Abstract
Background
Distal femur replacement is frequently used for limb salvage after bone tumor resections. It is also used in patients with severe bone loss because of traumatic conditions or revision TKA. Some studies on distal femur replacement reported on revision-free survival without distinguishing between patients with oncologic diagnoses and those without, although these patients might be incomparable because of their differences in important patient- and disease-specific characteristics. This may lead to an inaccurate and undifferentiated interpretation of the results of survival analyses.
Questions/purposes
(1) What is the overall cumulative incidence of revision surgery after cemented and cementless distal femoral replacement, as determined with a competing risk analysis? (2) Does the cumulative incidence of revision surgery change over time? (3) Are there differences in the cumulative incidence of revision surgery between patients with oncologic conditions and those without who are treated with cemented or cementless distal femoral replacement?
Methods
A total of 403 patients were possible candidates for distal femoral replacement. Of these, 56 patients elected to undergo different procedures, 83 were excluded because an expendable growing prosthesis was implanted, and 28 were lost to follow-up. Therefore, 229 patients who underwent distal femoral replacement for oncologic or non-oncologic reasons between 1983 and 2016 were retrospectively included in this study. The type of fixation method (cemented or cementless) was obtained from the patients’ medical records, operation reports, and radiographic analyses from plain radiographs. All radiographs were standardized and obtained at standard time intervals in our institution. No algorithm regarding the fixation approach was followed. According to our data, patients receiving cementless fixation were younger and therefore likely to be more active than those receiving cemented fixation. The median follow-up duration of the overall cohort was 85 months (range 0.1-391 months). Patients who died or had revision surgery before the 2-year minimum follow-up interval were adequately considered using competing risk calculation. The reasons for revision surgery were classified using the classification system proposed by the International Society for Limb Salvage. A competing risk analysis was performed to estimate the cumulative incidence function of revision, accounting for death as a competing event. To evaluate the influence of potential prognostic factors, including diagnosis (oncologic versus non-oncologic), fixation (cemented versus cementless), year of distal femoral replacement, age, and sex on the occurrence of revision surgery, univariate and multivariable Fine and Gray models were applied.
Results
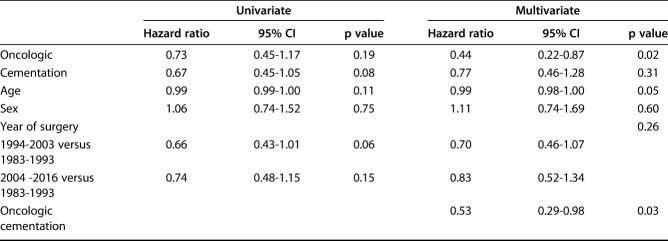
The competing risks analysis revealed cumulative incidences of revision surgery for any cause (Types 1 to 5) of 26% (95% CI, 20.3%-31.9%) at 12 months, 37.9% (95% CI, 31.3%-44.4%) at 24 months, 52.6% (95% CI, 45.1%-59.5%) at 5 years, and 58.2% (95% CI, 50.1%-65.4%) at 10 years for all patients. Rotating hinge-type prostheses showed a lower cumulative incidence of revision surgery (41.6%; 95% CI, 31.8%-51%) than fixed-hinge prostheses did (64%; 95% CI, 50.5%-74.5% ) at 5 years (Gray’s test: p = 0.01). According to the multivariate Fine and Gray model, the year of surgery did not have any effect on the risk of revision surgery (1994 to 2003: hazard ratio 0.70; 95% CI, 0.46-1.07); 2004 to 2016: HR 0.83; 95% CI, 0.52-1.34; p = 0.26). The multivariate analysis, adjusted for disease, sex, age, cementation, and year of surgery, revealed a difference in the risk of revision surgery between patients with oncologic disease and those with non-oncologic disease (HR 0.44 for oncologic versus non-oncologic; 95% CI, 0.22-0.87; p = 0.02) and a reduction in the risk of overall revision with cemented fixation in patients with oncologic disease (HR 0.53; 95% CI, 0.29-0.98; p = 0.03).
Conclusion
This study indicates that even with newer implants, there was a high incidence of revision surgery after distal femoral replacement. According to our analysis, patients with oncologic diagnoses have a lower likelihood of revision when the stem is cemented whereas the type of fixation did not impact patients with non-oncologic diagnoses. Because of differences in patient demographics (age, etiology of disease, and use of chemotherapy) and outcomes of fixation, oncologic and non-oncologic patients should be analyzed separately in survival studies about distal femoral replacement.
Level of Evidence
Level III, therapeutic study.
Introduction
Endoprosthetic replacement of the distal femur is a common procedure for limb salvage in patients who undergo bone resection for neoplastic bone diseases [1, 3, 17–19]. With the use of newer implant designs, in particular the transition from using fixed-hinge to rotating-hinge models, distal femoral replacement can lead to high levels of patient satisfaction and return to some sports activities in patients with bone cancer [17]. The use of distal femoral replacement is not solely restricted to limb salvage after tumor resections. These systems are also used for non-oncologic reasons such as revision arthroplasty after primary TKA in patients with severe bone loss [11, 36] or periprosthetic fractures [14], or after distal femur fractures with traumatic bone loss [4, 12]. Although the evolution of early distal femoral replacement designs to contemporary implants has brought major advantages regarding patient satisfaction and implant longevity, there are still high complication rates [8, 20, 22, 26, 36]. Adequate implant fixation is vitally important for sufficient implant survivorship, but the decision to use cemented or cementless fixation highly depends on patient-specific factors such as age, sex, and diagnosis leading to distal femoral replacement. Therefore, there is still a lack of consensus on which fixation method achieves the best implant survivorship [10]. According to the consensus of the International Society of Limb Salvage Meeting in 2011, Henderson et al. [13] published a classification system to define common types of failure after limb salvage procedures with endoprosthetic reconstruction.
Several publications have reported on the outcome of distal femoral replacement; however, they did not distinguish between patients with oncologic conditions and patients without [15, 16, 22–24, 31, 34, 35, 37], even though these groups differ in important patient- and disease-related factors that might have a substantial impact on surgical decisions and revision-free survival after distal femoral replacement. Competing risk calculation has been shown to be more accurate than conventional survival analysis methods [29]. In contrast to Kaplan-Meier survival analyses, a competing risk calculation includes death as a competing event, which gives a more realistic estimation of the cumulative incidence of revision surgery for patients undergoing distal femoral replacement [29].
