Abstract
Objectives
Symptom Screening in Pediatrics Tool (SSPedi) is a validated approach to measuring bothersome symptoms for English-speaking and Spanish-speaking children with cancer and paediatric haematopoietic stem cell transplantation (HSCT) recipients. Objectives were to translate SSPedi into French, and among French-speaking children receiving cancer treatments, to evaluate understandability and cultural relevance.
Methods
We conducted a multiphase, descriptive study to translate SSPedi into French. Forward translation was performed by four medical translators. After confirming that back translation was satisfactory, we enrolled French-speaking children with cancer and paediatric HSCT recipients at four centres in France and Canada.
Primary and secondary outcome measures
Understandability was evaluated by children themselves who self-reported degree of difficulty, and by two adjudicators who rated incorrectness. Assessment of cultural relevance was qualitative. Participants were enrolled in cohorts of 10.
Results
There were 30 children enrolled. Participants were enrolled from Marseille (n=10, 33%), Ottawa (n=1, 3%), Quebec City (n=11, 37%) and Toronto (n=8, 27%). No child reported that it was hard or very hard to complete French SSPedi in the last cohort of 10 participants. Changes to the instrument itself were not required. After enrolment of 30 respondents, the French translation of SSPedi was considered finalised based on self-reported difficulty with understanding, adjudicated incorrect understanding and cultural relevance.
Conclusions
We translated and finalised SSPedi for use by French-speaking children and adolescents receiving cancer treatments. Future work should begin to use the translated version to conduct research and to facilitate clinical care.
Keywords: paediatric oncology, bone marrow transplantation, paediatric oncology
Strengths and limitations of this study.
Multicentre conduct.
Multiple approaches to assessing understandability.
Use of external adjudicators.
Limited by conduct in only two countries.
Background
Children with cancer and paediatric haematopoietic stem cell transplantation (HSCT) recipients commonly experience severely bothersome symptoms.1–3 The Symptom Screening in Pediatrics Tool (SSPedi) is a reliable and valid approach to measuring bothersome symptoms in English-speaking children 8–18 years of age receiving cancer treatments.4 SSPedi was developed because of the need for a short and simple symptom screening and assessment tool for clinical utilisation in children receiving cancer treatments.5 SSPedi requires about 2–3 min to complete and it includes the following 15 symptoms considered most important to children and their guardians: disappointed or sad, scared or worried, cranky or angry, problems thinking, body or face changes, tiredness, mouth sores, headache, other pain, tingling or numbness, throwing up, hunger changes, taste changes, constipation and diarrhoea. SSPedi also allows children to record additional bothersome symptoms not already listed.
We conducted a multicentre study in Canada and the USA to evaluate the psychometric properties of SSPedi. SSPedi was reliable (internal consistency and test retest and inter-rater reliability), valid (construct validity) and responsive to change in 502 English-speaking children 8–18 years of age receiving cancer therapies.4 More specifically, the intraclass correlation coefficients were 0.88 (95% CI 0.82 to 0.92) for test retest reliability, and 0.76 (95% CI 0.71 to 0.80) for inter-rater reliability between children and parents. Mean difference in SSPedi scores between groups hypothesised to be more and less symptomatic was 7.8 (95% CI 6.4 to 9.2; p<0.001).4 Construct validity was demonstrated as all hypothesised relationships among measures were observed. SSPedi was responsive to change; those who reported they were much better or worse on a global symptom change scale had significantly changed from their baseline score (mean absolute difference 5.6, 95% CI 3.8 to 7.5; p<0.001).
We previously translated SSPedi into Spanish (personal communication, Lillian Sung, 9 January 2020) and clarified the procedures we would adopt generically for SSPedi translation and evaluation. Canada is a bilingual (French and English) country. We therefore next chose to translate SSPedi into French. Objectives were to translate SSPedi into French, and among French-speaking children receiving cancer treatments, to evaluate understandability and cultural relevance of the translation.
Methods
We conducted a multiphase, descriptive study to translate SSPedi into French. Written informed consent and assent were obtained from all study participants. For children providing assent, guardians also provided informed consent.
