Abstract
Squamous cell carcinomas (SCCs) are among the most prevalent human cancers. SCC comprises a wide range of tumours originated from diverse anatomical locations that share common genetic mutations and expression of squamous differentiation markers. SCCs arise from squamous and non-squamous epithelial tissues. Here, we discuss the different studies in which the cell of origin of SCCs has been uncovered by expressing oncogenes and/or deleting tumour suppressor genes in the different cell lineages that compose these epithelia. We present evidence showing that the squamous differentiation phenotype of the tumour depends on the type of mutated oncogene and the cell of origin, which dictate the competence of the cells to initiate SCC formation, as well as on the aggressiveness and invasive properties of these tumours.
Introduction
Squamous cell carcinomas (SCCs) are among the most frequent solid cancers in humans1 and represent a major cause of death worldwide. Their incidence is sharply rising owing to increased exposure to carcinogens, such as ultraviolet radiation related to sun exposure, smoking, alcohol consumption or human papilloma virus (HPV) infection1,2. SCCs are classified according to the location where they appear, being frequently found in skin, head and neck, oesophagus, lung and cervix2–6 and more rarely in pancreas, thyroid, bladder and prostate7–10.
Glossary.
Squamous cell carcinomas
(SCCs). Cancers that present with squamous differentiation, which is visible by the presence of keratin materials.
Lineage tracing
A method involving experiments that allow the labelling of a cell or a group of cells and assess the fate of these labelled cells and their progeny overtime.
Stem cells
Cells that are at the top of the cellular hierarchy and are characterized by long-term self-renewing capacity and give rise to progenitors, transit-amplifying cells and differentiated cells.
Progenitors
Cells that can self-renew and give rise to terminally differentiated cells. Depending on the proportion of asymmetric renewal and symmetric differentiation upon division, progenitors can live long term or short term.
Stratified squamous epithelium
Epithelium composed of a layer of basal proliferative cells and several suprabasal layers of differentiated cells that express keratins and progressively flatten near the surface, eventually presenting as enucleated cells that are shed from the surface. These amorphous keratinized ghost cells are known as squames. The inner surface of the body is lined with non-keratinized stratified squamous epithelium, which is characterized by superficial cells that are flattened and nucleated.
Keratin pearls
Keratin-derived amorphous materials arising from the differentiation of tumour cells.
Transit-amplifying cells
Cells that divide a finite number of times and then terminally differentiate.
Clonal analysis
The study of the fate, renewal and long-term maintenance of single isolated cells over time.
Secretory cells
Cells found throughout the body that secrete components to affect cell; secretory cells of the airway system (also known as Clara cells) produce mucins and antimicrobial peptides.
Ciliated cells
Cells that contain tiny hair-like structures on their surface, found in the airway system of mammals and the fallopian tube of female mammals; ciliated cells of the airway system propel debris and dirty mucus out of the respiratory tract through the movement of their cilia.
Type 1 cells
(AT1 cells). Cells of the alveolar epithelium that allow gas exchange.
Type 2 cells
(AT2 cells). Cells of the alveolar epithelium that produce surfactant, which helps the alveolar structure to stay open and thus allows gas exchange.
Lineage ablation
The selective killing of a cell lineage, which is usually performed by inducing expression of a toxin or a toxin receptor in a cell of interest and then administering that toxin.
Dedifferentiation
A process that occurs when committed or differentiated cells revert to a less committed state.
During the past decades, great efforts have been made to elucidate the cell of origin of different malignancies11. Lineage tracing studies allowed the identification of the cellular hierarchies and lineage segregation that mediate homeostasis and repair of the different tissues from which cancer arises12 (FIG. 1). Many cancers arise from tissues maintained by the presence of stem cells and progenitors that self-renew and differentiate into the different cell lineages that compose these tissues. Depending on the turnover, differentiated cells and progenitors usually present a shorter lifespan, while stem cells reside long term, sometimes throughout the life of the animals. Upon tissue damage, the cellular hierarchy that governs epithelial tissue homeostasis can be altered, and more committed progenitors and even differentiated cells can acquire stem cell potential and contribute to tissue repair13. With oncogenic hits, both stem cells and progenitors can serve as the cells of origin in cancer (BOX 1).
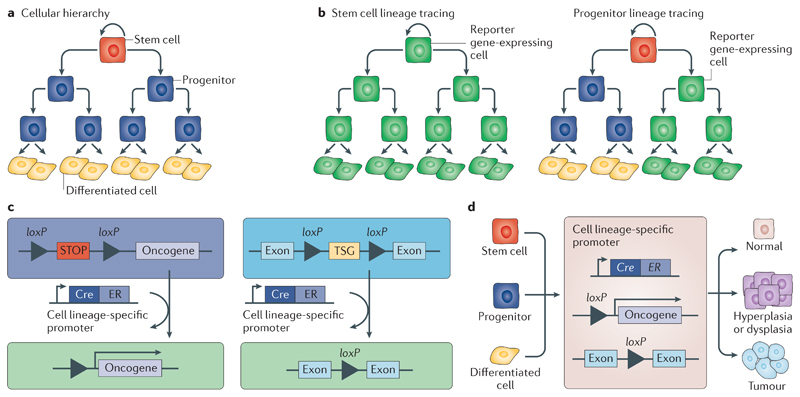
Fig 1. Lineage tracing and the cells of origin of cancer.
Many cancers arise from epithelial tissues, which are maintained by stem cells and their progeny. Stem cells are at the top of the cellular hierarchy and have the ability to self-renew and generate progenitors, which can self-renew and give rise to progenitors and terminally differentiated cells (part a). Tamoxifen-induced activation of the Cre recombinase under a cell lineage-specific promoter, for example, a stem cell-specific or progenitor-specific promoter, leads to the elimination of the STOP cassette between loxP sites, resulting in the expression of the reporter gene (part b), the expression of the oncogene or the deletion of the tumour suppressor gene (TSG) between loxP sites (part c) in the respective cell population and its progeny (parts b and c). If a progenitor-specific promoter is used, the reporter gene is expressed in progenitors and their progeny and in differentiated cells but not in the stem cell population. A progenitor usually has a limited lifetime, and thus, the reporter expression may be lost over time (part b, right panel). Conditional activation of oncogenes or deletion of TSGs has allowed the identification of the cells of origin in different mouse tumour models (part d). ER, oestrogen receptor.
Box 1. Cellular origin of cancer: stem cells versus committed cells.
Stem cell theory
Stem cells typically persist for a longer duration in the tissue, where they self-renew and give rise to progenitors and differentiated cells. It has been suggested that the longer a cell persists in a tissue, the higher the likelihood that this cell will accumulate the necessary mutations required to become tumorigenic. For this reason, stem cells are commonly considered the cells of origin of cancer146. Recent studies have suggested that the rate of stem cell turnover of a given human tissue is correlated with the probability of this tissue to develop cancer147–149.
Committed cell contribution
It has been suggested that the plasticity observed in epithelial tissues during tissue repair plays a role during tumour initiation. Extrinsic cues and/or oncogenic mutations can confer the ability to induce tumour development to already committed cells. In the intestine, activation of the WNT signalling pathway through the expression of a constitutively active form of catenin beta 1 (Ctnnb1) together with nuclear factor-κB (NF-κB) activation (through deletion of NF-κB inhibitor alpha (Nfkbia)) or simultaneous expression of constitutively active forms of Ctnnb1 and KrasG12D in committed epithelial intestinal cells, using the X-box-binding protein 1 (XBP1) promoter fused with a Cre recombinase and oestrogen receptor (Xbp1–Cre–ER), led to the dedifferentiation of previously committed cells into stem-like cells and to tumour development144. In the skin interfollicular epidermis, progenitors targeted by involucrin (Ivl)–Cre–ER mice require two hits (overexpression of a constitutively activated form of smoothened, frizzled class receptor (SmoM2) and Trp53 deletion) to induce basal cell carcinoma, whereas stem cells require only SmoM2 expression for tumour development27.
The nature of the cells at the cancer origin has also been proposed to determine the differentiation characteristics and aggressiveness of tumours. Tumours arising from progenitors may show lineage-restricted differentiation, while tumours arising from stem cells could present multi-lineage differentiation potential. However, multipotent differentiation of a tumour could also result from plasticity of tumour cells induced by their oncogenic mutations or by their microenvironment or by neighbouring cells.
In this Review, we discuss recent studies that define the cell of origin of cutaneous, head and neck, oesophageal and lung SCCs, which represent the most common SCCs. We first describe the architecture and cellular hierarchy present in the different epithelia from which the different SCCs arise. Then, we discuss how lineage tracing strategies have been instrumental to identifying the cell of origin in these SCCs. Finally, we discuss how oncogenic mutations and the cell of origin cooperate in determining the differentiation, aggressiveness and metastatic potential of SCCs.
Architecture of tissues of SCC origin
SCCs arise from epithelial tissues that can be classified as stratified squamous epithelium (which includes epithelia of skin, oesophagus and oral cavity) and non-squamous epithelia (which include airway epithelium). The different types of SCCs have common histological features, such as the presence of squamous differentiation visible by the formation of keratin pearls.
Skin compartments are maintained by their own resident stem cells
The skin epidermis acts as the first defensive line to isolate and protect our bodies from the external environment. The mammalian epidermis is a stratified epithelium composed by the interfollicular epidermis (IFE), hair follicles, sebaceous glands and sweat glands14. Lineage tracing experiments in mice have shown that during homeostasis, the distinct skin compartments are maintained by their own pool of lineage-restricted stem cells15–19 (FIG. 2). However, during tissue regeneration such as wound healing, epithelial cells acquire plasticity, and the different lineage-restricted stem cells, such as hair follicles and infundibulum, get activated, migrate towards the wounded region and differentiate into suprabasal cells of the IFE19–22.
