Abstract
Purpose
To systematically evaluate and summarize evidence across multiple systematic reviews (SRs) examining interventions addressing polypharmacy.
Summary
MEDLINE, the Cochrane Database of Systematic Reviews, and the Database of Abstracts of Reviews of Effects (DARE) were searched for SRs evaluating interventions addressing polypharmacy in adults published from January 2004 to February 2017. Two authors independently screened, appraised, and extracted information. SRs with Assessment of Multiple Systematic Reviews (AMSTAR) scores below 8 were excluded. After extraction of relevant conclusions from each SR, evidence was summarized and conclusions compared. Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology was used to assess evidence quality. Six SRs met the inclusion criteria, 4 of which used meta-analytic pooling. Five SRs focused on older adults. Four were not restricted to any specific disease type, whereas 1 focused on proton pump inhibitors and another focused on patients with severe dementia. Care settings and measured outcomes varied widely. SRs examining the impact on patient-centered outcomes, including morbidity, mortality, patient satisfaction, and utilization, found inconsistent evidence regarding the benefit of polypharmacy interventions, but most concluded that interventions had either null or uncertain impact. Two SRs assessing medication appropriateness found very low-quality evidence of modest improvements with polypharmacy interventions.
Conclusion
An overview of SRs of interventions to address polypharmacy found 6 recent and high-quality SRs, mostly focused on older adults, in which both process and outcome measures were used to evaluate interventions. Despite the low quality of evidence in the underlying primary studies, both SRs that assessed medication appropriateness found evidence that polypharmacy interventions improved it. However, there was no consistent evidence of any impact on downstream patient-centered outcomes such as healthcare utilization, morbidity, or mortality.
Keywords: systematic review, review, polypharmacy, deprescriptions, aged
KEY POINTS
Six high-quality systematic reviews of interventions addressing polypharmacy were identified.
The 2 systematic reviews considering the outcome of medication appropriateness found improvements with use of polypharmacy interventions; however, the underlying evidence assessed in these reviews was of low or very low quality.
No discernible impact of polypharmacy interventions on more downstream and patientrelevant outcomes (e.g., mortality, symptoms, adverse drug events, hospitalizations) was apparent from the reviewed evidence.
The sickest patients in the community are recently hospitalized elders. A substantial component of their morbidity and mortality is adverse drug events (ADEs).1–3 Moreover, the oldest, sickest patients are at highest risk for ADEs; they have the most complex and hazardous medication regimens but the fewest social and economic resources and the least physiologic reserve.4 This dangerous milieu frequently contributes to avoidable healthcare resource utilization, morbidity, and even mortality.5
As part of a larger plan to create a toolkit of evidence-based practices to improve medication management for recently hospitalized elders, we sought first to systematically review interventions in 3 domains encompassing much of medication management: postdischarge medication reconciliation, polypharmacy, and medication adherence. We address polypharmacy here; findings for the other 2 domains will be published subsequently as separate systematic overviews.
Polypharmacy is a major contributor to ADEs among frail elders, especially among those recently hospitalized. The most common definition of polypharmacy is strictly numerical, referring to the use of multiple medications daily.6 It has been argued, however, that a specific number of drugs does not indicate appropriateness of therapy, as all drugs may be necessary and appropriate for treatment.6 Therefore, there has been a shift toward the term inappropriate polypharmacy, which describes treatment where a patient has multiple morbidities and/or a complex condition that is being managed with more than 1 medicine and where the potential harms outweigh the potential benefits.7
Because polypharmacy is an area of intense interest, interventions addressing polypharmacy have generated hundreds of primary studies and dozens of systematic reviews (SRs). Elucidating the central findings of this literature can be unwieldy due not only to its volume but also because findings may differ by study setting and population, intervention characteristics, outcomes measured, analytic methods, sample sizes, and even differing interpretations. SRs have gained acceptance as a robust methodology to efficiently distill and summarize prior findings. However, because SRs may themselves be subject to the aforementioned concerns, especially in areas in which several SRs have been conducted, some researchers have encouraged the use of systematic overviews of SRs. With dozens of existing SRs on polypharmacy already published, we applied this systematic overview methodology. This approach allowed us to capitalize on both the accepted methodology of systematically evaluating literature and a large body of secondary literature.
