Abstract
Objective
In 2001, Chinese guidelines for the care of acute myocardial infarction (AMI) included a new recommendation against the routine use of magnesium. We studied temporal trends and institutional variation in the use of intravenous magnesium sulfate in nationally representative samples of individuals hospitalised with AMI in China between 2001 and 2015.
Methods
In an observational study (China PEACE—Retrospective Study) of AMI care, we used a two-stage, random sampling strategy to create a nationally representative sample of 28 208 patients with AMI at 162 Chinese hospitals in 2001, 2006, 2011 and 2015. The main outcome is use of intravenous magnesium sulfate over time.
Results
We identified 24 418 patients admitted for AMI, without hypokalaemia, in the four study years. Over time, there was a significant initial decrease in intravenous magnesium sulfate use, from 32.1% in 2001 to 17.1% in 2015 (p<0.001 for trend). The decline was greater in the Eastern (from 33.3% to 16.5%) and Western (from 34.8% to 17.2%) regions, as compared with the Central region (from 25.9% to 18.1%), with little difference between rural and urban areas. The proportion of hospitals using intravenous magnesium sulfate did not change over time (from 81.3% to 77.9%). The median ORs, representing hospital-level variation, were 6.03 in 2001, 3.86 in 2006, 4.26 in 2011 and 4.72 in 2015. Intravenous magnesium sulfate use was associated with cardiac arrest at admission and receipt of reperfusion therapy, but no hospital-specific characteristics.
Conclusions
Despite recommendations against its use, intravenous magnesium sulfate is used in about one in six patients with AMI in China. Our findings highlight the need for more efficient mechanisms to stop using ineffective therapies to improve patients’ outcomes and reduce medical waste.
Trial registration number
ClinicalTrials.gov (NCT01624883)
Keywords: acute myocardial infarction, magnesium sulfate, quality of healthcare
Strengths and limitations of this study.
This is the first large nationally representative registry demonstrating intravenous magnesium sulfate is still used in about one in six patients with acute myocardial infarction (AMI) in China, despite recommendations against its use since 2000s.
The study assessed the 15-year trend in the use of intravenous magnesium sulfate among patient with AMI in China.
The study first reported both patient level and hospital level resulted in the use of intravenous magnesium sulfate use, which could provide more targeted information for efficient mechanisms to stop using this ineffective therapy.
The study adopted standardised procedures for abstraction of medical records that ensure the reliability of our results in describing the use pattern of magnesium sulfate in the real world.
The very low prevalence of patients with some indications, such as magnesium sulfate deficiency would have little influence on the reliability of the results.
Introduction
The history of intravenous magnesium sulfate use for acute myocardial infarction (AMI) is convoluted. Once lauded in small, early trials as safe and highly effective,1–3 it was later demonstrated to be ineffective, and even harmful, in two large clinical trials (MAGIC [Early adminsitration of intravenous magnesium to high-risk patients with acute myocardial infarction in the Magnesium in Coronaries] and ISIS-4 [Fourth International Study of Infarct Survival]) and in a subsequent meta-analysis.4 5 Beginning in the early 2000s, AMI practice guidelines in the USA have specifically recommended against its routine use (Class III, level of evidence: C).6 7 Similarly, China published guidelines in 2001 recommending against the use of intravenous magnesium sulfate in patients with AMI, except in the setting of hypomagnesaemia or polymorphic ventricular tachycardia.8
Although several studies have evaluated the introduction and uptake of new therapies,9–11 few have examined deadoption of ineffective therapy in clinical practice.12–14 The deadoption of therapy is particularly important because the situation may involve greater resistance and barriers to discontinuing long-standing practices than simply introducing new and promising therapies into practice.15 Characterising the use of magnesium sulfate for AMI in clinical practice offers an opportunity to assess the speed with which providers stop using a therapy when new evidence has overturned prior dogma.
Accordingly, our objectives were to assess the trends and variation of regional and hospital-level use of intravenous magnesium sulfate among patient with AMI using data from the China PEACE—Retrospective AMI Study between 2001 and 2015. These data, from a nationally representative network of hospitals throughout China, provided a unique opportunity to examine the trend for discontinuing routine intravenous magnesium sulfate over time and to describe the variations across hospitals in its discontinuation.
Methods
Design overview
The design and methods of the China PEACE—Retrospective AMI Study have been previously published.16 In addition to a nationally representative sample of patients admitted for AMI in China during 2001, 2006 and 2011 created in the China PEACE—Retrospective AMI Study, we also included a more recent sample of patients admitted in 2015 using the same two-stage random sampling process. Briefly, in the first stage, we identified hospitals using a simple random sampling procedure within five economic-geographic regions: Eastern rural, Central rural, Western rural, Eastern urban and Central/Western urban. We stratified on both location and urban–rural classifications because economic development and clinical capacities differed across these categories. We sampled representative hospitals from 2011 to reflect current practices and used the same hospitals for the 2006, 2001 and 2015 so as to describe temporal trends. In the second stage, we sampled AMI cases from hospital databases in 2001, 2006, 2011 and 2015 using random sampling procedures.
