Abstract
Purpose
Tacrolimus is a nephrotoxic immunosuppressant historically monitored via enzyme-based immunoassay (IA). After 2011, the 2 largest laboratory companies in the United States implemented tacrolimus quantification by liquid chromatography–mass spectrometry (LC-MS); this method excludes metabolites, potentially resulting in lower quantified drug concentrations. We sought to determine if tacrolimus therapeutic drug monitoring via LC-MS, as performed using trough targets originally derived from IA values, influences clinical outcomes.
Methods
In a single-center retrospective cohort study of lung transplant recipients, risks of acute kidney injury, acute renal failure, and new-onset diabetes after transplantation, as well as chronic lung allograft dysfunction–free survival, were compared in 82 subjects monitored by LC-MS and 102 subjects monitored by IA using Cox proportional hazard models adjusted for age, sex, baseline renal function, and race.
Results
LC-MS–based monitoring was associated with a greater risk of acute kidney injury (adjusted hazard ratio, 1.65; 95% confidence interval, 1.02–2.67). No statistically significant differences in risks of acute renal failure and new-onset diabetes after transplantation were observed.
Conclusion
Although LC-MS provides a more accurate representation of the blood concentration of the parent compound tacrolimus exclusive of metabolite, established cut points for tacrolimus dosing may need to be adjusted to account for the increased risk of renal injury.
Keywords: immunoassay, immunosuppression, kidney injury, lung transplantation, liquid chromatography, mass spectrometry, tacrolimus
KEY POINTS.
The discrepancy between tacrolimus trough concentrations measured by liquid chromatography–mass spectrometry (LC-MS) versus immunoassay is clinically meaningful.
In lung transplant recipients who undergo therapeutic drug monitoring via LC-MS, the adoption of the immunoassay dosing adjustment scale leads to higher rates of acute kidney injury.
Established cut points for dosing of tacrolimus to achieve targeted trough concentrations require adjustment for the type of assay used in therapeutic drug monitoring.
Tacrolimus is a calcineurin inhibitor used for immunosuppression in solid organ transplantation.1 In lung transplantation, over 90% of patients are maintained on tacrolimus.2 Given the drug’s narrow therapeutic index, tacrolimus use requires therapeutic drug monitoring to ensure adequate immunosuppression while preventing drug toxicity.3 Acute nephrotoxicity, a major target organ toxic effect of tacrolimus, is believed to be driven by dose-related arteriolar vasoconstriction and tubular dysfunction.4 Another well-recognized tacrolimus complication is worsening glycemic control and diabetes mellitus.5 Target tacrolimus trough concentrations are both organ and center dependent. The algorithms that transplant centers use for tacrolimus dose adjustments are based on clinical experience, typically informed by tacrolimus troughs historically measured using an enzyme-based immunoassay (IA) method.6,7
The IA method for tacrolimus measurement captures both the parent drug and its major metabolites, including both immunologically active and inactive breakdown products.8,9 Liquid chromatography–mass spectrometry (LC-MS) tacrolimus assays have the advantage of precisely measuring the parent drug without being affected by metabolite cross-detection.10,11 As expected, when compared directly in testing of the same sample, IA systematically yields higher values than LC-MS.12–15 This effect is observed across different solid organ transplant types and persists with more recent versions of commercial IA products.16,17 Despite this phenomenon, the differences between assays may be underappreciated by clinicians, as reflected by the lack of published protocols for routine dose adjustment based on assay type. Further, as tacrolimus monitoring with LC-MS becomes more prevalent, it is important to determine the impact of monitoring tacrolimus trough concentrations with LC-MS on patient outcomes.
Between 2011 and 2013, the 2 largest commercial laboratory companies in the United States switched from IA to LC-MS as the primary method of measuring tacrolimus levels. We hypothesized that patients who undergo tacrolimus therapeutic drug monitoring via LC-MS may be exposed to doses of drug higher than intended because our tacrolimus adjustment protocols were developed for use in IA-based therapeutic drug monitoring. Since tacrolimus is nephrotoxic, we sought to evaluate whether tacrolimus therapeutic drug monitoring via LC-MS, as performed using trough targets originally derived from IA values, might be associated with renal injury. We also sought to evaluate whether LC-MS–based monitoring is associated with an increased risk of new-onset diabetes after transplantation (NODAT), similarly due to a higher concentration of the parent drug. Finally, we assessed whether applying IA drug adjustment algorithms to LC-MS measurements might adversely affect chronic lung allograft dysfunction (CLAD)–free survival. It was our hypothesis that as a reaction to renal injury, providers would reduce the dose of or discontinue tacrolimus to prevent further decline in renal function and, thus, that subjects would have less effective immunosuppression and an increased risk of CLAD.