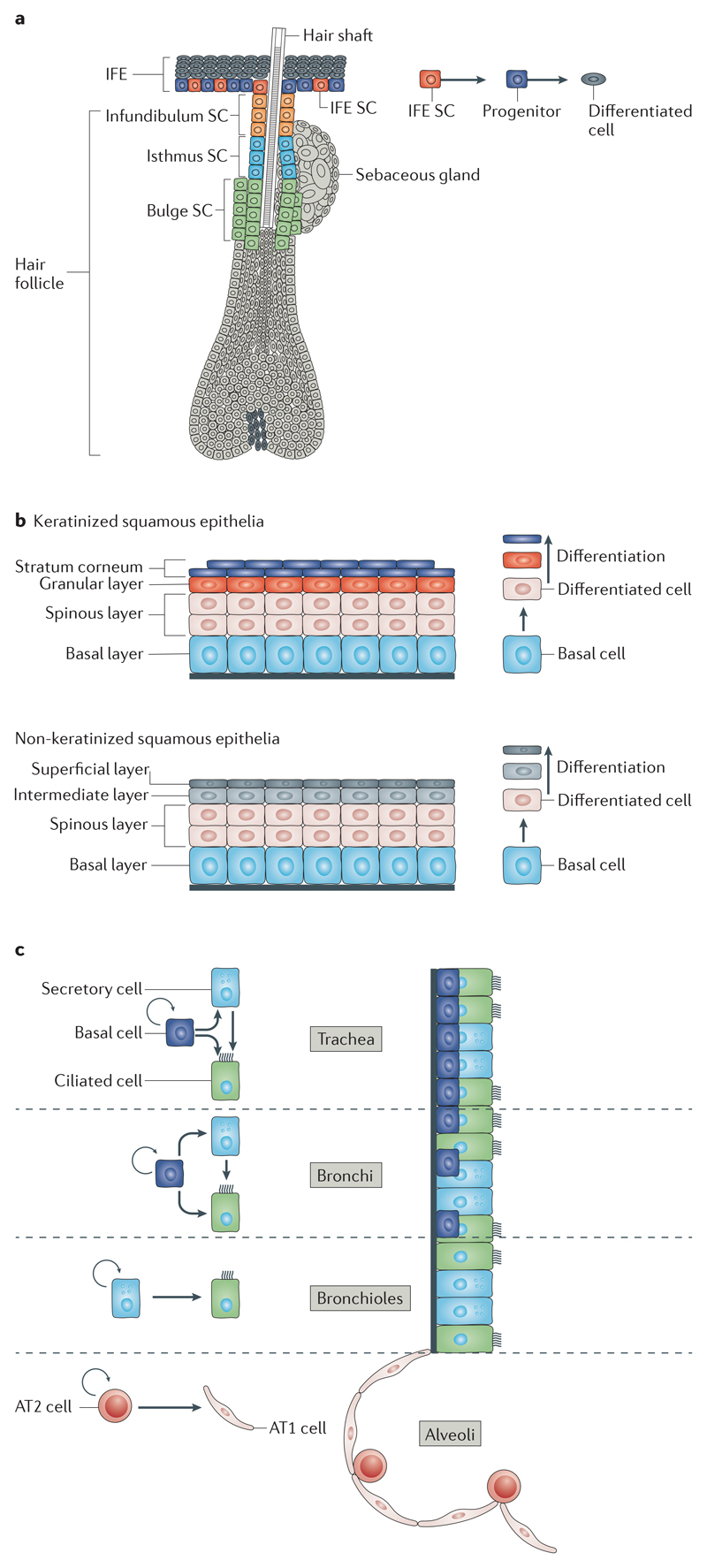
Fig 2. Architecture and cellular hierarchy present in the tissues from which SCC arise.
a | The different skin compartments are maintained by their own resident stem cells (SCs). The epidermis is composed of the interfollicular epidermis (IFE), hair follicles and sebaceous glands. The different anatomical regions that form the hair follicle, namely, bulge, infundibulum, isthmus and sebaceous glands, have their own pool of SCs. The hair follicle SCs are slow-cycling cells, residing below the sebaceous gland, in the permanent region of hair follicles. During physiological conditions, hair follicle SCs sustain the cyclic production of the hair, giving rise to transit-amplifying progenitors that rapidly divide and differentiate into the different concentric hair follicles lineages. b | The squamous epithelia of the skin, oral cavity, head and neck and oesophagus are composed of a layer of basal proliferative cells and several suprabasal layers of differentiated cells that progressively flatten before being lost. In keratinized squamous epithelium (for example, oesophageal epithelium), the differentiated cells are enucleated and shed from the surface; these amorphous keratinized ghost cells are called squames. The inner surface of the body is lined with non-keratinized stratified squamous epithelium (for example, oral epithelium), which is characterized by superficial cells that are flattened and nucleated. c | The different lung compartments are maintained by their own pool of SCs during homeostasis. Multipotent basal SCs maintain the mouse trachea and bronchi and give rise to secretory and ciliated cells. In the bronchioles, secretory cells represent a bipotent population of SCs that self-renew and give rise to ciliated cells. In the alveoli, bipotent type 2 (AT2) SCs self-renew and give rise to type 1 (AT1) and AT2 cells.
The IFE is a stratified squamous epithelium maintained by the existence of basal cells with high self-renewing capacities that balance proliferation and differentiation. How precisely the balance between renewal and differentiation is achieved to sustain the homeostasis of the epidermis remains a matter of discussion and may vary between the different parts of mouse skin (ear, paw, tail, ventral and dorsal skin)23–29. It was initially hypothesized that the epidermis is maintained by the existence of many small units of proliferation called epidermal proliferative units, which contain slow-cycling stem cells that generate transit-amplifying cells, which, after a defined number of cell divisions, give rise to terminally differentiated cells30. Lineage tracing using Ah–Cre–ER, a construct that combines the cytochrome P450, family 1, subfamily a, polypeptide 1 (Cyp1a1) promoter with a Cre recombinase fused to the oestrogen receptor (Cre–ER) and leads to expression in a largely ubiquitous manner following the administration of beta-naphthoflavone, has been used to assess the mode of epidermal homeostasis28,29. The Cre–ER allows control of the activity of Cre by the administration of tamoxifen, which mediates the translocation of Cre to the nucleus, the recombination of loxP sites and the expression of the reporter gene (FIG. 1). Clonal analysis of Ah–Cre–ER lineage tracing data in the IFE demonstrated that the clone size does not converge on a precise number of basal cells, suggesting that the unit of proliferation has no predefined fixed size. The clone size distribution could be explained by the presence of a single population of equipotent progenitors that balance renewal and differentiation in a stochastic manner28,29. More recent studies have demonstrated that epidermal cells are more heterogeneous than initially anticipated and have illustrated that different Cre–ER mice target different stem and progenitor cells with distinct proliferation rates and survival capacities, different long-term renewing capacities and different abilities to mediate long-term skin repair24–27. The population of progenitor cells targeted by the involucrin (Ivl)–Cre–ER in the mouse tail IFE is identical to the one targeted by the Ah–Cre–ER25,28. By contrast, keratin 14 (K14; also known as Krt14)–Cre–ER (K14–Cre–ER) targets progenitors and stem cells that have long-term survival and divide asymmetrically to give rise to progenitors25,27(FIG. 2a,b).
Distinct anatomical regions compose the hair follicles, including the infundibulum, isthmus, sebaceous glands and lower hair follicle regions that produce the hair shaft. These different epidermal regions are maintained by their own pool of resident stem cells15–19,31 (FIG. 2a). Mouse hair follicle stem cells can be analysed by using the K15 promoter-driven, the K19 promoter-driven or leucine-rich repeat-containing G protein-coupled receptor 5 (Lgr5) promoter-driven Cre-inducible constructs K15–Cre–PR18, K19–Cre–ER31 and Lgr5–Cre–ER15, respectively. In Cre–PR constructs, Cre is fused to the progesterone receptor (PR), and administration of RU468, a PR ligand, promotes the translocation of Cre to the nucleus and its activity (FIG. 1).
The oral epithelium is maintained by a proliferative basal compartment
The oral epithelium contains keratinized areas composed of keratinized and non-keratinized stratified squamous epithelium (FIG. 2b). The oral cavity and pharynx are coated with non-keratinized stratified epithelium. The masticatory mucosa is keratinized. The larynx is lined by ciliated pseudostratified columnar epithelium, except the vocal cords, which are covered by stratified squamous epithelium. In all these epithelia, cell proliferation is restricted to the basal layer.
Very little is known concerning the identity of stem cells in head and neck epithelia, the location of stem cell niches or how stem cells and progenitors balance proliferation and differentiation. Pulse–chase tritiated thymidine and 5-bromodeoxyuridine (BrdU) experiments revealed the existence of slow-cycling label-retaining cells in mouse oral epithelia, suggesting the existence of a pool of slow-cycling basal stem cells32. Lineage-tracing experiments in mice using the SRY-box 2 (Sox2)–Cre–ER and K14–Cre–ER transgenic systems, which drive Cre-inducible reporter gene expression in SOX2-expressing or K14-expressing basal cells, showed that cells located in the basal compartment present long-term maintenance and can give rise to the different cell types that form the tongue and soft palate33, 34.
The oesophagus is maintained by basal progenitors
The mouse and rat oesophagus has a keratinized squamous epithelium composed of 4–5 cell layers with a rapid turnover, which is maintained by the presence of proliferative basal cells that are able to self-renew, differentiate and migrate towards the lumen, giving rise to suprabasal layers of terminally differentiated cells35 (FIG. 2b).
The cellular hierarchy that mediates the homeostasis in mouse oesophageal epithelium remains a matter of intense debate33,36–42. Clonal analysis using Ah–Cre–ER transgenic mice suggests that the mouse oesophageal epithelium is maintained by a single, equipotent, committed progenitor cell population that balances renewal and differentiation37. By contrast, lineage tracing studies using Sox2–Cre–ER or K15–Cre–PR transgenic mice labelled a subpopulation of mouse basal cells with long-term maintenance and the ability to give rise to the differentiated cells, suggesting that the cells targeted by SOX2 promoter-inducible or K15 promoter-inducible Cre mark a progenitor and/or stem cell population with higher self-renewal potential than that of committed progenitors33,41. Other studies using fluorescence-activated cell sorting isolation, colony forming assays and 3D organoid assays further suggested the presence of heterogeneity within oesophageal epithelium in terms of marker expression, proliferation kinetics and ability to reform oesophageal epithelium39,40. Side by side comparison using the different Cre systems will be important to resolve this apparent discrepancy.
The human oesophagus has a non-keratinized squamous epithelium composed of several layers and two anatomical compartments, the papillae and the interpapillary region. A study in human oesophageal epithelium suggested the existence of two anatomically different compartments, one populated by a relatively quiescent stem cell population (interpapillary region) and the second populated by transit-amplifying cells that give rise to terminally differentiated cells (papillae)38. However, in another study, the quiescent cells expressing the stem cell marker CD34 were found at the tip of the papillae, and the highly proliferative cells were found at the interpapillary region42.
Lung compartments are maintained by their own resident stem cells
Two main compartments constitute mammalian lungs: the airways and the alveoli. The respiratory system allows gas exchange and protects lung epithelia from microorganisms and dust particles that are constantly inhaled. All different lung compartments (trachea, bronchi, bronchioles and alveoli) are maintained by their own resident stem cells during homeostasis43–47. Upon tissue damage, differentiated cells present some plasticity and can revert back to a basal stem cell fate48.
The trachea and bronchi are lined with a pseudostratified epithelium49, which consists of basal cells, secretory cells, ciliated cells and rare neuro-endocrine cells. The murine bronchioles are lined with a simple columnar epithelium composed mainly of secretory and ciliated cells, containing some neuroendocrine cells and no basal cells (FIG. 2c).
The airway epithelium has low renewal activity under steady-state conditions. In the mouse trachea, lineage tracing experiments using K5– Cre–ER, which targets basal cells of the trachea, showed that these cells contain self-renewing multipotent stem cells that give rise to basal, secretory and ciliated cells during postnatal growth and homeostasis and upon injury43. Lineage tracing in secretoglobin, family 1A, member 1 (Scgb1a1)–Cre–ER transgenic mice, which drives the inducible Cre in secretory cells in the trachea, bronchi and bronchioles and in cells of the alveolar epithelium, showed that Scgb1a1+ cells are progressively lost and replaced over time, suggesting that secretory cells in the trachea and bronchi represent a transit-amplifying cell population. However, in the bronchioles, Scgb1a1+ cells self-renew for longer periods of time without being lost and generate ciliated cells, thus representing a population of bipotent stem cells44. Lineage tracing in forkhead box J1 (Foxj1)–Cre–ER transgenic mice, a system that drives Cre-inducible reporter gene expression in ciliated cells, showed that ciliated cells are terminally differentiated cells45 that usually arise from secretory cells but upon injuries can arise from the direct differentiation of basal cells50 (FIG. 2c).
The alveolar epithelium contains two cell types: thin type 1 cells (AT1 cells) and cuboidal type 2 cells (AT2 cells). Lineage tracing experiments using surfactant associated protein C (Sftpc)–Cre–ER transgenic mice, a system that drives Cre-inducible reporter gene expression in AT2 cells, demonstrated that AT2 cells can self-renew for long periods of time and give rise to AT1 cells, suggesting that they represent a bipotent population of alveolar stem cells46,47 (FIG. 2c).
