Abstract
Background
To mitigate the possibility of infection after arthroplasty, intraoperative irrigation is essential to remove contaminating bacteria. Previous studies have demonstrated that irrigation with an EDTA solution before wound closure is superior to irrigation with normal saline in removing contaminating bacteria in a rat model of open fractures. However, the effectiveness of an EDTA solution in a model with a contaminated intra-articular implant remains unclear.
Questions/purposes
(1) Does irrigation with an EDTA solution decrease the proportion of culture-positive joints compared with normal saline, benzalkonium chloride, and povidone iodine? (2) Is an EDTA solution toxic to cells resident in joints including chondrocytes, osteoblasts, and synovial fibroblasts? (3) Does irrigation with an EDTA solution have adverse effects including arthrofibrosis and hypocalcemia?
Methods
We first established a model of contaminated intra-articular implants. Female Sprague-Dawley rats (n = 30 for each treatment group) underwent knee arthrotomy and implantation of a femoral intramedullary wire with 1 mm of intra-articular communication. To simulate bacterial contamination, the inserted wire was inoculated with either Staphylococcus aureus or Escherichia coli. After 1 hour, the wound and implant were irrigated with normal saline, benzalkonium chloride, povidone iodine, or an EDTA solution (1 mM). The animals were euthanized 1 week later, and the distal femur, knee capsule, and implanted wire were harvested for bacterial culture using standard techniques. In this study, we used a well-established animal model of an intra-articular implant and inoculated the implant to simulate the clinical setting of intraoperative contamination. The proportion of culture-positive joints in normal saline, benzalkonium chloride, povidone-iodine, and EDTA groups were compared. The viable cell numbers (chondrocytes, osteoblasts, and synovial fibroblasts) were counted and compared after treatment with either solution. Measurement of blood calcium level and histological examination of the joint were performed to rule out hypocalcemia and arthrofibrosis after EDTA irrigation.
Results
With S. aureus inoculation, EDTA irrigation resulted in fewer culture-positive joints than normal saline (37% [11 of 30] versus 70% [21 of 30]; p = 0.019), benzalkonium chloride (83% [25 of 30]; p < 0.001), and povidone iodine (83% [25 of 30]; p < 0.001) irrigation. Likewise, infection rates for implant inoculation with E. coli were also lower in the EDTA irrigation group (13% [four of 30]) than in the normal saline (60% [18 of 30]; p < 0.001), benzalkonium chloride (77% [23 of 30]; p < 0.001), and povidone iodine (80% [24 of 30]; p < 0.001) groups. Between normal saline control and EDTA, there were no differences in cell viability in chondrocytes (normal saline: 98% ± 18%; EDTA: 105% ± 18%; p = 0.127), osteoblasts (normal saline: 102 ± 19%, EDTA: 103 ± 14%; p = 0.835), and synovial fibroblasts (normal saline: 101% ± 21%, EDTA: 110% ± 13%; p = 0.073). EDTA irrigation did not result in hypocalcemia (before irrigation: 2.21 ± 0.32 mmol/L, after irrigation: 2.23 ± 0.34 mmol/L; p = 0.822); and we observed no arthrofibrosis in 30 histologic samples.
Conclusions
In a rat model of a bacteria-contaminated intra-articular implants, intraoperative irrigation with 1 mmol/L of an EDTA solution was superior to normal saline, 0.03% benzalkonium chloride, and 0.3% povidone iodine in preventing surgical-site infection and caused no adverse effects including death of resident cells, arthrofibrosis, and hypocalcemia. Future studies should seek to replicate our findings in other animal models, perhaps such as dog and goat.
Clinical Relevance
If other animal models substantiate the efficacy and safety of the EDTA solution, clinical trials would be warranted to determine whether the use of an EDTA irrigation solution might reduce the risk of periprosthetic joint infections in patients compared with traditional irrigation solutions.
Introduction
Surgical wound irrigation is an effective strategy to prevent and eliminate surgical field contamination because irrigation is able to physically remove contaminating bacteria. The practice of using antiseptic irrigation to eliminate intraoperative contamination has a long history, although the debate as to whether antiseptics are beneficial continues. For instance, there is an international consensus to support the recommendation from the CDC and WHO that advocates the use of povidone iodine instead of normal saline for wound irrigation during surgical procedures [3]. However, recent studies showed that intraoperative povidone iodine irrigation did not lead to a substantial reduction in the risk of periprosthetic infection irrigation after THA and TKA at 3 and 12 months after surgery [9, 10].