Therefore, we asked the following research questions: (1) What is the overall cumulative incidence of revision surgery after cemented and cementless distal femoral replacement, as determined with a competing risk analysis? (2) Does the cumulative incidence of revision surgery change over time? (3) Are there differences in the cumulative incidence of revision surgery between patients with oncologic conditions and those without who are treated with cemented or cementless distal femoral replacement?
Patients and Methods
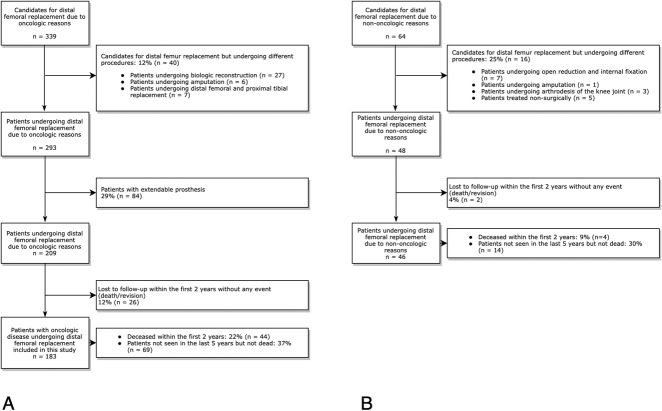
After receiving approval for this study from the local ethics committee, we conducted a retrospective cohort study reviewing all patients undergoing distal femoral replacement at our department for oncologic or non-oncologic reasons from 1983 to 2016. Patients who underwent distal femoral replacement because of tumor resection were identified using the Vienna Bone and Soft Tissue Tumor registry. Initially, 56 of 403 patients who were possible candidates for distal femoral replacement underwent different procedures (Fig. 1). Of 339 patients with oncologic disease who were possible candidates for distal femoral replacement, 12% (40 of 339) were excluded because they chose a different treatment strategy (biologic reconstruction, n = 27; amputation, n = 6; and combined distal femur and proximal tibia replacement, n = 7). Patients receiving an extendable prosthesis for distal femoral replacement (29%, 84 of 293) were also excluded from this study given the inherent higher prevalence of revision surgery for limb lengthening and immaturity of the skeletal system [28]. Twelve percent (26 of 209) of patients were lost to follow-up.
Fig. 1.
This study flowchart shows patients who were excluded because of different therapeutic approaches and loss to follow-up and included patients who underwent distal femoral replacement for (A) oncologic and (B) non-oncologic reasons between 1983 and 2016.
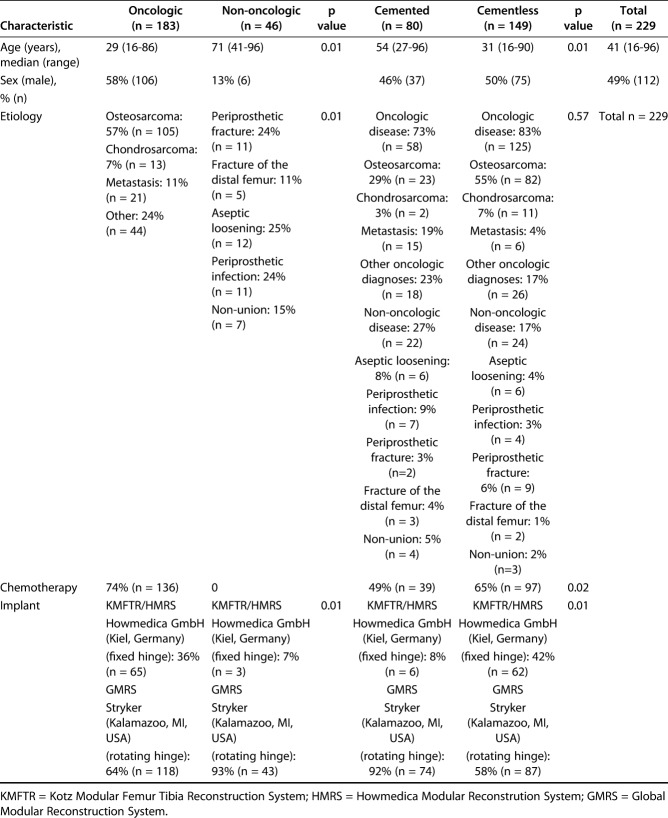
Therefore, we included 183 patients undergoing distal femoral replacement for oncologic reasons who had a minimum follow-up duration of 2 years in this study. Oncologic diagnoses leading to tumor resection and distal femoral replacement were osteosarcoma in 57% (105 of 183), chondrosarcoma in 7% (13), metastatic disease in 11% (21 of 183: renal cell cancer, nine patients; melanoma, three patients; lung cancer, two patients; breast cancer, four patients; prostate cancer, two patients; and colon cancer, one patient), other sarcomas in 19% (35 of 183), giant cell tumor in 3% (five of 183), and hematologic malignancies in 2% (four of 183) (Table 1). Patients with non-oncologic conditions undergoing distal femoral replacement were identified using our electronic hospital database. Twenty-five percent of possible candidates (16 of 64) received an alternative treatment (open reduction and internal fixation, seven patients; amputation, one patient; arthrodesis of the knee joint, three patients; and a non-surgical treatment because the patients’ health was poor, five patients). Two patients were lost to follow-up. Therefore, 46 patients who underwent distal femoral replacement for non-oncologic reasons with a minimum follow-up period of 2 years were included in this study. The reasons for distal femoral replacement in this cohort were a periprosthetic fracture in 24% (11 of 46), fracture of the distal femur in 11% (five of 46), aseptic loosening in 25% (12 of 46), periprosthetic infection in 24% (11 of 46), and non-union after distal femur fracture in 15% (seven of 46) (Table 1).
Table 1.