To test that hypothesis, we performed a single-center retrospective cohort study of lung transplant recipients assessing the risks of acute kidney injury (AKI), acute renal failure (ARF), NODAT, and CLAD-free survival in subjects who underwent therapeutic drug monitoring via IA and subjects who underwent therapeutic drug monitoring via LC-MS. Some of the data reported here were previously reported in abstract form.18
Methods
Study population
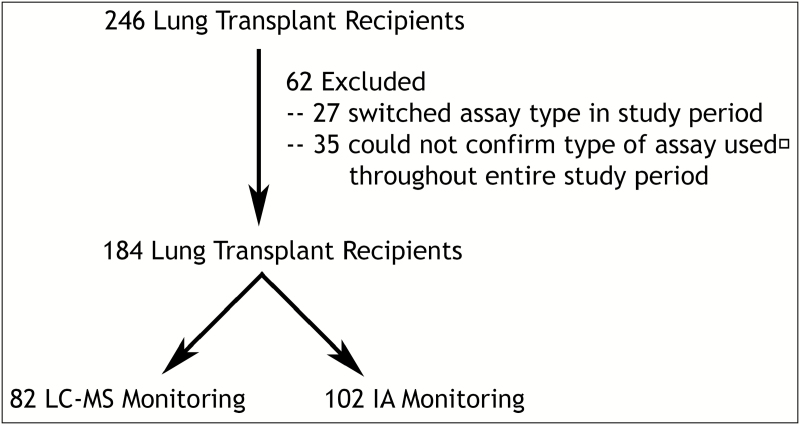
We performed a retrospective cohort study of all adults who underwent lung transplantation between February 2011 and October 2016 at our university hospital (Figure 1). This end date marked the point at which our lung transplant program became aware of the differences in assays. Therapeutic drug monitoring of tacrolimus was performed using a variety of clinical laboratories depending on the patient’s home location and insurance. The local laboratories used for tacrolimus monitoring included facilities run by Quest Diagnostics (switched from IA to LC-MS in July 2013), LabCorp (switched from IA to LC-MS in February 2011), the university’s clinical laboratory (IA), the Kaiser Permanente Clinical Laboratory (IA), and other, smaller clinical laboratories (varied use of assay type). We excluded subjects who underwent home laboratory monitoring at a smaller clinical laboratory due to the inability to verify the assay type used, as well as subjects who switched the type of laboratory monitoring (i.e., those who switched from our university’s clinical laboratory to LabCorp or Quest Diagnostic facilities and were transplanted before 2013) during the study period. Subjects were categorized into those who underwent laboratory monitoring via LC-MS (local laboratory monitoring via Quest Diagnostics after 2013 or LabCorp since 2011) and those who underwent laboratory monitoring via IA (local laboratory monitoring via the university’s clinical laboratory or Kaiser Permanente Clinical Laboratory since 2011). This study was approved through our university’s institutional review board (study #13-10738).
Figure 1.
CONSORT diagram of cohort creation. Exclusion criteria were applied to exclude (1) subjects who switched type of assay used during the study period and (2) subjects who underwent therapeutic drug monitoring at a small laboratory and for whom, as a result, use of the same type of assay through the entire study period could not be confirmed. Subjects were split into 2 groups according to type of assay used for tacrolimus therapeutic drug monitoring, liquid chromatography–mass spectrometry (LC-MS) or immunoassay (IA).
Clinical setting of therapeutic drug monitoring
All subjects started with the same initial immunosuppression regimen of tacrolimus, mycophenolate mofetil, and prednisone. After discharge from transplantation surgery, patients were required to stay in the immediate area surrounding our hospital for 6 weeks, during which time they received high-frequency follow-up. During this time, patients underwent twice-weekly monitoring of tacrolimus trough concentrations at the university’s clinical laboratory. Once the patients completed their postdischarge high-frequency follow-up, they were allowed to return home with less frequent follow-up. During this period, they continued therapeutic drug monitoring at a local laboratory. Follow-up monitoring initially occurred weekly, then once every 2 weeks, and then monthly thereafter.
Regardless of the laboratory used for therapeutic drug monitoring, the results were directly uploaded into our clinical laboratory review software, TITUS Presidio (TeleResults Corporation, San Francisco, CA). Tacrolimus dosing adjustments for all patients were performed by a transplant pulmonologist using the following trough goals: 10–14 ng/mL (posttransplant months 0–3), 10–12 ng/mL (months 3–6), 8–10 ng/mL (months 6–24), and 6–8 ng/mL (after 24 months). These goals were maintained for all patients unless nephrotoxicity or malignancy developed, at which point the trough goal could be lowered at the discretion of the provider.
Baseline demographics
Age at transplant, sex, race, diagnostic indication for transplantation (as categorized by the Lung Allocation Score19), presence of diabetes mellitus, and baseline renal function were abstracted from electronic medical records. Baseline renal function was defined as the median of all creatinine values collected between the time of posttransplant hospital discharge and the time of transitioning to local laboratory monitoring (typically 6 weeks after hospital discharge). We calculated the baseline estimated glomerular filtration rate using the Chronic Kidney Disease Epidemiology Collaboration equation.20 The presence of diabetes mellitus at baseline was defined by a glycated hemoglobin (HbA1c) concentration of ≥6.5%, 2 random blood glucose measurements of ≥200 mg/dL in the period before transitioning to local laboratory monitoring (excluding inpatient blood glucose measurements during the first 14 days after lung transplantation), or use of an antihyperglycemic before transplantation.21
Tacrolimus dosing and concentration
In order to assess differences in tacrolimus dosing between the LC-MS and IA groups, we used a data set of immunosuppressant and antifungal dosing values previously abstracted from the records of a randomly sampled cohort of 142 lung transplant recipients.22 For the subjects included in the study described here, complete dosing information was available for 103 subjects. For these subjects, we also abstracted all tacrolimus trough concentrations from the electronic medical record.