Adhesins are a group of bacterial proteins on the cell membrane that establish adhesion with host tissues [4]. Calcium, zinc, and magnesium ions at multiple ion-binding sites of adhesins are required for proper function [12, 21]. EDTA is a clinically-used chelating agent for metal ions that could competitively deprive adhesins of these essential ions [5, 15]. In an animal model of open fracture, irrigation with an EDTA solution increased the removal efficacy of attached bacteria and caused no additional toxicity compared with irrigation with normal saline [23]. As a result, EDTA irrigation reduced the infection risk in contaminated open-fracture wounds and enhanced infection control in infected wounds [23]. Recently, another study investigated the efficacy of EDTA irrigation in implant-exposed wounds involving a variety of bacterial species and consistent results have been reported [6]. Because of its poor vascularity, the joint capsule is more vulnerable to contaminated bacteria than a wound in soft tissue [13]. Thus, it is unknown whether intraoperative EDTA irrigation would also be more effective against bacterial contamination of intra-articular implants.
Therefore, we asked: (1) Does irrigation with an EDTA solution decrease the proportion of culture-positive joints compared with normal saline, benzalkonium chloride, and povidone iodine? (2) Is an EDTA solution toxic to cells resident in joints including chondrocytes, osteoblasts, and synovial fibroblasts? (3) Does irrigation with an EDTA solution have adverse effects including arthrofibrosis and hypocalcemia?
Materials and Methods
Study Overview
In this study, we used a well-established animal model of intra-articular implants [7] and inoculated the implant to simulate the clinical setting of intraoperative contamination. We then irrigated the surgical field, a routine surgical procedure in clinical arthroplasty, with different irrigation solutions to compare their efficacy. Based on a previous report [7], we established a slightly modified model (in an inoculated bacteria amount due to the different strains) of bacterial contamination of an intra-articular implant. Female Sprague-Dawley rats, weighing between 225 g and 275 g and aged between 10 to 12 weeks, were randomly assigned to one of five irrigation treatment groups: control (no irrigation, n = 60), normal saline (n = 60), benzalkonium chloride (n = 60), povidone iodine (n = 60), and an EDTA solution (n = 60). Female rats were chosen because fighting among cage companions is rare in contrast to their male counterparts. This is important because any wounds caused by the fighting might be potential routes for bacterial invasion. Two commonly used, well-characterized laboratory pathogens served as experimental bacterial inoculums of the surgical site and experimental implant. Equal numbers (n = 30) of animals from the five experimental groups received the two bacterial pathogens (Staphylococcus aureus and Escherichia coli). Bacterial bone cultures at the implant site, the inserted implant, and joint capsule were performed 1 week after surgery to determine the presence of periprosthetic joint infection (PJI). All animal experiments were conducted in accordance with protocols approved by the Institutional Animal Care and Use Committee of our hospital. All animals were kept in specific-pathogen-free grade animal facilities. Plastic-base cages bedded with wood shavings housed four rats a piece in this study. Animals were held in a temperature- and humidity-controlled environment (20 to 23° C; 50%) on a 12-hour light-dark schedule. Free access to food and tap water was provided. To investigate the safety of an EDTA solution in intra-articular irrigation, we assessed the cytotoxicity of four investigated irrigation solutions on human chondrocytes, osteoblasts, and synovial fibroblasts. In addition, we examined whether an EDTA irrigation would cause arthrofibrosis and hypocalcemia in our animal model.
Bacterial Inoculum Preparation
Bacteria were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). S. aureus (ATCC 29213) and E. coli (ATCC 25922) served as representative gram-positive and gram-negative pathogens, respectively, in this experiment. We maintained a stock culture of these two bacteria on tryptic soy agar containing 5% sheep blood (BD™ Trypticase™ Soy Agar II [tryptic soy agar II] with 5% sheep blood, Becton Dickinson, Franklin Lakes, NJ, USA), using standard culturing techniques. Fresh cultures of the two bacteria were prepared 24 hours before surgery. Stock solutions of the inoculums were prepared by separately collecting a sample of bacteria from the culture plates using a sterile cotton swab, washing the collected cells three times in normal saline, and adjusting the concentration to 4 × 108 colony-forming units per mL. A standard optical density curve was used to determine the concentration. Each rat would receive a 25 μL of bacterial inoculum (1 × 107 colony forming units). The inoculated volume and concentration were determined upon being able to reliably cause PJI in all rats.