Trained personnel at the national coordinating centres abstracted data from the medical records using standardised data definitions. Data abstraction quality was rigorously monitored by randomly auditing 5% of the medical records, in a process that ensured that the overall variable accuracy exceeded 98%.16 We also obtained information on the organisational learning culture of hospital in 2013 through questionnaires completed by the director and a physician of the Cardiology Department in each participating hospital (see online supplementary appendix).17
bmjopen-2019-033269supp001.pdf (1.3MB, pdf)
The Ethics Committee at the National Center for Cardiovascular Diseases approved the study. All collaborating hospitals either accepted central ethics approval or obtained local ethics approval by their ethics committees. Given the retrospective nature of the data and the lack of personal identifiers, patient-level consent was not required. The study was registered with ClinicalTrials.gov.
Study sample
Among the randomly sampled patients hospitalised for AMI in 2001, 2006, 2011 and 2015, only patients with a definite discharge diagnosis of AMI were included. We were unable to exclude patients with hypomagnesaemia, because magnesium levels were not collected. However, we excluded patients with chart-documented hypokalaemia during their hospitalisation, which could also represent an indication for magnesium repletion. In hospital-level analysis, only hospitals with 10 or more cases in a study year were included.
Variables
Receipt of intravenous magnesium sulfate was ascertained from the medical record. Patient-level characteristics abstracted from the medical records included demographics (age, gender), medical history (hypertension, diabetes, dyslipidaemia, current smoking, and history of myocardial infarction; coronary heart disease; ischaemic stroke; coronary artery bypass grafting; or primary coronary intervention (PCI)), clinical presentation (chest discomfort, heart rate, systolic blood pressure on admission and left bundle branch block on ECG), as well as in-hospital complications (cardiac arrest, cardiogenic shock and acute stroke) and year of hospitalisation (2001, 2006, 2011, 2015). The outcomes included: (1) in-hospital mortality or withdrawal from treatment due to a terminal status at discharge and (2) in-hospital composite of major complications (including death, withdrawal from treatment, reinfarction, shock, ischaemic stroke or congestive heart failure (online supplementary appendix). Hospital characteristics included teaching status, PCI capability, economic geographic regions and urban or rural location.
Organisational learning culture was measured with Learning Organization Survey (LOS-27, an abbreviated version of the original Garvin et al Learning Organization Survey).18 The LOS-27 consists of 27 questions, grouped into seven domains of organisational learning characteristics, including supportive learning environment, time for reflection, leadership that reinforces learning, experimentation, training, knowledge acquisition and performance monitoring.
Statistical analysis
To examine the trends at both the population and hospital levels across different study periods, p-values for trends were reported using the Cochran-Armitage test. We described the hospital-level distribution of the intravenous magnesium sulfate use among the hospitals with at least 10 patients with AMI in the study years. To further understand the hospital-level variation in intravenous magnesium sulfate use, we quantified interhospital variation using the median OR (MOR), by constructing generalised estimating equations in 2001, 2006, 2011 and 2015, respectively. MOR represents the average (median) OR for receiving intravenous magnesium sulfate for two patients with AMI with similar clinical characteristics admitted to two randomly selected hospitals.
To understand the most current pattern in intravenous magnesium sulfate use, we constructed multivariable models using the data from 2015, which also adopted generalised estimating equations to account for the clustering of patients within hospitals. Factors were selected based on clinical judgement and literature review,10 11 including patient and hospital characteristics. All covariates showed in table 1, except those with frequencies below 1%, were included in the multivariable model. We transformed continuous variables (eg, age and heart rate) into categorical variables using clinically meaningful cut-off values, and then created dummy variables. From the multivariable model in 2015, we then computed risk-standardised rates for each hospital separately. The risk-standardised rate was calculated as the ratio of observed to predicted outcomes, multiplied by the overall unadjusted rate, a form of indirect standardisation. Regarding the different dosage of intravenous magnesium sulfate, we conducted a sensitivity analysis to compare patients receiving multiple doses to those receiving a single dose of or no intravenous magnesium sulfate.
Table 1.