Common pathways in SCCs
SCCs are thought to result from a multistep process in which the sequential accumulation of genetic mutations leads to the generation of preneoplastic lesions that progress into invasive carcinomas2–5. The development of next-generation sequencing allowed the identification of new driver mutations responsible for tumour initiation and progression51. Recent studies have reported the mutational landscape of human SCCs, including cutaneous SCC (CSCC)52–54, oesophageal SCC (ESCC)55–58, head and neck SCC (HNSCC)59–64, lung SCC (LSCC)65–67 and cervical SCC68,69. These studies demonstrated that SCCs from different tissues present mutations in a common set of genes, suggesting that common mechanisms regulate SCC initiation across different tissues (FIG. 3).
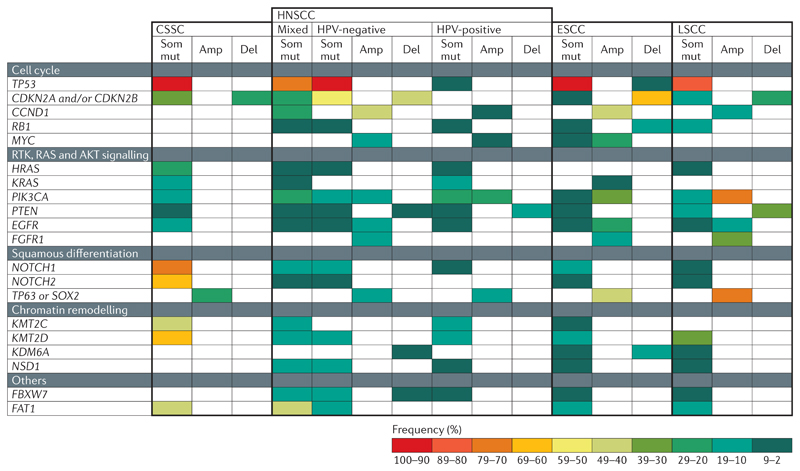
Fig 3. Common genetic alterations found in the different types of SCC.
Squamous cell carcinomas (SCCs) from different body locations (cutaneous SCC (CSCC)52–54, oesophageal SCC (ESCC)55–58, head and neck SCC (HNSCC)59–64 and lung SCC (LSCC)65–67) have somatic mutations (Som mut), amplifications (Amp) and deletions (Del) in genes controlling the cell cycle, the receptor tyrosine kinase (RTK), RAS and AKT signalling pathways, squamous differentiation and chromatin remodelling. The colour code represents the frequency of a given alteration among patients with each disease subtype. The white fields indicate that Del, Amp or Som mut have not been described for a given gene and SCC type. CCND1, cyclin D1; CDKN2, cyclin-dependent kinase inhibitor 2; EGFR, epidermal growth factor receptor; FAT1, FAT atypical cadherin 1; FBXW7, F-box and WD repeat domain-containing 7; FGFR1, fibroblast growth factor receptor 1; HPV, human papilloma virus; KDM6A, lysine demethylase 6A; KMT2C, lysine methyltransferase 2C; PIK3CA, phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit-α; NSD1, nuclear receptor-binding SET domain protein 1.
Cell cycle regulators
The tumour suppressor gene TP53, which induces cell cycle arrest and apoptosis70, is the most commonly mutated gene in SCCs originating from the different body locations (FIG. 3). Deletions and somatic mutations in cyclin-dependent kinase inhibitor 2A (CDKN2A), CDKN2B and RB1 are also frequent in the different types of SCCs. CDKN2A and CDKN2B encode p16-INK4A, and p15-INK4B respectively, which both control cell cycle arrest and activate the tumour suppressor genes TP53 and RB1. RB1 regulates G1 to S phase progression71. Moreover, amplifications of the cyclin D1 (CCND1) and MYC genes are frequently found in HNSCCs59–64 and ESCCs55–58 (FIG. 3). CCND1 binds to cyclin-dependent kinase 4 (CDK4) as well as CDK6 and promotes G1 to S phase progression by inhibiting RB72. MYC overexpression promotes cell cycle progression and contributes to cell transformation7
RAS, AKT and receptor tyrosine kinase signalling
Alterations in components of the RAS, AKT and receptor tyrosine kinase (RTK) signalling pathways, which regulate cell proliferation and survival74–76, are commonly found in SCCs (FIG. 3). HRAS was the first discovered oncogene associated with mouse and human CSCC77,78. Mutations in both HRAS and KRAS are frequently found in CSCC52–54 and to a lesser extent in HNSCC59–64 and LSCC65–67 (FIG. 3). Activation of the AKT pathway via phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit-α (PIK3CA) gene mutation or amplification or PTEN gene mutation or deletion is found in SCCs from different body locations. HNSCCs59–64, ESCCs55–58 and LSCCs65–67 have amplifications in epidermal growth factor receptor (EGFR) and fibroblast growth factor receptor 1 (FGFR1) (FIG. 3).
Squamous cell differentiation pathways
The NOTCH signalling pathway is involved in regulating cell fate decisions in various tissues during development and homeostasis including skin, oral, oesophageal and bronchial epithelia79,80. Four NOTCH receptors (NOTCH1–NOTCH4) exist in mammals. NOTCH1 is expressed in the skin in homeostatic conditions, predominantly in the suprabasal layers of the IFE, where it promotes keratinocyte differentiation and acts as a tumour suppressor81–83. Deletion of Notch1 in mouse epidermis accelerates and increases skin tumorigenesis81. Mutations in NOTCH1 and NOTCH2 genes are found in around half of human CSCCs52–54 and to a lesser extent in HNSCCs59–64, ESCCs55–58 and LSCCs65–67 (FIG. 3).
Tumour protein p63 (TP63) and SOX2 are frequently co-amplified in SCCs owing to their adjacent chromosomal localization (3q)84 (FIG. 3). TP63 is a member of the TP53 gene family and is expressed in the basal compartment of the skin, oesophagus, lung airways and larynx during development and homeostasis85,86. In skin, TP63 is required for epidermal stratification during development and to maintain the proliferative potential of basal keratinocytes during homeostasis87. A reciprocal negative regulation of TP63 expression and NOTCH activity has been described in the skin, where TP63 expressed by basal cells represses NOTCH activity, controlling the switch between proliferation and differentiation88. SOX2 is a transcription factor that controls pluripotency in embryonic stem cells, where it acts together with octamer-binding protein 4 (OCT4; also known as POU5F1)84. In contrast, in LSCC and ESCC, SOX2 acts together with TP63 to regulate the expression of genes involved in squamous carcinogenesis84.
SOX2 gene expression is upregulated in the majority of mouse and human CSCCs89,90 (FIG. 3). Deletion of Sox2 before chemical-induced carcinogenesis (DMBA followed by 12-O-tetradecanoylphorbol-13-acetate (TPA) application) prevents CSCC formation, showing the essential role of SOX2 during CSCC initiation89. Moreover, SOX2-expressing CSCC cells showed higher clonogenic potential upon subcutaneous injection into immunodeficient mice. Sox2 genetic lineage ablation, which allows the selective killing of Sox2-expressing cells in mice with CSCC, leads to tumour regression, consistent with the notion that SOX2 marks skin cancer stem cells89. Deletion of Sox2 impairs tumour propagation and induces CSCC regression89. Altogether, these studies demonstrated that SOX2 is essential for skin tumour initiation and progression and that it marks and regulates the function of cutaneous cancer stem cells, suggesting the presence of a continuum between tumour initiation and progression in skin SCCs89.
Epigenetic regulators
Mutations in chromatin-modifying enzymes leading to DNA and histone modifications have been described in numerous cancers91. Loss-of-function mutations in the histone 3 lysine 4 (H3K4) methyltransferases lysine methyltransferase 2C (KMT2C) and KMT2D have been described in the different types of SCCs52,55,57–59,61–63,65,66. In aggressive CSCC, KMT2C mutations are associated with poor outcome and increased bone invasion52, suggesting that KMT2C functions as a tumour suppressor gene. Additionally, HNSCCs59–64 and ESCCs55–58 carry loss-of-function mutations for nuclear receptor-binding SET domain protein 1 (NSD1), an H3K36 methyltransferase. Individuals carrying inactivating mutations in NSD1 often have overgrowth syndromes (Soto syndrome) and are more prone to cancer, including SCC92. Moreover, human HPV-negative HNSCC with NSD1 inactivating mutations showed decreased expression of genes involved in epithelial differentiation, indicating that NSD1 may act as a tumour suppressor by promoting differentiation93. Histone demethylase (such as lysine demethylase 6A (KDM6A)) inactivating mutations or deletions have been reported in HNSCC59–64, ESCC55–58 and LSCC65–67,94 (FIG. 3). However, the mechanisms by which mutations in these epigenetic regulators promote tumour initiation and progression remain poorly understood95.
Cell of origin in SCCs
The activation of oncogenes or deletion of tumour suppressor genes in specific cell populations using Cre expressed under different promoters has allowed the identification of the cells of origin of the different SCCs. Before the development of lineage tracing techniques, it was often believed that the tumour cells and the cells of origin expressed the same markers and therefore that the cells of origin could be inferred by the marker expression in tumour cells. However, lineage tracing studies have demonstrated that the expression of differentiation markers by tumour cells is sometimes misleading when used to extrapolate or determine their cellular origin (that is, basal cell carcinoma originating from the IFE expresses markers of hair follicle)31,96,97.
Cutaneous SCC
CSCC is one of the most common cancers in humans, accounting for over 700,000 new patients per year in the United States98. CSCCs rarely metastasize (5%), but metastasis is associated with a poor prognosis, with a patient survival of 10–20% over 10 years2. Sun exposure, chronic wounds and immunosuppression are the major risk factors of CSCC. CSCC also occurs in patients receiving BRAF inhibitors for the treatment of melanoma99.
Actinic keratosis (human) and papilloma (mouse) represent benign squamous lesions that progress into malignant CSCCs. The most extensively used mouse model for CSCC is a carcinogen-induced protocol consisting of a topical application of DMBA, a mutagen, followed by administration of TPA, which stimulates epidermal proliferation and inflammation100. TPA administration one year after the last DMBA application induced papilloma formation101,102, suggesting that DMBA-induced mutations occur in long-term epidermal stem cells or that DMBA-induced mutations immortalize a fraction of progenitors and confer them with stem cell properties. DMBA treatment followed by repeated dermo-abrasion, which removes IFE cells, leads to papilloma and carcinoma formation, although it does occur with reduced frequency103, suggesting that different epidermal lineages, including hair follicles and IFE, act as the cells of origin in CSCC. In addition, hair follicle stem cell lineage tracing demonstrates the direct contribution of hair follicle stem cells to DMBA–TPA-induced benign skin tumours104,105.
DMBA–TPA-induced CSCC is almost invariably (>90%) associated with mutations in RAS family members, most frequently Hras, followed by Kras and Rras2 (REFS77,106–108. Overexpression of Hras in differentiated epidermal cells using the K10 promoter leads to the generation of papilloma only at sites of wounding or irritation109, suggesting that either differentiated cells revert back to a stem-like state during wound healing or that wounding represents the second hit in K10+ cells already carrying and expressing the Hras mutant allele. In addition, Hras mutant allele expression in basal cells, using a truncated form of the K5 promoter results in papilloma and CSCC formation in mice110, indicating that CSCC originates from basal cells in the absence of wounding.