Animal Model and Surgical Procedures
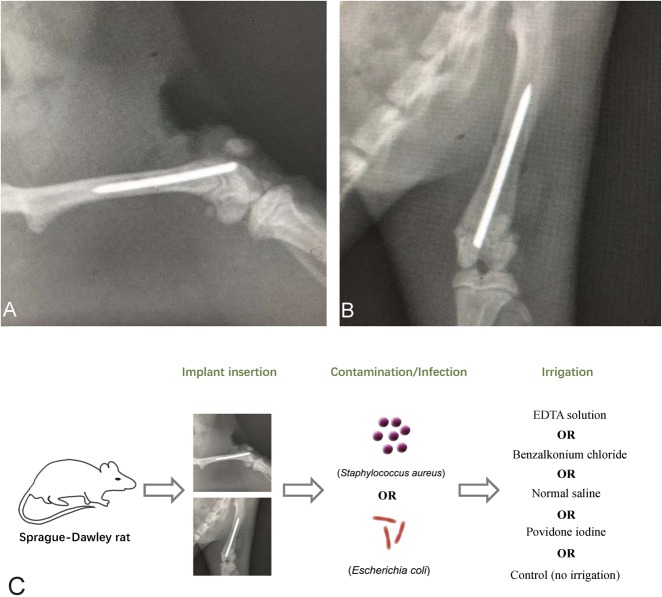
The rats were first anesthetized using 2.5% inhalational isoflurane. Sterile techniques were used throughout. After anesthetization, disinfection of the surgical site (povidone iodine and alcohol swabs), and placement of a sterile drape, a 3-cm longitudinal skin incision was made over the knee. Suprapatellar arthrotomy was performed to expose the articular surface of the distal femur. A 22-gauge sterile needle was inserted into the femoral canal in a retrograde fashion through the intercondylar notch. The tract was then reamed with sequentially larger needles until an 18-gauge needle was successfully inserted. A surgery-grade K-wire (0.088 mm in diameter and 20 mm in length) was inserted into the femoral canal, and 1 mm of the protruding part of the wire was positioned in the knee (Fig. 1). The arthrotomy site was temporarily closed with interrupted 4-0 sutures. Based on our pilot study, to reliably produce a surgical-site infection in this model, each rat was then injected at the surgical site with 25 μL of one of the inoculum solutions (1 × 107 colony forming units), and the pathogens were allowed to attach to the host tissue and implant for 1 hour. The rats remained anesthetized during the waiting period. The surgical site was reopened, and the irrigation procedure was conducted with one of the solutions. After irrigation, the wound was again closed with interrupted 4-0 sutures, and the animals were allowed to recover. All surgical procedures in this study were performed by the same researcher.
Fig. 1.
Representative radiographs show a surgically inserted K-wire in the femoral canal in (A) lateral and (B) AP views. The protruding part of the wire (1 mm) was positioned in the intra-articular prominence of the knee in this rat model. (C) A schematic diagram for model establishment.
Irrigation Solution Preparation and Procedure
Sterile normal saline (0.9%) was obtained from the clinical storage stock at our hospital. The EDTA solution was prepared by dissolving EDTA (ThermoFisher, Waltham, MA, USA) at a concentration of 1 mmol/L in normal saline; the pH was then adjusted to 7.4. The benzalkonium chloride (Macklin, Shanghai, China) solution was prepared by adding 1.76 mL of 17% benzalkonium chloride to 1 L of normal saline (0.03%). The povidone iodine (Merck, Darmstadt, Germany) solution was diluted with normal saline to 0.3% before use. The concentrations are the clinically used ones in our hospital. The surgical site was thoroughly irrigated with a standardized volume (300 mL) of the respective solutions using a 50-mL syringe bulb.
Bacterial Culture and Identification and Blood Cultures
The rats were euthanized 1 week after surgery to harvest surgical-site samples. The knee was prepared and draped in a fashion identical to that done for the initial surgery. We used sterile instruments in all of the following procedures. Surgical-site samples, including samples of the joint capsule, distal femur (20 mm in length from the articular surface), and K-wire, were harvested. Bone, joint capsule and K-wires were transferred separately into three sterile centrifuge tubes after harvesting. We added 3 mL of normal saline solution to each tube. The bone and joint capsule were homogenized with a tissue grinder (Merck, Darmstadt, Germany). K-wires were sonicated to release any adherent bacteria from the wire biofilm. Then, we incubated 30 µL of supernatant (presumably containing surgical-site bacteria, if they existed) on tryptic soy agar containing 5% sheep blood (tryptic soy agar II, Becton Dickinson) at 37° C for 24 hours. Positive results were defined as formation of at least one bacterial colony after 24 hours of culturing. Culture plates were photographically documented using a digital camera. Bacterial colonies were quantified using ImageJ analysis. Bacterial identification was confirmed with 16S ribosomal DNA sequencing. We used a MicroSeq 500 microbial identification system (ThermoFisher) according to the manufacturer’s instruction. Briefly, a bacterial colony was harvested to isolate DNA by suspending the cells in 100 µL of PrepMan™ Ultra Sample Preparation Reagent (ThermoFisher). We then heated the sample for 10 minutes at 100° C and centrifuged the sample to obtain the supernatant. We then amplified the 16S rDNA region with Master Mix (ThermoFisher) and purified extension products with MicroSEQ™ ID Ultra Sequencing-strips Kit (ThermoFisher). The electrophoresis of sample was then performed and analyzed with Applied Biosystems™ 3500 Series Genetic Analyzers (ThermoFisher).
Blood samples were also collected via cardiac puncture when the animals were euthanized to rule out the possibility that the original inoculation caused a systemic infection and that hematogenous bacteria were the source of subsequent intra-articular infection. These samples were cultured and analyzed using the same procedures as those used for the bone and implant experiments.
