Abstract
Background
Hydroxychloroquine (HCQ) is a recommended drug in systemic lupus erythematosus (SLE). It has a long terminal half-life, making it an attractive target for therapeutic drug monitoring. The aim of this study was to establish a relationship between blood HCQ concentration and lupus nephritis activity.
Methods
We conducted a retrospective observational study with data collected from clinical and laboratory records. Inclusion criteria were patients followed in the lupus clinic with biopsy-proven International Society of Nephrology/Renal Pathology Society Classes III, IV or V lupus nephritis on HCQ for at least 3 months (200–400 mg daily) and with HCQ levels measured during treatment. Exclusion criteria were patients on renal replacement therapy at baseline or patients lost to follow-up.
Results
In 171 patients, the HCQ level was measured in 1282 samples. The mean HCQ blood level was 0.75 ± 0.54mg/L and it was bimodally distributed. An HCQ level <0.20 mg/L [232 samples (18.1%)] appeared to define a distinct group of abnormally low HCQ levels. For patients in complete or partial remission at baseline compared with those remaining in remission, patients with renal flare during follow-up had a significantly lower average HCQ level (0.59 versus 0.81 mg/L; P= 0.005). Our data suggest an HCQ target level to reduce the likelihood of renal flares >0.6 mg/L (600 ng/mL) in those patients with lupus nephritis.
Conclusion
HCQ level monitoring may offer a new approach to identify non-adherent patients and support them appropriately. We propose an HCQ minimum target level of at least 0.6 mg/L to reduce the renal flare rate, but this will require a prospective study for validation.
Keywords: disease activity, glomerulonephritis, lupus nephritis, systemic lupus erythematosus, treatment
Introduction
Lupus nephritis is a major complication of systemic lupus erythematosus (SLE), occurring in up to two-thirds of SLE patients. Renal remission is important, with those patients that enter complete remission having a higher renal and overall survival compared with those patients in whom remission is not achieved [1]. Even a partial remission in lupus nephritis is associated with a significantly better patient and renal survival compared with no remission [2–5]. Renal flares average ~8 per 100 patient-years for the first 5 years after attaining remission [6]. Severe disease at baseline, a delay in reaching remission and attainment of partial compared to complete remission are some of the factors predisposing to renal flares [6–8]. Non-adherence to lupus nephritis treatment is the most important factor for non-remission or renal relapses [9–11].
Hydroxychloroquine (HCQ) is currently recommended long term for all patients with SLE provided no contraindications exist. There is evidence of multiple beneficial effects, including control of disease activity by its immunomodulatory effects, reduction of cardiovascular events, lipid- and glucose-lowering effects, antithrombotic effects and improved survival [12–16].
HCQ has a long terminal half-life of >40 days and a steady-state level in blood with small daily fluctuation [17], making it an attractive target for therapeutic drug monitoring to evaluate adherence to medication as well as efficacy.
A pharmacokinetic/pharmacodynamic relation for blood HCQ concentration has been found in rheumatoid arthritis and cutaneous lupus [18–20]. For SLE, low whole-blood HCQ concentrations suggest non-adherence and may predict disease exacerbations, with one study showing that a cut-off of 1 mg/L had a negative predictive value of 96% for a systemic flare during follow-up [21]. There is also evidence of a protective effect of HCQ in retarding renal damage occurrence in SLE [22], but there are no reports, to our knowledge, looking specifically at HCQ blood levels in patients with lupus nephritis.
The aim of this study was to establish a relationship between blood HCQ concentration and lupus nephritis activity, including its relation with time to remission and risk of renal flares.
Materials and Methods
Study design
This is a retrospective observational study with data collected from clinical and laboratory records from January 2011 to October 2015. This study was designed, implemented and reported in accordance with International Council for Harmonisation guidelines for good practice and the Declaration of Helsinki (1975, revised in 1989). Since the standard clinical protocol was followed for patient management, it was decided in discussion with the chair of the local research ethics committee that there was no requirement for formal ethical review. The manuscript was prepared in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology guidelines for observational studies.
Setting
Patients were recruited from those attending the Imperial College Healthcare National Health Service Trust Lupus Centre outpatient and inpatients services, Hammersmith Hospital, London, UK. Patients had follow-up appointments according to clinical indications. We currently follow up ~400 patients with lupus (the majority of whom have had at least one episode of lupus nephritis), not all of whom are on HCQ.
Inclusion criteria
All patients ≥18 years of age with biopsy-proven International Society of Nephrology/Renal Pathology Society (ISN/RPS) Classes III, IV or V lupus nephritis on HCQ for at least 3 months and with HCQ levels measured during treatment were included.
Exclusion criteria
Patients on renal replacement therapy at baseline or lost to follow-up were excluded.
Outcome variables
The baseline of the study was defined as the date of first measurement of HCQ concentration in the blood. The definitions for complete remission, partial remission and renal relapse were taken from the study by Condon et al. [23] previously performed in this centre. Complete remission was defined by the combination of a urine protein:creatinine ratio (uPCR) <50 mg protein/mmol creatinine and serum creatinine no >15% of baseline. Partial remission was defined by the combination of uPCR < 300 mg/mmol with >50% reduction from baseline and serum creatinine not >15% of baseline. Renal relapse was defined as a persistent increase of >30% in proteinuria and/or serum creatinine requiring renal biopsy or an increase/change in immunosuppression. Outcome variables were assessed at baseline and at each follow-up visit.