Baseline characteristics of using intravenous magnesium sulfate
| Characteristics | Overall, N (%) | Use, n (%) | Non-use, n (%) | P value |
| Patient characteristics | ||||
| Age (years) | 0.234 | |||
| <55 | 5262 (21.5) | 938 (21.3) | 4324 (21.6) | |
| 55–64 | 5821 (23.8) | 1072 (24.4) | 4749 (23.7) | |
| 65–74 | 6989 (28.6) | 1290 (29.4) | 5699 (28.5) | |
| ≥75 | 6346 (26.0) | 1094 (24.9) | 5252 (26.2) | |
| Gender | 0.144 | |||
| Female | 7257 (29.7) | 1346 (30.6) | 5911 (29.5) | |
| Male | 17 161 (70.3) | 3048 (69.4) | 14 113 (70.5) | |
| Hypertension | 12 551 (51.4) | 2247 (51.1) | 10 304 (51.5) | 0.7 |
| Diabetes | 4758 (19.5) | 768 (17.5) | 3990 (19.9) | <0.001 |
| Dyslipidaemia | 1588 (6.5) | 235 (5.3) | 1353 (6.8) | <0.001 |
| Currently smoking | 8084 (33.1) | 1496 (34.0) | 6588 (32.9) | 0.144 |
| Prior ischaemic stroke | 2706 (11.1) | 546 (12.4) | 2160 (10.8) | 0.002 |
| Prior myocardial infarction | 2504 (10.3) | 416 (9.5) | 2088 (10.4) | 0.057 |
| Prior CABG/PCI | 713 (2.9) | 104 (2.4) | 609 (3.0) | 0.016 |
| Chest discomfort | 22 211(91) | 4021 (91.5) | 18 190 (90.8) | 0.161 |
| Left branch block at presentation | 342 (1.4) | 65 (1.5) | 277 (1.4) | 0.624 |
| Cardiac arrest at presentation | 271 (1.1) | 81 (1.8) | 190 (0.9) | <0.001 |
| Cardiogenic shock at presentation | 1436 (5.9) | 279 (6.3) | 1157 (5.8) | 0.145 |
| Acute stroke at presentation | 530 (2.2) | 77 (1.8) | 453 (2.3) | 0.036 |
| Heart rate at presentation, beats per minute | 0.052 | |||
| <50 | 1019 (4.2) | 177(4.0) | 842 (4.2) | |
| 50–110 | 21 760 (89.1) | 3886 (88.4) | 17 874 (89.3) | |
| >110 | 1639 (6.7) | 331 (7.5) | 1308 (6.5) | |
| SBP at presentation, mm Hg | 0.004 | |||
| <120 | 8181 (33.5) | 1565 (35.6) | 6616 (33.0) | |
| 120–139 | 7534 (30.9) | 1299 (29.6) | 6235 (31.1) | |
| 140–159 | 5041 (20.6) | 913 (20.8) | 4128 (20.6) | |
| ≥160 | 3662 (15.0) | 617 (14.0) | 3045 (15.2) | |
| New onset of heart failure | 2506 (10.3) | 569 (12.9) | 1937 (9.7) | <0.001 |
| Medication within 24 hours | ||||
| Aspirin | 13 742 (56.3) | 2688 (61.2) | 11 054 (55.2) | <0.001 |
| ACE inhibitors or angiotensin receptor blockers | 13 662(56.0) | 2541 (57.8) | 11 121 (55.5) | 0.006 |
| β-blockers | 10 051 (41.2) | 1768 (40.2) | 8283 (41.4) | 0.169 |
| Clopidogrel | 10 572 (43.3) | 1845(42.0) | 8727 (43.6) | 0.054 |
| Statins | 13 031 (53.4) | 2398 (54.6) | 10 633 (53.1) | 0.076 |
| Reperfusion therapies | <0.001 | |||
| No reperfusion | 18 720 (76.7) | 3130 (71.2) | 15 590 (77.9) | |
| Fibrinolytic therapy | 3136 (12.8) | 746 (17.0) | 2390 (11.9) | |
| Primary PCI | 2562 (10.5) | 518 (11.8) | 2044 (10.2) | |
| Hospital characteristics | ||||
| Teaching hospital | 19 081 (78.1) | 3462 (78.8) | 15 619 (78.0) | 0.252 |
| PCI-capable hospital | 15 876 (65.0) | 2768 (63.0) | 13 108 (65.5) | 0.002 |
| Hospital level | 0.075 | |||
| Secondary or lower | 9045 (37.0) | 1576 (35.9) | 7469 (37.3) | |
| Tertiary hospital | 15 373 (63.0) | 2818 (64.1) | 12 555 (62.7) | |
| Economic geographic region | 0.01 | |||
| Eastern | 13 614 (55.8) | 2360 (53.7) | 11 254 (56.2) | |
| Central | 5886 (24.1) | 1115 (25.4) | 4771 (23.8) | |
| Western | 4918 (20.1) | 919 (20.9) | 3999 (20.0) | |
| Urban/rural | 0.003 | |||
| Rural | 10 064 (41.2) | 1724 (39.2) | 8340 (41.7) | |
| Urban | 14 354 (58.8) | 2670 (60.8) | 11 684 (58.3) | |
CABG, coronary artery bypass grafting; PCI, primary coronary intervention; SBP, systolic blood pressure.
To compare the outcomes between patients with and without intravenous magnesium sulfate, we applied propensity score matching to adjust differences in observed characteristics between them. We obtained the log odds of the probability that patients received intravenous magnesium sulfate with modelling a function of all the variables in table 1. Then we performed a one-to-one no replacement match between the two groups based on the estimated propensity score. The no intravenous magnesium sulfate patients was matched if patient had the closest score with a randomly selected intravenous magnesium sulfate patient and were considered eligible to match if the estimated logit was within 0.6 SD of the selected intravenous magnesium sulfate patient. This matching interval has been shown to eliminate approximately 90% of the bias in observed confounders (online supplementary appendix).19
For the questionnaire with LOS-27 (online supplementary appendix), we analysed the responses at the hospital level by calculating the average of the two responses to each question. Responses were categorised as positive if they were ≥5 on a 7-point scale or ≥4 on a 5-point scale. We then calculated the positive response rate at each hospital as the proportion of questions that had a positive response by the hospital, and demonstrated the correlations between positive response rate and risk-standardised rate of intravenous magnesium sulfate use in 2015, as well as the reduction in intravenous magnesium sulfate use from 2011 to 2015.
All comparisons were two-sided, with statistical significance defined as p-value less than 0.05. Statistical analysis was done with SAS software, V.9.4, and R software, V.3.3.1.