Patient characteristics distinguished by the reason for distal femoral replacement (oncologic versus non-oncologic) and by the type of fixation of distal femoral replacement (cemented versus cementless)
The median age of the overall cohort (n = 229; oncologic, 183 patients; non-oncologic, 46 patients) was 41 years (range 16 to 96 years). Patients undergoing distal femoral replacement for oncologic reasons (median age 29 years [range 16-86 years]) were younger than patients without oncologic conditions (median age 71 years [range 41-96 years]; p = 0.001) (Table 1). Fifty-one percent (117 of 209) of all patients, 42% (77 of 183) of patients with oncologic disease, and 87% (40 of 46) of patients with non-oncologic condition were women. There were more female than male patients in the non-oncologic cohort (p = 0.001) (Table 1).
The decision regarding whether patients received cemented or cementless distal femoral replacement was not based on a strict algorithm. The type of fixation was chosen for each patient based on patient-specific factors. Because of the long observational period, we could not consistently trace which parameters led to the decision about which fixation method was chosen. However, we strongly believe the indications for cemented or cementless fixation in distal femoral replacement have not changed dramatically in our institution over time. Usually, cementless fixation is preferred in young, active patients and primarily in those with primary bone tumors, whereas cemented fixation is mainly used in older patients with expected poor bone quality or metastatic bone lesions. However, bone quality and patient activity level were not assessed for the entire patient cohort. The type of fixation method (cemented or cementless) was determined by using the patients’ medical records, surgery protocols, and radiographic analyses. Regardless of which distal femoral replacement system was used, it always consisted of a cemented or cementless modular component that fit the metaphyseal and diaphyseal portion of the distal femur.
In our study, the 149 patients receiving cementless fixation were younger (median age 31 years [range 16-55 years]) than the 80 patients with cemented distal femoral replacement (median age: 54 years [range 27-72 years]; p = 0.001). Male and female patients were equally distributed between the cemented and cementless cohorts. Endoprosthetic systems used in this study were a fixed-hinge type of prosthesis (Howmedica Modular Replacement System, Kiel, Germany) and, beginning in 1999, a modified rotating-hinge version of the fixed-hinge type (Global Modular Replacement System, Stryker, Kalamazoo, MI, USA) (Table 1). Both systems underwent several design changes and technical modifications over time. The fixed-hinge system was regularly used until 2002. After 2002, the rotating-hinge type was the main system used for distal femoral replacement.
Revision procedures were defined as any reoperation linked to the previous distal femoral replacement. Complications causing revision surgery were classified with the classification system proposed by the International Society of Limb Salvage [13].
The median follow-up duration in the overall cohort was 85 months (range 0.1-391 months). Thirty-two percent (58 of 183) of oncologic patients and 48% (22 of 46) of non-oncologic patients underwent cemented distal femoral replacement, representing 35% of the overall cohort. Thirty-seven percent (69 of 183) of the patients with oncologic disease and 30% (14 of 46) of the patients with non-oncological disease who were included in this study have not been seen in the past 5 years but are not known to have died in the meantime. During the observation period, 30% of the patients (69 of 229) died after distal femoral replacement, 64 of whom were in the oncologic cohort. One oncologic patient died within 3 days after distal femoral replacement because of a pulmonary embolism. Most patients (42 of 64) died because of oncologic disease. Twenty-one oncologic patients died of unrelated reasons. Of the five non-oncologic patients, one patient died because of a thromboembolic complication. The remaining four patients died of reasons unrelated to distal femoral replacement. According to the International Society of Limb Salvage’s classification system, complications leading to revision surgery during the observation period (1983 to 2016) were soft-tissue failure (Type 1) in 24 patients (16 with oncologic disease), aseptic loosening (Type 2) in 35 patients (30 with oncologic disease), structural failure (Type 3) in 19 patients (17 with oncologic disease), infection (Type 4) in 33 patients (27 with oncologic disease), and tumor progression (Type 5) in five patients. Because the type of distal femoral replacement and the designs of these systems have changed over time, we wanted to observe whether technical modifications have led to a difference in the cumulative incidence of revision surgery. Because of the low number of fixed-hinge prostheses in the group of patients with non-oncologic diagnoses (three), we were only able to perform a competing risk analysis for the type of prosthesis in patients with oncologic disease. Fixed-hinge prostheses showed a higher cumulative incidence of revision than did the rotating-hinge type. The cumulative incidence of revision surgery for fixed-hinge prostheses was 48% (95% CI, 35.3%-59.7%) at 2 years and 64% (95% CI, 50.5%-74.5%) at 5 years, whereas that for the rotating-hinge prosthesis was 29.4% (95% CI, 21.2%-38%) at 2 years and 41.6% (95% CI, 31.8%-51%) at 5 years (Gray’s test: p = 0.01).
Statistical Analysis
The inverse Kaplan-Meier method [27] was used to quantify the median follow-up time. To estimate the cumulative incidence of revision surgery (the primary outcome), a competing risk analysis was performed. This method was used instead of a conventional survival analysis (for example, Kaplan-Meier or Cox proportional hazard regression) to correctly account for the competing event of death, whose occurrence precluded the event of interest (revision surgery). Gray’s test [9] was used to test for statistically significant differences in the cumulative incidence functions for revision surgery between patient groups. To evaluate the influence of potential prognostic factors, including diagnosis (oncologic versus non-oncologic), fixation type (cemented versus cementless), year of distal femoral replacement, age, and sex on the occurrence of revision surgery, univariate and multivariate Fine and Gray [6] models were applied. To test for a potential difference in the effect of the prognostic factor of fixation between patients with an oncologic disease and those without, with respect to the occurrence of revision, the interaction term fixation*group was tested in the multivariate model. Gray’s test was used to calculate differences in the cumulative incidence of revision surgery in patients with oncologic disease regarding their implanted distal femoral replacement design (fixed hinge versus rotating hinge). Only three patients with non-oncologic disease had a fixed hinge-type prosthesis; therefore, this factor was only calculated for the group of patients with oncologic disease. To explore potential differences in the most frequent modes of failure (aseptic loosening and infection) between patient groups, separate competing risks analyses were performed. Death was considered a competing event, as were revision surgeries for other causes of failure. The cumulative incidence functions were estimated, and differences between patient groups with respect to the cumulative incidence functions were tested using Gray’s test. Additionally, separate univariate Fine and Gray models were used to quantify the influence of potential prognostic factors on the occurrence of the two failure reasons (aseptic loosening and infection, respectively).