Exposure variables
All patients were on HCQ and the standard clinical practice was to administer 400 mg in one or two divided doses. The formulation was not designated and many patients take generic HCQ. The dose of the drug was decreased by the treating physician if drug toxicities occurred. The HCQ blood level was tested at least 3 months after starting the drug. Ophthalmological testing was undertaken on a case-by-case basis and drug toxicities were recorded as and when reported. Concomitant immunosuppression was continued as per local standard of care regimens.
Predictor variables
Demographic, clinical, laboratory and pharmacokinetic data were collected at baseline and at follow-up visits. Demographic variables were age, gender and race. Clinical variables were weight, ISN/RPS class of lupus nephritis, comorbidities, duration and time of diagnosis of SLE and lupus nephritis and use of immunosuppression. Laboratory variables included uPCR, serum creatinine, serum albumin, haemoglobin, total leucocyte count, C-reactive protein and SLE serology (levels of anti-dsDNA antibody, C3 and C4). Pharmocokinetic variables were HCQ dosing, HCQ blood levels and concomitant mycophenolic acid (MPA) blood levels if available.
Data sources
Demographic, clinical and HCQ pharmacokinetic details were collected from patient records, the hospital database and from the Renal Clinical Leslie Brent Laboratory. Data were collected for all patients for all follow-up visits during the study period.
Whole blood HCQ concentrations were determined by an adaptation of our previously published liquid chromatography–tandem mass spectrometry method using HCQ-d3 as the internal standard [24]. Total imprecision was 8.3% at 0.25 mg/L, 4.2% at 0.90 mg/L and 4.1% at 2.1 mg/L (n = 72). The measurement of whole blood rather than plasma HCQ is important. Whole blood concentrations are approximately five times the plasma concentrations, are more precise and are favoured for pharmacokinetic measurements.
Study sample size
The number of consecutive cases that met the inclusion criteria within the study period (January 2011–October 2015) determined the sample size.
Statistical methods
Individual HCQ levels are presented as histograms for inspection. Undetectable HCQ levels (< 0.1 mg/L) were recorded as 0.05 mg/L for analysis. Within-patient variability in HCQ level was assessed by a regression model with patient as an independent predictor variable. The average HCQ level for each patient was used for outcome analysis, with Student’s t-test and chi-square test as appropriate for numerical and categorical comparisons.
Ethical approval information
Since the standard clinical protocol was followed for patient management, it was decided in discussion with the chair of the local research ethics committee that there was no requirement for formal ethical review.
Results
Participants
A total of 171 patients >18 years of age were included with ISN/RPS Classes III, IV and V on HCQ for at least 3 months and with HCQ blood levels measured during treatment. The mean follow-up period was 26.9 ± 15.7 months.
Descriptive data
The baseline demographics and clinical and laboratory parameters are summarized in Table 1. The majority of patients were women [n = 147 (86%)] and the mean age was 39.8 ± 15.6 years. A total of 73 patients (42.7%) were of Southeast Asian origin from the Indian subcontinent.
Table 1. Baseline demographics and clinical and laboratory parameters of the population studied.
| Parameters | Total |
|---|---|
| Gender, M:F | 24:147 (14.0 versus 86.0%) |
| Age (years) | 39.8 ± 15.6 (range 17–72) |
| Weight (kg) | 68.7 ± 15.6 |
| Indian subcontinent ancestry, n (%) | 73 (42.7) |
| Black African/African-Caribbean ancestry, n (%) | 43 (25.1) |
| Caucasian, n (%) | 27 (15.8) |
| Duration SLE (years) | 9.7 ± 8.6 |
| Duration lupus nephritis (years) | 6.8 ± 7.5 |
| Duration HCQ (months) | 48.6 ± 47.9 (range 3–186) |
| Use of immunosuppressive therapya, n (%) | 160 (93.6%) |
| Prednisolone daily dose (mg)a | 3.3 ± 5.0 (range 0–25) |
| Use of iv CYPa, n (%) | 4 (2.3%) |
| Use of MMFa, n (%) | 113 (66.1%) |
| Use of RTXa, n (%) | 4 (2.3%) |
| dsDNA (IU/mL) | 102.1 ± 181.9 |
| Albumin (g/L) | 35.0 ± 5.7 |
| Complement C3 (g/L) | 0.99 ± 0.30 |
| Complement C4 (g/L) | 0.40 ± 1.12 |
| Total leucocyte count (×109 cells/L) | 7.3 ± 13.5 |
| B-cell counts (cells/uL) | 121.1 ± 215.9 |
M, male; F, female; CYC, cyclophosphamide; RTX, rituximab; iv, intravenous.
Current treatment at time of baseline measurement of HCQ.
At baseline out of 171 patients, 91 (53.2%) were in complete remission from their prior lupus nephritis, 36 (21.1%) were in partial remission and 39 (22.8%) had active lupus nephritis.
