously received regorafenib. At screening, following the complete tumour assessment, only the presence of brain and liver metastases was recorded. A CT scan of the brain at screening was optional.
The study was carried out in accordance with the guidelines of the Declaration of Helsinki and good clinical practice. All participants provided written informed consent.
Procedures
Patients were assigned to receive oral regorafenib 160 mg once daily for 3 weeks on/1 week off in 4-week cycles until disease progression, death, unacceptable toxicity, patient withdrawal or investigator decision to discontinue treatment. A patient with disease progression could continue treatment if the investigator judged that continuing regorafenib would provide clinical benefit. Delaying or reducing the regorafenib dose (to 120 mg or 80 mg per day) was permitted to manage clinically significant toxicities. A reduced dose could be re-escalated to a maximum of 160 mg/day once the toxicities resolved to baseline.
The primary endpoint was safety. Safety was monitored and evaluated continuously throughout treatment until the end of a 30-day post-treatment follow-up period. TEAEs were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) V.4.0. Each TEAE was assessed for seriousness. A TEAE was classified as serious if it resulted in death, was life-threatening, required inpatient hospitalisation or prolongation of existing hospitalisation (except if the admission resulted in a hospital stay of <12 hours, was pre-planned or was not associated with the TEAE), resulted in persistent or significant disability/incapacity, was a congenital abnormality/birth defect or was medically significant as judged by the investigator. Laboratory parameters, including complete blood count, and AST, ALT and bilirubin levels were monitored every 2 weeks for the first two cycles and thereafter every 2 to 4 weeks (at the discretion of the investigator). Deaths were recorded during the study through the 30-day follow-up period. Information about deaths occurring after the 30-day follow-up period was not formally collected.
Progression-free survival (PFS) was the only efficacy endpoint assessed, and was defined as the time from treatment assignment (date of first treatment) to the first observed disease progression or death due to any cause. Tumour measurements were assessed by the investigator at intervals and with methods consistent with the best standard of care of each institution. Only the date of disease progression was recorded. Progression was evaluated radiologically, or by clinical progression based on the judgement of the investigator if radiographic imaging was not possible.
Statistical analyses
It was planned to enrol approximately 100 patients based on the demand for the study drug and the available supply. Considering an ongoing global study (CONSIGN) in which Turkey was not a participant, study drug was allocated for 100 patients in Turkey. No statistical assumptions were made. Statistical analyses were performed using SAS release 9.4 or higher (SAS Institute Inc., Cary, North Carolina, USA). Safety analyses were performed on all patients who received at least one dose of regorafenib. The efficacy analysis was performed on all patients assigned to treatment. PFS and its 95% CI were estimated by the Kaplan–Meier method. PFS for patients without disease progression or death before or at the last visit was censored at the date of the last visit.
Patient and public involvement
The study was planned based on the unmet need for treatment options among patients in Turkey with mCRC who had disease progression after receiving standard therapies. Many patients in Turkey and their families requested access to regorafenib, and the open-label study design of REGARD meant that all enrolled patients received regorafenib treatment. No patient was involved in the design or conduct of the study. The results of the study were not disseminated to participants because patient data were anonymised.
Results
Patient disposition and baseline characteristics
Patients were enrolled and treated between July 2013 and April 2015 at 11 centres in Turkey. In total, 139 patients were screened, of whom 38 did not meet the eligibility requirements and one prematurely discontinued (online supplementary figure 1). A total of 100 patients were assigned to treatment and received at least one dose of regorafenib (population for safety and efficacy analyses). At the time of data cut-off for this analysis (24 April 2015), 97 patients (97%) had discontinued treatment. The most common reason for treatment discontinuation was progressive disease—radiological progression (50%), followed by adverse event not associated with disease progression (20%), patient withdrawal (14%) and adverse event associated with disease progression (12%) (online supplementary figure 1). At the cut-off date, three patients were still receiving treatment.
bmjopen-2018-027665supp001.pdf (984.1KB, pdf)
The median age of patients was 56.5 years (range 31 to 78) and 22% of patients were aged ≥65 years (table 1). More than half of patients (59%) had ECOG PS 0 and 53% had KRAS mutations. Seventy-eight per cent of patients had liver metastases and 2% had brain metastases. All patients had received prior treatment with fluoropyrimidine analogues, oxaliplatin, irinotecan and monoclonal antibodies (bevacizumab, and cetuximab/panitumumab if KRAS wild-type).