Outcome variables
Outcome variables of interest included AKI, ARF, NODAT, and CLAD-free survival. We included CLAD-free survival as an outcome because development of allograft dysfunction can be impacted by immunosuppressive regimen.23 Further, we hypothesized that treatment of subjects who developed renal injury may deviate from our standard immunosuppressive protocol in terms of tacrolimus dose reduction or discontinuation due to attempts to prevent further renal dysfunction. AKI was defined as a doubling of the baseline creatinine value as per the RIFLE (risk, injury, failure, loss, and end-stage renal disease) criteria and the Acute Kidney Injury Network (AKIN) criteria.24,25 ARF was defined as a tripling of the baseline creatinine value as per the RIFLE and AKIN criteria. NODAT was defined by a new diagnosis of diabetes in the period after transitioning to local laboratory monitoring.21 CLAD was defined as a drop in the forced expiratory volume in 1 second (FEV1) to ≤80% of the best posttransplant FEV1.26 Spirometry was performed on all patients at our university’s pulmonary function laboratory at regular intervals.
Paired comparison of tacrolimus concentration by assay type
One of the commercial clinical laboratories that used LC-MS to quantify tacrolimus concentrations still allowed for the quantification of tacrolimus via IA. When our program first became aware of the change in assay, blood from the same venipuncture draw was sent for tacrolimus quantification by both LC-MS and IA for a subset of patients to determine if we could replicate the discrepancies reported by others.13,16 In 22 unique subjects, 142 paired samples were analyzed (a total of 284 tacrolimus quantifications).
Analytic approach
Baseline variables were compared between the LC-MS group and the IA group using t tests for continuous variables and chi-square tests for categorical variables. The mean daily dose of tacrolimus and the mean trough concentration while not on antifungal therapy were calculated for each of the 103 subjects with complete dosing information. A Wilcoxon–Mann–Whitney test was used to compare the mean daily dose per subject, mean trough concentration per subject, and mean concentration-to-dose ratio per subject between the LC-MS and IA groups. Differences between the LC-MS and IA groups in the cumulative incidence of AKI, ARF, and NODAT were tested by log-rank. Differences between the LC-MS and IA groups in time until AKI, ARF, or NODAT were tested in unadjusted and multivariate Cox proportional hazards models, with the latter adjusted for age, sex, black race, and baseline renal function. We tested each model individually to ensure that the assumptions of proportional hazard were met. Black race was included given the previously reported increased risks of renal insufficiency and diabetes in black patients relative to those in other racial or ethnic groups.27–29 Subjects with a diagnosis of diabetes prior to their transition to locally based monitoring were excluded from the model assessing risk of NODAT. As a secondary analysis for AKI and ARF, death was included as a competing risk in unadjusted and adjusted competing risk regression models. CLAD-free survival was assessed by log-rank test and unadjusted and multivariate Cox proportional hazards models adjusted for age, sex, black race, and baseline renal function. For the 22 subjects with paired IA and LC-MS trough values, we tested differences in the reported concentrations using the Wilcoxon signed-rank test. We also tested the association between the results yielded by the 2 assays with Pearson correlation and assessed mean bias by Bland–Altman plot. For all analyses, a p value of <0.05 was deemed statistically significant.
Analyses were conducted using Stata 15 (StataCorp LLC, College Station, TX).
Results
Of the 246 subjects who underwent lung transplantation during the study period, 62 were excluded because they either switched assay type or because we could not verify that they stayed on the same assay throughout the study period. The remaining 184 subjects comprised the study cohort (Figure 1). Of the 184 subjects, 82 underwent therapeutic drug monitoring by LC-MS (45%) and 102 by IA (55%). Demographics and baseline renal function were similar between the monitoring groups (all p values ≥0.12) except that a higher proportion of subjects in the IA group were identified as being black (p = 0.05) (Table 1). In our nested cohort of subjects with complete dosing information, the mean daily dose of tacrolimus was higher in the LC-MS group than in the IA group (p = 0.03). There was no difference in mean tacrolimus concentrations between groups. The mean concentration:dose ratio was lower in the LC-MS group (p = 0.02) (Table 2).
Table 1.
Baseline Demographic and Clinical Characteristics of Study Cohort, by Monitoring Methoda
| Characteristic | LC-MS (n = 82) | Immunoassay (n = 102) | p b |
|---|---|---|---|
| Age, mean ± S.D., yr | 58.8 ± 12.2 | 56.1± 10.5 | 0.12 |
| Male sex, no. (%) | 46 (56) | 60 (59) | 0.71 |
| Diabetes, no. (%) | 42 (51) | 48 (47) | 0.58 |
| Race/ethnicity, no. (%) | 0.27 | ||
| Caucasian | 53 (65) | 53 (52) | |
| Black | 3 (4) | 12 (12) | |
| Hispanic | 12 (15) | 11 (11) | |
| Other | 14 (17) | 26 (25) | |
| Black race vs. other race | 3 (4) | 12 (12) | 0.05 |
| LAS diagnostic group, no. (%) | 0.36 | ||
| Group A: COPD | 15 (15) | 15 (18) | |
| Group B: pulmonary arterial hypertension | 4 (4) | 2 (2) | |
| Group C: cystic fibrosis | 10 (10) | 3 (4) | |
| Group D: idiopathic pulmonary fibrosis | 73 (71) | 63 (76) | |
| Creatinine concentration, mean ± S.D., mg/dL | 0.98 ± 0.43 | 1.00 ± 0.43 | 0.80 |
| eGFR, mean ± S.D., L/hr | 80.3 ± 21.4 | 81.6 ± 24.4 | 0.70 |
aLC-MS = liquid chromatography–mass spectrometry, LAS = Lung Allocation Score, COPD = chronic obstructive pulmonary disease, eGFR = estimated glomerular filtration rate.
bContinuous variables were compared via t test. Categorical variables were compared via chi-square test.