Outcome data
The HCQ level was measured in 1282 samples from the 171 patients and the mean HCQ blood level was 0.75 ± 0.54 mg/L.
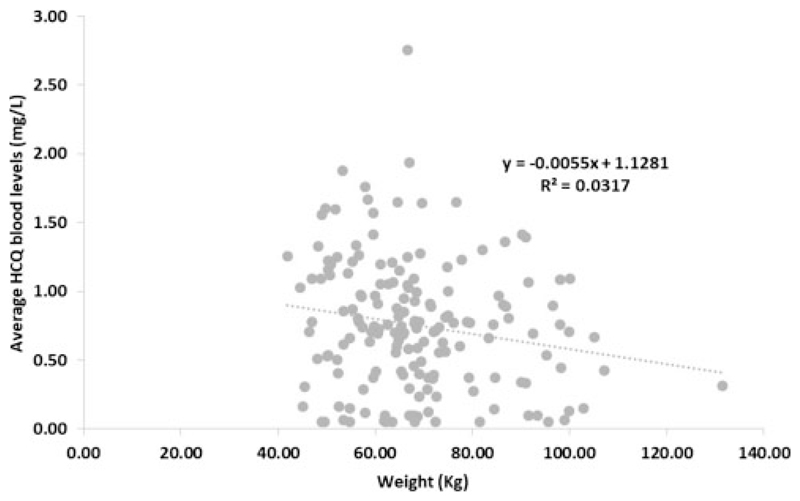
We have looked at patient factors affecting HCQ levels and found lower HCQ concentrations in obese patients (R = –0.179, P = 0.019) (Figure 1) but no relation to age, gender or ethnicity (data not shown).
Figure 1.
Variation in hydroxychloroquine levels according to patient weight.
Levels while taking 200 mg/day were lower than while taking 400 mg/day (0.58 ± 0.47 versus 0.79 ± 0.54 mg/L; P < 0.001). Over the study period, 74.9% of patients took 400 mg/day throughout, 14.0% took ≤200 mg/day, with the remaining 11.1% taking 200 or 400 mg/day during different periods. There was no defined protocol for dose selection, but those on <400 mg/day for part of the study were on lower baseline doses of prednisolone (2.2 versus 3.7 mg/day; P = 0.020) and mycophenolate mofetil (MMF) (0.62 versus 1.01 g/day; P = 0.005) and had lower estimated glomerular filtration rate (eGFR; 68 versus 78 mL/min/1.73 m2; P = 0.020), but they were not different in terms of age, gender, ethnicity, body weight or serum albumin.
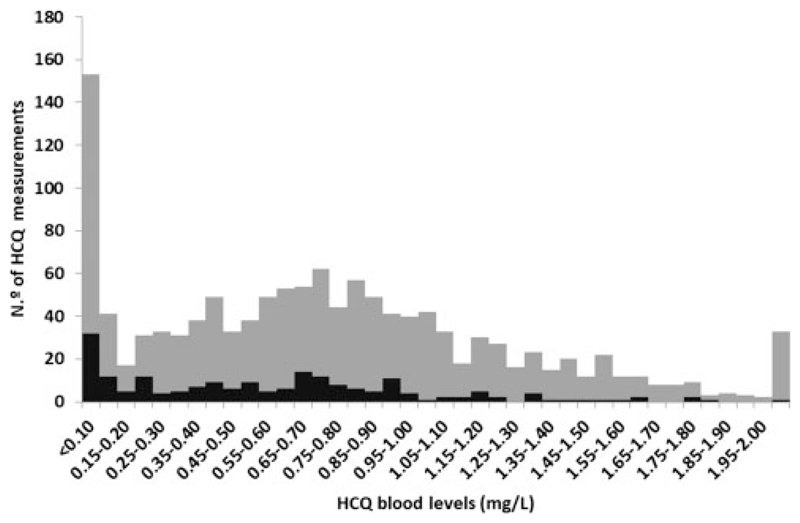
HCQ levels were bimodally distributed
An HCQ level <0.20 mg/L [232 samples (18.1%)] appeared to define a distinct group of abnormally low HCQ levels, which suggests a definition of non-adherence to the treatment, though in some cases it might be explained by insufficient dosing, as an effect of dose was noted (Figure 2).
Figure 2.
Distribution of total HCQ blood levels. Black: daily HCQ dosage of 200 mg; grey: daily HCQ dosage of 400 mg.
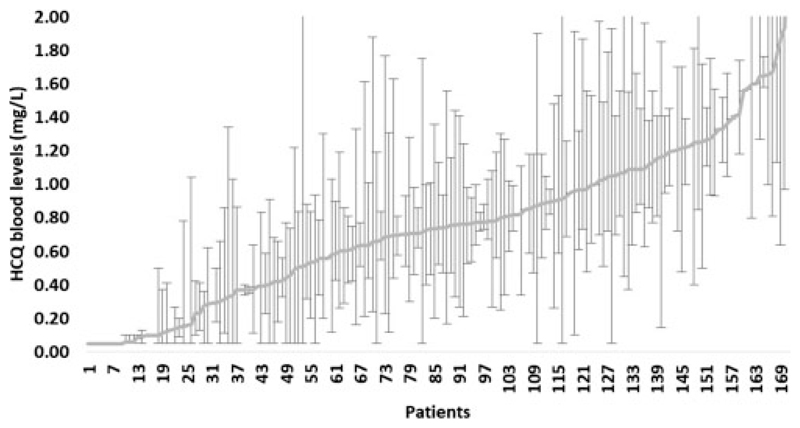
Less than 40% of total variation in HCQ levels occurred within individuals, suggesting a consistency of HCQ level over time (Figure 3). In those patients [n = 26 (15.2%)] with an average HCQ level <0.2 mg/L (poorly adherent patients), 88.7% of individual samples were also <0.2 mg/L. The average HCQ level for each patient was therefore used for clinical correlations.