Table 1.
Baseline characteristics
| Regorafenib (N=100) | |
| Male, n (%) | 58 (58) |
| Age, years | |
| Median (range) | 56.5 (31–78) |
| ≥65 years, n (%) | 22 (22) |
| ECOG PS, n (%) | |
| 0 | 59 (59) |
| 1 | 41 (41) |
| Time from initial diagnosis to treatment assignment | |
| Median (range), months | 33.6 (7.4–138.1) |
| <18 months, n (%) | 11 (11) |
| ≥18 months, n (%) | 89 (89) |
| Time from diagnosis of metastatic disease to treatment assignment | |
| Median (range), months | 29.3 (6.9–138.1) |
| <18 months, n (%) | 17 (17) |
| ≥18 months, n (%) | 83 (83) |
| Primary site of disease, n (%) | |
| Colon | 55 (55) |
| Rectum | 24 (24) |
| Colon and rectum | 21 (21) |
| KRAS status, n (%) | |
| Wild-type | 43 (43) |
| Mutant | 53 (53) |
| Unknown | 4 (4) |
| BRAF status, n (%) | |
| Wild-type | 6 (6) |
| Mutant | 0 |
| Unknown | 94 (94) |
| Brain metastases, n (%) | |
| No | 77 (77) |
| Yes | 2 (2) |
| Unknown | 21 (21) |
| Liver metastases, n (%) | |
| No | 22 (22) |
| Yes | 78 (78) |
ECOG PS, Eastern Cooperative Oncology Group performance status.
Dosing and treatment duration
The median overall time on treatment (including interruptions and delays) was 2.5 months (range 0.1 to 20.6; online supplementary table 1). Patients received a median of three cycles (range 1 to 22) of regorafenib. Twenty-nine per cent of patients received ≥5 cycles. The mean actual daily dose, which includes only days when a dose was given, was 150.8 mg (SD 15.1). Almost two-thirds of patients (64%) had a treatment interruption or delay and one-third (33%) had a dose reduction. Despite treatment interruptions and reductions, patients received a mean of 87.3% (SD 16.4) of the initial planned dose of 160 mg daily.
bmjopen-2018-027665supp002.pdf (67.3KB, pdf)
Safety
Almost all patients (96%) had at least one TEAE, and 80% of patients had TEAEs considered regorafenib related (table 2). Regorafenib-related TEAEs of grade 3 or 4 severity occurred in 48% of patients. Serious TEAEs occurred in 36% of patients and were regorafenib related in 15%. The most common regorafenib-related TEAEs were HFSR (27%), fatigue (18%), hypertension (16%) and anorexia (16%) (table 3). The most common grade 3 or 4 regorafenib-related TEAEs were hypophosphataemia (11%), fatigue (8%) and hyperbilirubinaemia (6%).
Table 2.
Overview of adverse events*
| Adverse events, n (%) | Regorafenib (N=100) |
||
| Treatment-emergent | Drug-related treatment-emergent | ||
| Any | 96 (96) | 80 (80) | |
| Worst grade | 1† | 6 (6) | 9 (9) |
| 2† | 13 (13) | 19 (19) | |
| 3 | 57 (57) | 42 (42) | |
| 4 | 10 (10) | 6 (6) | |
| 5 (death) | 10 (10) | 4 (4)‡ | |
| Serious | 36 (36) | 15 (15) | |
| Leading to dose modification§ | 70 (70) | 55 (55) | |
| Leading to dose reduction | 30 (30) | NA | |
| Leading to dose interruption | 62 (62) | NA | |
| Leading to permanent discontinuation | 28 (28) | 17 (17) | |
Severity graded by NCI-CTCAE V.4.0.
*Includes events occurring during treatment through the 30-day post-treatment follow-up period.
†The number of drug-related TEAEs can be larger than the number of TEAEs for a given grade because a patient is counted only once for each category. In the overall summary of TEAEs, a patient is counted once in the category of worst grade regardless of relationship to study drug. To find the drug-related TEAEs, a subset is first generated for any TEAEs that are drug related, and then the patient is counted once in the worst grade category. For a given patient, the worst grade of drug-related TEAEs may be different than the worst grade of overall TEAEs.