Table 2.
Tacrolimus Doses and Concentrations in Nested Cohort, by Monitoring Methoda
| Variable | LC-MS (n = 42) | Immunoassay (n = 61) | p d |
|---|---|---|---|
| Daily dose, mean ± S.D., mgb | |||
| All time points | 6.66 ± 3.54 | 5.19 ± 2.71 | 0.03 |
| Day of discharge–6 mo | 7.19 ± 4.91 | 6.86 ± 3.81 | 0.92 |
| 6 mo–2 yr | 6.25 ± 3.39 | 5.17 ± 2.88 | 0.10 |
| >2 yr | 5.35 ± 2.17 | 3.81 ± 2.17 | 0.12 |
| Concentration, mean ± S.D., ng/mLc | |||
| All time points | 8.99 ± 1.47 | 9.30 ± 1.33 | 0.21 |
| Day of discharge–6 mo | 9.49 ± 2.74 | 9.51 ± 1.82 | 0.82 |
| 6 mo–2 yr | 9.24 ± 1.48 | 9.61 ± 1.41 | 0.14 |
| >2 yr | 7.64 ± 1.53 | 7.60 ± 1.21 | 0.51 |
| Concentration:dose ratio, mean ± S.D.c | |||
| All time points | 1.84 ± 1.18 | 2.24 ± 1.09 | 0.02 |
| Day of discharge–6 mo | 1.75 ± 1.02 | 1.85 ± 1.07 | 0.73 |
| 6 mo–2 yr | 1.95 ± 1.18 | 2.36 ± 1.26 | 0.07 |
| >2 yr | 2.19 ± 1.89 | 2.43 ± 1.23 | 0.16 |
aLC-MS = liquid chromatography–mass spectrometry.
bMean of mean per subject daily dose.
cTime periods are as designated by trough goal dosing protocol. Days under observation included all time points for which subjects were not receiving antifungal therapy.
dWilcoxon–Mann–Whitney test was used to compare mean doses, trough concentrations, and concentration:dose ratios.
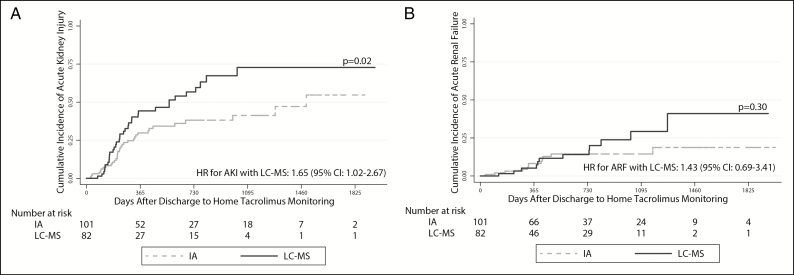
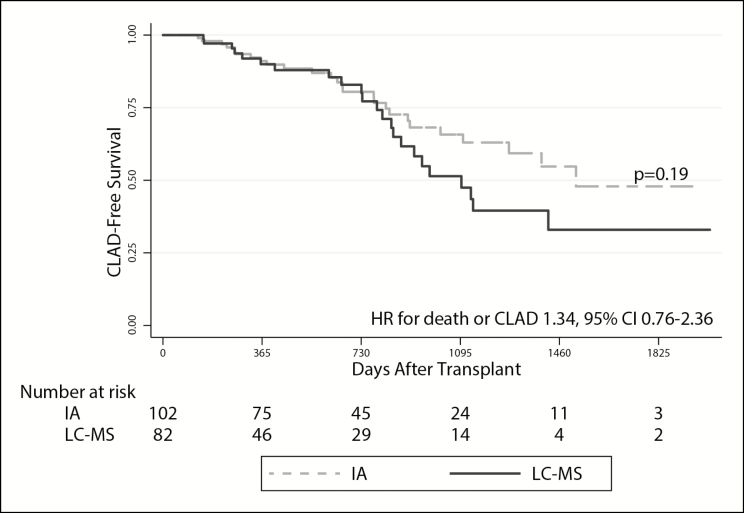
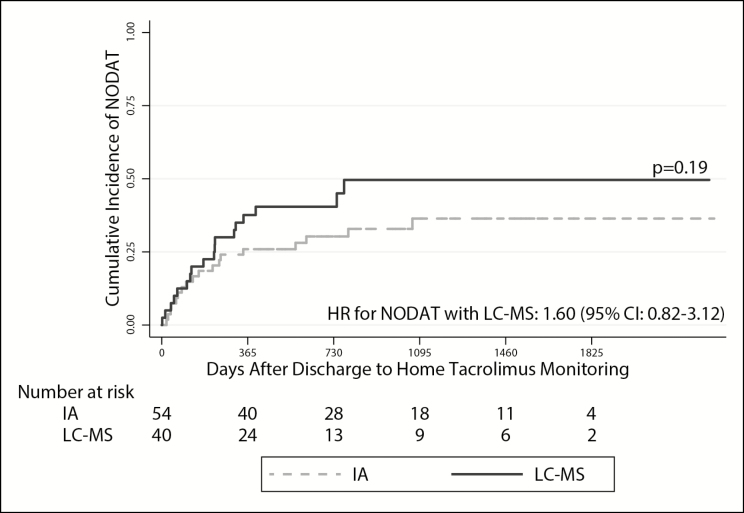
In unadjusted analyses, LC-MS–based monitoring was associated with a greater risk of AKI (hazard ratio [HR], 1.78; 95% confidence interval [CI], 1.10–2.87). This estimate was not substantively impacted after adjusting for age, sex, baseline renal function, and race (HR, 1.65; 95% CI, 1.02–2.67) (Table 3; Figure 2, panel A). We observed a similar relationship when death was modeled as a competing risk (unadjusted subdistribution HR, 1.91 [95% CI, 1.18–3.09]; adjusted subdistribution HR, 1.81 [95% CI, 1.14–2.90]). The point estimates for risk of ARF with LC-MS–based monitoring were similar to those observed for AKI in unadjusted models (HR, 1.53; 95% CI, 0.69–3.41), in adjusted models (HR, 1.43; 95% CI, 0.69–3.41), and in models that included death as a competing risk (unadjusted subdistribution HR, 1.73 [95% CI, 0.77–3.92]; adjusted subdistribution HR, 1.63 [95% CI, 0.70–3.82]), although none of these estimates achieved standard statistical significance (Table 3; Figure 2, panel B). LC-MS–based monitoring did not yield a statistically significant difference in CLAD-free survival (unadjusted HR for death or CLAD, 1.45 [95% CI, 0.83–2.55]; adjusted HR, 1.34 [95% CI, 0.76–2.36]) (Figure 3) or in the risk of NODAT (unadjusted HR, 1.54 [95% CI, 0.80–2.97]; adjusted HR, 1.60 [95% CI, 0.82–3.12]) (Figure 4). However, the point estimates of risk for both these outcomes were similar to those observed for AKI.
Table 3.
Hazard Ratios for Acute Kidney Injury and Acute Renal Failure With Use of LC-MS vs. Immunoassay for Tacrolimus Monitoringa
| Analysisb | Acute Kidney Injury | Acute Renal Failure |
|---|---|---|
| Unadjusted | HR, 1.78 (1.10–2.87) | HR, 1.53 (0.69–3.41) |
| Adjusted | HR, 1.65 (1.02–2.67) | HR, 1.43 (0.69–3.41) |