Translation of SSPedi from English to French
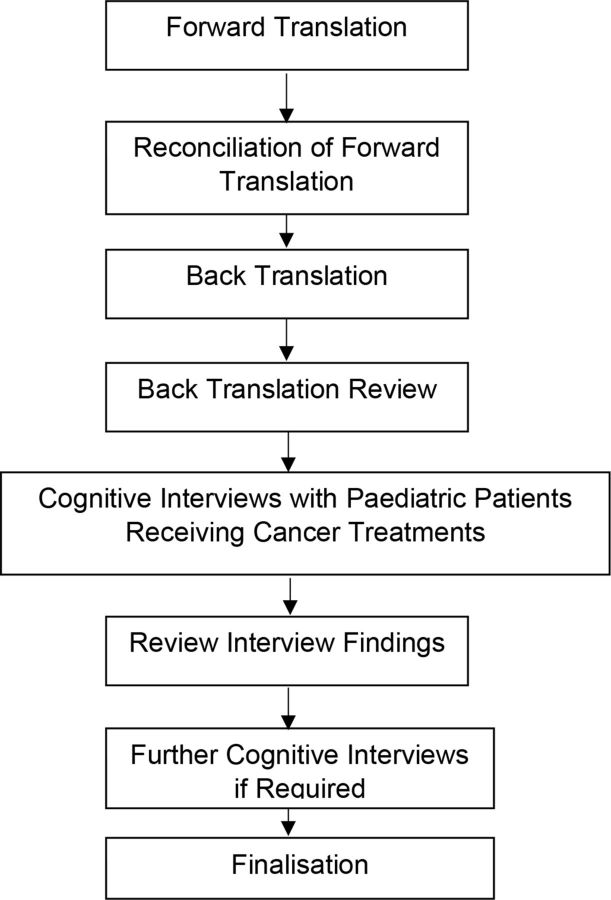
Translation of SSPedi into French included forward translation, reconciliation, back translation and back translation review, as outlined in figure 1. Methods followed the principles for the translation and cultural adaptation process from The Professional Society for Health Economics and Outcomes Research Task Force.6
Figure 1.
Standard approach for translation, validation and finalisation of Symptom Screening in Pediatrics Tool.
We convened a translation panel composed of the Toronto-based research team (RL, EP, LD, LS), the four forward translators and the investigators and interviewers from enrolment sites where the translation was tested (VL, GR-R, DJ, PG, OA). The Toronto-based research team included one paediatric oncologist, one paediatric pharmacist, one clinical research manager and one research student.
The initial forward translation of SSPedi was performed independently by four professional medical translators who are native French speakers. We planned to have two translators from each country in which the translation would be tested. Two translators had previously resided in France while the other two had always resided in Canada. Two were currently residing in Quebec (primary provincial language is French) and two were currently residing in Ontario (primary provincial language is English). In addition to translating SSPedi, the translators also translated the synonym list, which provides alternative words for each SSPedi symptom. The translation panel met through WebEx meetings to reconcile the four forward translations, with the goal of producing a single translated version of the tool. Discrepancies between the translated versions of SSPedi were identified and resolved by consensus, with input from French-speaking investigators.
Once the panel was satisfied with the translated version of the tool, it was sent to a new, independent translator for back translation. The back translation was performed by a bilingual native English-speaker with no previous knowledge of the original English version of SSPedi. The Toronto-based research team verified that the back translation did not contain mistranslations or inaccuracies. Next, this version was approved by all members of the translation panel prior to testing with patients.
Cognitive interviewing to evaluate understandability and cultural relevance
Eligible participants were native French-speaking children with cancer and paediatric HSCT recipients who were 8–18 years of age at the time of the interview. We excluded those who were not able to participate in the interview because of cognitive, visual or hearing limitations as judged by a member of the patient’s healthcare team.
The evaluation of translated SSPedi was performed using in-person interviews. All interviews were conducted by trained personnel who are fluent in the target language. All interviews were audio-recorded and adjudicated by the Toronto-based team. The goals of cognitive interviewing were to determine whether children self-reported that SSPedi items (introduction, response scale and individual symptoms) were hard to understand, whether children were incorrect in their understanding of SSPedi items as adjudicated by an external rater, and whether translated SSPedi was culturally appropriate.