The classical transgenic approach described before, in which the K5 or K14 promoter is used to express an oncogene, leads to the constitutive expression (non-inducible) of the oncogene in all basal cells from the early stages of epidermal development to adulthood110. Therefore, this strategy cannot be used to determine from which parts of the epidermis tumours arise. To assess from which adult epidermal compartments CSCCs arise, different groups have used Cre–ER or Cre–PR transgenic mice to conditionally express oncogenic KrasG12D in different epidermal compartments at physiological levels in mice (FIG. 4a). Papilloma was observed following activation of KrasG12D expression in K19+ or K15+ hair follicle stem cells and their progeny (using K19–Cre–ER and K15–Cre–PR transgenic mice)96,97. By contrast, activation of the same oncogenes in the rapidly dividing hair follicle transit-amplifying cells using a Cre-inducible sonic hedgehog (Shh) promoter-driven system (Shh–Cre–ER) did not lead to benign or malignant tumour formation96,97, suggesting that tumours arise from long-lived hair follicle progenitors and/or stem cells rather than transit-amplifying cells (FIG. 4a). Expression of KrasG12D in K14+ basal cells or IVL+ progenitors using K14–Cre–ER or Ivl–Cre–ER transgenic mice leads to papilloma formation96. Combined KrasG12D expression and Trp53 deletion in mice are required to initiate malignant SCCs96,97. Interestingly, whereas K14–Cre–ER-targeted IFE basal cells can give rise to SCC upon KrasG12D expression and Trp53 deletion, Ivl–Cre–ER IFE progenitors cannot (C.B., unpublished observations). Altogether, these studies indicate that hair follicle and IFE stem cells represent the cells of origin of mouse CSCCs (FIG. 4a).
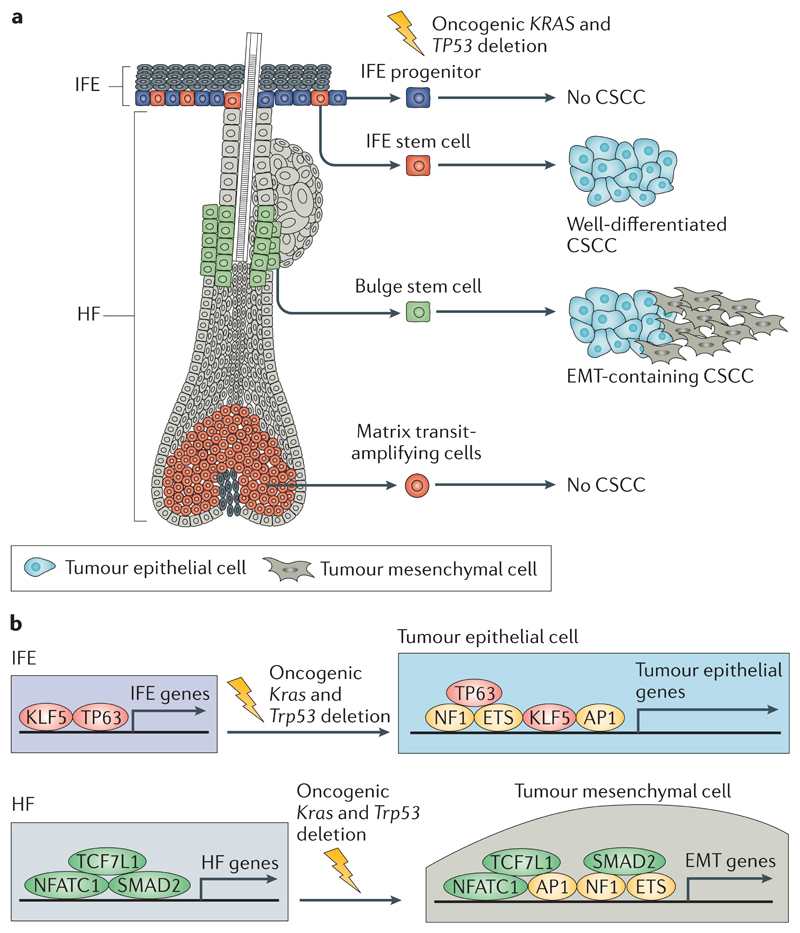
Fig 4. The cells at the origin of CSCC.
a | Activation of oncogenic Kras and deletion of Trp53 in interfollicular epidermis (IFE) and hair follicle (HF) stem cells lead to cutaneous squamous cell carcinoma (CSCC) formation, whereas these gene alterations in IFE progenitors or matrix transit-amplifying cells do not lead to CSCC formation. Oncogenic activation of Kras and deletion of Trp53 in IFE stem cells lead to the generation of well-differentiated CSCCs, whereas activation of the same oncogenic hits in HF stem cells leads to the generation of CSCCs with epithelial to mesenchymal transition (EMT) features. b | The transcriptional and epigenetic landscape of the cell of origin influences tumour differentiation. Upon oncogenic Kras expression and Trp53 deletion, a core of transcription factors (including members of the adaptor protein 1 (AP1), E26 transformation-specific (ETS) and nuclear factor 1 (NF1) families) promote tumour gene expression independently of the cell of origin. In addition to this core of transcription factors, lineage-specific transcription factors controlled by the cells of origin of CSCCs influence the specific differentiation of the tumours. Tumour protein 63 (TP63) and Krüppel-like factor 5 (KLF5) promote the expression of IFE genes and the development of well-differentiated squamous cell carcinomas (SCCs), whereas SMAD family member 2 (SMAD2), nuclear factor of activated T cell, cytoplasmic 1 (NFATC1) and transcription factor 7-like 1 (TCF7L1) promote the expression of HF genes and the development of SCCs in which EMT occurs.
Human CSCCs present a high degree of cellular heterogeneity, varying from well-differentiated to poorly differentiated tumours, which present a higher rate of recurrence and lower rate of cure after treatment2. To determine whether the cells of origin control tumour heterogeneity in CSCCs, intertumoural and intratumoural heterogeneity was assessed in SCCs arising from the same oncogenic hits in different cell lineages of the mouse epidermis111. Whereas conditional oncogenic KrasG12D expression and Trp53 deletion specifically in IFE basal cells, using K14–Cre– ER, lead to well-differentiated tumours in mice, activation of the same oncogenic hits in hair follicle lineages using Lgr5–Cre–ER leads to more invasive and less differentiated tumours with features of epithelial to mesenchymal transition (EMT) or purely mesenchymal-like tumours resembling spindle cell carcinoma111 (FIG. 4). These data demonstrate that oncogene-targeted hair follicle cells are primed to undergo EMT during tumorigenesis. Cancer cells derived from hair follicle lineages that underwent EMT have much higher clonogenic and metastatic potential, suggesting that the cells of origin in CSCC influence tumour stemness, local invasion and lung metastasis. The transcriptional and chromatin landscape of the cells of origin and the presence of different populations of tumour cells revealed that the different epidermal stem cells are epigenetically primed to undergo different differentiation programmes upon oncogenic transformation, giving rise to tumours with different degrees of squamous differentiation and EMT111 (FIG. 4b). Trp63 overexpression restricts EMT in oncogene-targeted hair follicle cells, leading to the formation of well-differentiated SCC, and this finding indicates that Trp63 is a key regulator of epithelial fate. Altogether, this study demonstrates the role of the cell lineages (hair follicle cells versus IFE cells) at the origin of CSCC in regulating EMT and identifies gene regulatory networks that promote squamous differentiation and EMT in primary skin SCCs111.
Head and neck SCC
HNSCCs are the sixth most common cancer worldwide112 and are associated with high mortality — approximately 50% of patients die of the disease. Smoking and alcohol consumption are the major risk factors in developing countries, and infection with HPV is a risk factor for HNSCC among non-smokers4. HPV serotype 16 is associated with increased risk of developing oropharyngeal SCC113 and cervical cancer114. Fanconi anaemia, a rare autosomal recessive disorder characterized by a high degree of genomic instability, predisposes to HNSCC115.
Oral leukoplakia is described to be the precursor lesion of HNSCC in humans116. Several chemical HNSCC carcinogen models have been developed in rodents, including DMBA-based and 4-nitroquinoline 1-oxide (4-NQO)-based methods, which recapitulate some features of human oral SCC (OSCC), including the progression of pre-malignant lesions to differentiated SCCs117. A combination of K14–Cre–ER lineage tracing with 4-NQO treatment in mice leads to the generation of papilloma and OSCC, providing evidence that basal cells represent the cells of origin in OSCC118.
RAS and TP53 genes are mutated in HNSCC59–64 (FIG. 3). The Cre-inducible expression of tumorigenic KrasG12D driven by the K14 or K15 promoter in basal cells of the oral mucosa in K14–Cre–ER and K5–Cre–ER transgenic mice consistently leads to the generation of papillomas in mouse oral mucosa and to hyperplasia of the tongue119,120. Combined KrasG12D activation and Trp53 deletion in mouse oral basal cells using the K14–Cre–ER system resulted in the generation of tongue SCC121 (FIG. 5a).
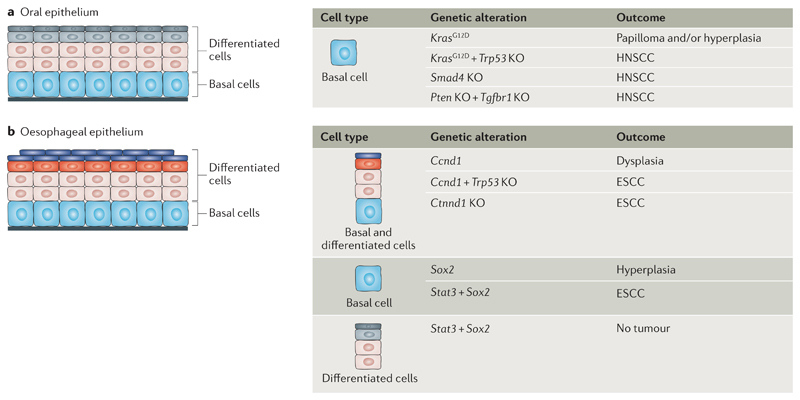
Fig 5. The cells of origin in HNSCC and ESCC.
a | The cells at the origin of head and neck squamous cell carcinoma (HNSCC) are shown. Basal cells of the oral epithelia can give rise to hyperplasia and/or papilloma formation upon oncogenic Kras activation. HNSCC can result from the activation of Kras, the combination of activation of Kras and deletion of Trp53, SMAD family member 4 (Smad4) deletion or double deletion of Pten and transforming growth factorbeta receptor type-1 (Tgfbr1) in basal cells. b | The cells at the origin of oesophageal squamous cell carcinoma (ESCC) are shown. Activation of cyclin D1 (Ccnd1) in combination with Trp53 deletion and deletion of catenin delta-1 (Ctnnd1) in oesophageal epithelial cells leads to ESCC formation. Expression of signal transducer and activator of transcription 3 (Stat3) and SRY-box 2 (Sox2) in basal cells but not in suprabasal cells promotes ESCC. KO, knockout.
Mutations in the transforming growth factor-β (TGFβ) and PTEN–AKT signalling pathways are frequently found in HNSCC59. Loss of function of SMAD family member 4 (Smad4) in mouse oral basal cells, using the Cre-inducible promoters K14–Cre–PR and K5–Cre–PR, leads to the generation of tumours ranging from moderately differentiated to poorly differentiated SCC with RAS activation and genomic instability122. Deletion of Pten or TGF-beta receptor type-1 (Tgfbr1) alone in basal cells using the Cre-inducible K14–Cre–ER promoter resulted in hyperproliferation and very few instances of HNSCC, whereas the combined deletion of Pten and Tgfbr1 in the same cells using K14–Cre–ER led to the formation of HNSCC123 (FIG. 5a). Altogether, these reports demonstrate that HNSCC can originate from oral basal cells.