‡The grade 5 regorafenib-related TEAEs were pulmonary embolism (1), malaise (as reported by the investigator) (1), sepsis (1) and thromboembolic event (1).
§Dose modifications include delays, reductions and interruptions.
NA, not available; NCI-CTCAE, National Cancer Institute Common Terminology Criteria for Adverse Events; TEAE, treatment-emergent adverse event.
Table 3.
Treatment-emergent adverse events occurring at any grade in ≥10 patients, and corresponding rates of drug-related treatment-emergent adverse events*
| Adverse events, % | Regorafenib (N=100) |
|||||
| Treatment-emergent | Drug-related treatment-emergent | |||||
| Any grade | Grade 3 | Grade 4 | Any grade | Grade 3 | Grade 4 | |
| Hand–foot skin reaction | 29 | 5 | NA | 27 | 5 | NA |
| Hyperbilirubinaemia | 25 | 13 | 1 | 13 | 5 | 1 |
| Anorexia | 21 | 7 | 0 | 16 | 5 | 0 |
| Fatigue | 20 | 9 | NA | 18 | 8 | NA |
| Hypertension | 20 | 7 | 0 | 16 | 5 | 0 |
| Weight loss | 20 | 1 | NA | 13 | 0 | NA |
| Hypophosphataemia | 19 | 15 | 0 | 15 | 11 | 0 |
| AST increased | 17 | 8 | 0 | 8 | 4 | 0 |
| Diarrhoea | 17 | 3 | 0 | 15 | 2 | 0 |
| Anaemia | 16 | 3 | 0 | 9 | 2 | 0 |
| Hoarseness | 13 | 0 | NA | 9 | 0 | NA |
| Hypothyroidism | 13 | 1 | 0 | 11 | 1 | 0 |
| Alkaline phosphatase increased | 12 | 6 | 0 | 3 | 1 | 0 |
| ALT increased | 12 | 5 | 0 | 7 | 5 | 0 |
Categories and severity by NCI-CTCAE V.4.0.
*Includes events occurring during treatment through the 30-day post-treatment follow-up period.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; NA, not applicable; NCI-CTCAE, National Cancer Institute Common Terminology Criteria for Adverse Events.
TEAEs regardless of relation to study drug led to dose reductions in 30% of patients and to permanent discontinuation in 28% (table 2). Regorafenib-related TEAEs led to permanent discontinuation in 17% of patients. The most common TEAEs (regardless of relation to study drug) leading to permanent discontinuation were hyperbilirubinaemia (10%), AST elevation (6%) and ALT elevation (5%) (online supplementary table 2). The most common TEAEs leading to dose reduction were HFSR (8%) and fatigue (7%) (online supplementary table 3).
bmjopen-2018-027665supp003.pdf (52.3KB, pdf)
bmjopen-2018-027665supp004.pdf (52.2KB, pdf)
Of the 10 patients with grade 5 TEAEs (fatal outcome), five TEAEs were associated with clinical disease progression, and one was a patient who was discharged at his own request and died the following day. Four patients died most likely secondary to regorafenib-related TEAEs: pulmonary embolism (n=1), malaise (as reported by the investigator; n=1), sepsis (n=1) and thromboembolic event (n=1).
The most common treatment-emergent grade 3 or 4 haematological or biochemical laboratory test abnormalities were hypophosphataemia (38%), hyperbilirubinaemia (17%), increased AST (9%), increased alkaline phosphatase (8%), increased lipase (8%) and hyponatraemia (8%) (online supplementary table 4). Most of these events were grade 3. Hepatobiliary disorders of any grade were reported in nine patients (9%) and were of grade 3 or 4 severity in four patients (4%). Two patients had grade 5 hepatobiliary disorders (hepatic failure); neither was attributed to study drug.