Initially, the child participant or their guardian completed a demographic questionnaire. Next, each participant was given time to complete the translated version of SSPedi in the presence of the interviewer. The entire tool or specific items could be read aloud if requested by the participant. Then the participant was asked how easy or hard SSPedi was to complete overall using a 5-point Likert scale ranging from 1=‘very hard’ to 5=‘very easy’. To assess cultural relevance, the participant was asked whether any of the questions did not make sense to them in thinking about their day-to-day life, as someone living in their country.
Next, the SSPedi instructions and the response options were presented and evaluated separately. The instructions were read aloud and the participant was asked to rate how easy or hard it was to understand them using the same 5-point Likert scale previously described. Next, using cognitive interviewing and prespecified probes, the interviewer assessed whether the participant was correct in their understanding of the instructions and, specifically, the concept of bother. Understanding of the degree of bother, in other words, the response options, was also assessed. Adjudicator-assessed understanding was rated on a 4-point Likert scale ranging from 1=‘completely incorrect’ to 4=‘completely correct’.
Then, each of the 15 SSPedi items was presented and evaluated separately. First, the individual SSPedi item was read aloud. Second, the participant was asked to rate how easy or hard that item was to understand using the same 5-point Likert scale previously described. We focused on the number who rated an item as very hard or hard to understand (score of 1 or 2 on the 5-point scale). Third, using cognitive interviewing and prespecified probes, the interviewer assessed whether the participant was correct in their understanding of each item using the 4-point Likert scale previously described. We focused on the number that were completely or mostly incorrect (score of 1 or 2 on the 4-point scale).
Inevaluable interviews were those where: (1) a participant could not understand the questions posed during cognitive interviews (not the SSPedi items themselves) or (2) the interviewer failed to probe the participant during the cognitive interview (thus not permitting evaluation of understanding). On completion of the interview, the audiotape was sent to Toronto. The Toronto-based adjudicator listened to the transcripts to identify inevaluable interviews and, for evaluable interviews, to independently rate the participant’s extent of understanding of translated SSPedi. Discrepancies between the assessments of the Toronto-based adjudicator and in-country interviewer were resolved by a third Toronto-based reviewer.
The Toronto-based research team met after each group of five interviews were completed to review participant responses and decide whether the translated version of SSPedi or the synonym list of terms required modification. Formal evaluation of outcomes was performed after each cohort of 10 participants and these occurred with the entire translation panel by WebEx meetings. Modification was required when at least two participants among the last cohort of 10 participants: (1) found an item hard or very hard to understand; (2) were completely or mostly incorrect in their understanding of an item; (3) other comments suggested changes were required, including those related to cultural relevance. To be finalised, the translated version of SSPedi must not have required any substantive changes in the last cohort of 10 participants interviewed. There was no attempt to compare findings between French-speaking children from Canada and France.
Patient and public involvement
No patients were involved in study design or conduct apart from being participants in the research.
Results
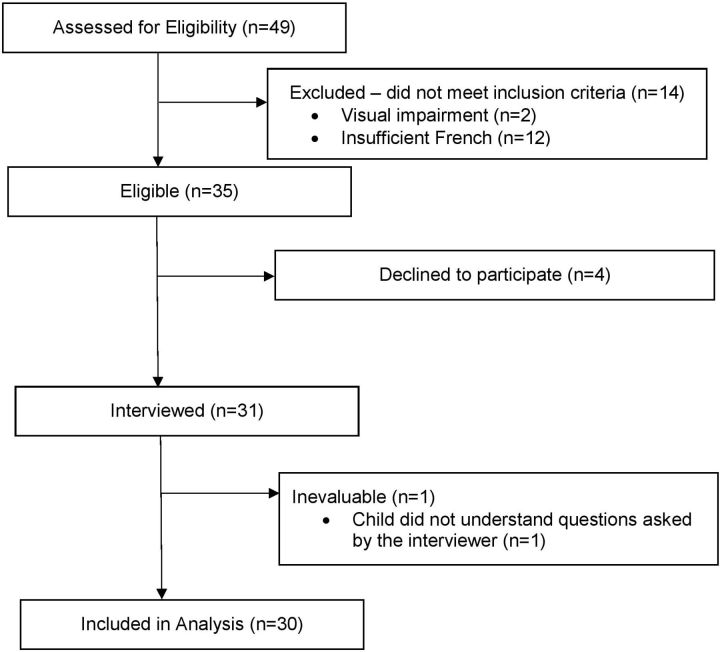
Between 24 September 2018 and 21 June 2019, we identified 49 children and enrolled 30 participants before the French translation of SSPedi was finalised. Figure 2 illustrates the flow diagram of participant identification and enrolment. Table 1 shows the demographic characteristics of the three cohorts of 10 participants enrolled to this study. The number of participants who were 8–10, 11–14 and 15–18 years of age were 8 (27%), 11 (37%) and 11 (37%), respectively. Participants were enrolled from Marseille, France (10, 33%), Ottawa, Canada (1, 3%), Quebec City, Canada (11, 37%) and Toronto, Canada (8, 27%).