To evaluate differences between groups of patients, the nonparametric Wilcoxon rank sum test (for continuous variables) and chi-square-test (for binary variables) were applied.
Two-sided p values < 0.05 were considered statistically significant. The statistical analysis was performed using SPSS software, version 23.0 (SPSS Inc., Chicago, IL, USA) and SAS, version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
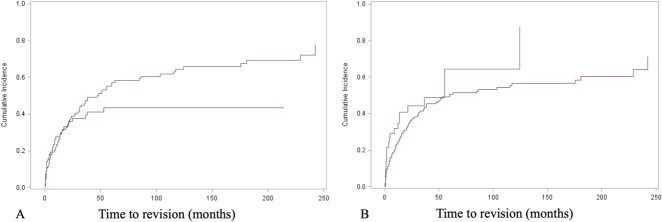
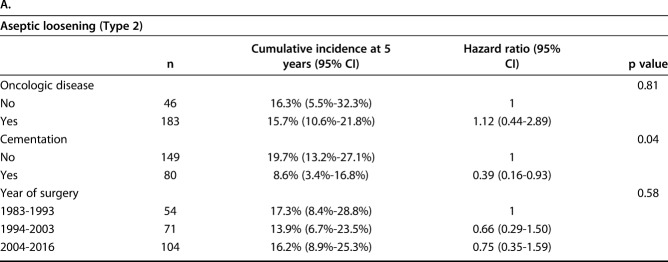
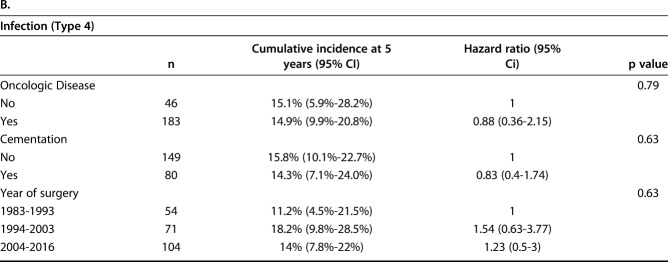
The competing risks analysis revealed cumulative incidences of revision of 26% (95% CI, 20.3%-31.9%) at 12 months, 37.9% (95% CI, 31.3%-44.4%) at 24 months, 52.6% (95% CI, 45.1%-59.5%) at 5 years, and 58.2% (95% CI, 50.1%-65.4%) at 10 years for all patients in this study (Fig. 2). Regarding the implant fixation method, no difference in the cumulative incidence of revision for any reason (Type 2 to Type 5) was found overall between the cemented (36.1%; 95% CI, 25.2%-47% at 2 years; 43.3%; 95% CI, 31.2%-54.9% at 5 years) and cementless cohorts (38.9%; 95% CI, 30.7%-47.% at 2 years; 57.3%; 95% CI, 47.8%-65.6% at 5 years; Gray’s test: p = 0.121) (Fig. 3A). Nevertheless, cementation was associated with a lower cumulative incidence of revision surgery for aseptic loosening (Type 2) than cementless was, with an incidence of 6.9% (95% CI, 2.5%-14.3%) at 2 years and 8.6% (95% CI, 3.4%-16.8%) at 5 years compared with 11.8% (95% CI, 7%-17.9%) and 19.7% (95% CI, 13.2%-27.1%; Gray’s test: p = 0.04) (Table 2A). There was no difference in the cumulative incidence of revision for infection (Type 4) between cemented and cementless fixation (cemented: 10.6%; 95% CI, 4.9%-18.8% at 2 years and 14.3%; 95% CI, 7.1%-24% at 5 years versus cementless: 11.9%; 95% CI, 7.2%-17.8% at 2 years and 15.8%; 95% CI, 10.1%-22.7% at 5 years; Gray’s test: p = 0.76) (Table 2B).
Fig. 2.
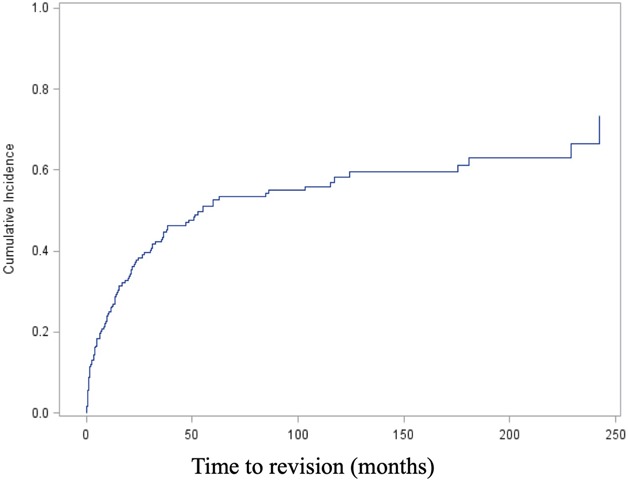
This figure shows a competing risk analysis of the overall cohort, including the cumulative incidence of revision, with death as a competing event.
Fig. 3.
(A) The competing risk analysis revealed no difference in the cumulative incidence of revision between cemented (red line) and cementless fixation (blue line) in the overall cohort (p = 0.121). (B) Additionally, no difference was found between patients with oncologic disease (red line) and those with non-oncologic disease (blue line) (p = 0.134).
Table 2.
The univariate Fine and Gray model was used to detect a potential influence of (A) aseptic loosening and (B) infection on the risk of revision surgery
We found that the cumulative incidence of revision did not vary by date of surgery during the studied time period. Patients undergoing distal femoral replacement were divided in three groups based on their date of surgery (1983 to 1993, 1994 to 2003, and 2004 to 2016). With a cumulative incidence of revision at 5 years of 59% (95% CI, 44.1%-71.2%) from 1983 to 1993, 48.4% (95% CI, 35.6%-60.1%) from 1994 to 2003, and 49.2% (95% CI, 37.3-60.1) from 2004 to 2016, Gray’s test did not reveal differences in the cumulative incidence among the three periods (p = 0.21). Using univariate and multivariate Fine and Gray models (adjusted for disease, age, sex, and cementation), the year of surgery did not have any effect on the risk of revision surgery (univariate: 1994 to 2003: hazard ratio 0.66; 95% CI, 0.43-1.01 versus 2004 to 2016: HR 0.74; 95% CI, 0.4801.12; p = 0.125; multivariate: 1994 to 2003: HR 0.70; 95% CI, 0.46-1.07 versus 2004 to 2016: HR 0.83; 95% CI, 0.52-1.34; p = 0.26) (Table 3).