Using this approach, we sought to understand and summarize existing evidence regarding the potential of interventions addressing polypharmacy to improve patient-centered outcomes for older adults, specifically after hospitalization. Studies have shown that transitions of care (e.g., into and out of the hospital) are a particularly dangerous time in terms of medication safety due to factors such as discontinuity of care, changes in medication regimens, the rushed nature of the discharge process, and inadequate patient and/or caregiver education.8 Although this overview provides a foundation for a toolkit targeting the postdischarge period, we considered interventions implemented across all time periods, with the idea that some successful interventions might be reconfigured for the postdischarge period, during which medication management is perhaps most challenging.
Methods
The systematic overview was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta Analyses (PRISMA) statement9; the PRISMA checklist used may be found in the supplementary material at www.ajhp.org (eAppendix A). For methodological guidance specific to systematic overviews of SRs, we also referred to published literature explicitly focused on this methodology.10–13
Data sources and searches
We performed a literature search in February 2017 using the databases MEDLINE, the Cochrane Database of Systematic Reviews, and the Database of Abstracts of Reviews of Effects (DARE). Two trained researchers developed search terms related to polypharmacy. The searches were limited to English-language articles published from January 2004 through February 2017, with a manual search of prior SR references to identify earlier or unpublished SRs. The search strategies are detailed in eAppendix B.
Selection of SRs
SRs (with or without meta-analyses) were eligible for review if they evaluated interventions addressing polypharmacy in adult patients. For the purposes of this overview, we considered an SR to be a summary of outcomes resulting from a detailed and comprehensive plan and search strategy for relevant evidence derived a priori.14 We included SRs of studies with any study design and outcome. We excluded reviews focusing exclusively on interventions implemented in low- to middle-income countries due to differences in care practices and healthcare infrastructure. We excluded SRs focused on interventions, conditions, or patients unlikely to inform readmission prevention among older adults, such as those focused on optimizing antipsychotic medications and antiretroviral regimens for patients with HIV infection. However, we did not restrict inclusion to the inpatient setting, as patients from other settings such as skilled nursing facilities and adult care homes may be relevant due to their age and comorbidities.
Two trained reviewers independently screened titles and abstracts using the prespecified inclusion and exclusion criteria. Next, 2 reviewers retrieved and examined full-text publications to determine eligibility. Research team members resolved discrepancies at the title-and-abstract and full-text screening levels by consensus in group meetings.
Quality evaluation
We assessed the methodological quality of each relevant SR using the validated Assessment of Multiple Systematic Reviews (AMSTAR) instrument.15 The tool contains 11 requisite items that are rated as present or absent, such that each SR may receive a score ranging from 0 to 11. Two reviewers independently applied the instrument. Discrepancies were reconciled through oral discussion. SRs with an AMSTAR score below 8 were excluded from the data synthesis, as that is a commonly applied threshold for high-quality SRs.
Data extraction
For included SRs, 2 research team members independently extracted data related to key characteristics using a standardized data extraction tool. Extracted variables included dates of literature search, number and design of included primary studies, intervention type(s), patient population(s), setting(s), primary outcome measure(s), presence of meta-analytic techniques and any pooled estimates, and major conclusions regarding intervention effectiveness. Reviewers compared extracted data and reconciled discrepancies through oral discussion.
Quality of evidence
We assessed the quality of evidence for each conclusion within each SR by applying Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology.16 We used objective criteria to assign a level of evidence in the following GRADE domains: study design; study quality; consistency; directness; and other modifying factors, including data imprecision and strength of effect estimates. We did not assess the quality of the individual studies within the SRs but reported the risk of bias of studies as documented in the SRs. One author assessed GRADE level of evidence for each SR.
Synthesis
We examined each SR’s major conclusions regarding the effectiveness of intervention strategies for the reported primary outcomes and classified authors’ conclusions into 1 of 4 distinct categories: (1) a positive association between intervention strategy and outcome, (2) a negative association between intervention strategy and outcome, (3) a null association between intervention strategy and outcome, and (4) preclusion from drawing conclusions due to limited or low-quality studies. We also documented whether conclusions were based on quantitative (meta-analytic) or qualitative assessments.