Patient and public involvement statement
Patients or public were not involved in the development of the study protocol.
Results
Study population
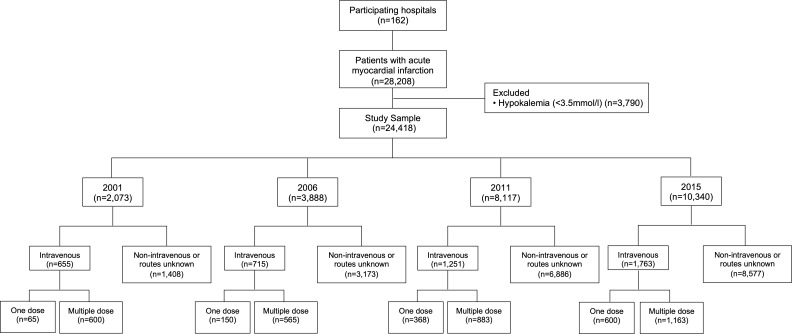
We identified 28 208 patients with AMI in 2001, 2006, 2011 and 2015 admitted to 162 hospitals. After excluding patients with hypokalaemia (<3.5 mmol/L, n=3790), 24 418 patients remained, including 2073 in 2001, 3888 in 2006, 8117 in 2011 and 10 340 in 2015 (figure 1). Almost half (41.2%) of the patients were hospitalised in rural areas. In the study population, the average age was 65.1±12.7 years, 29.7% were female, almost three quarters had at least one cardiac risk factors (hypertension, diabetes, dyslipidaemia or smoking) and about 10% had has a prior myocardial infarction or ischaemic stroke (table 1).
Figure 1.
Flow chart of study cohort. AMI, acute myocardial infarction; IV, intravenous.
Temporal trends and regional variations in intravenous magnesium sulfate use
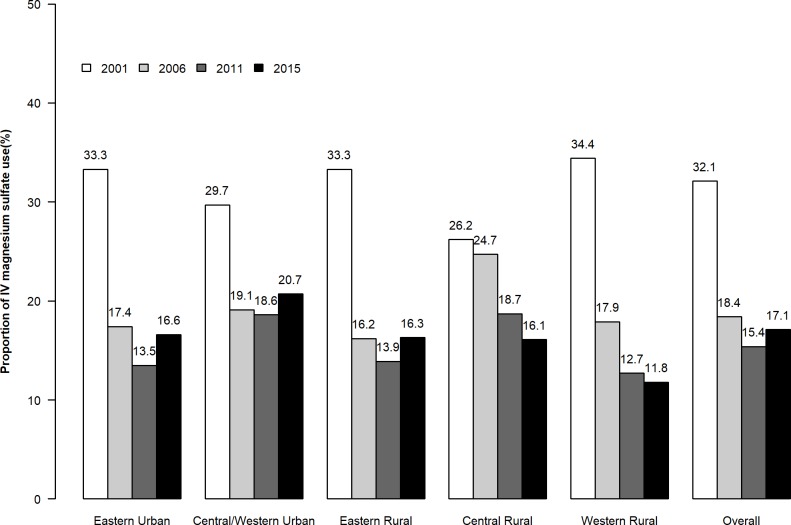
Over time, there was a significant initial decrease in the use of intravenous magnesium sulfate, from 32.1% (665) in 2001 to 18.4% (715) in 2006, 15.4% (1251) in 2011 and 17.1% (1763) in 2015 (p<0.001 for trend) (figure 2). There was significant variation in the temporal trends of use of intravenous magnesium sulfate across the five strata (p<0.001 for interaction). In general, the decline was greater in the Eastern region (16.8% (from 33.3% in 2001 to 16.5% in 2015), p<0.001) and Western region (16.6% (from 34.8% in 2001 to 17.2% in 2015), p<0.001), compared with the Central regions (7.8% (from 25.9% in 2001 to 18.1% in 2015), p<0.001). There was a more modest difference between rural areas (16.3% (from 31.6% to 15.3%), p<0.001) than in urban areas (13.9% (from 32.4% to 18.5%), p<0.001). No significant association was found between the positive response rate of LOS-27 in 2013 and the hospital-level reduction in intravenous magnesium sulfate use from 2011 to 2015 (R2=0.011, p=0.237) (online supplementary appendix).
Figure 2.
Trends of intravenous (IV) magnesium sulfate therapy in 2001, 2006, 2011 and 2015 in five economic-geographic regions.