| Unadjusted, with death as competing risk | SHR, 1.91 (1.18–3.09) | SHR, 1.73 (0.77–3.92) |
| Adjusted, with death as competing risk | SHR, 1.81 (1.14–2.90) | SHR, 1.63 (0.70–3.82) |
aData are hazard ratio (HR) or subdistribution hazard ratio (SHR) with 95% confidence interval. LC-MS = liquid chromatography–mass spectrometry.
bCompeting risks regression was used to model death as a competing risk. Adjusted models included age, sex, baseline renal function, and race.
Figure 2.
Time to first acute kidney injury (AKI) (panel A) and time to first acute renal failure (ARF) (panel B) in the cohort of lung transplant recipients. The solid and dashed lines represent incidence rates in patients whose tacrolimus levels were monitored via liquid chromatography–mass spectrometry (LC-MS) and immunoassay (IA), respectively. AKI was defined as doubling of the baseline creatinine value per the RIFLE criteria. ARF was defined as a tripling of the baseline creatinine per the RIFLE criteria. Log-rank testing was used to assess for differences in cumulative incidence rates (expressed as p values). Hazard ratio (HR) values with 95% confidence intervals (CIs) represent the risk of AKI and/or ARF development in subjects monitored via LC-MS versus IA in multivariate Cox proportional hazards models adjusted for age, sex, baseline renal function, and race.
Figure 3.
Likelihood of chronic lung allograft dysfunction (CLAD)–free survival over time in the cohort of lung transplant recipients. The solid and dashed lines represent survival in patients whose tacrolimus levels were monitored via liquid chromatography–mass spectrometry (LC-MS) and immunoassay (IA), respectively. CLAD was defined as a 20% decrease from peak posttransplant forced expiratory volume in 1 second (FEV1). Log-rank testing was used to assess for the between-group difference in CLAD-free survival (expressed as p value). The hazard ratio (HR) with 95% confidence interval (CI) represents the risk of death or development of CLAD in subjects monitored via LC-MS versus IA in multivariate cox proportional hazards models adjusted for age, sex, baseline renal function, and race.
Figure 4.
Time to development of new-onset diabetes after transplant over time (NODAT) in the cohort of lung transplant recipients. The solid and dashed lines represent survival in patients whose tacrolimus levels were monitored via liquid chromatography–mass spectrometry (LC-MS) and immunoassay (IA), respectively. NODAT was defined as a glycated hemoglobin (HbA1c) concentration of ≥6.5% or 2 separate random blood glucose values of ≥200 mg/dL. Subjects with diabetes before discharge to home tacrolimus monitoring were excluded from this analysis. Log-rank testing was used to assess for the difference in cumulative incidence values (expressed as a p value). The hazard ratio (HR) with 95% confidence interval (CI) represents the risk of developing NODAT in subjects monitored via LC-MS versus IA in multivariate Cox proportional hazards models adjusted for age, sex, baseline renal function, and race. Fifty-four subjects (29 in the LC-MS group and 25 in the IA group) were excluded from the NODAT analysis due to diagnosis of diabetes before transitioning to local laboratory monitoring.