Oesophageal SCC
Oesophageal cancer is the eighth most common cancer and the sixth leading cause of cancer death worldwide112. Oesophageal cancer includes two major histological subtypes: ESCC and oesophageal adenocarcinoma5. ESCC accounts for the majority (around 90%) of the cases of oesophageal carcinomas and is particularly common in certain regions of Asia. ESCC predominates in the upper and mid-oesophagus and is associated with smoking and alcohol exposure in Western countries. Adenocarcinoma generally occurs in the lower oesophagus near the gastric junction and is associated with gastric reflux and obesity5.
Several studies using the Epstein–Barr virus ED-L2 promoter, which is expressed in both basal and suprabasal compartments of the oraloesophageal squamous epithelia, identified the combination of genetic alterations that lead to ESCC development in mice124–127 (FIG. 5b). TP53 loss of function and CCND1 amplification represent the most frequent genetic alterations in ESCC58. Overexpression of Ccnd1 in mouse oesophageal squamous epithelia using the ED-L2 promoter leads to dysplasia, the precursor lesion of ESCC124. The combined overexpression of Ccnd1 and deletion of Trp53 driven by the ED-L2 promoter resulted in invasive oral-oesophageal cancer, leading to lymph node metastasis in 25% of mice with ESCC125 (FIG. 5b).
Inflammation was suggested to play an important role in ESCC carcinogenesis by fostering a microenvironment favourable for tumour initiation. Kr pel-like factor 4 (Klf4) overexpression in the oesophageal epithelia using the ED-L2 promoter leads to the production of proinflammatory cytokines (tumour necrosis factor (TNF), CXC-chemokine ligand 5 (CXCL5), granulocyte colony-stimulating factor (G-CSF) and interleukin-1α (IL-1α)) by the oesophageal basal cells, resulting in the recruitment of inflammatory cells and SCC formation in mice126. Deletion of catenin δ1 (Ctnnd1) in the tongue, oesophagus and forestomach using ED-L2–Cre mice led to immune cell infiltration, increased proliferation of basal cell, defects in differentiation and generation of ESCC127.
Lineage tracing experiments in a chemical-induced mouse model of oesophageal cancer, consisting of diethylnitrosamine (DEN) and sorafenib application, revealed that high-grade dysplasias resulted from a small bias towards symmetrically renewing division over asymmetrical division or symmetrical differentiation in oesophageal progenitors128. In invasive murine SCCs, which were induced by DEN treatment followed by KrasG12D expression in oesophageal cells of Ah–Cre–ER mice and following sorafenib application, the tumour expansion was driven by a larger bias towards self-renewing division in basal tumour cells128, suggesting that unbalanced cell fate is associated with ESCC development.
Genes controlling squamous cell differentiation, such as NOTCH or SOX2, are frequently altered in human ESCC55–58 (FIG. 3). Inactivation of the NOTCH signalling pathway in the oesophageal epithelium (using a dominant negative mutant of mastermind-like transcriptional coactivator 1 (Maml1) expressed in oesophageal cells of Ah–Cre–ER mice) in mice resulted in the replacement of wild-type epithelium by mutant cells, but this was not sufficient to promote dysplasia or ESCC formation, suggesting that NOTCH inhibition is not sufficient to initiate ESCC carcinogenesis129. SOX2 is amplified in human LSCCs and ESCCs130. Sox2 overexpression in murine oesophageal K5+ basal cells and their progeny (using K5–Cre–ER) leads to the expansion of the basal compartment, defects in squamous differentiation and hyperplasia131. When these Sox2-overexpressing cells are infected with lentivirus expressing constitutively activated signal transducer and activator of transcription 3 (STAT3), they give rise to SCC upon transplantation into immunodeficient mice. However, differentiated suprabasal cells co-expressing Sox2 and Stat3 did not lead to SCC upon transplantation. These findings demonstrate that activated STAT3 and SOX2 overexpression in murine oesophageal basal progenitors, but not differentiated suprabasal cells, leads to ESCC formation, indicating that oesophageal progenitors and/or stem cells represent the cells of origin in ESCC131 (FIG. 5b). Moreover, this study suggested that SOX2 overexpression represents a tumour-initiating event in ESCC and that cooperation with inflammation-mediated STAT3 activation is required for SOX2-driven ESCC tumorigenesis. Altogether, these reports suggest that ESCC originates from oesophageal progenitors and/or stem cells and that cell fate imbalance in the cells of origin is an important feature of tumour initiation.
Lung SCC
Lung cancer is one of the leading causes of cancer-related mortality, resulting in an estimated 1.4 million deaths per year worldwide132. Non-small-cell lung carcinoma and small-cell lung carcinoma are the two most frequent lung cancers. Non-small-cell lung carcinomas account for more than 85% of lung cancer cases and are classified into lung adenocarcinomas (50%), LSCCs (30–40%) and large cell carcinomas. Adenocarcinomas and SCCs present distinct molecular abnormalities65,133,134 and are thought to arise from distinct cells of origin135. Adenocarcinomas and large cell carcinomas usually arise peripherally (from the small bronchi, bronchioles or alveoli), and LSCCs usually arise proximally (from the main bronchi)3. Smoking and chronic inflammation are the major risk factors of LSCCs135.
The combination of KrasG12D expression and serine/threonine kinase 11 (Lkb1; also known as Stk11) deletion in the mouse lung epithelium using inoculation of adenovirus-derived Cre intranasally leads to the generation of a large spectrum of lung tumour types including LSCC136. Combined Pten and Lkb1 deletion in lung epithelia leads to the formation of highly penetrant, well-differentiated, rarely metastatic LSCCs, which appeared in both proximal and distal murine lungs, suggesting that different cell lineages are at the origin of LSCC137. SOX2 is one of the most frequently amplified genes (23%) and is highly expressed in human LSCCs130. SOX2 is expressed physiologically during the development of the airway system and in the adult trachea138. Conditional deletion of Sox2 in the developing airway epithelium using NK2 homeobox 5 (Nkx2-5)–Cre mice resulted in mouse perinatal death due to defects in the airway system and lung development138, including an excess of mucus-producing cells and a decrease of basal, ciliated and secretory cells138. Deletion of Sox2 in adult lung epithelial cells using the ubiquitously expressed cyto-megalovirus-derived Cre–ER promoter leads to a decrease of their renewal potential and repair capacity during tissue injuries138. These data demonstrate the essential role of SOX2 during airway system development and maintenance in adults. Overexpression of Sox2 combined with Lkb1 deletion in murine lung cells, using intranasal inhalation of a lentivirus engineered to overexpress Sox2 under the ubiquitous beta-actin (Actb) promoter and a lentivirus expressing Cre under the ubiquitous phosphoglycerate kinase (Pgk) promoter (Pgk–Cre), leads to the generation of LSCC139 (FIG. 6). By contrast, lentivirus expression of Sox2 in combination with Trp53 deletion or Trp53 and Rb1 double deletion in mouse lung epithelium, using Pgk–Cre lentivirus to delete Trp53 andRb1, leads to the generation of adenocarcinomas139.
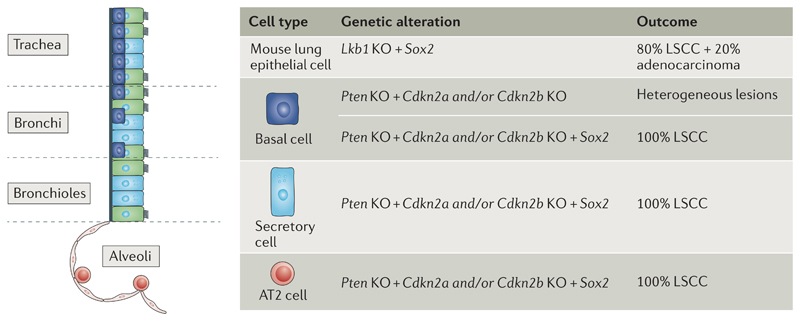
Fig 6. SOX2 promotes LSCC differentiation irrespective of the cell of origin.
Transcription factor SRY-box2 (SOX2) promotes squamous cell fate in lung tumours regardless of the cell of origin. Sox2 overexpression and serine/threonine kinase 11 (Lkb1 deletion in cells of the lung epithelium lead to lung squamous cell carcinoma (LSCC) and adenocarcinoma. In the absence of Sox2 overexpression, Pten and cyclin-dependent kinase inhibitor 2A (Cdkn2a) and/or Cdkn2b deletion in basal cells leads to heterogeneous lesions including adenocarcinoma and LSCCs. Sox2 overexpression and Pten and Cdkn2a and/or Cdkn2b deletion in basal, secretory or type 2 (AT2) cells lead to the formation of LSCCs. KO, knockout.
The previous studies define the combination of mutations required for LSCC development but do not define the lung lineages from which LSCCs arise. Overexpression of Sox2 using Scgb1a1–Cre–ER in mice, which targets secretory cells and rare AT2 cells located in the bronchioalveolar duct junction that display stem cell properties140, leads to the metaplastic transformation of pseudostratified epithelium of the bronchioles and alveoli into columnar cells expressing TP63. Some of these lesions progress into adenocarcinoma-like tumours but express squamous markers141. Oncogenic KrasG12D expression in secretory cells and rare AT2 cells using Scgb1a1–Cre–ER leads to the formation of alveolar adenocarcinomas, whereas the expression of KrasG12D combined with NOTCH inhibition by expression of the dominant negative Maml1 mutant in the same cells resulted in Sox2 expression and alveolar hyperplastic lesions that expressed squamous markers142, suggesting that NOTCH and SOX2 drive squamous differentiation in Kras-induced lung tumours. To gain insight into the cell of origin of LSCCs, the tumour suppressor genes (that is, Pten and Cdkn2a and/or Cdkn2b) or putative amplified oncogenes (that is, Fgfr1 and Sox2) in LSCCs in different lung cell types were deleted or overexpressed143 (FIG. 6). Deletion of Pten and Cdkn2a and/or Cdkn2b in mouse lung basal cells using intratracheal administration of adenovirus-expressing Cre under the K5 promoter (adeno-K5–Cre) or adeno-K14–Cre resulted in low-penetrance tumours of mixed histology. Deletion of Pten and Cdkn2a and/or Cdkn2b in combination with Fgfr1 overexpression in basal cells using intratracheal administration of adeno-K5–Cre or adeno-K14–Cre leads to heterogeneous tumours with sporadic squamous differentiation. By contrast, overexpression of Sox2 and deletion of Pten and Cdkn2a and/or Cdkn2b in basal cells resulted in the generation of multiple proximal LSCCs ranging from moderately differentiated to well-differentiated SCCs, providing evidence that basal cells can represent the cells of origin in LSCCs and that Sox2 overexpression promotes squamous differentiation. Interestingly, Sox2 overexpression in the context of Pten and Cdkn2a and/or Cdkn2b deletion in secretory and AT2 cells (using intratracheal administration of adeno-Scgb1a1–Cre and adeno-Spcc–Cre, respectively) leads to the formation of distal LSCCs, demonstrating that bronchus, bronchiole and alveolus cells can also act as the cells of origin of LSCC143. This study nicely illustrates that basal, secretory and AT2 cells represent different possible cells of origin for LSCCs in mice143. Moreover, it demonstrates that Sox2 overexpression is a key promoter of squamous cell differentiation in LSCCs, as previously reported in CSCCs89.