Hospital-level distributions in intravenous magnesium sulfate use
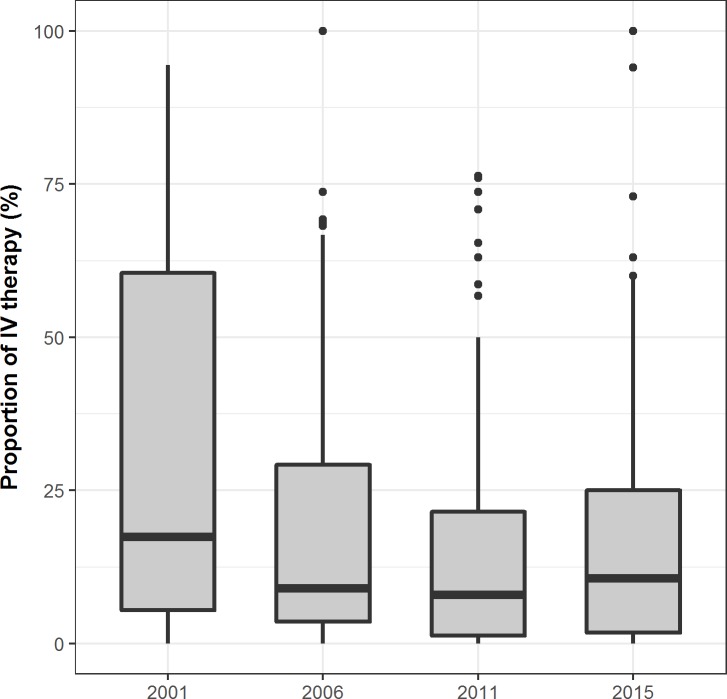
We examined hospital-level rates of intravenous magnesium sulfate use among hospitals with 10 or more cases per year, and observed a downward trend in the median, from 17.4% in 2001, 9.1% in 2006, 8.0% in 2011 to 10.7% in 2015 (figure 3). However, the proportion of hospitals still using magnesium sulfate were 81.3% in 2001, 84.8% in 2006, 76.6% in 2011 and 77.9% in 2015, with no significant decline (p for trend=0.26). Even in 2015, a quarter of hospitals had rates of intravenous magnesium sulfate use exceeding 25%. The MORs (95% CI) of each year characterised similar degrees of hospital-level variation (6.03 (3.93 to 8.52) in 2001, 3.86 (3.00 to 4.77) in 2006, 4.26 (3.38 to 5.20) in 2011 and 4.72 (3.70 to 5.83) in 2015).
Figure 3.
Intravenous (IV) magnesium sulfate use in 2001, 2006, 2011 and 2015 among all hospitals.
Patient and hospital characteristics associated with intravenous magnesium sulfate use
In univariate analysis, patients receiving intravenous magnesium sulfate were more likely to not have diabetes, dyslipidaemia or a prior revascularisation were more likely to have had a prior ischaemic stroke or cardiac arrest at presentation. They were more likely to receive reperfusion therapy, be at urban hospital or be in Central or Western regions (table 1). In the multivariable model, presence of cardiac arrest at admission (OR 3.38, 95% CI 2.50 to 5.82, p<0.001), receipt of aspirin within 24 hours (1.43 (1.22 to 1.67), statin use (1.33 (1.13 to 1.57), reperfusion therapy (1.67 (1.35 to 1.90) for fibrinolytic therapy, 1.69 (1.44 to 1.98) for primary PCI, both p<0.0001) and onset of heart failure (OR 1.69, 95% CI 1.34 to 2.09, p<0.001) were positively associated with intravenous magnesium sulfate use (online supplementary appendix). No significant difference was identified across the teaching status, economic geographic region and rural/urban of hospitals (table 1). The risk-standardised rate of intravenous magnesium sulfate use in 2015 was not associated with the positive response rate of LOS-27 (R2=0.027, p=0.04) (online supplementary appendix).
In-hospital outcomes of patients with and without intravenous magnesium sulfate use
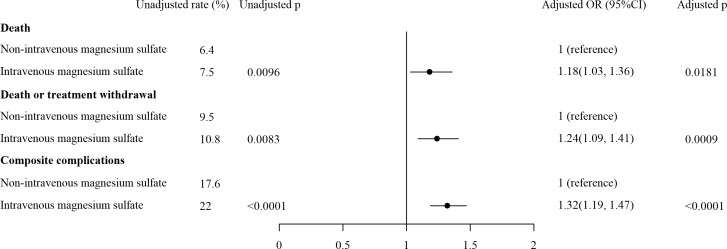
In the patients treated with intravenous magnesium sulfate, the crude rates of in-hospital death (7.5% vs 6.4%) (figure 4), in-hospital death or treatment withdraw (10.8% vs 9.5%) and in-hospital composite of major complications (22.0% vs 17.6%) were higher than in patients without intravenous magnesium sulfate therapy (p<0.01 for all). After adjusted for hospital characteristics, patient risk profiles, medication and reperfusion therapies, using propensity score matching, the patients treated with intravenous magnesium sulfate had still higher risk for in-hospital death (OR 1.18,95% CI 1.03 to 1.36, p=0.01), in-hospital death or treatment withdraw (OR 1.24, 95% CI 1.09 to 1.41, p=0.001) and in-hospital composite of major complications (OR 1.32, 95% CI 1.19 to 1.47, p<0.001).
Figure 4.
In-hospital outcomes between patients with and without intravenous (IV) magnesium sulfate.
Different dose of intravenous magnesium sulfate
We hypothesised that that magnesium sulfate prescribed more than once was more likely to be a routine administration than the single dose that is commonly used for repletion or arrhythmias. Thus, we conducted a sensitivity analysis focusing on multiple doses. The sensitivity analysis showed that there was also a significant decrease in the multiple doses of intravenous magnesium sulfate, from 28.9% in 2001 to 14.5% in 2006, 10.9% in 2011 and 11.31% in 2015 (p<0.001 for trend). Nearly identical predictors of intravenous magnesium sulfate use were found when we compared patients receiving multiple doses with those without receiving intravenous magnesium sulfate (online supplementary appendix).