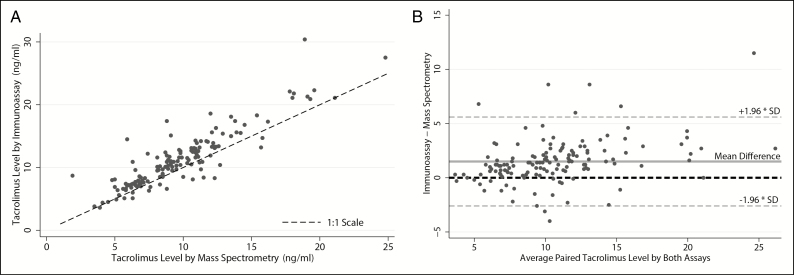
Direct comparisons of the 142 paired samples assayed on both LC-MS and IA platforms demonstrated that the 2 assays yielded different tacrolimus concentrations (p < 0.001). The 2 assays were strongly correlated (r = 0.89). The Bland–Altman plot demonstrated that IA had an estimated 1.5-ng/mL mean bias for higher tacrolimus trough concentrations compared to LC-MS (Figure 5). Of note, this directional bias was not universally observed.
Figure 5.
Tacrolimus concentrations for 142 paired blood samples from 22 subjects with trough concentrations measured by liquid chromatography–mass spectrometry and immunoassay. Panel A is a scatter plot of tacrolimus concentration by assay type. The black dashed line represents a 1:1 relationship. Panel B is a Bland–Altman plot. The solid grey line represents the mean difference in reported values for the 2 assay types. The black dashed line represents no difference between assays. The orange dashed lines represent the 95% confidence interval for the mean difference.
Discussion
In a cohort of lung transplant recipients undergoing therapeutic drug monitoring of tacrolimus via 2 different assays, subjects monitored via LC-MS required higher doses of tacrolimus than subjects monitored via IA to maintain similar tacrolimus trough concentrations. We also found that monitoring with LC-MS using trough targets originally derived from IA is associated with a nearly 80% increased risk of AKI. This heightened risk remained despite adjusting for covariates and accounting for death as a competing risk. There was also a nonsignificant trend towards increased risks of ARF and NODAT and decreased CLAD-free survival in the LC-MS–based monitoring group. As in prior studies, we found that reported drug concentrations with use of LC-MS and with use of IA were strongly correlated and that IA generally yielded higher trough concentrations than LC-MS. Importantly, we also found that these observed higher concentrations with IA were not uniform; in fully 15% of samples tested, LC-MS yielded higher concentrations than IA. This last finding has important implications for developing straightforward conversion estimates for existing IA-based dosing protocols.
The most likely explanation for the increased risk of AKI in patients monitored by LC-MS assays is that monitoring with LC-MS led to higher doses of tacrolimus administered; this was because our protocol of dosing adjustments to maintain certain trough goals was developed from our historical experience with IA. This difference between the 2 tests is underappreciated in clinical practice, and a similar practice of using an IA scale to adjust the dose of tacrolimus according to trough concentrations determined via LC-MS may be present in other centers and may apply to other organ transplant types. While there was no statistically significant increase in the risk of ARF within the LC-MS–based monitoring group, that the point estimate for risk was similar to that for AKI and the cumulative incidence curves for ARF separated after 2 years is concerning. That the CLAD-free survival curves separated around the same time (although not to a statistically significant degree) is also concerning (Figure 2, panel B; Figure 3). We speculate that the trend towards decreased CLAD-free survival in the LC-MS–based monitoring group may have been driven by tacrolimus dose reduction in attempts to prevent further calcineurin inhibitor–induced renal toxicity.
Multiple studies have directly compared LC-MS and IA for assessing tacrolimus concentrations.12–15 These studies, which included lung transplant recipients, consistently showed an overestimation of tacrolimus concentrations with use of IA.17 Our study adds to the existing literature by extending these well-described differences to include clinically relevant consequences. While the LC-MS assay accurately measures parent drug independent of metabolites, it remains unclear if the parent drug concentration is a better representation of immunosuppression than that provided by IA, which measures both parent drug and metabolites.10,11 Some of the tacrolimus metabolites are believed to be active. In rat models, the 31-O-desmethyl and the 15-O-desmethyl metabolites have immunosuppressive activity similar to that of the parent drug.30,31 A recent study in renal transplant recipients demonstrated that higher concentrations of the 15-O-desmethyl metabolite, but not the 13-O-desmethyl metabolite, are associated with renal toxicity, myelosuppression, and increased infections.32 As such, it may be beneficial for laboratories that use LC-MS–based quantification to include the concentrations of major immunologically active metabolites in assay reports.33 Furthermore, future human studies using LC-MS–based monitoring should include methods to detect the major metabolites, to determine if there is variation in the metabolite levels dependent on type of transplant, and if there are genetic polymorphisms contributing to the different by-products produced.
Our study had limitations. It was a single-center retrospective study with a modest sample size. While we were able to detect changes in AKI, the study may have been underpowered to detect differences in risks of ARF, CLAD-free survival, and NODAT. While our assessment of NODAT included measurements of glucose and HbA1c, we did not measure dapsone exposure, potentially resulting in underestimation of the incidence of NODAT.34 Further, it is possible that unmeasured confounders such as initiation of new potentially nephrotoxic agents influenced our findings. Lastly, it is not clear if our findings can be generalized to other centers that may use different dosing strategies.