Figure 2.
Flow diagram of participant identification and enrolment.
Table 1.
Demographic characteristics of participants evaluating the French translation of SSPedi
| Cohort 1 (n=10) |
Cohort 2 (n=10) |
Cohort 3 (n=10) |
|
| Age in years | |||
| 8–10 | 1 | 3 | 4 |
| 11–14 | 6 | 2 | 3 |
| 15–18 | 3 | 5 | 3 |
| Male sex | 6 | 6 | 7 |
| Diagnosis | |||
| Leukaemia | 2 | 0 | 1 |
| Lymphoma | 2 | 0 | 1 |
| Solid tumour | 3 | 2 | 5 |
| Brain tumour | 3 | 8 | 3 |
| Metastatic disease | 5 | 5 | 3 |
| On active treatment | 6 | 9 | 9 |
| Haematopoietic stem cell transplantation | 1 | 0 | 0 |
| Inpatient at interview | 4 | 5 | 2 |
| Attending school | 9 | 5 | 8 |
| Sites of enrolment | |||
| Marseille, France | 5 | 1 | 4 |
| Ottawa, Canada | 0 | 0 | 1 |
| Québec City, Canada | 0 | 8 | 3 |
| Toronto, Canada | 5 | 1 | 2 |
| Confident speaking French | |||
| Not at all | 0 | 0 | 0 |
| Not very | 0 | 0 | 0 |
| Somewhat | 0 | 1 | 1 |
| Confident | 1 | 2 | 0 |
| Very confident | 9 | 7 | 9 |
| Confident reading French | |||
| Not at all | 0 | 0 | 0 |
| Not very | 0 | 0 | 0 |
| Somewhat | 0 | 2 | 0 |
| Confident | 4 | 4 | 1 |
| Very confident | 6 | 4 | 9 |
SSPedi, Symptom Screening in Pediatrics Tool.
Table 2 shows understandability by SSPedi item in terms of self-reported difficulty with understanding (number finding an item hard or very hard to understand) and adjudicated incorrect understanding (number interpreting an item mostly or completely incorrectly). Changes made during the first two cohorts were only modifications to the synonym list; the instrument itself did not require modification. For the last cohort of 10 participants interviewed, none of the respondents reported that it was hard or very hard to complete French SSPedi overall. One found a single item hard to understand (changes in how your body or face look) and one was incorrect in their understanding of an item (mouth sores). Among all 30 participants, no issues in terms of cultural relevance were raised. None of the participants identified important missing symptoms from SSPedi. The finalised version of the French translation of SSPedi is shown as figure 3.
Table 2.
Self-reported difficulty and rater-adjudicated incorrectness in understanding the French translation of SSPedi
| SSPedi item | Cohort 1 (n=10) |
Cohort 2 (n=10) |
Cohort 3 (n=10) |
|||
| Hard* | Incorrect† | Hard* | Incorrect† | Hard* | Incorrect† | |
| SSPedi instructions | 0 | 0 | 1 | 0 | 0 | 0 |
| SSPedi items | ||||||
| Feeling disappointed or sad | 0 | 0 | 1 | 0 | 0 | 0 |
| Feeling scared or worried | 0 | 0 | 0 | 0 | 0 | 0 |
| Feeling cranky or angry | 1 | 0 | 0 | 0 | 0 | 0 |
| Problems with thinking or remembering things | 0 | 0 | 0 | 0 | 0 | 0 |
| Changes in how your body or face look | 0 | 0 | 1 | 0 | 1 | 0 |
| Feeling tired | 0 | 0 | 0 | 0 | 0 | 0 |
| Mouth sores | 0 | 0 | 1 | 2 | 0 | 1 |
| Headache | 0 | 0 | 0 | 0 | 0 | 0 |
| Hurt or pain (other than headache) | 0 | 0 | 0 | 0 | 0 | 0 |
| Tingly or numb hands or feet | 0 | 0 | 0 | 1 | 0 | 0 |
| Throwing up or feeling like you may throw up | 0 | 0 | 0 | 0 | 0 | 0 |
| Feeling more or less hungry than you usually do | 0 | 0 | 0 | 0 | 0 | 0 |
| Changes in taste | 0 | 0 | 0 | 0 | 0 | 0 |
| Constipation (hard to poop) | 0 | 1 | 0 | 1 | 0 | 0 |
| Diarrhoea (watery, runny poop) | 0 | 0 | 0 | 0 | 0 | 0 |
| Response scale | NA | 0 | NA | 0 | NA | 0 |
*How hard or easy each section was to understand as rated by participants—the number who rated the section as hard or very hard to understand is shown.