Table 3.
Univariate and multivariate Fine and Gray model displaying prognostic factors for the risk of revision surgery for any cause (Types 1 to 5) after distal femoral replacement
With the numbers we had, we could not show a difference in the cumulative incidence of revision for any cause (Type 1 to Type 5) between the cohort with oncologic disease (36.4%; 95% CI, 29.2%-43.5% at 2 years; 50.7%; 95% CI, 42.7%-58.2% at 5 years) and the cohort with non-oncologic disease (44.4%; 95% CI, 27.7%-59.8% at 2 years; 64.4%; 95% CI, 36.7%-82.5% at 5 years; Gray’s test: p = 0.134) (Fig. 3B). The multivariate analysis (adjusted for disease, sex, age, cementation, and year of surgery) revealed a difference in the risk of revision surgery between patients with oncologic disease and those without (HR 0.44 for oncologic versus non-oncologic; 95% CI, 0.22-0.87; p = 0.02) (Table 2). Additionally, the multivariate Fine and Gray model yielded an interaction between the two prognostic factors of oncologic disease and cementation (p = 0.03), revealing a reduction in the risk of revision with cemented fixation in patients with oncologic disease (HR 0.53; 95% CI, 0.29-0.98) (Fig. 4). In patients treated for oncologic disease, the cumulative incidence of revision surgery for any cause (Type 1 to Type 5) for the cemented cohort was estimated as 28.3% (95% CI, 17.1%-41%) at 2 years and 35.7% (95% CI, 22.5%-49.1%) at 5 years. In comparison, the cementless cohort had a cumulative incidence of revision of 40.1% (95% CI, 31.2%-48.8%) at 2 years and 57.1% (95% CI, 47.3%-65.8%) at 5 years (Gray’s test: p = 0.012). On the other hand, in patients treated for non-oncologic conditions, no relevant effect of cementation was found (HR 1.79; 95% CI, 0.73-4.40), with no difference between the cemented and non-cemented cohorts in terms of the cumulative incidence of revision at 2 years (cemented: 61.4%; 95% CI, 33.2%-65.8% versus cementless: 29.3%; 95% CI, 11.4%-50%) and at 5 years (cemented: 68.5%; 95% CI, 37.4%-86.5% versus cementless: 76.4%; 95% CI, 3.4%-97.9%; Gray’s test: p = 0.15).
Fig. 4.
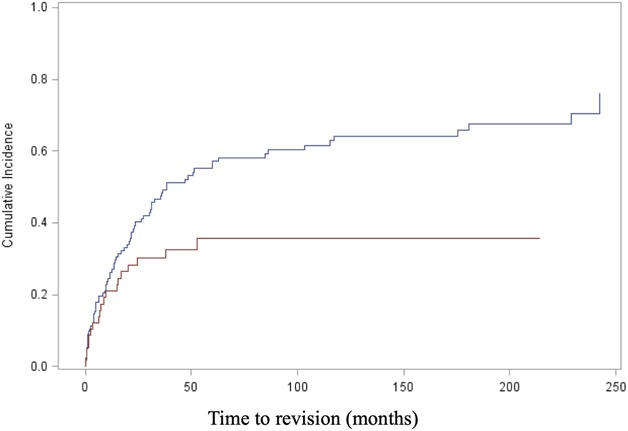
Cementation (red line; cementless = blue line) was associated with a difference in the cumulative incidence of revision surgery for any reason (Type 1 to Type 5) in patients with oncologic disease, as seen in the competing risk analysis (p = 0.012).
Discussion
Distal femoral replacement is often considered the standard therapeutic strategy for limb salvage in bone tumor resections or revision arthroplasties. However, success rates after distal femoral replacement have varied, depending on the statistical method used for calculating survivorship, the observational period, and the patient-specific characteristics of the study cohorts. A number of studies reported on implant survivorship after distal femoral replacement without distinguishing between oncologic and non-oncologic reasons for implantation or the method of implant fixation [15, 23, 24, 34, 35], even though these factors might have a critical impact on the risk of revision surgery and might not reflect the actual implant survivorship for specific patient populations. This study aimed to analyze whether the cumulative incidence of revision surgery has changed over time and to further elucidate whether there are possible differences in the cumulative incidence and risk of revision surgery between patients with oncologic disease and those without who underwent cemented or cementless distal femoral replacement. This study revealed that the incidence of revision surgeries after distal femoral replacement did not decrease dramatically over time. With the use of a multivariate analysis, we were unable to detect differences in the risk of revision surgery between patients with oncologic disease and those without, and we found that implant fixation plays a critical role in these differences, but only in patients treated for oncologic reasons. With the use of the multivariate analysis, we were not able to detect differences in the risk of revision surgery between patients with oncologic disease and those without. According to our results, implant fixation may have a major impact on the risk of revision surgery in patients with oncologic disease.
Limitations
This study has several limitations. This was a retrospective cohort study with a long observational period, and it was prone to substantial selection bias. In this context, indications for distal femoral replacement and the use of cement might have changed over time. Although no specific algorithm for the use of distal femoral replacement is followed, we believe that especially in the non-oncologic setting, indications for distal femoral replacement have changed over time. With the application of metal augments and modern, hinged revision TKA designs, currently, distal femoral replacement might be restricted to treating severe distal bone loss and/or severe osteopenia. The indications for distal femoral replacement for oncologic and non-oncologic diagnoses in this study were heterogenous. For patients with oncologic disease, we included patients with primary malignant bone tumors, metastatic bone disease, and aggressive, benign bone tumors. Not all of these patients received chemotherapy, and life expectancy might have varied dramatically among patients. Because of the long observational period, the investigated treatment strategies and implant designs may not reflect the current standards for treating primary bone tumors and performing revision arthroplasty of the knee. However, analyzing the three time periods (1983 to 1993, 1994 to 2003, and 2004 to 2016) regarding the cumulative incidence of revision surgery did not reveal any differences. Narrowing the observation period may also have decreased the study’s power by reducing the number of included patients. Additionally, the study lacked a potential control group of patients who underwent reconstruction with other methods. However, we believe that by including all indications during the observational period, we increased the generalizability of the results. By using the competing risk analysis, we reduced the risk of misinterpreting our results because of the variability of life expectancy.