Discussion
In this large nationally representative study, we found that despite an initial decline in the use of intravenous magnesium sulfate for patients with AMI in China after 2001, about one in six patients continued to be treated with it through 2015. Furthermore, there was substantial variation in the use of intravenous magnesium sulfate use across hospitals. No hospital characteristics were associated with intravenous magnesium sulfate use after adjusting for patient factors, including cardiac arrest and use of reperfusion therapy during hospitalisation.
Our study is the first, to our knowledge, to characterise the rate of deadoption of magnesium sulfate in patients with AMI in China. The only real-word study on the use of magnesium sulfate to treat AMI, which was based on data from the National Registry of Myocardial Infarction (NRMI-2) in the USA, found that the use rate of magnesium sulfate in patients within first 24 hours after AMI was 5.1% in the years 2001–2005 after the US guideline recommended against the use of magnesium sulfate.20 Questionnaire for chief cardiologist from 2500 hospital in China in 1998 revealed that 47% of physician would prescribe magnesium sulfate for patients with AMI.21 In contrast in 2015, threefold more Chinese patients with AMI were receiving magnesium sulfate. This is congruent with a survey among cardiologists in 2012, where over one-fifth reported that they were routinely using magnesium sulfate in patients with acute coronary syndrome.22
Several patient characteristics were identified to be associated with the use of intravenous magnesium sulfate for AMI. It was plausible that the presence of cardiac arrest or reperfusion therapy may spur some physicians to use magnesium sulfate to prevent arrhythmias, according to prior studies in both China and other countries.16 23–26 These explanations, even though not recommended by the guidelines, highlighted the gaps in physicians’ practice and highlights the needs for targeted education in the future.
The hospital-level and regional variations in intravenous magnesium sulfate highlights the marked variability with which different hospitals adopted new evidence about the lack of benefit from intravenous magnesium sulfate use. On the one hand, magnesium sulfate use in 2015 was neither associated with hospital-specific characteristics, nor different across geographic or socioeconomic regions. The teaching status or tertiary level did not translate into the better performance in this measure, which underscores the widespread need for continued education and evaluation of clinical practice. On the other hand, the regional variation in deadoption of magnesium sulfate during the 15-year period seemed not directly related to the regional socioeconomic development status that might be assumed to affect the resources available for acquiring and implementing guideline recommendation. Moreover, no evidence connects organisational learning culture with high performance, even much has been observed in studies of US hospitals.27 Given our findings, more research is needed to better understand current practice patterns that cause some hospitals to still use ineffective therapies.
Our findings raise several questions about the dissemination and implementation of evidence and guidelines in China, particularly regarding education for physicians when long-standing therapies are demonstrated to be non-beneficial, and need to be deadopted. We hypothesised that several factors may explain why the rate of magnesium sulfate use has remained relatively high in China. First, few actions have been taken to disseminate guidelines—after China published the guideline against intravenous magnesium sulfate for AMI in 2001,8 the textbook used in all Chinese medical colleges had not stopped recommending intravenous magnesium sulfate use in patients with AMI until 2009.28 Second, China’s hospital system is short for mechanisms to facilitate the implementation of guideline recommendations, and systematic approaches for monitoring the performance of hospitals and physicians in following the guidelines are lacking in China.29
The successful deadoption of non-beneficial or potentially harmful therapies for corresponding disease, which could reduce costs and potentially prevent complications, requires more than increased efforts from the part of guideline developers.12 After the dissemination of the guideline, more complicated issues need to be addressed, including how to develop tools reminding and alerting physicians when non-recommended therapies are ordered, how to establish a system to report feedback periodically on the appropriateness of treatment by practitioners and hospitals, and how to design an accountability-oriented mechanism to prohibit ineffective regimen being prescribed.30 These issues could only be properly addressed through collaborations with researchers, educators, policy-makers and other stakeholders.31 32
This study has several limitations that warrant consideration. First, we could not exclude patients with some indications, such as hypomagnesaemia and episodes of Torsade de pointes. However, we estimate that the influence is relatively small given low prevalence of these conditions previously reported.33 34 Second, we did not have the ability to prospectively ask clinicians why they were prescribing intravenous magnesium sulfate, which limited our capability to gain better understanding of the use pattern and influencing factors. Third, our data were acquired retrospectively through medical record abstraction. Thus, the quality of our data depends on the accuracy and completeness of prior documentation and abstraction. Nevertheless, the standardised procedures for abstraction of medical records ensure the reliability of our results in describing the use pattern of magnesium sulfate in the real world. Also, we analysed the data at the hospital level and were not able to determine whether the observed patterns were due to only a few physicians or were common throughout a hospital’s staff. Finally, residual confounding of measured or unmeasured variables might affect the observed results about in-hospital outcomes of patients with and without intravenous magnesium sulfate use.
In conclusion, the deadoption of magnesium sulfate for patients with AMI is suboptimal; moreover, the decrease of rate was slowing down recently, and steady at an unacceptably high level. Our findings highlight the need for more efficient mechanisms to translate evidence-based therapies into clinical practice in China to improve patients’ outcomes and reduce medical waste.