Results
Study selection
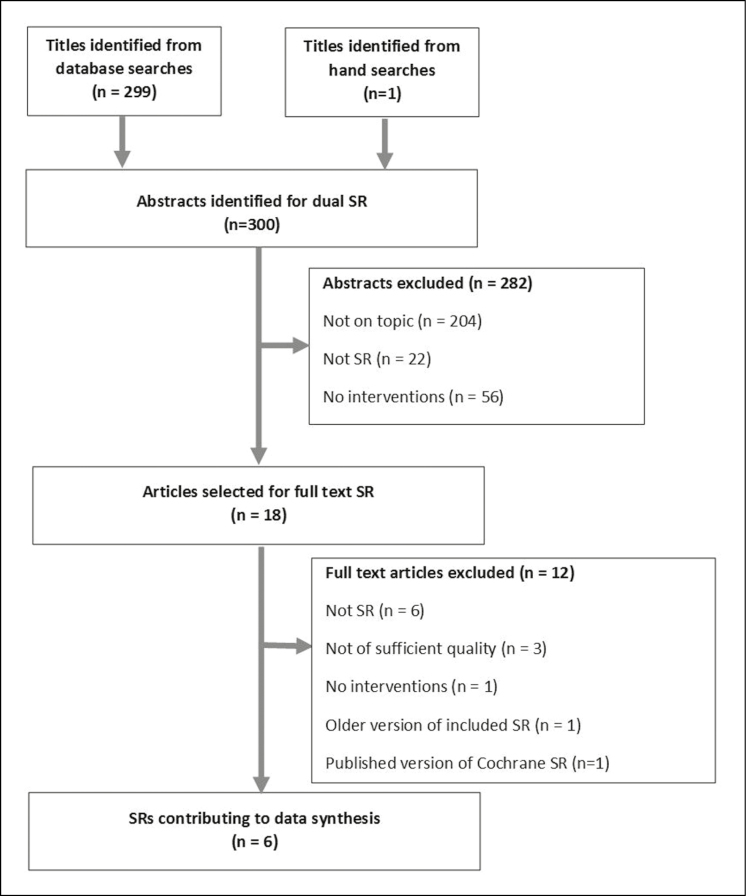
Our literature search identified 300 articles (Figure 1). After screening titles and abstracts, we selected 18 citations for full-text SR review. After reviewing the full-text versions of these articles, we identified 11 articles that met the inclusion criteria.17–27 Of these 11 articles, 1 was an older version of a more recent Cochrane SR26 and 1 was a peer-reviewed journal version of a Cochrane SR.27 To avoid redundancy, we classified these 2 articles as duplicates. We then assessed the methodological quality of the remaining 9 SRs.17–20,22,24,26,27 Six of these SRs received an AMSTAR score of 8 or higher. We reported on and synthesized the findings of these 6 SRs.17–20,22,24
Figure 1.
PRISMA flow diagram.
Study characteristics
Table 1 shows the major characteristics of included SRs. All 6 SRs were published during the period 2014–2017.17–20,22,24 Half (n = 3) of the SRs were published as Cochrane SRs,17,18,24 whereas the remainder were published in peer-reviewed journals.19,20,22 Four of the SRs included meta-analytic techniques for pooling outcome data.18,19,22,24 Five SRs restricted study populations to older adults,17,19,20,22,24 whereas 1 included only individuals with gastroesophageal reflux disease taking proton pump inhibitors.18 Of the 5 SRs focused on older adults, 4 were not restricted to patients of a specific disease type,17,19,22,24 while 1 focused on patients with severe dementia.20 The care settings discussed in the SRs varied widely; 2 SRs included only studies in nursing or care homes,17,20 1 included studies in an outpatient setting only,18 and 3 included studies in mixed settings such as hospitals, care facilities, and outpatient or primary care.19,22,24
Table 1.