Figure 3.
Consistency of HCQ blood levels over time per patient.
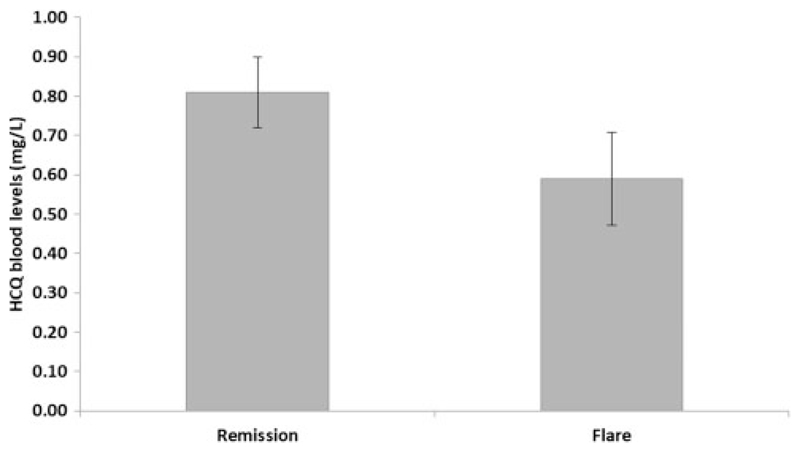
Regarding clinical outcome, for those with active nephritis at baseline average HCQ levels in those who subsequently achieved remission were similar to those who remained active (P = 0.23). In contrast, for patients in complete or partial remission at baseline compared with those remaining in remission, patients with renal flare during follow-up had significantly lower average HCQ levels (0.59 versus 0.81 mg/L; P = 0.005) (Figure 4).
Figure 4.
Average HCQ blood level by follow-up status (complete remission versus flare during follow-up in those in complete or partial remission at baseline).
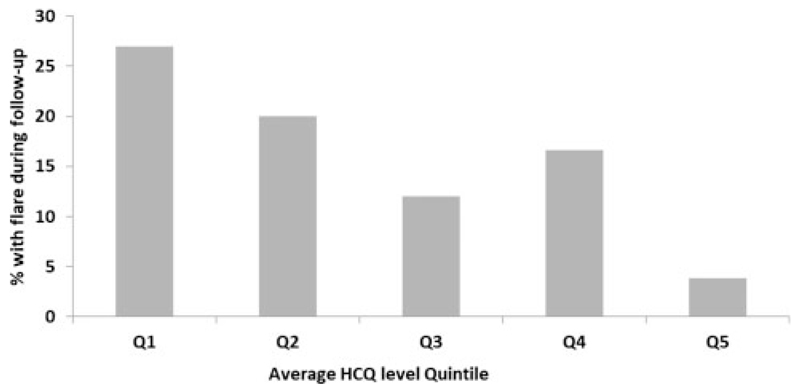
Considering only the patients in complete or partial remission at baseline, the proportion with disease flare according to quintile of average HCQ level is shown in Figure 5 (Q1: <0.31,Q2: 0.31–0.62, Q3: 0.63–0.81, Q4: 0.81–1.12, Q5: >1.12 mg/L). The P-value (chi-square test) is 0.2, but a significant difference is seen when comparing Q1–Q2 with Q3–Q5 (P = 0.041). The Q2–Q3 boundary is 0.62 mg/L, which can be considered the lower end of the desirable HCQ blood target level.
Figure 5.
Percentage of lupus nephritis flare during follow-up for patients in complete/partial remission at baseline per average HCQ blood level quintiles. Q1:<0.31 mg/L, Q2: 0.31–0.62 mg/L, Q3: 0.63–0.81 mg/L, Q4: 0.81–1.12 mg/L, Q5: >1.12 mg/L.
An HCQ level >0.6 mg/L was achieved in 68.8% of patients taking 400 mg/day throughout, and 46.5% of patients on lower doses for part of the time. Therefore a starting dose of 400 mg/day seems reasonable, although reductions or increases may be appropriate for a significant number.
An HCQ levels were left skewed, with the highest quintile ranging from 1.1 to 3.6 mg/L. Toxicity had not been systematically recorded so cannot be quantified: the most common problem noted was skin pigmentation, but a prospective study will be required to relate this to drug levels. No major toxicity was recorded.