Supplementary Material
Acknowledgments
We appreciate the multiple contributions made by study teams at National Clinical Research Center of Cardiovascular Diseases and Yale-New Haven Hospital Center for Outcomes Research and Evaluation in study design and operations, particularly the data collection by Yi Pi, Jiamin Liu, Wuhanbilige Hundei, Haibo Zhang, Lihua Zhang, Wenchi Guan, Xiaofang Yan, Yuan Yu, Xiqian Huo, Xin Zheng and Yuanlin Guo. We appreciate the editing by Aoxi Tian. We are grateful for the support provided by the Chinese government.
Footnotes
Twitter: @hmkyale
XW and XD contributed equally.
Contributors: XL, JL and HMK conceived the China PEACE study and take responsibility for all aspects of it. XW, XD, JL, FAM, JS, JL, HMK and XL designed the study. XW and XD wrote the first draft of the article, with further contributions from HY, EB, ND, JAS, FAM, JL, WG, HMK and XL. SH, YG and XB did statistical analysis. XL had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors interpreted data and approved the final version of the article.
Funding: This project was partly supported by the National Key Research and Development Program (2017YFC1310803, 2015BAI12B01) from the Ministry of Science and Technology of China, the Major Public Health Service Project from the Ministry of Finance and National Health and Family Planning Commission of China, the 111 Project from the Ministry of Education of China (B16005). HMK is supported by grant U01 HL105270-05 (Center for Cardiovascular Outcomes Research at Yale University). The sponsors had no role in the conduct of the study; in the collection, management, analysis and interpretation of the data; or in the preparation or approval of the manuscript.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Extra data are available by emailing xi.li@fwoxford.org.
References
- 1.Horner SM. Efficacy of intravenous magnesium in acute myocardial infarction in reducing arrhythmias and mortality. meta-analysis of magnesium in acute myocardial infarction. Circulation 1992;86:774–9. 10.1161/01.CIR.86.3.774 [DOI] [PubMed] [Google Scholar]
- 2.Teo KK, Yusuf S, Collins R, et al. . Effects of intravenous magnesium in suspected acute myocardial infarction: overview of randomised trials. BMJ 1991;303:1499–503. 10.1136/bmj.303.6816.1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thögersen AM, Johnson O, Wester PO. Effects of magnesium infusion on thrombolytic and non-thrombolytic treated patients with acute myocardial infarction. Int J Cardiol 1993;39:13–22. 10.1016/0167-5273(93)90292-O [DOI] [PubMed] [Google Scholar]
- 4.ISIS-4: a randomised factorial trial assessing early oral captopril, oral mononitrate, and intravenous magnesium sulphate in 58,050 patients with suspected acute myocardial infarction. ISIS-4 (fourth International study of infarct survival) Collaborative group. Lancet 1995;345:669–85. [PubMed] [Google Scholar]
- 5.Magnesium in Coronaries (MAGIC) Trial Investigators Early administration of intravenous magnesium to high-risk patients with acute myocardial infarction in the magnesium in coronaries (magic) trial: a randomised controlled trial. Lancet 2002;360:1189–96. 10.1016/S0140-6736(02)11278-5 [DOI] [PubMed] [Google Scholar]
- 6.Antman EM, Anbe DT, Armstrong PW, et al. . ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction). Circulation 2004;110:588–636. 10.1161/01.CIR.0000134791.68010.FA [DOI] [PubMed] [Google Scholar]
- 7.Ryan TJ, Anderson JL, Antman EM, et al. . ACC/AHA guidelines for the management of patients with acute myocardial infarction. A report of the American College of Cardiology/American heart association Task force on practice guidelines (Committee on management of acute myocardial infarction). J Am Coll Cardiol 1996;28:1328–428. 10.1016/s0735-1097(96)00392-0 [DOI] [PubMed] [Google Scholar]
- 8.China Society of Cardiology of Chinese Medical Association EBoCJoC Guideline for diagnosis and treatment of patients with acute myocardial infarction. Chinese Circulation Journal 2001;16:407–22. [Google Scholar]
- 9.Gambassi G, Forman DE, Lapane KL, et al. . Management of heart failure among very old persons living in long-term care: has the voice of trials spread? the SAGE Study Group. Am Heart J 2000;139:85–93. 10.1016/S0002-8703(00)90313-2 [DOI] [PubMed] [Google Scholar]
- 10.Kim N, Gross C, Curtis J, et al. . The impact of clinical trials on the use of hormone replacement therapy. A population-based study. J Gen Intern Med 2005;20:1026–31. 10.1111/j.1525-1497.2005.0221.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stafford RS, Furberg CD, Finkelstein SN, et al. . Impact of clinical trial results on national trends in alpha-blocker prescribing, 1996-2002. JAMA 2004;291:54–62. 10.1001/jama.291.1.54 [DOI] [PubMed] [Google Scholar]