Summary of Included Systematic Reviews (n = 6)a
| Authors (Yr Published) | Literature Search Coverage | Number of Articles Included and Study Designs | Population | Setting(s) | Intervention Type(s) | Primary Outcome Measure(s) | Pooled Effect Estimate(s) | Major Conclusion(s) | GRADE Level of Evidence |
|---|---|---|---|---|---|---|---|---|---|
| Publications on Deprescribing Interventions | |||||||||
| Boghossian et al.18 (2017) | Database inception–Nov 2016 | 6 RCTs or quasi-RCTs | Adults taking proton pump inhibitors | Outpatient | Deprescribing of chronic PPI therapy vs. continuous PPI use | GI symptoms, drug burden, cost/resource use, withdrawal events, participant satisfaction | GI symptom control: RR, 1.71; 95% CI, 1.31–2.21, favoring continuous use Drug burden: mean difference, –3.79, 95% CI, -4.73 to -2.84, favoring deprescribing Participant satisfaction: RR, 1.82; 95% CI, 1.26–2.65, favoring continuous use |
Deprescribing led to significant increases in GI symptoms, reduction in pill burden, and decline in participant satisfaction. There was insufficient data to make a conclusion regarding long-term benefits and harms of PPI discontinuation, cost, or withdrawal events. | Low (GI symptoms); moderate (drug burden); very low (participant satisfaction) |
| Page et al.22 (2016) | Database inception–Feb 2015 | 115 studies (56 RCTs, 22 comparative studies with concurrent control, and 37 comparative studies without concurrent control) | Adults age ≥65 yr | 14 hospitals, 29 residential aged care facilities, 73 community settings | Deprescribing of ≥1 medication | Mortality | Mortality in non-randomized studies: OR, 0.32; 95% CI, 0.17–0.60 Mortality in randomized studies: OR, 0.82; 95% CI, 0.61–1.11 Mortality with patient-specific interventions in randomized studies: OR, 0.62; 95% CI, 0.43–0.88 Mortality with generalized educational programs in randomized studies: OR, 1.21; 95% CI, 0.86–1.69 |
Mortality was significantly reduced in nonrandomized studies and in studies of patient-specific interventions; mortality was not significantly reduced in randomized studies and studies of generalized education programs. | Low (generalized education interventions); low (patient-specific interventions) |
| Publications on Interventions Aimed at Optimizing Prescribing | |||||||||
| Alldred et al.17 (2016) | Database inception–May 2015) | 12 RCTs | Adults age ≥65 yr | Care homes | Interventions to optimize overall prescribing; authors reported on medication review (n = 10), multidisciplinary case conferencing (n = 4), provider education (n = 5), and clinical decision support (n = 1) | ADEs, hospitalizations, mortality | NA | Authors were precluded from drawing conclusions due to variability in design, interventions, outcomes, and results. | Low (ADEs); low (hospitalizations); low (mortality) |
| Johansson et al.19 (2016) | Database inception–July 2015) | 25 studies (21 RCTs and 4 non-RCTs) | Adults age ≥65 yr taking ≥4 drugs | 15 primary care settings, 3 hospital settings, and 7 nursing home settings | Interventions to optimize overall prescribing; authors reported on pharmacist-led (n = 13), physician-led (n = 4), and multidisciplinary team-led interventions (n = 8) | Mortality, hospitalizations, drug use | All-cause mortality in all studies: OR, 1.02; 95% CI, 0.84–1.23 All-cause mortality in RCTs only: OR, 1.05; 95% CI, 0.85–1.29 |
There was no convincing evidence that the strategies assessed were effective in reducing mortality, hospitalizations, or drug use. | Low (mortality); very low (hospitalizations); low (drug-use) |
| Kroger et al.20 (2015) |
Database inception–Dec 2013) | 35 studies (15 RCTs, 20 non-RCTs) | Older adults with severe dementia | Nursing homes | Interventions to optimize overall prescribing; authors reported on medication review (n = 21), medication reconciliation (n = 8), education or training (n = 16), and use of interdisciplinary teams (n = 15) | Medication appropriateness | NA | The most effective elements of successful interventions included medication review, provider education, and use of multidisciplinary teams. | Very low (medication review); very low (provider education); very low (multidisciplinary teams) |
| Patterson et al.24 (2014) | Database inception–Nov 2013) | 12 studies (8 RCTs, 2 cluster RCTs, 2 controlled before-and-after studies) | Adults ≥65 yr taking ≥4 drugs | 3 hospital outpatient clinics, 3 hospital inpatient settings, 3 nursing homes, 2 primary care settings, 1 hospital–home care interface | Interventions to optimize overall prescribing: authors reported on clinical decision support (n = 1), pharmacist care approaches (n = 11) | Medication appropriateness | Medication appropriateness (summed MAI score): pooled mean difference, –3.88; 95% CI, –5.40 to –2.35 Medication appropriateness (change in MAI score): pooled mean difference, –6.78; 95% CI, –12.34 to –1.22) Medication appropriateness (number of drugs listed in Beers criteria): pooled mean difference, –0.1; 95% CI, –0.28 to 0.09) |
Overall, the included interventions improved appropriate polypharmacy. | Low (medication appropriateness, summed MAI score); low (medication appropriateness, change in MAI score); very low (medication appropriateness, number of drugs listed in Beers criteria) |
aGRADE = Grading of Recommendations Assessment, Development and Evaluation; RCT = randomized, controlled trial; PPI = proton pump inhibitor; GI = gastrointestinal; RR = relative risk; CI = confidence interval; OR = odds ratio; ADE = adverse drug event; NA = Not applicable;.