All harvested bacteria were positively identified as the inoculated strains. No polymicrobial colonization occurred, indicating that there was no contamination from extra-experimental bacteria. All blood culture results were negative, excluding the possibility that surgical-site inoculation inadvertently caused systemic infection and then PJI postoperatively. No postoperative deaths were observed.
Cytotoxicity Assessment of Irrigation Solutions
To assess the cytotoxicity of each solution, we used a Cell Counting Kit-8 (Zomanbio, China) to count cells surviving after treatment with irrigation solution. Human chondrocytes, osteoblasts, and synovial fibroblasts were obtained from the human cell bank of our department. All cell lines were cultured in alpha-minimum essential media (Corning, Corning, NY, USA) supplemented with 10% fetal bovine serum (Gibco, ThermoFisher). Culture media were replaced with fresh media every 2 days. Cells were passaged in a 1:3 ratio once they reached full confluence. The cytotoxicity test was conducted with cells in Passage 3. Cells were then seeded into 96-well plates at a cell density of 2 × 103 cells per well. After attachment, cells were treated with 200 μL of each irrigation solution for 5 minutes and then washed with fresh media three times. After culturing for 12 hours, the cultured media were removed and 10 μL of Cell Counting Kit-8 working solution and 200 μL of fresh media were added to each well. After 3 hours of incubation, the absorbance at 450 nm for each well (positively correlated to the number of viable cells) was read on a spectrophotometer.
Blood Calcium Measurement and Histologic Analysis After EDTA Irrigation
To exclude the possibility that EDTA irrigation can cause hypocalcemia, we measured blood calcium in naïve rats before and after prolonged intra-articular irrigation with an increased volume of EDTA solution (15 minutes, 3L) with a chemical analyzer.
The knees were harvested after 5 days and the sections were fixed with formalin, decalcified, and finally embedded into paraffin blocks. The sections were cut at a 4-μm thickness and attached to slides. Sections were stained with hematoxylin and eosin or toluidine blue using standard protocols. The arthrofibrosis was characterized by the formation of fibrotic scar tissue in the joint [20]. The diagnosis of arthrofibrosis was determined mainly by two independent pathologists in our hospital. If the results given by the two pathologists were inconsistent, the section would be examined by the third pathologist to determine the final result. No inconsistent results were encountered in this study.
Sample Size Calculation, Randomization and Blinding
The sample size was based on Type I error (α) set at 0.05, with an 80% power (1-β). Superiority margin was set at 0.1. According to our pilot study (n = 5 with S. aureus), detection of a 40% difference between EDTA (20%) and normal saline groups (20%) required 28 rats. Considering the possible loss of rats, we finally set the sample size at 30 for each group. Simple randomization was performed in a 1:1:1:1:1 ratio to assign all rats into five treatment groups. Although the groups were blinded to the irrigation procedures, the blinding method was only effective for the normal saline and EDTA groups. The povidone iodine and benzalkonium chloride solution could be identified easily by observation.
Statistical Analyses