bmjopen-2018-027665supp005.pdf (65.1KB, pdf)
Efficacy
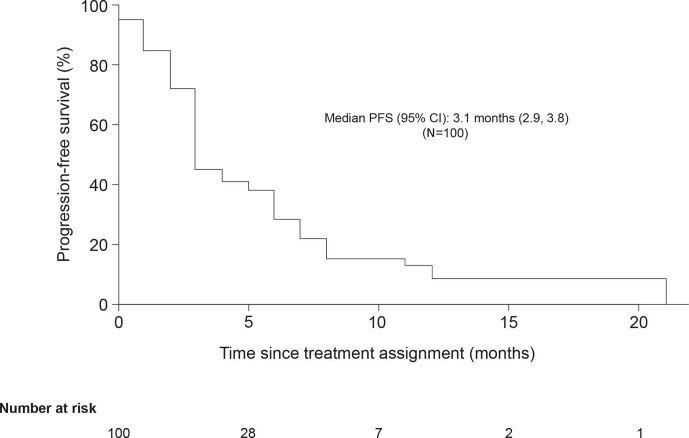
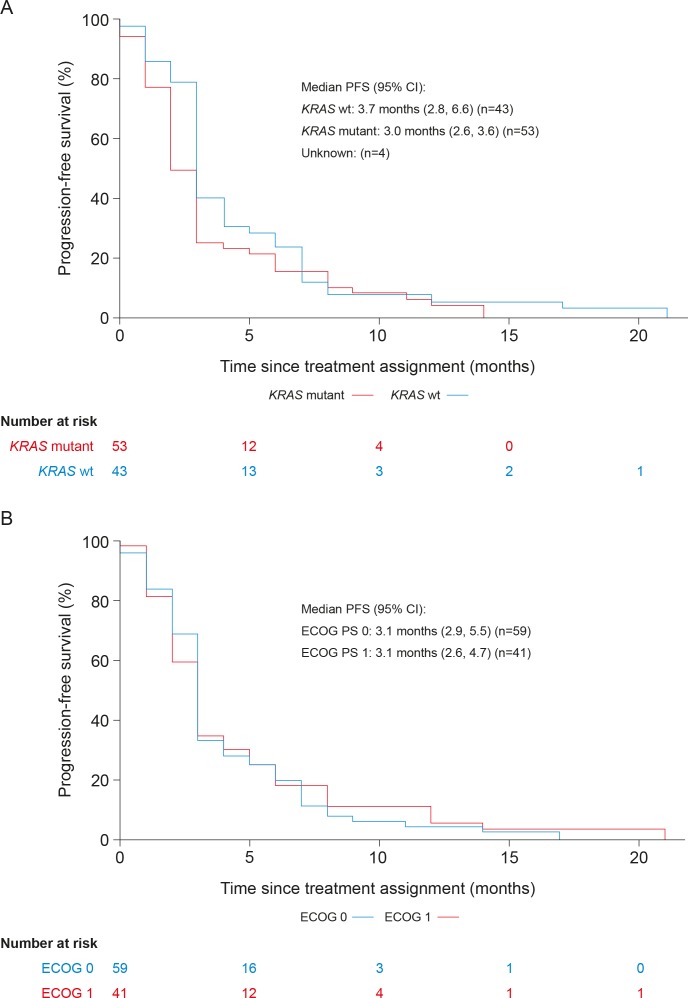
The median PFS (95% CI) for all patients was 3.1 months (2.9 to 3.8) (figure 1). For patients with KRAS-mutant tumours, the median PFS (95% CI) was 3.0 months (2.6 to 3.6), and for those with KRAS wild-type tumours, it was 3.7 months (2.8 to 6.6) (figure 2a). Among patients with baseline ECOG PS 0 and ECOG PS 1, median PFS (95% CI) was 3.1 months (2.9 to 5.5) and 3.1 months (2.6 to 4.7), respectively (figure 2b). The estimated PFS rate (95% CI) at 6 months was 30% (20% to 40%).
Figure 1.
Kaplan–Meier plot of progression-free survival (full analysis set). PFS, progression-free survival.
Figure 2.
Kaplan–Meier plot of progression-free survival by KRAS mutation status (A) and baseline ECOG PS (B) (full analysis set). ECOG PS, Eastern Cooperative Oncology Group performance status; PFS, progression-free survival; wt, wild type.