All 6 SRs focused broadly on 1 of 2 major categories of polypharmacy interventions: (1) deprescribing18,22 and (2) any intervention aimed at optimizing prescribing.17,19,20,24 Among the 2 SRs focused on deprescribing,18,22 one focused on the deprescribing of proton pump inhibitors18 and the other assessed the deprescribing of 1 or more medications.22 Interventions for the deprescribing of proton pump inhibitors included on-demand deprescribing and abrupt stopping of medication.18 Deprescribing interventions for 1 or more medications included both patient-specific efforts led by a doctor, pharmacist, nurse, or multidisciplinary team, often incorporating medication review, and generalized education programs aimed at doctors and nurses.22 Medication optimization interventions implemented in adult care homes consisted of medication review by pharmacists and doctors, multidisciplinary case conferencing, provider education, and clinical decision support.17,20 Interventions aimed at medication optimization in primary and inpatient care settings included pharmacist-led medication review using tools such as the Medication Appropriateness Index and the Screening Tool of Older Persons’ Potentially Inappropriate Prescriptions (STOPP)/Screening Tool), pharmacist-provided patient education, provider education, multidisciplinary team–led medication review, and computerized decision support.19,24
Major study conclusions
Primary outcomes assessed by the SRs were extremely varied (Table 2). The 2 SRs evaluating deprescribing interventions assessed mortality, symptoms, drug use, and patient satisfaction. Page et al.22 conducted a meta-analysis of 116 studies of patient-specific interventions and reported that mortality was significantly reduced in nonrandomized studies (pooled odds ratio [OR], 0.32; 95% confidence interval [CI], 0.17–0.60) and in randomized studies (pooled OR, 0.62; 95% CI, 0.43–0.88); however, non–patient-specific interventions had a null effect on mortality in both randomized studies (pooled OR, 0.82; 95% CI, 0.61–1.11) and randomized studies (pooled OR, 1.21; 95% CI, 0.86–1.69). The GRADE quality of evidence on which these conclusions were based was low. In the other SR focused on deprescribing, Boghossian et al.18 reported that on-demand deprescribing of proton pump inhibitors could reduce pill burden, measured as pill use per week per patient (pooled mean difference with intervention versus continued use, –3.79 pills; 95% CI, –4.73 to –2.84 pills) but also noted a statistically significant increase in symptoms (pooled risk ratio [RR], 1.71; 95% CI, 1.31–2.21) and decreased patient satisfaction (pooled RR, 1.82; 95% CI, 1.26–2.65). The quality of evidence for the outcomes of pill burden, symptoms, and patient satisfaction were assessed as moderate, low, and very low, respectively.18
Table 2.