Differences in the proportion of positive cultures among the groups were compared using Fisher’s exact test. Significance was evaluated using the nonparametric Wilcoxon rank sum test to compare colony counts between different treatments. SPSS version 24.0 (SPSS Inc, Chicago, IL, USA) was used for statistical analyses. Continuous variables are expressed as the mean ± SD. All statistical significance was defined as p < 0.05.
Results
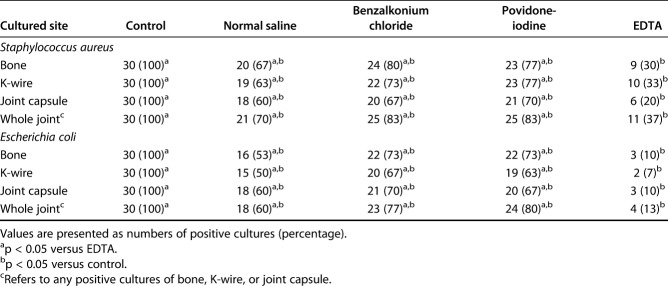
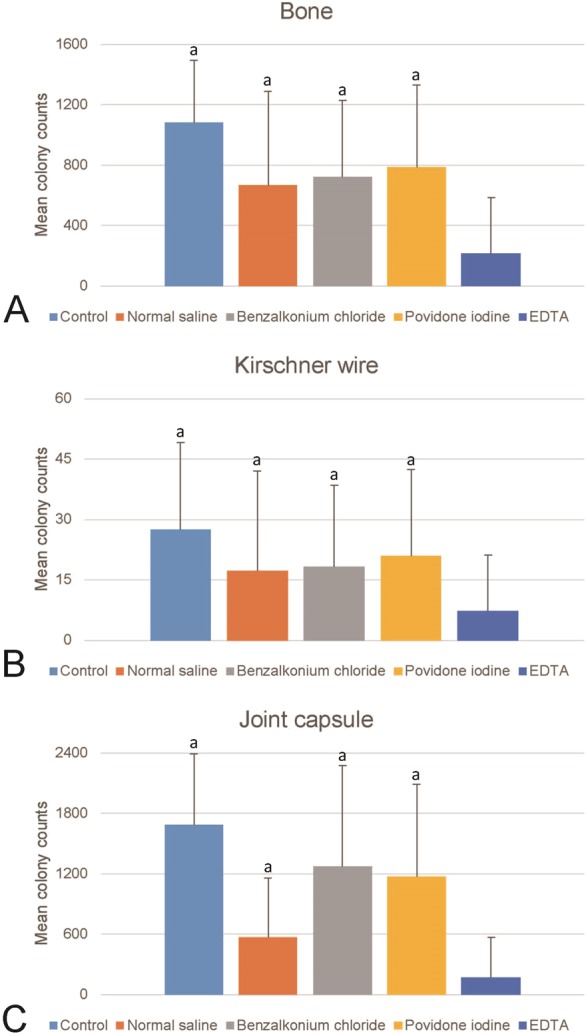
With S. aureus inoculation, EDTA irrigation resulted in fewer positive culture-positive implants than normal saline (37% [11 of 30] versus 70% [21 of 30]; p = 0.019), benzalkonium chloride (83% [25 of 30]; p < 0.001), and povidone iodine (83% [25 of 30]; p < 0.001) irrigation (Table 1). Likewise, infection rates for implant inoculation with E. coli were also lower in the EDTA irrigation group (13% [four of 30]) than in the normal saline (60% [18 of 30]; p < 0.001), benzalkonium chloride (77% [23 of 30]; p < 0.001), and povidone iodine (80% [24 of 30]; p < 0.001) groups. We observed the same pattern of results when we quantified the number of bacterial colonies after implant contamination. Intraoperative EDTA irrigation resulted in lower mean S. aureus colony counts in cultured samples (Fig. 2) than normal saline, benzalkonium chloride, and povidone iodine did (bone: EDTA 217 ± 367, normal saline 666 ± 622, benzalkonium chloride 723 ± 503, povidone iodine 787 ± 545; p < 0.001; K-wire: EDTA 7 ± 14, normal saline 17 ± 25, benzalkonium chloride 18 ± 20, povidone iodine 21 ± 21; p = 0.002; joint capsule: EDTA 168 ± 399, normal saline 567 ± 588, benzalkonium chloride 1268 ± 1004, povidone iodine 1170 ± 914; p < 0.001). As with S. aureus implant contamination, we observed that intraoperative EDTA irrigation was superior to the other irrigation treatments in reducing the number of E. coli colonies from surgical-site samples harvested from joints inoculated with E. coli. The mean E. coli colony counts for EDTA irrigation were lower (Fig. 3) than those of normal saline, benzalkonium chloride, and povidone iodine (bone: EDTA 27 ± 86, normal saline 445 ± 533, benzalkonium chloride 475 ± 483, povidone iodine 488 ± 534; p < 0.001; K-wire: EDTA 1 ± 4, normal saline 16 ± 17, benzalkonium chloride 24 ± 19, povidone iodine 23 ± 16; p < 0.001; joint capsule: EDTA 27 ± 88, normal saline 774 ± 720, benzalkonium chloride 1015 ± 774, povidone iodine 1048 ± 805; p < 0.001).
Table 1.
Proportions of positive cultures by specimen type and treatment group
Fig. 2.
These graphs show the mean colony forming unit counts of cultures derived from surgical-site samples contaminated with S. aureus and irrigated with 300 mL of EDTA solution, normal saline, benzalkonium chloride, or povidone iodine in (A) bone, (B) K-wire, and (C) the joint capsule in this rat model. Error bars indicate the SD. aHigher colony counts than for EDTA (p < 0.05).
Fig. 3.
These graphs shows the mean colony forming unit counts of cultures derived from surgical-site samples contaminated with E. coli and then irrigated with 300 mL of EDTA solution, normal saline, benzalkonium chloride, or povidone iodine in (A) bone, (B) K-wire, and (C) the joint capsule in this rat model. Error bars indicate the SD. aDifferent from EDTA.
Between normal saline control and EDTA, there were no differences in cell viability in chondrocytes (normal saline: 98% ± 18% versus EDTA: 105% ± 18%; p = 0.127) (Fig. 4), osteoblasts (normal saline: 102 ± 19% versus EDTA: 103 ± 14%; p = 0.835), and synovial fibroblasts (normal saline: 101% ± 21% versus EDTA: 110% ± 13%; p = 0.073).
Fig. 4.