Patients in the top quintile of average HCQ level experienced fewer flares during follow-up, and no flattening of the level–outcome slope was observed. It is therefore not possible to suggest an upper limit for a target range based on this study. When faced with high levels and possible toxicity, however, clinicians may find it helpful to know that an average HCQ level >1.6, 1.8 and 2.0 mg/L was seen in 4.7%, 1.8% and 0.6% of patients (8, 3 and 1 patient), respectively. The HCQ benefits observed in this study were therefore achieved with levels lower than these in the majority of patients. Thus our data suggest that an HCQ target level to prevent flares should be at least 0.6 mg/L (600 ng/mL).
Discussion
HCQ is recommended long term for all patients with SLE provided no contraindications exist [12–16]. Its mechanisms of action are still under investigation, but there is evidence that HCQ, by blocking the toll-like receptors 7 and 9 in plasmacytoid dendritic cells, inhibits interferon-α production, which plays a crucial role in SLE pathogenesis [25]. It has a long terminal half-life of >40 days, with small daily fluctuations of blood levels, making it an attractive target for therapeutic drug monitoring to evaluate adherence to medication as well as efficacy.
The blood HCQ concentration varies widely among patients with SLE. However, current information on factors influencing this variability is still controversial. A retrospective analysis of data from the Plaquenil Lupus Systemic (PLUS) study found that high body mass index, no treatment with corticosteroids, increased delay between the last tablet intake and measurement of blood HCQ concentration, low platelet and neutrophil counts and high estimated creatinine clearance are associated with low blood HCQ concentration [26]. The authors did not find an association of ethnicity or smoking and blood HCQ concentration and no pharmacokinetic drug–drug interaction with antacids or inhibitors and inducers of cytochrome P450 enzymes [26]. In contrast, another study did not find a relation between HCQ levels and eGFR, but only 14% of patients had Stage 4 or 5 CKD [27]. This and other studies also did not find any significant relationship between HCQ levels and gender, body weight, body mass index, renal function or chronic smoking [27, 28, 21]. On the other hand, a recent Asian study reported that HCQ levels are related to CYP2D6 polymorphisms in Korean lupus patients taking oral HCQ, which may explain why there is wide variation in blood HCQ concentrations [29]. In our study, HCQ levels were lower in obese patients but unaffected by age, gender or ethnicity. It was also interesting to see that some patients on lower daily doses of HCQ (200 mg daily) achieved adequate serum levels of HCQ, as shown in Figure 2, highlighting the variability between patients. Hence, for some patients, 400 mg daily would be a higher dose than necessary.
Poor adherence to therapeutic regimens is a common problem in patients with chronic diseases, including SLE, and is associated with a higher risk of flares, morbidity, hospitalizations and poor renal outcome. The rates of non-adherence in SLE patients range from 3 to 76% depending on the assessment methods, which are all subject to limitations [30]. Costedoat-Chalumeau et al. [31] reported in 2007 a total non-adherence with HCQ of 7% (undetectable levels). Reasons for non-adherence included both those attributed to HCQ characteristics (concern about potential side effects, perceived inefficacy of the drug compared with other treatments, adverse side effects attributed to the drug) and related to patient characteristics (failure to accept the disease and forgetfulness). In a cohort of 70 patients with childhood-onset SLE, only 32% of patients were sufficiently adherent to HCQ [32]. A cross-sectional study described that 13% of SLE patients had whole-blood HCQ levels of < 15 ng/mL (complete non-adherence) [28]. A more recent study found a proportion of total non-adherence of 11%, defined as an HCQ serum level of <10 ng/mL [27]. Our study showed that an HCQ level <0.2 mg/L (200 ng/mL) can be a useful indicator of poor adherence, which may reflect a more generalized adherence problem involving other disease-modifying agents. Using this definition, we found non-adherence with HCQ consistently occurs in at least 15.6% of our lupus nephritis population. However, we cannot exclude that in some cases these low levels were a result of underdosing or differences in HCQ pharmacokinetics instead of non-adherence. Direct comparison between studies is confounded by different cut-off values used to define non-adherence and differences in the HCQ assay. For patients identified as being poorly adherent by therapeutic monitoring, counselling may improve medication adherence. In the study by Costedoat-Chalumeau et al. [31], adherence improved after patients were informed of the results and interviewed about their adherence to treatment in a non-judgmental discussion. Additionally, there is evidence from a cross-sectional study that counselling patients with low HCQ levels led to an increase in its concentration, where only 56% of the patients had levels >500 ng/mL (0.5 mg/L) at their first follow-up visit versus 80% at their last follow-up in those who attended three visits or more [28].
We collected MPA levels on a number of the patients included in this study. However, whereas HCQ levels are little affected by the time of the last dose, this is not the case for MPA, and few levels were true troughs. So, from a retrospective data collection, where timing of dose was not routinely collected, it is impossible to draw any firm conclusions as to whether there is generalized or specific non-adherence. Some patients appear to take neither their MMF or HCQ (repeatedly undetectable levels of both), whereas some definitely take one and not the other. We have reviewed the baseline MPA and HCQ levels—102 patients had simultaneous MPA and HCQ levels. Of those with HCQ levels <0.2 (n = 17), just 1 (5.9%) had an undetectable level of MPA, 9 (52.9%) had levels <1.0 (which could well represent a long trough) and 10 (58.8%) had levels below the minimum therapeutic level (1.4). In the 85 patients with HCQ levels ≥ 0.2, none had undetectable MPA levels, 38 (44.7%) had levels <1.0 and 57 (67.1%) had levels <1.4. Conversely, 41.2% of those with undetectable HCQ had therapeutic levels of MPA, versus just 32.9% of those with HCQ levels >0.2. This suggests that non-adherence is not necessarily uniform.