Additionally, although we used multivariate statistical models with important patient-related factors such as age, sex, oncologic state, and year of surgery, we were not able to include other important factors such as bone quality or patient activity level because of the retrospective nature of the study. The lack of these important variables needs to be considered to interpret our data. We were also not able to include any functional outcome data or patient-reported outcome measurements because they were not collected from all patients owing to the long observation period of the study. The incidence of patients with oncologic disease who were lost to follow-up was also relatively high (12%). Our department is a specialized center for musculoskeletal oncology and patients from other countries are referred for surgical treatment, but postoperative care and follow-up visits are conducted in the patients’ home countries. Unfortunately, a higher number of patients were lost to follow-up immediately postoperatively.
Overall Survivorship of Cemented and Cementless Distal Femoral Replacement
We found the overall incidence of revision increased over time to 58% at 10 years when both patient groups were included in the competing risk analysis. Cementation did not have any effect on the cumulative incidence of revision surgery for any cause in our overall cohort. However, cemented distal femoral replacements were less frequently revised because of aseptic loosening than cementless endoprostheses in patients who underwent surgery for oncologic indications. With almost 12% of aseptic loosening of cementless implants occurring within the first 2 years, we believe that initial implant fixation or osseointegration was not achieved in patients with oncologic disease. Possible reasons for failed osseointegration in patients with cementless distal femoral replacement are discussed in detail below. Our results are comparable to other reports, especially when patients are not distinguished based on oncologic and non-oncologic diagnoses. Pala et al. [22] described a failure rate of 29.8% after a median of 1.3 years after implantation using the same type of implant in patients with oncologic diagnoses and those with non-oncologic diagnoses. The 5-year implant survival estimate was similar to our results, but the authors did not use a competing risk analysis to calculate survivorship. Toepfer et al. [35] also showed comparable results between patients with oncologic diagnoses and those without. The authors reported a failure rate of 47% after 5 years. Similar to our results, the authors were not able to detect differences between patients with oncologic disease and those without by solely comparing implant survivorship.
Was There a Change in the Incidence of Revisions During the Observation Period (1983 to 2016)?
We found that the cumulative incidence of revision did not vary by date of surgery during the studied time period. Despite the evolution of surgical techniques and designs for distal femoral replacement, we found a high overall complication rate in this long-term observation study. Complications were assessed using the International Society of Limb Salvage’s classification system for modes of failure of limb salvage procedures [13]. The advantage of this system is that it not only summarizes all major complications that might occur after distal femoral replacement, but it also arranges complications in ascending order according to their severity (from Type 1 to Type 5). However, a limitation of this classification system in this particular study is that Type 5 complications (tumor progression) only occurred in the oncologic cohort. Fortunately, Type 5 complications occurred in only 3% of the oncologic cohort and it was therefore the least frequently observed complication. These findings suggest that future studies on this topic should distinguish between oncologic and non-oncologic patients. The rate of aseptic loosening was the highest in the oncologic cohort. In oncologic patients, cementless fixation may be associated with aseptic loosening. Because patients with oncologic indications for distal femoral replacement were generally much younger than patients with non-oncologic indications, patients with high expectations in terms of sports activity may be more likely to receive cementless distal femoral replacement. This leads to greater loading forces that may result in aseptic loosening. Unfortunately, we cannot give any statements regarding sports activity in this cohort. Furthermore, our results show that evolution of the design of the prosthesis from a fixed hinge to a rotating hinge led to a decrease in the cumulative incidence of revision surgery. With the transition from fixed-hinge to rotating-hinge designs, efforts have been made to reduce the risk of bushing wear (Type 3) and aseptic loosening (Type 2) [19]. Unfortunately, with the sample size we had, we were not able to calculate the risk of revision surgery for each complication individually. Despite the beneficial effects of the rotating-hinge design, we were not able to detect a reduction in the risk of revision surgery from 1983 to 2016. We assume that the high revision rate is more likely because of the complexity of the operations and comorbidities and conditions of the patients than because of the endoprosthetic design alone.
Survivorship by Oncologic versus Non-oncologic Indications
The cumulative incidence of revision surgery did not differ between oncologic and non-oncologic patients. However, the multivariate analysis adjusted for important variables such as age, sex, disease (oncologic versus non-oncologic), cementation, and year of surgery clearly showed that patients with oncologic diagnoses have a lower risk of undergoing revision surgery than patients with non-oncologic diagnoses. These findings are surprising because the patients with oncologic disease were younger and tended to be more active, and therefore were expected to have a higher risk of revision surgery than those who did not have oncologic disease. However, because patients undergoing distal femoral replacement for non-oncologic reasons often had undergone several prior revision surgeries, the risk of revision after distal femoral replacement might have been increased.