Limitations of the SCC mouse models
Although some progress has been made in the characterization of the cell of origin of SCCs using murine models, many questions still need to be resolved. The cellular hierarchy present in some tissues from which SCC arises (cervical, oral cavity and head and neck tissues) is poorly characterized. The distinction between stem cells, progenitors and differentiated cells within the basal layer of these epithelia remains complicated. The generation of new specific Cre mice allowing targeting of these different populations will be essential to refine the respective contribution of stem cells versus progenitors in cancer initiation. The mechanisms that confer the competence or resistance of a given cell population to SCC initiation should be further studied to design new strategies to prevent and treat SCCs.
Moreover, caution is needed when extrapolating the results obtained in mice to the human setting, especially where histological differences exist between the two species. This is the case of the oesophageal epithelium, which is keratinized in mice but non-keratinized in humans. It has been suggested that keratinization in mouse oesophagus protects it from injuries and ESCC development. Additionally, the transition from squamous epithelia (oesophagus) to columnar epithelia (gastric cardia) occurs at the gastro-oesophageal junction (between the oesophagus and stomach) in humans, whereas in mice, it occurs within the stomach. Another important difference is observed in the lung; basal cells and cartilage rings extend from the trachea to the bronchioles in humans, whereas in mice, they are limited to the trachea and bronchi (FIG. 2).
Conclusions and future directions
Comparisons across different SCCs illustrate that SCCs are characterized by very similar mutation landscapes, including alterations in the TP53, SOX2, TP63, CDNK2A (P16-INK4A), NOTCH1, KMT2D, PIK3CA and PTEN genes. Comparative genomic studies have reported that LSCCs and ESCCs share more commonly mutated genes with other types of SCCs from other body locations than with adenocarcinomas originating from the same tissue56,94,134. The type and number of oncogenic mutations that occur in cells of origin determine SCC invasiveness, differentiation and EMT features. In mouse models, more than one mutation in an oncogene or a tumour suppressor gene is usually required for the formation of invasive SCCs. TP53 deletion is the most frequent tumour suppressor gene mutation in human and mouse SCCs, and Trp53 deletion is usually sufficient to convert benign tumours into malignant SCCs when another oncogene (Ras or Ccnd1) is expressed in mouse models of SCC96,97,125. In addition to these key oncogenic mutations, cell fate determinants, such as SOX2 or TP63 overexpression, are required to promote the squamous fate of SCCs143.
The different studies that investigate the cells of origin in SCCs highlight another common feature — the cells of origin arise from mutations in proliferative basal cells that are characterized by their ability to self-renew and to generate terminally differentiated cells. No clear demonstration that oncogenic mutations can induce the reversion of non-proliferative epithelial cells to a stem cell-like state and consequently drive SCCs initiation has been provided. Very often, despite their rapid turnover, transit-amplifying progeny seem unable to initiate malignant SCCs, although in some cases, committed progenitors are able to initiate benign squamous tumours. It will be interesting to study whether additional oncogenic mutations in more committed progenitors can lead to SCC formation, as described in basal cell carcinoma or intestinal tumours144,27. However, when mouse tumours arise upon experimental introduction of oncogenic mutations in stem cells, it remains unclear whether the cancer originates directly from the stem cell or from the more committed progeny.
Next-generation sequencing techniques allowed the identification of new drivers in the different types of SCC. New SCC mouse models could be generated to better model the diversity and tumour heterogeneity found in SCCs from different tissues and to better understand how the order and combination of different genetic alterations modify the cell at the origin of cancer and tumour progression. Moreover, a better understanding of the transcriptional and chromatin landscape of the different types of SCCs will uncover common and tissue-specific determinants that regulate tumour initiation and squamous differentiation programmes across different SCCs. In addition, it would be interesting to assess whether the cells of origin in SCCs can be inferred from the DNA methylation patterns of cancer cells, as suggested in CSCC145. Moreover, understanding how the cell of origin controls the generation and properties of cancer stem cells would be essential to designing new strategies to prevent tumour progression and metastasis and to decrease resistance to therapy.
Acknowledgements
C.B. is an investigator of WELBIO. A.S.-D. is supported by a fellowship of the Belgian Fund for Scientific Research (FNRS). CB is supported by the FNRS, the Fondation contre le Cancer, the Université Libre de Bruxelles Fondation, the Fondation Baillet Latour, Worldwide Cancer Research and a consolidator grant from the European Research Council.
Footnotes
Author contributions
Both authors read the literature, discussed the contents of the Review and wrote the article.
Competing interests
The authors declare no competing financial interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dotto GP, Rustgi AK. Squamous cell cancers: a unified perspective on biology and genetics. Cancer Cell. 2016;29:622–637. doi: 10.1016/j.ccell.2016.04.004. [This is a landmark review summarizing the aetiology and genetic determinants of SCCs arising from different body locations.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alam M, Ratner D. Cutaneous squamous-cell carcinoma. N Engl J Med. 2001;344:975–983. doi: 10.1056/NEJM200103293441306. [DOI] [PubMed] [Google Scholar]
- 3.Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359:1367–1380. doi: 10.1056/NEJMra0802714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011;11:9–22. doi: 10.1038/nrc2982. [DOI] [PubMed] [Google Scholar]
- 5.Rustgi AK, El-Serag HB. Esophageal carcinoma. N Engl J Med. 2014;371:2499–2509. doi: 10.1056/NEJMra1314530. [DOI] [PubMed] [Google Scholar]
- 6.Bray F, et al. Trends in cervical squamous cell carcinoma incidence in 13 European countries: changing risk and the effects of screening. Cancer Epidemiol Biomarkers Prev. 2005;14:677–686. doi: 10.1158/1055-9965.EPI-04-0569. [DOI] [PubMed] [Google Scholar]
- 7.Malik RD, et al. Squamous cell carcinoma of the prostate. Rev Urol. 2011;13:56–60. [PMC free article] [PubMed] [Google Scholar]
- 8.Tunio MA, Al Asiri M, Fagih M, Akasha R. Primary squamous cell carcinoma of thyroid: a case report and review of literature. Head Neck Oncol. 2012;4:8. doi: 10.1186/1758-3284-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ben Kridis W, et al. Primary squamous cell carcinoma of the pancreas: a report of two cases and review of the literature. Intern Med. 2015;54:1357–1359. doi: 10.2169/internalmedicine.54.4091. [DOI] [PubMed] [Google Scholar]
- 10.Martin JW, et al. Squamous cell carcinoma of the urinary bladder: systematic review of clinical characteristics and therapeutic approaches. Arab J Urol. 2016;14:183–191. doi: 10.1016/j.aju.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blanpain C. Tracing the cellular origin of cancer. Nat Cell Biol. 2013;15:126–134. doi: 10.1038/ncb2657. [DOI] [PubMed] [Google Scholar]
- 12.Van Keymeulen A, Blanpain C. Tracing epithelial stem cells during development, homeostasis, and repair. J Cell Biol. 2012;197:575–584. doi: 10.1083/jcb.201201041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blanpain C, Fuchs E. Stem cell plasticity. Plasticity of epithelial stem cells in tissue regeneration. Science. 2014;344 doi: 10.1126/science.1242281. 1242281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blanpain C, Fuchs E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol Cell Biol. 2009;10:207–217. doi: 10.1038/nrm2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaks V, et al. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat Genet. 2008;40:1291–1299. doi: 10.1038/ng.239. [DOI] [PubMed] [Google Scholar]
- 16.Jensen KB, et al. Lrig1 expression defines a distinct multipotent stem cell population in mammalian epidermis. Cell Stem Cell. 2009;4:427–439. doi: 10.1016/j.stem.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horsley V, et al. Blimp1 defines a progenitor population that governs cellular input to the sebaceous gland. Cell. 2006;126:597–609. doi: 10.1016/j.cell.2006.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morris RJ, et al. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol. 2004;22:411–417. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- 19.Page ME, Lombard P, Ng F, Gottgens B, Jensen KB. The epidermis comprises autonomous compartments maintained by distinct stem cell populations. Cell Stem Cell. 2013;13:471–482. doi: 10.1016/j.stem.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levy V, Lindon C, Zheng Y, Harfe BD, Morgan BA. Epidermal stem cells arise from the hair follicle after wounding. FASEB J. 2007;21:1358–1366. doi: 10.1096/fj.06-6926com. [DOI] [PubMed] [Google Scholar]
- 21.Ito M, et al. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med. 2005;11:1351–1354. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- 22.Aragona M, et al. Defining stem cell dynamics and migration during wound healing in mouse skin epidermis. Nat Commun. 2017;8 doi: 10.1038/ncomms14684. 14684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rompolas P, et al. Spatiotemporal coordination of stem cell commitment during epidermal homeostasis. Science. 2016;352:1471–1474. doi: 10.1126/science.aaf7012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sada A, et al. Defining the cellular lineage hierarchy in the interfollicular epidermis of adult skin. Nat Cell Biol. 2016;18:619–631. doi: 10.1038/ncb3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mascre G, et al. Distinct contribution of stem and progenitor cells to epidermal maintenance. Nature. 2012;489:257–262. doi: 10.1038/nature11393. [DOI] [PubMed] [Google Scholar]
- 26.Gomez C, et al. The interfollicular epidermis of adult mouse tail comprises two distinct cell lineages that are differentially regulated by Wnt, Edaradd, and Lrig1. Stem Cell Rep. 2013;1:19–27. doi: 10.1016/j.stemcr.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanchez-Danes A, et al. Defining the clonal dynamics leading to mouse skin tumour initiation. Nature. 2016;536:298–303. doi: 10.1038/nature19069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clayton E, et al. A single type of progenitor cell maintains normal epidermis. Nature. 2007;446:185–189. doi: 10.1038/nature05574. [DOI] [PubMed] [Google Scholar]
- 29.Doupe DP, Klein AM, Simons BD, Jones PH. The ordered architecture of murine ear epidermis is maintained by progenitor cells with random fate. Dev Cell. 2010;18:317–323. doi: 10.1016/j.devcel.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 30.Potten CS. Cell replacement in epidermis (keratopoiesis) via discrete units of proliferation. Int Rev Cytol. 1981;69:271–318. doi: 10.1016/s0074-7696(08)62326-8. [DOI] [PubMed] [Google Scholar]
- 31.Youssef KK, et al. Identification of the cell lineage at the origin of basal cell carcinoma. Nat Cell Biol. 2010;12:299–305. doi: 10.1038/ncb2031. [DOI] [PubMed] [Google Scholar]
- 32.Hume WJ, Potten CS. The ordered columnar structure of mouse filiform papillae. J Cell Sci. 1976;22:149–160. doi: 10.1242/jcs.22.1.149. [DOI] [PubMed] [Google Scholar]