The benefits of HCQ treatment in SLE have been demonstrated in a randomized, double-blind, placebo-controlled study of 47 SLE patients in which the risk of clinical SLE flares rose 2.5-fold during a 6-month period after discontinuation of the drug [33]. There is evidence that whole blood HCQ concentrations suggest non-adherence and may predict disease exacerbations, with one study showing that a cut-off of 1000 ng/mL (1 mg/L) had a negative predictive value of 96% for flare during follow-up [21]. This cut-off was in the same range as those able to block intracellular toll-like receptors in vitro, currently considered the key activity of HCQ in SLE [25]. In contrast to these results, two other studies did not find a significant concentration–effect relationship for HCQ in the treatment of SLE [27, 34]. However, in the first study, consistently high enough blood HCQ concentrations were not achieved [34]. In contrast, in the second study, in a subgroup of patients with serological and clinical remission and having therapeutic HCQ levels (defined as >500 ng/mL), a trend of lower disease activity and incidence of flares was indeed observed [27]. In 2013, Costedoat-Chalumeau et al. [35] showed that low HCQ concentration is associated with higher SLE activity, but adjusting the HCQ dose to a target level in blood did not reduce flares over a 7-month follow-up. However, the maintenance of HCQ >1000 ng/mL (1 mg/L) during the 7-month follow-up was difficult to achieve [35].
There is evidence of a protective effect of HCQ in retarding renal damage occurrence in SLE and protecting against renal insufficiency [22]. Additionally, HCQ was previously shown to be an independent predictor of complete renal remission in SLE patients treated with MMF for membranous lupus nephritis [36]. However, there are no reports to our knowledge looking specifically at HCQ blood levels in patients with lupus nephritis as in our study. We have shown that for patients with active nephritis at baseline, average HCQ levels in those who subsequently achieved remission were similar to those who remained active. In contrast, for patients in complete or partial remission at baseline compared with those remaining in remission, patients with renal flare during follow-up had significantly lower average HCQ levels (0.59 for patients who flared versus 0.81 mg/L for patients who maintained remission). Therapeutic drug monitoring may therefore assist in maintaining remission in lupus nephritis. Of course, it is possible that remission was maintained by adherence to other maintenance immunosuppression in those who also took their HCQ. But this does not diminish its value in evaluating adherence, whether specific or more generalized.
Our retrospective data also provide a suggestion for a therapeutic target range of HCQ levels. Renal flares were significantly fewer in those with HCQ levels >0.6 mg/L, which can be considered the lower end of the desirable HCQ target level. It is not possible from our study to define an ideal upper level, as toxicity was not systematically monitored, a limitation of our study.
In general, HCQ is well tolerated and rarely needs to be discontinued for an adverse reaction [16]. Gastrointestinal intolerance, pruritus and other cutaneous manifestations are not rare, but usually disappear with dose reduction. Potential severe side effects include retinal, neuromuscular and cardiac manifestations. Although these are rare, they require immediate discontinuation of treatment. In our study, no significant adverse effects were found, even for high HCQ levels, but further studies are necessary to confirm whether the high levels seen in some are necessary. The most common problem noted in our study was skin pigmentation, but a prospective study will be required to relate this to drug levels.
Another limitation of this study includes the fact that HCQ samples were collected when patients attended the clinic, so intervals between levels were not consistent between patients. That may result in ascertainment bias, as patients with poor outcomes are likely overrepresented in the analysis of the cohort as a whole. However, all patients with available HCQ blood levels were included, even if only one measurement was available, and there was significant consistency in levels over time for patients tested more than once, minimizing its effect.
Finally, the retrospective and observational nature of this study is the main limitation to our conclusions. A prospective study is necessary to validate our results.
In conclusion, non-adherence remains a major cause of poor outcomes in patients with lupus nephritis and our study suggests it consistently occurs in at least 15.6% of our lupus nephritis population, at least with respect to HCQ. HCQ level monitoring offers a new approach to identify such patients and support them more appropriately. We propose a minimum HCQ target level to prevent flares of 0.6 mg/L (600 ng/mL), but this will require a prospective study for validation.
Acknowledgements
The authors would like to thank Nathalie Costedoat-Chalumeau for advice and support, especially with developing the assay.
Funding
This work was supported by the National Institute for Health Research Imperial Biomedical Research Centre.
Footnotes
Authors’ Contributions
L.L. conceived the idea. G.C. and J.L. developed and validated the assay. C.C. and S.A. contributed equally to collecting data and drafting the manuscript. All authors contributed to the study design, data analysis and manuscript preparation.
Conflict of Interest Statement
None declared.