†Participant understanding of each section as rated by the in-country interviewer and a Toronto-based adjudicator—the number who were rated as mostly or completely incorrect is shown
NA, not assessed; SSPedi, Symptom Screening in Paediatrics Tool.
Figure 3.
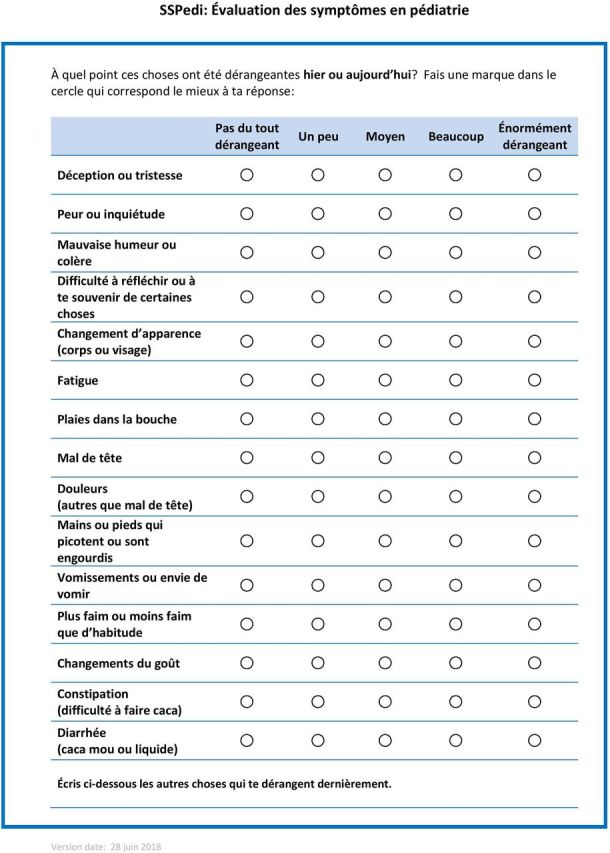
French translation of Symptom Screening in Pediatrics Tool (SSPedi).
Discussion
We reported the process for translating and evaluating the French version of SSPedi. The final version was well-understood by French-speaking children receiving cancer treatments. The translation of patient-reported outcomes to other languages is important to reduce disparities and ensure all children can benefit from approaches to improve quality of life.
We enrolled 30 participants in this study and required that modifications not be required among the last 10 participants evaluating the translated version of SSPedi. Although several instruments have been translated and validated using fewer participants,7–9 we felt it was important to enrol a modest number to increase confidence in the assessment of understandability. We also used at least two adjudicators of understanding to improve the reliability of this assessment.
While translation of a self-report symptom assessment tool for children receiving cancer treatments 8–18 years of age is important, it will also be important to extend translation to other French-speaking respondents. These include proxy-respondents in the setting of children 8–18 years of age with illness acuity or impairments that preclude self-reporting of symptoms. Such an instrument is available in English.10 Similarly, translation of a symptom screening tool for younger children is also important. While such a tool has been developed for children 4–7 years of age,11 it has not yet been validated in English.
The strengths of this research include its multicentre conduct and multiple approaches to assessing understandability. Audio-recording interviews and use of an external adjudicator is another strength that enhances rigour of the research. However, the study is limited by its conduct in only two Francophone countries; evaluation in other French-speaking nations may not necessarily yield the same results. In addition, only one HSCT recipient was included and thus, further evaluation in this population is warranted.