Major Conclusions Reported in Included Systematic Reviews and GRADE Level of Supporting Evidencea
| Reported Primary Outcomes | ||||||||
|---|---|---|---|---|---|---|---|---|
| Publication (Yr Published) | Population | Mortality | Symptoms | Drug Use | Medication Appropriateness | Adverse Drug Events | Hospitalizations | Patient Satisfaction |
| Publications on Deprescribing Interventions | ||||||||
| Boghossian et al.18 (2017) | Adults taking proton pump inhibitors |
 Lowb Lowb
|
 Moderate Moderate |
 Very lowb Very lowb
|
||||
| Page et al.22 (2016) | Older adults | (Patient-specific)  Lowb Lowb
|
||||||
(Education)  Lowb Lowb
|
||||||||
| Publications on Interventions Aimed at Optimizing Prescribing | ||||||||
| Alldred et al.17 (2016) | Older adults in care homes |
 Very low Very low |
 Very low Very low |
 Very low Very low |
||||
| Johansson et al.19 (2016) | Older adults taking ≥4 drugs |
 Lowb Lowb
|
 Lowb Lowb
|
 Very lowb Very lowb
|
||||
| Kroger et al.20 (2015) | Nursing home residents with severe dementia | (Multidisciplinary teams)  Very low Very low |
||||||
(Provider education)  Very low Very low |
||||||||
(Medication review)  Very low Very low |
||||||||
| Patterson et al.24 (2014) | Older adults taking ≥4 drugs |
 Very low to lowb Very low to lowb
|
aThe plus and minus symbols denote improved and worsened outcomes, respectively; the equal sign denotes that outcome was assessed and investigators reported no effect; the question mark symbol denotes outcome was assessed and investigators were precluded from drawing conclusions due to limited or low-quality studies.
bConclusion based on meta-analytic data pooling.
In the 2 SRs that examined the effectiveness of polypharmacy interventions aimed at optimizing prescribing,17,19,20,24 the primary outcomes assessed varied widely and included mortality, drug use, medication appropriateness, ADEs, and hospitalizations. Neither of 2 SRs assessing the effect on mortality found that interventions reduced it.17,19 Of the 2 SRs reporting on medication appropriateness,20,24 the first used meta-analytic pooling to conclude that polypharmacy interventions, such as pharmaceutical care, have been effective at improving medication appropriateness; however, this conclusion was based on low-quality or very low-quality evidence.24 The second SR reported that multidisciplinary teams, medication review, and provider education were the most effective intervention components for improving medication appropriateness; quality of evidence for these conclusions was very low.20 The following additional outcomes were assessed in this subset of SRs, but no evidence for the effectiveness of prescribing-focused polypharmacy interventions was found: medication-related problems, including ADEs17,24; drug use19; medication adherence24; quality of life24; and hospitalizations.17,19
Quality evaluations
The quality assessments of the included SRs using the AMSTAR instrument are described in eAppendix C. The median score was 10.5 (interquartile range, 9.5–11.0).
Discussion
In summary, we found 6 high-quality SRs on interventions addressing polypharmacy, all of which were published after 2013. Five of these SRs focused on older adults. Four SRs focused on interventions that optimized prescribing, whereas 2 concentrated on deprescribing exclusively. Both SRs considering the outcome of medication appropriateness found improvements. However, these SRs were based on low-quality or very low-quality evidence. Furthermore, the clinical significance of improvements in medication appropriateness was noted to be “unclear” in one review.24 With respect to patient-centered outcomes (mortality, morbidity, and healthcare resource utilization), there was little evidence of benefit except for 1 SR reporting significant reductions in drug use with deprescribing interventions.
The only other SR that presented evidence of more downstream, patient-centered benefit was that of Page et al., which found in a subanalysis that mortality was “significantly reduced when patient-specific deprescribing interventions were applied in [randomized controlled trials].” 22 This SR was notable for its liberal inclusion of primary studies. In all, it considered results from 132 publications describing 116 studies. Upon applying GRADE criteria, the level of evidence for this SR conclusion was assessed as low. In light of this low quality of evidence, we are hesitant to accept this conclusion without further study. Furthermore, in seeking to isolate which specific interventions might reduce mortality, it was disappointing that none of the component studies achieved statistical significance alone or clearly stood out as driving the pooled estimate.
Nonetheless, there is face validity to the idea that patient-specific deprescribing interventions would be more successful than less tailored interventions (e.g., generalized educational campaigns). Face validity is an accepted criterion for determining which predictors to include in a model, and we would advocate for its use in this context of low-quality evidence. Our major practical insight from this overview is a recommendation that provider organizations interested in addressing polypharmacy concentrate first on patient-specific deprescribing interventions. Such specificity might be achieved via clinical decision support, via pharmacy personnel, or by other means. One example of such an intervention is found in the SR by Kroger et al.,20 wherein Verrue et al.28 found that medication review conducted by pharmacists using the Beers criteria (including 11 patient-specific advisories for potentially hazardous drug–disease and drug–syndrome interactions) resulted in increased medication appropriateness, as measured by several instruments.