References
- 1.Korbet SM, Lewis EJ, Schwartz MM, et al. Factors predictive of outcome in severe lupus nephritis. Am J Kidney Dis. 2000;35:904–914. doi: 10.1016/s0272-6386(00)70262-9. [DOI] [PubMed] [Google Scholar]
- 2.Tamirou F, Lauwerys BR, Dall’Era M, et al. A proteinuria cut-off level of 0.7 g/day after 12 months of treatment best predicts long-term renal outcome in lupus nephritis: data from the MAINTAIN Nephritis Trial. Lupus Sci Med. 2015;2:e000123. doi: 10.1136/lupus-2015-000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dall’Era M, Cisternas MG, Smilek DE et al. Predictors of long-term renal outcome in lupus nephritis trials: lessons learned from the Euro-Lupus Nephritis cohort. Arthritis Rheum. 2015;67:1305–1313. doi: 10.1002/art.39026. [DOI] [PubMed] [Google Scholar]
- 4.Dall’Era M, Stone D, Levesque V, et al. Identification of biomarkers that predict response to treatment of lupus nephritis with mycophenolate mofetil or pulse cyclophosphamide. Arthritis Care Res. 2011;63:351–357. doi: 10.1002/acr.20397. [DOI] [PubMed] [Google Scholar]
- 5.Korbet SM, Lewis EJ. Severe lupus nephritis: the predictive value of a ≥50% reduction in proteinuria at 6 months. 2013. Nephrol Dial Transplant. 2013;28:2313–2318. doi: 10.1093/ndt/gft201. [DOI] [PubMed] [Google Scholar]
- 6.Grootscholten C, Berden JHM. Discontinuation of immunosuppression in proliferative lupus nephritis: is it possible? Nephrol Dial Transplant. 2006;21:1465–1469. doi: 10.1093/ndt/gfl208. [DOI] [PubMed] [Google Scholar]
- 7.Sidiropoulos PI, Kritikos HD, Boumpas DT. Lupus nephritis flares. Lupus. 2005;14:49–52. doi: 10.1191/0961203305lu2059oa. [DOI] [PubMed] [Google Scholar]
- 8.Llei GG, Takada K, Parkin D, et al. Renal flares are common in patients with severe proliferative lupus nephritis treated with pulse immunosuppressive therapy: long-term follow-up of a cohort of 145 patients participating in randomized controlled studies. Arthritis Rheum. 2002;37:1158–1163. doi: 10.1002/art.10142. [DOI] [PubMed] [Google Scholar]
- 9.Ward MM. Access to care and the incidence of endstage renal disease due to systemic lupus erythematosus. J Rheumatol. 2010;37:1158–1163. doi: 10.3899/jrheum.091199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ward MM. Changes in the incidence of endstage renal disease due to lupus nephritis in the United States, 1996–2004. J Rheumatol. 2009;36:63–67. doi: 10.3899/jrheum.080625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruce IN, Gladman DD, Urowitz MB. Factors associated with refractory renal disease in patients with systemic lupus erythematosus: the role of patient nonadherence. Arthritis Care Res. 2000;13:406–408. [PubMed] [Google Scholar]
- 12.Tang C, Godfrey T, Stawell R, et al. Hydroxychloroquine in lupus: emerging evidence supporting multiple beneficial effects. Intern Med J. 2012;42:968–978. doi: 10.1111/j.1445-5994.2012.02886.x. [DOI] [PubMed] [Google Scholar]
- 13.Ruiz-Irastorza G, Khamashta MA. Hydroxychloroquine: the cornerstone of lupus therapy. Lupus. 2008;17:271–273. doi: 10.1177/0961203307086643. [DOI] [PubMed] [Google Scholar]
- 14.Costedoat-Chalumeau N, Leroux G, Piette J-C, et al. Why all systemic lupus erythematosus patients should be given hydroxychloroquine treatment? Joint Bone Spine. 2010;77:4–5. doi: 10.1016/j.jbspin.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Petri M. Use of hydroxychloroquine to prevent thrombosis in systemic lupus erythematosus and in antiphospholipid antibody-positive patients. Curr Rheumatol Rep. 2011;13:77–80. doi: 10.1007/s11926-010-0141-y. [DOI] [PubMed] [Google Scholar]
- 16.Costedoat-Chalumeau N, Dunogué B, Morel N, et al. Hydroxychloroquine: a multifaceted treatment in lupus. Presse Med. 2014;43:e167–e180. doi: 10.1016/j.lpm.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 17.Furst DE. Pharmacocynetics of hydroxychloroquine and chloroquine during treatment of rheumatic disease. Lupus. 1996;5(Suppl 1):S11–S15. [PubMed] [Google Scholar]
- 18.Tett SE, Day RO, Cutler DJ. Concentration-effect relationship of hydroxychloroquine in rheumatoid arthritis—a cross sectional study. J Rheumatol. 1993;20:1874–1879. [PubMed] [Google Scholar]
- 19.Munster T, Gibbs JP, Shen D, et al. Hydroxychloroquine concentrationresponse relationships in patients with rheumatoid arthritis. Arthritis Rheum. 2002;46:1460–1469. doi: 10.1002/art.10307. [DOI] [PubMed] [Google Scholar]