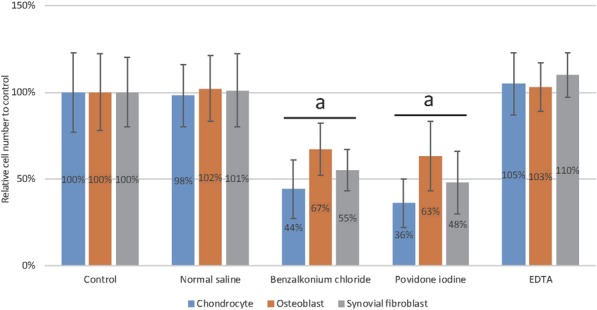
This graph shows the mean percentage of viable human chondrocytes, osteoblasts, and synovial fibroblasts after they were treated with EDTA solution, normal saline, benzalkonium chloride, or povidone iodine. After 12 hours, the viable cells were counted with the Cell Counting Kit-8 and standardized according to control (no treatment). Error bars indicate the SD. The numbers listed on the bars were the mean percentages. aDifferent from control.
EDTA irrigation did not result in hypocalcemia (before irrigation: 2.21 ± 0.32 mmol/L; after irrigation: 2.23 ± 0.34 mmol/L; p = 0.822), and arthrofibrosis was not observed in histologic samples (see Figure, Supplemental Digital Content 1, http://links.lww.com/CORR/A288).
Discussion
PJI is one of the most severe complications of joint arthroplasty [14, 19]. Intraoperative irrigation is an effective strategy to prevent and eliminate surgical field contamination in arthroplasty. Previous animal studies have revealed that EDTA irrigation is superior to normal saline in eliminating contaminating pathogens and preventing infection in open-fracture wounds [6, 23]. However, the intra-articular environment is quite different from an open-fracture wound in terms of resident cell types and the enrichment of blood supply. Thus, the efficacy of EDTA for intra-articular procedures remains unclear. In the present animal study, we modelled an arthroplasty procedure we experimentally contaminated with either E. coli or S. aureus. We showed that irrigation with an EDTA solution is superior to normal saline, benzalkonium chloride, and povidone iodine in reducing the number of positive S. aureus and E. coli cultures derived from surgical-site tissue and implant samples. We then assessed the toxicity of EDTA to the joint-resident cells including chondrocytes, osteoblasts, and synovial fibroblasts and found no additional toxicity to these cells compared with normal saline. In addition, EDTA irrigation did not result in hypocalcemia and arthrofibrosis. These findings set the stage for a larger-animal model, and if EDTA works comparably there as it did in our study, perhaps clinical studies in humans thereafter should be performed to translate these findings into clinical practice.
The current work had several limitations. First, we used benzalkonium and povidone iodine solution at our hospital’s clinically-used concentration. The concentration we used was also identical or close to that adopted by several previous studies [6, 9, 10, 16]. Thus, we believe the concentration we used is clinically representative for these two agents. Second, we used S. aureus and E. coli as pathogens because they represent gram-positive and gram-negative bacteria commonly found in PJI; however, many other pathogens also cause PJI, and the efficacy of EDTA solution might be slightly different for those pathogens. Third, to establish PJI reliably in rats, we inoculated the joint at a dose of 1 × 107 colony forming units which might be much higher than the bacterial level of clinical surgical field. Normal saline, benzalkonium chloride, and povidone iodine might be as effective as EDTA solution in a real-world setting with less bacterial burden. Third, the study was conducted in a rat model, and rats clearly have a different response to bacterial infection compared with humans. Different animal species are not equally susceptible to PJI even after adjusting for their sizes and weights [11]. Similar studies conducted with animals possessing immune systems that are more similar to the human immune system might be an important steppingstone before performing clinical trials with human participants. Finally, we had a relatively short follow-up period for arthrofibrosis. Although a previous study has revealed that arthrofibrosis could develop 5 days after certain stimuli [22], it is still possible that arthrofibrosis might develop in the extended follow-up.
An EDTA solution outperformed antiseptic solutions and normal saline. Antiseptic solutions for irrigation are preferred among some surgeons and the efficacy of these solutions remains controversial mainly because of their toxicity. In an animal model of complex musculoskeletal wounds contaminated with Pseudomonas aeruginosa, the bacterial counts initially decreased but increased to similar or even greater levels after irrigation with bacitracin, castile soap, and benzalkonium chloride solutions compared with pretreatment baseline in only 48 hours. In contrast, when it was performed with normal saline, the bacterial burden reduced to 29% of the pretreatment level immediately and remained lower than the pretreatment (68%) level at 48 hours [16]. Consistently, in a S. aureus-contaminated rat model, antiseptic solutions led to bacterial rebound to pretreatment level due to their host-tissue toxicity [17]. The FLOW trial demonstrated that patients irrigated with castile soap (14.8%) had a higher reoperation rate compared with normal saline (11.6%; hazard ratio, 1.32 [95% CI 1.06 to 1.66]; p = 0.01) [2]. All these previous and current studies have suggested a fact that local tissue toxicity caused by the irrigation solution might diminish their enhanced efficacy in bacterial removal.