- 33.Arnold K, et al. Sox2(+) adult stem and progenitor cells are important for tissue regeneration and survival of mice. Cell Stem Cell. 2011;9:317–329. doi: 10.1016/j.stem.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okubo T, Clark C, Hogan BL. Cell lineage mapping of taste bud cells and keratinocytes in the mouse tongue and soft palate. Stem Cells. 2009;27:442–450. doi: 10.1634/stemcells.2008-0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marques-Pereira JP, Leblond CP. Mitosis and differentiation in the stratified squamous epithelium of the rat esophagus. Am J Anat. 1965;117:73–87. doi: 10.1002/aja.1001170106. [DOI] [PubMed] [Google Scholar]
- 36.Leblond CP, Clermont Y, Nadler NJ. The pattern of stem cell renewal in three epithelia. (esophagus, intestine and testis) Proc Can Cancer Conf. 1967;7:3–30. [PubMed] [Google Scholar]
- 37.Doupe DP, et al. A single progenitor population switches behavior to maintain and repair esophageal epithelium. Science. 2012;337:1091–1093. doi: 10.1126/science.1218835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seery JP, Watt FM. Asymmetric stem-cell divisions define the architecture of human oesophageal epithelium. Curr Biol. 2000;10:1447–1450. doi: 10.1016/s0960-9822(00)00803-4. [DOI] [PubMed] [Google Scholar]
- 39.Croagh D, Phillips WA, Redvers R, Thomas RJ, Kaur P. Identification of candidate murine esophageal stem cells using a combination of cell kinetic studies and cell surface markers. Stem Cells. 2007;25:313–318. doi: 10.1634/stemcells.2006-0421. [DOI] [PubMed] [Google Scholar]
- 40.DeWard AD, Cramer J, Lagasse E. Cellular heterogeneity in the mouse esophagus implicates the presence of a nonquiescent epithelial stem cell population. Cell Rep. 2014;9:701–711. doi: 10.1016/j.celrep.2014.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giroux V, et al. Long-lived keratin 15+ esophageal progenitor cells contribute to homeostasis and regeneration. J Clin Invest. 2017;127:2378–2391. doi: 10.1172/JCI88941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barbera M, et al. The human squamous oesophagus has widespread capacity for clonal expansion from cells at diverse stages of differentiation. Gut. 2015;64:11–19. doi: 10.1136/gutjnl-2013-306171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rock JR, et al. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci USA. 2009;106:12771–12775. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rawlins EL, et al. The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell. 2009;4:525–534. doi: 10.1016/j.stem.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rawlins EL, Hogan BL. Ciliated epithelial cell lifespan in the mouse trachea and lung. Am J Physiol Lung Cell Mol Physiol. 2008;295:L231–L234. doi: 10.1152/ajplung.90209.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barkauskas CE, et al. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest. 2013;123:3025–3036. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Desai TJ, Brownfield DG, Krasnow MA. Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature. 2014;507:190–194. doi: 10.1038/nature12930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tata PR, et al. Dedifferentiation of committed epithelial cells into stem cells in vivo. Nature. 2013;503:218–223. doi: 10.1038/nature12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rock JR, Hogan BL. Epithelial progenitor cells in lung development, maintenance, repair, and disease. Annu Rev Cell Dev Biol. 2011;27:493–512. doi: 10.1146/annurev-cellbio-100109-104040. [DOI] [PubMed] [Google Scholar]
- 50.Pardo-Saganta A, et al. Injury induces direct lineage segregation of functionally distinct airway basal stem/progenitor cell subpopulations. Cell Stem Cell. 2015;16:184–197. doi: 10.1016/j.stem.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vogelstein B, et al. Cancer genome landscapes. Science. 2013;339:1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pickering CR, et al. Mutational landscape of aggressive cutaneous squamous cell carcinoma. Clin Cancer Res. 2014;20:6582–6592. doi: 10.1158/1078-0432.CCR-14-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.South AP, et al. NOTCH1 mutations occur early during cutaneous squamous cell carcinogenesis. J Invest Dermatol. 2014;134:2630–2638. doi: 10.1038/jid.2014.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang NJ, et al. Loss-of-function mutations in Notch receptors in cutaneous and lung squamous cell carcinoma. Proc Natl Acad Sci USA. 2011;108:17761–17766. doi: 10.1073/pnas.1114669108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.The Cancer Genome Atlas Research, N et al. Integrated genomic characterization of oesophageal carcinoma. Nature. 2017;541:169–175. doi: 10.1038/nature20805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Song Y, et al. Identification of genomic alterations in oesophageal squamous cell cancer. Nature. 2014;509:91–95. doi: 10.1038/nature13176. [DOI] [PubMed] [Google Scholar]
- 57.Lin DC, et al. Genomic and molecular characterization of esophageal squamous cell carcinoma. Nat Genet. 2014;46:467–473. doi: 10.1038/ng.2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gao YB, et al. Genetic landscape of esophageal squamous cell carcinoma. Nat Genet. 2014;46:1097–1102. doi: 10.1038/ng.3076. [DOI] [PubMed] [Google Scholar]
- 59.The Cancer Genome Atlas, N. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517:576–582. doi: 10.1038/nature14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Agrawal N, et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science. 2011;333:1154–1157. doi: 10.1126/science.1206923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seiwert TY, et al. Integrative and comparative genomic analysis of HPV-positive and HPV-negative head and neck squamous cell carcinomas. Clin Cancer Res. 2015;21:632–641. doi: 10.1158/1078-0432.CCR-13-3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stransky N, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333:1157–1160. doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lechner M, et al. Targeted next-generation sequencing of head and neck squamous cell carcinoma identifies novel genetic alterations in HPV+ and HPV- tumors. Genome Med. 2013;5:49. doi: 10.1186/gm453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pickering CR, et al. Integrative genomic characterization of oral squamous cell carcinoma identifies frequent somatic drivers. Cancer Discov. 2013;3:770–781. doi: 10.1158/2159-8290.CD-12-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.The Cancer Genome Atlas Research, N. Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489:519–525. doi: 10.1038/nature11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim Y, et al. Integrative and comparative genomic analysis of lung squamous cell carcinomas in East Asian patients. J Clin Oncol. 2014;32:121–128. doi: 10.1200/JCO.2013.50.8556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li C, et al. Whole exome sequencing identifies frequent somatic mutations in cell-cell adhesion genes in chinese patients with lung squamous cell carcinoma. Sci Rep. 2015;5 doi: 10.1038/srep14237. 14237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.The Cancer Genome Atlas Research, N et al. Integrated genomic and molecular characterization of cervical cancer. Nature. 2017;543:378–384. doi: 10.1038/nature21386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ojesina AI, et al. Landscape of genomic alterations in cervical carcinomas. Nature. 2014;506:371–375. doi: 10.1038/nature12881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen J. The cell-cycle arrest and apoptotic functions of p53 in tumor initiation and progression. Cold Spring Harb Perspect Med. 2016;6 doi: 10.1101/cshperspect.a026104. a026104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weinberg RA. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 72.Baldin V, Lukas J, Marcote MJ, Pagano M, Draetta G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993;7:812–821. doi: 10.1101/gad.7.5.812. [DOI] [PubMed] [Google Scholar]
- 73.Meyer N, Penn LZ. Reflecting on 25 years with MYC. Nat Rev Cancer. 2008;8:976–990. doi: 10.1038/nrc2231. [DOI] [PubMed] [Google Scholar]
- 74.Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3:11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- 75.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 76.Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141:1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Quintanilla M, Brown K, Ramsden M, Balmain A. Carcinogen-specific mutation and amplification of Ha-ras during mouse skin carcinogenesis. Nature. 1986;322:78–80. doi: 10.1038/322078a0. [DOI] [PubMed] [Google Scholar]
- 78.Spencer JM, Kahn SM, Jiang W, DeLeo VA, Weinstein IB. Activated ras genes occur in human actinic keratoses, premalignant precursors to squamous cell carcinomas. Arch Dermatol. 1995;131:796–800. [PubMed] [Google Scholar]
- 79.Koch U, Lehal R, Radtke F. Stem cells living with a Notch. Development. 2013;140:689–704. doi: 10.1242/dev.080614. [DOI] [PubMed] [Google Scholar]
- 80.Nowell CS, Radtke F. Notch as a tumour suppressor. Nat Rev Cancer. 2017;17:145–159. doi: 10.1038/nrc.2016.145. [DOI] [PubMed] [Google Scholar]
- 81.Nicolas M, et al. Notch1 functions as a tumor suppressor in mouse skin. Nat Genet. 2003;33:416–421. doi: 10.1038/ng1099. [DOI] [PubMed] [Google Scholar]
- 82.Blanpain C, Lowry WE, Pasolli HA, Fuchs E. Canonical notch signaling functions as a commitment switch in the epidermal lineage. Genes Dev. 2006;20:3022–3035. doi: 10.1101/gad.1477606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rangarajan A, et al. Notch signaling is a direct determinant of keratinocyte growth arrest and entry into differentiation. EMBO J. 2001;20:3427–3436. doi: 10.1093/emboj/20.13.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Watanabe H, et al. SOX2 and p63 colocalize at genetic loci in squamous cell carcinomas. J Clin Invest. 2014;124:1636–1645. doi: 10.1172/JCI71545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Crum CP, McKeon FD. p63 in epithelial survival, germ cell surveillance, and neoplasia. Annu Rev Pathol. 2010;5:349–371. doi: 10.1146/annurev-pathol-121808-102117. [DOI] [PubMed] [Google Scholar]
- 86.Melino G, Memmi EM, Pelicci PG, Bernassola F. Maintaining epithelial stemness with p63. Sci Signal. 2015;8:re9. doi: 10.1126/scisignal.aaa1033. [DOI] [PubMed] [Google Scholar]
- 87.Blanpain C, Fuchs E. p63: revving up epithelial stem-cell potential. Nat Cell Biol. 2007;9:731–733. doi: 10.1038/ncb0707-731. [DOI] [PubMed] [Google Scholar]
- 88.Dotto GP. Notch tumor suppressor function. Oncogene. 2008;27:5115–5123. doi: 10.1038/onc.2008.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Boumahdi S, et al. SOX2 controls tumour initiation and cancer stem-cell functions in squamous-cell carcinoma. Nature. 2014;511:246–250. doi: 10.1038/nature13305. [DOI] [PubMed] [Google Scholar]
- 90.Siegle JM, et al. SOX2 is a cancer-specific regulator of tumour initiating potential in cutaneous squamous cell carcinoma. Nat Commun. 2014;5 doi: 10.1038/ncomms5511. 4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dawson MA, Kouzarides T. Cancer epigenetics: from mechanism to therapy. Cell. 2012;150:12–27. doi: 10.1016/j.cell.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 92.Fickie MR, et al. Adults with Sotos syndrome: review of 21 adults with molecularly confirmed NSD1 alterations, including a detailed case report of the oldest person. Am J Med Genet A. 2011;155A:2105–2111. doi: 10.1002/ajmg.a.34156. [DOI] [PubMed] [Google Scholar]