Although we are unaware of any other overview of SRs in this area with which to compare our findings, the different component SRs are themselves perhaps the best comparators. The 3 Cochrane SRs, which are known for their excellent methodological standards, all noted the poor quality of existing evidence and the need for more research. The other included SRs tended to include more primary studies but were no more likely to find interventions to be effective.
In organizing and assessing SR-level evidence of polypharmacy interventions, our overview helped to map out existing evidence on the effectiveness of such interventions by population, measured outcomes, and intervention types. Our findings suggest that there is significant interest in interventions to improve polypharmacy, with the published literature assessing a wide variety of patient outcomes. We hope that our work provides decision makers, as well as physicians and other healthcare professionals, with a clear understanding of the evidence available in this area and helps direct readers to more targeted information. Beyond summarizing and enhancing the accessibility of existing literature, our overview highlights the absence of high-quality evidence to inform high-quality SRs. Although our overview identified 6 SRs that employed high-quality methodology, there were few strategies for which high-quality evidence of effectiveness was found.
Our work has several limitations. As with all reviews of existing literature, a central limitation of our review was the quality and scope of existing evidence. Just as SRs often address quality concerns by focusing on high-quality primary literature, we focused on high-quality SRs. Although this quality-based filtering tended to exacerbate scope deficiencies, the included SRs offered a range of strict to lenient methodological perspectives, such that results from a variety of primary studies were incorporated. Furthermore, even though our clinical area of interest involved older adults at care transitions, we included SRs focusing on other care settings. This broad scope was intentional and stemmed from an idea that polypharmacy interventions found to be successful in other (e.g., outpatient) settings might also offer benefit at care transitions. Because few studies in the polypharmacy literature focus on care transitions, limiting a search to this care setting would have required extreme compromises in scope or quality.
A second limitation involved our distance from the primary literature. Although we chose to conduct an overview of SRs to capitalize on prior work, we also recognized that this methodology may miss some of the nuance appreciable in an SR or by conducting primary research. Finally, the fact that all of the identified SRs were published after 2013 suggests that polypharmacy is an emerging area, with a literature base that may still be rapidly evolving. Further high-quality studies are needed to assess the impact of efforts of reduce polypharmacy on patient care, especially among older adults undergoing transitions of care.
Conclusion
An overview of SRs of interventions to address polypharmacy found 6 recent and high-quality SRs, mostly focused on older adults, in which both process and outcome measures were used to evaluate interventions. Despite the low quality of evidence in the underlying primary studies, both SRs that assessed medication appropriateness found evidence that polypharmacy interventions improved it. However, there was no consistent evidence of any impact on downstream patient-centered outcomes such as healthcare utilization, morbidity, or mortality.
Disclosures
This research was supported by the American Society of Health-System Pharmacists (ASHP) Research and Education Foundation and the National Institute on Aging of the National Institutes of Health under awards K23AG049181 (JMP) and R01AG058911 (JMP). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Supplementary Material
Contributor Information
Members of the PHARM-DC group:
References
- 1. Forster AJ, Murff HJ, Peterson JF et al. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003; 138(3):161-7. [DOI] [PubMed] [Google Scholar]
- 2. Kripalani S, Roumie CL, Dalal AK et al. Effect of a pharmacist intervention on clinically important medication errors after hospital discharge: a randomized trial. Ann Intern Med. 2012; 157(1):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tsilimingras D, Schnipper J, Duke A et al. Post-discharge adverse events among urban and rural patients of an urban community hospital: a prospective cohort study. J Gen Intern Med. 2015; 30(8):1164-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burns JM, Sneddon I, Lovell M et al. Elderly patients and their medication: a post-discharge follow-up study. Age Ageing. 1992; 21(3):178-81. [DOI] [PubMed] [Google Scholar]
- 5. Ernst FR, Grizzle AJ. Drug-related morbidity and mortality: updating the cost-of-illness model. J Am Pharm Assoc. 2001; 41(2):192-9. [DOI] [PubMed] [Google Scholar]
- 6. Masnoon N, Shakib S, Kalisch-Ellett L, Caughey GE. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017; 17(1):230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Best Practice Advocacy Centre New Zealand. Polypharmacy in primary care: managing a clinical conundrum (2014) https://bpac.org.nz/BPJ/2014/October/docs/BPJ64-polypharmacy.pdf (accessed 2019 Jul 20).