Early laboratory animal studies favored the use of antiseptic solutions mainly based on bacterial counts immediately after irrigation [1, 18]. This design underestimates the effect of cytotoxicity and subsequent rebound of bacteria. Previous studies have revealed that EDTA could effectively eliminate bacteria without causing cytotoxicity [23]. No additional toxicity to endothelial cells and fibroblasts compared with normal saline was observed in vitro [23]. Supporting this result, wound healing was delayed after daily irrigation with castile soap, benzalkonium chloride, and bacitracin solutions compared with EDTA and normal saline in a skin defect model [6]. Notably, the types of resident cells in a joint cavity are clearly different from those of a wound. Thus, in this study, we assessed the toxicity of EDTA against chondrocytes, synovial fibroblasts, and osteoblasts and found no additional toxicity of EDTA.
There are several potential adverse effects of an EDTA irrigation. First, EDTA is a widely-used chelating agent for medical purposes in the treatment of heavy metal poisoning. It chelates ions including calcium, zinc, and magnesium to form a complex [5, 15]. Thus, EDTA irrigation would deprive calcium ion from the host. Here, we measured the blood calcium before and after irrigation and found no difference. However, translation of this finding needs to be cautious because the joint cavities of larger subjects are accordingly larger. More calcium might be lost due to irrigation in larger animals and humans. Second, the incidence of arthrofibrosis after knee arthroplasty is estimated to reach more than 10% [8]. Its pathogenesis is related to intra-articular inflammation caused by surgical damage and prosthetic irrigation [20]. Intra-articular irrigation with cytotoxicity would clearly cause damage to joint-resident cells and subsequent inflammation. Considering the toxicity of the EDTA solution, such damage would be very mild; we did not observe any scar formation in our study period.
Conclusions
After evaluating the efficacy of various intraoperative irrigation solutions in a rat model of a contaminated intra-articular implant, we concluded that an EDTA solution is superior to normal saline, 0.03% benzalkonium chloride, and 0.3% povidone iodine in reducing bacterial infection and colonization in our laboratory model. The EDTA solution showed no additional toxicity compared with normal saline and no adverse effects even after prolonged irrigation. If other animal models substantiate the efficacy and safety of the EDTA solution, clinical trials would be warranted to determine whether the use of an EDTA irrigation solution might reduce the risk of PJIs in patients compared with traditional irrigation solutions.
Acknowledgments
We thank Xiaolin Liu PhD, for providing the bacterial strains used in this study, and we thank Yang Wang PhD, and Shumin Zhou PhD, for their constructive suggestions.
Footnotes
This study was supported by the National Natural Science Foundation of China (Grant No. 81672144, 81974331) and a Shanghai Municipal Education Commission-Gaofeng Clinical Medicine Grant (Grant No. 20161429).
Each author certifies that neither he or she, nor any member of his or her immediate family, has funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his or her institution approved the animal protocol of this investigation and that all investigations were conducted in conformity with ethical principles of research.
Two of the authors (HZ, BB) contributed equally to this work.
References
- 1.Anglen JO, Apostoles S, Christensen G, Gainor B. The efficacy of various irrigation solutions in removing slime-producing Staphylococcus. J Orthop Trauma. 1994;8:390-396. [DOI] [PubMed] [Google Scholar]
- 2.Bhandari M, Jeray KJ, Petrisor BA, Devereaux PJ, Heels-Ansdell D, Schemitsch EH, Anglen J, Della Rocca GJ, Jones C, Kreder H, Liew S, McKay P, Papp S, Sancheti P, Sprague S, Stone TB, Sun X, Tanner SL, Tornetta P, 3rd, Tufescu T, Walter S, Guyatt GH. A trial of wound irrigation in the initial management of open fracture wounds. N Engl J Med. 2015;373:2629-2641. [DOI] [PubMed] [Google Scholar]
- 3.Blom A, Cho J, Fleischman A, Goswami K, Ketonis C, Kunutsor SK, Makar G, Meeker DG, Morgan-Jones R, Ortega-Pena S, Parvizi J, Smeltzer M, Stambough JB, Urish K, Ziliotto G. General assembly, prevention, antiseptic irrigation solution: proceedings of International Consensus on Orthopedic Infections. J Arthroplasty. 2019;34:S131-s138. [DOI] [PubMed] [Google Scholar]
- 4.Carvalho E, Ching Ching AT, Estima Abreu PA, Ho PL, Barbosa AS. Breaking the bond: recent patents on bacterial adhesins. Recent Pat DNA Gene Seq. 2012;6:160-171. [DOI] [PubMed] [Google Scholar]