Additionally, we found that the type of fixation affected the cumulative incidence of revision surgery in patients with oncologic disease, whereas in non-oncologic patients, cementation did not lead to any changes. With the numbers we had and the variables included in our multivariate analysis, we detected a lower cumulative incidence and decreased risk of revision surgery for cemented distal femoral replacement in the oncologic cohort. However, as mentioned, we were not able to include additional essential variables such as bone quality and activity level in our analysis. A possible reason for superior revision-free survival in the cemented oncologic cohort is that chemotherapy negatively influences osseointegration. In a recent study by Elalfy et al. [5], patients treated with chemotherapy had a higher revision rate and impaired new-bone formation in cementless implants with compression fixation. However, several other studies have shown similar negative effects on bone formation after chemotherapy [7, 21, 33]. In particular, Pugh et al. [25] found greater survival and lower revision rates in oncologic patients with cemented endoprostheses than in those with uncemented endoprostheses. They also found a higher revision rate in patients undergoing chemotherapy than in patients without chemotherapy. Myers et al. [19] emphasized using a cemented diaphyseal stem with a hydroxyapatite-coated collar in oncologic patients undergoing distal femoral replacement. Nonetheless, that study investigated a different distal femoral replacement system; therefore, it may be more difficult to compare the implant survivorship with that of the present study. Other reports using the same distal femoral replacement systems had similar observations to our study, despite the use of different classifications of failure mechanisms [19, 32]. Additionally, our results are comparable to the mid- to long-term results of another study using similar distal femoral replacement systems [22] and to those of other institutions using different distal femoral replacement systems [2, 3, 30]. There is still debate about fixation methods in distal femoral replacement. Although we found greater survivorship for cemented distal femoral replacement in patients with oncologic disease, supporting and contradictory results can be found in the reports of others [5, 10, 15, 19]. For example, Hu et al. [15] detected better survival in patients with cementless fixation than in those with cemented fixation. However, unlike our study, the authors did not distinguish between oncologic and non-oncologic patients. We believe there are three possible explanations for these contradictory results. First, the differences in implant survivorship between cemented and uncemented fixation are negligible, and differences in implant survival regarding cementation are only individual study-related phenomena. This might be why some reports on the fixation method in distal femoral replacement could not detect any difference between cemented and cementless fixation [1, 10, 18]. Second, only a few studies have used a competing risks analysis to determine revision-free survival in patients undergoing distal femoral replacement. A competing risks analysis gives a more accurate estimation of revision-free survival because a conventional survival analysis does not adequately consider competing events [29]. Given the high morbidity of these patients, patients who die of their disease or comorbidities are no longer at risk of having complications. Third, owing to the heterogeneity of previous studies, it may not be feasible to compare their results in terms of implant survivorship. Therefore, to decrease confounding factors, the study population should remain as homogenous as possible.
Conclusion
This study indicates that even with improvements in surgical techniques and implant designs, distal femoral replacement still shows a high revision rate and reduced implant survival. Because of differences in patient demographics and the cumulative incidence of revision surgery regarding fixation methods, we cannot make any definitive conclusions, but the implant survival of the cohorts with and without oncologic disease with respect to distal femoral replacement seems comparable. We may have an insufficient number of patients to confirm there is no real difference in revision rates between these two groups, but we recommend that future studies examining the results of distal femoral replacement evaluate these two indications separately.
Acknowledgments
We thank Mrs. Mag. Zettel and Mrs. Gangl for managing data in the Vienna Bone and Soft Tissue Tumor registry.
Footnotes
One author (RW) receives personal fees in an amount more than USD 10,000 from DePuy Synthes (Warsaw, IN, USA) and personal fees in an amount more than USD 10,000 from Stryker (Kalamazoo, MI, USA), outside the submitted work.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at the Medical University of Vienna, Vienna, Austria.
References
- 1.Ahlmann ER, Menendez LR, Kermani C, Gotha H. Survivorship and clinical outcome of modular endoprosthetic reconstruction for neoplastic disease of the lower limb. J Bone Joint Surg Br . 2006;88:790-795. [DOI] [PubMed] [Google Scholar]
- 2.Bhangu AA, Kramer MJ, Grimer RJ, O’Donnell RJ. Early distal femoral endoprosthetic survival: cemented stems versus the Compress® implant. Int Orthop. 2006;30:465-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Capanna R, Scoccianti G, Frenos F, Vilardi A, Beltrami G, Campanacci DA. What was the survival of megaprostheses in lower limb reconstructions after tumor resections? Clin Orthop Relat Res . 2015;473:820-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clement ND, MacDonald D, Moran M, Burnett R, Howie CR, Patton JT. Mega prosthetic distal femoral arthroplasty for non-tumour indications: Does the indication affect the functional outcome and survivorship? Knee Surg Sports Traumatol Arthrosc . 2015;23:1330-1336. [DOI] [PubMed] [Google Scholar]
- 5.Elalfy MA, Boland PJ, Healey JH. Chemotherapy curtails bone formation from compliant compression fixation of distal femoral endoprostheses. Clin Orthop Relat Res . 2019;477:206-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association . 1999;94:496-509. [Google Scholar]
- 7.Friedlaender GE, Tross RB, Doganis AC, Kirkwood JM, Baron R. Effects of chemotherapeutic agents on bone. I. Short-term methotrexate and doxorubicin (adriamycin) treatment in a rat model. J Bone Joint Surg Am . 1984;66:602-607. [PubMed] [Google Scholar]
- 8.Gosheger G, Gebert C, Ahrens H, Streitbuerger A, Winkelmann W, Hardes J. Endoprosthetic reconstruction in 250 patients with sarcoma. Clin Orthop Relat Res . 2006;450:164-171. [DOI] [PubMed] [Google Scholar]
- 9.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat . 1988;16:1141-1154. [Google Scholar]