Discussion
REGARD is the first study to assess regorafenib in a large number of patients in Turkey with mCRC who had disease progression on standard therapy. The safety results presented here are consistent with the well-established safety profile of regorafenib based on prior prospective studies in patients with mCRC, in which over 3000 patients have been treated.5–7 In addition, the efficacy results of REGARD show that PFS with regorafenib was in the range of that previously reported.5–7
Patients in REGARD were generally similar to regorafenib-treated patients with mCRC in previous studies. The median age of patients in REGARD (56.5 years) was similar to that of regorafenib-treated patients in CONCUR (57.5 years), and slightly younger than that of regorafenib-treated patients in CORRECT (61 years) and CONSIGN (62 years).5–7 A higher proportion of patients in REGARD had ECOG PS 0 (59%) compared with proportions in CORRECT (regorafenib arm, 52%), CONCUR (regorafenib arm, 26%) and CONSIGN (47%). The proportion of patients with liver metastases in REGARD (78%) was similar to that in CONSIGN (77%).7 All patients received prior treatment with targeted agents (bevacizumab, and cetuximab/panitumumab if KRAS wild-type), similar to the patient population of CORRECT.5 Most patients (83%) had been diagnosed with metastatic disease for at least 18 months before starting treatment, similar to regorafenib-treated patients in CORRECT (82%) and CONSIGN (82%).5 7
The duration of regorafenib treatment in REGARD is also consistent with that previously reported. Patients were treated for a median of 2.5 months, slightly longer than the median duration of regorafenib in CORRECT (1.7 months), and similar to that in CONCUR (2.4 months) and CONSIGN (2.5 months). The mean per cent of planned dose in REGARD (87%) was slightly higher than what was observed in CORRECT (79%) and CONSIGN (75%).5 7
Most patients in REGARD (80%) experienced a regorafenib-related TEAE, as was seen in prior studies, and the rate of grade ≥3 regorafenib-related TEAEs (52%) was similar to the rates reported with regorafenib in CORRECT (54%), CONCUR (53%) and CONSIGN (57%).5–7 The most common regorafenib-related TEAEs in REGARD included HFSR, fatigue, anorexia and hypertension, consistent with results from the earlier studies. However, the frequencies of HFSR, fatigue and hypertension were generally lower in REGARD than in CORRECT, CONCUR and CONSIGN.5–7 Since the median duration of regorafenib treatment, the mean daily dose and the per cent of planned dose administered in REGARD were similar to or higher than the values for these parameters in the earlier regorafenib studies, the lower rates of some TEAEs in REGARD were not likely due to patients receiving less treatment.5–7 The observed lower rates may be due to differences in local management practices or to the close follow-up of patients by investigators because REGARD was the first clinical experience with regorafenib in Turkey; no patients from Turkey were randomised or treated in the CORRECT, CONCUR or CONSIGN trials. Other common regorafenib-related TEAEs in REGARD included hyperbilirubinaemia, hypophosphataemia and increased ALT and AST. The incidence rates of laboratory test abnormalities were consistent with the clinical trial experience for regorafenib.8 Overall, no new safety concerns were identified.
Dose modifications were common in REGARD, with 55% of patients having a dose modification due to a regorafenib-related TEAE. However, only 17% permanently discontinued treatment due to a regorafenib-related TEAE, suggesting that dose modifications enabled patients to remain on therapy. This pattern of a high rate of dose modifications and a relatively low rate of treatment-related discontinuations was also reported in previous studies.5–7 9 In REGARD, approximately one-third of patients (30%) had a TEAE leading to dose reduction, and HFSR was the most frequently reported TEAE leading to dose reduction (8% of patients), consistent with what was observed in CORRECT and CONCUR.5 6 No patients in REGARD discontinued treatment due to HFSR.
The frequent use of dose modifications with regorafenib, as was observed in REGARD and in earlier trials, motivated a recent randomised, phase II trial (ReDOS) comparing a dose-escalation strategy with standard regorafenib dosing in patients with mCRC.10 In ReDOS, a higher proportion of patients in the dose-escalation group (who started regorafenib at 80 mg daily and increased the daily dose by 40 mg per week as tolerated to 160 mg) initiated cycle 3 compared with the proportion who started treatment at the standard 160 mg dose (43% vs 26%, p=0.043), and efficacy results suggested improved overall survival in the dose-escalation group, although the difference was not statistically significant. The results show that administering regorafenib using a dose-escalation strategy is an option, and might allow more patients to remain on treatment and potentially derive clinical benefit.