- 93.Papillon-Cavanagh S, et al. Impaired H3K36 methylation defines a subset of head and neck squamous cell carcinomas. Nat Genet. 2017;49:180–185. doi: 10.1038/ng.3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hoadley KA, et al. Multiplatform analysis of 12 cancer types reveals molecular classification within and across tissues of origin. Cell. 2014;158:929–944. doi: 10.1016/j.cell.2014.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu F, Wang L, Perna F, Nimer SD. Beyond transcription factors: how oncogenic signalling reshapes the epigenetic landscape. Nat Rev Cancer. 2016;16:359–372. doi: 10.1038/nrc.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lapouge G, et al. Identifying the cellular origin of squamous skin tumors. Proc Natl Acad Sci USA. 2011;108:7431–7436. doi: 10.1073/pnas.1012720108. [This study demonstrates that only stem cells and not transit-amplifying cells from the epidermis are able to form CSCC.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.White AC, et al. Defining the origins of Ras/p53-mediated squamous cell carcinoma. Proc Natl Acad Sci USA. 2011;108:7425–7430. doi: 10.1073/pnas.1012670108. [References 96 and 97 report that only stem cells and not hair follicle transit-amplifying cells are able to give rise to CSCC.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J Am Acad Dermatol. 2013;68:957–966. doi: 10.1016/j.jaad.2012.11.037. [DOI] [PubMed] [Google Scholar]
- 99.Arnault JP, et al. Keratoacanthomas and squamous cell carcinomas in patients receiving sorafenib. J Clin Oncol. 2009;27:e59–61. doi: 10.1200/JCO.2009.23.4823. [DOI] [PubMed] [Google Scholar]
- 100.Abel EL, Angel JM, Kiguchi K, DiGiovanni J. Multi-stage chemical carcinogenesis in mouse skin: fundamentals and applications. Nat Protoc. 2009;4:1350–1362. doi: 10.1038/nprot.2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Van Duuren BL, Sivak A, Katz C, Seidman I, Melchionne S. The effect of aging and interval between primary and secondary treatment in two-stage carcinogenesis on mouse skin. Cancer Res. 1975;35:502–505. [PubMed] [Google Scholar]
- 102.Morris RJ. Keratinocyte stem cells: targets for cutaneous carcinogens. J Clin Invest. 2000;106:3–8. doi: 10.1172/JCI10508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Argyris TS, Slaga TJ. Promotion of carcinomas by repeated abrasion in initiated skin of mice. Cancer Res. 1981;41:5193–5195. [PubMed] [Google Scholar]
- 104.Li S, et al. A keratin 15 containing stem cell population from the hair follicle contributes to squamous papilloma development in the mouse. Mol Carcinog. 2013;52:751–759. doi: 10.1002/mc.21896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Morris RJ, Tryson KA, Wu KQ. Evidence that the epidermal targets of carcinogen action are found in the interfollicular epidermis of infundibulum as well as in the hair follicles. Cancer Res. 2000;60:226–229. [PubMed] [Google Scholar]
- 106.Balmain A, Ramsden M, Bowden GT, Smith J. Activation of the mouse cellular Harvey-ras gene in chemically induced benign skin papillomas. Nature. 1984;307:658–660. doi: 10.1038/307658a0. [DOI] [PubMed] [Google Scholar]
- 107.Bizub D, Wood AW, Skalka AM. Mutagenesis of the Ha-ras oncogene in mouse skin tumors induced by polycyclic aromatic hydrocarbons. Proc Natl Acad Sci USA. 1986;83:6048–6052. doi: 10.1073/pnas.83.16.6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nassar D, Latil M, Boeckx B, Lambrechts D, Blanpain C. Genomic landscape of carcinogen-induced and genetically induced mouse skin squamous cell carcinoma. Nat Med. 2015;21:946–954. doi: 10.1038/nm.3878. [DOI] [PubMed] [Google Scholar]
- 109.Bailleul B, et al. Skin hyperkeratosis and papilloma formation in transgenic mice expressing a ras oncogene from a suprabasal keratin promoter. Cell. 1990;62:697–708. doi: 10.1016/0092-8674(90)90115-u. [DOI] [PubMed] [Google Scholar]
- 110.Brown K, Kristopaitis T, Yong S, Chejfec G, Pickleman J. Cystic glucagonoma: A rare variant of an uncommon neuroendocrine pancreas tumor. J. Gastrointest Surg. 1998;2:533–536. doi: 10.1016/s1091-255x(98)80053-x. [DOI] [PubMed] [Google Scholar]
- 111.Latil M, et al. Cell-Type-Specific Chromatin States Differentially Prime Squamous Cell Carcinoma Tumor-Initiating Cells for Epithelial to Mesenchymal Transition. Cell Stem Cell. 2017;20:191–204.e5. doi: 10.1016/j.stem.2016.10.018. [This study demonstrates for the first time that the cancer cell of origin determines the tumour phenotype, occurrence of EMT and aggressiveness in CSCCs.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 113.Andrews E, Seaman WT, Webster-Cyriaque J. Oropharyngeal carcinoma in non-smokers and non-drinkers: a role for HPV. Oral Oncol. 2009;45:486–491. doi: 10.1016/j.oraloncology.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 114.zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2:342–350. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 115.Kutler DI, et al. High incidence of head and neck squamous cell carcinoma in patients with Fanconi anemia. Arch Otolaryngol Head Neck Surg. 2003;129:106–112. doi: 10.1001/archotol.129.1.106. [DOI] [PubMed] [Google Scholar]
- 116.Califano J, et al. Genetic progression model for head and neck cancer: implications for field cancerization. Cancer Res. 1996;56:2488–2492. [PubMed] [Google Scholar]
- 117.Hawkins BL, et al. 4NQO carcinogenesis: a mouse model of oral cavity squamous cell carcinoma. Head Neck. 1994;16:424–432. doi: 10.1002/hed.2880160506. [DOI] [PubMed] [Google Scholar]
- 118.Tang XH, Scognamiglio T, Gudas LJ. Basal stem cells contribute to squamous cell carcinomas in the oral cavity. Carcinogenesis. 2013;34:1158–1164. doi: 10.1093/carcin/bgt021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Caulin C, et al. Inducible activation of oncogenic K-ras results in tumor formation in the oral cavity. Cancer Res. 2004;64:5054–5058. doi: 10.1158/0008-5472.CAN-04-1488. [DOI] [PubMed] [Google Scholar]
- 120.Raimondi AR, Vitale-Cross L, Amornphimoltham P, Gutkind JS, Molinolo A. Rapid development of salivary gland carcinomas upon conditional expression of K-ras driven by the cytokeratin 5 promoter. Am J Pathol. 2006;168:1654–1665. doi: 10.2353/ajpath.2006.050847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Raimondi AR, Molinolo A, Gutkind JS. Rapamycin prevents early onset of tumorigenesis in an oral-specific K-ras and p53 two-hit carcinogenesis model. Cancer Res. 2009;69:4159–4166. doi: 10.1158/0008-5472.CAN-08-4645. [DOI] [PubMed] [Google Scholar]
- 122.Bornstein S, et al. Smad4 loss in mice causes spontaneous head and neck cancer with increased genomic instability and inflammation. J Clin Invest. 2009;119:3408–3419. doi: 10.1172/JCI38854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bian Y, et al. Loss of TGF-beta signaling and PTEN promotes head and neck squamous cell carcinoma through cellular senescence evasion and cancer-related inflammation. Oncogene. 2012;31:3322–3332. doi: 10.1038/onc.2011.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nakagawa H, et al. The targeting of the cyclin D1 oncogene by an Epstein-Barr virus promoter in transgenic mice causes dysplasia in the tongue, esophagus and forestomach. Oncogene. 1997;14:1185–1190. doi: 10.1038/sj.onc.1200937. [DOI] [PubMed] [Google Scholar]
- 125.Opitz OG, et al. A mouse model of human oral-esophageal cancer. J Clin Invest. 2002;110:761–769. doi: 10.1172/JCI15324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tetreault MP, et al. Klf4 overexpression activates epithelial cytokines and inflammation-mediated esophageal squamous cell cancer in mice. Gastroenterology. 2010;139:2124–2134.e9. doi: 10.1053/j.gastro.2010.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Stairs DB, et al. Deletion of p120-catenin results in a tumor microenvironment with inflammation and cancer that establishes it as a tumor suppressor gene. Cancer Cell. 2011;19:470–483. doi: 10.1016/j.ccr.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Frede J, Greulich P, Nagy T, Simons BD, Jones PH. A single dividing cell population with imbalanced fate drives oesophageal tumour growth. Nat Cell Biol. 2016;18:967–978. doi: 10.1038/ncb3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Alcolea MP, et al. Differentiation imbalance in single oesophageal progenitor cells causes clonal immortalization and field change. Nat Cell Biol. 2014;16:615–622. doi: 10.1038/ncb2963. [This study reports that equipotent oesophageal progenitors are the cells of origin of ESCC by cell fate imbalance.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bass AJ, et al. SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nat Genet. 2009;41:1238–1242. doi: 10.1038/ng.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Liu K, et al. Sox2 cooperates with inflammation-mediated Stat3 activation in the malignant transformation of foregut basal progenitor cells. Cell Stem Cell. 2013;12:304–315. doi: 10.1016/j.stem.2013.01.007. [This study reports that oesophageal basal cells but not differentiated cells lead to ESCC upon activation of STAT3 and overexpression of SOX2.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jemal A, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. (2011) [DOI] [PubMed] [Google Scholar]
- 133.The Cancer Genome Atlas Research, N. Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511:543–550. doi: 10.1038/nature13385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Campbell JD, et al. Distinct patterns of somatic genome alterations in lung adenocarcinomas and squamous cell carcinomas. Nat Genet. 2016;48:607–616. doi: 10.1038/ng.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chen Z, Fillmore CM, Hammerman PS, Kim CF, Wong KK. Non-small-cell lung cancers: a heterogeneous set of diseases. Nat Rev Cancer. 2014;14:535–546. doi: 10.1038/nrc3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ji H, et al. LKB1 modulates lung cancer differentiation and metastasis. Nature. 2007;448:807–810. doi: 10.1038/nature06030. [DOI] [PubMed] [Google Scholar]
- 137.Xu C, et al. Loss of Lkb1 and Pten leads to lung squamous cell carcinoma with elevated PD-L1 expression. Cancer Cell. 2014;25:590–604. doi: 10.1016/j.ccr.2014.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Que J, Luo X, Schwartz RJ, Hogan BL. Multiple roles for Sox2 in the developing and adult mouse trachea. Development. 2009;136:1899–1907. doi: 10.1242/dev.034629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mukhopadhyay A, et al. Sox2 cooperates with Lkb1 loss in a mouse model of squamous cell lung cancer. Cell Rep. 2014;8:40–49. doi: 10.1016/j.celrep.2014.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kim CF, et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 141.Lu Y, et al. Evidence that SOX2 overexpression is oncogenic in the lung. PLoS ONE. 2010;5:e11022. doi: 10.1371/journal.pone.0011022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Xu X, et al. The cell of origin and subtype of K-Ras-induced lung tumors are modified by Notch and Sox2. Genes Dev. 2014;28:1929–1939. doi: 10.1101/gad.243717.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ferone G, et al. SOX2 is the determining oncogenic switch in promoting lung squamous cell carcinoma from different cells of origin. Cancer Cell. 2016;30:519–532. doi: 10.1016/j.ccell.2016.09.001. [This study demonstrates that three different lung lineages (basal, secretory and AT2 cells) represent the cell of origin of LSCC and that SOX2 restricts tumour lineage to SCC.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schwitalla S, et al. Intestinal tumorigenesis initiated by dedifferentiation and acquisition of stem-cell-like properties. Cell. 2013;152:25–38. doi: 10.1016/j.cell.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 145.Rodriguez-Paredes M, et al. Methylation profiling identifies two subclasses of squamous cell carcinoma related to distinct cells of origin. Nat Commun. 2018;9 doi: 10.1038/s41467-018-03025-1. 577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Barker N, et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–611. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- 147.Tomasetti C, Li L, Vogelstein B. Stem cell divisions, somatic mutations, cancer etiology, and cancer prevention. Science. 2017;355:1330–1334. doi: 10.1126/science.aaf9011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tomasetti C, Vogelstein B. Cancer risk: role of environment-response. Science. 2015;347:729–731. doi: 10.1126/science.aaa6592. [DOI] [PubMed] [Google Scholar]
- 149.Zhu L, et al. Multi-organ Mapping of Cancer Risk. Cell. 2016;166:1132–1146.e7. doi: 10.1016/j.cell.2016.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]