In conclusion, we translated and finalised SSPedi for use by French-speaking children and adolescents receiving cancer treatments. Future work should begin to use the translated version to conduct research and to facilitate clinical care.
Supplementary Material
Acknowledgments
We thank all the translators who worked with us on this project and whose expertise and insights greatly assisted the translation and evaluation process.
Footnotes
Contributors: LD and LS developed the study concept and design. EP, RL, GR-R, PG, OA and J-LT were involved in data collection. LS drafted the manuscript. All authors VL, GR-R, DJ, OA, PG, RL, J-LT, EP, LD and LS participated in data interpretation, reviewed, revised and approved the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication: Not required.
Ethics approval: This study was approved by The Hospital for Sick Children’s Research Ethics Board (#1000057560), the Children’s Hospital of Eastern Ontario Research Ethics Board (18/156X), the CHU de Québec-Université Laval Research Ethics Board (MP-20-2019-4436) and the Committee for the Protection of People at the Hôpital Timone (#2018-A02299-46).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request. The datasets used or analysed during the current study are available from the corresponding author on reasonable request.
References
- 1.Baggott C, Dodd M, Kennedy C, et al. . Changes in children's reports of symptom occurrence and severity during a course of myelosuppressive chemotherapy. J Pediatr Oncol Nurs 2010;27:307–15. 10.1177/1043454210377619 [DOI] [PubMed] [Google Scholar]
- 2.Miller E, Jacob E, Hockenberry MJ. Nausea, pain, fatigue, and multiple symptoms in hospitalized children with cancer. Oncol Nurs Forum 2011;38:E382–93. 10.1188/11.ONF.E382-E393 [DOI] [PubMed] [Google Scholar]
- 3.Pöder U, Ljungman G, von Essen L. Parents' perceptions of their children's cancer-related symptoms during treatment: a prospective, longitudinal study. J Pain Symptom Manage 2010;40:661–70. 10.1016/j.jpainsymman.2010.02.012 [DOI] [PubMed] [Google Scholar]
- 4.Tomlinson D, Dupuis LL, Gibson P, et al. . Initial development of the symptom screening in pediatrics tool (SSPedi). Support Care Cancer 2014;22:71–5. 10.1007/s00520-013-1945-x [DOI] [PubMed] [Google Scholar]
- 5.Dupuis LL, Ethier M-C, Tomlinson D, et al. . A systematic review of symptom assessment scales in children with cancer. BMC Cancer 2012;12:430. 10.1186/1471-2407-12-430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wild D, Grove A, Martin M, et al. . Principles of good practice for the translation and cultural adaptation process for patient-reported outcomes (pro) measures: report of the ISPOR Task force for translation and cultural adaptation. Value Health 2005;8:94–104. 10.1111/j.1524-4733.2005.04054.x [DOI] [PubMed] [Google Scholar]
- 7.Beck I, Olsson Möller U, Malmström M, et al. . Translation and cultural adaptation of the integrated palliative care outcome scale including cognitive interviewing with patients and staff. BMC Palliat Care 2017;16:49. 10.1186/s12904-017-0232-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y, Hinds PS, Wang J, et al. . Translation and linguistic validation of the pediatric patient-reported outcomes measurement information system measures into simplified Chinese using cognitive interviewing methodology. Cancer Nurs 2013;36:368–76. 10.1097/NCC.0b013e3182962701 [DOI] [PubMed] [Google Scholar]
- 9.Mapi Research Institute Linguistic validation of the PedsQL - a Quality of Life Questionnaire. Lyon, France, 2002. [Google Scholar]
- 10.Hyslop S, Dupuis LL, Baggott C, et al. . Validation of the proxy version of symptom screening in pediatrics tool in children receiving cancer treatments. J Pain Symptom Manage 2018;56:107–12. 10.1016/j.jpainsymman.2018.03.025 [DOI] [PubMed] [Google Scholar]
- 11.Tomlinson D, Hyslop S, Stein E, et al. . Development of mini-SSPedi for children 4-7 years of age receiving cancer treatments. BMC Cancer 2019;19:32. 10.1186/s12885-018-5210-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.