Unlike in the phase III CORRECT and CONCUR trials, in REGARD, there was no control arm, overall survival was not assessed and PFS was based on investigator evaluation with the frequency of assessments and the response criteria defined by each institution’s best standard of care. REGARD was designed as a single-arm study primarily to assess safety because prior to its initiation, the randomised, controlled CORRECT trial showed that regorafenib significantly improved overall survival compared with placebo.5 The lack of standardised PFS assessments across study sites in REGARD and the lack of an overall survival endpoint limit interpretation of the efficacy results. However, the median PFS observed in REGARD (3.1 months) was in the range of median PFS values reported in several other trials of regorafenib in mCRC: CORRECT (1.9 months); CONCUR (3.2 months); CONSIGN (2.7 months), the large (N=2864) international trial with a study design similar to that of REGARD; and CORRELATE (2.9 months), a recent large prospective observational study.5–7 11
In addition to regorafenib, other agents that have shown efficacy in patients with mCRC and disease progression after standard therapy include TAS-102 (trifluridine/tipiracil) and anti-PD-1 immune checkpoint inhibitors.12–15 TAS-102 treatment is associated with haematological adverse events and can be given before or after regorafenib; the optimal sequence for regorafenib and TAS-102 has not been established.1 12 The PD-1 immune checkpoint inhibitors pembrolizumab and nivolumab have shown efficacy in the small percentage of patients with mCRC and high microsatellite instability.13–15 In patients with treatment-refractory mCRC and HER2 gene amplification, which conveys resistance to anti-epidermal growth factor receptor therapy, anti-HER2 agents appear to be active.16 17 However, none of these newer agents are currently approved for the treatment of mCRC in Turkey.
In conclusion, the REGARD study enabled patients in Turkey with mCRC whose disease had progressed on all available standard therapies the opportunity to receive an additional line of treatment prior to market authorisation. The safety and tolerability profile of regorafenib and the PFS observed in this single-arm, open-label study were consistent with previous reports of regorafenib in patients with mCRC. The high rate of dose modifications observed in REGARD is also consistent with prior reports, emphasising the importance of managing treatment-related TEAEs with established dosing recommendations.
Supplementary Material
Acknowledgments
We thank the patients who participated in the REGARD trial and their families. Statistical analysis was provided by Christian Kappeler (Bayer) and Mehmet Berktas (KAPPA). Editorial assistance in the preparation of this manuscript was provided by Jennifer Tobin of OPEN Health Medical Communications (Choice), with financial support from Bayer.
Footnotes
Contributors: Study conception: FD, KO. Study design: FD, ŞY, KO. Data acquisition: FD, ŞY, MB, NFA, IY, MÖ, TE, AS, HŞC, UAS, IOK, PFY. Data assembly and quality control of data and algorithms: KO. Data analysis and interpretation: FD, ŞY, KO. Statistical analysis: FD, KO. Drafting and critical review of the manuscript for important intellectual content: FD, KO, ŞY, MB, NFA, IY, MÖ, TE, AS, HŞC, UAS, IOK, PFY. All authors approved the submission of the manuscript for publication. All authors agree to be accountable for all aspects of the work, including resolving any questions arising concerning the accuracy or integrity of any part of the work.
Funding: This work was supported by Bayer. Bayer provided the study medication and collaborated with the authors to design the study. Bayer worked with the investigators on the collection, analysis and interpretation of the data, and on the preparation of this report. The authors made the final decision to submit the article for publication.
Competing interests: KO is an employee of Bayer HealthCare Pharmaceuticals; ŞY has received personal fees from Bayer, Roche, Sanofi, Amgen, Eli Lilly, Merck Serono, and has received grants from Celgene; FD, MB, NFA, İY, MÖ, TE, AS, HŞC, UAS, IOK and PFY have no conflicts of interest to declare.
Patient consent for publication: Not required.
Ethics approval: The study protocol was approved by a Central Ethics Committee of the Turkish Ministry of Health, consistent with Turkish regulations.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request. Availability of the data underlying this publication will be determined according to Bayer’s commitment to the EFPIA/PhRMA 'Principles for responsible clinical trial data sharing'. This pertains to scope, time point and process of data access. As such, Bayer commits to sharing upon request from qualified scientific and medical researchers patient-level clinical trial data, study-level clinical trial data and protocols from clinical trials in patients for medicines and indications approved in the USA and European Union (EU) as necessary for conducting legitimate research. This applies to data on new medicines and indications that have been approved by the EU and US regulatory agencies on or after 1 January 2014. Interested researchers can use www.clinicalstudydatarequest.com to request access to anonymised patient-level data and supporting documents from clinical studies to conduct further research that can help advance medical science or improve patient care. Information on the Bayer criteria for listing studies and other relevant information is provided in the 'Study sponsors section' of the portal. Data access will be granted to anonymised patient-level data, protocols and clinical study reports after approval by an independent scientific review panel. Bayer is not involved in the decisions made by the independent review panel. Bayer will take all necessary measures to ensure that patient privacy is safeguarded.