- 5.Chappell LT. Should EDTA chelation therapy be used instead of long-term clopidogrel plus aspirin to treat patients at risk from drug-eluting stents? Altern Med Rev. 2007;12:152-158. [PubMed] [Google Scholar]
- 6.Deng Z, Liu F, Li C. Therapeutic effect of ethylenediaminetetraacetic acid irrigation solution against wound infection with drug-resistant bacteria in a rat model: an animal study. Bone Joint Res. 2019;8:189-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edelstein AI, Weiner JA, Cook RW, Chun DS, Monroe E, Mitchell SM, Kannan A, Hsu WK, Stulberg SD, Hsu EL. Intra-articular vancomycin powder eliminates methicillin-resistant S. aureus in a rat model of a contaminated intra-articular implant. J Bone Joint Surg Am. 2017;99:232-238. [DOI] [PubMed] [Google Scholar]
- 8.Gollwitzer H, Burgkart R, Diehl P, Gradinger R, Buhren V. [Therapy of arthrofibrosis after total knee arthroplasty]. [in German] Orthopade. 2006;35:143-152. [DOI] [PubMed] [Google Scholar]
- 9.Hart A, Hernandez NM, Abdel MP, Mabry TM, Hanssen AD, Perry KI. Povidone-iodine wound lavage to prevent infection after revision total hip and knee arthroplasty: an analysis of 2,884 cases. J Bone Joint Surg Am. 2019;101:1151-1159. [DOI] [PubMed] [Google Scholar]
- 10.Hernandez NM, Hart A, Taunton MJ, Osmon DR, Mabry TM, Abdel MP, Perry KI. Use of povidone-iodine irrigation prior to wound closure in primary total hip and knee arthroplasty: an analysis of 11,738 cases. J Bone Joint Surg Am. 2019;101:1144-1150. [DOI] [PubMed] [Google Scholar]
- 11.Jie K, Deng P, Cao H, Feng W, Chen J, Zeng Y. Prosthesis design of animal models of periprosthetic joint infection following total knee arthroplasty: A systematic review. PLoS One. 2019;14:e0223402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krishnan V, Narayana SV. Crystallography of gram-positive bacterial adhesins. Adv Exp Med Biol. 2011;715:175-195. [DOI] [PubMed] [Google Scholar]
- 13.Moriarty TF, Harris LG, Mooney RA, Wenke JC, Riool M, Zaat SAJ, Moter A, Schaer TP, Khanna N, Kuehl R, Alt V, Montali A, Liu J, Zeiter S, Busscher HJ, Grainger DW, Richards RG. Recommendations for design and conduct of preclinical in vivo studies of orthopedic device-related infection. J Orthop Res. 2019;37:271-287. [DOI] [PubMed] [Google Scholar]
- 14.Olsen AS, Wilson A, O'Malley MJ, Urish KL, Klatt BA. Are sonication cultures of antibiotic cement spacers useful during second-stage reimplantation surgery for prosthetic joint infection? Clin Orthop Relat Res. 2018;476:1986-1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ouyang P, Gottlieb SH, Culotta VL, Navas-Acien A. EDTA chelation therapy to reduce cardiovascular events in persons with diabetes. Curr Cardiol Rep. 2015;17:96. [DOI] [PubMed] [Google Scholar]
- 16.Owens BD, White DW, Wenke JC. Comparison of irrigation solutions and devices in a contaminated musculoskeletal wound survival model. J Bone Joint Surg Am. 2009;91:92-98. [DOI] [PubMed] [Google Scholar]
- 17.Penn-Barwell JG, Murray CK, Wenke JC. Comparison of the antimicrobial effect of chlorhexidine and saline for irrigating a contaminated open fracture model. J Orthop Trauma. 2012;26:728-732. [DOI] [PubMed] [Google Scholar]
- 18.Rosenstein BD, Wilson FC, Funderburk CH. The use of bacitracin irrigation to prevent infection in postoperative skeletal wounds. An experimental study. J Bone Joint Surg Am. 1989;71:427-430. [PubMed] [Google Scholar]
- 19.Sambri A, Cadossi M, Giannini S, Pignatti G, Marcacci M, Neri MP, Maso A, Storni E, Gamberini S, Naldi S, Torri A, Zannoli S, Tassinari M, Fantini M, Bianchi G, Donati D, Sambri V. Is treatment with dithiothreitol more effective than sonication for the diagnosis of prosthetic joint infection? Clin Orthop Relat Res. 2018;476:137-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Usher KM, Zhu S, Mavropalias G, Carrino JA, Zhao J, Xu J. Pathological mechanisms and therapeutic outlooks for arthrofibrosis. Bone Res. 2019;7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vengadesan K, Narayana SV. Structural biology of Gram-positive bacterial adhesins. Protein Sci. 2011;20:759-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watson RS, Gouze E, Levings PP, Bush ML, Kay JD, Jorgensen MS, Dacanay EA, Reith JW, Wright TW, Ghivizzani SC. Gene delivery of TGF-beta1 induces arthrofibrosis and chondrometaplasia of synovium in vivo. Lab Invest. 2010;90:1615-1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu H, Bao B, Zheng X. Is NS-EDTA effective in clearing bacteria from infected wounds in a rat model? Clin Orthop Relat Res. 2018;476:1083-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]