- 10.Haijie L, Dasen L, Tao J, Yi Y, Xiaodong T, Wei G. Implant survival and complication profiles of endoprostheses for treating tumor around the knee in adults: a systematic review of the literature over the past 30 years. J Arthroplasty. 2018;33:1275-1287. [DOI] [PubMed] [Google Scholar]
- 11.Harrison RJ, Thacker MM, Pitcher JD, Temple HT, Scully SP. Distal femur replacement is useful in complex total knee arthroplasty revisions. Clin Orthop Relat Res . 2006;446:113-120. [DOI] [PubMed] [Google Scholar]
- 12.Hart GP, Kneisl JS, Springer BD, Patt JC, Karunakar MA. Open reduction vs distal femoral replacement arthroplasty for comminuted distal femur fractures in the patients 70 years and older. J Arthroplasty . 2017;32:202-206. [DOI] [PubMed] [Google Scholar]
- 13.Henderson ER, O’Connor MI, Ruggieri P, Windhager R, Funovics PT, Gibbons CL, Guo W, Hornicek FJ, Temple HT, Letson GD. Classification of failure of limb salvage after reconstructive surgery for bone tumours : a modified system Including biological and expandable reconstructions. Bone Joint J . 2014;96:1436–1440. [DOI] [PubMed] [Google Scholar]
- 14.Hoellwarth JS, Fourman MS, Crossett L, Goodman M, Siska P, Moloney GB, Tarkin IS. Equivalent mortality and complication rates following periprosthetic distal femur fractures managed with either lateral locked plating or a distal femoral replacement. Injury 2018;49:392-397. [DOI] [PubMed] [Google Scholar]
- 15.Hu C-C, Chen S-Y, Chen C-C, Chang Y-H, Ueng SW-N, Shih H-N. Superior survivorship of cementless vs cemented diaphyseal fixed modular rotating-hinged knee megaprosthesis at 7 years’ follow-up. J Arthroplasty . 2017;32:1940-1945. [DOI] [PubMed] [Google Scholar]
- 16.Kagan R, Adams J, Schulman C, Laursen R, Espana K, Yoo J, Doung Y-C, Hayden J. What factors are associated with failure of compressive osseointegration fixation? Clin Orthop Relat Res . 2017;475:698-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lang NW, Hobusch GM, Funovics PT, Windhager R, Hofstaetter JG. What sports activity levels are achieved in patients with modular tumor endoprostheses of osteosarcoma about the knee? Clin Orthop Relat Res . 2015;473:847-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mittermayer F, Windhager R, Dominkus M, Krepler P, Schwameis E, Sluga M, Kotz R, Strasser G. Revision of the Kotz type of tumour endoprosthesis for the lower limb. J Bone Joint Surg Br . 2002;84:401-406. [DOI] [PubMed] [Google Scholar]
- 19.Myers GJ, Abudu AT, Carter SR, Tillman RM, Grimer RJ. Endoprosthetic replacement of the distal femur for bone tumours. J Bone Joint Surg Br . 2007;89:521-526. [DOI] [PubMed] [Google Scholar]
- 20.Natarajan MV, Sivaseelam A, Ayyappan S, Bose JC, Sampath Kumar M. Distal femoral tumours treated by resection and custom mega-prosthetic replacement. Int Orthop . 2005;29:309-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishio K, Tanabe A, Maruoka R, Nakamura K, Takai M, Sekijima T, Tunetoh S, Terai Y, Ohmichi M. Bone mineral loss induced by anticancer treatment for gynecological malignancies in premenopausal women. Endocr Connect . 2013;2:11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pala E, Henderson ER, Calabrò T, Angelini A, Abati CN, Trovarelli G, Ruggieri P. Survival of current production tumor endoprostheses: complications, functional results, and a comparative statistical analysis. J Surg Oncol . 2013;108:403-408. [DOI] [PubMed] [Google Scholar]
- 23.Pala E, Trovarelli G, Angelini A, Maraldi M, Berizzi A, Ruggieri P. Megaprosthesis of the knee in tumor and revision surgery. Acta Biomed . 2017;88:129-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pala E, Trovarelli G, Angelini A, Ruggieri P. Distal femur reconstruction with modular tumour prostheses: a single Institution analysis of implant survival comparing fixed versus rotating hinge knee prostheses. Int Orthop . 2016;40:2171-2180. [DOI] [PubMed] [Google Scholar]
- 25.Pugh LR, Clarkson PW, Phillips AE, Biau DJ, Masri BA. Tumor endoprosthesis revision rates increase with peri-operative chemotherapy but are reduced with the use of cemented implant fixation. J Arthroplasty . 2014;29:1418-1422. [DOI] [PubMed] [Google Scholar]
- 26.Ruggieri P, Bosco G, Pala E, Errani C, Mercuri M. Local recurrence, survival and function after total femur resection and megaprosthetic reconstruction for bone sarcomas. Clin Orthop Relat Res . 2010;468:2860-2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials . 1996;17:343-346. [DOI] [PubMed] [Google Scholar]
- 28.Schinhan M, Tiefenboeck T, Funovics P, Sevelda F, Kotz R, Windhager R. Extendible prostheses for children after resection of primary malignant bone tumor. J Bone Joint Surg Am . 2015;97:1585-1591. [DOI] [PubMed] [Google Scholar]
- 29.Schuh R, Kaider A, Windhager R, Funovics PT. Does competing risk analysis give useful information about endoprosthetic survival in extremity osteosarcoma? Clin Orthop Relat Res . 2015;473:900-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwartz AJ, Kabo JM, Eilber FC, Eilber FR, Eckardt JJ. Cemented distal femoral endoprostheses for musculoskeletal tumor: improved survival of modular versus custom implants. Clin Orthop Relat Res . 2010;468:2198-2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scoccianti G, Frenos F, Beltrami G, Campanacci DA, Capanna R. Levels of silver ions in body fluids and clinical results in silver-coated megaprostheses after tumour, trauma or failed arthroplasty. Injury . 2016;47:11-16. [DOI] [PubMed] [Google Scholar]
- 32.Sharma S, Turcotte RE, Isler MH, Wong C. Experience with cemented large segment endoprostheses for tumors. Clin Orthop Relat Res . 2007;459:54-59. [DOI] [PubMed] [Google Scholar]
- 33.Silbermann R, Roodman GD. Bone effects of cancer therapies. Curr Opin Support Palliat Care . 2011;5:251-257. [DOI] [PubMed] [Google Scholar]
- 34.Toepfer A, Harrasser N, Petzschner I, Pohlig F, Lenze U, Gerdesmeyer L, von Eisenhart-Rothe R, Mühlhofer H, Suren C. Is total femoral replacement for non-oncologic and oncologic indications a safe procedure in limb preservation surgery? A single center experience of 22 cases. Eur J Med Res . 2018;23:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toepfer A, Harrasser N, Schwarz P-R, Pohlig F, Lenze U, Mühlhofer HML, Gerdesmeyer L, von Eisenhart-Rothe R, Suren C. Distal femoral replacement with the MML system: a single center experience with an average follow-up of 86 months. BMC Musculoskelet Disord . 2017;18:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vertesich K, Puchner SE, Staats K, Schreiner M, Hipfl C, Kubista B, Holinka J, Windhager R. Distal femoral reconstruction following failed total knee arthroplasty is accompanied with risk for complication and reduced joint function. BMC Musculoskelet Disord . 2019;20:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zimel MN, Farfalli GL, Zindman AM, Riedel ER, Morris CD, Boland PJ, Healey JH. Revision distal femoral arthroplasty with the Compress® prosthesis has a low rate of mechanical failure at 10 years. Clin Orthop Relat Res . 2016;474:528-536. [DOI] [PMC free article] [PubMed] [Google Scholar]