Despite these limitations, our study had several strengths. To our knowledge, it was the first to evaluate clinically important outcomes comparing 2 different but highly prevalent approaches to monitoring tacrolimus. Further, highlighting the potential risks of using an IA-based dosing scale for patients who undergo therapeutic drug monitoring via LC-MS has important clinical implications. This knowledge has changed our own clinical practice, as we are now more comfortable with tacrolimus dose reduction to below our historical trough goals in subjects monitored via LC-MS. Notably, we and clinicians in other programs may need to develop experience or evidenced-based protocols for tacrolimus dosing in the era of LC-MS quantification; this is especially pressing since the largest laboratory monitoring company in the United States stopped routinely offering IA-based monitoring of tacrolimus in 2018. Given that LC-MS provides higher accuracy with lower long-term costs, we anticipate that LC-MS–based monitoring will become more prevalent worldwide.35 Lastly, given the widespread use of tacrolimus outside of lung transplantation, our findings are likely relevant to other organ transplant populations.
LC-MS–based monitoring of tacrolimus drug concentrations is becoming more prevalent due to its reliability and lower cost. Better awareness of the differences between LC-MS and IA is essential because of the potential risks of making dosing adjustments based on IA scales in patients monitored with LC-MS.
Conclusion
Although LC-MS provides a more accurate representation of the blood concentration of the parent compound tacrolimus exclusive of metabolite, established cut points for tacrolimus dosing may need to be adjusted to account for the increased risk of renal injury.
Disclosures
Dr. Greenland has received grants from Astellas Pharma Global Development for work outside the research described here. Dr. Singer’s contribution to this research was funded by R01 HL134851, and Dr. Kolaitis’s contribution to this research was funded by T32 HL 7185-42. The other authors have declared no potential conflicts of interest.
References
- 1. Kaufman DB, Shapiro R, Lucey MR et al. Immunosuppression: practice and trends. Am J Transplant. 2004; 4(suppl 9):38-53. [DOI] [PubMed] [Google Scholar]
- 2. Chambers DC, Yusen RD, Cherikh WS et al. The registry of the International Society for Heart and Lung Transplantation: thirty-fourth adult lung and heart-lung transplantation report—2017; focus theme: allograft ischemic time. J Heart Lung Transplant. 2017; 36(10):1047-59. [DOI] [PubMed] [Google Scholar]
- 3. Sikma MA, van Maarseveen EM, van de Graaf EA et al. Pharmacokinetics and toxicity of tacrolimus early after heart and lung transplantation. Am J Transplant. 2015; 15(9):2301-13. [DOI] [PubMed] [Google Scholar]
- 4. Issa N, Kukla A, Ibrahim HN. Calcineurin inhibitor nephrotoxicity: a review and perspective of the evidence. Am J Nephrol. 2013; 37(6):602-12. [DOI] [PubMed] [Google Scholar]
- 5. Fan Y, Xiao YB, Weng YG. Tacrolimus versus cyclosporine for adult lung transplant recipients: a meta-analysis. Transplant Proc. 2009; 41(5):1821-4. [DOI] [PubMed] [Google Scholar]
- 6. Taylor DO, Barr ML, Meiser BM et al. Suggested guidelines for the use of tacrolimus in cardiac transplant recipients. J Heart Lung Transplant. 2001; 20(7):734-8. [DOI] [PubMed] [Google Scholar]
- 7. Garrity ER Jr, Hertz MI, Trulock EP et al. Suggested guidelines for the use of tacrolimus in lung-transplant recipients. J Heart Lung Transplant. 1999; 18(3):175-6. [DOI] [PubMed] [Google Scholar]
- 8. Murthy JN, Davis DL, Yatscoff RW, Soldin SJ. Tacrolimus metabolite cross-reactivity in different tacrolimus assays. Clin Biochem. 1998; 31(8):613-7. [DOI] [PubMed] [Google Scholar]
- 9. Wei TQ, Zheng YF, Dubowy M, Sharma M. Sandwich assay for tacrolimus using 2 antitacrolimus antibodies. Clin Chem. 2014; 60(4):621-30. [DOI] [PubMed] [Google Scholar]
- 10. McShane AJ, Bunch DR, Wang S. Therapeutic drug monitoring of immunosuppressants by liquid chromatography-mass spectrometry. Clin Chim Acta. 2016; 454:1-5. [DOI] [PubMed] [Google Scholar]
- 11. Taylor PJ, Jones A, Balderson GA et al. Sensitive, specific quantitative analysis of tacrolimus (FK506) in blood by liquid chromatography-electrospray tandem mass spectrometry. Clin Chem. 1996; 42(2):279-85. [PubMed] [Google Scholar]
- 12. Brown NW, Gonde CE, Adams JE, Tredger JM. Low hematocrit and serum albumin concentrations underlie the overestimation of tacrolimus concentrations by microparticle enzyme immunoassay versus liquid chromatography-tandem mass spectrometry. Clin Chem. 2005; 51(3):586-92. [DOI] [PubMed] [Google Scholar]
- 13. Lower DR, Cropcho L, Rosendorff A. Comparison of CEDIA FK506 assay with HPLC/MS/MS in a large cohort of pediatric patients. Am J Clin Pathol. 2013; 139(6):788-92. [DOI] [PubMed] [Google Scholar]