- 8. Kripalani S, Jackson AT, Schnipper JL, Coleman EA. Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007; 2(5):314-23. [DOI] [PubMed] [Google Scholar]
- 9. Moher D, Liberati A, Tetzlaff J, Altman DG, for the PRISMA Group Preferred Reporting Items for Systematic reviews and Meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009; 62(10):1006-12. [DOI] [PubMed] [Google Scholar]
- 10. Whitlock EP, Lin JS, Chou R et al. Using existing systematic reviews in complex systematic reviews. Ann Intern Med. 2008; 148(10):776-82. [DOI] [PubMed] [Google Scholar]
- 11. Smith V, Devane D, Begley CM, Clarke M. Methodology in conducting a systematic review of systematic reviews of healthcare interventions. BMC Med Res Methodol. 2011; 11(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pollock A, Campbell P, Brunton G et al. Selecting and implementing overview methods: implications from five exemplar overviews. Syst Rev. 2017; 6(1):145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hunt H, Pollock A, Campbell P et al. An introduction to overviews of reviews: planning a relevant research question and objective for an overview. Syst Rev. 2018; 7(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Uman LS. Systematic reviews and meta-analyses. J Can Acad Child Adolesc Psychiatry. 2011; 20(1):57-9. [PMC free article] [PubMed] [Google Scholar]
- 15. Shea BJ, Grimshaw JM, Wells GA et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007; 7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Atkins D, Best D, Briss PA et al. Grading quality of evidence and strength of recommendations. BMJ. 2004; 328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alldred DP, Kennedy MC, Hughes C et al. Interventions to optimise prescribing for older people in care homes. Cochrane Database Syst Rev. 2016; 2:CD009095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Boghossian TA, Rashid FJ, Thompson W et al. Deprescribing versus continuation of chronic proton pump inhibitor use in adults. Cochrane Database Syst Rev. 2017; 3:CD011969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Johansson T, Abuzahra ME, Keller S et al. Impact of strategies to reduce polypharmacy on clinically relevant endpoints: a systematic review and meta-analysis. Br J Clin Pharmacol. 2016; 82(2):532-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kroger E, Wilchesky M, Marcotte M et al. Medication use among nursing home residents with severe dementia: identifying categories of appropriateness and elements of a successful intervention. J Am Med Dir Assoc. 2015; 16(7):629.e1-17. [DOI] [PubMed] [Google Scholar]
- 21. Loganathan M, Singh S, Franklin BD et al. Interventions to optimise prescribing in care homes: systematic review. Age Ageing. 2011; 40(2):150-62. [DOI] [PubMed] [Google Scholar]
- 22. Page AT, Clifford RM, Potter K et al. The feasibility and effect of deprescribing in older adults on mortality and health: a systematic review and meta-analysis. Br J Clin Pharmacol. 2016; 82(3):583-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Paquin AM, Zimmerman K, Rudolph JL. Risk versus risk: a review of benzodiazepine reduction in older adults. Expert Opin Drug Saf. 2014; 13(7):919-34. [DOI] [PubMed] [Google Scholar]
- 24. Patterson SM, Cadogan CA, Kerse N et al. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database Syst Rev. 2014(10):CD008165. [DOI] [PubMed] [Google Scholar]
- 25. Van der Cammen TJ, Rajkumar C, Onder G et al. Drug cessation in complex older adults: time for action. Age Ageing. 2014; 43(1):20-5. [DOI] [PubMed] [Google Scholar]
- 26. Alldred DP, Raynor DK, Hughes C et al. Interventions to optimise prescribing for older people in care homes. Cochrane Database Syst Rev. 2013; ( 2):CD009095. [DOI] [PubMed] [Google Scholar]
- 27. Cooper JA, Cadogan CA, Patterson SM, Kerse N. Interventions to improve the appropriate use of polypharmacy in older people: a Cochrane systematic review. Cochrane Database Syst Rev. 2015; 5(12):E009235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Verrue C, Mehuys E, Boussery K et al. A pharmacist-conducted medication review in nursing home residents: impact on the appropriateness of prescribing. Acta Clin Belg. 2012; 67(6):423-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.