References
- 1.National Comprehensive Cancer Network Clinical practice guidelines in oncology (NCCN guidelines): colon cancer. version 4, 2018. Available: http://www.nccn.org/professionals/physician_gls/pdf/colon.pdf [Accessed 30 Sep 2019].
- 2.Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 2016;27:1386–422. 10.1093/annonc/mdw235 [DOI] [PubMed] [Google Scholar]
- 3.Abou-Elkacem L, Arns S, Brix G, et al. Regorafenib inhibits growth, angiogenesis, and metastasis in a highly aggressive, orthotopic colon cancer model. Mol Cancer Ther 2013;12:1322–31. 10.1158/1535-7163.MCT-12-1162 [DOI] [PubMed] [Google Scholar]
- 4.Wilhelm SM, Dumas J, Adnane L, et al. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer 2011;129:245–55. 10.1002/ijc.25864 [DOI] [PubMed] [Google Scholar]
- 5.Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:303–12. 10.1016/S0140-6736(12)61900-X [DOI] [PubMed] [Google Scholar]
- 6.Li J, Qin S, Xu R, et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2015;16:619–29. 10.1016/S1470-2045(15)70156-7 [DOI] [PubMed] [Google Scholar]
- 7.Van Cutsem E, Martinelli E, Cascinu S, et al. Regorafenib for patients with metastatic colorectal cancer who progressed after standard therapy: results of the large, single-arm, open-label phase IIIB CONSIGN study. Oncologist 2019;24:185–92. 10.1634/theoncologist.2018-0072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.STIVARGA (regorafenib) tablets Prescribing information. Whippany, NJ 07981 USA: Bayer HealthCare Pharmaceuticals Inc, 2017. [Google Scholar]
- 9.Falcone A, Van Cutsem E, Sobrero A, et al. Regorafenib dose modifications in patients with metastatic colorectal cancer in the phase III CORRECT study. Eur J Cancer 2013;49:S546. [Google Scholar]
- 10.Bekaii-Saab TS, Ou F-S, Ahn DH, et al. Regorafenib dose-optimisation in patients with refractory metastatic colorectal cancer (ReDOS): a randomised, multicentre, open-label, phase 2 study. Lancet Oncol 2019;20:1070–82. 10.1016/S1470-2045(19)30272-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ducreux M, Petersen LN, Öhler L, et al. Safety and effectiveness of regorafenib in patients with metastatic colorectal cancer in routine clinical practice in the prospective, observational CORRELATE study. Eur J Cancer 2019;123:146–54. 10.1016/j.ejca.2019.09.015 [DOI] [PubMed] [Google Scholar]
- 12.Mayer RJ, Van Cutsem E, Falcone A, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med 2015;372:1909–19. 10.1056/NEJMoa1414325 [DOI] [PubMed] [Google Scholar]
- 13.Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015;372:2509–20. 10.1056/NEJMoa1500596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357:409–13. 10.1126/science.aan6733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol 2017;18:1182–91. 10.1016/S1470-2045(17)30422-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinelli E, Troiani T, Sforza V, et al. Sequential HER2 blockade as effective therapy in chemorefractory, HER2 gene-amplified, RAS wild-type, metastatic colorectal cancer: learning from a clinical case. ESMO Open 2018;3:e000299 10.1136/esmoopen-2017-000299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sartore-Bianchi A, Trusolino L, Martino C, et al. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol 2016;17:738–46. 10.1016/S1470-2045(16)00150-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2018-027665supp001.pdf (984.1KB, pdf)
bmjopen-2018-027665supp002.pdf (67.3KB, pdf)
bmjopen-2018-027665supp003.pdf (52.3KB, pdf)
bmjopen-2018-027665supp004.pdf (52.2KB, pdf)
bmjopen-2018-027665supp005.pdf (65.1KB, pdf)