- 20.Francés C, Cosnes A, Duhaut P, et al. Low blood concentration of hydroxychloroquine in patients with refractory cutaneous lupus erythematosus: a French multicenter prospective study. Arch Dermatol. 2012;148:479–484. doi: 10.1001/archdermatol.2011.2558. [DOI] [PubMed] [Google Scholar]
- 21.Costedoat-Chalumeau N, Amoura Z, Hulot J-S, et al. Low blood concentration of hydroxychloroquine is a marker for and predictor of disease exacerbations in patients with systemic lupus erythematosus. Arthritis Rheum. 2006;54:3284–3290. doi: 10.1002/art.22156. [DOI] [PubMed] [Google Scholar]
- 22.Pons-Estel GJ, Alarcón GS, McGwin G, et al. Protective effect of hydroxychloroquine on renal damage in patients with lupus nephritis: data from LUMINA, a multiethnic U.S.cohort. Arthritis Rheum. 2009;61:830–839. doi: 10.1002/art.24538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Condon MB, Ashby D, Pepper RJ, et al. Prospective observational single-centre cohort study to evaluate the effectiveness of treating lupus nephritis with rituximab and mycophenolate mofetil but no oral steroids. Ann Rheum Dis. 2013;72:1280–1286. doi: 10.1136/annrheumdis-2012-202844. [DOI] [PubMed] [Google Scholar]
- 24.Chusney G, Lightstone L, Cairns T, et al. Quantification of hydroxychloroquine in whole blood samples by LC-MS/MS. Ther Drug Monit. 2011;33:500. (Abstract number P074) [Google Scholar]
- 25.Lafyatis R, York M, Marshak-Rothstein A. Antimalarial agents: closing the gate on toll-like receptors? Arthritis Rheum. 2006;54:3068–3070. doi: 10.1002/art.22157. [DOI] [PubMed] [Google Scholar]
- 26.Jallouli M, Galicier L, Zahr N, et al. Determinants of hydroxychloroquine blood concentration variations in systemic lupus erythematosus. Arthritis Rheum. 2015;67:2176–2184. doi: 10.1002/art.39194. [DOI] [PubMed] [Google Scholar]
- 27.Mok CC, Penn HJ, Chan KL, et al. Hydroxychloroquine serum concentrations and flares of systemic lupus erythematosus: a longitudinal cohort analysis. Arthritis Care Res. 2016;68:1295–1302. doi: 10.1002/acr.22837. [DOI] [PubMed] [Google Scholar]
- 28.Durcan L, Clarke WA, Magder LS, et al. Hydroxychloroquine blood levels in systemic lupus erythematosus: clarifying dosing controversies and improving adherence. J Rheum. 2015;42:2092–2097. doi: 10.3899/jrheum.150379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JY, Vinayagamoorthy N, Han K, et al. Association of polymorphisms of cytochrome P450 2D6 with blood hydroxychloroquine levels in patients with systemic lupus erythematosus. Arthritis Rheum. 2016;68:184–190. doi: 10.1002/art.39402. [DOI] [PubMed] [Google Scholar]
- 30.Costedoat-Chalumeau N, Pouchot J, Guettrot-Imbert G, et al. Adherence to treatment in systemic lupus erythematosus patients. Best Pract Res Clin Rheumatol. 2013;27:329–340. doi: 10.1016/j.berh.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Costedoat-Chalumeau N, Amoura Z, Hulot JS, et al. Very low blood hydroxychloroquine concentration as an objective marker of poor adherence to treatment of systemic lupus erythematosus. Ann Rheum Dis. 2007;66:821–824. doi: 10.1136/ard.2006.067835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ting TV, Kudalkar D, Nelson S, et al. Usefulness of cellular text messaging for improving adherence among adolescents and young adults with systemic lupus erythematosus. J Rheumatol. 2012;39:174–179. doi: 10.3899/jrheum.110771. [DOI] [PubMed] [Google Scholar]
- 33.Canadian Hydroxychloroquine Study Group. A randomized study of the effect of withdrawing hydroxychloroquine sulfate in systemic lupus erythematosus. N EnglJ Med. 1991;324:150–154. doi: 10.1056/NEJM199101173240303. [DOI] [PubMed] [Google Scholar]
- 34.Carmichael SJ, Day RO, Tett SE. A cross-sectional study of hydroxychloroquine concentrations and effects in people with systemic lupus erythematosus. Intern Med J. 2013;43:547–553. doi: 10.1111/imj.12100. [DOI] [PubMed] [Google Scholar]
- 35.Costedoat-Chalumeau N, Galicier L, Aumaˆıtre O, et al. Hydroxychloroquine in systemic lupus erythematosus: results of a French multicentre controlled trial (PLUS study) Ann Rheum Dis. 2013;7:1786–1792. doi: 10.1136/annrheumdis-2012-202322. [DOI] [PubMed] [Google Scholar]
- 36.Kasitanon N, Fine DM, Haas M, et al. Hydroxychloroquine use predicts complete renal remission within 12 months among patients treated with mycophenolate mofetil therapy for membranous lupus nephritis. Lupus. 2006;15:366–370. doi: 10.1191/0961203306lu2313oa. [DOI] [PubMed] [Google Scholar]