- 12.Hauptman PJ, Schnitzler MA, Swindle J, et al. . Use of nesiritide before and after publications suggesting drug-related risks in patients with acute decompensated heart failure. JAMA 2006;296:1877–84. 10.1001/jama.296.15.1877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sauro K, Bagshaw SM, Niven D, et al. . Barriers and facilitators to adopting high value practices and de-adopting low value practices in Canadian intensive care units: a multimethod study. BMJ Open 2019;9:e024159 10.1136/bmjopen-2018-024159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niven DJ, McCormick TJ, Straus SE, et al. . Identifying low-value clinical practices in critical care medicine: protocol for a scoping review. BMJ Open 2015;5:e008244 10.1136/bmjopen-2015-008244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niven DJ, Rubenfeld GD, Kramer AA, et al. . Effect of published scientific evidence on glycemic control in adult intensive care units. JAMA Intern Med 2015;175:801–9. 10.1001/jamainternmed.2015.0157 [DOI] [PubMed] [Google Scholar]
- 16.Dharmarajan K, Li J, Li X, et al. . The China patient-centered Evaluative assessment of cardiac events (China peace) retrospective study of acute myocardial infarction: study design. Circ Cardiovasc Qual Outcomes 2013;6:732–40. 10.1161/CIRCOUTCOMES.113.000441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yin ES, Downing NS, Li X, et al. . Organizational culture in cardiovascular care in Chinese hospitals: a descriptive cross-sectional study. BMC Health Serv Res 2015;15:569 10.1186/s12913-015-1211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singer SJ, Moore SC, Meterko M, et al. . Development of a short-form Learning Organization Survey: the LOS-27. Med Care Res Rev 2012;69:432–59. 10.1177/1077558712448135 [DOI] [PubMed] [Google Scholar]
- 19.Xing SG, Rosenbaum PR. Comparison of multivariate matching methods: structures, distances, and algorithms. Journal of Computational & Graphical Statistics 1993;2:405–20. [Google Scholar]
- 20.Ziegelstein RC, Hilbe JM, French WJ, et al. . Magnesium use in the treatment of acute myocardial infarction in the United States (observations from the second National Registry of Myocardial Infarction). Am J Cardiol 2001;87:7–10. 10.1016/S0002-9149(00)01263-7 [DOI] [PubMed] [Google Scholar]
- 21.LX. J, ZM. C, JX. X, et al. . Suvery of hospital of myocardial infarction in China. Linchuang XIn Xue Guan Bing Za Zhi 2002:417–20. [Google Scholar]
- 22.Chen Y, Jiang L, Zhang Q, et al. . Doctor-reported hospital management of acute coronary syndrome in China: a nationwide survey of 1029 hospitals in 30 provinces. World J Cardiovasc Dis 2012;02:168–76. 10.4236/wjcd.2012.23029 [DOI] [Google Scholar]
- 23.Abraham AS, Rosenmann D, Kramer M, et al. . Magnesium in the prevention of lethal arrhythmias in acute myocardial infarction. Arch Intern Med 1987;147:753–5. 10.1001/archinte.1987.00370040135023 [DOI] [PubMed] [Google Scholar]
- 24.Rapaport E, ACC/AHA American College of Cardiology/American Heart Association . Guidelines for the acute coronary syndromes. Curr Cardiol Rep 2001;3:289–96. 10.1007/s11886-001-0082-1 [DOI] [PubMed] [Google Scholar]
- 25.Raghu C, Peddeswara Rao P, Seshagiri Rao D. Protective effect of intravenous magnesium in acute myocardial infarction following thrombolytic therapy. Int J Cardiol 1999;71:209–15. 10.1016/S0167-5273(99)00125-4 [DOI] [PubMed] [Google Scholar]
- 26.Zhou H, Sun M. Suppresion of thrombolysis-induced reperfusion arrhythmias in acute myocardial infarction by magnesium sulfate. Journal of Clinical Cardiology 2000;16:156–8. [Google Scholar]
- 27.Bradley EH, Curry LA, Spatz ES, et al. . Hospital strategies for reducing risk-standardized mortality rates in acute myocardial infarction. Ann Intern Med 2012;156:618–26. 10.7326/0003-4819-156-9-201205010-00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu Z, Zhong N. Internal medicine (the seventh edition. Beijing: People's Medical Publishing House, 2009. [Google Scholar]
- 29.Jiang L, Krumholz HM, Li X, et al. . Achieving best outcomes for patients with cardiovascular disease in China by enhancing the quality of medical care and establishing a learning health-care system. Lancet 2015;386:1493–505. 10.1016/S0140-6736(15)00343-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Niven DJ, Mrklas KJ, Holodinsky JK, et al. . Towards understanding the de-adoption of low-value clinical practices: a scoping review. BMC Med 2015;13:255 10.1186/s12916-015-0488-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carrese JA, Malek J, Watson K, et al. . The essential role of medical ethics education in achieving professionalism: the Romanell report. Acad Med 2015;90:744–52. 10.1097/ACM.0000000000000715 [DOI] [PubMed] [Google Scholar]
- 32.Grimshaw J, Eccles M, Tetroe J. Implementing clinical guidelines: current evidence and future implications. J Contin Educ Health Prof 2004;24 Suppl 1:S31–7. 10.1002/chp.1340240506 [DOI] [PubMed] [Google Scholar]
- 33.Kafka H, Langevin L, Armstrong PW. Serum magnesium and potassium in acute myocardial infarction. Influence on ventricular arrhythmias. Arch Intern Med 1987;147:465–9. [PubMed] [Google Scholar]
- 34.Romano TJ. Serum magnesium levels may not indicate low tissue magnesium levels. Arch Intern Med 1997;157:460 10.1001/archinte.1997.00440250120014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2019-033269supp001.pdf (1.3MB, pdf)