- 14. Saitman A, Metushi IG, Mason DS, Fitzgerald RL. Evaluation of the Waters MassTrak LC-MS/MS assay for tacrolimus and a comparison to the Abbott Architect immunoassay. Ther Drug Monit. 2016; 38(3):300-4. [DOI] [PubMed] [Google Scholar]
- 15. Sallustio BC, Noll BD, Morris RG. Comparison of blood sirolimus, tacrolimus and everolimus concentrations measured by LC-MS/MS, HPLC-UV and immunoassay methods. Clin Biochem. 2011; 44(2-3): 231-6. [DOI] [PubMed] [Google Scholar]
- 16. Polledri E, Mercadante R, Ferraris Fusarini C et al. Immunosuppressive drugs in whole blood: validation of a commercially available liquid chromatography/tandem mass spectrometry kit and comparison with immunochemical assays. Rapid Commun Mass Spectrom. 2017; 31(13):1111-120. [DOI] [PubMed] [Google Scholar]
- 17. Salm P, Rutherford DM, Taylor PJ et al. Evaluation of microparticle enzyme immunoassay against HPLC-mass spectrometry for the determination of whole-blood tacrolimus in heart- and lung-transplant recipients. Clin Biochem. 2000; 33(7):557-62. [DOI] [PubMed] [Google Scholar]
- 18. Kolaitis NA, Calabrese D, Ahern P et al. Tacrolimus trough monitoring by mass spectroscopy is associated with acute kidney injury in lung transplant recipients. J Heart Lung Transpl. 2018; 37(4):S308-9. [Google Scholar]
- 19. Egan TM, Murray S, Bustami RT et al. Development of the new lung allocation system in the United States. Am J Transplant. 2006; 6(5, pt 2):1212-27. [DOI] [PubMed] [Google Scholar]
- 20. Levey AS, Stevens LA, Schmid CH et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009; 150(9):604-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2019. Diabetes Care. 2019; 42(suppl 1):S13-28. [DOI] [PubMed] [Google Scholar]
- 22. Calabrese DR, Florez R, Dewey K et al. Genotypes associated with tacrolimus pharmacokinetics impact clinical outcomes in lung transplant recipients. Clin Transplant. 2018; 32(8):e13332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gallagher HM, Sarwar G, Tse T et al. Erratic tacrolimus exposure, assessed using the standard deviation of trough blood levels, predicts chronic lung allograft dysfunction and survival. J Heart Lung Transplant. 2015; 34(11):1442-8. [DOI] [PubMed] [Google Scholar]
- 24. Bellomo R, Ronco C, Kellum JA et al. , for the Acute Dialysis Quality Initiative Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004; 8(4):R204-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mehta RL, Kellum JA, Shah SV et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007; 11(2):R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cooper JD, Billingham M, Egan T et al. A working formulation for the standardization of nomenclature and for clinical staging of chronic dysfunction in lung allografts. International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 1993; 12(5):713-6. [PubMed] [Google Scholar]
- 27. Albertus P, Morgenstern H, Robinson B, Saran R. Risk of ESRD in the United States. Am J Kidney Dis. 2016; 68(6):862-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mathioudakis NN, Giles M, Yeh HC et al. Racial differences in acute kidney injury of hospitalized adults with diabetes. J Diabetes Complications. 2016; 30(6):1129-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Valdez R, Yoon PW, Liu T, Khoury MJ. Family history and prevalence of diabetes in the U.S. population: the 6-year results from the National Health and Nutrition Examination Survey (1999-2004). Diabetes Care. 2007; 30(10):2517-22. [DOI] [PubMed] [Google Scholar]
- 30. Iwasaki K, Shiraga T, Nagase K et al. Isolation, identification, and biological activities of oxidative metabolites of FK506, a potent immunosuppressive macrolide lactone. Drug Metab Dispos. 1993; 21(6):971-7. [PubMed] [Google Scholar]
- 31. Iwasaki K. Metabolism of tacrolimus (FK506) and recent topics in clinical pharmacokinetics. Drug Metab Pharmacokinet. 2007; 22(5):328-35. [DOI] [PubMed] [Google Scholar]
- 32. Zegarska J, Hryniewiecka E, Zochowska D et al. Tacrolimus metabolite M-III may have nephrotoxic and myelotoxic effects and increase the incidence of infections in kidney transplant recipients. Transplant Proc. 2016; 48(5):1539-42. [DOI] [PubMed] [Google Scholar]
- 33. Dubbelboer IR, Pohanka A, Said R et al. Quantification of tacrolimus and three demethylated metabolites in human whole blood using LC-ESI-MS/MS. Ther Drug Monit. 2012; 34(2):134-42. [DOI] [PubMed] [Google Scholar]
- 34. Albright ES, Ovalle F, Bell DS. Artificially low hemoglobin A1c caused by use of dapsone. Endocr Pract. 2002; 8(5):370-2. [DOI] [PubMed] [Google Scholar]
- 35. Brandhorst G, Oellerich M, Maine G et al. Liquid chromatography-tandem mass spectrometry or automated immunoassays: what are the future trends in therapeutic drug monitoring? Clin Chem. 2012; 58(5):821-5. [DOI] [PubMed] [Google Scholar]